Abstract
Commercially available diagnostic tools for the detection of lactate dehydrogenase elevating virus (LDV) infection have been restricted to measurement of serum lactate dehydrogenase (LDH) activity levels and detection of the viral genome by RT-PCR assays. Serologic diagnosis of LDV infection has not been widely adopted due to the belief that the formation of antigen–antibody complexes and B-cell polyclonal activation may confound interpretation of results. In the current study, we inoculated BALB/c, C57BL/6, and Swiss Webster mice with LDV to compare the diagnostic reliability of a commercially available multiplex fluorescent immunoassay for the detection of antiLDV antibodies with that of the LDH enzyme assay. The serologic assay was vastly more sensitive and specific than was the LDH enzyme assay. Moreover, the serologic assay detected antiviral antibodies throughout the 3-mo time course of this study. These results suggest that antigen–antibody complex formation and polyclonal B-cell activation had little effect on assay performance.
Abbreviation: LDV, lactate dehydrogenase elevating virus; MFI, multiplex fluorescent immunoassay
Lactate dehydrogenase elevating virus (LDV) is an RNA virus within the family Arteriviridae that initially was identified as a transmissible contaminant of tumors in laboratory mice.41 Previous reports have demonstrated that after experimental infection, circulating virus levels peak by 12 to 24 h, stabilize by 1 to 2 wk after infection, and persist for the lifetime of the mouse.36,38-40 Although clinical disease is rare and only seen in immunocompromised strains, viral infection has many implications for biomedical research, including changes in tumor growth rate,3,26,31,49 differences in immunologic function,21,29,32,33,35,44,45 polyclonal B-cell activation,12 and altered serum enzyme levels.34,39
Laboratory mouse colonies are commonly screened for viral pathogens by serology, but commercial diagnostic methods for LDV have been limited to RT-PCR assays10,20,47,48 and measurement of serum lactate dehydrogenase (LDH) activity. Although RT-PCR assays are a very reliable diagnostic method, it is costly and time intensive. Historically, measurement of serum or plasma LDH activity has been used to detect infection, but at least one study has determined that this assay has poor sensitivity and specificity.48 LDV infection causes a 5- to 10-fold elevation of LDH within 3 d of exposure, and, due to decreased clearance rates, LDH levels can remain elevated for as long as 10 mo after exposure.1 Enzyme activity is assayed by the coupled reaction catalyzed by lactate dehydrogenase:
LDH activity (in units) is quantified as the change in optical density due to the oxidation of NADH or reduction of NAD+.18 When using LDH activity levels for diagnostic purposes, false positives may result from cellular damage and leakage of LDH due to repeated venipuncture16 or hemolysis.50 Differences in baseline plasma levels of LDH between sexes and ages of inbred mice may confound the interpretation of results also.19 One published diagnostic method, not currently commercially available, is the detection of viral particles in plasma by using a latex agglutination assay.29 However, this assay was used to detect acute LDV infection, and its utility for detecting chronic infection is unknown. In light of the weaknesses of the RT-PCR and LDH activity assays and the unavailability of the latex agglutination assay, we reexamined the suitability of a serologic assay for the detection of LDV.
Serologic diagnosis of LDV infection has not received wide acceptance due to potentially confounding polyclonal B-cell activation and antigen–antibody complex formation.17,37 Although polyclonal B-cell activation and antigen–antibody complex formation in LDV-infected mice are well described in the literature,12,13,35 their effect on antiLDV antibody detection is unclear. Immunocompetent mice mount a robust immune response, but only a small fraction of the antibodies formed are specific for LDV proteins.15 AntiLDV antibodies are directed toward 2 of the 3 structural proteins: the 14-kDa nucleocapsid protein and the 24- to 42-kDa glycosylated envelope protein.5,14 Questions about the sensitivity and specificity of antiLDV antibody detection arose when multiple studies demonstrated the presence of low-molecular–weight complexes in LDV immune serum that bound to ELISA plates not coated with antigen.6,25 These complexes are believed to comprise auto-antibodies and cellular proteins rather than antiLDV antibodies and LDV viral proteins.25,43 Application of a blocking agent to ELISA plates not coated with antigen reduced, but did not completely prevent, the binding of nonspecific antibodies to uncoated wells.25 This result suggests that nonspecific antibodies may interfere with the detection and quantification of antiLDV antibodies. However, to our knowledge, there have been no controlled studies to show that these nonspecific antibodies compromise the sensitivity or specificity of antiLDV antibody detection in murine serum samples. Contrary to the suggested interference of these low-molecular–weight complexes, many reports have shown that LDV-infected mice mount a demonstrable antibody response, as detected by ELISA and immunofluorescent assays, as early as 1 wk after infection and which remains elevated for at least 1 y.5,7,14,30,40 These methods are not routinely used in high-throughput screening laboratories because of the relative inefficiency of cultivating LDV: this virus does not replicate well in cell culture.46
The objective of the current study was to compare the diagnostic reliability of a commercially available multiplex fluorescent immunoassay (MFI) for the detection of antiLDV antibodies with that of the LDH activity assay in laboratory mice experimentally infected with LDV. To this end, we inoculated 2 inbred strains and a single outbred stock of laboratory mice with LDV or a sham inoculum and evaluated serum longitudinally over a 3-mo period for LDH enzyme activity, antiLDV antibodies by MFI, and LDV genomic DNA by RT-PCR assay. To confirm the presence or absence of viral genome in each mouse, spleen samples were evaluated by using an RT-PCR assay, the ‘gold standard.’ Our results demonstrate that the serologic diagnosis of LDV infection by using MFI was more sensitive and specific than was the LDH activity assay in female Swiss Webster, C57BL/6, and BALB/c mice at 2, 4, 8, and 12 wk after infection.
Materials and Methods
Animal husbandry.
Female Swiss Webster, C57BL/6, and BALB/c mice (age, 4 wk) were obtained from the Frederick Cancer Research and Development Center (Frederick, MD). One mouse per strain was used to confirm that study subjects were free from serum antibodies to mouse hepatitis virus, minute virus of mice, mouse parvovirus types 1 through 3, mouse norovirus, Theiler meningoencephalitis virus, epizootic diarrhea of infant mice, Sendai virus, Mycoplasma pulmonis, pneumonia virus of mice, reovirus 3, lactate dehydrogenase elevating virus, lymphocytic choriomeningitis virus, ectromelia virus, mouse adenovirus 1 and 2, and polyoma virus by serology performed at IDEXX RADIL (Columbia, MO).
Control and experimentally infected mice were housed separately in autoclaved individually ventilated cages (Thoren Caging Systems, Hazelton, PA) with soft-texture pelleted bedding (Paperchips, Shepherd Specialty Papers, Watertown, TN). Mice were provided with irradiated feed (5053 PicoLab Rodent Diet 20, PMI Nutrition International, Brentwood, MO) and acidified, autoclaved water ad libitum. All cage changes took place in a laminar flow hood. All animal procedures were performed in accordance with federal regulations and guidelines and were approved by the University of Missouri's IACUC.
Viral inoculation.
Aliquots of frozen heparinized plasma (15 U heparin per 1 mL whole blood) from LDV-infected mice during peak viremia (approximately 18 to 24 h after infection) were a gift from Kim J Hasenkrug (Rocky Mountain Laboratories, Hamilton, MT). Inoculum was prepared by diluting plasma 1:50 in PBS. Experimental mice were inoculated intraperitoneally with 0.25 mL diluted plasma. Control mice were given 0.25 mL sterile PBS intraperitoneally. Analysis of the diluted plasma inoculum by quantitative RT-PCR assay determined that each mouse was inoculated with approximately 3.9 × 105 viral copies.
Kinetics of seroconversion and determination of viral copy number in serum by quantitative RT-PCR assay.
Swiss Webster experimental and control groups (n = 8) were euthanized by CO2 inhalation at 2, 4, 8, and 12 wk after infection. Blood samples were collected by cardiocentesis, diluted 1:2 in normal saline, allowed to clot for 1 h at 4 °C, and centrifuged at 4000 × g for 6 min. Serum samples were submitted to IDEXX RADIL for MFI and LDH activity testing. MFI detected antibodies against the LDV viral protein encoded by ORF7;14 MFI fluorescence values greater than 2500 were considered positive for antiLDV antibody. Each serum sample also was evaluated for reactivity to nonantigen proteins by testing serum samples for reactivity against lysates from A9 (mouse fibroblast) and BHK (baby hamster kidney) cell lines. LDH enzyme activity levels greater than 2500 IU/L were considered positive for LDV.
To confirm infection status, the spleen was collected aseptically, snap-frozen, stored at −80 °C until use, and submitted for LDV RT-PCR assay (IDEXX RADIL). RT-PCR primers were designed to amplify a portion of ORF7 of the LDV genome. Aliquots (140 μL) of diluted serum were stored at −80 °C until processing for LDV quantitative RT-PCR.
Assessment of seroconversion in inbred mouse strains.
Blood samples (approximately 20 μL) for MFI were collected from the lateral saphenous vein of LDV- and sham-inoculated BALB/c and C57BL/6 mice (n = 8 each group) at 2, 4, and 8 wk after infection. Antemortem samples were submitted for detection of LDV antibodies by MFI. All mice were euthanized at 12 wk after inoculation. Blood samples were collected and submitted as described earlier for MFI and LDH activity testing. Spleen samples were collected as described earlier and submitted for LDV RT-PCR analysis.
Statistical analysis.
Viral copy number at 2, 4, 8, and 12 wk infection was compared in Swiss Webster mice experimentally inoculated with LDV to determine whether significant differences were present between groups. A logarithmic (base 2) transformation was applied to normalize the data set. The data were analyzed by using one-way ANOVA and Student–Newman–Keuls posttests.
Differences in reactivity to nonantigen proteins in Swiss Webster mice were compared with consideration to 2 factors: between treatment groups (sham-inoculated compared with experimentally inoculated) and over time (2, 4, 8, and 12 wk). The data were analyzed by using 2-way ANOVA and Student–Newman–Keuls posttests. Differences were considered statistically significant when the P value was less than 0.05.
κ statistics were used to determine the degree of correlation between the results of the MFI and LDH assays. All statistical analyses were performed by using SigmaPlot 11.0 (Systat Software, San Jose, CA).
Results
Kinetics of seroconversion.
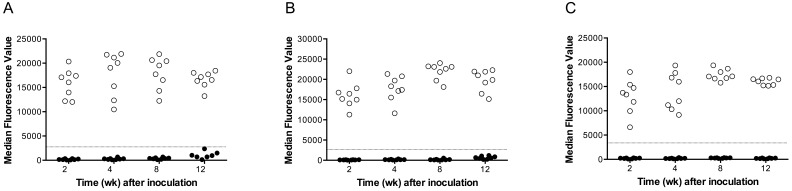
To determine when seroconversion occurs in LDV-infected mice and whether this result can be used to detect LDV infection, serum samples were collected from Swiss Webster mice at 2, 4, 8, and 12 wk after infection. Viral genome was detected by RT-PCR in the spleen of all experimental mice and none of the control mice. The MFI had 100% specificity and sensitivity at 2, 4, 8, and 12 wk after infection (Table 1, Figure 1). However, the LDH enzyme assay had 25% sensitivity at 2 wk, 50% sensitivity at 4 wk, 25% sensitivity at 8 wk, and 63% sensitivity at 12 wk after infection (Table 1). The LDH activity assay showed 100% specificity.
Table 1.
Kinetics of seroconversion for LDV infection in Swiss Webster female mice
| LDH enzyme assay |
||||
| Time (wk) after inoculation | Group | No. positivea | No. of IU/Lb | No. positive by MFIa |
| 2 | ||||
| Sham | 0 | 788 ± 421 | 0 | |
| LDV | 2 | 2188 ± 310 | 8 | |
| 4 | ||||
| Sham | 0 | 1089 ± 594 | 0 | |
| LDV | 4 | 2569 ± 653 | 8 | |
| 8 | ||||
| Sham | 0 | 700 ± 127 | 0 | |
| LDV | 2 | 2086 ± 443 | 8 | |
| 12 | ||||
| Sham | 0 | 1006 ± 333 | 0 | |
| LDV | 5 | 3283 ± 1060 | 8 | |
8 mice tested per group. Results were considered positive for LDV if serum LDH activity exceeded 2500 IU/L.
Values reported as mean ± 1 SD.
Figure 1.
Distribution of LDV MFI median fluorescence values of serum samples (n = 8 per group) from sham (filled circles) and LDV (open circles) -inoculated (A) Swiss Webster, (B) C57BL/6, and (C) BALB/c female mice at 2, 4, 8, and 12 wk after infection. MFI fluorescence values greater than 2500 were considered positive for antiLDV antibody.
An additional experiment was performed to determine whether dilution of whole-blood samples affected LDH activity assay results. To this end, Swiss Webster control (n = 4) and LDV-inoculated (n = 16) mice were euthanized at 2 wk after inoculation. Whole blood collected from each mouse was separated into 2 samples, one undiluted and the other diluted 1:2 in normal saline. Samples were allowed to clot for 2 h at room temperature and centrifuged, and serum was submitted for LDH enzyme activity testing. The LDH enzyme assay had 56% and 31% sensitivity in the diluted and undiluted samples, respectively. Further, the Cohen κ statistic showed strong agreement between sampling techniques (κ, 0.68; SE, 0.22).
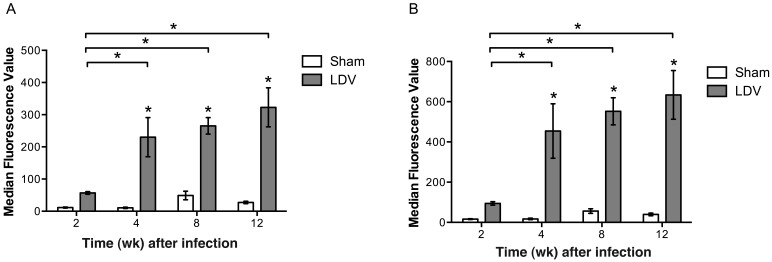
Serum samples from LDV-infected animals demonstrated significantly (P < 0.05) increased reactivity to nonantigen proteins, ranging from 5- to 20-fold increases when comparing sham with LDV-infected mice at 4, 8, and 12 wk after infection (Figure 2). LDV-infected mice showed significant (P < 0.05) 4- to 6-fold increases in protein reactivity between the 2-wk time point and each later point (Figure 2).
Figure 2.
Comparison of nonspecific MFI reactivity (mean ± SE; n = 8 per group) to (A) A9 and (B) BHK cell lysates in sham and LDV-infected serum samples. Nonspecific reactivity was significantly (*, P < 0.05; 2-way ANOVA, Student–Newman–Keuls) increased in LDV-infected samples compared with sham samples at 4, 8, and 12 wk after inoculation. Nonspecific reactivity among LDV-infected samples was significantly (*, P < 0.05; 2-way ANOVA, Student–Newman–Keuls) different between the 2-wk time point and each subsequent time point.
Determination of viral copy number in serum by quantitative RT-PCR.
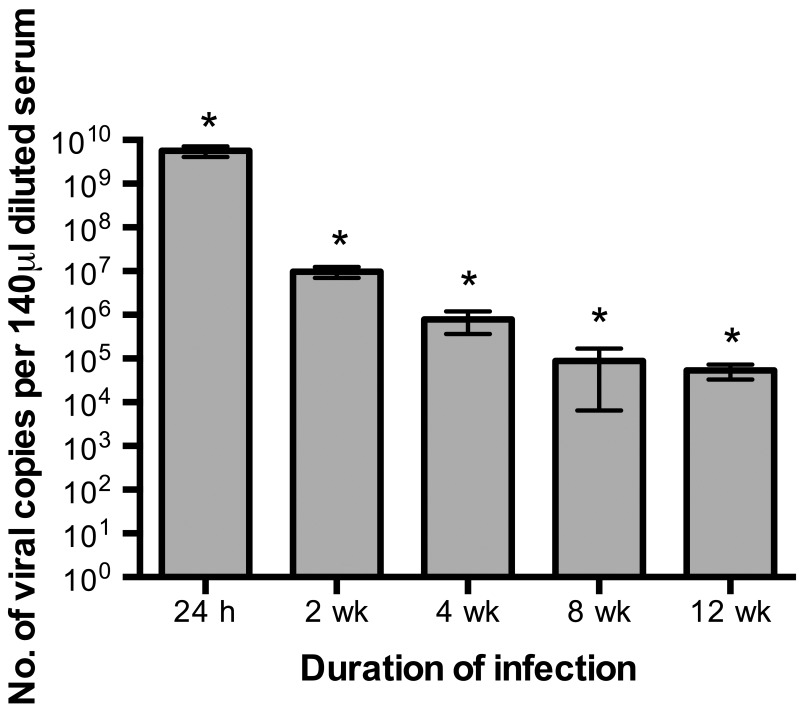
Quantitative RT-PCR assays were performed on serum samples from Swiss Webster mice to determine whether variability in levels of circulating virus contributed to inconsistent results from the LDH enzyme assay (Figure 3). As described in the literature,34 peak viremia occurred at the 24-h time point, and circulating virus levels were significantly (P < 0.05) lower at each time point thereafter. The magnitude of viremia in LDV-infected mice did not appear to affect the ability of the LDH enzyme assay to detect infection (Table 1). For example, although more virus was present at 2 wk than 8 wk after infection, the LDH enzyme assay had the sensitivity (25%) at these 2 time points. At 12 wk, when the lowest level of circulating virus occurred, the sensitivity of the LDH enzyme assay was highest (63%). These results suggest that sensitivity of the LDH activity assay is not dependent on high viral copy numbers in serum.
Figure 3.
Determination of viral copy number (mean ± SE.; n = 8 for all groups except 24 h [n = 10]) in serum of LDV-inoculated Swiss Webster mice at 24 h and 2,4,8, and 12 wk after inoculation by quantitative RT-PCR assay. Viral copy number was significantly (*, P < 0.05; one-way ANOVA, Student–Newman–Keuls) different at each time point.
Assessment of seroconversion in inbred mouse strains.
To determine whether seroconversion can be used to detect LDV infection in inbred mouse strains with disparate immunologic responses, serum samples were collected from C57BL/6 and BALB/c mice at 2, 4, 8, and 12 wk after infection. Viral genome was detected by RT-PCR in the spleens of all experimental mice, and all control mice tested negative. Antemortem serum samples collected at 2, 4, and 8 wk after infection were used only to test for antiLDV antibodies. At all 3 time points, MFI identified infected C57BL/6 and BALB/c mice but showed no reactivity for control mice. Serum samples collected at 12 wk after infection were tested by MFI for antiLDV antibodies and by the LDH activity assay. The MFI had 100% sensitivity and specificity; and the assay was able to detect infected C57BL/6 and BALB/c mice at 12 wk postinfection, but showed no reactivity for control mice (Table 2). In comparison, the LDH enzyme assay had 13% sensitivity in BALB/c mice and 50% sensitivity in C57BL/6 mice at the 12-wk time point (Table 2). The LDH enzyme assay had 100% specificity at the 12-wk time point in both inbred strains.
Table 2.
Comparison of LDV diagnostic assays in 2 inbred mouse strains at 3 mo after inoculation
| LDH enzyme assay |
||||
| Strain | Group | No. positivea | No. of IU/Lb | No. positive by MFI |
| BALB/c | ||||
| Sham | 0 | 954 ± 97 | 0 | |
| LDV | 1 | 1746 ± 544 | 8 | |
| C57BL/6 | ||||
| Sham | 0 | 813 ± 146 | 0 | |
| LDV | 4 | 2650 ± 642 | 8 | |
8 mice tested per group. Results were considered positive for LDV if serum LDH activity exceeded 2500 IU/L.
Values reported as mean ± 1 SD.
Degree of correlation between LDH enzyme assay and MFI results.
Analysis of the results from the MFI and LDH enzyme assays yielded a κ value of 0.23, indicating fair to poor agreement between these 2 diagnostic tests.
Discussion
Evaluation of serum for the presence of antiviral antibodies has not been used widely for diagnosis of LDV infection. Anecdotal evidence suggested that the formation of antigen–antibody complexes and B-cell polyclonal activation might confound the interpretation of serologic data. In the current study, a commercially available MFI correctly identified LDV- and sham-infected mice at multiple time points during infection in 2 inbred strains and an outbred stock. Although screening laboratory mouse colonies for antiLDV antibodies has not been performed routinely in light of reported interference from antigen–antibody complexes and B-lymphocyte polyclonal activation, our results show that these potential confounders had no effect on MFI performance.
One potential mechanism through which antigen–antibody complexes might decrease the sensitivity of antiLDV antibody detection is consumption of virus-specific antibodies. We speculated that this effect, if present, would be exacerbated during early infection, when viral levels are highest (Figure 3) and antiLDV antibodies are just beginning to rise.8 Instead, MFI sensitivity was uniformly 100% at every time point examined (Table 1). These results suggest that consumption of viral-specific antibodies did not affect the ability of the MFI to detect infected mice during our study.
Another potential mechanism to decrease the specificity of antiLDV antibody detection is the binding of nonspecific antibodies to the MFI antigen as a consequence of polyclonal B-cell activation. In LDV-infected mice, total plasma IgG steadily increases for as long as 1 y after infection even though antiLDV antibody production plateaus at 2 mo postinfection,8 suggesting that nonspecific antibodies are being produced also. Ultimately, MFI correctly identified all uninfected and LDV-infected mice. Compared with those from sham-inoculated mice, serum samples from LDV-infected mice demonstrated increased reactivity to nonantigen proteins at 4, 8, and 12 wk after infection (Figure 2). In addition, among LDV-infected mice, reactivity to nonLDV antigens was increased after 2 wk of infection (Figure 2). These observations are consistent with B-cell polyclonal activation. Nonspecific reactivity in the LDV-infected serum samples had no effect on the ability of the serologic MFI assay to detect the presence of antiLDV antibodies.
To further challenge the robustness of the MFI, we tested samples from 2 inbred mouse strains with known disparate immunologic responses. When challenged with an infectious agent, T lymphocytes from C57BL/6 mice are more likely to mount a TH1 response, characterized by proinflammatory cytokines such as IFNγ.23 This cytokine activates macrophages, which are essential for elimination of intracellular pathogens. During infection, LDV replicates within macrophages.9,42,46 Although IFNγ generally is believed to inhibit humoral immunity, experimental LDV infections in IFNγ-deficient mice with a B6 background produced no significant differences in viremia or total antiLDV IgG as compared with those of LDV-infected wildtype B6 mice.4 Results from the same study showed that IFNγ deficient mice produced fewer low-molecular–weight (nonspecific) antigen–antibody complexes, indicating at least a contributory role for IFNγ in the production of these nonspecific antibodies.4 In contrast, T lymphocytes from BALB/c mice are more likely to produce a TH2 response, characterized by the cytokines IL4 and IL5, which stimulate humoral immunity, which is thought to be crucial in the elimination of extracellular pathogens.11 Given this information, we suspected that both B6 mice and BALB/c mice might demonstrate nonspecific reactivity in the LDV MFI. Despite this concern, the MFI correctly identified LDV-infected inbred mice and showed no reactivity toward control mice at each time point.
Unlike antibody detection, the LDH enzyme assay had low sensitivity. Given the widespread use of this assay in experimental studies, these results were unexpected. Although LDH levels were at least 2 times higher in LDV-infected mice than control mice (Tables 1 and 2), the differences between groups were not as pronounced as those seen with the MFI (Figure 1). One potential reason for the absence of elevated LDH activity levels beyond the threshold is variability in circulating viral load at the various time points examined (Tables 1 and 2, Figure 3). However, the LDH enzyme assay showed equivalent sensitivity (25%) at the 2- and 8-wk time points, despite a significantly decreased serum viral level at 8 wk after infection. Ultimately, the sensitivity of the LDH activity assay remained poor regardless of serum viral copy number. Interstrain variability of baseline LDH19 and LDH elevation in response to LDV infection22 have been reported. At least one other study has reported low sensitivity for the LDH activity assay after 3 wk of experimental infection in C3H/HeN mice.48 In summary, the LDH enzyme assay may have limited value for the detection of LDV infection in laboratory mice.
An additional finding from our current experiments was that serum can be used as a diagnostic specimen for LDV RT-PCR assays. Previous reports have suggested that inhibitory factors present in serum lead to false-negative results.28 However, we did not encounter this problem. Using serum as a diagnostic specimen for LDV RT-PCR may prove to be a useful antemortem diagnostic test for immunocompromised mice or as a secondary testing method for serum samples that demonstrate nonspecific reactivity.
The serological MFI is a new diagnostic method for LDV infection. Multiple reports have demonstrated that using MFI has several advantages over ELISA, including equal or greater sensitivity and specificity, use of a smaller volume of sample, and the ability to detect antibodies against multiple infectious agents in a single reaction well.2,24,27 For these reasons, MFI will be extremely useful for direct testing of experimental mice, given that small volume blood samples can be collected for antemortem diagnosis. However, because LDV is transmitted primarily through experimental procedures or fight wounds, the LDV MFI is not likely to be useful for assessing colony health status from dirty-bedding sentinels. Another potential scenario in which the MFI may lack utility is during the acute period between infection and seroconversion. During the experimental inoculations, seroconversion occurred during the first 2 wk of infection. How long seroconversion can reliably be detected may differ depending on the inoculating dose of virus, viral strain, and immune status of the host. In acutely infected mice, RT-PCR may still be the best diagnostic method. Given the ubiquitous use of biologic materials in mice used for biomedical research, the LDV MFI offers laboratory animal veterinarians an improved serologic diagnostic tool for the detection of LDV infection.
Acknowledgment
Matthew H Myles is employed by IDEXX RADIL, an organization with direct commercial interest in this subject matter.
We thank Jason Evans and Giedre Turner for their excellent technical assistance. This work was supported by residency funds from the Department of Veterinary Pathobiology, by the University of Missouri College of Veterinary Medicine, by IDEXX RADIL, and by a Mission Enhancement grant from the state of Missouri.
References
- 1.Bailey JM, Clough J, Stearman M. 1964. Clearance of plasma enzymes in normal and LDH-agent–infected mice. Proc Soc Exp Biol Med 117:350–354 [DOI] [PubMed] [Google Scholar]
- 2.Biagini RE, Sammons DL, Smith JP, MacKenzie BA, Striley CA, Semenova V, Steward-Clark E, Stamey K, Freeman AE, Quinn CP, Snawder JE. 2004. Comparison of a multiplexed fluorescent covalent microsphere immunoassay and an enzyme-linked immunosorbent assay for measurement of human immunoglobulin G antibodies to anthrax toxins. Clin Diagn Lab Immunol 11:50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinton-Darnell M, Brand I. 1977. Delayed foreign-body tumorigenesis in mice infected with lactate dehydrogenase elevating virus: brief communication. J Natl Cancer Inst 59:1027–1029 [DOI] [PubMed] [Google Scholar]
- 4.Cafruny WA, Bradley SE, Rowland RR. 1999. Regulation of immune complexes during infection of mice with lactate dehydrogenase elevating virus: studies with interferon-γ gene knockout and tolerant mice. Viral Immunol 12:163–173 [DOI] [PubMed] [Google Scholar]
- 5.Cafruny WA, Chan SP, Harty JT, Yousefi S, Kowalchyk K, McDonald D, Foreman B, Budweg G, Plagemann PG. 1986. Antibody response of mice to lactate dehydrogenase elevating virus during infection and immunization with inactivated virus. Virus Res 5:357–375 [DOI] [PubMed] [Google Scholar]
- 6.Cafruny WA, Heruth DP, Jaqua MJ, Plagemann PG. 1986. Immunoglobulins that bind to uncoated ELISA plate surfaces: appearance in mice during infection with lactate dehydrogenase elevating virus and in human antinuclear-antibody–positive sera. J Med Virol 19:175–186 [DOI] [PubMed] [Google Scholar]
- 7.Cafruny WA, Plagemann PG. 1982. Immune response to lactate dehydrogenase elevating virus: isolation of infectious virus–immunoglobulin G complexes and quantitation of specific antiviral immunoglobulin G response in wild-type and nude mice. Infect Immun 37:1001–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cafruny WA, Plagemann PG. 1982. Immune response to lactate dehydrogenase elevating virus: serologically specific rabbit neutralizing antibody to the virus. Infect Immun 37:1007–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan SP, Onyekaba CO, Harty JT, Plagemann PG. 1989. Persistent infection of mice by lactate dehydrogenase elevating virus: transient virus replication in macrophages of the spleen. Virus Res 14:317–326 [DOI] [PubMed] [Google Scholar]
- 10.Chen Z, Plagemann PG. 1997. Detection of lactate dehydrogenase elevating virus in transplantable mouse tumors by biological assay and RT-PCR assays and its removal from the tumor cell. J Virol Methods 65:227–236 [DOI] [PubMed] [Google Scholar]
- 11.Corry DB, Folkesson HG, Warnock ML, Erle DJ, Matthay MA, Wiener-Kronish JP, Locksley RM. 1996. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med 183:109–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coutelier JP, Coulie PG, Wauters P, Heremans H, van der Logt JT. 1990. In vivo polyclonal B-lymphocyte activation elicited by murine viruses. J Virol 64:5383–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coutelier JP, van der Logt JT, Heessen FW, Vink A, van Snick J. 1988. Virally induced modulation of murine IgG antibody subclasses. J Exp Med 168:2373–2378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coutelier JP, Van Roost E, Lambotte P, Van Snick J. 1986. The murine antibody response to lactate dehydrogenase elevating virus. J Gen Virol 67:1099–1108 [DOI] [PubMed] [Google Scholar]
- 15.Coutelier JP, Van Snick J. 1985. Isotypically restricted activation of B lymphocytes by lactic dehydrogenase virus. Eur J Immunol 15:250–255 [DOI] [PubMed] [Google Scholar]
- 16.Dillberger JE, Monroy P, Altman NH. 1987. The effect of 3 bleeding techniques on lactic dehydrogenase levels in mice: implications for lactic dehydrogenase virus bioassay. Lab Anim Sci 37:356–359 [PubMed] [Google Scholar]
- 17.Fox J, Anderson LC, Loew FM, Quimby FW. 2002. Laboratory animal medicine, 2nd ed. San Diego (CA): Academic Press. [Google Scholar]
- 18.Fox J, Barthold SW, Davisson MT, Newcomer CE, Quimby FW, Smith AL. 2007. The Mouse in biomedical research: diseases. New York (NY): Elsevier. [Google Scholar]
- 19.Frith CH, Suber RL, Umholtz R. 1980. Hematologic and clinical chemistry findings in control BALB/c and C57BL/6 mice. Lab Anim Sci 30:835–840 [PubMed] [Google Scholar]
- 20.Goto K, Takakura A, Yoshimura M, Ohnishi Y, Itoh T. 1998. Detection and typing of lactate dehydrogenase elevating virus RNA from transplantable tumors, mouse liver tissues, and cell lines, using PCR. Lab Anim Sci 48:99–102 [PubMed] [Google Scholar]
- 21.Hayashi T, Iwata H, Hasegawa T, Ozaki M, Yamamoto H, Onodera T. 1991. Decrease in neutrophil migration induced by endotoxin and suppression of interleukin 1 production by macrophages in lactic dehydrogenase virus-infected mice. J Comp Pathol 104:161–170 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi T, Ozaki M, Mori I, Saito M, Itoh T, Yamamoto H. 1992. Enhanced clearance of lactic dehydrogenase 5 in severe combined immunodeficiency (SCID) mice: effect of lactic dehydrogenase virus on enzyme clearance. Int J Exp Pathol 73:173–181 [PMC free article] [PubMed] [Google Scholar]
- 23.Heinzel FP, Sadick MD, Holaday BJ, Coffman RL, Locksley RM. 1989. Reciprocal expression of interferon gamma or interleukin 4 during the resolution or progression of murine leishmaniasis. Evidence for expansion of distinct helper T cell subsets. J Exp Med 169:59–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu CC, Franklin C, Riley LK. 2007. Multiplex fluorescent immunoassay for the simultaneous detection of serum antibodies to multiple rodent pathogens. Lab Anim (NY) 36:36–38 [DOI] [PubMed] [Google Scholar]
- 25.Hu B, Even C, Plagemann PG. 1992. Immune complexes that bind to ELISA plates not coated with antigen in mice infected with lactate dehydrogenase elevating virus: relationship to IgG2a- and IgG2b-specific polyclonal activation of B cells. Viral Immunol 5:27–38 [DOI] [PubMed] [Google Scholar]
- 26.Isakov N, Feldman M, Segal S. 1981. Effect of lactic dehydrogenase virus infection on tumor induction and tumor growth. Cancer Res 41:667–672 [PubMed] [Google Scholar]
- 27.Khan IH, Kendall LV, Ziman M, Wong S, Mendoza S, Fahey J, Griffey SM, Barthold SW, Luciw PA. 2005. Simultaneous serodetection of 10 highly prevalent mouse infectious pathogens in a single reaction by multiplex analysis. Clin Diagn Lab Immunol 12:513–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipman NS, Henderson K, Shek W. 2000. False-negative results using RT-PCR for detection of lactate dehydrogenase elevating virus in a tumor cell line. Comp Med 50:255–256 [PubMed] [Google Scholar]
- 29.Markine-Goriaynoff D, Hulhoven X, Cambiaso CL, Monteyne P, Briet T, Gonzalez MD, Coulie P, Coutelier JP. 2002. Natural killer cell activation after infection with lactate dehydrogenase elevating virus. J Gen Virol 83:2709–2716 [DOI] [PubMed] [Google Scholar]
- 30.McDonald TL, Donnelly T, Weber A, Quenette L. 1983. Antibody classes and subclasses in circulating immune complexes isolated from mice infected with lactic dehydrogenase virus. Immunology 48:511–517 [PMC free article] [PubMed] [Google Scholar]
- 31.Michaelides MC, Schlesinger S. 1974. Effect of acute or chronic infection with lactic dehydrogenase virus (LDV) on the susceptibility of mice to plasmacytoma MOPC-315. J Immunol 112:1560–1564 [PubMed] [Google Scholar]
- 32.Monteyne P, Van Broeck J, Van Snick J, Coutelier JP. 1993. Inhibition by lactate dehydrogenase-elevating virus of in vivo interleukin 4 production during immunization with keyhole limpet haemocyanin. Cytokine 5:394–397 [DOI] [PubMed] [Google Scholar]
- 33.Morimoto M, Iwata H, Hayashi T. 1999. Lactic dehydrogenase virus infection inhibits allergic eosinophil reaction and IL5 gene expression in vivo. Int Arch Allergy Immunol 120:78–84 [DOI] [PubMed] [Google Scholar]
- 34.Notkins AL. 1971. Enzymatic and immunologic alterations in mice infected with lactic dehydrogenase virus. Am J Pathol 64:733–746 [PMC free article] [PubMed] [Google Scholar]
- 35.Notkins AL, Mergenhagen SE, Rizzo AA, Scheele C, Waldmann TA. 1966. Elevated γ-globulin and increased antibody production in mice infected with lactic dehydrogenase virus. J Exp Med 123:347–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Notkins AL, Shochat SJ. 1963. Studies on the multiplication and the properties of the lactic dehydrogenase agent. J Exp Med 117:735–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Percy D, Barthold SW. 2007. Pathology of laboratory rodents and rabbits, 3rd ed. Ames (IA): Blackwell Publishing Professional. [Google Scholar]
- 38.Plagemann PG, Gregory KF, Swim HE, Chan KKW. 1963. Plasma lactic dehydrogenase elevating agent of mice: distribution in tissues and effect on lactic dehydrogenase isozyme patterns. Can J Microbiol 9:75–86 [Google Scholar]
- 39.Plagemann PG, Watanabe M, Swim HE. 1962. Plasma lactic dehydrogenase elevating agent of mice: effect on levels of additional enzymes. Proc Soc Exp Biol Med 111:749–754 [DOI] [PubMed] [Google Scholar]
- 40.Porter DD, Porter HG, Deerhake BB. 1969. Immunofluorescence assay for antigen and antibody in lactic dehydrogenase virus infection of mice. J Immunol 102:431–436 [PubMed] [Google Scholar]
- 41.Riley V, Lilly F, Huerto E, Bardell D. 1960. Transmissible agent associated with 26 types of experimental mouse neoplasms. Science 132:545–547 [DOI] [PubMed] [Google Scholar]
- 42.Ritzi DM, Holth M, Smith MS, Swart WJ, Cafruny WA, Plagemann GW, Stueckemann JA. 1982. Replication of lactate dehydrogenase elevating virus in macrophages. 1. Evidence for cytocidal replication. J Gen Virol 59:245–262 [DOI] [PubMed] [Google Scholar]
- 43.Rowland RR, Even C, Anderson GW, Chen Z, Hu B, Plagemann PG. 1994. Neonatal infection of mice with lactate dehydrogenase elevating virus results in suppression of humoral antiviral immune response but does not alter the course of viraemia or the polyclonal activation of B cells and immune complex formation. J Gen Virol 75:1071–1081 [DOI] [PubMed] [Google Scholar]
- 44.Snodgrass MJ, Lowrey DS, Hanna MG., Jr 1972. Changes induced by lactic dehydrogenase virus in thymus and thymus-dependent areas of lymphatic tissue. J Immunol 108:877–892 [PubMed] [Google Scholar]
- 45.Stevenson MM, Rees JC, Meltzer MS. 1980. Macrophage function in tumor-bearing mice: evidence for lactic dehydrogenase-elevating virus-associated changes. J Immunol 124:2892–2899 [PubMed] [Google Scholar]
- 46.Stueckemann JA, Holth M, Swart WJ, Kowalchyk K, Smith MS, Wolstenholme AJ, Cafruny WA, Plagemann PG. 1982. Replication of lactate dehydrogenase elevating virus in macrophages. 2. Mechanism of persistent infection in mice and cell culture. J Gen Virol 59:263–272 [DOI] [PubMed] [Google Scholar]
- 47.van der Logt JT, Kissing J, Melchers WJ. 1994. Enzymatic amplification of lactate dehydrogenase elevating virus. J Clin Microbiol 32:2003–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner AM, Loganbill JK, Besselsen DG. 2004. Detection of lactate dehydrogenase elevating virus by use of a fluorogenic nuclease RT-PCR assay. Comp Med 54:288–292 [PubMed] [Google Scholar]
- 49.Weiland E, Grossmann A, Thiel HJ, Weiland F. 1990. Lactate dehydrogenase elevating virus induces antibodies reactive with a surface antigen of aetiologically unrelated murine cell transformants. J Gen Virol 71:1233–1236 [DOI] [PubMed] [Google Scholar]
- 50.Yucel D, Dalva K. 1992. Effect of in vitro hemolysis on 25 common biochemical tests. Clin Chem 38:575–577 [PubMed] [Google Scholar]