Abstract
Effective gene therapy for heart failure has not yet been achieved clinically. The aim of this study is to quantitatively assess the cardiac isolation efficiency of the molecular cardiac surgery with recirculating delivery (MCARD™) and to evaluate its efficacy as a means to limit collateral organ gene expression. 1014 genome copies (GC) of recombinant adeno-associated viral vector 6 encoding green fluorescent protein under control of the cytomegalovirus promoter was delivered to the nine arrested sheep hearts. Blood samples were assessed using real-time quantitative polymerase chain reaction (RT QPCR). Collateral organ gene expression was assessed at four-weeks using immunohistochemical staining. The blood vector GC concentration in the cardiac circuit during complete isolation trended from 9.59±0.73 to 9.05±0.65 (log GC/cm3), and no GC were detectable in the systemic circuit (P<0.001). The washing procedure performed prior to relinquishing the cardiac circuit decreased the systemic blood vector GC concentration >800-fold (P<0.001), consistent with >99% isolation efficiency. Conversely, incomplete isolation resulted in equalization of vector GC concentration in the circuits, leading to robust collateral organ gene expression. MCARD™ is an efficient, clinically translatable myocardial delivery platform for cardiac specific gene therapy. The cardiac surgical techniques utilized are critically important to limit collateral organ gene expression.
Keywords: Cardiac surgery, Cardiac gene therapy, Organ gene expression, Adeno-associated virus, Green fluorescent protein
1. Introduction
Gene therapy to ameliorate left ventricular dysfunction offers a potential therapeutic strategy for patients with heart failure. Molecular cardiac surgery with recirculating delivery (MCARD™) is a novel cardiac-specific delivery platform via the use of cardiopulmonary bypass (CPB). Although viewed as technically complex, we and others have demonstrated effective use of CPB, with minimal procedure-related complications and very efficient cardiac gene delivery [1–4]. Taken together, these studies indicate the potential for an efficient CPB-based cardiac-specific vector-mediated gene delivery platform combined with a promising therapeutic vector/transgene to be utilized in select patients with cardiac failure.
In this study, we aim to quantitatively assess the cardiac isolation efficiency of the MCARD™ procedure and to elucidate the extent of collateral gene expression in distant organs. We further review the main technical features of the MCARD™ to evaluate the potential for clinical application.
2. Materials and methods
2.1. Animal use and surgical protocol
All animals received humane care in compliance with the NIH and guidelines established by the University of Pennsylvania. Nine Dorsett male sheep (approx. one-year-old), weighing 38.6±3.4 kg, were the basis for the results presented here.
2.2. Details of the surgical technique with complete and incomplete cardiac isolation
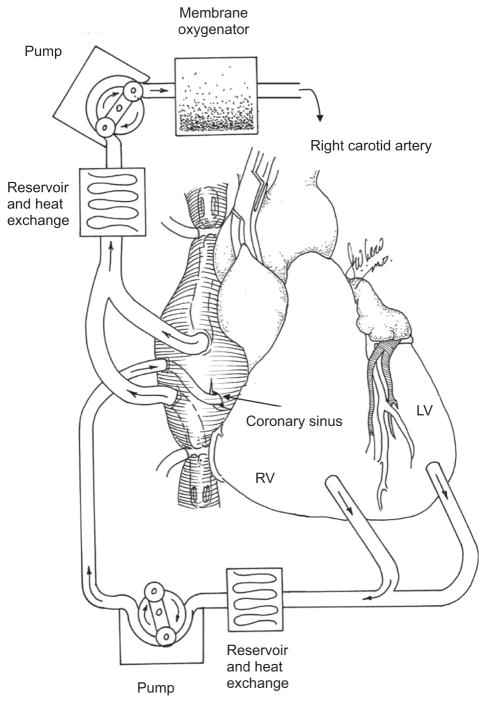
After initiating CPB, the right and the left azygous veins are ligated; a temporary snare is place around the middle cardiac vein; vent cannulas are placed into the left ventrical (LV) through the apex and into the right ventrical (RV) through the outflow tract. The LV, RV and aortic root vents are connected via a Y connector to the venous limb of the cardiac venous return circuit. The arterial limb of the cardiac circuit is connected to the coronary sinus catheter. The aorta is cross-clamped and cold crystalloid cardioplegia is delivered antegrade. The heart is isolated by tightening the superior vena cava (SVC) and inferior vena cava (IVC) snares, cross-clamping the pulmonary artery and tightening the snare around the middle cardiac vein (Fig. 1). With the heart fully decompressed, cardiac circuit flow is initiated until the coronary sinus pressure equals 60–80 mmHg. Flow was about 60–100 m/min average. The virus solution is injected into the retrograde catheter and allowed to dwell for 10 minutes and recirculation commences for 20 minutes. The coronary circuit is then flushed antegrade with 1000 ml of hetastarch to washout residual vector. Rewarming is initiated and the animal is weaned from CPB and closed in standard fashion. During incomplete cardiac isolation (n=1), after cross-clamping, the snares on the SVC and IVC were not tightened, resulting in communication between the cardiac and systemic circuits.
Fig. 1.
Molecular cardiac surgery with recirculating gene delivery (MCARD™). Cardiac isolated perfusion circuit and dual bypass configuration.
2.3. Vector design and production
1×1014 genome copies (GC) of single-stranded or self-complimentary recombinant adeno-associated virus 6 vectors, encoding green fluorescent protein (GFP) under the control of the human cytomegalovirus promoter immediate early enhancer/promoter with a splice donor/acceptor sequence and polyadenylation signal from the human globin gene are produced [5].
2.4. Real-time quantitative polymerase chain reaction (RT QPCR)
RT PCR was performed using the MyiQ RT PCR Detection System (Bio-Rad Corporation, CA, USA) and analyzed using the MyiQ software package (Bio-Rad Corporation, CA, USA). QPCR analysis was performed in optical 96-well plates using iQ SYBRGreen Supermix (Bio-Rad Laboratories, Hercules, CA, USA).
2.5. Histological analysis
Hematoxylin and eosin and Masson’s trichrome staining were performed on microscopic sections.
2.6. Immunostaining analysis
GFP expression was detected in frozen tissue sections by direct fluorescence and by immunostaining. For colorimetric detection of GFP we used polyclonal rabbit anti-GFP antibody as a primary and HRP-conjugated goat anti-rabbit IgG (Invitrogen, CA, USA) as a secondary antibodies according to manufacture’s protocol.
2.7. Statistical methods
Comparisons between the blood vector GC cardiac and systemic concentrations at specific time points were performed using two-sided paired Student’s t-tests. One way analysis of variance (ANOVA) was used to compare blood QPCR in the systemic and cardiac circulation at the different time points.
3. Results
3.1. Genome biodistribution
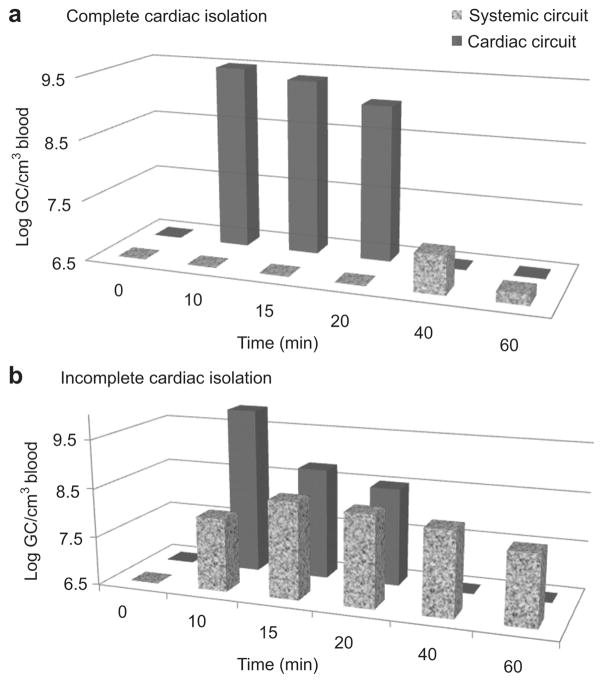
In these experiments, we chose to illustrate the observed GC on a logarithm (log) scale to facilitate ease of interpretation of the data. With complete cardiac isolation, the blood vector GC concentration trended from 9.59±0.73 to 9.05±0.65 (log GC/cm3). No GC were detectable in the systemic circuit during 10 minutes of dwelling and 20 minutes of recirculation (P<0.001). After completion of the recirculation interval and the washing procedure, the initial systemic blood vector GC concentration significantly decreased to 6.68±1.08 log GC/cm3 or more than 800 times less than the starting cardiac circuit concentration (P<0.001) (Fig. 2a).
Fig. 2.
(a) Biodistribution of genome copies over time in blood samples taken from systemic and cardiac circuits during complete cardiac isolation. The MCARD procedure excludes vector genomes access to the systemic circulation during recirculation, consistent with 99.65% isolation efficiency. (b) Biodistribution of genome copies over time in blood samples taken from systemic and cardiac circuits during incomplete cardiac isolation showing equalization of the biodistribution of vector genomes between systemic and cardiac circuits over time. log, logarithm.
Conversely, during incomplete cardiac isolation, there was a gradual equalization of the vector genome concentrations in both the cardiac and systemic circuits. Postrecirculation washout of the circuit did not lead to any notable change in the systemic biodistribution of vector genomes, suggesting that equilibrium had already been reached (Fig. 2b).
3.2. MCARD™ efficiency
The initial blood concentration of vector in the cardiac circuit is determined by calculating the dilution factor (e.g. 1×1014 divided by the real time cardiac circuit volume plus 40 ml vial content). The cardiac circuit volume typically was 280±40 ml. Here, we define cardiac isolation efficiency as (maximum cardiac circuit vector concentration –maximum systemic vector concentration)/(maximum cardiac circuit vector concentration)×100=99.65%. Repeated bio-distribution studies determined insignificant amounts GC (<0.002%) in various circuit components. Although GC distribution graphically appears large secondary to the log scale representation, the absolute amount of GC remaining in the systemic circuit postrecirculation is relatively low when converted to absolute integer values. The systemic circuit volume is estimated at 75 ml per kg of animal weight. Converted to the linear scale, 6.68 (log GC/cm3) is 4,786,000 GC/ml. Therefore, after conversion to an absolute integer value, it is clear that only 0.002% of 1×1014 GC injected was detectable in the systemic circulation.
3.3. Collateral organ gene expression
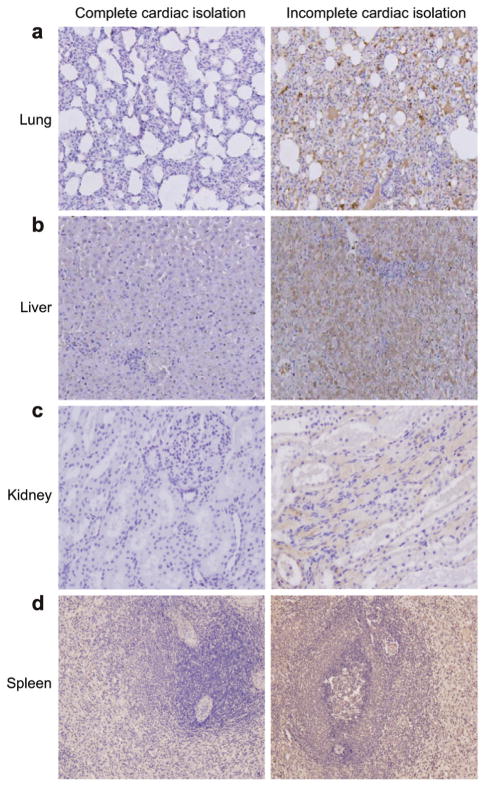
In the case with incomplete cardiac isolation, immunohistochemical staining revealed significant reporter gene expression in the lungs, liver, kidneys and spleen likely resulting from the relatively high systemic biodistribution of AAV6 vector genomes. In contrast, there was no immunohistochemical evidence of GFP expression evident in collateral organs four weeks after the MCARD™ procedure with complete cardiac isolation. Furthermore, the surrounding tissue of all main organs after MCARD™ showed no evidence of significant inflammation or tissue damage which might have resulted as an untoward consequence of viral transfection (Fig. 3a–d).
Fig. 3.
Representative green fluorescent protein (GFP) immunohistochemical sections (magnification ×100; ×200) are displayed for MCARD™ with complete and incomplete cardiac isolation. The brown color represents cells with GFP expression and the blue color represents intact cells. Lung (a), liver (b), kidney (c), and spleen (d) cells are shown after histochemical assay for GFP activity. This assay reveals significant collateral organ gene expression after incomplete cardiac isolation. In contrast, there is minimal if any evidence of collateral organ gene expression after complete cardiac isolation. These photomicrographs were taken from animals killed four weeks after the MCARD™ procedure was performed.
3.4. Operative results
All of the animals in this series survived to euthanasia at four weeks. Average bypass and cross-clamp times were 128.9±11.1 minutes and 67.9±9.6 minutes, respectively. All animals were weaned from CPB with mild inotropic support and were extubated within three to five hours of surgery.
Signs of inflammation and evidence of myocardial edema, necrosis or fibrosis were absent in all histological samples procured, following euthanasia at four weeks.
4. Discussion
4.1. Antegrade vs. retrograde route of gene delivery
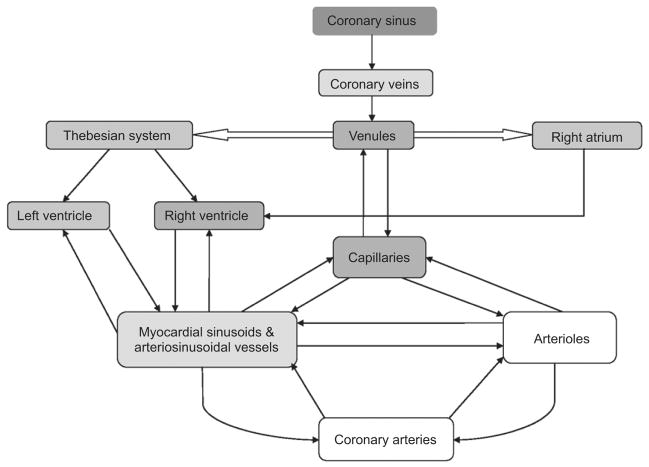
In our initial experiments, we used antegrade intracoronary viral gene delivery [6]. Subsequently, we utilized gene transfer using retrograde coronary delivery [2] resulting in more efficient myocardial gene transfer. These results were also confirmed by Boekstegers et al. [7]. Retrograde cardioplegia compared to antegrade provides a more uniform distribution of cardioplegia in the presence of coronary artery disease. Conversely, the quantity of cardioplegia administered retrograde that traverses the capillary beds is significantly lower than for the antegrade delivery method due to shunting through the thebesian and arteriosinusoidal channels into the ventricular chambers (Fig. 4). Parlington et al. [8] demonstrated that nutritive flow in the LV averaged 65% by retrograde perfusion vs. 87% when given antegrade. Disadvantages of retrograde cardioplegia include relatively poor perfusion of the RV. We and others believe that the coronary venous infusion allows for prolonged adhesion time of the vector to cardiac endothelium and results both in an increase in endothelial permeability as well as a higher pressure gradient across the interstitial capillaries and venules, promoting filtration of macromolecular (nanoparticle-sized) vectors into the interstitium of the heart [1].
Fig. 4.
Schematic of the retrograde coronary veno–arterial system.
4.2. Coronary venous and arterial anatomy
In sheep, the coronary venous system is similar to the human. A prominent left azygous vein enters on the left side of the ovine heart and drains into the coronary sinus. This anatomical feature is the main difference between ovine and human coronary venous anatomy [9]. Therefore, ligation of the left azygous vein is a necessary condition for complete cardiac isolation in the ovine model. The coronary venous system not only connects with myocardial capillaries but also interconnects with other coronary veins by way of veno-venous collaterals and with the ventricular and atrial cavities directly through the thebesian vessels. The anatomy of the thebesian veins in sheep is similar to that of the human heart and is divided into three components. The first part drains blood from the capillary bed into the ventricles; the second part drains blood from arteries into the ventricles without traversing capillary beds; and the third part forms direct connections with the coronary veins, shunting blood from these vessels into the ventricles [10].
To understand how retrograde perfusion of virus might facilitate global and efficient transduction of cardiac myocytes, the interrelationship of the coronary venous and arterial anatomy must be considered. Arterioles give rise directly to capillaries and/or metarterioles. Just proximal to their origin from arterioles, capillaries have a precapillary sphincter. Blood flow through capillary channels is designed for exchange of nutrients and metabolites, referred to as nutrient flow. In some parts of the microcirculation, blood flow bypasses the capillary bed, moving through a connection called an arterio–venous shunts, which directly connects an arteriole and a venule. This type of blood flow is called non-nutrient flow because it does not allow for nutrient exchange. When there is recirculation of vector genomes in the (retrograde) direction, high venous pressure is directly transferred to the venule and capillary where nutrient flow (filtration) takes place. In the antegrade direction, precapillary sphincters are likely to greatly diminish the electromotive force (pressure gradient), inhibiting the filtration of macromolecular assemblies, such as the transgene construct.
4.3. Coronary sinus balloon position and occlusion of coronary sinus vein
Two principles that are fundamental to the MCARD™ procedure are internal occlusion of the coronary sinus by balloon inflation and the external temporal occlusion of any coronary veins that drain into the coronary sinus proximal to the balloon position with temporary snares. It has been determined that coronary sinus balloon occlusion during retrograde infusion improves cardioplegia delivery and also results in a doubling of nutrient flow [11]. When the retrograde catheter is mal-positioned, the loss of regurgitated volume through the veno-venous anastomosis can range up to 36% [12]. To reduce the loss of input virus, and to avoid decompression of the coronary venous system, we temporarily use a snare to occlude the middle cardiac vein. We saw no complications resulting with this technique in any of the cases.
4.4. Coronary sinus pressure and flow
It has been previously demonstrated in several studies that an increased perfusion pressure in the coronary arteries enhances myocardial gene transfer [1]. Does this premise hold true for the coronary venous system? In general, the coronary sinus pressure is limited to 40–60 mmHg to prevent coronary sinus injury, peripheral vascular edema and hemorrhage. In the canine model, it was noted that damage to the capillaries and venules occurred when the perfusion pressure in the coronary sinus rose above 60 mmHg [13]. Conversely, a study of Eke et al. [14] showed that in the vented arrested heart, the coronary sinus pressure could safely be increased to up to 120 mmHg. In this study we have never observed histological evidence of myocardial edema or any other injury at the four-week time point for coronary sinus pressures <100 mmHg. During coronary sinus pressure elevation, venous flow more readily enters the capillary channels and is associated with a faster and more uniform distribution of nutrient flow [15], possibly due to distending the veins thus rendering valves incompetent.
5. Limitations of the study
The present experiments were performed with a relatively small number of animals, and normal sheep were used, which were without evidence of coronary artery disease or heart failure. Although coronary venous and arterial anatomy of the sheep is similar to that of humans, this study cannot necessarily be extrapolated to patients. Coronary sinus pressure and flow used in these experiments were constant, in efforts to evaluate transduction efficiency under controlled conditions.
MCARD™ is utilized as a title trademark to avoid confusion of this methodology with other competing platforms.
6. Conclusions
MCARD™ is a cardiac vector-mediated gene delivery platform that is 99.6% efficient in restricting vector genomes to the coronary circulation. This method uniquely allows for high-efficiency recirculating gene delivery to the coronary circulation of large animals while minimizing collateral organ expression and is potentially clinically translatable for heart failure gene therapy.
Acknowledgments
We thank Marina Sumaroka, Catherine Tomasulo, Jennifer White, and Danielle Thesier for contribution of all of the molecular genetic studies conducted in this work. Joseph Rabinowitz, Julie Johnston, and Lili Wang deserve thanks as well for the vector construct preparation. We would like to extend acknowledgments to Michail Petrov, David Holt, Charles Yarnall, Alice Isidro, Rose Nolen-Walston, and JanLee Jensen, for their excellent technical assistance and postoperative care. Further, we thank the animal care staff and technicians for excellent handling of the animals in the Biomedical Research Building Vivarium – University of Pennsylvania.
References
- 1.Katz MG, Swain JB, White JD, Low D, Stedman H, Bridges CR. Cardiac gene therapy: optimization of gene delivery techniques in vivo. Hum Gene Ther. 2010;21:371–380. doi: 10.1089/hum.2009.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bridges CR, Gopal K, Holt DE, Yarnall C, Cole S, Anderson RB, Yin X, Nelson A, Kozyak BW, Wang Z, Lesniewski J, Su LT, Thesier DM, Sundar H, Stedman HH. Efficient myocyte gene delivery with complete cardiac surgical isolation in situ. J Thorac Cardiovasc Surg. 2005;130:1364–1370. doi: 10.1016/j.jtcvs.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 3.Jones JM, Wilson KH, Koch WJ, Milano CA. Adenoviral gene transfer to the heart during cardiopulmonary bypass: effect of myocardial protection technique on transgene expression. Eur J Cardiothorac Surg. 2002;21:847–852. doi: 10.1016/s1010-7940(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Davidson MJ, Jones JM, Emani SM, Wilson KH, Jaggers J, Koch WJ, Milano CA. Cardiac gene delivery with cardiopulmonary bypass. Circulation. 2001;104:131–133. doi: 10.1161/01.cir.104.2.131. [DOI] [PubMed] [Google Scholar]
- 5.Zolotuchin S, Byrne BJ, Mason E, Zolotuchin I, Potter M, Chestnut K, Summerford C, Samulski RJ, Muzyczka N. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther. 1999;6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 6.Bridges CR, Burkman JM, Malekan R, Konig SM, Chen H, Yarnall CB, Gardner TJ, Stewart AS, Stecker MM, Patterson T, Stedman HH. Global cardiac-specific transgene expression using cardiopulmonary bypass with cardiac isolation. Ann Thorac Surg. 2002;73:1939–1946. doi: 10.1016/s0003-4975(02)03509-9. [DOI] [PubMed] [Google Scholar]
- 7.Boekstegers P, von Degenfeld G, Giehrl W, Heinrich D, Hullin R, Kupatt C, Steinbeck G, Baretton G, Middeler G, Katus H, Franz WM. Myocardial gene transfer by selective pressure-regulated retroinfusion of coronary veins. Gene Ther. 2000;7:232–240. doi: 10.1038/sj.gt.3301079. [DOI] [PubMed] [Google Scholar]
- 8.Parlington MT, Acar C, Buckberg GD, Kofsky ER, Bugyi HI. Studies of retrograde cardioplegia. I. Capillary blood flow distribution to myocardium supplied open and occluded arteries. J Thorac Cardiovasc Surg. 1989;97:605–612. [PubMed] [Google Scholar]
- 9.Besoluk K, Tipirdamaz S. Comparative macroanatomic investigations of the venous drainage of the heart in Akkaraman sheep and Angora goats. Anat Histol Embryol. 2001;30:249–252. doi: 10.1046/j.1439-0264.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- 10.Ansari A. Anatomy and clinical significance of ventricular thebesian veins. Clin Anat. 2001;14:102. doi: 10.1002/1098-2353(200103)14:2<102::AID-CA1018>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Rudis E, Gates RN, Laks H, Drinkwater DC, Ardehali A, Aharon A, Chang P. Coronary sinus ostial occlusion during retrograde delivery of cardioplegic solution significantly improves cardioplegic distribution and efficacy. J Thorac Cardiovasc Surg. 1995;109:941–947. doi: 10.1016/S0022-5223(95)70320-9. [DOI] [PubMed] [Google Scholar]
- 12.Tosson R, Kuschkowitz F, Dasbach G, Lacskovics A. Relationship between of the coronary sinus catheter and distribution of cardioplegia. J Heart Valve Dis. 1999;8:120–123. [PubMed] [Google Scholar]
- 13.Hammond GL, Davies AL, Austen WG. Retrograde coronary sinus perfusion: a method of myocardial protection in dog during left coronary artery occlusion. Ann Surg. 1967;166:39–50. doi: 10.1097/00000658-196707000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eke CC, Gundry SR, Fukushima N, Bailey LL. Is there a safe limit to coronary sinus pressure during retrograde cardioplegia? Am Surg. 1997;63:417–420. [PubMed] [Google Scholar]
- 15.Mohl W, Kajgana I, Bergmeister H, Rattay F. Intermittent pressure elevation of coronary venous system as a method to protect ischemic myocardium. Interact CardioVasc Thorac Surg. 2005;4:66–69. doi: 10.1510/icvts.2004.095364. [DOI] [PubMed] [Google Scholar]