Abstract
Objective
To evaluate changes in both the N-terminal (arginine vasopressin; AVP) and C-terminal (copeptin) fragments of the vasopressin prohormone before, during, and after an ultramarathon race and to assess vasopressin and copeptin concentrations in runners with and without hyponatremia.
Design
Observational study.
Setting
Three trials (2 sodium balance and 1 hyponatremia treatment) in 2 separate approximately 160-km footraces [Western States Endurance Run (WSER) and Javelina Jundred Mile Race (JJ100)].
Participants
Six hyponatremic and 20 normonatremic runners; 19 finishers with 7 completing 100 km.
Main Outcome Measures
Plasma AVP ([AVP]p), copeptin ([copeptin]p), sodium ([Na+]p), and protein (%plasma volume change; %PV) concentrations.
Results
In the WSER Sodium Trial, a 3-fold prerace to postrace increase in both [AVP]p (0.7 ± 0.4 to 2.7 ± 1.9 pg/mL; P < 0.05) and [copeptin]p (10.3 ± 12.5 to 28.2 ± 16.3 pmol/L; nonsignificant) occurred, despite a 2 mEq/L decrease in [Na+]p (138.7 ± 2.3 to 136.7 ± 1.6 mEq/L; NS). A significant correlation was noted between [AVP]p and [copeptin]p postrace (r = 0.82; P < 0.05). In the WSER Treatment Trial, despite the presence of hyponatremia pre-treatment versus posttreatment ([Na+]p = 130.3 vs 133.5 mEq/L, respectively), both [AVP]p (3.2 vs 2.1 pg/mL) and [copeptin]p (22.5 vs 24.9 pmol/L) were well above the detectable levels. A significant correlation was noted between [AVP]p and [copeptin]p 60 minutes after treatment (r = 0.94; P < 0.05). In the JJ100 Sodium Trial, significant correlations were found between [copeptin]p change and %PV change (r = −0.34; P < 0.05) and between [AVP]p change and [Na+]p change (r = 0.39; P < 0.05) but not vice-versa.
Conclusions
[Copeptin]p seems to be a reliable surrogate of stimulated [AVP]p during exercise. Nonosmotic vasopressin stimulation occurs during ultradistance running. [Copeptin]p may better reflect chronic (%PV) vasopressin secretion under conditions of endurance exercise.
Keywords: exercise, ultramarathon, antidiuretic hormone, SIADH
INTRODUCTION
Exercise-associated hyponatremia (EAH) is thought to be a variant of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), but direct evidence supporting nonosmotic arginine vasopressin (AVP) secretion as a primary pathophysiological mechanism in individuals with EAH is limited and somewhat equivocal.1–3 A major scientific hurdle to defining a clear relation between EAH and SIADH is the inherent difficulty in measuring circulating levels of bioactive AVP in the blood. The short half-life (10–20 minutes)4,5 and moderate amount of plasma (0.5–1.0 mL) required to measure bioactive AVP makes appropriate blood collection and processing difficult, particularly in field settings.2,3,6–8 Additionally, the technical difficulties and labor intensive process of accurately quantifying AVP within physiologically meaningful ranges (0–5 pg/mL) makes both the cost and the availability of AVP measurement prohibitive to many scientists performing research at athletic events.
Recent studies of copeptin, the C-terminal fragment of the vasopressin prohormone, suggest that measurement of this peptide may serve as a surrogate marker of AVP secretion,6,9,10 which could potentially reduce the multiple difficulties associated with AVP measurement in athletes who develop EAH. The stability of this 39–amino acid glycosylated peptide has enabled the development of assays for copeptin that have several distinct advantages: (1) Only small blood samples (50 μL) are required; (2) either serum or plasma can be used; (3) samples can be stored at room temperature for 7 days with < 20% loss of recovery; and (4) the assay takes hours6,9 rather than days11 to complete. Therefore, the potential use of measuring copeptin to evaluate the presence (or absence) of nonosmotic AVP secretion during prolonged endurance exercise might enhance the capability to study, diagnose, and treat what is becoming a common, and sometimes fatal, dysnatremia during endurance exercise.
To critically evaluate exercise-induced changes in AVP secretion, we simultaneously measured both copeptin and bioactive AVP prospectively in runners competing at extreme distances (approximately 160 km) because the incidence of EAH in finishers of races of this distance has previously been documented to be as high as 44% to 51%.12,13 Three separate study trials were performed in 2 different approximately 160-km races to evaluate copeptin and AVP levels in runners with and without EAH. Due to the differential stability of the short-peptide (AVP) and long-peptide (copeptin) fragments of provasopressin in plasma, we hypothesized that copeptin would be a better surrogate measure of chronic AVP secretion during prolonged endurance running, whereas bioactive AVP would better reflect more acute changes in plasma osmolality.
PATIENTS AND METHODS
Study Design and Population
All studies were approved by the Arizona State University Institutional Review Board, and subjects voluntarily gave written informed consent before participating. Three study trials were performed during 2 different approximately 160-km trail footraces in 2009: The Western States 100-Mile Endurance Run (WSER; 161 km) and the Javelina Jundred Mile Race (JJ100; 162 km). Two separate trials were simultaneously conducted during the WSER race to identify both normonatremic (Sodium Balance Trial) and hyponatremic (Hyponatremia Treatment Trial) runners. At both races, the research station was located directly adjacent to race finish, with spotters identifying and leading research subjects directly to the research area as soon as each runner crossed the finish line. Postrace blood sampling was obtained immediately after race completion (< 5 minutes) in all WSER participants before food and/or fluid were allowed and well within the half-life of bioactive AVP to ensure accurate postrace plasma concentrations. The JJ100 race was a 25-km loop course, where all runners stopped at the research station for less than 5 minutes during the race for serial blood draws and measurements. Blood was also obtained immediately upon race finish in the JJ100 cohort (< 3 minutes). Data were consolidated from 3 separate study trials to ensure that a more comprehensive evaluation of copeptin versus AVP concentrations would be evaluated in both differential states of natremia (hypo and normo) and during steady-state (prerace) versus non–steady-state conditions (mid-race and postrace).
The WSER Sodium Balance Trial
Blood (5 mL) was drawn from an antecubital vein both before and immediately upon race finish for measurement of plasma sodium concentration ([Na+]p), copeptin concentration ([copeptin]p), AVP concentration ([AVP]p), and plasma protein concentration (PP).
The WSER Hyponatremia Treatment Trial
A 5-mL blood sample was obtained in runners with a [Na+]p below 135 mEq/L (hyponatremia) immediately before and 60 minutes after the administration of hypertonic saline for analysis of [Na+]p, [copeptin]p, [AVP]p, and PP. No food or fluid intake was allowed during the 60-minute treatment period.
The JJ100 Sodium Balance Trial
Blood (5 mL) was drawn from an antecubital vein at specified intervals: prerace, 50 km, 100 km, and 162 km (postrace) for measurement of [Na+]p, [copeptin]p, [AVP]p, and PP.
Laboratory Assessment
[Na+]p was measured onsite using an I-Stat portable analyzer (Abbott, Princeton, New Jersey). All venous blood samples for [copeptin]p, [AVP]p, and PP were immediately placed on ice and centrifuged within 10 minutes at 3000 rpm. Separated plasma was immediately stored on dry ice until the samples could be frozen at −80°C. All samples remained frozen until further analysis was performed. Changes in plasma volume (PV) were estimated by comparing prerace and postrace measurements of PP using a clinical refractometer14 (Clinical Refractometer 5711–2020; Schuco, Tokyo, Japan).
[Copeptin]p was measured in a blinded fashion in a single batch using a commercial sandwich immunoluminometric assay (LUMItest CT-proAVP; B.RA.M.H.S. AG, Berlin, Germany), as described previously9 Since that original publication, the assay was modified as follows: the capture antibody was replaced by a murine monconal antibody directed to amino acids 137–144 (GPAGAL) of proAVP This modification improved the sensitivity of the assay. The lower detection limit was 0.4 pmol/L, and the functional assay sensitivity (< 20% interassay coefficient of variation) was < 1.0 pmol/L.
[AVP]p was measured in a blinded fashion in a single batch by specific radioimmunoassay after acetone-ether extraction, as described previously11 The standard curve for AVP was linear between 0.5 and 10.0 pg per tube with the use of a synthetic AVP standard (Bachem Americas, Inc, Torrance, California). The minimum detectable concentration of AVP in extracted plasma was 0.1 pg/mL, with an intraassay coefficient of variation 6.4% and an interassay coefficient of 8.8%. Plasma AVP concentrations are expressed throughout the text in picogram per milliliter. The equimolar conversion to picomoles per milliliter entails multiplying the picogram per milliliter value by a factor of 1.08.
Data Analyses
Paired t test and regression analysis were performed using Statistica version 9 (StatSoft, Tulsa, Oklahoma) and GraphPad Prism version 5 (La Jolla, California), respectively. Data are expressed as means ± SD. P values < 0.05 were considered significant.
RESULTS
A significant positive correlation was noted between [AVP]p and [copeptin]p when data from both WSER and JJ100 Trials were combined (r = 0.55; P < 0.0001; N = 74); WSER trials were combined (r = 0.67; P < 0.001); and JJ100 serial testing points combined (r = 0.31; P < 0.05).
The WSER Sodium Balance Trial
Six runners (2 women) presented at the finish line with a mean finishing time of 26 hours and 42 minutes and a mean age of 47 ± 5 years. There was a 3-fold increase in both [AVP]p and [copeptin]p from prerace to postrace, despite a 2.0 mEq/L decrease in [Na+]p (Table). Prerace (baseline) plasma concentrations of both AVP and copeptin for the normona-tremic WSER runners were lower than the pretreatment and posttreatment values for the 6 hyponatremic WSER runners enrolled into the Treatment Trial, despite their higher [Na+]p (Table). A significant positive correlation was noted between [AVP]p and [copeptin]p postrace but not prerace in normona-tremic runners participating in the WSER Sodium Balance trial (Figure 1).
TABLE.
Plasma AVP, Copeptin, and Sodium Concentrations in Hyponatremic and Normonatremic Ultradistance Runners Participating in 3 Separate Trials
| Trial Measurement (N) | [AVP]p, Mean ± SD (Minimum-Maximum), pg/mL | [Copeptin]p, Mean ± SD (Minimum-Maximum), pmol/L | [Na+]p, Mean ± SD (Minimum-Maximum), mEq/L |
|---|---|---|---|
| WSER Sodium Balance Trial, prerace (6 normonatremic) | 0.7 ± 0.4 (0.2–1.1) | 10.3 ± 12.5 (2.1–34.9) | 138.7 ± 2.3 (136–142) |
| WSER Sodium Balance Trial, postrace (6 normonatremic) | 2.7 ± 1.9 (1.4–6.4) | 28.2 ± 16.2 (3.6–52.1) | 136.7 ± 1.6 (135–139) |
| WSER Hyponatremia Trial, pretreatment (6 hyponatremic) | 3.2 ± 2.9 (0.3–6.9) | 22.5 ± 27.5 (2.9–73.0) | 130.3 ± 2.6 (126–133) |
| WSER Hyponatremia Trial, posttreatment (6 hyponatremic) | 2.1 ± 2.5 (0.5–7.1) | 24.9 ± 39.7 (2.8–105.0) | 133.5 ± 3.4 (127–136) |
| JJ100 Sodium Balance Trial, prerace14 | 0.8 ± 0.5 (0.5–2.4) | 5.0 ± 2.9 (1.4–10.4) | 141.4 ± 2.2 (137–145) |
| JJ100 Sodium Balance Trial, 50 km14 | 2.7 ± 2.0 (0.8–7.1) | 17.8 ± 13.3 (1.2–47.5) | 143.4 ± 3.5 (137–149) |
| JJ100 Sodium Balance Trial, 100 km14 | 1.2 ± 0.6 (0.3–2.1) | 16.5 ± 13.8 (2.2–35.8) | 139.6 ± 3.2 (132–144) |
| JJ100 Sodium Balance Trial, 162 km7 | 1.8 ± 1.1 (0.4–4.0) | 19.4 ± 17.3 (3.1–51.8) | 138.9 ± 2.0 (137–143) |
FIGURE 1.
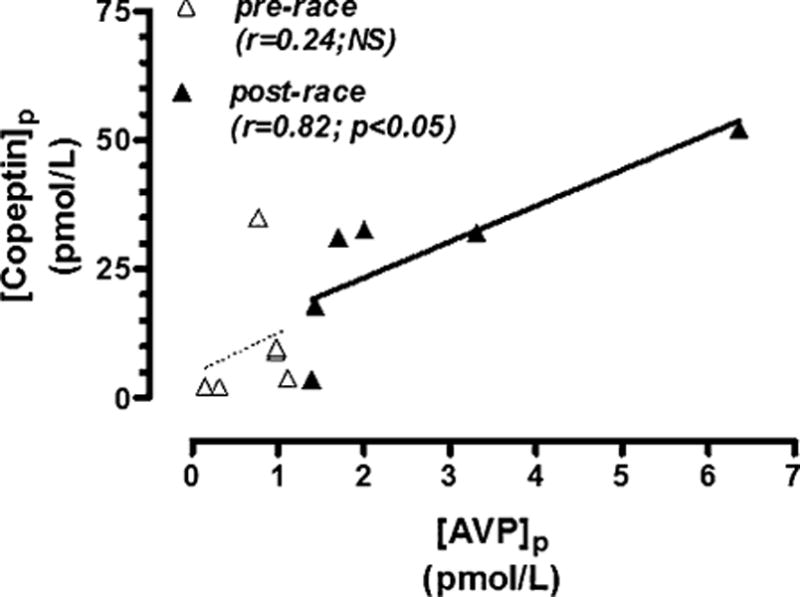
Plasma AVP versus copeptin concentrations prerace and postrace in 6 normonatremic runners completing > 150 km in the WSER Sodium Balance trial (normonatremic).
The WSER Hyponatremia Treatment Trial
Six hyponatremic runners (2 women) with paired data finished the race. Their mean finishing time was 26 hours and 15 minutes and their mean age 44 ± 9 years. Despite the presence of hyponatremia both before and after the treatment (130.3 vs133.5 mEq/L, respectively), both [AVP]p (3.2 vs 2.1 pg/mL) and [copeptin]p (22.5 vs 24.9 pmol/L) were well above detectable levels (Table). A significant positive correlation between [AVP]p and [copeptin]p was noted 60 minutes after the treatment with 100 mL of 3% NaCl but not pretreatment (Figure 2). The pretreatment to posttreatment average increase in [Na+]p (+3.2 mEq/L) was statistically significant (P < 0.05), but the corresponding pretreatment to posttreatment decrease in [AVP]p (−1.1 pg/mL) and increase in [copeptin]p (+2.4 pmol/L) were not.
FIGURE 2.
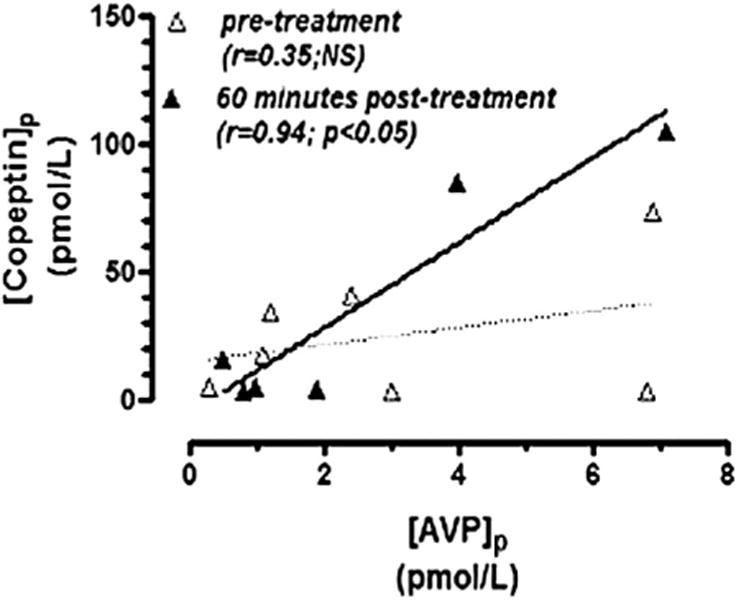
Plasma AVP versus copeptin concentrations pre-treatment and posttreatment with 100 mL of 3% NaCl in 6 hyponatremic WSER runners.
The JJ100 Sodium Trial
Seven of 16 consenting runners completed the 162-km race with a mean finishing time of 27 hours 36 minutes. This group had a mean age of 50 ± 10 years. Fourteen runners completed 100 km (Table). There were no significant differences between finishers and nonfinishers with respect to [AVP]p, [copeptin]p, and [Na+] through 100 km, so data were combined for these analyses. Only 1 female runner presented with hyponatremia ([Na+]p = 132 mEq/L) at 1 point (100 km), and her corresponding levels of [copeptin]p and [AVP]p were 2.2 pmol/L and 0.7 pg/mL, respectively. All remaining runners were normonatremic at all points tested.
The lowest mean values for both [AVP]p (0.8 ± 0.5 pg/mL) and [copeptin]p (5.0 ± 2.9 pmol/L) were recorded prerace (race registration), whereas the lowest mean [Na+]p (138.9 ± 2.0 mEq/L) was recorded at race finish when mean [copeptin]p (19.4 ± 17.3 pmol/L), but not [AVP]p (1.8 ± 1.1 pg/mL), levels were highest (Table).
A significant positive correlation was noted between [AVP]p and [copeptin]p when all JJ100 data were combined and at 100 km (r = 0.58; P < 0.05) and 162 km (r = 0.91; P < 0.01); however, significant correlations were not noted at either prerace or 50-km testing points (Figure 3). Combined data from all checkpoints revealed a statistically significant inverse correlation between [copeptin]p change and percent change in PV (r = −0.34; P < 0.05) but not between [AVP]p change and percent change in PV (Figures 4A, B). Conversely, a statistically significant positive correlation was noted between [AVP]p change and [Na+]p change (r = 0.39; P < 0.05) but not between [copeptin]p change and [Na+]p change (Figures 4C, D).
FIGURE 3.
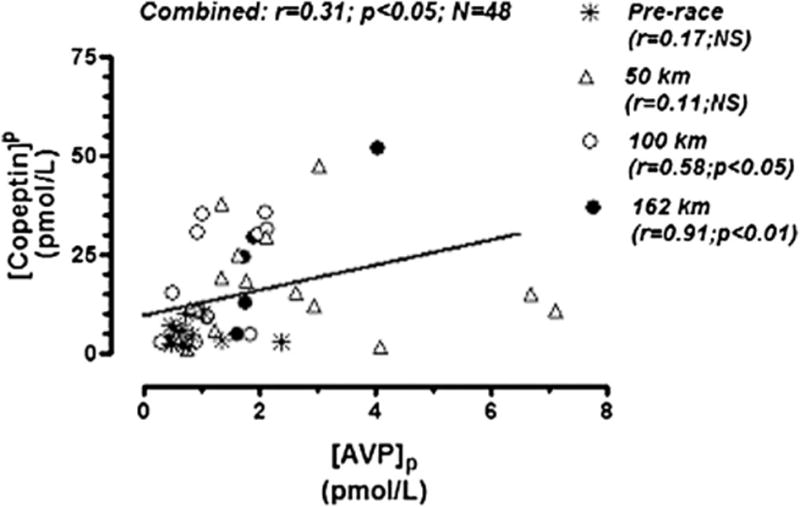
Plasma AVP versus copeptin concentrations from combined data taken from 4 serial measurements (prerace, at 50 km, at 100 km, and at 162 km) at the Javelina Jundred Mile Race.
FIGURE 4.
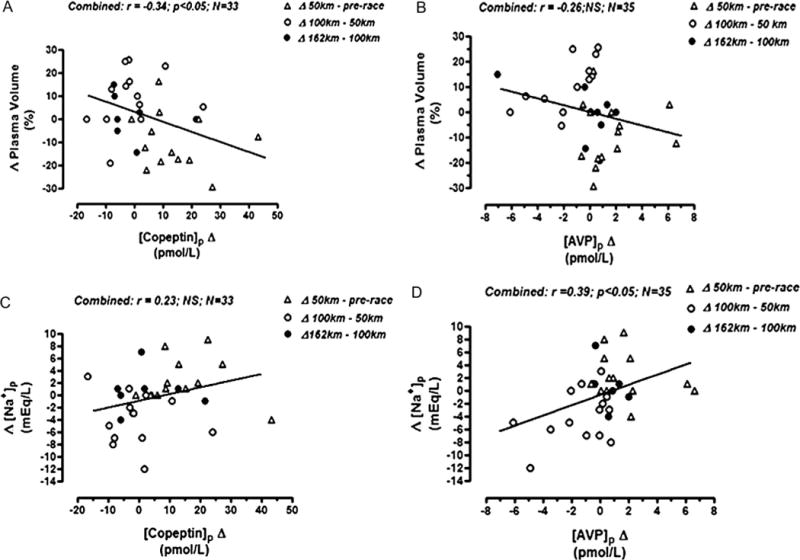
Javelina Jundred Mile Race relationships from combined serial data. A, Percent plasma volume change versus plasma copeptin concentration change. B, Percent PV change versus plasma AVP concentration change. C, Plasma sodium concentration change versus plasma copeptin concentration change. D, Plasma sodium concentration change versus plasma AVP concentration change.
DISCUSSION
Collective data from all 3 approximately 160-km field investigations support 3 major findings summarized as the following points and detailed sequentially below: (1) Both AVP and copeptin levels are significantly increased during and at the end of extreme distance runs despite decreases in [Na+]p, confirming nonosmotic simulation of AVP secretion during this sustained exercise activity; (2) significant correlations between these 2 independent measures of AVP secretion indicate the validity of using copeptin as a surrogate marker of AVP secretion in exercise studies; and (3) differences in the measured correlations of these peptides with changes in plasma [Na+]p and volume raise the possibility that exercise-induced changes in [copeptin]p may better reflect more chronic sustained changes in PV, whereas exercise-induced changes in [AVP]p may better reflect more acute changes in plasma sodium/osmolality status.
Nonosmotic Vasopressin Secretion Occurs in Normonatrmic and Hyponatremic Runners
On average, there was a 2-fold to 3-fold increase in [AVP]p and a 3-fold to 4-fold increase in [copeptin]p fromprerace to postrace in both the WSER and JJ100 trials. Exercise-induced increases in both copeptin9,15 and AVP16 have been well documented. Direct relationships between exercise intensity and PV contraction and corresponding increases in blood sodium concentration have been documented during short-duration exercise.17 In these studies of more prolonged exercise, the increases in AVP and copeptin levels occurred despite 2.0 and 2.5 mEq/L decreases in [Na+]p in each trial, respectively. More importantly for the pathophysiology of EAH, the pretreatment and posttreatment [AVP]p and [copeptin]p in the 6 hyponatremic runners enrolled into the WSER Treatment Trial were significantly elevated compared with prerace (baseline) plasma concentrations of both AVP and copeptin in the normonatremic runners in both the WSER and JJ100 trials, despite their higher [Na+]p. These results therefore confirm the conclusions of previous studies that have suggested the presence of a nonosmotic stimulus to AVP secretion under conditions of prolonged endurance exercise.1,3
In 6 hyponatremic runners enrolled into the WSER Treatment Trial, 60 minutes after the administration of a bolus of 3% NaCl, there was a mean absolute decrease in [AVP]p (−1.2 ± 2.1 pg/mL; nonsignificant) and a mean absolute increase in [copeptin]p (2.4 ± 15.9 pmol/L; nonsignificant). The more pronounced variability in [AVP]p change may be due to increased metabolic liability of the smaller peptide fragment or increased stability of the larger [copeptin]p fragment. Additionally, datum obtained from the single case of hyponatremia confirmed at the 100-km mark of the JJ100 race demonstrates that at this nadir in [Na+]p (132 mEq/L), there was a concomitant nadir in [AVP]p but not [copeptin]p. Regardless of the apparent divergence in the mean [AVP]p and [copeptin]p changes after the treatment, the magnitude of both hormone concentrations were well above detectable levels for both assays and add further confirmation of the presence of nonosmotic AVP stimulation in WSER hyponatremic runners. Whether nonsuppressed AVP levels were secondary to the SIADH or sodium depletion in hyponatremic runners cannot be fully ascertained from these studies.18
Copeptin Is a Valid Surrogate Marker for AVP During Exercise Studies
Significant positive correlations were noted between [AVP]p and [copeptin]p in both the WSER and JJ100 trials. Interestingly, the correlations were much higher for levels obtained at the end of each race (and in the JJ100 trial at the later time points) than for prerace levels, or when all data were combined. In addition, the correlation between [copeptin]p and [AVP]p were not statistically significant both (1) immediately postrace in hyponatremic runners before administration of hypertonic saline (pretreatment; Figure 1A), and (2) at the 50km point during the JJ100 race (Figure 3). Several potential explanations could explain these findings. In prolonged endurance exercise, an apparent dissociation between these variables may occur as mechanisms serving to maintain both PV19 and sodium/osmolality20 become simultaneously activated. The percentage of dysnatremia within the cohorts was greatest during these 2 testing points (100% hyponatremia in the pretreatment WSER cohort and 21% hypernatremia in the 50-km JJ100 cohort). Therefore, the lack of observed correlations at these 2 time points may have been secondary to highly volatile and competing nonosmotic (PV) and osmotic ([Na+]p) stimuli when osmotic disruption was most pronounced.
Alternatively, the lack of correlation between [copeptin]p and [AVP]p at rest (prerace) in normonatremic runners before both the WSER (Figure 1A) and JJ100 (Figure 3) races may be explained by the wide individual variability that exists in “unstimulated” AVP levels. Weak correlations between the 2 stoichiometrically released fragments have been previously demonstrated in healthy controls, which has been attributed to the suggestion that AVP and copeptin are more difficult to detect reliably at lower levels.9,10 Although this is true, both peptide fragments reported in this study were well within the detectable limits for each assay and both PV and sodium/ osmolality should have been most stable during baseline, nonexercising, testing conditions.
Despite the positive correlations between AVP and copeptin levels during prolonged exercise, the differences in the relative magnitudes of the plasma levels of these peptides are notable. Because both AVP and copeptin are released into the bloodstream by the neurohypophysis in equimolar amounts, the measured differences in the molar concentrations of these peptides would appear most likely to be due to differences in clearance and/or metabolism of either or both cleavage products of provasopressin. The molar ratio of [copeptin]p/[AVP]p measured immediately after prolonged endurance exercise in normonatremic race finishers was 11.2 ± 5.7 in the WSER Sodium Balance Trial and 9.7 ± 4.5 in the JJ100 Sodium Balance Trial, closely approximating the ratio of 12.0 ± 7.4 reported in patients with sepsis.10 It has been previously documented that the average resting [copeptin]p value in 153 healthy male subjects is 5.5 ± 2.4 pmol/L,21 whereas the mean [AVP]p value in a separate cohort of 18 healthy males is 5.5 ± 0.4 pg/mL.21 Therefore, it would be reasonable to predict that during resting (unstimulated) conditions, the molar ratio of [copeptin]p/[AVP]p would be approximately 1 (equimolar).
However, this was not the case in our runners because “unstimulated” prerace ratios were similar to the above-mentioned “stimulated” vasopressin ratios in both the WSER (14.9 ± 15.4) and JJ100 (7.4 ± 4.8) cohorts. Because the preservation of the linear association between [AVP]p and [copeptin]p has been demonstrated in this study and multiple previous studies10,22 despite obvious discrepancies in the corresponding magnitudes ([copeptin]p ≈ 10 × [AVP]p), these data do support the assumption that both peptides are cleared at different but fairly constant rates regardless of whether the plasma concentrations are low or high.5
It is well documented in both animals and humans that irreversible AVP clearance from plasma occurs predominantly, but not exclusively,23 in both the liver and kidney,5 but studies have been yet to be performed exploring the mechanism(s) of copeptin clearance. Because the stability of the copeptin fragment has potential application as a surrogate measure of AVP secretion, additional investigations clarifying how this peptide is cleared from plasma are warranted. It is important to note that exercise induces a 36% to 42% reduction in renal blood flow,24 induces a 28% to 53% reduction in hepatic clearance (dependant on ambient temperature and exercise intensity),25,26 and alters effective PV and distribution,27 all of which may contribute to alterations in the clearance rate of AVP and/or copeptin. Conversely, limited findings from animal studies demonstrate a positive linear relationship between plasma osmolality and plasma concentrations of cystine aminopeptidase (an enzyme that deactivates AVP via proteolytic cleavage) independently of the osmotic stimulus used28 suggesting that other factors may likely affect the metabolism of one or both peptides when homeostasis is perturbed.
The Long and Short Half-lives of Copeptin Versus AVP, Respectively, May Potentially Delineate Chronic Versus Acute Stimuli to Vasopressin Release During Prolonged Endurance Exercise
In runners participating in the JJ100 Sodium Balance Trial, serial data seem to support the hypothesis that [copeptin]p may be more reflective of more chronic (in our case, volemic) stimuli, whereas [AVP]p may be more reflective of acute (osmotic) stimuli. This premise is mathematically suggested by the significant inverse association between [copeptin]p change and percent PV change and positive association between [AVP]p change and blood [Na+] change—but not vice versa (Figure 4)—both of which support differential clearance of these 2 peptides.
It should be recognized that this analysis does not imply that (1) changes in bioactive [AVP]p are insensitive to acute changes in PV or (2) changes in [copeptin]p are unresponsive to changes in plasma osmolality, as previous investigations have clearly supported such relationships at rest.4,18,29 Rather, these data raise the possibility that due to the apparent differences in stability in plasma, which hypothetically are related to differential clearance rates of either or both peptides, [copeptin]p and [AVP]p may differentially reflect chronic and acute stimuli to AVP secretion during non-steady-state conditions. The predominant “chronic” nonosmotic stimuli suggested by our findings are exercise-induced PV contraction, although contributions from other potential nonosmotic stimuli (heat, stress, nausea/vomiting, etc) may likely have similar lag effects on the more stable [copeptin]p. This differential may be especially useful for studies of exercise because during periods of heightened and sustained physical stress, dynamic alterations to multiple competing stimuli occur simultaneously and are difficult to monitor through single point testing. The availability of 2 different biomarkers that assess the secretion of a single hormone could offer clear advantages during unstable physiological situations. This would particularly be true in pathological states, where analysis of both short-term (bioactive AVP) and long-term (cumulative copeptin) measurements may yield increased insights to differentiate AVP physiology from pathophysiology.
Summary
Simultaneous measurement of both the N-terminal nonpeptide AVP and the C-terminal 39-amino acid copeptin products of the vasopressin prohormone suggests that exercise-induced nonosmotic stimuli to AVP secretion commonly override osmotic regulation during competitive long distance running events. Furthermore, these studies suggest the possible utility of using [copeptin]p and bioactive [AVP]p differentially to delineate predominant stimuli to AVP secretion during non–steady-state conditions, although these data are limited in size and strength. The opportunity to choose either a long-term measure (copeptin) and/or a short-term measure (AVP) may offer a more flexible approach toward differentially studying and monitoring disorders of AVP secretion, including treatment and surveillance, in clinical care and exercise settings.
Limitations
We acknowledge that the small sample size(s) and observational design limits the overall strength of these 3 hypothesized conclusions. Most specifically, novel speculation that copeptin levels may better reflect persistent stimuli (PV changes) and vice versa may be further open to debate secondary to our chosen technique for measuring PV change. Our estimations of percent PV change (using plasma proteins) have been systematically evaluated against both changes in hematocrit and plasma water in animal models.14 In that comparative analysis,14 values for percent PV change comparing plasma protein (4.8%–6.4%) and plasma water (3.9%–6.3%) were much lower than the PV change observed using hematocrit (7.4%–9.2%) presumably due to the influx of red blood cells into the central circulation in response to hypovolemia.14 Hence, no current method for determination of percent PV change is currently without limitation (including direct measures of Evans blue dye or indocyanine green), particularly during prolonged endurance exercise, whereas further comparisons between the different methodologies needs to be more critically addressed.
Acknowledgments
The authors are humbly indebted to the help of Ulrike Haagen at the Thermo Fisher Scientific/Brahms for arranging the copeptin measurements from Berlin; Greg Soderland, members of the WSER Board, and WSER volunteers whose support made the ambitious work possible; Jamil and Nathan Coury from the Javelina Jundred race who instigated the work at short notice with overwhelming support and enthusiasm; Nik Sharma and Qin Xu from the Georgetown University Medical Center’s Endocrine Laboratory who ran these samples with care, expertise, and timeliness; Lulu Weschler, Ginger Hook, Ben Holexa, and Bill Butler whose integrity and commitment to research and field support are beyond measure; and finally the noble and esteemed participants whose benevolence and sacrifice to science as well as their fellow runners, who are truly admirable.
Supported by a grant from the Western States Endurance Run Foundation. Copeptin measurements were performed and supported by Thermo Fisher Scientific/Brahms.
Footnotes
Nils G. Morgenthaler is employed by Thermo Fisher Scientific.
References
- 1.Hew-Butler T, Jordaan E, Stuempfle KJ, et al. Osmotic and non-osmotic regulation of arginine vasopressin during prolonged endurance exercise. J Clin Endocrinol Metab. 2008;93:2072–2078. doi: 10.1210/jc.2007-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hew-Butler TD, Ayus JC, Kipps C, et al. Statement of the second international exercise-associated hyponatremia consensus development conference, New Zealand, 2007. Clin J Sport Med. 2008;18:111–121. doi: 10.1097/JSM.0b013e318168ff31. [DOI] [PubMed] [Google Scholar]
- 3.Siegel AJ, Verbalis JG, Clement S, et al. Hyponatremia in marathon runners due to inappropriate arginine vasopressin secretion. Am J Med. 2007;120:461.e11–e17. doi: 10.1016/j.amjmed.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Verbalis JG. Disorders of body water homeostasis. Best Pract Res Clin Endocrinol Metab. 2003;17:471–503. doi: 10.1016/s1521-690x(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 5.Lauson HD. Metabolism of neurohypophyseal hormones. In: Greep RO, Astwood EB, Knobil E, et al., editors. Handbook of Physiology. Washington, DC: American Physiological Society; 1974. pp. 287–393. [Google Scholar]
- 6.Morgenthaler NG, Struck J, Jochberger S, et al. Copeptin: clinical use of a new biomarker. Trends Endocrinol Metab. 2008;19:43–49. doi: 10.1016/j.tem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Nelson PB, Ellis D, Fu F, et al. Fluid and electrolyte balance during a cool weather marathon. Am J Sports Med. 1989;17:770–772. doi: 10.1177/036354658901700608. [DOI] [PubMed] [Google Scholar]
- 8.Dessypris A, Wagar G, Fyhrquist F, et al. Marathon run: effects on blood cortisol—ACTH, iodothyronines—TSH and vasopressin. Acta Endocrinol (Copenh) 1980;95:151–157. doi: 10.1530/acta.0.0950151. [DOI] [PubMed] [Google Scholar]
- 9.Morgenthaler NG, Struck J, Alonso C, et al. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 10.Jochberger S, Morgenthaler NG, Mayr VD, et al. Copeptin and arginine vasopressin concentrations in critically ill patients. J Clin Endocrinol Metab. 2006;91:4381–4386. doi: 10.1210/jc.2005-2830. [DOI] [PubMed] [Google Scholar]
- 11.Verbalis JG, McHale CM, Gardiner TW, et al. Oxytocin and vasopressin secretion in response to stimuli producing learned taste aversion in rats. Behav Neurosci. 1986;100:466–475. doi: 10.1037//0735-7044.100.4.466. [DOI] [PubMed] [Google Scholar]
- 12.Stuempfle KJ, Lehmann DR, Case HS, et al. Hyponatremia in a cold weather ultraendurance race. Alaska Med. 2002;44:51–55. [PubMed] [Google Scholar]
- 13.Lebus DK, Casazza GA, Hoffman MD, et al. Can changes in body mass and total body water accurately predict hyponatremia following a 161-km running race? Clin J Sport Med. 2010;20:193–199. doi: 10.1097/JSM.0b013e3181da53ea. [DOI] [PubMed] [Google Scholar]
- 14.Stricker EM. Some physiological and motivational properties of the hypovolemic stimulus for thirst. Physiol Behav. 1968;3:379–385. [Google Scholar]
- 15.Maeder MT, Staub D, Brutsche MH, et al. Copeptin response to clinical maximal exercise tests. Clin Chem. 2010;56:674–676. doi: 10.1373/clinchem.2009.136309. [DOI] [PubMed] [Google Scholar]
- 16.Hew-Butler T, Noakes T, Soldin S, et al. Acute changes in endocrine and fluid balance markers during high intensity, steady-state and prolonged endurance running: unexpected increases in oxytocin and brain natriuretic peptide during exercise. Eur J Endocrinol. 2008;159:729–737. doi: 10.1530/EJE-08-0064. [DOI] [PubMed] [Google Scholar]
- 17.Wilkerson JE, Horvath SM, Gutin B, et al. Plasma electrolyte content and concentration during treadmill exercise in humans. J Appl Physiol. 1982;53:1529–1539. doi: 10.1152/jappl.1982.53.6.1529. [DOI] [PubMed] [Google Scholar]
- 18.Fenske W, Stork S, Blechschmidt A, et al. Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab. 2009;94:123–129. doi: 10.1210/jc.2008-1426. [DOI] [PubMed] [Google Scholar]
- 19.Senay LC, Jr, Pivarnik JM. Fluid shifts during exercise. Exerc Sport Sci Rev. 1985;13:335–387. [PubMed] [Google Scholar]
- 20.Hew-Butler T, Collins M, Bosch A, et al. Maintenance of plasma volume and serum sodium concentration despite body weight loss in Ironman triathletes. Clin J Sport Med. 2007;17:116–122. doi: 10.1097/JSM.0b013e3180326836. [DOI] [PubMed] [Google Scholar]
- 21.Aylward PE, Floras JS, Leimbach WN, et al. Effects of vasopressin on the circulation and its baroreflex control in healthy men. Circulation. 1986;73:1145–1154. doi: 10.1161/01.cir.73.6.1145. [DOI] [PubMed] [Google Scholar]
- 22.Jochberger S, Dorler J, Luckner G, et al. The vasopressin and copeptin response to infection, severe sepsis, and septic shock. Crit Care Med. 2009;37:476–482. doi: 10.1097/CCM.0b013e3181957532. [DOI] [PubMed] [Google Scholar]
- 23.Share L, Kimura T, Matsui K, et al. Metabolism of vasopressin. Fed Proc. 1985;44:59–61. [PubMed] [Google Scholar]
- 24.Radigan LR, Robinson S. Effects of environmental heat stress and exercise on renal blood flow and filtration rate. J Appl Physiol. 1949;2:185–191. doi: 10.1152/jappl.1949.2.4.185. [DOI] [PubMed] [Google Scholar]
- 25.Rowell LB, Blackmon JR, Martin RH, et al. Hepatic clearance of indocyanine green in man under thermal and exercise stresses. J Appl Physiol. 1965;20:384–394. doi: 10.1152/jappl.1965.20.3.384. [DOI] [PubMed] [Google Scholar]
- 26.Rowell LB, Blackmon JR, Bruce RA. Indocyanine green clearance and estimated hepatic blood flow during mild to maximal exercise in upright man. J Clin Invest. 1964;43:1677–1690. doi: 10.1172/JCI105043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swartz RD, Sidell FR. Effects of heat and exercise on the elimination of pralidoxime in man. Clin Pharmacol Ther. 1973;14:83–89. doi: 10.1002/cpt197314183. [DOI] [PubMed] [Google Scholar]
- 28.Silveira PF, Irazusta J, Gil J, et al. Interactions among challenges of hydromineral balance, angiotensin-converting enzyme, and cystine aminopeptidase. Peptides. 2001;22:2137–2144. doi: 10.1016/s0196-9781(01)00556-3. [DOI] [PubMed] [Google Scholar]
- 29.Szinnai G, Morgenthaler NG, Berneis K, et al. Changes in plasma copeptin, the c-terminal portion of arginine vasopressin during water deprivation and excess in healthy subjects. J Clin Endocrinol Metab. 2007;92:3973–3978. doi: 10.1210/jc.2007-0232. [DOI] [PubMed] [Google Scholar]


