Abstract
Glucocorticoids including betamethasone (BM) are routinely administered to women entering into early preterm labor to facilitate fetal lung development and decrease infant mortality; however, fetal steroid exposure may lead to deleterious long term consequences. In a sheep model of fetal programming, BM-exposed (BMX) offspring exhibit elevated mean arterial pressure (MAP) and decreased baroreflex sensitivity (BRS) for control of heart rate by 0.5-years of age associated with changes in the circulating and renal renin-angiotensin systems (RAS). In the brain solitary tract nucleus, angiotensin (Ang) II actions through the AT1 receptor oppose the beneficial actions of Ang-(1-7) at the Mas receptor for BRS regulation. Therefore, we examined Ang peptides, angiotensinogen (Aogen), and receptor expression in this brain region of exposed and control offspring of 0.5- and 1.8-years of age. Mas protein expression was significantly lower (>40%) in the dorsal medulla of BMX animals at both ages; however, AT1 receptor expression was not changed. BMX offspring exhibited a higher ratio of Ang II to Ang-(1-7) (2.30 ± 0.36 versus 0.99 ± 0.28; p<0.01) and Ang II to Ang I at 0.5-years. Although total Aogen was unchanged, Ang I-intact Aogen was lower in 0.5-year BMX animals (0.78 ± 0.06 vs. 1.94 ± 0.41; p<0.05) suggesting a greater degree of enzymatic processing of the precursor protein in exposed animals. We conclude that in utero BM exposure promotes an imbalance in the central RAS pathways of Ang II and Ang-(1-7) that may contribute to the elevated MAP and lower BRS in this model.
Keywords: fetal programming, Renin Angiotensin System, brain, sheep, hypertension
1. Introduction
Antenatal glucocorticoid (GC) therapy decreases respiratory distress syndrome and infant mortality when administered to women at risk for preterm delivery [22]. Numerous randomized, controlled trails confirm the efficacy of this therapy [8, 38], and organizations such as the National Institutes of Health and the American College of Obstetricians and Gynecologists have recommended antenatal GC treatment for women at risk for delivery before 34 weeks of gestation [1].
The long term consequences of fetal GC exposure are not well characterized, particularly their influence on cardiovascular events. At 14 years of age, preterm children exposed to GCs exhibit higher blood pressure than children born preterm with no exposure [9]. Experimental studies by our group and others have begun to elucidate the potential mechanisms for altered blood pressure associated with GC exposure [2, 5, 40]. These mechanisms include alterations in kidney development [41], a significant reduction in nephron number [2, 44], impaired neural control [28, 30, 33], and alterations to the circulating and local renin-angiotensin systems (RAS) [2, 5, 29, 40]. In the present study, pregnant ewes were exposed to a clinically relevant dose of BM during the early third trimester, a critical window of kidney and brain development in the fetus. This time corresponds to the period at which GC therapy is administered to women entering into preterm labor [29]. Therefore, we investigated the role of both BM exposure and age on the expression of the brain RAS. We hypothesize that the balance between Ang II and Ang-(1-7) pathways within the brain are altered in a way that is consistent with the chronic elevation in blood pressure and reduction in BRS in this sheep model of fetal programming.
2. Materials and methods
2.1 Animals
Sheep received saline or betamethasone acetate: phosphate 1:1 mixture (IM, 2 doses of 0.17 mg/kg, 24 hours apart) at the 80th day of gestation. After term delivery, animals were farm raised and weaned at 3 months of age. At 0.5- or 1.8-years, male offspring were brought to our Association for Assessment of Laboratory Animals Care (ACUC) approved facility, where they were maintained on a normal diet, with free access to tap water and a 12-hour light/dark cycle (lights on 7 AM to 7 PM). Sheep were anesthetized with ketamine and isoflurane and euthanized by exsanguination. Brain medullas were removed and immediately covered in Clear Frozen Section Compound (VWR West Chester, PA) and stored at −80°C. Tissue from a total of 21 animals was used in this study. These procedures were approved by the Wake Forest University School of Medicine ACUC for animal care.
2.2 Western Blot Analysis
Brain medullas were cut 4 mm rostral and 2 mm caudal to the obex and divided in half along the dorsoventral axis to isolate the dorsal medulla including the NTS. Isolated membrane or cytoplasmic fractions of brain dorsal medulla (10 and 35 μg, respectively) were added to Laemmli buffer containing β-mercaptoethanol. Proteins were separated on 12% SDS polyacrylamide gels for 80 min at 120 V in Tris-glycine buffer and electrophoretically transferred onto polyvinylidene difluoride membranes. Immunodetection was performed on blots blocked for 1 h with 5% dry milk (Bio-Rad, Hercules, CA) and Tris-buffered saline containing 0.05% Tween and probed with antibodies against Mas (1:250 dilution; Alomone AAR-013, Jerusalem, Israel), AT1 receptor (1:200; Alomone AAR-011) and both total and Ang I-intact forms of rat angiotensinogen (Aogen: 1:2,000). Mas and AT1 receptor antibodies were probed against proteins separated using the Criterion Cell and Blotter (Bio-Rad) on 12% Tris-HCl 26 lane gels (Bio-Rad 345-0016). Specificity of Mas and AT1 receptor antibodies was validated by preabsorption of the antibody with the immunizing peptide (ratio of 1μg peptide to 1μg antibody) on proteins run on 12% Mini-PROTEAN TGX gels. The two Aogen antibodies were raised against residues 25–34 [Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu-Cys*, Ang I sequence] and residues 42–57 [Cys-Ala-Gln-Leu-Glu-Asn-Pro-Ser-Val-Glu-Thr-Leu-Pro-Glu-Pro-Thr] of the rat protein [18]. An additional cysteine residue (C*) was added for covalent coupling of the Ang I peptide to keyhole limpet hemocyanin to enhance antigenicity. Both rat and sheep contain the identical Ang I sequence while the sheep 42–57 sequence [Cys-Asp-Gln-Leu-Glu-Lys-Pro-Ser-Val-Glu-Thr-Ala-Pro-Asp-Pro-Thr] shares similar identity to the rat [18]. Aogen antibodies were probed on 12% Mini-PROTEAN TGX gels. Reactive proteins were detected with PerkinElmer ECL substrate (Waltham, MA) and exposed to Amersham Hyperfilm enhanced chemiluminescence (Piscataway, NJ). Gels were stripped and probed with β-Actin (Sigma-Aldrich A5441) as a loading control. Band density was calculated using MCID Elite 7.0 (Cambridge, England)
2.3 Angiotensinogen Measurement
Renin isolated from sheep kidney cortex (100 mg) was added to a cocktail of inhibitors (aprotinin, bestatin, PCMB, soybean trypsin inhibitor, 10 μM each) in the presence or absence of aliskiren (10 μM) on ice. Nephrectomized sheep plasma as the source of intact Aogen was added and the reaction was transferred to a 37°C water bath. Aliquots of the reactions were removed at 30, 60, 120, and 240 minutes, added to Laemmli buffer containing β-mercaptoethanol, and put on ice. All samples were boiled and loaded on a gel for Western blot analysis. Separate gels were run and the blots probed with antibodies against the Ang I sequence (Ang I-intact or AI-Aogen) and the internal sequence (Int-Aogen), representing both intact and Ang I-cleaved forms of angiotensinogen (total Aogen).
2.4 Peptide Measurement
Ang peptides in the medullary tissues were measured in the Hypertension Center Core Assay Laboratory utilizing multiple radioimmunoassays (RIAs) [4, 6, 29]. Frozen medullas were homogenized in acid ethanol (80% vol/vol 0.1 N HCl) containing the peptidase inhibitors EDTA, phenanthroline, phenylmethylsulfonyl fluoride (PMSF), p-Chloromercuribenzoic acid (PCMB), and a renin inhibitor [4]. Total protein content was analyzed in aliquots from the acid ethanol homogenate using the Bradford protein assay with BSA as a standard. Homogenates were centrifuged at 30,000 g for 20 min, and supernatant was decanted and acidified with 1% heptafluorobutyric acid. The solution was precipitated overnight at 4°C and centrifuged at 30,000 g for 20 min. The supernatants were concentrated in a vacuum centrifuge and applied to activated Sep-Paks C18 columns (Waters, Milford, MA), washed with 0.1% HFBA, and eluted with 5 ml of 80% methanol, and 0.1% HFBA. Recovery of Ang peptides was determined by addition of 125I-Ang-(1–7) to homogenates by comparing total counts applied to the Sep-Pak to that recovered in the eluate [6, 29]. The Ang peptide content of each fraction was determined by separate RIAs for Ang I, Ang II, and Ang-(1-7) that fully recognize each peptide but cross-react less than 0.01% with each other [29]. Minimum detection levels for the assays are 1 fmol/ml, 0.8 fmol/ml, and 2.8 fmol/ml for Ang I, Ang II, and Ang-(1-7), respectively. Peptide content in the medulla is expressed as fmol/mg protein.
2.5 Statistics
Data are expressed as mean ± SEM. Unpaired t tests and two-way ANOVA with Bonferroni posttests were used for the statistical analysis of the data with GraphPad Prism 5.01 (GraphPad Software, San Diego, CA). The criterion for statistical significance was set at *P < 0.05. We are able to detect a difference of 45% between group means with an N=4 in each group, and a difference of 55% between group means for N=3 with a standard deviation equal to 15% of the total value, and a Beta error of 80%.
3. Results
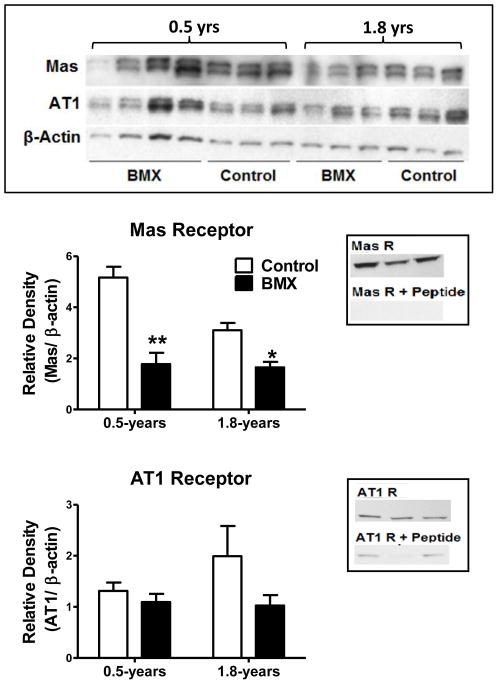
The protein expression for Mas and AT1 receptors was determined by Western blot analysis normalized to β-Actin. Both antibodies revealed double bands at the expected molecular weights (Mas antibody = 50 kDa, AT1 receptor antibody = 40 kDa) using the Criterion Cell apparatus and 12% Tris-HCl gels (Figure 1, upper panel). Direct comparisons between control and BMX animals as well as for 0.5- and 1.8-years of age were achieved with a 26 lane gel. As shown in Figure 1 (middle panel, left), Mas receptor expression was significantly lower in the BMX animals (p < 0.05) at both 0.5- and 1.8-years of age. In contrast, there was no difference in AT1 receptor protein expression in homogenates of the dorsal medulla at 0.5-years (Figure 1, lower panel, left). We noted a large variability in AT1 receptor expression at 1.8-years; however, there was no trend towards increased AT1 receptor expression in BMX animals, indicating that altered AT1 receptor expression may not contribute to the phenotype at this time. Preabsorption of the primary antibody with the appropriate immunizing peptide was performed on tissue extracts separated on 12% Mini-PROTEAN TGX gels. This abolished the protein band for the Mas receptor, and attenuated expression of the AT1 receptor band (Figure 1, middle and lower panels, right).
Figure 1.
The AT7/Mas receptor protein expression was significantly reduced in the dorsal medulla of 0.5- and 1.8-Year old betamethasone exposed (BMX) animals. Upper panel: Western blots for Mas and AT1 receptors, as well as β-Actin expression in the dorsal medulla from 0.5 and 1.8 year olds. Quantification of receptor expression revealed reduced levels of the Mas protein at both ages (middle panel, left) and the protein band was abolished by preabsorption of the primary antibody with the antigenic peptide (middle panel, right). There was no reduction in AT1 receptor expression (lower panel, left) and the protein band was diminished by preabsorption with the antigenic peptide (lower panel, right). Mas and AT1 receptor densities were normalized to β-Actin. Mas protein was detected at 50 kDa, AT1 at 40 kDa, and β-Actin at 42 kDa. Data are mean ± SEM [0.5-Year: N = 4 BMX; N = 3 control and 1.8-Year: N = 3 BMX; N = 3 control]; *P < 0.05 vs. control
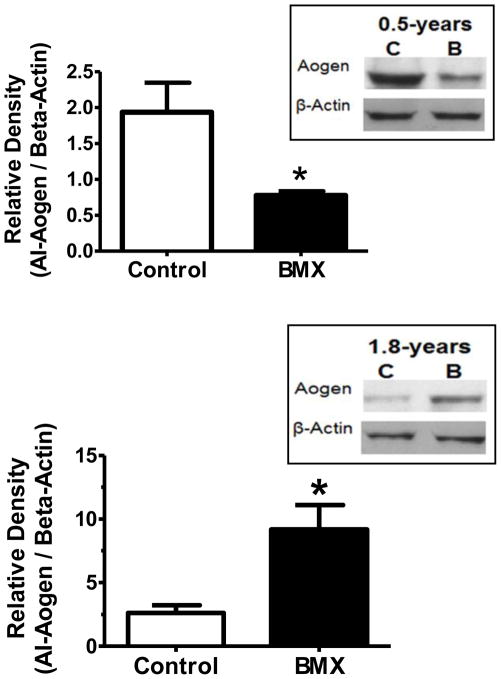
The specificity of the Aogen antibodies in sheep was validated by measuring AI-Aogen and total Aogen in samples with and without the renin inhibitor aliskerin. The cytoplasm from kidney cortex homogenates provided active renin, and plasma from nephrectomized sheep was the source of the intact AI-Aogen substrate. The upper blot of Figure 2 reveals a time dependent decrease in the AI-Aogen samples lacking the renin inhibitor. Addition of aliskiren essentially abolished the disappearance of the AI-Aogen band up to the 4 hour time point (240 mins). In contrast, the lower blot probed with the Int-Aogen antibody demonstrates no change in the band over time regardless of the presence of aliskiren suggesting this antibody measures both Ang I and des-Ang I forms of Aogen (total Aogen). Utilizing these antibodies, we then quantified the relative expression of Aogen in the brain medulla. At 0.5-years of age, there was no difference in total Aogen protein expression between control and BMX animals (data not shown); however, AI-Aogen was 44% lower in the BMX animals (Figure 3, upper panel). These data suggest that greater processing of Aogen may occur in the dorsal medulla of exposed sheep at this age. In contrast, AI-Aogen was 280% higher in the dorsal medulla of BMX animals (Figure 3 lower panel) with no change in total Aogen levels (data not shown)at 1.8-years of age.
Figure 2.
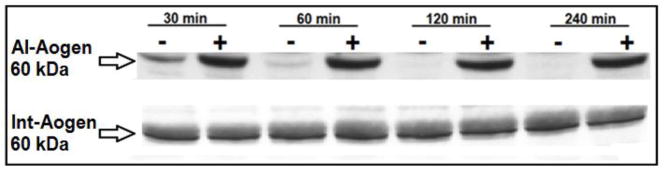
The renin inhibitor aliskiren preserves Ang I-intact angiotensinogen (AI-Aogen). Upper blot: immunoblot of samples without (−) or with (+) the renin inhibitor aliskiren probed with the AI-Aogen antibody. Lower blot: immunoblot of samples (−/+ aliskerin) probed with the Int-Aogen antibody. Samples contained nephrectomized sheep plasma incubated with the cytosolic fraction of sheep renal cortex from 30 to 240 minutes at 37°C. The 60 kDa band is shown.
Figure 3.
AI-Aogen expression was decreased in betamethasone exposed (BMX) animals at 0.5-years of age. Western blot analysis of AI-Aogen in dorsal medulla of 0.5- (upper panel) and 1.8- (lower panel) year old animals is shown. AI-Aogen protein was detected at 60 kDa. Band density was normalized to β-Actin. Data are mean ± SEM [0.5-Year N = 4 control; N = 4 BMX and 1.8-Year N = 3 control; N = 3 BMX]; * P < 0.05 vs. control.
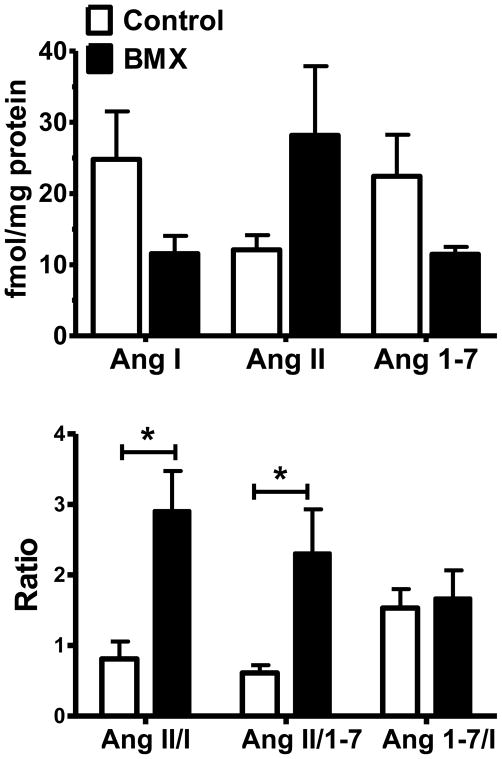
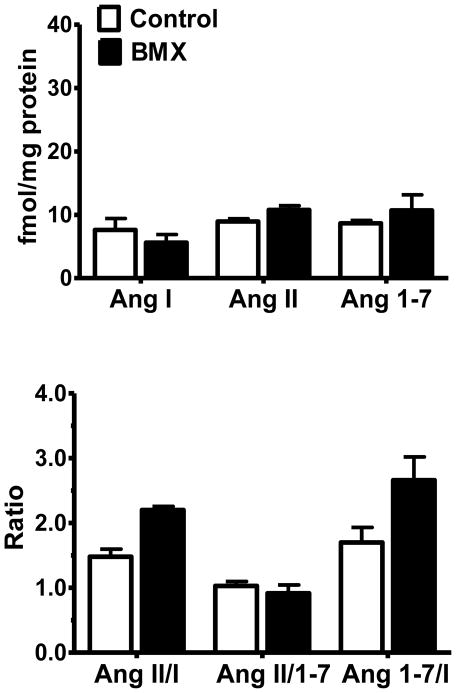
Tissue concentrations of Ang I, Ang II, and Ang-(1-7) were determined in the dorsal medulla of both 0.5- (Figure 4, upper panel) and 1.8- (Figure 5, upper panel) year old animals. Although there were no statistical differences between BMX and control animals for any of the individual peptides, expression of the data as peptide ratios revealed significant differences between control and exposed animals. At 0.5-years of age, BMX animals exhibit a significantly higher ratio of Ang II to Ang-(1-7) as well as a higher Ang II/Ang I (Figure 4, lower panel). The ratio of Ang-(1-7)/Ang I was not different between groups. Moreover, there were no differences in the peptide content or ratios between BMX and control animals at the 1.8-year time point (Figure 5, lower panel).
Figure 4.
Angiotensin tissue content and the peptide ratios in control and betamethasone exposed (BMX) animals at 0.5-Years of age. The tissue content of Ang I, Ang II, and Ang-(1-7) in dorsal medulla of 0.5-year old animals was not significantly different between control and BMX groups (upper panel). Significantly higher peptide ratios of Ang II: Ang-(1-7) and Ang II: Ang I but not Ang-(1-7):Ang I were evident in BMX animals compared to controls (lower panel). Data are mean ± SEM [N = 5 control; N = 4 BMX]; *P < 0.05 vs. control.
Figure 5.
Angiotensin tissue content and the peptide ratios in control and betamethasone exposed (BMX) animals at 1.8-Years of age. Tissue content of angiotensin peptide (upper panel) and the peptide ratios (lower panel) were not significantly different between BMX and control groups at this age. Data are mean ± SEM [N = 3 control; N = 4 BMX].
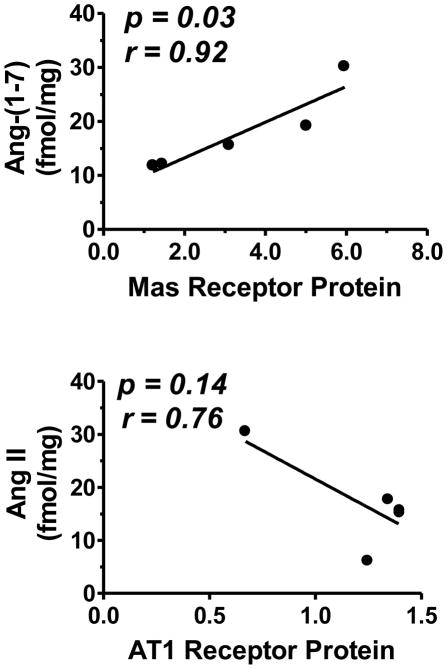
As shown in Figure 6, there was a significant positive correlation between Ang-(1-7) peptide levels and the Mas receptor (p = 0.02; r = 0.92) at 0.5-years, while the levels of Ang II peptide trended towards a negative correlation with the AT1 receptor at this age (p = 0.07; r = −0.84).
Figure 6.
Correlation of Mas protein expression with Ang-(1-7) peptide levels in the brain dorsal medulla. Control and betamethasone exposed (BMX) animals are both used in this correlation. There was a positive correlation between Mas receptor and Ang-(1-7) peptide concentration (upper panel), and a trend for negative correlation between AT1 receptor and Ang II peptide concentration (lower panel). Correlation analysis was performed using GraphPad Prism 5.01 plotting and statistical software. Data are mean ± SEM [N = 5 in each group of control and BMX sheep].
4. Discussion
In the sheep model of GC-induced fetal programming, antenatal BM exposure is associated with impaired BRS as early as 6-weeks of age, with reduced BRS and increased MAP by 0.5-years of age [28, 30, 33]. Furthermore, microinjection of the AT1 receptor antagonist Candesartan (CV) into the nucleus tractus solitarius improved BRS in both control and BMX animals at 6-weeks of age [33]. In contrast, Ang-(1-7) receptor blockade with the selective Mas antagonist D-Ala7-Ang-(1-7) (D-Ala, A779) inhibits the reflex only in the unexposed animals [30, 31, 33]. In BMX sheep at 0.5- and 1.8-years of age, AT1 receptor blockade also improved the reflex and lowered arterial blood pressure to that of controls [30, 34]. However, there was little effect of bilateral microinjection of D-Ala on BRS or blood pressure in BMX sheep [30]. Overall, these data suggest an imbalance in the actions of Ang II and Ang-(1-7) for baroreflex control of heart rate in the brain medulla that occurs as early as 6-weeks and persists to adulthood [32]. The current study demonstrates that lower expression of the Mas receptor in the dorsal medulla at both 0.5- and 1.8- years of age. At 0.5-years of age there is also a higher ratio of Ang II to Ang-(1-7), suggesting decreased Ang-(1-7) tone in this brain region may contribute to the impaired BRS and dysregulation of blood pressure. Indeed, these data reflect the functional imbalance between Ang II and Ang-(1-7) in the kidney of BMX animals. We previously showed that young male BMX sheep exhibit a decreased renal vascular response to Ang-(1-7) [37]. Ang-(1-7) infusion inhibits sodium reabsorption in control males, but prenatal BM also attenuated the natriuretic response to Ang-(1-7) [37]. Binding studies also revealed a greater proportion of AT1 sites in the renal cortex of adult BMX sheep, as well as a reduced proportion of AT7 or D-Ala sensitive sites [17]. Thus, BMX may induce the loss of Ang-(1-7) receptors resulting in the inability of the kidney to produce a natriuretic response to Ang-(1-7) [37].
In addition to altered receptor expression, AI-Aogen expression was lower in the BMX animals at 0.5-years, suggesting an enhanced degree of Aogen processing to form Ang peptides. At the same age, the Ang II: Ang-(1-7) and Ang II: Ang I ratios were higher in BMX animals, but there was no difference in the ratio of Ang-(1-7) to Ang I. This suggests an increased role for Ang II in 0.5-year BMX animals relative to Ang-(1-7) that may likely contribute to the suppression of BRS at this age [34]. Moreover, the greater extent of Aogen processing in the medulla of BMX sheep may contribute to higher levels of Ang II without alterations in the tissue content of Ang-(1-7). Thus, it is possible that changes in ACE and ACE2 activities further influence the relative levels of the peptides. The balance between Ang II and Ang I may reflect higher ACE activity, while the balance between Ang II and Ang-(1-7) may reflect both ACE and ACE2 activities. Our previous study demonstrated that the ratio of ACE to ACE2 activity was significantly higher in the circulation of the BMX sheep reflecting changes in both peptidase activities [29]. Proximal tubular and urinary ACE2 activities were also reduced in the kidneys of the exposed animals [35]. Studies are in progress to assess the enzyme activities of ACE and ACE2 in the dorsal medullary tissues of control and BMX animals.
At 1.8-years of age, there is less AI-Aogen processing in BMX compared to control animals. While administration of CV reduces blood pressure and enhances BRS in 1.8-year old BMX animals [28, 29], we now report that Ang II content and AT1 receptor expression are no different than controls. It is possible that a reduction in the Mas receptor may lead to less Ang-(1-7) tone in the functional antagonism of the Ang II-AT1 receptor axis. Kostenis et al [20] show that the Mas receptor heterodimerizes with the AT1 receptor and significantly impairs the Ang II mediated elevation of intracellular Ca2+ with no change in AT1 protein expression or pharmacological characteristics of the receptor. Therefore, the loss of the Mas receptor may result in greater AT1 receptor signaling in the absence of overall changes in the AT1 receptor protein or Ang II levels. This may explain the sensitivity of the BMX sheep to acute administration of the AT1 antagonist CV at 1.8 years of age [28, 29].
Finally, we correlated the Ang II and Ang-(1-7) peptide levels to the AT1 and Mas receptors, in the dorsal medullas of 0.5-year old animals. This analysis revealed a trend for the negative correlation between Ang II and the AT1 receptor consistent with previous reports that Ang II attenuates expression of the AT1 receptor [21, 24]. Interestingly, we found a positive correlation between Ang-(1-7) and expression of the Mas receptor. Indeed, several studies have documented changes in the Mas receptor in various pathological conditions [10, 12, 17]. There are conflicting studies regarding the effect of Ang-(1-7) on Mas receptor expression; however, our study would support the findings of Tan et al [27, 36] that demonstrate the feed forward or positive regulation of the Mas receptor by Ang-(1-7). Therefore, a reduction in tissue levels of Ang-(1-7) may attenuate expression of Mas and lead to an imbalance favoring greater Ang II-AT1 receptor tone. In this regard, the nitric oxide response to Ang-(1-7) was reduced in the renal cortex of BMX sheep at 1.8-years of age which was associated with a lower proportion of D-Ala-sensitive binding sites [16, 17, 18, 25]. Moreover, our studies find that the Ang-(1-7) antagonist D-Ala increases blood pressure and inhibits the BRS in control but not BMX sheep at 0.5-years of age [30]. These data suggest that attenuated expression of Ang-(1-7) and the Mas receptor may contribute to the cardiovascular phenotype of BMX sheep.
GCs are strong regulators of fetal growth and development which may influence a myriad of target proteins including growth factors, cytoarchitectural proteins, receptors, binding proteins, as well as various components of cell signaling pathways [13]. Overexposure to GCs during fetal development influences numerous organ systems and predisposes the individual to disease states later in life [7, 41]. However, we find that as early as 6-weeks of age, BM exposure is associated with an altered BRS despite the fact that blood pressure is not changed. Therefore, it is possible that the cardiovascular centers in the brain may be an early and key target for programming events following BM exposure in utero. In this regard, maternal protein deprivation in late gestation also resulted in increased mRNA expression of Aogen and ACE, but reduced levels of AT2 receptor mRNA levels in fetal rat brains [15]. These data lend additional support to the concept that the brain undergoes almost immediate alterations in response to a stressful in utero environment. Further characterization of the timing of changes that take place in 6-week old brains is required. Importantly, this time period will allow for the assessment of the RAS components when BRS is altered but MAP is not changed between control and BMX animals.
In addition to the influence on the RAS, in utero overexposure to GCs reduced placental expression of 11β-hydrosteroid dehydrogenase (11β-HSD2), an enzyme which oxidizes active GCs to their inactive derivates [39]. The enzyme plays a protective role in pregnancy, where 11β-HSD2 is highly concentrated in the placenta and shields the fetus from overexposure to the circulating maternal GCs [43]. During development the expression of 11β-HSD2 is evident throughout the brain, suggesting that the enzyme may protect sensitive tissues from overexpression to GCs before the placental barrier has been fully established [26]. While 11β-HSD2 expression decreases greatly after birth, recent studies have revealed 11β-HSD2 mRNA in the NTS of adult rat brains using histochemistry and RT-PCR [26]. The effect of prenatal GC exposure on brain 11β-HSD2 activity has not been well characterized; however, studies in the kidney reveal lower 11β-HSD2 expression in rats exposed to maternal low protein diet [42]. It is possible that fetal exposure to GCs alters 11β-HSD2 activity in the brain, thus allowing an excess of active steroids to reach key brain areas regulating blood pressure and the stress response.
Alterations in GC content and receptor expression may contribute to fetal programming through epigenetic mechanisms. Both hypertension and fetal programming exhibit altered methylation patterns and modified histones in the brain. Goyal et al [15] found that antenatal maternal protein deprivation leads to epigenetic changes and alterations in the RAS within fetal mouse brains. In this model, the mRNA levels of both Aogen and the AT2 receptors were higher in exposed offspring. Moreover, maternal protein deprivation was associated with decreased methylation of CpG islands in the promoter regions of the ACE-1 gene, and upregulation of miRNAs that regulate ACE-1 mRNA translation in the fetal brain. DNA methylation at CpG islands and histone acetylation are also known to limit nephron development [23]. Reduced nephron number during development or shortly after birth is correlation to the development of essential hypertension later in life [19]. The 11βHSD-2 gene is under epigenetic control and plays an important regulatory role in fetal exposure to maternal glucocorticoids [3]. High 11βHSD-2 promoter methylation is associated with hypertension in patients treated with glucocorticoids [11, 14]. It is possible that epigenetic changes such as histone modification or DNA methylation play a role in the development of the functional changes evident in this model of fetal programming.
Conclusion
It is widely accepted that events that take place in utero have the ability to impact the long term cardiovascular health of an individual. The current study uses a GC induced model of fetal programming to investigate the role of the brain RAS in the development and maintenance of hypertension. These data provide evidence that BMX sheep undergo programming events that alter the receptor levels and peptide ratios in the brain dorsal medulla that may functionally change the balance between Ang II and Ang-(1-7). Targeted therapies that restore the balance of these two peptidergic systems within the brain RAS may be clinically important in the fetal programming of cardiovascular disease.
Highlights.
BM exposure decreases Mas receptor expression in medulla at 0.5- and 1.8-years
Decreased Mas partially explains previous reports of decreased autonomic function
Aogen processing is augmented at 0.5- years and reduced at 1.8-years in BMX sheep
Peptides ratios are shifted in favor of the Ang II in BMX animals at 0.5- years
Ang(1-7) peptide levels positively correlate to mas receptor at 0.5- years
Acknowledgments
This work was supported by the National Institutes of Health (HD-047584, HD-017644, and HL-51952), the Groskert Heart Fund, and Wake Forest Venture Fund. Additionally, the authors gratefully acknowledge Ellen Tommasi, Nancy Pirro and Eric LeSaine for their technical and surgical support.
Glossary of terms
- Aogen
Angiotensinogen
- Ang
Angiotensin
- Ang I
[Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8-His9-Leu10]
- Ang II
[Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7-Phe8]
- Ang-(1-7)
[Asp1-Arg2-Val3-Tyr4-Ile5-His6-Pro7]
- AI-Aogen
antibody against angiotensinogen residues 25–34
- Int Aogen
antibody against angiotensinogen residues 42–57
- GC
glucocorticoid
- BM
Betamethasone
- BMX
Betamethasone Exposed
- RAS
renin-angiotensin system
Footnotes
Conflict of interests
The authors declare that there are no competing financial interests in the work described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. JAMA. 1995;273:413–8. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 2.Alexander BT. Fetal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1–R10. doi: 10.1152/ajpregu.00417.2005. [DOI] [PubMed] [Google Scholar]
- 3.Alikhani-Koopaei R, Fouladkou F, Frey FJ, Frey BM. Epigenetic regulation of 11 beta-hydroxysteroid dehydrogenase type 2 expression. J Clin Invest. 2004;114:1146–57. doi: 10.1172/JCI21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allred AJ, Chappell MC, Ferrario CM, Diz DI. Differential actions of renal ischemic injury on the intrarenal angiotensin system. Am J Physiol Renal Physiol. 2000;279:F636–45. doi: 10.1152/ajprenal.2000.279.4.F636. [DOI] [PubMed] [Google Scholar]
- 5.Bikkavilli RK, Tsang SY, Tang WM, Sun JX, Ngai SM, Lee SS, et al. Identification and characterization of surrogate peptide ligand for orphan G protein-coupled receptor mas using phage-displayed peptide library. Biochem Pharmacol. 2006;71:319–37. doi: 10.1016/j.bcp.2005.10.050. [DOI] [PubMed] [Google Scholar]
- 6.Chappell MC, Brosnihan KB, Diz DI, Ferrario CM. Identification of angiotensin-(1-7) in rat brain. Evidence for differential processing of angiotensin peptides. J Biol Chem. 1989;264:16518–23. [PubMed] [Google Scholar]
- 7.Dodic M, Moritz K, Koukoulas I, Wintour EM. Programmed hypertension: kidney, brain or both? Trends Endocrinol Metab. 2002;13:403–8. doi: 10.1016/s1043-2760(02)00693-8. [DOI] [PubMed] [Google Scholar]
- 8.Doran TA, Swyer P, MacMurray B, Mahon W, Enhorning G, Bernstein A, et al. Results of a double-blind controlled study on the use of betamethasone in the prevention of respiratory distress syndrome. Am J Obstet Gynecol. 1980;136:313–20. doi: 10.1016/0002-9378(80)90855-8. [DOI] [PubMed] [Google Scholar]
- 9.Doyle LW, Ford GW, Davis NM, Callanan C. Antenatal corticosteroid therapy and blood pressure at 14 years of age in preterm children. Clin Sci (Lond) 2000;98:137–42. [PubMed] [Google Scholar]
- 10.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, et al. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–82. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrari P, Sansonnens A, Dick B, Frey FJ. In vivo 11beta-HSD-2 activity: variability, salt-sensitivity, and effect of licorice. Hypertension. 2001;38:1330–6. doi: 10.1161/hy1101.096112. [DOI] [PubMed] [Google Scholar]
- 12.Filho AG, Ferreira AJ, Santos SH, Neves SR, Silva Camargos ER, Becker LK, et al. Selective increase of angiotensin(1-7) and its receptor in hearts of spontaneously hypertensive rats subjected to physical training. Exp Physiol. 2008;93:589–98. doi: 10.1113/expphysiol.2007.014293. [DOI] [PubMed] [Google Scholar]
- 13.Fowden AL, Forhead AJ. Endocrine mechanisms of intrauterine programming. Reproduction. 2004;127:515–26. doi: 10.1530/rep.1.00033. [DOI] [PubMed] [Google Scholar]
- 14.Friso S, Pizzolo F, Choi SW, Guarini P, Castagna A, Ravagnani V, et al. Epigenetic control of 11 beta-hydroxysteroid dehydrogenase 2 gene promoter is related to human hypertension. Atherosclerosis. 2008;199:323–7. doi: 10.1016/j.atherosclerosis.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 15.Goyal R, Goyal D, Leitzke A, Gheorghe CP, Longo LD. Brain renin-angiotensin system: fetal epigenetic programming by maternal protein restriction during pregnancy. Reprod Sci. 2010;17:227–38. doi: 10.1177/1933719109351935. [DOI] [PubMed] [Google Scholar]
- 16.Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, et al. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296:F1484–93. doi: 10.1152/ajprenal.90766.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwathmey TM, Shaltout HA, Rose JC, Diz DI, Chappell MC. Glucocorticoid-induced fetal programming alters the functional complement of angiotensin receptor subtypes within the kidney. Hypertension. 2011;57:620–6. doi: 10.1161/HYPERTENSIONAHA.110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwathmey TM, Westwood BM, Pirro NT, Tang L, Rose JC, Diz DI, et al. Nuclear angiotensin-(1-7) receptor is functionally coupled to the formation of nitric oxide. Am J Physiol Renal Physiol. 2010;299:F983–90. doi: 10.1152/ajprenal.00371.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoy WE, Hughson MD, Bertram JF, Douglas-Denton R, Amann K. Nephron number, hypertension, renal disease, and renal failure. J Am Soc Nephrol. 2005;16:2557–64. doi: 10.1681/ASN.2005020172. [DOI] [PubMed] [Google Scholar]
- 20.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, et al. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111:1806–13. doi: 10.1161/01.CIR.0000160867.23556.7D. [DOI] [PubMed] [Google Scholar]
- 21.Lassegue B, Alexander RW, Nickenig G, Clark M, Murphy TJ, Griendling KK. Angiotensin II down-regulates the vascular smooth muscle AT1 receptor by transcriptional and post-transcriptional mechanisms: evidence for homologous and heterologous regulation. Mol Pharmacol. 1995;48:601–9. [PubMed] [Google Scholar]
- 22.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25. [PubMed] [Google Scholar]
- 23.Millis RM. Epigenetics and hypertension. Curr Hypertens Rep. 2011;13:21–8. doi: 10.1007/s11906-010-0173-8. [DOI] [PubMed] [Google Scholar]
- 24.Ouali R, Berthelon MC, Begeot M, Saez JM. Angiotensin II receptor subtypes AT1 and AT2 are down-regulated by angiotensin II through AT1 receptor by different mechanisms. Endocrinology. 1997;138:725–33. doi: 10.1210/endo.138.2.4952. [DOI] [PubMed] [Google Scholar]
- 25.Pendergrass KD, Gwathmey TM, Michalek RD, Grayson JM, Chappell MC. The angiotensin II-AT1 receptor stimulates reactive oxygen species within the cell nucleus. Biochem Biophys Res Commun. 2009;384:149–54. doi: 10.1016/j.bbrc.2009.04.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seckl JR. 11beta-Hydroxysteroid dehydrogenase in the brain: a novel regulator of glucocorticoid action? Front Neuroendocrinol. 1997;18:49–99. doi: 10.1006/frne.1996.0143. [DOI] [PubMed] [Google Scholar]
- 27.Shah A, Oh YB, Lee SH, Lim JM, Kim SH. Angiotensin-(1-7) attenuates hypertension in exercise-trained renal hypertensive rats. Am J Physiol Heart Circ Physiol. 2012;302:H2372–80. doi: 10.1152/ajpheart.00846.2011. [DOI] [PubMed] [Google Scholar]
- 28.Shaltout HA, Chappell MC, Rose JC, Diz DI. Exaggerated sympathetic mediated responses to behavioral or pharmacological challenges following antenatal betamethasone exposure. Am J Physiol Endocrinol Metab. 2011;300:E979–85. doi: 10.1152/ajpendo.00636.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaltout HA, Figueroa JP, Rose JC, Diz DI, Chappell MC. Alterations in circulatory and renal angiotensin-converting enzyme and angiotensin-converting enzyme 2 in fetal programmed hypertension. Hypertension. 2009;53:404–8. doi: 10.1161/HYPERTENSIONAHA.108.124339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaltout HA, Figueroa JP, Rose JC, Chappell MC, Averill DB, Diz DI. Evidence of Ang-(1-7) deficiency in antenatal betamethasone-treated young adult sheep. Abstract Hypertension. 2008;52:E107. [Google Scholar]
- 31.Shaltout HA, Rose JC, Chappell MC, Diz DI. Antenatal betamethasone exposure attenuates the functional role of angiotensin-(1-7) in the NTS. Hypertension. 2010;56:e103. [Google Scholar]
- 32.Shaltout HA, Rose JC, Chappell MC. Diz DI Ang-(1-7) Deficiency and Impairment of Baroreflex for Control of Heart Rate Precede the Antenatal Betamethasone Exposure-Induced Elevation in Blood Pressure. Abstract Hypertension. 2011;58:163. doi: 10.1161/HYPERTENSIONAHA.111.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaltout HA, Rose JC, Chappell MC, Diz DI. Angiotensin-(1-7) deficiency and baroreflex impairment precede the antenatal Betamethasone exposure-induced elevation in blood pressure. Hypertension. 2012;59:453–8. doi: 10.1161/HYPERTENSIONAHA.111.185876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaltout HA, Rose JC, Figueroa JP, Chappell MC, Diz DI, Averill DB. Acute AT(1)-receptor blockade reverses the hemodynamic and baroreflex impairment in adult sheep exposed to antenatal betamethasone. Am J Physiol Heart Circ Physiol. 2010;299:H541–7. doi: 10.1152/ajpheart.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, et al. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292:F82–91. doi: 10.1152/ajprenal.00139.2006. [DOI] [PubMed] [Google Scholar]
- 36.Tan Z, Wu J, Ma H. Regulation of angiotensin-converting enzyme 2 and Mas receptor by Ang-(1-7) in heart and kidney of spontaneously hypertensive rats. J Renin Angiotensin Aldosterone Syst. 2011;12:413–9. doi: 10.1177/1470320311402109. [DOI] [PubMed] [Google Scholar]
- 37.Tang L, Bi J, Valego N, Carey L, Figueroa J, Chappell M, et al. Prenatal betamethasone exposure alters renal function in immature sheep: sex differences in effects. Am J Physiol Regul Integr Comp Physiol. 2010;299:R793–803. doi: 10.1152/ajpregu.00590.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Teramo K, Hallman M, Raivio KO. Maternal glucocorticoid in unplanned premature labor. Controlled study on the effects of betamethasone phosphate on the phospholipids of the gastric aspirate and on the adrenal cortical function of the newborn infant. Pediatr Res. 1980;14:326–9. doi: 10.1203/00006450-198004000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Vackova Z, Vagnerova K, Libra A, Miksik I, Pacha J, Staud F. Dexamethasone and betamethasone administration during pregnancy affects expression and function of 11 beta-hydroxysteroid dehydrogenase type 2 in the rat placenta. Reprod Toxicol. 2009;28:46–51. doi: 10.1016/j.reprotox.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Wang Z, Takahashi T, Saito Y, Nagasaki H, Ly NK, Nothacker HP, et al. Salusin beta is a surrogate ligand of the mas-like G protein-coupled receptor MrgA1. Eur J Pharmacol. 2006;539:145–50. doi: 10.1016/j.ejphar.2006.03.064. [DOI] [PubMed] [Google Scholar]
- 41.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol. 2005;289:R955–62. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- 42.Wyrwoll CS, Mark PJ, Waddell BJ. Developmental programming of renal glucocorticoid sensitivity and the renin-angiotensin system. Hypertension. 2007;50:579–84. doi: 10.1161/HYPERTENSIONAHA.107.091603. [DOI] [PubMed] [Google Scholar]
- 43.Yang K. Placental 11 beta-hydroxysteroid dehydrogenase: barrier to maternal glucocorticoids. Rev Reprod. 1997;2:129–32. doi: 10.1530/ror.0.0020129. [DOI] [PubMed] [Google Scholar]
- 44.Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod Sci. 2010;17:186–95. doi: 10.1177/1933719109351098. [DOI] [PMC free article] [PubMed] [Google Scholar]