Abstract
Management of pain in research swine used for studies involving painful procedures is a considerable challenge. Here we assessed whether a regional anesthesia method is effective for pain control of hindlimb injuries in pigs used for research in bone fracture healing. For this randomized controlled study, we administered regional anesthesia before an experimental femoral injury was produced. Using ultrasound guidance, we placed sterile infusion catheters near the sciatic and femoral nerves and administered local anesthetic (bupivacaine) for the first 24 h after surgery. We evaluated various behavioral and physiologic parameters to test the hypothesis that this regional anesthesia would provide superior analgesia compared with systemic analgesia alone. We also collected blood samples to evaluate serum levels of cortisol and fentanyl postoperatively. At the end of the study period, we collected sciatic and femoral nerves and surrounding soft tissues for histopathologic evaluation. Treatment pigs had lower subjective pain scores than did control animals. Control pigs had a longer time to first feed consumption and required additional analgesia earlier in the postoperative period than did treatment pigs. Ultrasound-guided regional anesthesia is a viable and effective adjunct to systemic analgesics for providing pain control in swine with experimental femoral fractures.
Abbreviation: VAS, visual analog scale
Experimentally induced injuries in research animals may cause pain that requires the use of analgesia to ensure the welfare of the animal and to avoid the profound physiologic effects of unalleviated pain that can confound research. Our institution used swine for an induced femoral fracture model to study bone healing. Because of the potentially painful nature of such injuries and the extended follow-up required, pain control was a considerable concern.
Pain in research swine is often alleviated by using systemic opioids. The most common opioid used in research swine is buprenorphine, a centrally acting mixed opioid receptor agonist–antagonist that derives most of its analgesic effect from its partial agonistic activity at the µ opioid receptor.25 However, the multiple parenteral injections that are necessary over the course of a day to achieve continuous analgesia can be distressing to pigs. Another opioid analgesic that has been used in swine is fentanyl, a centrally acting pure µ opioid receptor agonist, which can be administered transdermally. However, transdermal fentanyl absorption in the pig is highly variable and may not reliably achieve effective levels in the blood.32,50 NSAID, such as meloxicam, act by inhibiting the cyclooxygenase enzymes, which are key components of inflammatory pain pathways.25 These drugs can be delivered parenterally or orally. However, oral analgesics may be difficult to deliver reliably in swine, and NSAID alone may not provide sufficient analgesia for moderate to severe pain.25 Furthermore, in addition to their analgesic and antiinflammatory effects, NSAID can affect renal function, platelet function, and the integrity of gastrointestinal mucosa, especially at higher doses and with long-term administration.25 Because their antiinflammatory and other effects may potentially alter research outcomes, NSAID analgesics are often contraindicated in animal research.
An alternative option for providing effective analgesia that can be administered with minimal stress to the animal is perineural infusion of a local anesthetic. Local anesthetics such as lidocaine or bupivacaine produce anesthesia by blocking the function of voltage-gated sodium channels in the neuronal cell membrane, thus inhibiting neuronal signal conduction.25 In this way, they can produce different degrees of anesthesia, analgesia, and motor blockade in a localized area, with minimal systemic effects. Perineural catheters allow administration of regional anesthesia over extended periods of time with minimal stress to the patient. These methods have seen much use in human patients and constitute an effective option for postoperative pain control.46
Successful delivery of regional anesthetic treatments can be facilitated by the use of ultrasound imaging. Regional anesthesia and ultrasound-guided regional anesthesia have been described extensively in the biomedical literature, and many reviews of these techniques are available.16,18,46,51,59,63-65 Ultrasound-guided methods of regional anesthesia have demonstrated distinct advantages over methods that involve blind injection or nerve stimulation. Reported advantages include increased success of the nerve block, use of less local anesthetic, faster procedure time, and faster onset.18,51,59
Regional anesthesia is commonly used for surgical procedures in veterinary patients.20,21,49,53,68 Continuous or intermittent infusion of local anesthetics for postoperative or persistent pain in animals has also been reported.19,69,71 Techniques for preprocedural single injection ultrasound-guided and nerve-stimulation–assisted regional anesthesia in dogs have been described.5,9,15,23 Swine have been used as a model for evaluating complications of regional anesthesia, such as intraneural injection and systemic and localized toxicity due to local anesthetics.1,10,12,48,72,73 However, there are no published studies in the literature on the use of ultrasonography for placement of peripheral perineural infusion catheters for treatment of postoperative pain in animals.
Anesthesia-related studies in swine are scarce. Peripheral nerve regional anesthesia with or without ultrasound guidance for analgesia of painful injuries in research pigs has not been reported. A few clinical trials in the literature describe pain assessment and control in pigs.26 This dearth of information is problematic given the common use of pigs as research models involving surgical procedures and the standards and legal requirements for alleviation of pain in laboratory animals. The development of advanced anesthetic techniques, such as the method we present here, can support advances in medical research and satisfy accepted animal welfare standards.
All pigs in this study received systemic opioids, including parenteral buprenorphine and transdermal fentanyl, and NSAID for analgesia. However, because of the nature of the injury, we recognized regional anesthesia as a viable option for additional anesthesia. We tested the hypothesis that ultrasound-guided regional anesthesia as an adjunct to systemic analgesics would reduce the need for systemic analgesics and improve subjective pain scores compared with systemic analgesics alone for postoperative management of painful femur fractures in research swine.
Materials and Methods
Animals.
This study was reviewed and approved by the Uniformed Services University of the Health Sciences IACUC and was performed in accordance with the Animal Welfare Act3 and the Guide for the Care and Use of Laboratory Animals.36 Our study evaluated 19 female Yorkshire swine (Sus scrofa domestica) that were being used in a study on bone healing. At time of surgery, median age was 109 d (range, 95 to 134 d), and mean weight was 38.3 kg (range, 33.0 to 45.5 kg). The pigs were obtained from an approved commercial source (Animal Biotech Industries, Danboro, PA) from a herd that is free of major pathogens. Pigs were received from the supplier in pairs and underwent physical examination by a veterinarian on their arrival at the facility.
Housing and husbandry.
Study animals were housed individually in pens equipped with automatic watering systems and were fed a standard swine diet (Teklad Miniswine Diet 8753, Harlan, Madison, WI) ad libitum. Primary enclosures were cleaned and sanitized twice daily by animal care staff. Each pig was allowed a minimum of 5 d to acclimate to the facility before use in the study.
Anesthesia and monitoring.
For analgesia, all pigs received a transdermal fentanyl patch (50 µg/h, Watson Laboratories, Corona, CA) approximately 1 d prior to surgery (mean, 21.3 h; 1 SD, 3.1 h). Hair was removed from the skin over the dorsal trunk by using a commercial depilatory agent (Nair, Church and Dwight, Princeton, NH). The site then was rinsed, dried, and wiped with isopropyl alcohol before the fentanyl patch was placed and a transparent film dressing (Tegaderm Film, 3M, St Paul, MN) was applied over the patch. On the day of surgery and before anesthesia, all pigs were premedicated with tiletamine–zolazepam (6 mg/kg IM; Telazol, Fort Dodge Animal Health, Overland Park, KS) in the neck. They then were intubated and maintained on 1.5% to 2.5% isoflurane anesthesia for the duration of the procedure. All pigs were maintained on an intravenous infusion of lactated Ringer solution at a rate of approximately 10 mL/kg/h, and a closed urine-collection system was used for the duration of the procedure. A trained anesthesia technician monitored and recorded intraoperative parameters, including electrocardiography, pulse oximetry, capnography, bispectral index, end-tidal and inspired isoflurane concentration, respiratory rate, body temperature, noninvasive blood pressure, and anesthetic depth.
Regional anesthesia administration.
Prior to surgery, one pig of each pair was randomly assigned to the treatment (bupivacaine, Hospira, Lake Forest, IL) group and the other to the control (saline, Hospira) group in a blinded randomized controlled trial design. Once the pigs were anesthetized, we used ultrasound guidance to locate the sciatic nerve (as it exits the pelvis through the greater sciatic foramen in the parasacral region) and the femoral nerve (in the proximal inguinal region). The skin over each site was marked for reference.
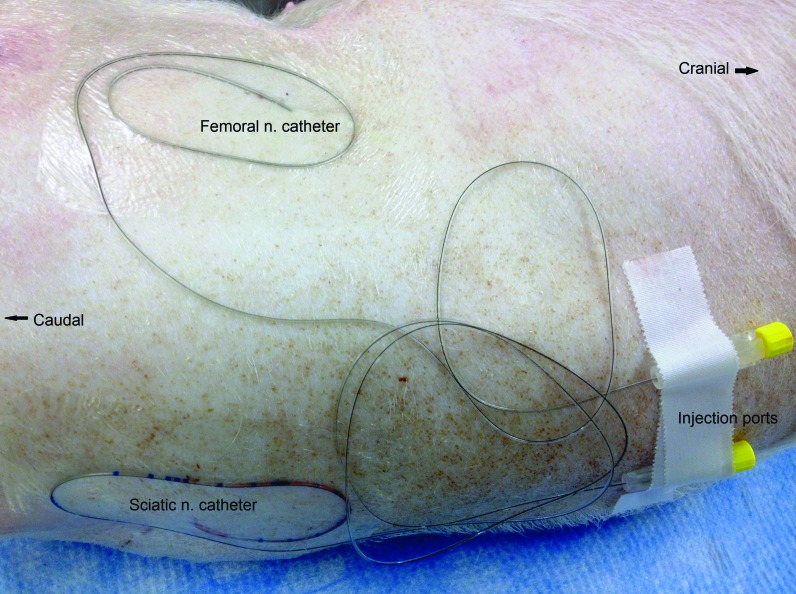
After antiseptic preparation and sterile draping of the right dorsal parasacral area, the ultrasound probe was placed in a sterile sleeve and applied to the injection site, which was coated with sterile ultrasound gel. After the sciatic nerve was located, a Tuohy needle (17 gauge; length, 9 cm) was inserted in plane with the probe with a short-axis view of the nerve (Figure 1). With ultrasound visualization, the needle was advanced until it was immediately adjacent to the nerve. A syringe containing 10 mL of either 0.5% bupivacaine (treatment group) or 0.9% sodium chloride (control group) was attached to the hub of the needle, and after aspiration and confirmation of the absence of blood, 1 mL was injected. If the ultrasound image showed correct placement of the injected fluid, the remaining volume was infused while the ultrasound image was observed. A 19-gauge closed-tip multiorifice polyamide infusion catheter (Perifix or Contiplex Tuohy Set, B Braun, Melsungen, Germany) then was threaded through the Tuohy needle and held in place while the needle was retracted. The catheter was secured by using tissue adhesive and transparent adhesive dressings with an accessible injection port.
Figure 1.
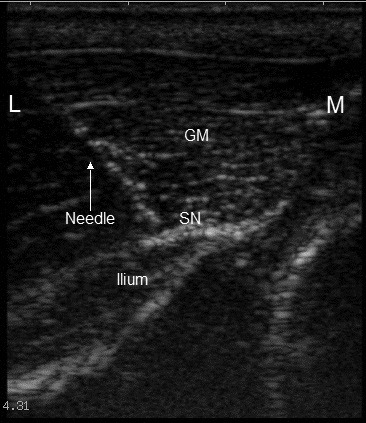
Ultrasound view of the parasacral sciatic nerve approach, showing the approximate position of the needle used for placement of the infusion catheter. At this level, the sciatic nerve (SN) lies deep to the gluteal musculature (GM) and on the surface of the ilium. The needle is directed ventromedially. L, lateral; M, medial.
For the femoral nerve, a short-axis view of the nerve was obtained in the inguinal area as far cranial as possible. After antiseptic preparation and draping of the lateral flank, the Tuohy needle was introduced through the skin of the lateral ventral flank area cranial to the thigh and directed medially and caudally in plane with the ultrasound probe toward the site of interest (where the femoral nerve exits the inguinal canal; Figure 2). Local anesthetic (0.5% bupivacaine) or saline (10 mL) was then infused, and the infusion catheter was placed and secured, as described for the sciatic nerve (Figure 3).
Figure 2.
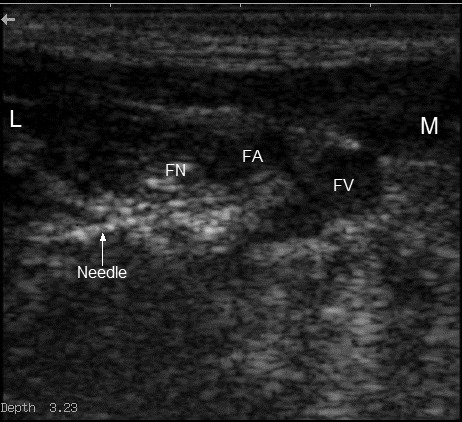
Ultrasound view of the femoral nerve approach, showing the approximate position of the needle used for placement of the infusion catheter. The femoral nerve (FN) lies immediately lateral (L) to the femoral artery (FA) and vein (FV). The needle is directed medially (M).
Figure 3.
Regional anesthesia infusion catheters after placement. Transparent adhesive dressing covers the entry sites, and injection ports are secured in an easily accessible location for administration of local anesthetic.
For ultrasound guidance of catheter placement, we used a UMS 700 Digital Ultrasonic Diagnostic Imaging System (model DP6600Vet) with a 7.5-MHz linear transducer (Universal Medical Systems, Bedford Hills, New York). With the first 4 pigs, we also used a peripheral nerve stimulator (Stimuplex HNS12, B Braun, Melsungen, Germany) to confirm the accuracy of ultrasonography in localizing the nerves of interest.
Injury.
After application of regional anesthesia and aseptic preparation of the right lateral thigh, femurs were prestabilized by using an external fixation device. A right-side midshaft femoral fracture then was produced by using a captive-bolt device on the lateral aspect of the thigh. All fractures were evaluated radiographically immediately after injury. The degree of injury was generally consistent across groups, with penetration of both cortices and fragmentation around the fracture site. After evaluation, the limb and fixation device were covered with a protective cast.
Postoperative monitoring and analgesia.
After surgery and before recovery from anesthesia, all pigs received a single dose of buprenorphine (0.05 mg/kg IM; Hospira) and a single dose of meloxicam (0.4 mg/kg SC; Boehringer Ingelheim Vetmedica, St Joseph, MO). Repeat doses of bupivacaine (0.25%, 10 mL at each site) or the same volume of sterile saline then were administered every 6 to 8 h for the first 24 h postoperatively, after which the pig was sedated with a combination of ketamine (10 mg/kg; Fort Dodge Animal Health) and xylazine (2 mg/kg IM; Vedco), and the perineural catheters were removed. In all, 4 doses of local anesthetic or saline were administered to each pig: one dose preoperatively and 3 doses postoperatively.
After full recovery from anesthesia on the day of surgery, pigs were monitored frequently, with full evaluations at least every 6 to 8 h during the first 24 h after surgery. Pigs were evaluated regularly throughout the study by a clinical veterinarian. Any animal assessed, according to the veterinarian's clinical judgment, to be in pain warranting treatment was given rescue analgesia (buprenorphine, 0.05 to 0.1 mg/kg IM). Feed was offered immediately after full recovery from anesthesia, and time to first consumption of any feed was recorded. We also evaluated several pain indicators before and after surgery, including heart rate as measured by direct cardiac auscultation, respiratory rate by observation, and subjective pain assessment. Activity levels were estimated by using a remote telemetry monitoring system (Data Sciences International, St Paul, MN), which counted physical movements by the subjects starting the day before surgery through the fourth postoperative day.
To obtain baseline measurements, all evaluations were performed at 2 time points preoperatively: once before and once after placement of a transdermal fentanyl patch. Evaluations were then repeated at 3 time points on the day of surgery: 2 and 4 h after recovery from anesthesia and again the night after surgery (approximately 10 to 11 h postsurgery). Each pig was then evaluated twice daily for the next 4 d: once each morning (AM) and afternoon (PM). In addition, each pig was weighed prior to surgery and weekly thereafter.
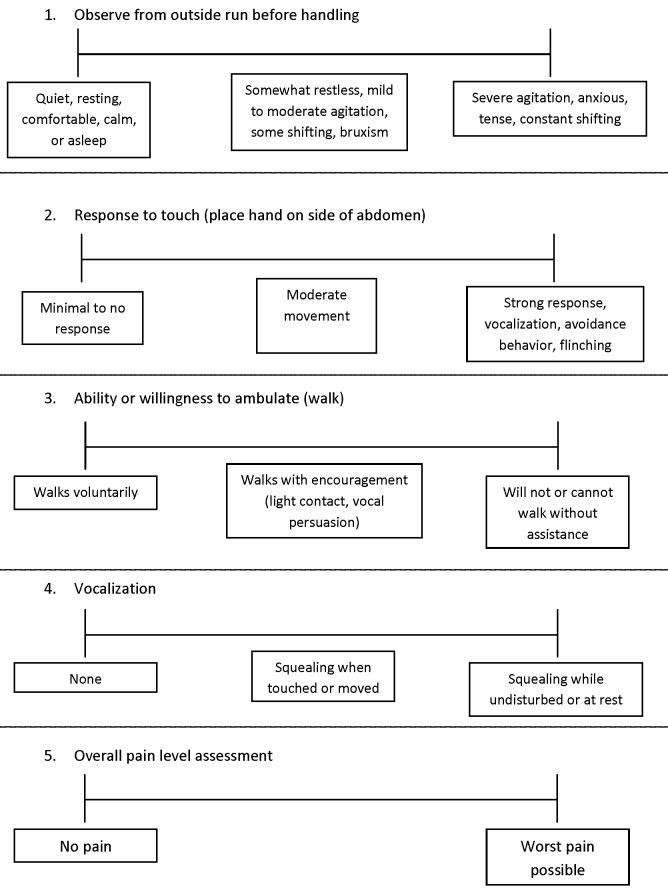
Five categories of subjective pain indicators were scored by using a modified visual analog scale (VAS): passive observation of the pig from outside its enclosure before entering (VAS1, observation); strength and character of response to physical contact when a hand was placed on the side of abdomen (VAS2, contact); impairment of ability or willingness to ambulate (VAS3, ambulation); nature and intensity of vocalization (VAS4, vocalization); and an overall dynamic and interactive subjective assessment of pain level after completion of the full evaluation (VAS5, overall). The VAS chart consisted of a 100-mm horizontal line for each category. After assessing the animal, the evaluator placed a mark on the line according to the assessed level of pain, with pain scores increasing from left to right on the scale (Figure 4). The VAS score then was derived by measuring the distance of the mark (in mm) from the left side of the scale. In all cases, a higher score indicated more severe impairment or pain.
Figure 4.
VAS scoring sheet used for subjective pain-level assessments. Evaluators were instructed to mark each line at the most appropriate position, using the descriptions in the boxes as guidelines. In general, the left side of the scale represents no pain, and the right side the highest level of pain.
All personnel involved in the study were blinded to treatment; the same person performed 90% of all evaluations. In cases where other evaluators were used, each pair of pigs was evaluated by the same person.
Blood sampling and analyses.
Blood samples were collected from anesthetized pigs intraoperatively and from heavily sedated pigs at approximately 24 h after surgery, to evaluate serum levels of cortisol and fentanyl. Additional blood samples were taken from sedated pigs at 1 wk after surgery, to measure serum fentanyl levels.
Hematology and serum biochemistry data were analyzed for changes consistent with pain or distress, such as hyperglycemia, altered neutrophil:lymphocyte ratios, and dehydration due to decreased water intake.
Histopathology.
At 3 wk after surgery, all pigs were euthanized and necropsied for collection of peripheral nerves and surrounding soft tissue for histopathologic analysis. Bilaterally, 5-cm portions of both femoral and sciatic nerves were dissected free, along with surrounding skeletal muscle, for evaluation. Tissue samples were fixed in 10% buffered formalin, embedded in paraffin wax, sectioned at approximately 5 µm, and submitted to the Armed Forces Radiobiology Research Institute Division of Comparative Pathology for evaluation. Sections were routinely stained with hematoxylin and eosin and examined by light microscopy in a blinded manner for histopathologic evaluation by a board-certified veterinary pathologist. Sections were evaluated for any pathologic change within the peripheral nervous system and surrounding soft tissues, with specific emphasis on degenerative or inflammatory changes within nerves.
Statistical analysis.
Physical activity data were collected and processed by using the Physiology Platform (DSI Ponema, Valley View, OH). Telemetry data containing counts of physical movements were divided into 12-h segments corresponding to the light (0700 to 1900) and dark (1900 to 0700) phase of the room light schedule. Total counts per hour for each segment were calculated and then compared between treatment and control groups by using a linear mixed-effects model as described following.
We evaluated data graphically for normality and used the Student t test to compare means and the Mann–Whitney U test to compare medians, for continuous independent variables. For data with repeated measures, we used the Student t test with no adjustment for multiple comparison to compare preoperative time points and postoperative time points that were beyond the expected period of analgesia (that is, day 2 and later). We used a linear mixed-effects model to compare overall differences between treatment groups and differences between treatment groups over time during the treatment period (that is, days 0 and 1). When the main effect of group or the group×time interaction was significant in the mixed model, individual time points were compared by using the Student t test with no adjustment for multiple comparisons. We used the log-rank test and Kaplan–Meier curves to compare data measuring time to an event, and we estimated hazard ratios by using Cox proportional hazards regression.
Statistical software packages were used to perform all statistical analyses (SPSS Statistics version 19, IBM, Chicago, IL) and to create graphs of study data (Prism 6, GraphPad, San Diego, CA). Unless otherwise indicated, data are expressed as mean ± SEM. All differences were considered significant if the 2-tailed P value was less than 0.05. Because this study was appended to an ongoing study, sample size was predetermined by the needs of the parent study.
Results
Three pigs were removed from the study early; 2 of these (one treatment and one control pig) were euthanized early because of inadequate stabilization of the femur fracture. Another pig in the treatment group died during the immediate postanesthetic period due to apparent laryngospasm after endotracheal tube removal. Compared with the treatment group, the control group had a significantly (P < 0.05) higher heart rate at the time of prestudy physical examination (Table 1). Weight, temperature, and respiratory rate did not differ significantly at any time point. No difference in pain control was noted between the initial 4 subjects in which nerve electrostimulation was used and the remaining pigs in which ultrasound alone was used for catheter placement.
Table 1.
Prestudy physical examination data for all pigs by treatment group
| Groupa | Mean ± SEM | P | |
| Weight (kg) | 0.90 | ||
| Treatment | 27.8 ± 2.3 | ||
| Control | 28.2 ± 2.8 | ||
| Temperature (°C) | 0.09 | ||
| Treatment | 38.9 ± 0.10 | ||
| Control | 39.2 ± 0.11 | ||
| Heart rate (bpm) | 0.02 | ||
| Treatment | 118.8 ± 6.9 | ||
| Control | 151.3 ± 11.0 | ||
| Respiratory rate (breaths per min) | 0.12 | ||
| Treatment | 43.2 ± 3.3 | ||
| Control | 50.7 ± 3.3 | ||
| Age (d) | 0.97 | ||
| Treatment | 82.2 ± 4.6 | ||
| Control | 82.4 ± 5.2 | ||
n = 10 for treatment group; n = 9 for control group.
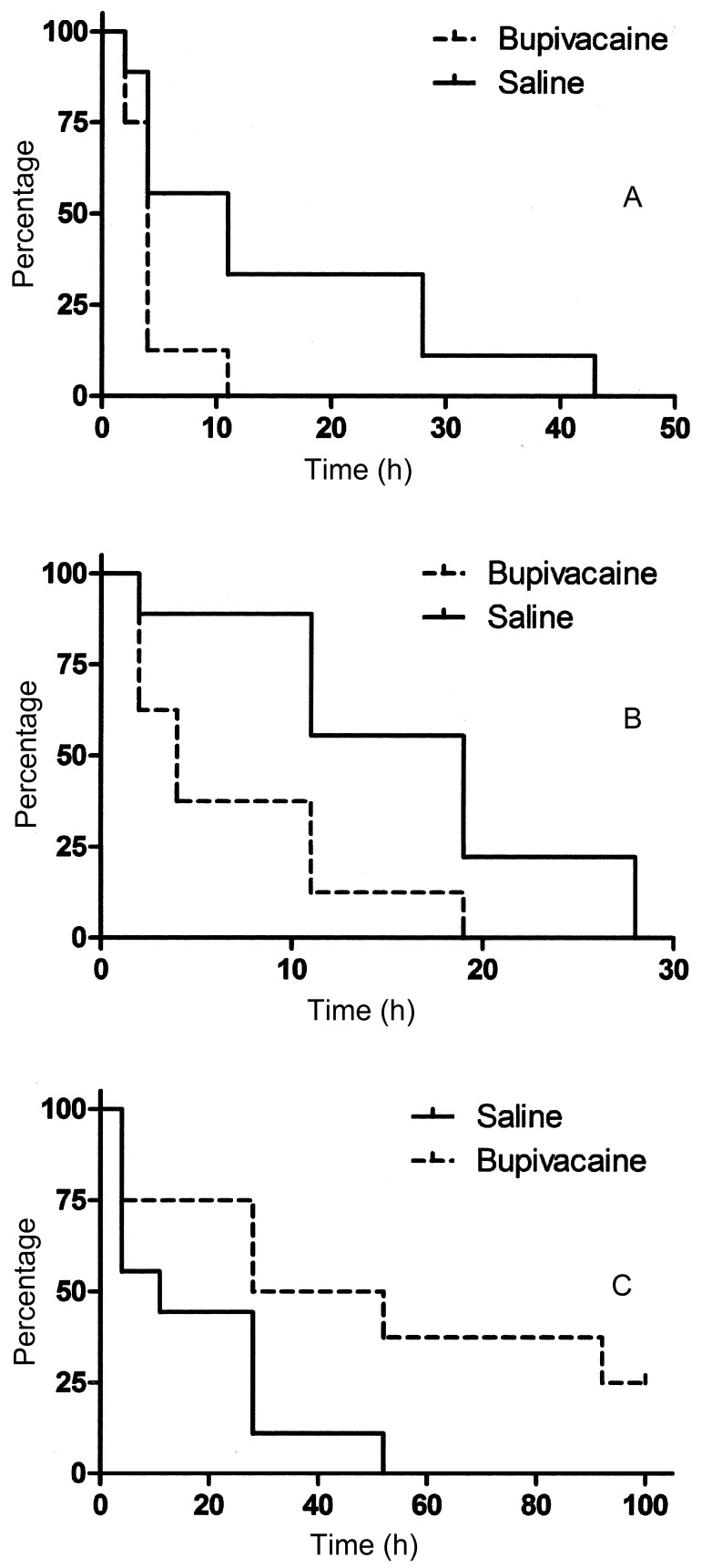
The median (± SE of the median) time at which the pigs ate their first meal was 4.0 ± 0.4 h for the treatment group and 11.0 ± 5.0 h for the control group (Figure 5). Median time to first defecation was 4.0 ± 1.4 h for the treatment group and 19 ± 3.3 h for the control group (Figure 5).
Figure 5.
Kaplan–Meier cumulative survival curves, showing the time (in h) to occurrence of the event of interest. (A) Time to first consumption of feed after surgery. Hazard ratio (95% confidence interval): 4.4 (1.0–18.7). The curves differ significantly (P = 0.048). (B) Time to first bowel movement after surgery. Hazard ratio (95% confidence interval): 5.0 (1.3–19.6). The curves differ significantly (P = 0.021). (C) Time to first administration of rescue analgesia after surgery. Hazard ratio (95% confidence interval): 3.9 (1.0–14.7). The survival curves differ significantly (P = 0.044).
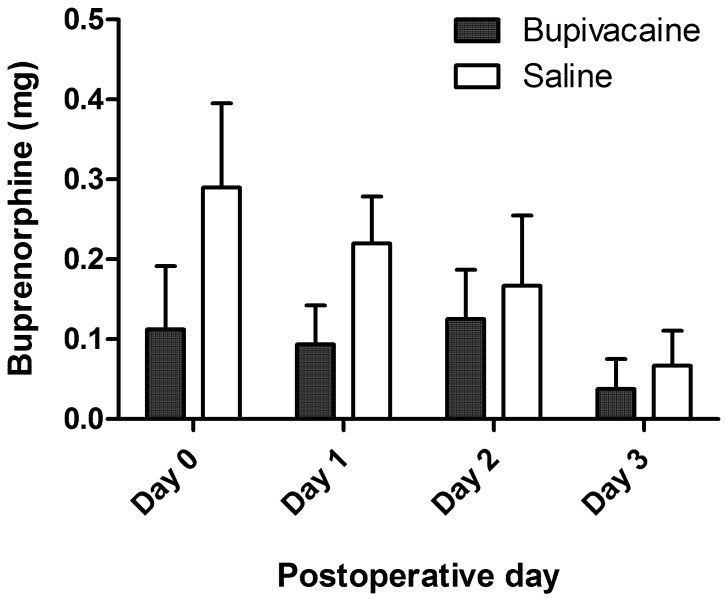
At each evaluation, the evaluator determined whether the pig was in sufficient pain to merit rescue analgesia. The median time of first intervention was 28 ± 22.6 h for the treatment group and 11 ± 10.4 h for the control group. (Figure 5) When the total quantity of buprenorphine administered postoperatively was compared, control subjects tended to receive greater quantities on average (Figure 6). However, differences did not achieve statistical significance at any time point.
Figure 6.
Average total daily quantity of buprenorphine administered to each pig on the day of surgery (day 0) and on each of the next 3 d after surgery. Error bar, ±1 SEM.
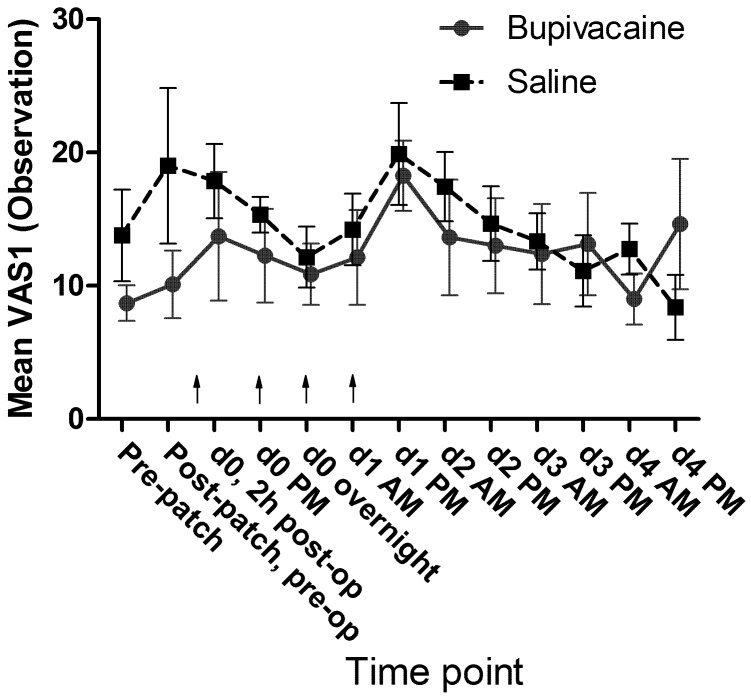
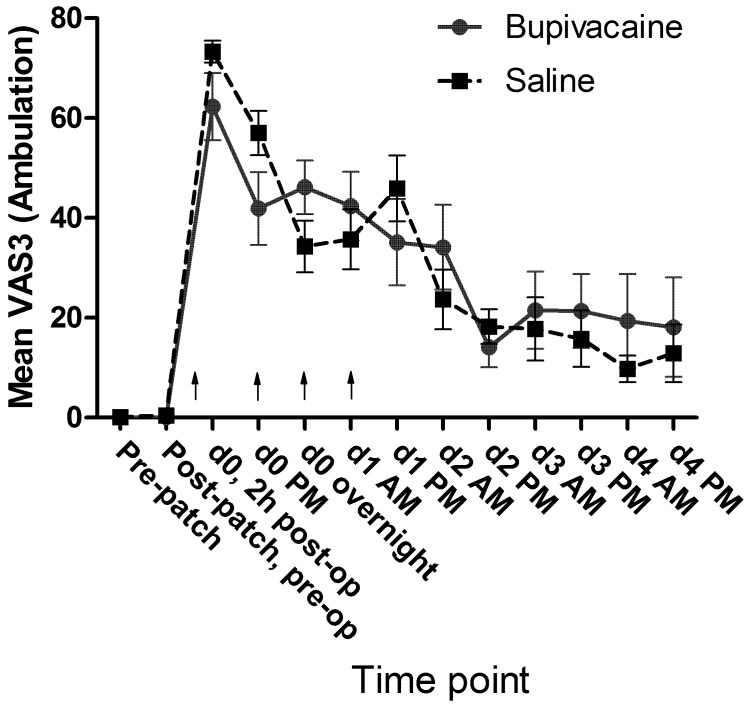
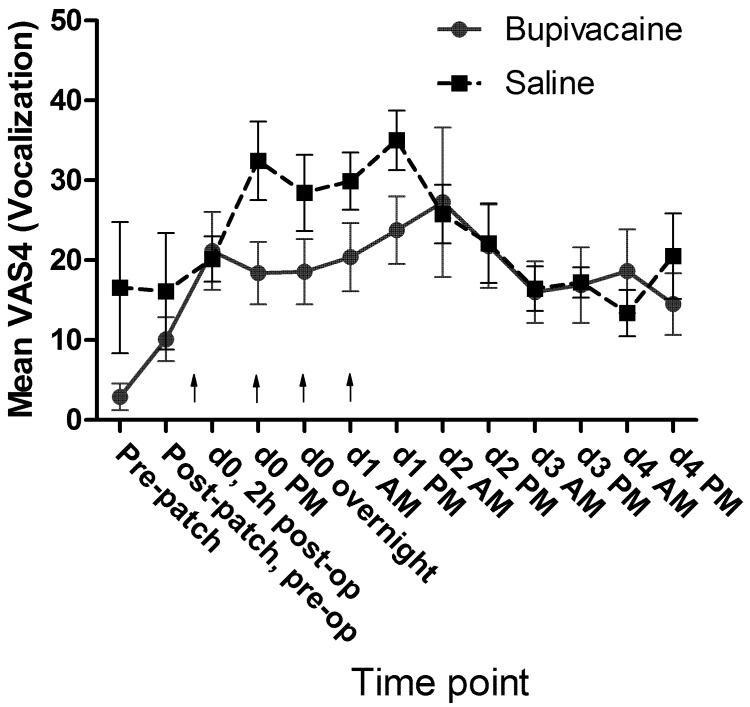
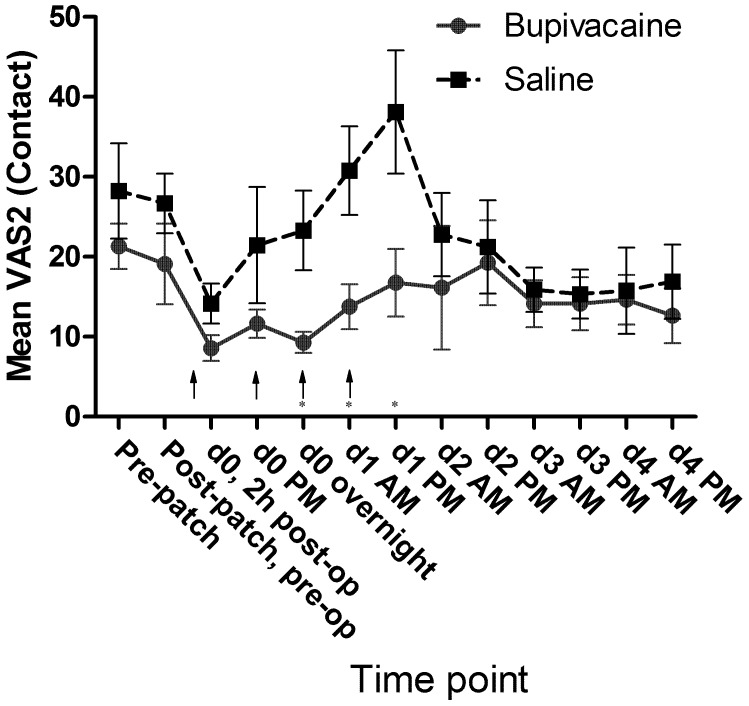
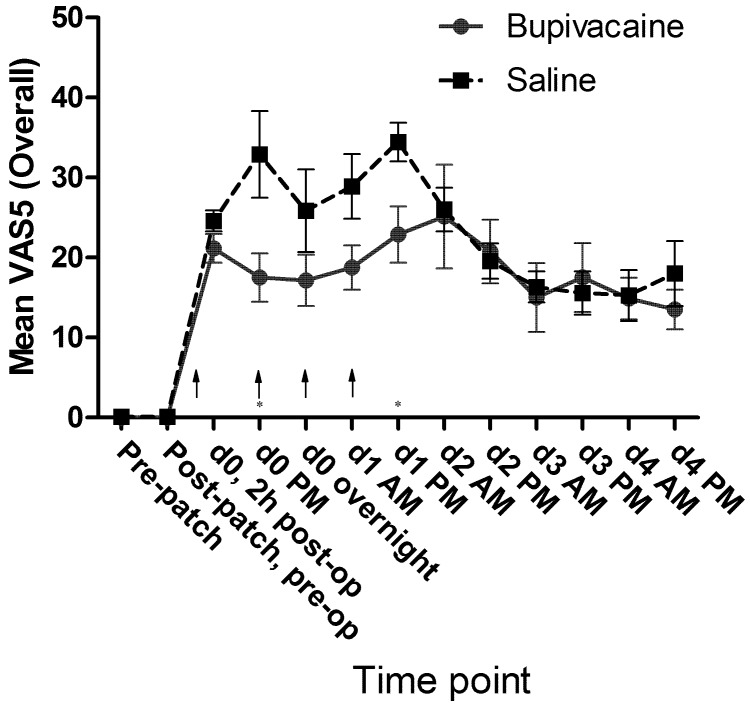
No significant differences in mean passive observation scores (VAS1, Figure 7), mean ambulation scores (VAS3, Figure 8), or mean vocalization scores (VAS4, Figure 9) were noted. Mean scores for response to contact (VAS2) were higher at all time points for the control group, and there was a significant (P = 0.009) difference in overall means between groups in the mixed-effects model. In the pairwise comparison, the difference achieved significance at 3 time points within the first 24 h of surgery (Figure 10). In the overall pain level assessment score (VAS5), there was a significant difference between groups in the mixed-effects model (P = 0.008), and pain assessment scores at 2 time points differed significantly (P < 0.05) in the pairwise comparison (Figure 11). None of the VAS parameters showed a significant interaction between treatment and time in the mixed-effects model.
Figure 7.
Mean VAS1 (observation) scores by treatment group. Arrows indicate doses of local anesthetic or saline (control). d0, day of surgery; d1, first day after surgery; and so forth. Error bar, ±1 SEM.
Figure 8.
Mean VAS3 (ambulation) scores by treatment group. Arrows indicate doses of local anesthetic or saline (control). d0, day of surgery; d1, first day after surgery; and so forth. Error bar, ±1 SEM.
Figure 9.
Mean VAS4 (vocalization) scores by treatment group. Arrows indicate doses of local anesthetic or saline (control). d0, day of surgery; d1, first day after surgery; and so forth. Error bar, ±1 SEM.
Figure 10.
Mean VAS2 (contact) scores by treatment group. Arrows indicate doses of local anesthetic or saline (control). Significant difference (Student t test) between groups on a particular day is indicated by an asterisk. d0, day of surgery; d1, first day after surgery; and so forth. Error bar, ±1 SEM.
Figure 11.
Mean VAS5 (overall) scores by treatment group. Arrows indicate doses of local anesthetic or saline (control). Significant difference (Student t test) between groups on a particular day is indicated by an asterisk. d0, day of surgery; d1, first day after surgery; and so forth. Error bar, ±1 SEM.
Physical examination parameters.
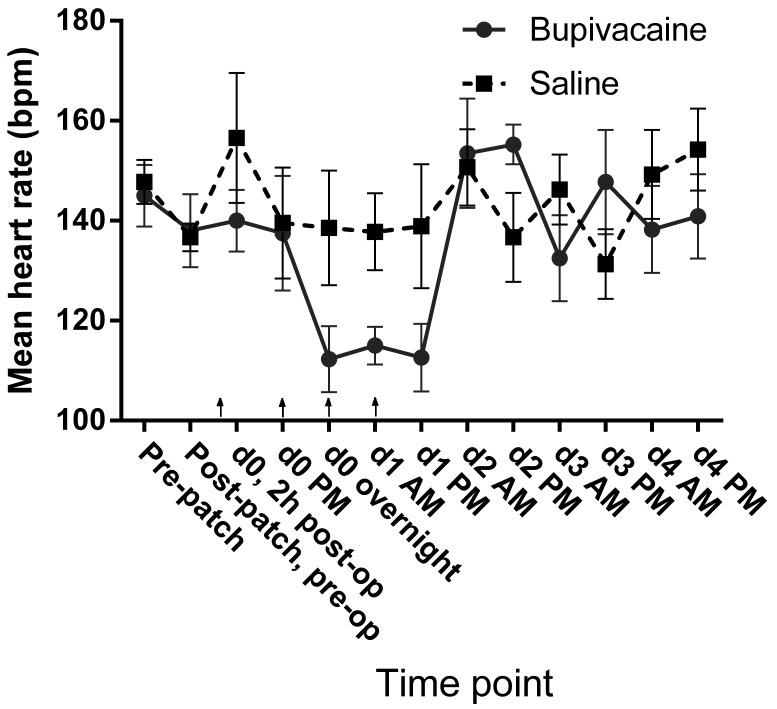
At each evaluation, the evaluator measured heart rate by cardiac auscultation with a stethoscope, and respiratory rate was measured by visual observation of thoracic movement. Heart rate decreased in the treatment group during the first 24 h after treatment but stayed relatively constant in the control group (Figure 12). However, the average heart rate did not differ significantly between treatment groups overall (P = 0.061) or between groups over time (P = 0.64) in the mixed-effects model.
Figure 12.
Mean heart rate (bpm) as measured by direct cardiac auscultation during physical examination. Arrows indicate doses of local anesthetic or saline (control). Error bar, ±1 SEM.
The mean respiratory rate was higher in the control group than in the treatment group during the first 24 h after surgery. However, in the mixed-effects model, the differences between treatment groups (P = 0.18) and between groups over time (P = 0.25) were not significant.
Activity levels, estimated as the frequency of physical movements or motion measured telemetrically during the postoperative period, dropped sharply after surgery relative to preoperative levels. Although activity levels tended to be slightly higher in the control group, we did not note a significant effect of treatment (P = 0.84) or a significant interaction between treatment and time (P = 0.99) in the mixed-effects model.
Clinical pathology.
Serum biochemistry values did not differ between treatment groups (Table 2). Of the hematology parameters measured, RBC count, Hgb, and Hct measured at 24 h were all significantly (P < 0.05) increased in the control group compared with the treatment group (Table 3). Blood samples were collected for serum fentanyl measurement at 3 approximate time points: 24, 48, and 72 h after application of a transdermal fentanyl patch (Figure 13). Overall fentanyl levels (mean ± SEM) for both groups combined were 0.31 ± 0.070 ng/mL at 24 h, 0.21 ± 0.045 ng/mL at 48 h, and 0.12 ± 0.023 ng/mL at 72 h. There were no significant differences between treatment and control groups in mean fentanyl levels at any of the time points (Table 4).
Table 2.
Serum chemistry data at 24 h after surgery
| Group | n | Mean ± SEM | Reference rangea | P | |
| Sodium (mmol/L) | 142–149 | 0.43 | |||
| Bupivacaine | 7 | 145.2 ± 4.7 | |||
| Saline | 7 | 145.0 ± 1.0 | |||
| Chloride (mmol/L) | 100–109 | 0.69 | |||
| Bupivacaine | 7 | 100.3 ± 2.6 | |||
| Saline | 7 | 101.3 ± 0.9 | |||
| Glucose (mg/dL) | 85–160 | 0.25 | |||
| Bupivacaine | 7 | 96.3 ± 8.3 | |||
| Saline | 7 | 113.3 ± 7.2 | |||
| BUN (mg/dL) | 6–30 | 0.24 | |||
| Bupivacaine | 7 | 7.6 ± 0.5 | |||
| Saline | 7 | 8.3 ± 0.6 | |||
| Creatinine (mg/dL) | 0.5–2.1 | 0.79 | |||
| Bupivacaine | 7 | 1.1 ± 0.1 | |||
| Saline | 7 | 1.2 ± 0.1 | |||
| Serum total protein (g/dL) | 6.1–7.5 | 0.11 | |||
| Bupivacaine | 8 | 5.0 ± 0.4 | |||
| Saline | 9 | 5.9 ± 0.3 | |||
| Cortisol (ng/mL) | — | 0.42 | |||
| Bupivacaine | 8 | 16.2 ± 0.7 | |||
| Saline | 9 | 17.1 ± 0.7 | |||
Reference ranges were provided by the commercial laboratory conducting the analyses; no reference range has been established for cortisol in swine.
Table 3.
Hematology results at 24 h after surgery
| Group | Mean ± SEM | P | Reference rangea | |
| Hct (%) | 0.011 | 36.9–55.0 | ||
| Bupivacaine | 27.4 ± 0.49 | |||
| Saline | 29.5 ± 0.50 | |||
| RBC count (×106/µL) | 0.024 | 5.5–8.2 | ||
| Bupivacaine | 5.4 ± 0.082 | |||
| Saline | 5.9 ± 0.14 | |||
| Hemoglobin (g/dL) | 0.012 | 12.6–19.4 | ||
| Bupivacaine | 8.9 ± 0.15 | |||
| Saline | 9.6 ± 0.19 | |||
| Neutrophil:lymphocyte ratiob | 0.91 | 0.7 | ||
| Bupivacaine | 1.8 ± 0.84 | |||
| Saline | 1.9 ± 0.56 | |||
Both groups contained 6 pigs. Samples were collected at 24 h from sedated subjects and placed in EDTA tubes.
Reference ranges for erythrocyte indices were provided by the reference laboratory.
Reference value was obtained from reference 38.
Figure 13.
Serum fentanyl levels (ng/mL) at time (h) since placement of a fentanyl patch (50 µg/h). Boxes represent bupivacaine-treated pigs; circles indicate saline controls. Blood sampling occurred at the following times (h; mean ± 1 SD) after fentanyl patch placement: first sampling, 21.1 ± 2.5 h; second sampling, 47.4 ± 3.5 h; and third sampling, 73.2 ± 2.7 h.
Table 4.
Fentanyl levels (ng/mL) by time point and treatment group
| Group | n | Mean ± SEM | P | |
| 24 h | 0.095 | |||
| Bupivacaine | 10 | 0.198 ± 0.060 | ||
| Saline | 9 | 0.431 ± 0.12 | ||
| 48 h | 0.85 | |||
| Bupivacaine | 10 | 0.199 ± 0.057 | ||
| Saline | 12a | 0.217 ± 0.071 | ||
| 72 h | 0.45 | |||
| Bupivacaine | 4 | 0.099 ± 0.041 | ||
| Saline | 4 | 0.137 ± 0.023 |
In some pigs, fentanyl patches began detaching and had to be replaced earlier than planned. Because blood draw time points were fixed already, some pigs had two 48-h measurements but no 72-h measurements.
Histopathology.
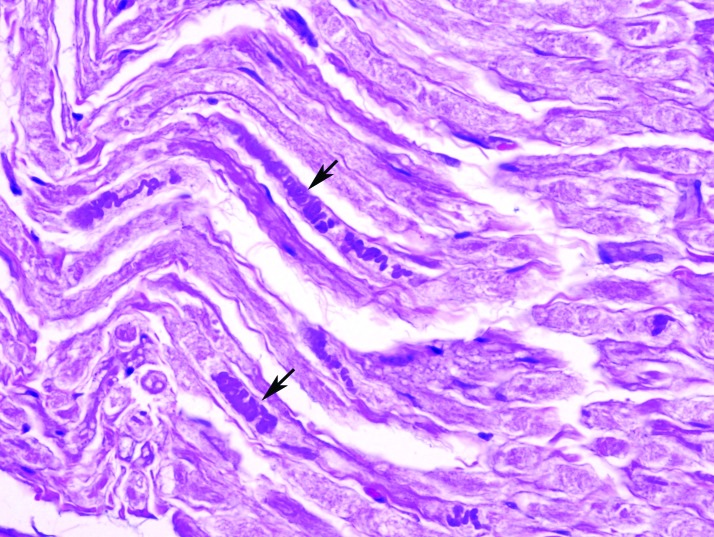
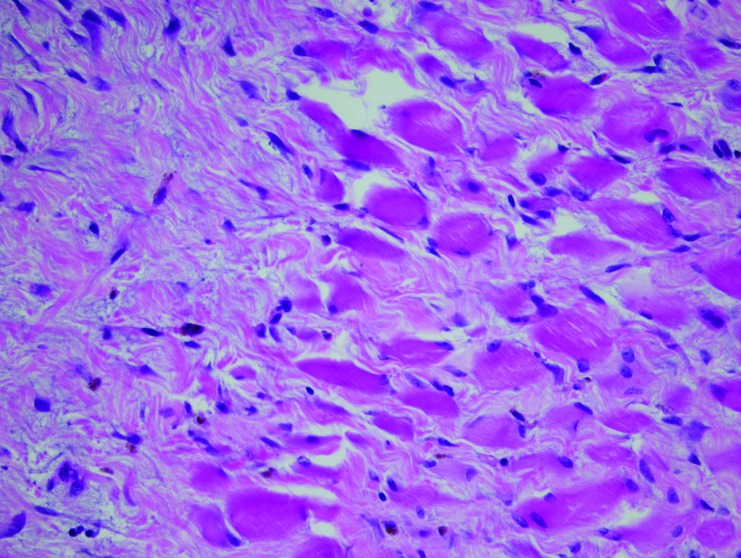
Histopathologic examination of all submitted tissues revealed a mix of lesions in both saline and bupivacaine cohorts. These findings included examples of degenerative radiculoneuropathy characterized by myelin sheath ectasia, fiber shrinking, angulation, hyperchromatism and loss (Figure 14), and focally extensive and severe rhabdomyocytic atrophy and loss with fibrosis (Figure 15).
Figure 14.
Right sciatic nerve of a bupivacaine-injected pig, showing axonal degeneration and loss characterized by hyperchromatic condensation of the axons (arrows). Hematoxylin and eosin stain; magnification, 600×.
Figure 15.
Skeletal muscle in the area of the right femoral nerve of a bupivacaine-injected pig, showing rhabdomyocytic atrophy and loss that is focally extensive and severe with abundant fibrosis. Hematoxylin and eosin stain; magnification, 400×.
Within the saline cohort, 89% of the pigs showed histopathologic evidence of pathology in at least one of the sampled nerves. Of these pigs, 75% had right-sided pathology, 75% had left-sided pathology, and 62.5% showed bilateral lesions. Within the bupivacaine cohort, 62.5% of pigs displayed nerve fiber pathology, of which 100% had right-sided lesions and 25% had bilateral lesions. However, none of the differences were statistically significant, and no correlation was observed between presence of histopathologic lesions and clinical signs of altered limb function.
Two lesions were classified as severe by the pathologist. Both were from muscle tissue around the right femoral nerves of bupivacaine-treated pigs. The first lesion was characterized as rhabdomyocytic loss that was focally extensive and severe, with fibrosis, hemosiderosis, perivascular lymphoplasmocytic cellulitis, and myelin sheath ectasia with axonal loss. The other lesion had rhabdomyocytic atrophy, degeneration, necrosis, loss, and fibrosis that was severe and multifocal to coalescing, with multifocal rare axonal swelling.
Discussion
This study provides substantial evidence for a beneficial effect of regional anesthesia in addition to traditional systemic analgesics for hindlimb pain in swine. Most notably, untreated pigs required rescue analgesia earlier after surgery than did treated pigs. Furthermore, treated pigs ate food and defecated earlier in the postoperative period than did their untreated counterparts. Because anorexia and decreased bowel function can occur with pain in animals, these differences provide evidence for an analgesic effect of the treatment. In addition, the observed effect on time to defecation may reflect greater inhibition of bowel function in control pigs because they received more postoperative opioid analgesics, which are known to decrease motility and increase transit time in the gastrointestinal tract.24
The results in some categories of subjective pain assessment suggest a beneficial effect of the treatment. Control pigs had higher pain scores even though they tended to receive rescue analgesia sooner than did treatment animals. The response to physical contact (VAS2) and the overall assessment of pain levels after examination (VAS5) appeared to best discriminate between groups. In addition to demonstrating a positive effect of the regional anesthetic treatment, this result shows that subjective assessment that includes response to physical contact may be helpful in assessing pain in swine.
In addition, VAS score trends over time are consistent with the expected time course of the regional anesthesia effects. Scores tended to be very similar between treatment groups at the first postoperative evaluation (2 h after surgery). At that time point, it might be expected that the residual effects of anesthesia and postoperative analgesics either controlled pain sufficiently or resulted in sufficient sedation to mask the effects of pain as assessed by the subjective observer. However, starting at the next time point (4 h after surgery), VAS scores in the control group tended to be higher than those in the treatment group, suggesting better pain control in the treatment group. This difference persisted through the treatment period and then regressed at approximately 8 to 24 h after the last bupivacaine treatment. It is noteworthy that simple observation of the pig from outside the pen—a common practice for pain assessment in laboratory animals—failed to detect any difference between groups. Furthermore, although ability to ambulate improved significantly in both groups over time, it did not differ between groups, suggesting that this parameter is not a reliable indicator of pain control in this model. Alternatively, the cast material on the legs may have impeded ambulation sufficiently in both groups to obscure any pain-related differences in ambulation. Furthermore, ambulation may have been inhibited by impairment of motor function in the treated limb due to the regional anesthesia, but this possibility was not assessed in the study.
The VAS pain scoring system, a commonly used, validated method for quantifying self-reported pain levels in human patients,6,28 was applied to the subjective assessment of pain in swine in our current study through the observation of behavioral and physiologic indicators of pain. Multiple studies report the use of subjective VAS assessments of pain in animals, with most studies done in dogs.7,11,13,33,34,44,60 The absolute values on the VAS scale do not necessarily correlate with any absolute pain level, and significant interobserver variation has been observed in subjective pain assessments in animals.34 However, the aim of the current study was not to determine absolute quantitative pain levels in individual pigs but rather to compare relative pain levels between pigs at equivalent time points and to determine how differences between groups change over time. Furthermore, we used a blinded, experienced observer to compare subjective pain levels in randomly assigned treatment and control pigs to eliminate possible bias.
At the time of entry into the facility prior to any experimental manipulation, average heart rate on physical examination was higher in the pigs that would form the control group than those in the treatment group. However, all pigs were assigned randomly to the treatment or control group, and other physical examination data (temperature, respiratory rate, weight) showed no other indications of baseline differences between treatment and control groups. Furthermore, after acclimation to the facility, subsequent pre- and postoperative examinations showed no significant difference in heart rate between groups. In light of these findings, we believe that this initial difference represents random variation in conditions and not any true baseline difference between groups.
A trend toward decreased heart rate in the treatment group was noted for about the first 24 h after surgery, though the difference between groups was not statistically significant. All pigs were evaluated at equivalent time points relative to surgery and anesthesia, so it unlikely that this apparent difference resulted from the effects of general anesthesia. Bupivacaine has been shown to affect heart rate in human patients undergoing regional anesthesia, with some studies reporting increased heart rates and others decreased heart rates.4,8,17,41 The decreased heart rates in bupivacaine-treated pigs in our current study may be a result of a direct effect of bupivacaine on heart function. However, decreased activity due to impaired mobility may result in lower heart rates, with a more pronounced effect in the group with enhanced pain control.
Erythrocyte measures (RBC count, Hct, and Hgb) differed between groups at the 24-h postoperative time point, with slightly lower indices in the treatment group. This difference at 24 h may represent random variation in RBC mass between the 2 groups or may indicate slight dehydration. In addition, we cannot rule out the possibility of a direct effect of bupivacaine on RBC in swine.
We assessed serum cortisol as an indirect measure of pain-induced sympathetic nervous system stimulation.30,58 Cortisol levels did not differ significantly between the treatment and control groups. This result may be due to lack of sensitivity of the assay, effects of handling and sedation for blood collection, or high stress in both groups due to immobility and handling.24,37,40,57
Blood glucose levels can be affected transiently by stress, fear, pain, feed consumption, and the use of sedatives.22,39,56 Blood glucose levels did not differ significantly between treatment and control groups in our study, and this result suggests that blood glucose may be an insensitive measure of pain in swine.
We measured serum fentanyl levels to ensure that the analgesic effect was similar between groups and to evaluate whether transdermal fentanyl delivery at the dose used resulted in consistently adequate analgesic levels for 72 h. Other studies have evaluated serum fentanyl levels in swine with transdermal fentanyl administration. For example, one study reported serum fentanyl levels at 24 h of 0.47 ng/mL (range, 0.17 to 1.0 ng/mL), with peak concentrations at around 12 to 24 h, by using an approximate dose of 2.0 μg/kg/h.50 Our study, which used a lower dose (1.3 μg/kg/h), found a proportionally lower mean serum fentanyl value (0.31 ng/mL). The authors of another study postulated based on extrapolation from other species that therapeutic plasma fentanyl levels in pigs may reasonably be assumed to fall within the approximate range of 0.2 to 3.0 ng/mL.70 If we assume the low end of this estimate (0.2 ng/mL), then 50%, 64%, and 100% of the pigs in our study were at subtherapeutic levels at 24, 48, and 72 h, respectively. This result suggests that the transdermal fentanyl dose used in the present study likely is insufficient to produce therapeutic levels in swine.
Some of the histopathologic lesions observed in our study were similar to the findings of others.67,72 However, we found no significant patterns of distribution between treatment and control groups or between left and right limbs. The prevalence of background lesions in the neural and perineural soft tissue obscures any potential correlation between lesions and treatments. Consequently, whether the lesions were incidental or related to injection of fluid, presence of bupivacaine, the bone injury, or prolonged recumbency is unclear.
This study faces several limitations. The small sample size and relatively high variability in some parameters limits the power to detect significant differences. Another limitation involves the difficulty in evaluating pain in animals such as pigs.2 Pigs commonly object strongly to human handling even in the absence of pain, which makes it difficult to discern whether avoidance, guarding, vocalization, or reactions to touch are indicators of pain or simply behavioral idiosyncrasies. Furthermore, depending on the nature and severity of pain, pigs may demonstrate increases or decreases in activity, movement, and responsiveness.25,26,35 Analgesics, such as fentanyl and buprenorphine, can cause sedation, which may confound interpretation of behavioral signs of pain. Lastly, our control pigs received rescue analgesia earlier than did those that were treated, and this variation may have abrogated some of the differences in pain-related parameters.
Another challenge for the current study is confirming continual efficacy of the regional block. After placement, the infusion catheter tip may migrate as the animal moves, resulting in some or all of the local anesthetic not reaching the desired site. If catheter migration occurred in some subjects, it could have artificially shortened the apparent duration of analgesia achieved. Repeat imaging of the catheter prior to its removal would have confirmed correct placement throughout the treatment period.
In the present study, pigs concurrently received a pure µ opioid receptor agonist (fentanyl) and a partial µ agonist (buprenorphine). Because buprenorphine has a stronger affinity for the µ opioid receptor, it has the potential to displace and thus decrease the effects of fentanyl.62 Prolongation or potentiation of the adverse effects of opioids and decreases in expected analgesic effects have been reported with concurrent use of buprenorphine and pure µ agonists.14,29,54 However, other studies have reported no effect of the combination therapy on adverse effects of opioids.27 Furthermore, some studies have shown additive or synergistic analgesic effects when combining buprenorphine and fentanyl in human and animal studies.42,43,62 It cannot be determined what effect, if any, resulted from combining buprenorphine and fentanyl in the pigs in our study. It is also unknown whether displacement of fentanyl from opioid receptors by buprenorphine altered the serum fentanyl levels that we measured.
The relative novelty of ultrasound-guided regional anesthesia necessitates additional research, including the use of alternative local anesthetics, such as ropivacaine, or local anesthetic adjuncts, such as vasoconstrictors,47,53,66 α2 adrenergic agonists, opioids, benzodiazepines, and corticosteroids.31,47,52,53,55,61,66 Future studies may examine the use of continuous-infusion devices to provide more stable regional anesthesia and different behavioral or physiologic parameters for assessing pain, such as quantified intensity and frequency of vocalization and neuroendocrine markers of pain.31,52
The current study presents a postoperative pain assessment method for use in swine and supports the hypothesis that ultrasound-guided regional anesthesia provides superior analgesia compared with systemic analgesics alone for management of pain in hindlimb injuries. The temporal patterns of treatment effects fit well with expected values, with maximal differences present during the postoperative treatment period and absent between 8 and 24 h after cessation of treatment; this pattern is consistent with the known duration of effect of bupivacaine.45 In conclusion, ultrasound-guided regional anesthesia represents an important refinement in pain management in laboratory animals and may realize superior analgesia with fewer potential systemic effects, thus improving both animal welfare and the validity of research outcomes.
Acknowledgments
This research project was supported by funding from the US Army Military Research and Materiel Command, Ft Detrick (W81XWH-09-2-0179) and by the Uniformed Services University of the Health Sciences, Bethesda (grants R0702A and CO22AA). We thank the Department of Laboratory Animal Medicine veterinary and animal care staff for animal care support, Ms K Brady and Dr D Larsen for technical assistance, Dr C Olsen for statistical consultation, and SSgt E Stewart and Mr B Johnson for their preparation of histopathologic specimens.
Disclaimer: The views of the authors do not purport to reflect the position of the Uniformed Services University or the Department of Defense.
References
- 1.Altermatt FR, Cummings TJ, Auten KM, Baldwin MF, Belknap SW, Reynolds JD. 2010. Ultrasonographic appearance of intraneural injections in the porcine model. Reg Anesth Pain Med 35:203–206 [DOI] [PubMed] [Google Scholar]
- 2.Anil SS, Anil L, Deen J. 2002. Challenges of pain assessment in domestic animals. J Am Vet Med Assoc 220:313–319 [DOI] [PubMed] [Google Scholar]
- 3.Animal Welfare Act as Amended. 2008. 7 USC §2131-2159.
- 4.Bardsley H, Gristwood R, Baker H, Watson N, Nimmo W. 1998. A comparison of the cardiovascular effects of levobupivacaine and rac-bupivacaine following intravenous administration to healthy volunteers. Br J Clin Pharmacol 46:245–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benigni L, Corr SA, Lamb CR. 2007. Ultrasonographic assessment of the canine sciatic nerve. Vet Radiol Ultrasound 48:428–433 [DOI] [PubMed] [Google Scholar]
- 6.Bijur PE, Silver W, Gallagher EJ. 2001. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med 8:1153–1157 [DOI] [PubMed] [Google Scholar]
- 7.Brodbelt DC, Taylor PM, Stanway GW. 1997. A comparison of preoperative morphine and buprenorphine for postoperative analgesia for arthrotomy in dogs. J Vet Pharmacol Ther 20:284–289 [DOI] [PubMed] [Google Scholar]
- 8.Bruelle P, LeFrant JY, de La Coussaye JE, Peray PA, Desch G, Sassine A, Eledjam JJ. 1996. Comparative electrophysiologic and hemodynamic effects of several amide local anesthetic drugs in anesthetized dogs. Anesth Analg 82:648–656 [DOI] [PubMed] [Google Scholar]
- 9.Campoy L, Bezuidenhout AJ, Gleed RD, Martin-Flores M, Raw RM, Santare CL, Jay AR, Wang AL. 2010. Ultrasound-guided approach for axillary brachial plexus, femoral nerve, and sciatic nerve blocks in dogs. Vet Anaesth Analg 37:144–153 [DOI] [PubMed] [Google Scholar]
- 10.Candela D, Louart G, Bousquet PJ, Muller L, Nguyen M, Boyer JC, Peray PA, Goret L, Ripart J, Lefrant JY, de La Coussaye JE. 2010. Reversal of bupivacaine-induced cardiac electrophysiologic changes by 2 lipid emulsions in anesthetized and mechanically ventilated piglets. Anesth Analg 110:1473–1479 [DOI] [PubMed] [Google Scholar]
- 11.Carroll GL, Narbe R, Kerwin SC, Taylor L, Peterson K, Hartsfield SM. 2011. Dose range finding study for the efficacy of meloxicam administered prior to sodium urate-induced synovitis in cats. Vet Anaesth Analg 38:394–406 [DOI] [PubMed] [Google Scholar]
- 12.Chan VW, Brull R, McCartney CJ, Xu D, Abbas S, Shannon P. 2007. An ultrasonographic and histological study of intraneural injection and electrical stimulation in pigs. Anesth Analg 104:1281–1284 [DOI] [PubMed] [Google Scholar]
- 13.Conzemius MG, Hill CM, Sammarco JL, Perkowski SZ. 1997. Correlation between subjective and objective measures used to determine severity of postoperative pain in dogs. J Am Vet Med Assoc 210:1619–1622 [PubMed] [Google Scholar]
- 14.Cook PJ, James IM, Hobbs KE, Browne DR. 1982. Controlled comparison of IM morphine and buprenorphine for analgesia after abdominal surgery. Br J Anaesth 54:285–290 [DOI] [PubMed] [Google Scholar]
- 15.Costa-Farre C, Blanch XS, Cruz JI, Franch J. 2011. Ultrasound guidance for the performance of sciatic and saphenous nerve blocks in dogs. Vet J 187:221–224 [DOI] [PubMed] [Google Scholar]
- 16.Cowlishaw PJ, Scott DM, Barrington MJ. 2012. The role of regional anaesthesia techniques in the management of acute pain. Anaesth Intensive Care 40:33–45 [DOI] [PubMed] [Google Scholar]
- 17.de Leeuw MA, Slagt C, Hoeksema M, Zuurmond WW, Perez RS. 2011. Hemodynamic changes during a combined psoas compartment–sciatic nerve block for elective orthopedic surgery. Anesth Analg 112:719–724 [DOI] [PubMed] [Google Scholar]
- 18.Delaunay L, Plantet F, Jochum D. 2009. [Ultrasound and regional anaesthesia]. Ann Fr Anesth Reanim 28:140–160 [Article in French] [DOI] [PubMed] [Google Scholar]
- 19.Driessen B, Scandella M, Zarucco L. 2008. Development of a technique for continuous perineural blockade of the palmar nerves in the distal equine thoracic limb. Vet Anaesth Analg 35:432–448 [DOI] [PubMed] [Google Scholar]
- 20.Duke T. 2000. Local and regional anesthetic and analgesic techniques in the dog and cat: part I. Pharmacology of local anesthetics and topical anesthesia. Can Vet J 41:883–884 [PMC free article] [PubMed] [Google Scholar]
- 21.Duke T. 2000. Local and regional anesthetic and analgesic techniques in the dog and cat: part II. Infiltration and nerve blocks. Can Vet J 41:949–952 [PMC free article] [PubMed] [Google Scholar]
- 22.Duncan JR, Prasse KW, Mahaffey EA. 1994. Veterinary laboratory medicine: clinical pathology. Ames (IA): Iowa State University Press. [Google Scholar]
- 23.Echeverry DF, Gil F, Laredo F, Ayala MD, Belda E, Soler M, Agut A. 2010. Ultrasound-guided block of the sciatic and femoral nerves in dogs: a descriptive study. Vet J 186:210–215 [DOI] [PubMed] [Google Scholar]
- 24.Fagundes A, Negrao J, da Silva R, Gomes J, de Oliveira Souza L, Fukushima R. 2008. Environmental temperature and serum cortisol levels in growing–finishing pigs. Braz J Vet Res Anim Sci 45:136–140 [Google Scholar]
- 25.Fish RE, Brown MJ, Danneman PJ, Karas AZ. 2008. Anesthesia and analgesia in laboratory animals. San Diego (CA): Academic Press. [Google Scholar]
- 26.Flecknell PA, Waterman-Pearson A. 2000. Pain management in animals. London (UK): WB Saunders. [Google Scholar]
- 27.Freye E, Hartung E, Levy JV. 2005. No potentiation of fentanyl by use of transdermal buprenorphine in patients undergoing fast-track anesthesia for open-heart surgery. J Opioid Manag 1:162–167 [DOI] [PubMed] [Google Scholar]
- 28.Gallagher EJ, Bijur PE, Latimer C, Silver W. 2002. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am J Emerg Med 20:287–290 [DOI] [PubMed] [Google Scholar]
- 29.Goyenechea Jaramillo LA, Murrell JC, Hellebrekers LJ. 2006. Investigation of the interaction between buprenorphine and sufentanil during anaesthesia for ovariectomy in dogs. Vet Anaesth Analg 33:399–407 [DOI] [PubMed] [Google Scholar]
- 30.Guay J. 2006. The benefits of adding epidural analgesia to general anesthesia: a metaanalysis. J Anesth 20:335–340 [DOI] [PubMed] [Google Scholar]
- 31.Hargreaves KM. 1990. Neuroendocrine markers of stress. Anesth Prog 37:99–105 [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey-Clark CJ, Gilespie K, Riggs KW. 2000. Transdermal fentanyl compared with parenteral buprenorphine in postsurgical pain in swine: a case study. Lab Anim 34:386–398 [DOI] [PubMed] [Google Scholar]
- 33.Hielm-Bjorkman AK, Kapatkin AS, Rita HJ. 2011. Reliability and validity of a visual analogue scale used by owners to measure chronic pain attributable to osteoarthritis in their dogs. Am J Vet Res 72:601–607 [DOI] [PubMed] [Google Scholar]
- 34.Holton LL, Scott EM, Nolan AM, Reid J, Welsh E, Flaherty D. 1998. Comparison of 3 methods used for assessment of pain in dogs. J Am Vet Med Assoc 212:61–66 [PubMed] [Google Scholar]
- 35.Institute for Laboratory Animal Research 2009. Recognition and alleviation of pain in laboratory animals. Washington (DC): National Academies Press. [PubMed] [Google Scholar]
- 36.Institute for Laboratory Animal Research 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 37.Kadarmideen HN, Janss LL. 2007. Population and systems genetics analyses of cortisol in pigs divergently selected for stress. Physiol Genomics 29:57–65 [DOI] [PubMed] [Google Scholar]
- 38.Kahn CM. The Merck veterinary manual. Whitehouse Station (NJ): Merck.
- 39.Kim MJ, Park CS, Jun MH, Kim MC. 2007. Antagonistic effects of yohimbine in pigs anaesthetised with tiletamine–zolazepam and xylazine. Vet Rec 161:620–624 [DOI] [PubMed] [Google Scholar]
- 40.Klemcke HG, Nienaber JA, Hahn GL. 1989. Plasma adrenocorticotropic hormone and cortisol in pigs: effects of time of day on basal and stressor-altered concentrations. Proc Soc Exp Biol Med 190:42–53 [DOI] [PubMed] [Google Scholar]
- 41.Knudsen K, Beckman Suurkula M, Blomberg S, Sjovall J, Edvardsson N. 1997. Central nervous and cardiovascular effects of IV infusions of ropivacaine, bupivacaine, and placebo in volunteers. Br J Anaesth 78:507–514 [DOI] [PubMed] [Google Scholar]
- 42.Kogel B, Christoph T, Strassburger W, Friderichs E. 2005. Interaction of µ-opioid receptor agonists and antagonists with the analgesic effect of buprenorphine in mice. Eur J Pain 9:599–611 [DOI] [PubMed] [Google Scholar]
- 43.Kress HG. 2009. Clinical update on the pharmacology, efficacy, and safety of transdermal buprenorphine. Eur J Pain 13:219–230 [DOI] [PubMed] [Google Scholar]
- 44.Lascelles BD, Cripps PJ, Jones A, Waterman AE. 1997. Post-operative central hypersensitivity and pain: the preemptive value of pethidine for ovariohysterectomy. Pain 73:461–471 [DOI] [PubMed] [Google Scholar]
- 45.Lemke KA, Dawson SD. 2000. Local and regional anesthesia. Vet Clin North Am Small Anim Pract 30:839–857 [DOI] [PubMed] [Google Scholar]
- 46.Liu SS, Ngeow JE, Yadeau JT. 2009. Ultrasound-guided regional anesthesia and analgesia: a qualitative systematic review. Reg Anesth Pain Med 34:47–59 [DOI] [PubMed] [Google Scholar]
- 47.Liu SS, Salinas FV. 2003. Continuous plexus and peripheral nerve blocks for postoperative analgesia. Anesth Analg 96:263–272 [DOI] [PubMed] [Google Scholar]
- 48.Lupu CM, Kiehl TR, Chan VW, El-Beheiry H, Madden M, Brull R. 2010. Nerve expansion seen on ultrasound predicts histologic but not functional nerve injury after intraneural injection in pigs. Reg Anesth Pain Med 35:132–139 [DOI] [PubMed] [Google Scholar]
- 49.Mahler SP, Adogwa AO. 2008. Anatomical and experimental studies of brachial plexus, sciatic, and femoral nerve—location using peripheral nerve stimulation in the dog. Vet Anaesth Analg 35:80–89 [DOI] [PubMed] [Google Scholar]
- 50.Malavasi LM, Augustsson H, Jensen-Waern M, Nyman G. 2005. The effect of transdermal delivery of fentanyl on activity in growing pigs. Acta Vet Scand 46:149–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marhofer P, Schrogendorfer K, Koinig H, Kapral S, Weinstabl C, Mayer N. 1997. Ultrasonographic guidance improves sensory block and onset time of 3-in-1 blocks. Anesth Analg 85:854–857 [DOI] [PubMed] [Google Scholar]
- 52.Marx G, Horn T, Thielbein J, Knubel B, von Borell E. 2003. Analysis of pain-related vocalization in young pigs. J Sound Vibrat 266:687–698 [Google Scholar]
- 53.Muir WW, Hubbell JA, Bednarski RM, Skarda RT. 2007. Handbook of veterinary anesthesia, 4th ed. St Louis (MO): Mosby Elsevier. [Google Scholar]
- 54.Muller H, Gerlach H, Gips H, Richter M, Borner U, Hempelmann G. 1986. [Intra- and postoperative interactions between the 2 opioids fentanyl and buprenorphine]. Anaesthesist 35:219–225 [Article in German] [PubMed] [Google Scholar]
- 55.Murphy DB, McCartney CJ, Chan VW. 2000. Novel analgesic adjuncts for brachial plexus block: a systematic review. Anesth Analg 90:1122–1128 [DOI] [PubMed] [Google Scholar]
- 56.Nishimura R, Kim HY, Matsunaga S, Hayashi K, Tamura H, Sasaki N, Takeuchi A. 1994. Effects of medetomidine–midazolam on plasma glucose and insulin concentrations in laboratory pigs. J Vet Med Sci 56:559–561 [DOI] [PubMed] [Google Scholar]
- 57.Perez MP, Palacio J, Santolaria MP, del Acena MC, Chacon G, Verde MT, Calvo JH, Zaragoza MP, Gascon M, Garcia-Belenguer S. 2002. Influence of lairage time on some welfare and meat quality parameters in pigs. Vet Res 33:239–250 [DOI] [PubMed] [Google Scholar]
- 58.Prunier A, Mounier AM, Hay M. 2005. Effects of castration, tooth resection, or tail docking on plasma metabolites and stress hormones in young pigs. J Anim Sci 83:216–222 [DOI] [PubMed] [Google Scholar]
- 59.Salinas FV. 2010. Ultrasound and review of evidence for lower extremity peripheral nerve blocks. Reg Anesth Pain Med 35:S16–S25 [DOI] [PubMed] [Google Scholar]
- 60.Slingsby LS, Waterman-Pearson AE. 1998. Comparison of pethidine, buprenorphine, and ketoprofen for postoperative analgesia after ovariohysterectomy in the cat. Vet Rec 143:185–189 [DOI] [PubMed] [Google Scholar]
- 61.Thornton PC, Grant SA, Breslin DS. 2010. Adjuncts to local anesthetics in peripheral nerve blockade. Int Anesthesiol Clin 48:59–70 [DOI] [PubMed] [Google Scholar]
- 62.Troster A, Ihmsen H, Singler B, Filitz J, Koppert W. 2012. Interaction of fentanyl and buprenorphine in an experimental model of pain and central sensitization in human volunteers. Clin J Pain 28:705–711 [DOI] [PubMed] [Google Scholar]
- 63.Tsui B, Suresh S. 2010. Ultrasound imaging for regional anesthesia in infants, children, and adolescents: a review of current literature and its application in the practice of extremity and trunk blocks. Anesthesiology 112:473–492 [DOI] [PubMed] [Google Scholar]
- 64.Tsui BC, Pillay JJ. 2010. Evidence-based medicine: assessment of ultrasound imaging for regional anesthesia in infants, children, and adolescents. Reg Anesth Pain Med 35:S47–S54 [DOI] [PubMed] [Google Scholar]
- 65.Tsui BC, Suresh S. 2010. Ultrasound imaging for regional anesthesia in infants, children, and adolescents: a review of current literature and its application in the practice of neuraxial blocks. Anesthesiology 112:719–728 [DOI] [PubMed] [Google Scholar]
- 66.Udelsmann A, Silva WA, Moraes AC, Dreyer E. 2009. Hemodynamic effects of ropivacaine and levobupivacaine intravenous injection in swine. Acta Cir Bras 24:296–302 [DOI] [PubMed] [Google Scholar]
- 67.Voelckel WG, Klima G, Krismer AC, Haslinger C, Stadlbauer KH, Wenzel V, von Goedecke A. 2005. Signs of inflammation after sciatic nerve block in pigs. Anesth Analg 101:1844–1846 [DOI] [PubMed] [Google Scholar]
- 68.Wagner AE, Mama KR, Ruehlman DL, Pelkey S, Turner AS. 2011. Evaluation of effects of sciatic and femoral nerve blocks in sheep undergoing stifle surgery. Lab Anim (NY) 40:114–118 [DOI] [PubMed] [Google Scholar]
- 69.Watts AE, Nixon AJ, Reesink HL, Cheetham J, Fubini SL, Looney AL. 2011. Continuous peripheral neural blockade to alleviate signs of experimentally induced severe forelimb pain in horses. J Am Vet Med Assoc 238:1032–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilkinson AC, Thomas ML, 3rd, Morse BC. 2001. Evaluation of a transdermal fentanyl system in Yucatan miniature pigs. Contemp Top Lab Anim Sci 40:12–16 [PubMed] [Google Scholar]
- 71.Wolfe TM, Bateman SW, Cole LK, Smeak DD. 2006. Evaluation of a local anesthetic delivery system for the postoperative analgesic management of canine total ear canal ablation—a randomized, controlled, double-blinded study. Vet Anaesth Analg 33:328–339 [DOI] [PubMed] [Google Scholar]
- 72.Zink W, Bohl JR, Hacke N, Sinner B, Martin E, Graf BM. 2005. The long-term myotoxic effects of bupivacaine and ropivacaine after continuous peripheral nerve blocks. Anesth Analg 101:548–554 [DOI] [PubMed] [Google Scholar]
- 73.Zink W, Seif C, Bohl JR, Hacke N, Braun PM, Sinner B, Martin E, Fink RH, Graf BM. 2003. The acute myotoxic effects of bupivacaine and ropivacaine after continuous peripheral nerve blockades. Anesth Analg 97:1173–1179 [DOI] [PubMed] [Google Scholar]