Abstract
Despite the conventional treatments of radiation therapy and chemotherapy, the 5-year survival rates for patients with advanced-stage cervical cancers remain low. Cancer immunotherapy has emerged as an alternative, innovative therapy that may improve survival. Here, we utilize a preclinical HPV-16 E6/E7-expressing tumor model, TC-1, and employ the chemotherapeutic agent cisplatin to generate an accumulation of CD11c+ dendritic cells in tumor loci making it an ideal location for the administration of therapeutic vaccines. Following cisplatin treatment, we tested different routes of administration of a therapeutic HPV vaccinia vaccine encoding HPV-16 E7 antigen (CRT/E7-VV). We found that TC-1 tumor-bearing C57BL/6 mice treated with cisplatin and intratumoral injection of CRT/E7-VV significantly increased E7-specific CD8+ T cells in the blood and generated potent local and systemic antitumor immune responses compared to mice receiving cisplatin and CRT/E7-VV intraperitoneally or mice treated with cisplatin alone. We further extended our study using a clinical grade recombinant vaccinia vaccine encoding HPV-16/18 E6/E7 antigens (TA-HPV). We found that intratumoral injection with TA-HPV following cisplatin treatment also led to increased E7-specific CD8+ T cells in the blood as well as significantly decreased tumor size compared to intratumoral injection with wild type vaccinia virus. Our study has strong implications for future clinical translation using intratumoral injection of TA-HPV in conjunction with the current treatment strategies for patients with advanced cervical cancer.
Keywords: Cisplatin, Vaccine, Vaccinia virus, Human papillomavirus, TA-HPV
Introduction
Cervical cancer is the third most common female cancer worldwide [1]. Advanced stages of cervical cancer are commonly treated by radiation therapy combined with cisplatin-based chemotherapy [2]. Despite the current treatment regimens, stage 3 and 4 cervical cancers have a 5-year survival rate of less than 35 % [3]. Therefore, alternative innovative therapies are required if improvements in survival rates are to be realized in this clinical context. Cancer immunotherapy represents one such promising treatment. This is because the adaptive immune system, once activated, is able to specifically recognize and target tumor cells while sparing normal cells. Furthermore, tumor-specific immunity may be able to recognize multiple tumor loci throughout the body. Thus, cancer immunotherapy represents a potentially promising approach that can be used in conjunction with conventional chemotherapy and radiotherapy to further improve the survival rate of patients with advanced-stage cervical cancer.
Cervical cancer is an ideal model for developing cancer immunotherapy methodologies because tumor-specific antigens are well defined. It is now clear that human papillomavirus (HPV) is the etiological factor for cervical cancer (for review, see [4]). Furthermore, two HPV-encoded oncogenic proteins, E6 and E7, are constantly expressed in the majority of HPV-associated cervical cancers and are responsible for the malignant transformation of pre-cancerous lesions to cervical cancer [5]. Thus, E6 and E7 are ideal targets for cancer immunotherapy aiming to generate an immune response to HPV in cervical cancer cells [6]. Recombinant vaccinia virus (VV) encoding E6 or E7 can efficiently deliver E6/E7 antigen in vivo. Therefore, the VV vector is an attractive methodology to generate an E6/E7-specific immune response to eliminate HPV infected tumor cells.
Our lab has previously created a VV vector expressing the chimeric protein consisting of calreticulin (CRT) linked to HPV-16 E7 (CRT/E7-VV) [7]. CRT has been shown to enhance CD8+ T cell–mediated immune responses by improving antigen processing associated with MHC class I antigen presentation [8, 9]. We have demonstrated that mice vaccinated with CRT/E7-VV generated the highest E7-specific CD8+ T-cell immune response compared to those vaccinated with VV expressing relevant control proteins [7]. The potent E7-specific CD8+ T-cell immune response generated by CRT/E7-VV translates into a powerful therapeutic antitumor effect. This indicates that the VV expressing the CRT/E7 chimeric protein represents an ideal vaccine for generating E7-specific antitumor immunity.
Although vaccination with CRT/E7-VV was found to generate an antitumor immune response, it is still critical to determine the optimal route of administration in order to effectively deliver CRT/E7-VV to professional antigen-presenting cells, such as dendritic cells (DCs). Recently, we found that treating mice with the chemotherapeutic agent cisplatin generated a significantly greater accumulation of CD11c+ DCs in tumor loci compared with untreated mice [10]. The increased accumulation of CD11c+ DCs in the tumor represents an ideal location for administration of therapeutic vaccines such as CRT/E7-VV.
In this study, we explored the treatment of E7-expressing tumor-bearing mice with cisplatin in combination with intratumoral injection of CRT/E7-VV or TA-HPV. We demonstrated that mice treated with this approach were capable of generating a potent systemic E7-specific CD8+ T-cell immune response as well as a strong therapeutic antitumor effect against E7-expressing tumors. We further extended our study using a clinical grade therapeutic HPV recombinant vaccinia vaccine, TA-HPV [6, 11–13]. TA-HPV carries modified E6 and E7 genes from HPV types 16 and 18 and has been safely administered to over 100 patients in the previously mentioned clinical trials [14]. However, these studies use intramuscular or percutaneous scarification for vaccine administration and do not take advantage of directly injecting the vaccine in an environment enriched with professional antigen-presenting cells. Our study indicated that intratumoral injection of TA-HPV also generated potent HPV antigen-specific CD8+ T-cell immune responses and antitumor effects against HPV-16 E6/E7 expressing tumors. Our study has strong implications for future clinical translation using TA-HPV as a vaccine in conjunction with the current treatment regimen for patients with advanced cervical cancer to improve survival.
Materials and methods
Mice
Five- to eight-week-old female C57BL/6 mice were purchased from the National Cancer Institute (Frederick, MD) and kept in the oncology animal facility of the Johns Hopkins Hospital (Baltimore, MD). All of the animal procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals by Johns Hopkins University Animal Care and Use Committee.
Cell lines
Briefly, TC-1 cells were obtained by co-transformation of primary C57BL/6 mouse lung epithelial cells with HPV-16 E6 and E7 and an activated ras oncogene as described previously [15]. TC-1 tumor cells were grown in RPMI 1640 supplemented with 10 % FBS, 50 U/ml penicillin/streptomycin, 2 mM l-glutamine, 1 mM sodium pyruvate, 2 mM nonessential amino acids, and 2-mercaptoethanol (2-ME, 50 μM) at 37 °C with 5 % CO2. The E7-specific CD8+ cell line has been previously described [16].
Characterization of CD11c+ cells derived from the tumor microenvironment
C57BL/6 mice (five per group) were injected with TC-1 cells s.c. at a dose of 1 × 105 per mouse. Eight days later, mice were treated with cisplatin twice at a 3-day interval by intraperitoneal (i.p.) injection at a dose of 5 mg/kg body weight, using methods similar to those described previously [17]. Two days later, the tumors were harvested and characterized for the presence of CD11c+ cells. Briefly, single-cell suspensions from TC-1 tumors were prepared by surgically excising TC-1 tumors using sterile technique and placing them in RPMI-1640 medium containing 100 U/ml penicillin and 100 μg/ml streptomycin. The solid tumors were then minced into 1- to 2-mm pieces and filtered through a 70-lm nylon filter mesh. The resultant single tumor cell suspensions and tumor-infiltrating lymphocytes were washed twice in phosphate-buffered saline (PBS) and stained for CD11c using a monoclonal antibody specific for CD11c followed by flow cytometry analysis.
Vaccinia and vaccination
The wild type (WT) VV and CRT/E7-expressing VV were prepared as described previously [18]. Briefly, pSCIIMCS2 encoding CRT/E7 was generated by cutting pcDNA3 plasmids encoding E7 vaccines by NotI/PmeI and cloned into NotI/SmaI sites of pSCIIMCS2. These constructs were transfected into WT-VV-infected CV-1 using Lipofectamine 2000, in order to facilitate heterologous recombination. The recombinant vaccinia viruses were isolated as described previously [15]. The WT-VV and CRT/E7-VV were amplified by infecting TK cells in vitro according to a standard protocol [18]. Titer was determined by plaque assay using BSC-1. The viral stocks were preserved at −70 °C prior to vaccination. Before use, the virus was thawed, trypsinized with 1/10 volume of trypsin/EDTA in a 37 °C water bath for 30 min, and diluted with minimal essential medium (MEM) containing 2.5 % fetal bovine serum to the final concentration of 1 × 108 plaque-forming units (PFU)/ml. Mice were immunized i.p. or intratumorally (i.t.) with 3 × 106 PFU of one of the vaccinia vaccines.
In vivo tumor treatment experiments
For in vivo treatment, 1 × 105 TC-1 tumor cells per mouse were injected s.c. into 5- to 8-week-old C57BL/6 mice. Tumor-bearing mice were treated with cisplatin twice at a 3-day interval by i.p. injection (5 mg/kg body weight) 8 or 12 days following tumor challenge (when tumor diameter was approximately 5 mm) [17]. The cisplatin-treated mice were further treated with or without CRT/E7-VV i.p. or i.t. at a dose of 3 × 106 PFU per mouse. Tumor-bearing mice treated with CRT/E7-VV injected i.p. or i.t. alone were included for comparison. Mice were monitored twice weekly by visual inspection and palpation. Tumor diameter was measured with digital calipers and tumor volume was calculated using the formula mm3 = 2(shortest diameter) × 0.5(longest diameter).
Antibodies
PE-conjugated CD11c, CD8α, and Gr1, and FITC-conjugated IFN-γ antibodies were purchased from BD Pharmingen (San Jose, CA, USA), and FITC-conjugated CD11b antibody was purchased from eBioscience (San Diego, CA, USA).
Tetramer analysis of E7-specific CD8+ T cells
Peripheral blood samples were depleted of red blood cells by ACK lysis and were stained with PE-conjugated HPV-16 H-2Db-RAHYNIVTF tetramer reagent (kindly provided by the National Institute of Allergy and Infectious Disease MHC Tetramer Core Facility) as described previously [19]. Tetramer staining was combined with surface staining using FITC-conjugated anti-CD8 (BDPharmingen). Cells were analyzed on a BD FACSCalibur.
Intracellular cytokine staining and flow cytometry analysis
For the characterization of the activation of E7-specific CD8+ T cells in vitro, control TC-1, wild type VV (MOI = 0.5)–treated TC-1, and CRT/E7-VV (MOI = 0.5)–treated TC-1 cells were incubated with E7-specific CD8+ T cells at a 1:1 ratio of tumor to T cells for 16 h in the presence of GolgiPlug (BDPharmingen). The stimulated CD8+ T cells were washed once with FACScan buffer and were stained with PE-conjugated monoclonal rat anti-mouse CD8α (clone 53.6.7). Cells were subjected to intracellular cytokine staining using the Cytofix/Cytoperm kit according to manufacturer’s instructions (BD Pharmingen). Intracellular IFN-γ was stained with FITC-conjugated rat anti-mouse IFN-γ. Flow cytometry analysis was performed using FACSCalibur with CellQuest software (BD Bioscience).
Characterization of CD11b+ Gr1+ myeloid-derived suppressor cells
C57BL/6 mice (5 per group) were inoculated subcutaneously with TC-1 tumor cells at a dose of 1 × 105. After 12 days, the mice were divided into six groups reflecting different treatment regimens: group 1 received only TC-1 tumor challenge, group 2 was treated with cisplatin by i.p. injection (5 mg/kg body weight) on days 12 and 15, group 3 was immunized with the CRT/E7-VV by i.p. injection, group 4 was immunized with CRT/E7-VV by i.t. injection, group 5 was i.p. immunized and treated with cisplatin, and group 6 was i.t. immunized and treated with cisplatin. Splenocytes were harvested on day 34 after tumor challenge and characterized for CD11b+ Gr1+ myeloid-derived suppressor cells (MDSCs) using antibodies specific for CD11b and Gr1 (BD Pharmingen). Flow cytometry analysis was performed using FACSCalibur with CellQuest software.
Statistical analysis
All data expressed as mean ± SE are representative of at least two independent experiments. Comparisons between individual data points were made using Student’s t test. The Kaplan–Meier method was used to analyze the survival rate and the difference in survival among groups was compared using the log-rank test. Statistical analyses were performed using SPSS version 17.0, with significance considered at p < 0.05.
Results
Cisplatin increases CD11c+ dendritic cells in the tumor locus
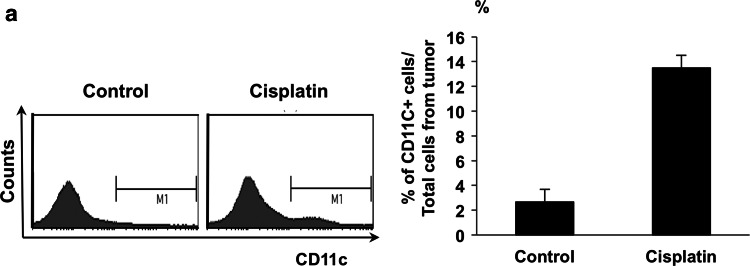
We first characterized the effect of cisplatin treatment on the presence of CD11c+ DCs in the tumor microenvironment. C57BL/6 mice were challenged subcutaneously with E7-expressing TC-1 tumor cells and later injected i.p. with cisplatin. Tumors were then harvested and characterized for the presence of CD11c+ DCs. As shown in Fig. 1, TC-1 tumor-bearing mice treated with cisplatin generated a considerably higher percentage of CD11c+ DCs in tumor loci compared to mice that did not receive cisplatin. This data suggests that in the tumor locus, following chemotherapy, a significant number of CD11c+ DCs accumulate, rendering it an ideal location for administration of therapeutic vaccines due to the abundance of professional antigen-presenting cells.
Fig. 1.
Characterization of the effect of cisplatin treatment on CD11c+ dendritic cells in the tumor locus. TC-1 tumor-bearing mice were given cisplatin (5 mg/kg) at day 8 and 11. Single-cell suspensions from TC-1 tumors were prepared 2 days after the last cisplatin treatment by surgically excising TC-1 tumors. The solid tumors were then minced into 1- to 2-mm pieces and filtered through a 70-lm nylon filter mesh. The resultant single-cell suspensions containing tumor cells and tumor-infiltrating lymphocytes were stained for CD11c. For flow cytometry analysis, the number of events was 1 × 105. a Right panel shows histograms of representative flow cytometry capturing 1 × 105 events. Left panel displays the % of CD11c+ cells among all cells from the tumor
Tumor-bearing mice treated with cisplatin followed by intratumoral injection of CRT/E7-expressing vaccinia virus generates a potent antitumor effect
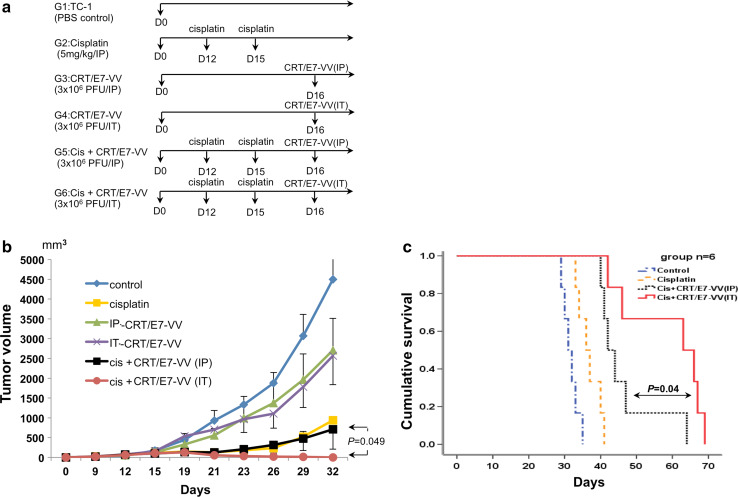
The presence of the large number of DCs in tumor loci creates an opportunity to deliver antigen to DCs through i.t. injection. Therefore, we examined the antitumor effect of cisplatin treatment alone and in combination with CRT/E7-VV administered via different routes using the TC-1 tumor model as shown in the schematic diagram in Fig. 2a. After tumor cell challenge, groups of TC-1 tumor-bearing mice were treated twice with cisplatin, three days apart, followed by administration of CRT/E7-VV 1 day after the final cisplatin treatment. Vaccinia virus treatments were administered via i.p. or i.t. injections. As shown in Fig. 2b, the combination of cisplatin and i.t. injection of CRT/E7-VV resulted in a significantly decreased tumor volume compared to cisplatin-treated mice receiving i.p. injection of CRT/E7-VV or cisplatin treatment alone. This significant therapeutic antitumor effect translated into an improved survival of treated tumor-bearing mice (Fig. 2c). These data suggest that the combination of cisplatin with i.t. injection of CRT/E7-VV generates the most potent therapeutic antitumor effect.
Fig. 2.
Cisplatin and intratumoral injection of CRT/E7-VV treatment has significant anti-tumor effect. Groups of C57BL/6 mice were s.c. challenged with 1 × 105 TC-1 tumor cells per mouse on day 0. Tumor-bearing mice were treated with cisplatin and/or CRT/E7-VV as indicated in the timeline. Cisplatin was given via intraperitoneal injection of 5 mg/kg body weight. VV was injected via intraperitoneal or intratumoral injection in the amount of 3 × 106 PFU/mouse. a Diagrammatic representation of the different treatment regimens of cisplatin and/or VV. b Line graph depicting the tumor volume in TC-1 tumor-bearing mice (five per group) treated with the different treatment regimens. c Kaplan–Meier survival analysis of TC-1 tumor-bearing mice (six per group) treated with the different treatment regimens. Points, mean; bars, SE
Combination treatment with cisplatin and intratumorally administered CRT/E7 expressing vaccinia virus increases E7-specific CD8+ T cell–mediated immune responses and reduces myeloid-derived suppressor cells systemically
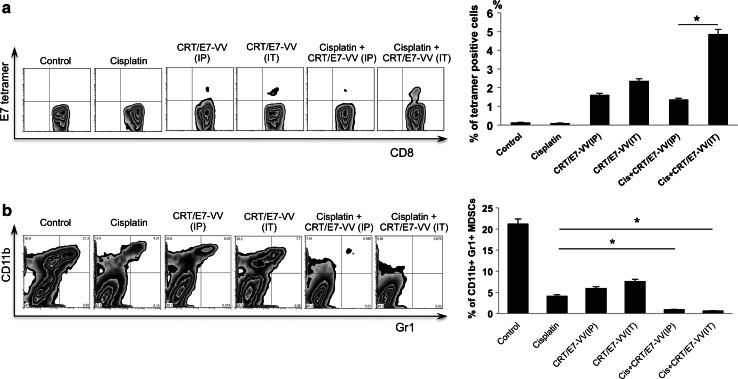
We further examined the effect of cisplatin treatment alone and in combination with i.p. or i.t. injection of CRT/E7-VV on the E7-specific CD8+ T cell–mediated immune response. Peripheral blood samples derived from each treatment group were tested for E7-specific CD8+ T cells by E7-tetramer and FITC-conjugated CD8 cells and quantified using flow cytometry. As shown in Fig. 3a, the combination treatment of cisplatin and i.t. injection of CRT/E7-VV generated the highest proportion of E7-specific CD8+ T cells among all treatment groups. Intratumoral injection of CRT/E7-VV generated significantly higher E7-specific CD8+ T cells compared to i.p. injection of CRT/E7-VV. These data indicate that the combination treatment of cisplatin with i.t. injection of CRT/E7-VV induced the most potent E7-specific CD8+ T-cell immune response. We further examined the effect of cisplatin alone and in combination with i.p. and i.t. injection with CRT/E7-VV on the number of immunosuppressive cells. Splenocytes were harvested 34 days after tumor challenge, isolated, and characterized for the presence of myeloid-derived suppressor cells (MDSCs) using flow cytometry analysis. Combination treatment with CRT/E7-VV injected i.p. and i.t. significantly decreased the number of MDSCs in the spleen compared to treatment with cisplatin alone (Fig. 3b). These data are suggestive of the importance of cisplatin treatment in combination with CRT/E7-VV injection to reduce MDSCs systemically. Taken together, this data indicates that the combination treatment is able to generate potent HPV-16 antigen-specific CD8+ T cell–mediated immune responses and reduce systemic MDSCs.
Fig. 3.
Characterization of E7-specific CD8+ T cells and myeloid-derived suppressor cells following various treatments. Peripheral blood samples (collected from the tail vein on day 10 after last treatment) were stained with PE-conjugated HPV16 H-2Db–RAHYNIVTF (E7) tetramer reagent and FITC-conjugated CD8+ antibody. a Left panel is representative flow cytometry analysis. Right panel is bar graph representing the percentage of E7-specific CD8+ T cells among all CD8+ T cells of peripheral blood. b Splenocytes were harvested on day 34 after tumor challenge. The cells isolated from spleens were then characterized for the presence of CD11b+ Gr1+ myeloid suppressor cells using flow cytometry analysis. Left panel is representative flow cytometry analysis. Right panel is a bar graph depicting the percentages of CD11b+ Gr1+ myeloid suppressor cells per 3 × 105 monocytes in the spleen. Data shown are representative of two experiments conducted. Columns, mean; bars, SE (*p < 0.05)
Intratumoral injection of CRT/E7 expressing vaccinia virus generates a greater E7-specific immune response than treatment with wild type vaccinia virus
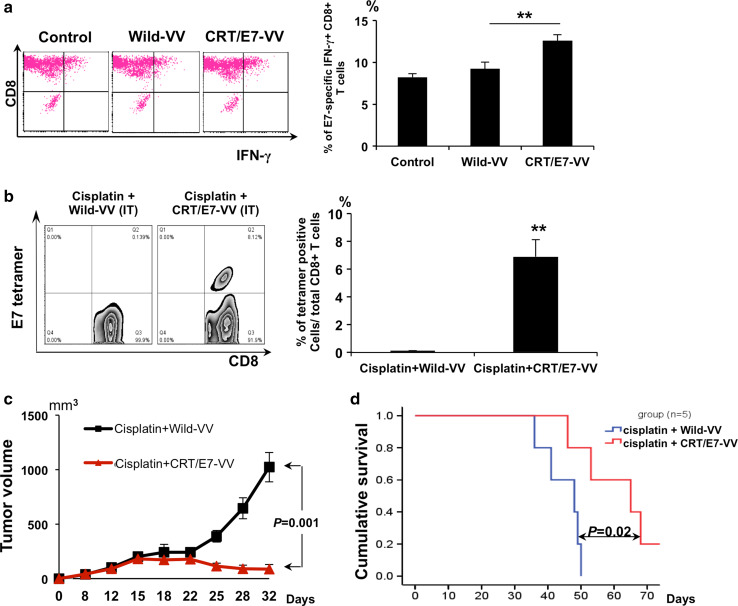
We further characterized the presentation of E7 peptide by MHC class I molecules in TC-1 tumor cells infected with CRT/E7-VV or wild type VV. We incubated TC-1 tumor cells infected with CRT/E7-VV or WT-VV with cells from an E7-specific CD8+ T-cell line and characterized the activation of the T cells using intracellular cytokine staining for IFN-γ. As shown in Fig. 4a, TC-1 cells infected with CRT/E7-VV had a significantly higher activation of E7-specific CD8+ T cells compared with TC-1 cells treated with WT-VV. This indicates that infection with CRT/E7-VV can lead to a significant increase in E7 peptide presentation via MHC class I molecules in tumor cells. Furthermore, TC-1 tumor-bearing mice treated with cisplatin followed by CRT/E7-VV generated a significantly higher number of E7-specific CD8+ T cells systemically compared to mice treated with cisplatin followed by WT-VV (Fig. 4b). We further characterized the therapeutic antitumor effect generated by i.t. injection with CRT/E7-VV or WT-VV in cisplatin-treated TC-1 tumor-bearing mice. Treatment with cisplatin and CRT/E7-VV resulted in decreased tumor volume and increased survival compared to treatment with cisplatin and WT-VV (Fig. 4c, d). Thus, the data show that compared to WT-VV, CRT/E7-VV generates a greater antitumor immune response by making tumor cells more susceptible to destruction by enhancing presentation of E7 antigen and boosting the E7-specific CD8+ T-cell response.
Fig. 4.
Characterization of TC-1 tumor cells following infection with various vaccinia viruses. a Intracellular cytokine staining and flow cytometry analysis to determine the number of IFN-γ–secreting E7-specific CD8+ T cells induced by non-infected TC-1 cells or wild type vaccinia virus–infected TC-1 cells or CRT/E7-VV-infected TC-1 cells (MOI = 0.5). TC-1 cells were incubated with cells from an E7-specific CD8+ T-cell line at 1:1 E:T ratio for 15 h. After incubation, cells were stained for CD8 and IFN-γ and analyzed by flow cytometry. Left panel is representative flow cytometry analysis. Right panel is a bar graph representing the percentage of IFN-γ secreting E7-specific CD8+ T cells among all CD8+ T cells. Data shown are mean of three experiments performed (**p < 0.01). b Peripheral blood samples (collected from the tail vein on day 10 after last treatment) were stained with PE-conjugated HPV16 E7 tetramer reagent and FITC-conjugated CD8+ cells. Left panel is representative flow cytometry analysis. Right panel is a bar graph representing the percentage of E7-specific CD8+ T cells among all CD8+ T cells of peripheral blood. Data shown are representative of two experiments performed. Columns, mean; bars, SE (*p < 0.05, **p < 0.01).c Groups of C57BL/6 mice (five per group) were s.c. challenged with 1 × 105 TC-1 tumor cells per mouse on day 0. Tumor-bearing mice were treated with cisplatin and wild type vaccinia virus or CRT/E7-VV according to the schedule in Fig. 1a. The line graph depicting the tumor volume in TC-1 tumor-bearing mice treated with cisplatin with wild type VV or CRT/E7-VV (mean ± SE) (p = 0.001). d Kaplan–Meier survival analysis of TC-1 tumor-bearing mice treated with the different treatment regimens
Combination treatment with cisplatin and intratumorally administered CRT/E7 expressing vaccinia virus elicits a systemic immunotherapeutic response
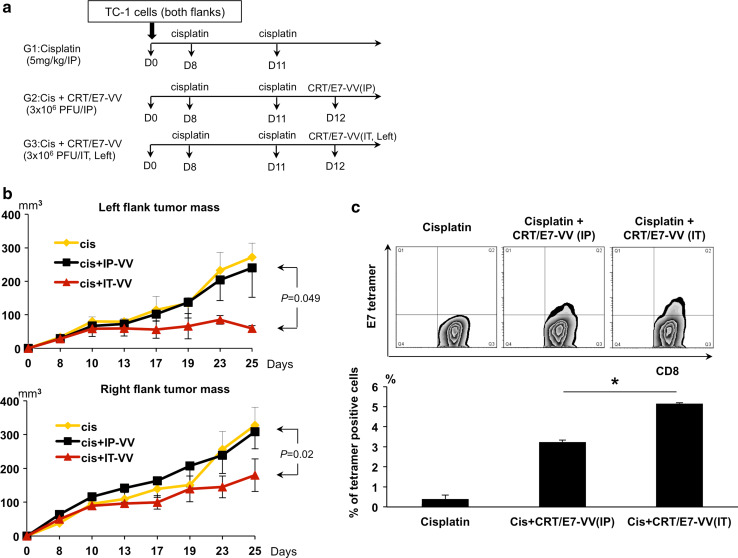
We further examined whether cisplatin would generate a systemic immunotherapeutic effect either alone or in combination with i.t. or i.p. injection with CRT/E7-VV. Mice were subcutaneously challenged with TC-1 tumor cells on both flanks. Mice were treated with cisplatin and subsequently injected with CRT/E7-VV i.p. or i.t. in the left flank tumor only as outlined in Fig. 5a. As shown in Fig. 5b, the right flank tumor volume was significantly lower in cisplatin-treated mice with the left flank tumor receiving i.t injection with CRT/E7-VV compared to those receiving i.p. injection of CRT/E7-VV. Furthermore, the percentage of E7-specific CD8+ T cells among tumor-infiltrating lymphocytes in the right flank tumor was highest in mice receiving treatment with cisplatin and i.t. injection of CRT/E7-VV compared to mice receiving treatment with cisplatin followed by i.p. injection of CRT/E7-VV (Fig. 5c). Additionally, the left flank tumor volume was significantly lower than the right flank tumor volume in mice treated with cisplatin and i.t. CRT/E7-VV (p = 0.002). Taken together, these data suggest that the therapeutic antitumor effects on the right flank tumor are contributed by the systemic distribution of the tumor-specific immunity generated by i.t. injection of CRT/E7-VV in the left flank tumor.
Fig. 5.
Cisplatin and intratumoral injection of CRT/E7-VV elicits a systemic anti-tumor effect and therapeutic immune response. a Schematic diagram of cisplatin and VV treatments. Groups of C57BL/6 mice (five per group) were s.c. challenged with 1 × 105TC-1 tumor cells per mouse on both flanks. TC-1 tumor-bearing mice were treated with cisplatin and/or CRT/E7-VV as indicated in the time line. Vaccinia virus was injected via intraperitoneal or intratumoral injection (left flank tumor) in the amount of 3 × 106 PFU/mouse. b Line graphs depicting the tumor volume in TC-1 tumors. Upper panel is left flank tumor volume. Lower panel is right flank tumor volume. Points, mean; bars, SE. c Tumor-infiltrating lymphocytes (collected from the right flank tumor mass) were stained with PE-conjugated HPV E7 tetramer reagent and FITC-conjugated CD8+ cells. Upper panel is representative flow cytometry analysis. Lower panel is a bar graph representing the percentage of E7-specific CD8+ T cells among all CD8+ T cells of tumor mass. Data shown are representative of two experiments performed. Columns, mean; bars, SE (*p < 0.05)
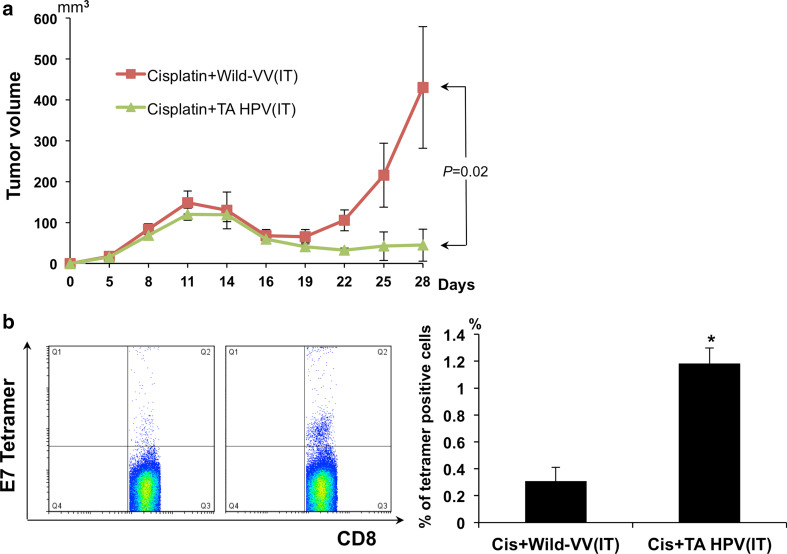
Cisplatin and TA-HPV vaccine treatment generates greater antitumor effect and E7-specific immune response than cisplatin and wild type vaccinia virus
We have demonstrated that intratumoral administration of CRT/E7-VV effectively improves survival, decreases tumor volume, and increases antigen-specific CD8+ T cells when administered following cisplatin treatment in a preclinical model. This encouraging preclinical data warrants further exploration of our treatment methodology in a clinical trial. In order to expeditiously apply this approach to the clinical arena to help patients suffering from advancer cervical cancer, it is important to consider a safe therapeutic recombinant HPV vaccinia vaccine that covers the high-risk HPV types. One such candidate is TA-HPV, a vaccine based on the Wyeth vaccine strain of vaccinia that carries modified E6 and E7 genes from HPV types 16 and 18. Therefore, we tested TA-HPV in a manner similar to the experiments outlined above using CRT/E7-VV. TC-1 tumor-bearing mice were treated with cisplatin followed by i.t. injection with WT-VV or TA-HPV as outlined in Fig. 2a. As shown in Fig. 6, TC-1 tumor-bearing mice injected i.t. with TA-HPV following cisplatin treatment generated a more potent E7-specific CD8+ T-cell response and better therapeutic antitumor effects compared to those treated with WT-VV. These data serve as an important foundation for future clinical translation of this treatment platform using TA-HPV.
Fig. 6.
Cisplatin and TA-HPV treatment generates greater anti-tumor effect and E7-specific immune response than cisplatin and wild typeVV. Groups of C57BL/6 mice (five per group) were s.c. challenged with 1 × 105 per mouse of TC-1 tumor cells on day 0. Tumor-bearing mice were treated with cisplatin and either wild type vaccinia virus or TA-HPV according to the schedule outlined in Fig. 1a. a Line graph depicts tumor volume over time. b Peripheral blood samples (collected from the tail vein on day 10 after last treatment) were stained with PE-conjugated HPV16 H-2Db–RAHYNIVTF (E7) tetramer reagent and FITC-conjugated CD8+ cells. Left panel is representative flow cytometry analysis. Right panel is a bar graph representing the percentage of E7-specific CD8+ T cells/3 × 104 CD8+ T cells of peripheral blood. Columns, mean; bars, SE (*p < 0.01)
Discussion
In this study, we found that treatment with cisplatin followed by i.t. injection of CRT/E7-VV in an in vivo murine cervical cancer model generates a potent antitumor effect. Mice receiving this treatment experienced inhibited tumor growth, prolonged survival, and an increased proportion of E7-specific CD8+ T cells systemically and in tumor loci compared to mice treated with cisplatin alone or cisplatin followed by i.p. injection of CRT/E7-VV. Moreover, i.t. injection with a clinical grade therapeutic HPV vaccinia vaccine, TA-HPV, also increased the number of E7-specific CD8+ T cells and generated potent antitumor effects in treated TC-1 tumor-bearing mice. The current data are consistent with our recent study in which we demonstrated that cisplatin induced accumulation of CD11c+ DCs in E7-expressing tumor loci [10]. We found that the increased number of CD11c+ DCs were capable of taking up the E7 antigen peptide administered by intratumor injection and migrating to the draining lymph node. In addition, we found that the antigen-loaded DCs had high level of expression of co-stimulatory molecules and efficiently activated E7-specific CD8+ T cells [10]. These data suggest that following the enrichment of DCs in tumor loci in response to chemotherapy, these DCs have the capacity to uptake tumor antigen, mature, travel to the draining lymph nodes, and prime antigen-specific CD8+ T cells. Taken together, our data indicate that the DC-rich tumor microenvironment induced by treatment with cisplatin may serve as a suitable environment for vaccination with different types of therapeutic HPV recombinant vaccinia vaccines [20] as well as other forms of cancer vaccines to result in better control of tumors.
Chemotherapy with cisplatin in conjunction with therapeutic HPV vaccine has previously been used for the control of HPV-16 E6/E7-expressing tumors. For example, Bae et al. examined the synergistic effects of cisplatin combined with an HPV-16 E7 protein subunit vaccination with CpG oligonucleotide adjuvant [21]. In addition, Tseng et al. have demonstrated that cisplatin can be used in conjunction with an E7-specific therapeutic HPV DNA vaccine to significantly enhance therapeutic antigen-specific antitumor effects [17]. However, neither of these studies employed intratumor injection of the therapeutic vaccine. In the current study, we found that intratumor injection is significantly better than other routes of administration for the recombinant vaccinia vaccine to generate therapeutic antitumor effects. Our data suggests that intratumor injection is a preferred route of administration to maximize antitumor effects following chemotherapy with cisplatin.
Successful treatment of tumor-bearing mice with cisplatin followed by i.t. injection of CRT/E7-VV or TA-HPV has significant clinical implications. TA-HPV has previously been widely tested in HPV-associated lesions [6, 11–13]. In addition, several clinical grade reagents for a therapeutic vaccine consisting of plasmid DNA expressing mutated HPV-16 E7 fused to CRT [22], or heat shock protein 70 [23], have already been created and tested in clinical trials. All of these clinical grade E6/E7 vaccine candidates can potentially be used for i.t. injection in conjunction with standard treatment regimens to improve outcomes in patients with advanced stages of cervical cancer.
Direct injection of CRT/E7-VV at the tumor site may generate therapeutic antitumor effects against TC-1 tumors through several different mechanisms. First, the vaccinia virus may contribute to the antitumor effect by triggering tumor oncolysis (for review, see Worschech et al. [24]). Secondly, destruction of tumor cells may potentially result in TC-1 tumor-specific CD8+ T-cell immunity through a cross-priming mechanism. Thirdly, CRT/E7-VV may directly infect CD11c+ DCs in the tumor loci contributing to the generation of E7-specific CD8+ T-cell immune responses. Finally, the expression of CRT/E7 by tumor cells infected by the vaccinia virus may also render tumor cells susceptible to CTL-mediated killing by E7-specific CD8+ T cells. Thus, multiple mechanisms may account for the observed potent therapeutic antitumor effect generated by i.t. injection of CRT/E7-VV. The generation of the tumor antigen-specific CD8+ T-cell immune response may also contribute to the systemic therapeutic antitumor effect. This is evident in the significant reduction in right flank tumor mass in response to i.t. injection of CRT/E7-VV in the left flank tumors, compared to i.p. injection of CRT/E7-VV (Fig. 5b). Thus, the combined effect of tumor oncolysis with a specific T cell–mediated immune response demonstrates the potency of i.t. injection of CRT/E7-VV as a therapeutic agent for HPV-associated cervical cancer.
In summary, we found that cisplatin treatment increased the population of CD11c+ dendritic cells in tumor loci making it an ideal locus for the administration of vaccine to elicit a tumor-specific immune response. Because HPV-associated cervical cancers are conventionally treated by cisplatin and these tumors are readily accessible for i.t. injection of a therapeutic HPV vaccine, the approach described in the current study may likely be applied for the control of HPV-associated advanced cervical cancers.
Acknowledgments
This work was supported by the Cervical Cancer SPORE, P50CA098252, the Head and Neck Cancer SPORE, P50DE019032, and 2RO1 CA114425-06 from the National Institutes of Health/National Cancer Institute.
Conflict of interest
The authors declare that no conflict of interests exist.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: A Cancer J Clin. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Thomas GM. Improved treatment for cervical cancer–concurrent chemotherapy and radiotherapy. New Engl J Med. 1999;340(15):1198–1200. doi: 10.1056/NEJM199904153401509. [DOI] [PubMed] [Google Scholar]
- 3.Society AC (2012) Cervical cancer: survival rates by stage. http://www.cancer.org/Cancer/CervicalCancer/DetailedGuide/cervical-cancer-survival. Accessed July 16 2012
- 4.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6(10):753–763. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65(1):473–478. doi: 10.1128/jvi.65.1.473-478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borysiewicz LK, Fiander A, Nimako M, Man S, Wilkinson GW, Westmoreland D, Evans AS, Adams M, Stacey SN, Boursnell ME, Rutherford E, Hickling JK, Inglis SC. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347(9014):1523–1527. doi: 10.1016/S0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 7.Hsieh CJ, Kim TW, Hung CF, Juang J, Moniz M, Boyd DA, He L, Chen PJ, Chen CH, Wu TC. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine. 2004;22(29–30):3993–4001. doi: 10.1016/j.vaccine.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 8.Spee P, Neefjes J. TAP-translocated peptides specifically bind proteins in the endoplasmic reticulum, including gp96, protein disulfide isomerase and calreticulin. Eur J Immunol. 1997;27(9):2441–2449. doi: 10.1002/eji.1830270944. [DOI] [PubMed] [Google Scholar]
- 9.Sadasivan B, Lehner PJ, Ortmann B, Spies T, Cresswell P. Roles for calreticulin and a novel glycoprotein, tapasin, in the interaction of MHC class I molecules with TAP. Immunity. 1996;5(2):103–114. doi: 10.1016/S1074-7613(00)80487-2. [DOI] [PubMed] [Google Scholar]
- 10.Kang TH, Mao CP, Lee SY, Chen A, Lee JH, Kim TW, Alvarez R, Roden RB, Pardoll DM, Hung CF, Wu TC. Chemotherapy acts as an adjuvant to convert the tumor microenvironment into a highly permissive state for vaccination-induced antitumor immunity. Cancer Res. 2013 doi: 10.1158/0008-5472.CAN-12-4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiander AN, Tristram AJ, Davidson EJ, Tomlinson AE, Man S, Baldwin PJ, Sterling JC, Kitchener HC. Prime-boost vaccination strategy in women with high-grade, noncervical anogenital intraepithelial neoplasia: clinical results from a multicenter phase II trial. Int J Gynecol Cancer. 2006;16(3):1075–1081. doi: 10.1111/j.1525-1438.2006.00598.x. [DOI] [PubMed] [Google Scholar]
- 12.Davidson EJ, Boswell CM, Sehr P, Pawlita M, Tomlinson AE, McVey RJ, Dobson J, Roberts JS, Hickling J, Kitchener HC, Stern PL. Immunological and clinical responses in women with vulval intraepithelial neoplasia vaccinated with a vaccinia virus encoding human papillomavirus 16/18 oncoproteins. Cancer Res. 2003;63(18):6032–6041. [PubMed] [Google Scholar]
- 13.Kaufmann AM, Stern PL, Rankin EM, Sommer H, Nuessler V, Schneider A, Adams M, Onon TS, Bauknecht T, Wagner U, Kroon K, Hickling J, Boswell CM, Stacey SN, Kitchener HC, Gillard J, Wanders J, Roberts JS, Zwierzina H. Safety and immunogenicity of TA-HPV, a recombinant vaccinia virus expressing modified human papillomavirus (HPV)-16 and HPV-18 E6 and E7 genes, in women with progressive cervical cancer. Clin Cancer Res. 2002;8(12):3676–3685. [PubMed] [Google Scholar]
- 14.Boursnell ME, Rutherford E, Hickling JK, Rollinson EA, Munro AJ, Rolley N, McLean CS, Borysiewicz LK, Vousden K, Inglis SC. Construction and characterisation of a recombinant vaccinia virus expressing human papillomavirus proteins for immunotherapy of cervical cancer. Vaccine. 1996;14(16):1485–1494. doi: 10.1016/S0264-410X(96)00117-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen CH, Suh KW, Ji H, Choti MA, Pardoll DM, Wu TC. Antigen-specific immunotherapy for human papillomavirus 16 E7-expressing tumors grown in the liver. J Hepatol. 2000;33(1):91–98. doi: 10.1016/S0168-8278(00)80164-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang TL, Ling M, Shih IM, Pham T, Pai SI, Lu Z, Kurman RJ, Pardoll DM, Wu TC. Intramuscular administration of E7-transfected dendritic cells generates the most potent E7-specific anti-tumor immunity. Gene Ther. 2000;7(9):726–733. doi: 10.1038/sj.gt.3301160. [DOI] [PubMed] [Google Scholar]
- 17.Tseng CW, Hung CF, Alvarez RD, Trimble C, Huh WK, Kim D, Chuang CM, Lin CT, Tsai YC, He L, Monie A, Wu TC. Pretreatment with cisplatin enhances E7-specific CD8+ T-Cell-mediated antitumor immunity induced by DNA vaccination. Clin Cancer Res. 2008;14(10):3185–3192. doi: 10.1158/1078-0432.CCR-08-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Earl PL, Moss B. Mutational analysis of the assembly domain of the HIV-1 envelope glycoprotein. AIDS Res Human Retroviruses. 1993;9(7):589–594. doi: 10.1089/aid.1993.9.589. [DOI] [PubMed] [Google Scholar]
- 19.Cheng WF, Hung CF, Lin KY, Ling M, Juang J, He L, Lin CT, Wu TC. CD8+ T cells, NK cells and IFN-gamma are important for control of tumor with downregulated MHC class I expression by DNA vaccination. Gene Ther. 2003;10(16):1311–1320. doi: 10.1038/sj.gt.3301982. [DOI] [PubMed] [Google Scholar]
- 20.Zurkova K, Babiarova K, Hainz P, Krystofova J, Kutinova L, Otahal P, Nemeckova S. The expression of the soluble isoform of hFlt3 ligand by recombinant vaccinia virus enhances immunogenicity of the vector. Oncol Rep. 2009;21(5):1335–1343. doi: 10.3892/or_00000359. [DOI] [PubMed] [Google Scholar]
- 21.Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clin Cancer Res. 2007;13(1):341–349. doi: 10.1158/1078-0432.CCR-06-1838. [DOI] [PubMed] [Google Scholar]
- 22.Medicine SKCCCJH (2009) Therapeutic vaccination for patients with HPV16+ cervical intraepithelial neoplasia (CIN2/3). National Library of Medicine (US). http://clinicaltrials.gov/ct2/show/NCT00988559. Accessed July 2 2012
- 23.Trimble CL, Peng S, Kos F, Gravitt P, Viscidi R, Sugar E, Pardoll D, Wu TC. A phase I trial of a human papillomavirus DNA vaccine for HPV16+ cervical intraepithelial neoplasia 2/3. Clin Cancer Res. 2009;15(1):361–367. doi: 10.1158/1078-0432.CCR-08-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worschech A, Haddad D, Stroncek DF, Wang E, Marincola FM, Szalay AA. The immunologic aspects of poxvirus oncolytic therapy. Cancer Immunol Immunother CII. 2009;58(9):1355–1362. doi: 10.1007/s00262-009-0686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]