Abstract
Large animal models of heart failure are essential in preclinical device testing. In sheep, catheter-based coil embolization of the left anterior descending and diagonal artery provides a minimally invasive and reproducible model of myocardial infarction (MI). Although widely used, this model has historically been plagued with a 30% mortality rate, both in the literature and in our own experience. Our study endeavored to decrease the mortality rate by targeting the most common cause of death, intractable arrhythmias, during creation of the ovine MI model. To this end, we evaluated 2 methods of managing perioperative antiarrhythmic therapy and cardiopulmonary resuscitation during model creation. The first group of sheep was managed at the discretion of the individual operator, whereas the second group was treated according to a standardized protocol that included mandatory pretreatment with amiodarone. Sheep experiencing life-threatening arrhythmias, most commonly ventricular fibrillation, were either resuscitated according to operator-driven instructions or the standardized protocol. By comparing these 2 treatment groups, we have shown that using a standardized protocol is advantageous in reducing mortality associated with the ovine MI model. Since implementing the standardized protocol, our laboratory has lowered the expected mortality rate to 10% during catheter-based induction of ovine MI and has greatly reduced the number of animals required for study needs. In addition, the standardized protocol has proven beneficial in training new staff members. By implementing this standardized method of model management, the outcomes of model creation have been optimized.
Abbreviation: CPR, cardiopulmonary resuscitation; LV, left ventricle; MI, myocardial infarction
Large animal models of heart failure are essential for the translation of basic science into clinical practice.3,4,6 The ovine model has been well characterized in literature3-6,9,12,14,15,17 as a crucial preclinical research model for studying the physiopathology of myocardial infarction (MI) and postMI left ventricular (LV) remodeling. This model provides the structural and biologic background to evaluate novel medical devices and cell based therapies for the treatment of heart failure. The ovine cardiovascular system is comparable to that of humans at the cellular level1,12 as well as in terms of cardiac metabolism, electrophysiology, and LV function.14 In Dorset sheep, the comparable anatomy of the heart and a coronary vasculature that lacks collateral circulation enables the creation of infarcts with predictable size and location.6,17 Furthermore, the docile nature, stable adult body size, and adequate size of the peripheral vasculature make sheep an exceedingly suitable model for chronic studies evaluating percutaneous catheter-based therapies.9
In our laboratory, a minimally invasive closed-chest approach is used to induce permanent coronary artery occlusion in sheep via catheter delivery of thrombogenic coils. This model has been useful in developing structural and functional impairment that simulates postinfarction LV dysfunction in humans.4 However, despite preventative pharmacologic therapy, perioperative arrhythmias are often refractory and may result in sudden death. The literature currently describes a 30% mortality rate associated with this model.5,9 Site and duration of occlusion, preprocedural pharmacologic treatment, anesthetic strategy, and management practices all play a role in minimizing mortality after surgical or interventional induction of MI in sheep. To minimize mortality associated with creating the ovine MI model, we targeted the primary cause of acute death, intractable arrhythmias. We hypothesized that implementing a standardized cardiopulmonary resuscitation (CPR) protocol would decrease the overall mortality associated with model creation.
Materials and Methods
Animals.
Animal use was approved by the Skirball Center for Cardiovascular Research IACUC for an ovine myocardial infarction model to be used in preclinical testing of cardiovascular therapies. Research was conducted in accordance with all applicable regulations and the Guide for the Care and Use of Laboratory Animals.11 Female Dorset sheep (Ovis aries; weight, 34 to 47 kg) were purchased from a local commercial vendor (Animal Biotech Industries, Danboro, PA). Sheep were tested and confirmed negative for Q-fever prior to purchase. All animals were vaccinated, dewormed, and acclimated for a minimum of 5 d prior to experimental procedures. Sheep were group-housed in large stainless steel cages with raised grid flooring. Sheep were provided water ad libitum and fed a dry commercial diet (Purina Rumilab 5508, WF Fisher and Son, Somerville, NJ) supplemented with alfalfa hay.
Study design.
A total of 44 sheep underwent transfemoral catheter-based embolization procedures with coronary coils for induction of MI leading to LV dysfunction and eventual chronic heart failure. The first 15 animals in the series were managed at the operator's discretion, with resuscitation of sheep experiencing life-threatening perioperative arrhythmias according to operator-driven instructions. The remaining 29 sheep were managed according to a standardized protocol (Figure 1). Importantly, all procedures were conducted in catheterization laboratories equipped with interventional radiology equipment (Innova 2100 or 3100, GE Healthcare, Milwaukee, WI) by the same 3 operators (GHY, GBC, and KM), who have extensive training in interventional cardiology. Likewise, the same experienced veterinary staff members (CF, AW, DRO, and PAM) were responsible for anesthesia, monitoring, and, when necessary, performing CPR in all 44 animals before and after implementation of the standardized protocol.
Figure 1.
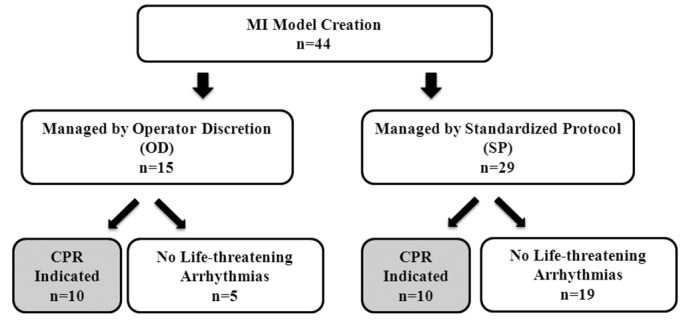
Overview of study design. Of the 44 sheep that underwent catheter-based coil embolization for the creation of a myocardial infarction model, 15 were managed according to operator discretion with regard to perioperative antiarrhythmic therapy and, if needed, resuscitation. The remaining 29 cases were managed according to a standardized protocol for antiarrhythmic therapy and cardiopulmonary resuscitation.
Management of the ovine MI model at the operator's discretion.
Prior to development and implementation of the standardized protocol, perioperative antiarrhythmic therapy and resuscitation efforts were led by the individual operator. Antiarrhythmic therapy included atenolol (25 mg PO beginning 2 d prior to procedure and continued until 2 to 3 d afterward; Butler Schein, Dublin, OH) in addition to metoprolol (Hospira Worldwide, Lake Forest, IL) and lidocaine (Hospira Worldwide), which were administered as needed at the operator's discretion for arrhythmias. Standard resuscitation drugs—atropine (American Regent, Shirley, NY), epinephrine (Hospira Worldwide), and lidocaine (Hospira Worldwide)— also were used, along with sodium bicarbonate (Hospira Worldwide) and calcium chloride (Hospira Worldwide). Although the choice of drugs used in perioperative management of arrhythmias and for resuscitation was consistent, the dosage and timing of administration varied among operators.
Development and implementation of a standardized protocol.
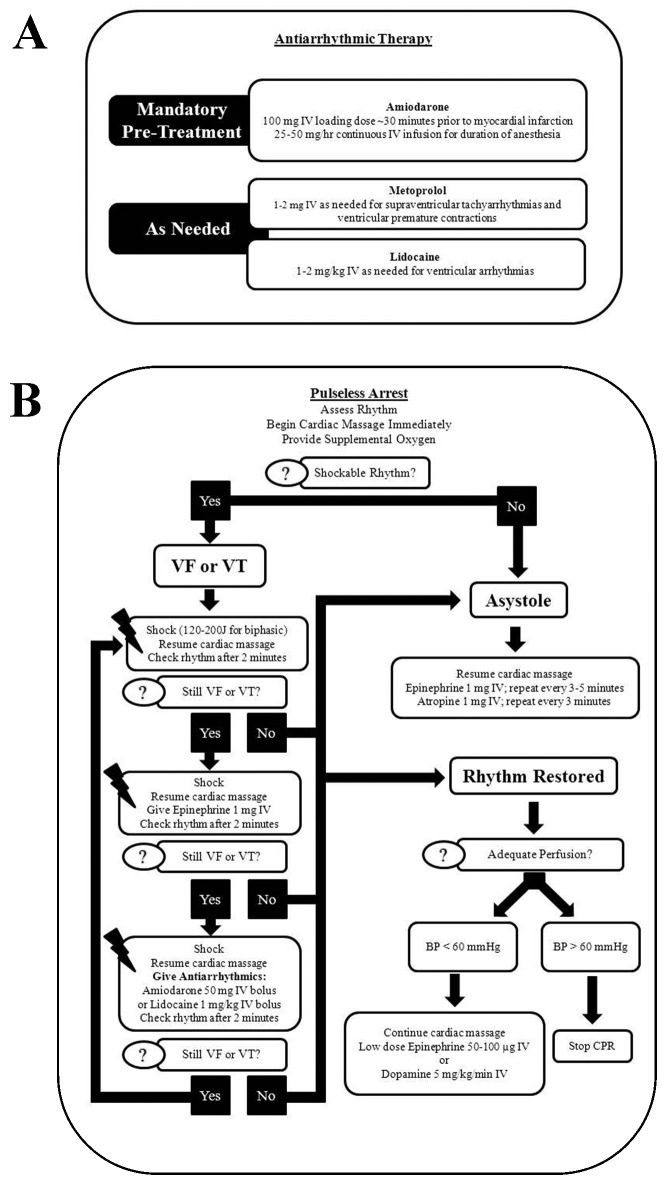
The standardized protocol (Figure 2) was developed by using published guidelines2,8,10,18 and the collective experience of cardiologists, catheterization specialists, and veterinary staff at our facility. After the protocol was developed and agreed upon, all veterinary and catheterization laboratory staff members were trained regarding the standardized protocol, with emphasis placed on rapid recognition of life-threatening arrhythmias and prompt initiation of CPR. For ease of reference, these guidelines were physically posted in catheterization laboratories and made digitally available to all staff. In addition, the standardized protocol included mandatory pretreatment of the sheep with amiodarone (100 mg IV [loading dose] approximately 30 min prior to coil embolization and then 25 to 50 mg/h continuous intraoperative intravenous infusion for the duration of anesthesia; Hospira Worldwide) and ad hoc pharmacologic treatment of arrhythmias with metoprolol (1 to 2 mg IV; Hospira Worldwide) or lidocaine (1 to 2 mg/kg IV; Hospira Worldwide) or both agents as indicated in the standardized protocol (Figure 2).
Figure 2.
Standardized protocol of (A) antiarrhythmic therapy for sheep undergoing catheter-based myocardial infarction procedures and (B) cardiopulmonary resuscitation for animals experiencing pulseless cardiac arrest. All veterinary and research staff received training according to these algorithms. In addition, the standardized protocol was posted within the catheterization laboratory and made readily available for review in digital format. VF, ventricular fibrillation; VT, ventricular tachycardia.
Ovine model of myocardial infarction.
After solid food had been withheld for 48 h, sheep were premedicated with glycopyrrolate (0.004 to 0.02 mg/kg IM; American Regent) and tiletamine–zolazepam (2 to 5 mg/kg IM; Telazol, Fort Dodge Animal Health, Fort Dodge, IA) then mask-induced with 3% to 5% isoflurane (Baxter Healthcare, Deerfield, IL). Once fully anesthetized, sheep were intubated, placed in dorsal recumbency, and connected to an anesthesia machine–mechanical ventilator. Anesthesia was maintained with 1.5% to 2.5% isoflurane in O2. Electrocardiographic and physiologic monitoring was initiated. The inguinal area was shaved and prepared for aseptic access of the femoral artery. Before catheterization, heparin (100 U/kg IV; Hospira) was given to achieve an activated clotting time of 250 s or greater; additional heparin boluses were administered as need to maintain the activated clotting time at 250 s or greater. After the appropriate depth of anesthesia was achieved, a 7-French vascular access sheath (Terumo Medical, Elkton, MD) was placed in the femoral artery percutaneously, by using the standard Seldinger technique or by surgical cutdown. Intracoronary nitroglycerin (100 μg; American Regent Shirley, NY) was prophylactically administered to prevent vasospasm. The left coronary artery was cannulated by using a 7-French guide catheter (Cordis, Miami, FL) under fluoroscopic guidance. Baseline coronary angiography and transthoracic echocardiography were performed.
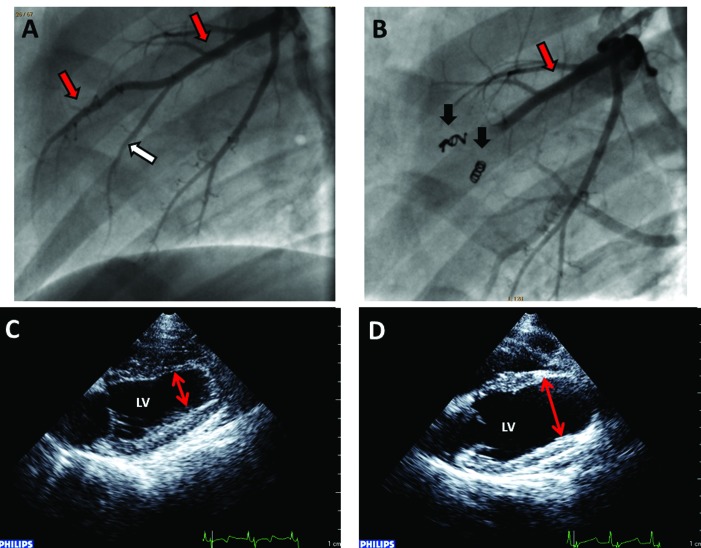
MI was induced by advancing a coronary infusion catheter into the middle left anterior descending artery (also known as the paraconal interventricular branch of the left coronary artery), where appropriately sized (diameter, 2.0 to 3.5 mm) coronary coils (Cook Medicine, Bloomington, IN) were delivered into the middle left anterior descending artery and adjacent diagonal arteries to block regional coronary blood flow. Coronary angiography was performed to confirm total occlusion and serial angiograms were taken every 15 to 20 min to ensure complete and persistent occlusion. After a 120-min period of continuous monitoring of electrocardiographic and hemodynamic parameters after coil implantation, final angiography was performed (Figure 3). All catheters and sheaths were removed, and surgical sites closed routinely. Postoperative care was provided by the veterinary staff and included continuous monitoring of vital parameters until sheep were fully recovered. Analgesia was preemptively provided by buprenorphine (0.01 to 0.02 mg/kg IM; Reckitt Benckiser Pharmaceutical, Richmond, VA) and flunixin meglumine (2 mg/kg IM; Phoenix Pharmaceutical, St Joseph, MO) given perioperatively in addition to the intraoperative use of bupivacaine as a regional anesthetic (1 mg/kg, intercostal nerve block; Hospira). Postoperative analgesia consisting of buprenorphine and flunixin meglumine was provided for an additional 1 to 2 d or longer as clinically indicated. To assess LV function, follow-up echocardiography was performed 6 wk after coil embolization (Figure 3).
Figure 3.
Representative angiographic images taken (A) before and (B) after coil implantation (thrombogenic coils, black arrows) to confirm occlusion of the left anterior descending artery (red arrows) and diagonal artery (white arrow). Vessel occlusion was confirmed by the lack of flow distal to implanted coils. Representative images of B-mode echocardiography of the LV obtained from the parasternal long-axis view (C) on day 0 and (D) at 6 wk postmyocardial infarction to assess LV function. Dilation of the LV and apical aneurysm (red double-headed arrows) was present at 6 wk after infarction.
Statistics.
Echocardiographic parameters are reported as mean ± 1 SD. Data were analyzed according to the unpaired Student t test and, when indicated, a Mann–Whitney rank sum test was used. Mortality data were analyzed by using a Fisher exact test. Differences among group means were considered significant when the P value was less than or equal to 0.05. All analyses were performed by using data analysis software system (Statistica version 10, StatSoft, Tulsa, OK).
Results
Analysis of MI and LV function.
Total occlusion of the left anterior descending artery and diagonal artery by thrombogenic coil was confirmed visually by angiography (Figure 3). The effect of myocardial infarction on LV function was assessed by echocardiography on day 0 and at 6 wk after coil embolization. In sheep surviving until 6 wk after the procedure, LV functional parameters revealed profound postMI cardiac dysfunction in both groups, as evidenced by metrics of cardiac dilatation (end-diastolic volume: 79 ± 21 mL in the operator discretion group compared with 71 ± 15 mL in the standardized protocol group, P = 0.26; end-systolic volume: 48 ± 19 mL in the operator discretion group compared with 45 ± 11 mL in the standardized protocol group, P = 0.56) and by reduction in ejection fraction (41% ± 8% in the operator discretion group compared with 38% ± 7% in the standardized protocol group, P = 0.26; Table 1).
Table 1.
Comparative analysis of LV function between groups at baseline and at 6 wk after MI
| Operator-driven instructions | Standardized protocol | P | ||
| Baseline | End-diastolic volume (mL) | 49 ± 8 | 50 ± 8 | 0.80 |
| End-systolic volume (mL) | 20 ± 4 | 22 ± 4 | 0.11 | |
| Ejection fraction (%) | 60 ± 3 | 55 ± 3 | 0.26 | |
| 6 wk after MI | End-diastolic volume (mL) | 79 ± 21 | 71 ± 15 | 0.26 |
| End-systolic volume (mL) | 48 ± 19 | 45 ± 11 | 0.56 | |
| Ejection fraction (%) | 41 ± 8 | 38 ± 7 | 0.26 |
Outcomes in sheep with life-threatening perioperative arrhythmias.
Of the 15 sheep that underwent catheter-based coil embolization and were treated according to operator discretion, 67% (10 of 15) experienced arrhythmias warranting CPR. In contrast, only 34% (10 of 29) of the sheep treated according to the standardized protocol developed life-threatening arrhythmias requiring CPR. Furthermore, the mortality rate of sheep requiring CPR differed between the 2 treatment groups. The mortality rate in the operator discretion group was 50% (5 of 10) whereas the standardized protocol group had the lower (P < 0.05) mortality rate of 30% (3 of 10). Regardless of study group, mortality was due to perioperative refractory arrhythmia, most commonly ventricular fibrillation, and typically occurred at 33 ± 10 min after coil embolization.
Effect of standardized protocol on mortality.
Overall mortality in the group treated according to operator-driven instructions was 33% (5 of 15 sheep), comparable to published information regarding this model.4 Presumably as a combined consequence of decreased frequency of life-threatening arrhythmias and improved rate of successful resuscitation, the overall mortality in sheep treated according to the standardized protocol was 10% (3 of 29). As mentioned earlier, a mortality rate of 30% occurred among animals requiring CPR that was administered according to the standardized protocol; this rate is radically reduced from the 50% mortality of sheep requiring CPR that were treated according to operator-driven instructions. The overall mortality rate associated with the standardized protocol (10%) is dramatically lower than the 33% overall mortality rate experienced prior to implementation of the standardized protocol.
Discussion
The ovine MI model is validated in the literature as a crucial step in the preclinical evaluation of therapies for human heart failure. Despite the model's many advantages and ability to create moderate MI with stable but decreased cardiovascular function, we had come to expect a mortality rate of approximately 30% during the creation of the model, a proportion that is concordant to what has been established in literature. Our current study was designed to decrease the mortality rate of the ovine middle left anterior descending artery catheter-based coil embolization procedure by targeting the most common cause of death, perioperative intractable arrhythmias. To this end, we evaluated the effect of 2 methods of CPR management on overall mortality.
When LV impairment, which corresponds to infarct size, was compared, both groups sustained similar levels of myocardial dysfunction. In the current study, we demonstrated a 20% reduction of mortality when MI model creation was managed according to a standardized protocol as compared with operator discretion. Despite higher mortality rates in the group managed according to operator discretion, a review of medical records by facility veterinarians did not reveal any inadequacies in resuscitation efforts led by individual operators. Although the reduction of mortality by using the standardized protocol was not statistically significant, we feel that animal welfare was improved notably and that the decrease in the number of animals needed for successful model creation was worthwhile.
The decreased mortality associated with the standardized protocol is likely multifactorial and may be attributed to factors that are difficult to quantify. Sheep in the standardized protocol group received dopamine to alleviate systemic hypotension; this treatment may have conferred a survival advantage. In addition, consistent pretreatment with amiodarone prior to MI induction may have played a role in reducing the susceptibility of infarcted hearts to succumbing to life-threatening arrhythmias and may have contributed to an improved success rate of CPR, but this benefit is unlikely to be solely responsible for the improved outcomes achieved. Conflicting conclusions have been reported regarding the effect of amiodarone with regard to the effectiveness of CPR efforts when administered acutely in porcine models of cardiac arrest without MI.13,20 Furthermore, in a recent report of a porcine endovascular model of myocardial infarction and reperfusion, mortality was 33% despite preoperative infusion of amiodarone.19
We mainly ascribe the success of the standardized protocol to the rapid initiation of CPR. Minimizing the preshock pause duration is known to improve the rate of successful termination of ventricular fibrillation.7 The initial seconds during cardiac arrest determine the chances of successful defibrillation. The importance of leadership in the catheterization laboratory and staff training is further emphasized by recent recommendations put forth by the American Heart Association21 and the Reassessment Campaign on Veterinary Resuscitation (RECOVER) initiative.16 In addition, after the institution of the standardized protocol, research and veterinary staff members appeared to demonstrate confidence in effective decision-making in a high-stress situation.
By implementing this protocol, we positively affected the overall success of MI model creation. Our laboratory has lowered the expected mortality rate to 10% during catheter based induction of myocardial infarction in sheep and reduced the overall number of animals required. In addition, the implementation of a standardized protocol has enhanced procedural efficiency and proven beneficial for training of veterinary and research staff. Given the results of the current study, the standardized approach to arrhythmia management that we presented here will continue to be used for this model and will be evaluated for utility in other species and studies.
References
- 1.Adler CP, Friedburg H, Herget GW, Neuburger M, Schwalb H. 1996. Variability of cardiomyocyte DNA content, ploidy level, and nuclear number in mammalian hearts. Virchows Archiv 429:159–164 [DOI] [PubMed] [Google Scholar]
- 2. Ali B, Zafari AM. 2007. Narrative review. Cardiopulmonary resuscitation and emergency cardiovascular care: review of the current guidelines. Ann Intern Med 147: 171–179. [DOI] [PubMed]
- 3.Borenstein N, Bruneval P, Behr L, Laborde F, Montarras D, Daurès JP, Derumeaux G, Pouchelon J-L, Chetboul V. 2006. An ovine model of chronic heart failure: echocardiographic and tissue Doppler-imaging characterization. J Card Surg 21:50–56 [DOI] [PubMed] [Google Scholar]
- 4.Charles CJ, Elliott JM, Nicholls MG, Rademaker MT, Richards M. 2000. Myocardial infarction with and without reperfusion in sheep: early cardiac and neurohumoral changes. Clin Sci 98:703–711 [PubMed] [Google Scholar]
- 5.Devlin G, Matthews K, McCracken G, Stuart S, Jensen J, Conaglen J, Bass J. 2000. An ovine model of chronic stable heart failure. J Card Fail 6:140–143 [DOI] [PubMed] [Google Scholar]
- 6.Dixon JA, Spinale FG. 2009. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail 2:262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edelson DP, Abella BS, Kramer-Johansen J, Wik L, Myklebust H, Barry AM, Merchant RM, Hoek TLV, Steen PA, Becker LB. 2006. Effects of compression depth and preshock pauses predict defibrillation failure during cardiac arrest. Resuscitation 71:137–145 [DOI] [PubMed] [Google Scholar]
- 8.Fletcher DJ, Boller M, Brainard BM, Haskins SC, Hopper K, McMichael MA, Rozanski EA, Rush JE, Smarick SD. 2012. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 7: clinical guidelines. J Vet Emerg Crit Care (San Antonio) 22:S102–S131 [DOI] [PubMed] [Google Scholar]
- 9.Geens JH, Trenson S, Rega FR, Verbeken EK, Meyns BP. 2009. Ovine models for chronic heart failure. Int J Artif Organs 32:496–506 [DOI] [PubMed] [Google Scholar]
- 10.Hopper K, Epstein SE, Fletcher DJ, Boller M. 2012. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 3: basic life support. J Vet Emerg Crit Care (San Antonio) 22:S26–S43 [DOI] [PubMed] [Google Scholar]
- 11.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 12.Ikeda Y, Yutani C, Huang Y, Masuda K, Yuasa T, Kawaguchi O, Hunyor SN. 2001. Histological remodeling in an ovine heart failure model resembles human ischemic cardiomyopathy. Cardiovasc Pathol 10:19–27 [DOI] [PubMed] [Google Scholar]
- 13.Ji XF, Li CS, Wang S, Yang L, Cong LH. 2010. Comparison of the efficacy of nifekalant and amiodarone in a porcine model of cardiac arrest. Resuscitation 81:1031–1036 [DOI] [PubMed] [Google Scholar]
- 14. Locatelli P, Olea FD, De Lorenzi A, Salmo F, Vera Janavel GL, Hnatiuk AP, Guevara E, Crottogini AJ. 2011. Reference values for echocardiographic parameters and indexes of left ventricular function in healthy, young adult sheep used in translational research: comparison with standardized values in humans. Int J Clin Exp Med 4: 258–264. [PMC free article] [PubMed]
- 15.Locatelli P, Olea FD, Mendiz O, Salmo F, Fazzi L, Hnatiuk A, Laguens R, Crottogini A. 2011. An ovine model of postinfarction dilated cardiomyopathy in animals with highly variable coronary anatomy. ILAR J 52:E16–E21 [DOI] [PubMed] [Google Scholar]
- 16.McMichael M, Herring J, Fletcher DJ, Boller M. 2012. RECOVER evidence and knowledge gap analysis on veterinary CPR. Part 2: preparedness and prevention. J Vet Emerg Crit Care (San Antonio) 22:S13–S25 [DOI] [PubMed] [Google Scholar]
- 17.Moainie SL, Gorman JH, Guy TS, Bowen FW, Jackson BM, Plappert T, Narula N, St John-Sutton MG, Narula J, Edmunds LH, Gorman RC. 2002. An ovine model of postinfarction dilated cardiomyopathy. Ann Thorac Surg 74:753–760 [DOI] [PubMed] [Google Scholar]
- 18.Nadkarni VM, Nolan JP, Billi JE, Bossaert L, Böttiger BW, Chamberlain D, Drajer S, Eigel B, Hazinski MF, Hickey RW, Jacobs I, Kloeck W, Montgomery WH, Morley PT, O'Connor RE, Okada K, Shuster M, Travers AH, Zideman D. 2010. Part 2: international collaboration in resuscitation science. 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 122:S276–S282 [DOI] [PubMed] [Google Scholar]
- 19.Pérez de Prado A, Cuellas-Ramón C, Regueiro-Purriños M, Gonzalo-Orden JM, Pérez-Martínez C, Altónaga JR, García-Iglesias MJ, Orden-Recio MA, García-Marín JF, Fernández-Vázquez F. 2009. Closed-chest experimental porcine model of acute myocardial infarction–reperfusion. J Pharmacol Toxicol Methods 60:301–306 [DOI] [PubMed] [Google Scholar]
- 20.Schwarz B, Mair P, Wagner-Berger H, Stadlbauer KH, Girg S, Wenzel V, Lindner KH. 2003. Neither vasopressin nor amiodarone improve CPR outcome in an animal model of hypothermic cardiac arrest. Acta Anaesthesiol Scand 47:1114–1118 [DOI] [PubMed] [Google Scholar]
- 21.Shuster M, Lim SH, Deakin CD, Kleinman ME, Koster RW, Morrison LJ, Nolan JP, Sayre MR. 2010. Part 7: CPR techniques and devices. 2010 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 122:S338–S344 [DOI] [PubMed] [Google Scholar]