Abstract
Objective
Uterine serous papillary carcinoma (USPC) is an aggressive variant of endometrial cancer characterized by an innate resistance to chemotherapy and poor prognosis. In this study, we evaluated the expression of αV-integrins in primary USPC cell lines and the in vitro ability of intetumumab (CNTO 95), a fully human monoclonal antibody against αV-integrins, to inhibit USPC cell adhesion and migration.
Materials and Methods
The surface expression of integrins belonging to the αV-family, including αVβ3, αVβ5, and αVβ6, was evaluated in 6 primary USPC cell lines using flow cytometry analysis. To test the ability of intetumumab to inhibit USPC cell adhesion and migration, adhesion assays in the presence of vitronectin and migration assays through an 8.0-μm pore polycarbonate membrane also were performed.
Results
We found high expression of the αV-subunit on the cell surface of all 6 primary USPC cell lines tested (100% positive cells; mean fluorescence intensity range, 13.1–39.5). When the expression of single heterodimeric integrins was evaluated, αVβ3, αVβ5, and αVβ6 were expressed on 37.5%, 32.0%, and 16.3% of cells (mean fluorescence intensity range, 6.5–16.2, 9.2–32.5, and 6.2–11.5, respectively). Importantly, in functional assays, low doses of intetumumab were effective in inhibiting adhesion (0.15 μg/mL, P = 0.003) and migration (1.25 μg/mL P = 0.02) of primary USPC cell lines.
Conclusions
The αV-integrins are overexpressed on the cell surface of primary USPC cell lines. Intetumumab may significantly inhibit USPC cell adhesion and migration pathways and may therefore represent a novel treatment option for patients harboring this rare but highly aggressive variant of endometrial cancer.
Keywords: Uterine serous papillary carcinoma, CNTO 95, Intetumumab, αV-integrin
Cancers of the uterine corpus are the most common gynecological cancers, with 43,470 estimated new cases and 7950 estimated deaths for 2010 in the United States.1 Although the majority of endometrial tumors are diagnosed at early stage and are characterized by a low-grade endometrioid histology (type I tumors), their rare counterparts (i.e., type II tumors, including serous papillary, clear cell, and poorly differentiated endometrioid carcinomas) are responsible for the most relapses and deaths from uterine cancer.2,3 Uterine serous papillary carcinoma (USPC) accounts for up to 10% of endometrial cancer and is characterized by an aggressive biological behavior because of its propensity for early lymphatic and intraperitoneal spread and recurrence.4,5 Thus, development of novel, potentially effective targeted therapies against USPC metastatic pathways remains a high priority.
Integrins constitute a family of proteins involved in many physiological and pathological functions, including cell functioning, embryonic implantation and development, tissue morphogenesis, angiogenesis, blood clotting, wound healing, oncogenesis, metastasis, thrombosis, and inflammation.6 As transmembrane glycoproteins, they are expressed in different combinations by all cells, including platelets, leukocytes, mesenchymal tissues, and epithelial cells.7 Integrins achieve a diversity of functions by the formation of heterodimers, always consisting of one α-subunit and one β-chain, with 24 distinct combinations known so far.8 The αV subunit can associate with many different β-subunits, whereas other α-subunits dimerize with only one kind of β-chain.8 Both chains participate in the binding of the ligand, in cell-cell or cell–extracellular matrix (ECM) protein interactions. The intracellular portion of the integrin undergoes different associations with cytoskeletal elements and phosphorylation, in association with changes in cell morphology and movement, and provides signals via secondary messengers.7 Previous research has demonstrated that integrins play a role in multiple steps in tumorigenesis, including cell spreading, invasion, and survival of several types of solid tumors, such as melanoma, breast, prostate, and oral squamous cell carcinomas.9,10 Unfortunately, however, limited information is currently available on expression of αV-integrins in endometrial carcinoma.11,12
The presence of integrins on the cell surface renders them accessible to antibody-based therapies. Intetumumab (formerly CNTO 95; Centocor, Inc, Malvern, PA) is a human monoclonal antibody (mAb) that recognizes αV-integrin with high affinity (Kd = 200 pM for purified αV-chain and Kd = 1-24 nM for αV-integrins on expressing cells13). Previous studies have shown that this antibody binds αVβ1, αVβ3, αVβ5, and αVβ6; blocks αVβ3 and αVβ5; and inhibits integrin-mediated tumor growth and angiogenesis in vitro and in vivo on different animal models (mice,13 rats,13,14 and monkeys15) and in several different types of human tumors, including melanoma,13 breast,16 and small cell lung cancers.14 Phase I clinical trials have been conducted, showing that it is well tolerated in patients with advanced solid tumors17 and in hormone-refractory prostate cancer patients when administered with docetaxel and prednisone.18
To our knowledge, no studies have specifically described the expression of αV-integrins in USPCs. In this report, in an attempt to fill this gap in knowledge, we carefully investigated the surface expression of multiple αV-containing integrins in primary USPC cell lines and evaluated for the first time the in vitro potential of intetumumab as a novel targeted agent against this biologically aggressive and chemotherapy-resistant variant of endometrial cancer.
MATERIALS AND METHODS
Establishment of USPC Cell Lines
Six primary USPC tumor cell lines were established, after sterile processing, from fresh tumor biopsy samples collected at the time of surgery after approval from the institutional review board, as described previously.19,20 Briefly, tissue was mechanically minced to less than 3 mm3 in an enzyme solution made of 0.07% collagenase type I (Sigma, St Louis, MO) and 0.005% DNAse 2000 KU/mg (Sigma) in RPMI 1640 and incubated in the same solution in a magnetic stirring apparatus for 1 hour at room temperature. Enzymatically dissociated cells were then washed twice in RPMI 1640 with 10% fetal bovine serum (FBS; Gemini Bio-products, Calabasas, CA) and maintained in RPMI 1640 supplemented with 10% FBS, 50 IU/mL of penicillin, 50 μg/mL of streptomycin (Cellgro; Mediatech, Manassas, VA), and 0.75 μg/mL Fungizone (GIBCO Cell Culture; Invitrogen Corporation, Grand Island, NY) at 37°C, 5% CO2. Purity and homogeneity of fresh tumor cultures were tested by morphology, immunohistochemical staining, and/or flow cytometry with antibodies against cytokeratins. Only primary cultures that had at least 95% viability and contained greater than 99% epithelial cells were used in the experiments described below. Briefly, after seeding on plastic ware (48–72 hours), nonadherent cells and contaminant inflammatory cells were gently removed from the culture by multiple washings with phosphate buffered saline. Table 1 shows the characteristics of the patients from which the cell lines have been obtained. Tumors were staged using the International Federation of Obstetrics and Gynecology 1988 endometrial cancer staging system.
TABLE 1.
Patient characteristics, from which the 6 USPC cell lines were established
| Patient | Age (yr) | Race | FIGO Stage | USPC Histopathology |
|---|---|---|---|---|
| USPC-ARK-1 | 62 | AA | IVA | Pure |
| USPC-ARK-2 | 63 | AA | IVB | Pure |
| USPC-ARK-3 | 59 | AA | IVB | Mixed |
| USPC-ARK-4 | 73 | W | IVB | Pure |
| USPC-ARK-5 | 73 | AA | IIIC | Pure |
| USPC-ARK-6 | 62 | W | IB | Mixed |
AA, African American; W, white; FIGO, International Federation of Gynecology and Obstetrics.
Flow Cytometry Analysis
Flow cytometry studies were performed using intetumumab, a fully humanized anti–αV-integrin IgG1κ mAb (Centocor, Inc). As isotype control antibody, the chimeric anti-CD20 IgG1 mAb rituximab (Rituxan; Genentech, San Francisco, CA) was used. A fluorescein isothiocyanate–conjugated goat anti-human F(ab’)2 immunoglobulin (BioSource International, Camarillo, CA) was used as a secondary reagent. Other mouse antihuman mAbs used to test the expression of αV-integrin family were as follows: mAb 1953Z (clone P3G8, anti-αV subunit), mAb 1951Z (clone P4G11, anti-β1 subunit), mAb 1976Z (clone LM609, anti-αVβ3), mAb 1961 (clone P1F6, anti-αVβ5), and mAb 2077Z (clone 10D5, anti-αVβ6) (all Chemicon International, Temecula, CA). A mouse IgG2a κ isotype control (BD Biosciences, San Diego, CA) and a fluorescein isothiocyanate–conjugated polyclonal goat antimouse IgG (BD Biosciences) were used for the staining. In dose titration studies, we found 2.5 μg/mL to be the optimal intetumumab concentration to stain USPC cells (data not shown). Therefore, all further analyses were performed using intetumumab, as well as all other primary antibodies, at a concentration of 2.5 μg/mL. Analysis was conducted with a FACSCalibur, using CellQuest software (BD Biosciences). Data were collected for 10,000 cells gated per sample and displayed as histograms plotting the number of cells counted versus the intensity of fluorescence.
Adhesion Assay
Microtest 96-well plates (BD Falcon, Franklin Lakes, NJ) were coated at 37°C for 2 hours with vitronectin (1 μg/mL; Promega, Madison, WI) and blocked for 1 hour with 2 mg/mL of bovine serum albumin in phosphate buffered saline. Cells were incubated at 2 × 105/mL density with various concentrations of intetumumab or rituximab at 37°C for 15 minutes. Approximately 100 μL of the cell-antibody mixture was added to each well and incubated for 20 minutes at 37°C. Non-adherent cells were removed by rinsing the plate with serum-free RPMI 1640, and adherent cells were fixed with 10% buffered formalin phosphate (Fisher Scientific, Fair Lawn, NJ) and stained with crystal violet (0.1% weight/volume [w/v] in ddH2O; Sigma) for 25 minutes at room temperature. After rinsing the plate in ddH2O, Triton X-100 (0.5% in ddH2O; Acros Organics, Fair Lawn, NJ) was added to the wells to allow solubilization of the dye overnight at room temperature, before reading the absorbance at 595 nm in a microtiter plate reader (VersaMax; Molecular Devices, Silicon Valley, CA).
Migration Assay
Cell migration assays were performed in 24-transwell chambers with polycarbonate membrane (6.5 mm diameter, 8.0-μm pore size; Corning Incorporated, Corning, NY). Cells were kept in starvation for 24 hours before performing the assay. Cells (100,000/200 μL) were added to the upper chambers in the presence or absence of antibodies, in serum-free RPMI 1640. Approximately 800 μL of RPMI 1640 with 10% FBS was added to the lower chambers as a chemoattractant. The plates were incubated at 37°C for 48 hours. Migration was terminated by fixing the membranes with 10% buffered formalin phosphate and staining with crystal violet (0.1% w/v in ddH2O) for 25 minutes at room temperature. After rinsing the filters in ddH2O, the interior of each insert was scrubbed with a cotton swab to remove unmigrated cells. The stained cells were lysed with Triton X-100 (0.5% in ddH2O) overnight at room temperature. The resulting colored mixture was transferred to a 96-well plate, and optical densities were read in triplicate at 595 nm in a VersaMax microtiter plate reader.
Statistical Analysis
Mann-Whitney U test was used to compare the behavior of cells in the presence versus absence of antibody in both adhesion and migration assays. Statistical analysis was performed using PASW Statistics version 18 (SPSS, Chicago, IL). A P < 0.05 was considered statistically significant.
RESULTS
αV-Integrin Is Expressed on the Surface of USPC Cell Lines
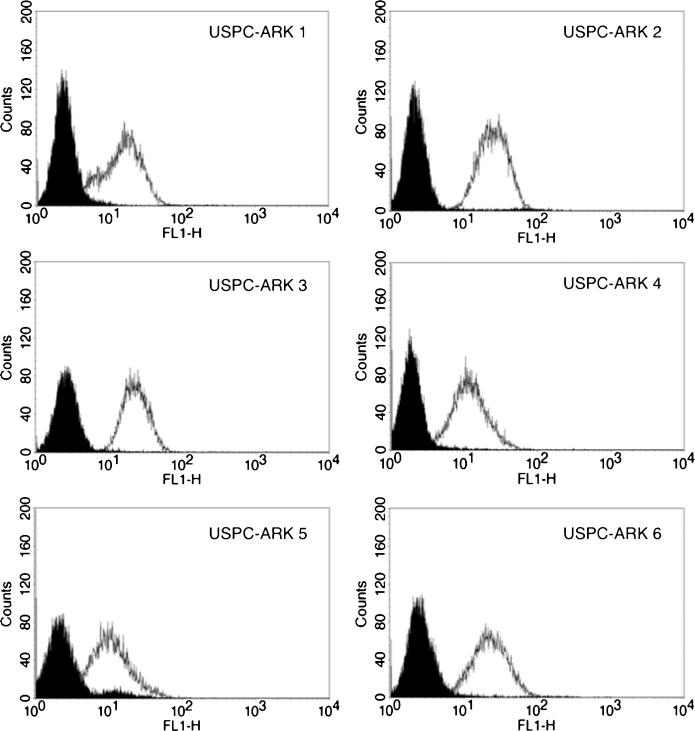
We evaluated the surface expression of αV-integrin by FACS analysis on 6 primary USPC cell lines using intetumumab, a fully human mAb that recognizes αV-integrin with high affinity. We found all USPC cell lines tested (ie, 6 of 6 [100%]) to be strongly positive (average positive gated cells, 100%) for αV-integrin expression on the cell surface, with a mean fluorescence intensity (MFI) of 28.4 (SD, ±9.55; range, 13.1–39.5) when using intetumumab (Fig. 1). The mAb 1953Z, the murine mAb anti-αV, demonstrated a similar positivity with an average (98.5%) of positive gated cells and an MFI of 32.2 (SD, ±8.27; range, 24.7–48.4).
FIGURE 1.
αV-integrin expression on cell surface by flow cytometry analysis. Representative expression of αV-integrins on the cell surface of 6 primary USPC cell lines when evaluated by flow cytometry. 2.5 μg/mL of intetumumab, as well as isotype control, were used to target αV-integrins. Rituximab anti-CD20 control antibody (solid black) and intetumumab (white peak).
Then, we checked the expression of multiple β-subunits to which the αV-chain can bind and heterodimerize in different combinations. Specifically, we tested the expression of β1-integrin alone and αVβ3, αVβ5, and αVβ6 heterodimers (Table 2). We found the β1-subunit to be expressed in 12.5% of the cells, with an MFI of 11.6 (SD, ±5.73; range, 7.3–22.1) (Table 2). The αVβ3 and αVβ5 showed a higher expression, with 37.5% and 32.0% of positive cells and an MFI of 12.3 (SD, ±4.02; range, 6.5–16.2) and 17.5 (SD, ±9.23; range, 9.2–32.5), respectively (Table 2). Finally, we found a lower expression of αVβ6 with only 16.3% of cells being positive and an MFI of 7.8 (SD, ±2.01; range, 6.2–11.5) (Table 2).
TABLE 2.
Cell surface expression analysis of integrins in a representative flow cytometry experiment
| Patient | Intetumumab | αV | β1 | αVβ3 | αVβ5 | αVβ6 | |
|---|---|---|---|---|---|---|---|
| USPC-ARK-1 | 100 | 97.2 | 15.6 | 36.5 | 28.1 | 9.1 | % Positive |
| 32.2 | 29.9 | 11.3 | 14.2 | 24.1 | 6.5 | MFI | |
| USPC-ARK-2 | 100 | 100 | 18.2 | 33.3 | 62.3 | 27.6 | % Positive |
| 34.9 | 29.7 | 7.9 | 8.0 | 9.2 | 7.1 | MFI | |
| USPC-ARK-3 | 100 | 100 | 8.4 | 12.0 | 48.2 | 35.7 | % Positive |
| 28.9 | 28.8 | 7.4 | 16.2 | 11.2 | 6.2 | MFI | |
| USPC-ARK-4 | 100 | 100 | 28.1 | 50.9 | 39.2 | 14.5 | % Positive |
| 21.9 | 31.6 | 7.3 | 6.5 | 10.5 | 6.8 | MFI | |
| USPC-ARK-5 | 100 | 94.0 | 2.4 | 35.3 | 5.7 | 2.7 | % Positive |
| 13.1 | 24.7 | 22.1 | 14.8 | 17.3 | 8.7 | MFI | |
| USPC-ARK-6 | 100 | 100 | 2.3 | 56.7 | 8.6 | 8.1 | % Positive |
| 39.5 | 48.4 | 13.5 | 14.2 | 32.5 | 11.5 | MFI |
MFI, Mean Fluorescence Intensity; USPC, Uterine Serous Papillary Carcinoma.
Intetumumab Inhibited Integrin-Mediated USPC Cell Adherence to a Vitronectin Substratum
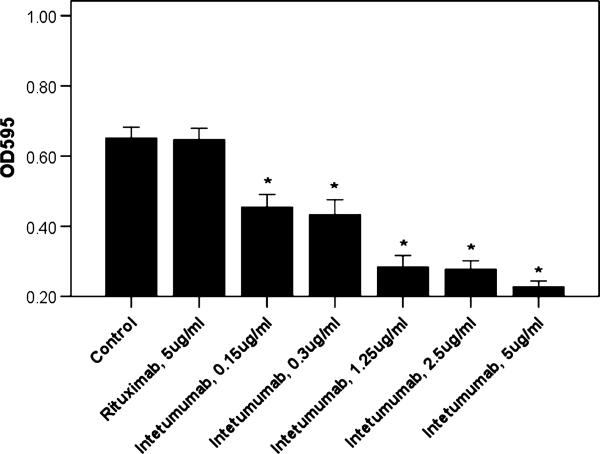
The αV-integrins are known receptors for ECM proteins, such as vitronectin, tenascin, and fibronectin.6 We therefore investigated the ability of intetumumab to inhibit the adhesion of USPC cell lines (ie, USPC-ARK-2, USPC-ARK-3, USPC-ARK-4, and USPC-ARK-6) to vitronectin-coated plates in vitro. As representatively shown in Figure 2 for 1 USPC primary cell line (ie, USPC-ARK-3), we found that different concentrations of intetumumab were able to specifically inhibit the αV-integrin–vitronectin interaction in a dose-dependent fashion in all cell lines tested. The range of inhibition with intetumumab was 30% to 65% when compared with control USPC cells where no mAb was added (P < 0.003 for each condition). No significant inhibition was reported in the presence of rituximab (1% inhibition, P = 0.717), independent of the concentration used.
FIGURE 2.
Representative adhesion assay on USPC-ARK 3. Increasing concentrations of intetumumab inhibit the adhesion of USPC-ARK-3 cells to the plate. Extent of cell adhesion in the presence of various concentrations of antibody is shown as absorbance of the solution after lysis of stained adherent cells. Data shown are mean (±SEM) of at least 3 different experiments. *P ≤ 0.003 versus the condition in the absence of any antibody.
Intetumumab Inhibited Serum-Induced Migration of USPC Cells
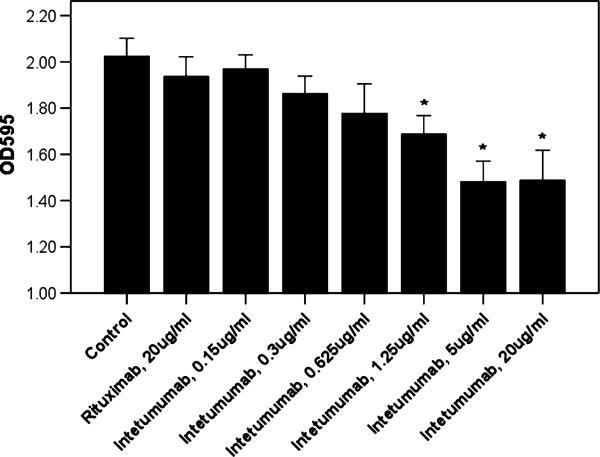
The ability of intetumumab to block the binding of αV-integrins to ECM protein vitronectin suggested potential intetumumab effects on cell motility because this event is known to be mediated by integrin-ECM interactions.6 We therefore investigated the ability of intetumumab to inhibit the migration of USPC cell lines (ie, USPC-ARK-2, USPC-ARK-4, and USPC-ARK-6) through an 8.0-μm pore polycarbonate membrane. We used FBS 10% as chemoattractant to stimulate the migration of tumor cells kept in starvation medium (serum free RPMI 1640) for 24 hours before performing the experiments. We found that intetumumab concentrations from 1.25 μg/mL up to 20 μg/mL were able to significantly inhibit the migration of 17% to 27% of USPC-ARK-2 cells through the membrane when compared with control cells with no mAb added (P < 0.03; Fig. 3). Lower doses of intetumumab (ie, 0.15 μg/mL, 0.3 μg/mL, or 0.625 μg/mL; inhibition range, 3%–12%; P values between 0.724 and 0.142) or rituximab (up to the highest dose, 20 μg/mL, inhibition of 4%; P = 0.724) did not significantly affect migration of tumor cells. Similar results were obtained using the USPC-ARK-4 and USPC-ARK-6 cell lines (data not shown).
FIGURE 3.
Representative migration assay on USPC-ARK 2. Increasing concentrations of intetumumab inhibit the migration of USPC-ARK-2 cells through an 8.0-μm pore polycarbonate membrane, mediated by a chemoattractant stimulus. Extent of cell migration in the presence of various concentrations of antibody is shown as absorbance of the solution after lysis of stained migrated cells. Data shown are mean (±SEM) of at least 3 different experiments. *P ≤ 0.03 versus the condition in the absence of any antibody.
DISCUSSION
Uterine serous papillary carcinoma histology is considered a prognostically unfavorable factor in endometrial cancer because of the high rates of chemotherapy resistance, recurrence, and mortality secondary to the diagnosis with this rare but highly aggressive variant of endometrial tumor.4,5 Importantly, because USPCs, similar to ovarian cancer, have the tendency to early spread throughout the peritoneal cavity, the discovery of novel, effective treatment options to inhibit USPC migration and metastases would be highly desirable. The αV-containing integrins are known to play a major role in tumor growth, adhesion, movement, angiogenesis, and metastasis formation in several types of tumors.6 Accordingly, in the current study, we have carefully evaluated the surface expression of αV-integrins in multiple primary USPC cell lines and analyzed for the first time the potential therapeutic effects of intetumumab, a fully human monoclonal antibody against αV-integrins, to inhibit USPC cell adhesion and migration in vitro.
We found significant αV-chain surface expression in all 6 primary USPC cell lines available to this study. Consistent with the results obtained for αV-chain with intetumumab, when single heterodimeric integrin expression was evaluated by flow cytometry with specific antibodies, integrins αVβ3, αVβ5, and αVβ6 also were found upregulated in primary USPC cell lines at different levels. Although limited information is currently available in the literature regarding expression of αV-integrins in endometrial cancer, our results in USPC are consistent with those of recent reports demonstrating significant expression of the integrins αVβ5 and αVβ6 in endometrial endometrioid tumors.11,12 Of interest, in the study by Hecht et al,12 although αVβ6 integrin was only weakly or not expressed in normal epithelium, it was found upregulated in most endometrial endometrioid carcinomas, especially high-grade tumors and those characterized by the presence of metastatic disease. These observations, combined with our results, are consistent with the possibility that high expression of αV-integrins in biologically aggressive tumors, such as poorly differentiated endometrioid tumors12 and USPC, may at least partially account for their enhanced invasive behavior and their rapid pattern of metastatic spread.
Importantly, because αV-chain expression was found in 100% of the primary USPC cell lines tested, targeting these receptors with intetumumab, a fully human mAb direct against αV-integrins, may represent a novel, potentially effective therapy to prevent USPC metastatic spread. Consistent with this hypothesis, we were able to demonstrate a significant inhibition of USPC cell adhesion to ECM proteins, such as vitronectin, using low doses of intetumumab. In this regard, vitronectin was the substrate chosen for our in vitro model because it is the specific ligand of αV-subunit, whereas fibrinogen and fibronectin also can be recognized by other α-integrins, that is, α3, α4, α5, α8, and αIIb.21 Importantly, our results are consistent with the data of other groups who have demonstrated intetumumab's ability to inhibit adhesion in other human cancers, such as melanoma13 and breast cancer.16
Next, to mimic the passage of tumor cells through the endothelium to reach the bloodstream and exit from it to spread throughout the body (ie, a key process in metastasis formation), we have tested the in vitro ability of intetumumab to inhibit the migration of USPC cells through an 8.0-μm pore polycarbonate membrane. We found intetumumab to significantly inhibit the migration of USPC cells through the membrane. These results clearly demonstrate that intetumumab may have the ability to inhibit the migration of USPC cells in vitro and potentially in vivo. Our data are therefore in agreement with previous reports demonstrating the ability of this fully human anti–αV-integrin antibody to inhibit migration and invasion and spontaneous metastasis in multiple human tumor models.13,16
Although not directly tested in the current work because of the lack of a suitable USPC immunocompetent animal model, multiple studies have previously demonstrated the in vivo activity of intetumumab to inhibit the growth of several human tumor models in animals when administered both intraperitoneally13,14 or intravenously.13,16 In this regard, rat and monkey models are the most suitable for in vivo studies because intetumumab has no reactivity against mouse αV-integrin, has limited cross-reactivity with rat αV-integrin (Kd = 220 nM), and can recognize cynomolgus macaque integrins with similar binding affinity as human cells (Kd = 5.1 and 5.2 nM, respectively).13 Of interest, intetumumab in combination with radiation therapy has been recently tested in rat models by Ning and colleagues,14 showing that it is active in tumor growth delay, better when administered just before the radiotherapy session and when used in maintenance therapy. In that study, to further assess the safety and tolerability of the antibody, they also checked the effect of intetumumab on intestinal crypt stem cells, which are sensitive to radiation, and showed that intetumumab did not affect the stem cells when administered in combination with radiation therapy.14
Importantly, intetumumab is currently under investigation in several clinical trials. Before proceeding with human clinical trials, the safety of long-term administration of intetumumab was tested on cynomolgus macaque, resulting in no signs of toxicity and no histopathological changes in any tissue.15 Furthermore, the effects of intetumumab on physiological angiogenesis have been tested in additional wound healing studies conducted on cynomolgus macaques.15 Two phase I studies have shown that intetumumab is generally active, safe, and well tolerated, both alone17 or when used in combination with docetaxel in patients with castrate-resistant metastatic prostate cancer.18 Major common adverse events were fever, headache, chills (responding to acetaminophen or ibuprofen),17 neutropenia, nausea, and neutropenic fever.18
In summary, our study with intetumumab in primary USPC cell lines demonstrates for the first time that αV-integrins are overexpressed on the cell surface of primary uterine serous tumors and that intetumumab is able to significantly inhibit USPC cell adhesion and migration pathways in vitro. On the basis of these findings, intetumumab may represent a novel treatment option to combine with other active regimens for the therapy of patients harboring this rare but highly aggressive variant of endometrial cancer.
ACKNOWLEDGMENTS
The authors thank Centocor, Inc, Malvern, Pa, for providing intetumumab (CNTO 95) free of charge for our studies.
This study was supported in part by grants from the Angelo Nocivelli, the Berlucchi, and the Camillo Golgi Foundation, Brescia, Italy, NIH R01 CA122728-01A2 to AS, and grants 501/A3/3 and 0027557 from the Italian Institute of Health (to A.D.S.). This investigation also was supported by NIH Research Grant CA-16359 from the National Cancer Institute.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistic, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Clement PB, Young RH. Endometrioid carcinoma of the uterine corpus: a review of its pathology with emphasis on recent advances and problematic aspects. Adv Anat Pathol. 2002;9:145–184. doi: 10.1097/00125480-200205000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Fadare O, Zheng W. Insight into endometrial serous carcinogenesis and progression. Int J Clin Exp Pathol. 2009;2:411–432. [PMC free article] [PubMed] [Google Scholar]
- 4.Bansal N, Yendluri V, Wenham RM. The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer Control. 2009;16:8–13. doi: 10.1177/107327480901600102. [DOI] [PubMed] [Google Scholar]
- 5.Kurman RJ, McConnell TG. Precursors of endometrial and ovarian carcinoma. Virchows Arch. 2010;456:1–12. doi: 10.1007/s00428-009-0824-9. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth JA, Nakada MT, Trikha M, et al. Alpha-v integrins as therapeutic targets in oncology. Cancer Invest. 2007;25:632–646. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- 7.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 8.Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9:804–820. doi: 10.1038/nrd3266. [DOI] [PubMed] [Google Scholar]
- 9.Rathinam R, Alahari SK. Important role of integrins in the cancer biology. Cancer Metastasis Rev. 2010;29:223–237. doi: 10.1007/s10555-010-9211-x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Lu H, Dazin P, et al. Squamous cell carcinoma cell aggregates escape suspension-induced, p53-mediated anoikis. Fibronectin and integrin αv integrin mediate survival signals through focal adhesion kinase. J Biol Chem. 2004;279:48342–48349. doi: 10.1074/jbc.M407953200. [DOI] [PubMed] [Google Scholar]
- 11.Choi DS, Kim H-J, Yoon J-H, et al. Endometrial cancer invasion depends on cancer-derived tumor necrosis factor-α and stromal derived hepatocyte growth factor. Int J Cancer. 2009;124:2528–2538. doi: 10.1002/ijc.24238. [DOI] [PubMed] [Google Scholar]
- 12.Hecht JL, Dolinski BM, Gardner HA, et al. Overexpression of the αvβ6 integrin in endometrial cancer. Appl Immunohistochem Mol Morphol. 2008;16:543–547. doi: 10.1097/PAI.0b013e31816bc5ee. [DOI] [PubMed] [Google Scholar]
- 13.Trikha M, Zhou Z, Nemeth JA, et al. CNTO 95, a fully human monoclonal antibody that inhibits αv integrins, has antitumor and antiangiogenic activity in vivo. Int J Cancer. 2004;110:326–335. doi: 10.1002/ijc.20116. [DOI] [PubMed] [Google Scholar]
- 14.Ning S, Tian J, Marshall DJ, et al. Anti-alphav integrin monoclonal antibody intetumumab enhances the efficacy of radiation therapy and reduces metastasis of human cancer xenografts in nude rats. Cancer Res. 2010;70:7591–7599. doi: 10.1158/0008-5472.CAN-10-1639. [DOI] [PubMed] [Google Scholar]
- 15.Martin PL, Jiao Q, Cornacoff J, et al. Absence of adverse effects in cynomolgus macaques treated with CNTO 95, a fully human anti-αv integrin monoclonal antibody, despite widespread tissue binding. Clin Cancer Res. 2005;11:6959–6965. doi: 10.1158/1078-0432.CCR-04-2623. [DOI] [PubMed] [Google Scholar]
- 16.Chen Q, Manning CD, Millar H, et al. CNTO 95, a fully human anti >v integrin antibody, inhibits cell signaling, migration, invasion, and spontaneous metastasis of human breast cancer cells. Clin Exp Metastasis. 2008;25:139–148. doi: 10.1007/s10585-007-9132-4. [DOI] [PubMed] [Google Scholar]
- 17.Mullamitha SA, Ton NC, Parker GJM, et al. Phase I evaluation of a fully human anti-αv integrin monoclonal antibody (CNTO 95) in patients with advanced solid tumors. Clin Cancer Res. 2007;13:2128–2135. doi: 10.1158/1078-0432.CCR-06-2779. [DOI] [PubMed] [Google Scholar]
- 18.Chu FM, Picus J, Fracasso PM, et al. A phase 1, multicenter, open-label study of the safety of two dose levels of a human monoclonal antibody to human αv integrins, intetumumab, in combination with docetaxel and prednisone in patients with castrate-resistant metastatic prostate cancer. Invest New Drugs. 2010 doi: 10.1007/s10637-010-9388-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Bellone S, Siegel ER, Cocco E, et al. Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer. Int J Gynecol Cancer. 2009;19:860–866. doi: 10.1111/IGC.0b013e3181a8331f. [DOI] [PubMed] [Google Scholar]
- 20.El-Sahwi K, Bellone S, Cocco E, et al. Overexpression of EpCAM in uterine serous papillary carcinoma: implications for EpCAM-specific immunotherapy with human monoclonal antibody adecatumumab (MT201). Mol Cancer Ther. 2010;9:57–66. doi: 10.1158/1535-7163.MCT-09-0675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker GC. Integrins: molecular targets in cancer therapy. Curr Oncol Rep. 2006;8:96–103. doi: 10.1007/s11912-006-0043-3. [DOI] [PubMed] [Google Scholar]