Abstract
Rationale
Mutations in the cardiac type 2 ryanodine receptor (RyR2) have been linked to catecholaminergic polymorphic ventricular tachycardia (CPVT). CPVT-associated RyR2 mutations cause fatal ventricular arrhythmias in young individuals during beta-adrenergic stimulation.
Objective
This study sought to determine the effects of a novel identified RyR2-G230C mutation and whether this mutation and RyR2-P2328S alter the sensitivity of the channel to luminal calcium (Ca2+).
Methods
Functional characterizations of recombinant human RyR2-G230C channels were performed under conditions mimicking stress. Human RyR2 mutant channels were generated by site-directed mutagenesis and heterologously expressed in HEK293 cells together with calstabin2 (FKBP12.6). RyR2 channels were measured in order to examine the regulation of the channels by cytosolic vs. luminal SR Ca2+.
Results
A 50 year-old Caucasian male with repeated syncopal episodes after exercise had a cardiac arrest and harbored the mutation RyR2-G230C. PKA-phosphorylated RyR2-G230C channels exhibited a significantly higher open probability (Po) at diastolic Ca2+ concentrations, associated with a depletion of calstabin2. The luminal Ca2+ sensitivities of RyR2-G230C and RyR2-P2328S channels were WT-like.
Conclusions
The RyR2-G230C mutant exhibits similar biophysical defects compared to previously characterized CPVT mutations: decreased binding of the stabilizing subunit calstabin2 and a leftward shift in the Ca2+-dependence for activation under conditions that simulate exercise, consistent with a “leaky” channel. Both RyR2-G230C and RyR2-P2328S channels exhibit normal luminal Ca2+ activation. Thus, diastolic SR Ca2+ leak caused by reduced calstabin2 binding and a leftward shift in the Ca2+-dependence for activation by diastolic levels of cytosolic Ca2+ is a common mechanism underlying CPVT.
Keywords: CPVT, sudden cardiac death, ryanodine receptor, calstabin
Introduction
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a rare inherited arrhythmogenic disease characterized by syncope or sudden death induced by emotional or physical stress. The mortality in untreated individuals ranges from 30 to 50% by the age of 40. Dominantly inherited forms of CPVT have been linked to mutations in the cardiac ryanodine receptor (RyR2) 1, 2. Currently more than 70 RyR2 mutations have been identified, and RyR2 mutations may account for 50% of diagnosed cases of CPVT 2, 3.
Here, we report a novel CPVT mutation in RyR2 in a 50 year-old Caucasian male with a history of repeated syncopal episodes after exercise, beginning at age 30. The proband had a cardiac arrest and was diagnosed with CPVT. RyR2 genetic analysis performed by direct sequencing of the patient’s DNA revealed a RyR2-G230C mutation. A brother died suddenly during exercise at age 16 and a son died suddenly at 12 years of age from cardiac arrest after swimming. A younger son was tested and does not carry the RyR2-G230C mutation.
Our group first elaborated a mechanism for CPVT by showing that CPVT and sudden cardiac death (SCD)-related RyR2 mutations decrease the binding of calstabin2 (FKBP12.6) to RyR2 which is further reduced by protein kinase A (PKA) phosphorylation during adrenergic stimulation 4-7. Moreover we showed that CPVT linked RyR2 mutations, including RyR2-S2246L, RyR2-R2474S, RyR2-R4497C, RyR2-P2328S, RyR2-Q4201R and RyR2-V4653F shift the sensitivity for cytosolic calcium (Ca2+)-dependent activation of the channel to the left resulting in channels that are inappropriately activated at low diastolic levels of cytosolic Ca2+ thereby causing a diastolic sarcoplasmic reticulum (SR) Ca2+ leak 4, 5, 7. Importantly, the leftward shift in the sensitivity for cytosolic Ca2+-dependent activation of the CPVT mutant channels compared to WT channels was only observed after PKA phosphorylation of the channels (conditions that mimic the effect of exercise or stress on the channel). This is in agreement with the clinical phenotype of CPVT patients who experience arrhythmias during exercise, but not at rest.
Another group has proposed that increased sensitivity to luminal SR Ca2+ is the mechanism underlying leaky CPVT mutant channels. This group reported that CPVT, SCD or arrhythmogenic right ventricular dysplasia (ARVD)-linked RyR2 mutations, including RyR2-E189D, RyR2-N4104K, RyR2-R4496C and RyR2-N4895D, exhibited increased sensitivity to activation by luminal Ca2+ leading them to conclude that store overload induced Ca2+ release (SOICR) is the cause of CPVT 8-10. A third group, using the CPVT mutant model RyR2-R2474S, suggested that CPVT and SCD-linked mutations induce defective inter-domain conformational changes which destabilize the closed state of the channel and enhance its sensitivity to Ca2+ 11,12. It has also been reported that RyR2-L433P and RyR2-N2386I mutant channels exhibit impaired sensitivity to Ca2+-dependent channel inhibition 13. More recently, three additional studies at the cellular level reported that the CPVT RyR2-R4496C mutant is abnormally Ca2+ sensitive and “leaky” and pointed out the importance of SR Ca2+ load in the genesis of Ca2+ waves and arrhythmias 14-16.
These disparate conclusions have impaired a clear understanding of the mechanism underlying CPVT and require further investigation to resolve the differences.
Here, we report a novel CPVT mutation RyR2-G230C and show that this mutation, like others we have reported 4-6, results in increased depletion of calstabin2 from the RyR2 macromolecular complex and enhanced sensitivity to cytosolic Ca2+ (left-ward shift in the Ca2+-dependence for activation of the channel) and under conditions that simulate exercise-induced stress (beta-adrenergic stimulation). Importantly, examined at the single channel level both RyR2-G230C and RyR2-P2328S mutant channels exhibited normal luminal Ca2+ sensitivity compared to WT, indicating that their threshold for luminal Ca2+ activation is the same as WT channels. Thus, increased sensitivity to cytosolic Ca2+ can explain the leaky channel behavior of CPVT mutant RyR2 channels and indicate that altered sensitivity to luminal Ca2+ (SOICR) is not required to explain CPVT.
Methods
Recombinant mutant channels were generated and expressed in HEK293 cells. HEK293 cell lines expressing RyR2 wt and mutants were generated using QuikChange II XL Site-Directed Mutagenesis Kit (Stratagene). Single channel measurements were performed to investigate the RyR2 channel properties in planar lipid bilayers. Measurements of FKBP12.6 binding to immunoprecipitated RyR2 were performed under PKA phosphorylation. A detailed Methods section can be found in the online data supplement available at http://circres.ahajournals.org.
Results
Phenotype
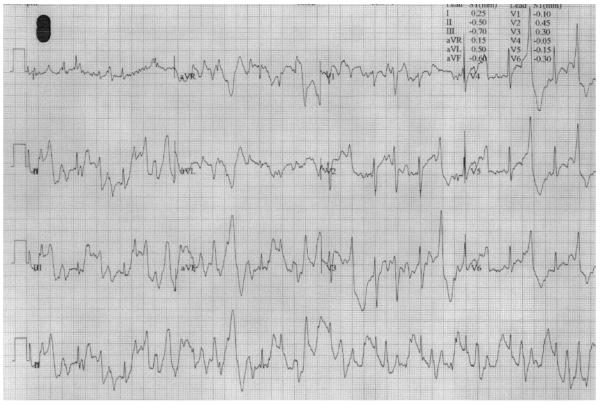
Clinical evaluation including cardiac catheterization revealed no cardiac structural abnormalities. ECG at rest showed normal sinus rhythm including a normal QT interval. Treadmill stress test (Bruce protocol) induced polymorphic premature ventricular beats (Figure 1).
Figure 1. Electrocardiogram of CPVT patient after treadmill stress test (Bruce Protocol).
Holter monitor recording of proband showing sinus tachycardia with polymorphic ventricular beats.
Molecular characterization of CPVT-linked RyR2 mutation
Blood samples were obtained by venipuncture, and genomic DNA was extracted from peripheral blood lymphocytes. We performed genetic testing of the proband’s genomic DNA and confirmed it by dideoxy sequencing method. We found on genetic testing that the proband has a missense mutation with a Guanine to Thymine substitution at the 688th base of the RyR2 gene nucleotide sequence (RYR2-688G>T) which resulted in a glycine to cysteine substitution at the 230th position (G230C) close to the amino-terminus of the RyR2 protein (Online Figure I). This CPVT-linked RyR2 mutation has not been previously reported.
Pedigree of index family
RM is a 50 year-old Caucasian male with history of repeated syncopal episodes after exercise beginning at age 30. He had a cardiac arrest and was diagnosed with CPVT. The proband’s brother died suddenly during exercise at age 16 and the proband’s son died suddenly at 12 years of age from cardiac arrest after swimming. A younger son was tested and does not carry the RyR2-G230C mutation (Online Figure II).
Functional characterization of CPVT-linked mutant RyR2-G230C channels
To study the effects of the CPVT-associated RyR2-G230C mutations on the channel function, we characterized the biophysical properties of the single channel activity (Figure 2). ER microsomes from HEK293 cells transiently co-expressing calstabin2 and either recombinant RyR2-WT or RyR2-G230C were fused into planar lipid bilayers. In order to test the functional properties of RyR2-G230C mutant channels under conditions simulating stress due to increased activity of the sympathetic nervous system we measured single channel activity of the RyR2-WT and RyR2-G230C channels from microsomes that were subjected to PKA phosphorylation in vitro using the planar lipid bilayer technique over a range of physiological Ca2+ concentrations, starting at 150 nmol/L up to full activation of the channel (~1-10 μmol/L Ca2+). At low cytosolic calcium concentration (i.e. 150 nmol/L free Ca2+) that corresponds to the resting diastolic Ca2+ level under basal conditions (no PKA treatment), the activity of RyR2-WT and RyR2-G230C channels were similar, displaying a very low open probability (Po) (mean Po of 0.007 ± 0.004 for RyR2-WT, n = 5; versus mean Po of 0.009 ± 0.004 for RyR2-G230C, n = 5, p=NS; Figure 2A and C). As the cis (cytosolic) Ca2+ concentration was increased, the Po of both channels increased to a similar degree (mean Po of 0.041/0.359/0.531 at 350/700/1000 nmol/L Ca2+ for RyR2-WT versus mean Po of 0.108/0.310/0.626 at 350/700/1000 nmol/L Ca2+ for RyR2-G230C, p = NS; Figure 2D).
Figure 2. Functional characterization of recombinant human RyR2-G230C mutant.
A-B: Representative single channel current traces of untreated RyR2-WT and RyR2-G230C channels (A) and PKA phosphorylated RyR2-WT and RyR2-G230C channels (B) measured at 150 nmol/L (nM) cytosolic [Ca2+]. Channel openings are shown as upward deflections; the closed (c −) state of the channel is indicated by horizontal bars in the beginning of each tracing. Example of channel activity is shown at two different time scales (5s for one upper trace and 500 ms for three lower traces) as indicated by dimension bars, and the Po, To (average open time) and Tc (average closed time) are shown above each 5 s trace. An amplitude histogram is shown on the right side of each representative single channel trace to illustrate two to three distinct peaks corresponding to fully open (~4 pA), subconductance (~2 pA) and closed (~0 pA) states of the channel. C: Bar graph summarizing single channel Po under 150 nmol/L cytosolic [Ca2+] in RyR2-WT (n = 5), RyR2-WT + PKA (n = 5), RyR2-G230C (n = 5) and RyR2-G230C + PKA (n = 7) channels. Data presented as mean +/− S.E.M; *p < 0.05. D: Single channel open probability of mutant and WT channels were measured at Ca2+ concentrations from 150 nmol/L to 10 μmol/L (full activation). Each data points represents the open probability calculated as an average from 3 to 7 independent experiments shown as mean ± SE. Ca2+ dependences of untreated RyR2-WT (open triangles, dotted line), PKA-phosphorylated RyR2-WT (open circles, dash-dotted line), untreated RyR2-G230C (full triangles, full line), PKA-phosphorylated RyR2-G230C (full circles, solid line) were fitted by the sigmoidal equation. At 150 nmol/L and 350 nmol/L, Po of PKA-phosphorylated RyR2-G230C is significantly higher (* indicates p < 0.05) compared with PKA-phosphorylated WT-RyR2 channels.
After PKA treatment, channel activity was increased for both RyR2-WT and RyR2-G230C. However, the Po of RyR2-G230C mutant channels was significantly higher compared to RyR2-WT channels at 150 nmol/L cytosolic Ca2+ (mean Po of 0.068 ± 0.020 for RyR2-WT, n = 5, versus mean Po of 0.182 ± 0.038 for RyR2-G230C, n = 7, p < 0.05; Figure 2B and C). The activity of RyR2-G230C channels was also higher with 350 nmol/L cytosolic Ca2+ (mean Po of 0.174 ± 0.064 for RyR2-WT, n = 5, versus mean Po of 0.338 ± 0.032 for RyR2-G230C, n = 7, p < 0.05; Figure 2D). It should be noted that the mean closed time was comparable (data not shown). Once the channel was activated with further cytosolic Ca2+ (> 700 nmol/L free Ca2+), no differences in Po were found between PKA-treated RyR2-G230C and WT channels (Figure 2D).
In addition to an increased sensitivity at low [Ca2+]cyt, amplitude histograms indicated an increased prevalence of sub-conductance state in the PKA phosphorylated RyR2-G230C channels (Figure 2B) as previously observed for RyR channels that were PKA phosphorylated and depleted of calstabin 5, 17, 18.
Taken together, these results indicate that under stress conditions (mimicked by PKA phosphorylation) the CPVT-associated RyR2 mutation RyR2-G230C results in increased sensitivity to cytosolic Ca2+ causing “leaky” channels. These results are consistent with the observed functional effect of previously characterized CPVT and SCD-related RyR2 mutations 4-6 and with the clinical observations that cardiac arrhythmias in CPVT patients occur during physical or emotional stress.
Biochemical characterization of RyR2-G230C mutation
Previous studies have shown that the depletion of calstabin2 from the RyR2 channel complex destabilizes the closed state of RyR2 4, 5. This destabilization leads to enhanced diastolic Ca2+ sensitivity manifested as an increased RyR2 Po in the presence of low activating [Ca2+] = 150 nmol/L 5, 17, 19. In support of this model, our group has demonstrated that recombinant CPVT and SCD-related RyR2 mutants, expressed in HEK293 cells, exhibit a reduced affinity for calstabin2 compared to WT channels 4, 5.
To determine whether the increased Po and gating changes observed for the PKA phosphorylated RyR2-G230C mutant channels were associated with depletion of the stabilizing subunit calstabin2 protein, RyR2 macromolecular complexes were immunoprecipitated from HEK293 cell membranes. While untreated channels revealed only a basal level of PKA phosphorylation, PKA-treated RyR2-WT and RyR2-G230C channels exhibited a comparable high level of PKA phosphorylation (Figure 3A and B) and significantly lower amounts of calstabin2 bound to the PKA phosphorylated RyR2-G230C mutant channels compared to the RyR2-WT treated channels (Figure 3A and C). The significantly lower amount of calstabin2 bound to the PKA phosphorylated RyR2-G230C mutant channels suggests that the CPVT-associated mutation decreases the binding affinity of calstabin2 to the mutant RyR2 as previously reported for other CPVT-linked RyR2 mutations 4, 5.
Figure 3. Biochemical characterization of recombinant human RyR2-G230C mutant.
A: Representative RyR2 immunoprecipitation and immunoblots. B-C: Bar graphs showing S2809 phosphorylation level (B) and depletion of calstabin2 (C) from RyR2-WT and RyR2-G230C ± PKA treatment. RyR2 immunoprecipitations were performed on ER vesicles. Levels of S2809 phosphorylation and levels of calstabin2 in the RyR2 complex were normalized to the total amount of RyR2 (A.U.). Data presented as mean +/− S.E.M; *p < 0.05.
CPVT linked RyR2-G230C and RyR2-P2328S mutations do not affect luminal Ca2+ sensitivity
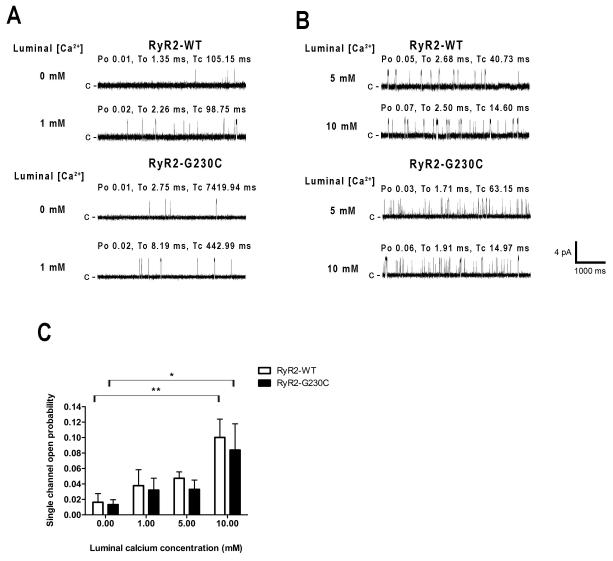
To study the effects of RyR2-G230C mutation on the luminal Ca2+ sensitivity, we performed single channel measurements using Ba2+ as the charge carrier with added Ca2+ to the luminal (trans) side at low cytosolic (cis) Ca2+ in absence of allosteric modulators (i.e. no PKA treatment, no Mg-ATP nor caffeine). Addition of 1 mmol/L Ca2+ to the luminal side did not significantly increase the single Po of recombinant RyR2-G230C and WT channels (mean Po of 0.016 ± 0.011 at 0 mmol/L luminal [Ca2+], n = 8, versus mean Po of 0.038 ± 0.021 at 1 mmol/L luminal [Ca2+] for RyR2-WT, n = 5, and mean Po of 0.013 ± 0.006 at 0 mmol/L luminal [Ca2+], n = 10, versus mean Po of 0.032 ± 0.015 at 1 mmol/L luminal [Ca2+] for RyR2-G230C, n = 8, p = NS; Figure 4A and C). Addition of 5 mmol/L luminal Ca2+ did not increase Po significantly in either recombinant channel groups (mean Po of 0.047 ± 0.008 for RyR2-WT, n = 5, versus mean Po of 0.033 ± 0.012 for RyR2-G230C, n = 7, p = NS; Figure 4B and C). Addition of 10 mmol/L luminal Ca2+ induced comparable significant increases in Po in both WT and CPVT channels when compared to the Po obtained at 0 mmol/L luminal Ca2+ (mean Po of 0.016 ± 0.011 at 0 mmol/L luminal [Ca2+], n = 8 versus mean Po of 0.100 ± 0.024 at 10 mmol/L luminal [Ca2+], n = 5, for RyR2-WT, p < 0.01, and mean Po of 0.013 ± 0.006 at 0 mmol/L luminal [Ca2+], n = 10, versus mean Po of 0.084 ± 0.034, n = 5, for RyR2-G230C, p < 0.05; Figure 4B and C).
Figure 4. Effects of luminal Ca2+ on recombinant human RyR2-G230C mutant channels.
A-B: Representative single channel current traces of recombinant RyR2-WT and RyR2-G230C channels measured at four different luminal Ca2+ concentrations as indicated on the left (0 and 1 mmol/L (mM) luminal [Ca2+], first and second rows in A, and 5 and 10 mmol/L luminal [Ca2+], first and second rows in B) with Ba2+ as the charge carrier at 0 mV. The cytosolic (cis) free [Ca2+] was 150 nmol/L. Channel openings are shown as upward deflections; the closed (c −) state of the channel is indicated by horizontal bars in the beginning of each tracing and the Po, To and Tc of the illustrative trace, calculated over 2 min of continuous recording, are shown above each trace. C: Bar graph summarizing single channel Po at 0, 1, 5 and 10 mmol/L luminal [Ca2+] in RyR2-WT (white bars) and RyR2-G230C (black bars) channels. Data presented as mean +/− S.E.M; *p < 0.05, **p < 0.01.
However, even at 10 mmol/L luminal [Ca2+], no difference in sensitivity to activation by luminal Ca2+ was observed between WT and RyR2-G230C recombinant channels (mean Po of 0.100 ± 0.024 for RyR2-WT, n = 5, versus mean Po of 0.084 ± 0.034 for RyR2-G230C, n = 5, p = NS; Figure 4C).
In order to reduce the baseline effect of divalent cations on the luminal side of the channel and to avoid any contaminating Ca2+ in the Ba2+ solution, we also tested the effect of luminal Ca2+ in absence of other divalent ions. Thus, we performed single channel measurements using Cs+ as the primary current charge carrier with added Ca2+ to the luminal (trans) side in presence of EGTA. In agreement with the above Ba2+ experiments, Po obtained with 0, 1, 5 and 10 mmol/L free Ca2+ to the luminal side were comparable between RyR2-G230C and WT (mean Po of 0.230/0.336/0.330/0.498 at 0/1/5/10 mmol/L Ca2+, n = 5 for each, for RyR2-WT versus mean Po of 0.262/0.288/0.400/0.317 at 0/1/5/10 mmol/L Ca2+, n = 4 for each, for RyR2-G230C, p = NS; Online Figure III).
Thus, our results indicate that the sensitivity of RyR2-G230C mutant channel to activation by luminal Ca2+ is the same as that of WT channels, demonstrating that there is no requirement to invoke the SOICR hypothesis to explain the mechanism of leak observed for the CPVT-linked RyR2-G230C channels.
RyR2-P2328S is a CPVT-linked missense mutation that is located in the central region of the RyR2 gene near the FKBP12.6 binding site 20. We first observed that recombinant human PKA-phosphorylated RyR2-P2328S mutant exhibits decreased binding of calstabin2 and have a significant gain-of-function defect consistent with a “leaky” channel at low cytosolic Ca2+ and with a rightward shift in the half-maximal inhibitory Mg2+ concentration (IC50) 7. The murine model of RyR2-P2328S mutation was then characterized by Goddard and colleagues who observed altered cellular Ca2+ handling linked to CPVT-linked arrhythmias with a more pronounced effect in the homozygote than the heterozygote 21.
To determine whether the RyR2-P2328S mutation exhibits a reduced threshold for luminal Ca2+, we measured the effects of increasing luminal [Ca2+] in the presence of 3 mmol/L Mg-ATP and 0.6 mmol/L MgCl2 at 150 nmol/L [Ca2+]cyt (Figure 5A and B).
Figure 5. Effects of luminal Ca2+ on recombinant human RyR2-P2328S mutant.
A-B: Representative single channel current traces of recombinant RyR2-WT and RyR2-P2328S channels measured at increasing luminal Ca2+ concentrations as indicated on the left (0 and 1 mmol/L (mM) luminal [Ca2+], first and second rows in A, and 5 and 10 mmol/L luminal [Ca2+], first and second rows in B) with Ba2+ as the charge carrier at 0 mV. The cytosolic (cis) side contained 3 mmol/L Mg-ATP, 0.6 mmol/L MgCl2 and 150 nmol/L free [Ca2+]. Channel openings are shown as upward deflections; the closed (c −) state of the channel is indicated by horizontal bars in the beginning of each tracing and the Po, To and Tc of the illustrative trace, calculated over 2 min of continuous recording, are shown above each trace. C: Luminal Ca2+ dependence of single channel Po in RyR2-WT (white bars) and RyR2-P2328S (black bars) channels. All Po measurements were made from 4 to 8 different channels at different luminal [Ca2+] concentrations (0, 0.02, 0.2, 0.6, 1, 2, 5 and 10 mmol/L).
Data were averaged and Po increased as luminal Ca2+ increased with no significant difference between RyR2-P2328S and WT recombinant channels (e.g. mean Po of 0.062 ± 0.037 at 0.2 mmol/L luminal [Ca2+], n = 5, versus mean Po of 0.110 ± 0.070 at 1 mmol/L luminal [Ca2+] for RyR2-WT, n = 4, and mean Po of 0.026 ± 0.022 at 0.2 mmol/L luminal [Ca2+], n = 4, versus mean Po of 0.042 ± 0.037 at 1 mmol/L luminal [Ca2+] for RyR2-P2328S, n = 4, p = NS; Figure 5A and C).
Increased [Ca2+] on the luminal side of the channel slightly increased single channel Po in both groups, with no significant differences between WT and RyR2-P2328S channels (e.g. mean Po of 0.160 ± 0.060 at 5 mmol/L luminal [Ca2+], n = 4, versus mean Po of 0.209 ± 0.086 at 10 mmol/L luminal [Ca2+] for RyR2-WT, n = 4, and mean Po of 0.161 ± 0.109 at 5 mmol/L luminal [Ca2+], n = 4, versus mean Po of 0.070 ± 0.036 at 10 mmol/L luminal [Ca2+] for RyR2-P2328S, n = 4, p = NS; Figure 5B and C). Thus, like the hRyR2-G230C channels, the CPVT-linked recombinant hRyR2-P2328S channels exhibit the same sensitivity to luminal [Ca2+] as WT RyR2 channels.
Discussion
In the present study we report a novel CPVT-linked RyR2 mutation that results in the substitution of cysteine for glycine at position 230, RyR2-G230C. The mutation is located in the disease-associated “hot spot” N-terminal domain of cardiac RyR2 20, 22-24. We co-expressed recombinant hRyR2 channels and the stabilizing subunit calstabin2. Our experiments indicate that the functional consequences of the RyR2-G230C mutation are a “leaky” RyR2 channel at diastolic Ca2+ (low cytosolic [Ca2+]) associated with reduced binding of calstabin2 to the RyR2 channel that occurs only under stress conditions. Moreover, because previous CPVT/ARVDRyR2 mutants were proposed to have increased luminal Ca2+ sensitivity 8-10, we compared the effects of luminal (trans) Ca2+ on RyR2-G30C mutant channel properties and did not find any differences in the CPVT channels compared to WT channels. Additionally, we also compared the luminal Ca2+ dependence of another CPVT mutant RyR2-P2328S, known to be hypersensitive to cytosolic Ca2+ 7, 21. Like the RyR2-G230C channels, the RyR2-P2328S mutant channels do not display any abnormalities in responsiveness to luminal [Ca2+]. These results show that the threshold for luminal Ca2+ activation is the same in WT and two CPVT mutant channels and therefore the SOICR mechanism is not a generalized mechanism for CPVT.
Overall, our results reinforce the molecular mechanism by which, under the beta-adrenergic stimulation, CPVT-associated RyR2 mutations lead to a pathologically increased cytosolic Ca2+ sensitivity associated with a depletion of calstabin2. Furthermore, our results indicate that the RyR2-G230C mutant channels exhibit increased cytosolic Ca2+ sensitivity that is not dependent on luminal Ca2+, and thus do not support the concept of store overload-induced Ca2+ release as a generalized mechanism for CPVT 8-10, 25.
In this report, we found a single base substitution in the RyR2 gene in a 50 year old man. The proband suffered from repeated syncope episodes after exercise beginning at age 30, had a cardiac arrest, and was diagnosed with CPVT. This single base substitution in Ryr2 resulted in a novel RyR2-G230C mutation close to the amino-terminus with similar biophysical defects as other mutations in the middle and carboxy-terminus of the RyR2 channel and is associated with decreased binding of the stabilizing subunit calstabin2.
Cardiac arrhythmia susceptibility genes that have been identified predominantly encode ion channels 26, 27. Our work strongly suggests that the single point mutation RyR2-G230C is linked to the cardiac pathological phenotype observed in the proband. A critical feature of CPVT is that triggering of an arrhythmogenic episode occurs in the absence of structural alterations to the heart and requires the presence of both a causal mutation and stressful conditions that induce beta-adrenergic stimulation. Our results support the model by which CPVT-associated mutations in the RyR2, under stress conditions (i.e. mimicked by the PKA phosphorylation of RyR2 in vitro), induce a significant increase in the cytosolic Ca2+ sensitivity and highlight the link with the calstabin2 binding from the RyR2 macromolecular complex.
Several studies have confirmed that CPVT and SCD-related RyR2 mutations lead to a cytosolic Ca2+ hypersensitivity, providng support for the Ca2+-induced Ca2+ release (CICR) mechanism rather than the SOICR hypothesis 4-6, 14. In line with this view, Fernandez-Velasco et al have observed an increase in Ca2+ spark frequency in RyR2-R4496C permeabilized cardiomyocytes, dependent on the diastolic intracellular Ca2+ concentration (i.e. nmol/L range of [Ca2+]) as well as an increase in cytosolic Ca2+ sensitivity in crude SR membrane of RyR2-R4496C using [3H] ryanodine binding experiments 14.
One explanation for the proposal that SOICR is the mechanism underlying CPVT is that it is technically difficult to distinguish the SOICR mechanism from the CICR mechanism because luminal Ca2+ may flow through the channel and modulate the cytosolic Ca2+ binding sites of RyR2 28, 29. Indeed, previous studies have shown that luminal Ca2+ regulates the cardiac RyR2 via direct feedback by binding to cytosolic Ca2+ activation and inactivation sites 30. Therefore, any experiments performed using high concentrations of luminal Ca2+, which passes through the channel will be subject to the limitation that this luminal Ca2+ can activate cytosolic sites after it passes through the channel. Therefore, if CPVT channels are more sensitive to cytosolic [Ca2+] than WT channels, luminal Ca2+ passing through the channel could result in increased Po in CPVT channels not because of a reduced threshold for activation by luminal Ca2+ (as proposed by the SOICR hypothesis) but rather because of increased sensitivity to cytosolic Ca2+ as we have shown previously 4-7. To circumvent this limitation, we used Ba2+ as the charge carrier in the luminal (trans) bilayer chamber and tightly controlled the free cytosolic (cis) [Ca2+] with EGTA in a range from ~ 150 nmol/L to 10 μmol/L at 0 mV. Although Ba2+ can pass through the RyR2 pore, Ba2+ ions cannot activate RyR2 channels and therefore cannot explain any increases of the channel Po. To determine whether the RyR2-G230C mutation increases the sensitivity to activation by luminal Ca2+ and thus increases the open probability of the channel, we examined the effects of Ca2+ added to luminal (trans) chamber, in presence of low cytosolic Ca2+ (i.e. 150 nmol/L free Ca2+) and in absence of any agonist (e.g. no ATP, no caffeine and no PKA phosphorylation) since the SOICR mechanism has been previously reported for some CPVT mutants, but only in absence of PKA phosphorylation of the channel. Adding Ca2+ on the luminal side of the channel induced a slight increase of Po, with no significant difference between RyR2-G230C and WT channels. Furthermore, we performed experiments on RyR2-P2328S channels testing increasing concentrations of luminal Ca2+ in the presence of the physiological channel modulators Mg-ATP and MgCl2 and did not find any differences in Po compared to WT channels. Our data show that SOICR is not a generalized mechanism for CPVT, however we cannot exclude that regulation of RyR2, by luminal SR proteins such as calsequestrin can modulate the Ca2+ sensitivity of the channel 28, 29. Thus, our results and those of others 4-6, 14 indicate that the CICR mechanism can explain CPVT and that there is no need to invoke SOICR as an alternative mechanism as recently explained 28.
The RyR2-G230C mutation is located in the N-terminal portion of the channel. The N-terminus of RyR2 is one of the three major regions that harbor disease-causing mutations 20, 22, 23, 31. Several biochemical and functional studies have indicated that disease-associated RyR mutations are located along the domain interface between the N-terminal and central regions of RyRs and weaken inter-domain interactions (the domain unzipping hypothesis) 11, 12, 32. A recent crystal structure provided an insight into the hotspot domain I of RyR and revealed the exact locations of more than 50 disease-associated mutations 24. Interestingly, these results indicated that Glycine 230 of hRyR2 is located in a critical subunit interface area that contains more than 19 disease-associated mutations in RyR1 and RyR2. Therefore, it is reasonable to propose that a single point mutation such as G230C may destabilize the contact and/or the folding of individual RyR2 domains, destabilizing the closed state of the channel. G230C mutation may also induce a decrease of the RyR2 protein level and/or the associated calstabin2. Our results show that the protein levels of immunoprecipitated recombinant RyR2-G230C and WT as well as the associated calstabin2 are similar. Because we use HEK293 cells heterologously expressing RyR2 and calstabin2, we cannot make any statement about RyR2 or calstabin2 protein levels in vivo. However, we have previously reported that knock-in mice expressing RyR2 harboring human CPVT mutations have normal levels of RyR2 and calstabin2 proteins 4. Our results also indicate that the RyR2-G230C mutation alters the channel activity only after PKA phosphorylation and leads to greater depletion of calstabin2 from the channel complex associated with a higher sensitivity to low cytosolic [Ca2+] compared to WT. Thus, using immunodetection of western blotted proteins, we do not observe any difference in the associated calstabin2 level between RyR2-G230C and WT when the channels are not PKA phosphorylated. Previous results from our group indicate that, using 35S-labeled FKBP12.6, CPVT-associated mutants RyR2-S2226L, RyR2-R2474S, and RyR2-R4497C have decreased basal affinity for FKBP12.6 5. We also previously reported that a transgenic mouse overexpressing calstabin2 can prevent leaky RyR2 and heart failure progression 33. Interestingly, the PKA phosphorylation level of pS2809 is similar between RyR2-G230C and WT. Thus, the consequences of G230C mutation on RyR2 might be dependent on allosteric or charge effects of the PKA phosphorylation that promote channel openings. These observations are in agreement with previous published results showing that CPVT-associated RyR2 mutations, located at different regions of the amino acid sequence (e.g. RyR2-R2474S and RyR2-V4653F) exacerbate the effect of PKA phosphorylation as evidenced by an increased depletion of calstabin2 from the RyR2 complex 5, 7.
While our results rule out the possibility that G230C mutation may affect the closed state of the channel (i.e. mean closed time was comparable), more experiments will be needed to investigate the Ca2+-dependent inhibition of RyR2-G230C channels since some CPVT-related RyR2 mutants exhibit sensitivity to Ca2+-dependent channel inhibition 13. Moreover, RyR has highly reactive cysteines able to form disulfide bonds 34. Therefore, the cysteine introduced by the CPVT mutation may interact with other cysteines in the channel, disturbing folding or inducing conformational changes to the channel. Further experiments under reducing conditions are needed to determine whether the substitution of cysteine for glycine induces conformational changes that destabilize the channel and increase sensitivity to cytosolic Ca2+. Our recent results from a murine model indicate that catecholamine treatment can result in oxidization of RyR2 in addition to causing PKA phosphorylation. The combination of oxidation and PKA phosphorylation can activate the channel and promote diastolic SR Ca2+ leak 35. The addition of an extra cysteine in RyR2-G230C could increase the effect of channel oxidation during stress. However, other CPVT mutations that do not involve addition of a cysteine, such as RyR2-P2328S, result in similar effects on channel properties.
In conclusion, we have demonstrated a novel functionally significant CPVT mutation in RyR2. The consequences of this mutation are an increased sensitivity to cytosolic Ca2+ under stress, associated with a depletion of calstabin2 from the RyR2 macromolecular complex, when the PKA phosphorylation level at serine 2809 is similar to the level in WT. These observations reinforce the model of “leaky” RyR2 channel via an altered calstabin2 binding as a mechanism by which CPVT-related RyR2 mutations cause arrhythmias.
Supplementary Material
Novelty and Significance.
What is known?
Individuals with catecholaminergic polymorphic ventricular tachycardia (CPVT)-linked mutations in the cardiac ryanodine receptor (RyR2) have exercise induced arrhythmias, and normal ECGs and no arrhythmias at rest.
Fatal ventricular arrhythmias are caused by intracellular calcium leak via mutant CPVT-linked RyR2 channels on the sarcoplasmic reticulum (SR).
CPVT mutations in the central and carboxy terminal domains of RyR2 cause decreased binding to the channel stabilizing subunit calstabin2 rendering the RyR2 channels leaky and the calcium leak is exacerbated by exercise leading to fatal arrhythmias.
What new information does this article contribute?
A new clinically significant mutation RyR2-G230C that leads to CPVT is reported.
The novel RyR2-G230C mutation near the amino terminus induces calcium leak by dereasing the binding of calstabin2 to the RyR2 macromolecular complex resulting in increased sensitivity of the channel to activation by diastolic calcium levels during stress.
CPVT-linked mutations, RyR2-G230C and RyR2-P2328S, do not affect the threshold for luminal calcium activation and do support an alternative mechanism called store overload-induced calcium release (SOICR).
In this article, we report the identification and characterization of a new CPVT-linked RyR2-G230C mutation. To investigate the functional properties of the RyR2-G230C mutation, we generated and expressed recombinant human RyR2 mutant channels together with calstabin2. We performed single channel experiments to investigate the biophysical properties of the mutant channels and to test the molecular mechanisms for CPVT. We show that RyR2-G230C mutant channels exhibit a calcium leak during stress because of decreased binding to calstabin2 which results in increased sensitivity of the mutant channels to activation by diastolic calcium levels. The results also indicate that RyR2-G230C, as well as a previously reported RyR2-P2328S CPVT-linked mutant channel, exhibit normal thresholds for activation by luminal SR calcium. These results show that there is no need to invoke the store overload-induced Ca2+ release mechanism. The present study shows that a CPVT-linked mutation located near the amino terminus of RyR2 shares a common mechanism for triggering arrhythmias by rendering the channel leaky due to decreased calstabin2 binding and increased sensitivity to activation by low levels of cytosolic calcium.
Acknowledgments
We would like to thank Jeffrey Rossi for his excellent technical assistance.
Sources of Funding
This work was supported by grants from the NHLBI to ARM.
Non-standard Abbreviations and Acronyms
- CPVT
catecholaminergic polymorphic ventricular tachycardia
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- PKA
cAMP-dependent protein kinase
- SCD
sudden cardiac death
- ARVD
arrhythmogenic right ventricular dysplasia
- SOICR
store overload induced Ca2+ release
- CICR
calcium-induced calcium release
Footnotes
Disclosures
ARM is a consultant for ARMGO Pharma, Inc. a biotech company targeting RyR2 treatment for prevention of CPVT.
Subject codes: [5] – [132]
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laitinen PJ, Brown KM, Piippo K, Swan H, Devaney JM, Brahmbhatt B, Donarum EA, Marino M, Tiso N, Viitasalo M, Toivonen L, Stephan DA, Kontula K. Mutations of the cardiac ryanodine receptor (RyR2) gene in familial polymorphic ventricular tachycardia. Circulation. 2001;103:485–490. doi: 10.1161/01.cir.103.4.485. [DOI] [PubMed] [Google Scholar]
- 2.Priori SG, Napolitano C, Tiso N, Memmi M, Vignati G, Bloise R, Sorrentino V, Danieli GA. Mutations in the cardiac ryanodine receptor gene (hRyR2) underlie catecholaminergic polymorphic ventricular tachycardia. Circulation. 2001;103:196–200. doi: 10.1161/01.cir.103.2.196. [DOI] [PubMed] [Google Scholar]
- 3.Priori SG, Napolitano C, Memmi M, Colombi B, Drago F, Gasparini M, DeSimone L, Coltorti F, Bloise R, Keegan R, Cruz Filho FE, Vignati G, Benatar A, DeLogu A. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 4.Lehnart SE, Mongillo M, Bellinger A, Lindegger N, Chen BX, Hsueh W, Reiken S, Wronska A, Drew LJ, Ward CW, Lederer WJ, Kass RS, Morley G, Marks AR. Leaky Ca2+ release channel/ryanodine receptor 2 causes seizures and sudden cardiac death in mice. J Clin Invest. 2008;118:2230–2245. doi: 10.1172/JCI35346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 6.Tester DJ, Dura M, Carturan E, Reiken S, Wronska A, Marks AR, Ackerman MJ. A mechanism for sudden infant death syndrome (SIDS): stress-induced leak via ryanodine receptors. Heart Rhythm. 2007;4:733–739. doi: 10.1016/j.hrthm.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lehnart SE, Wehrens XH, Laitinen PJ, Reiken SR, Deng SX, Cheng Z, Landry DW, Kontula K, Swan H, Marks AR. Sudden death in familial polymorphic ventricular tachycardia associated with calcium release channel (ryanodine receptor) leak. Circulation. 2004;109:3208–3214. doi: 10.1161/01.CIR.0000132472.98675.EC. [DOI] [PubMed] [Google Scholar]
- 8.Jiang D, Jones PP, Davis DR, Gow R, Green MS, Birnie DH, Chen SR, Gollob MH. Characterization of a novel mutation in the cardiac ryanodine receptor that results in catecholaminergic polymorphic ventricular tachycardia. Channels (Austin) 2010;4:302–310. doi: 10.4161/chan.4.4.12666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SR. Enhanced store overload-induced Ca2+ release and channel sensitivity to luminal Ca2+ activation are common defects of RyR2 mutations linked to ventricular tachycardia and sudden death. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 10.Jiang D, Xiao B, Yang D, Wang R, Choi P, Zhang L, Cheng H, Chen SR. RyR2 mutations linked to ventricular tachycardia and sudden death reduce the threshold for store-overload-induced Ca2+ release (SOICR) Proc Natl Acad Sci U S A. 2004;101:13062–13067. doi: 10.1073/pnas.0402388101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laver DR, Honen BN, Lamb GD, Ikemoto N. A domain peptide of the cardiac ryanodine receptor regulates channel sensitivity to luminal Ca2+ via cytoplasmic Ca2+ sites. Eur Biophys J. 2008;37:455–467. doi: 10.1007/s00249-007-0238-z. [DOI] [PubMed] [Google Scholar]
- 12.Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res. 2010;106:1413–1424. doi: 10.1161/CIRCRESAHA.109.209312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas NL, Lai FA, George CH. Differential Ca2+ sensitivity of RyR2 mutations reveals distinct mechanisms of channel dysfunction in sudden cardiac death. Biochem Biophys Res Commun. 2005;331:231–238. doi: 10.1016/j.bbrc.2005.02.194. [DOI] [PubMed] [Google Scholar]
- 14.Fernandez-Velasco M, Rueda A, Rizzi N, Benitah JP, Colombi B, Napolitano C, Priori SG, Richard S, Gomez AM. Increased Ca2+ sensitivity of the ryanodine receptor mutant RyR2R4496C underlies catecholaminergic polymorphic ventricular tachycardia. Circ Res. 2009;104:201–209. doi: 10.1161/CIRCRESAHA.108.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashimura T, Briston SJ, Trafford AW, Napolitano C, Priori SG, Eisner DA, Venetucci LA. In the RyR2(R4496C) mouse model of CPVT, beta-adrenergic stimulation induces Ca waves by increasing SR Ca content and not by decreasing the threshold for Ca waves. Circ Res. 2010;107:1483–1489. doi: 10.1161/CIRCRESAHA.110.227744. [DOI] [PubMed] [Google Scholar]
- 16.Sedej S, Heinzel FR, Walther S, Dybkova N, Wakula P, Groborz J, Gronau P, Maier LS, Vos MA, Lai FA, Napolitano C, Priori SG, Kockskamper J, Pieske B. Na+-dependent SR Ca2+ overload induces arrhythmogenic events in mouse cardiomyocytes with a human CPVT mutation. Cardiovasc Res. 2010;87:50–59. doi: 10.1093/cvr/cvq007. [DOI] [PubMed] [Google Scholar]
- 17.Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR. Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 18.Carter S, Pitt SJ, Colyer J, Sitsapesan R. Ca(2)+-dependent phosphorylation of RyR2 can uncouple channel gating from direct cytosolic Ca(2)+ regulation. J Membr Biol. 2011;240:21–33. doi: 10.1007/s00232-011-9339-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiao RP, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H. The immunophilin FK506-binding protein modulates Ca2+ release channel closure in rat heart. J Physiol. 1997;500:343–354. doi: 10.1113/jphysiol.1997.sp022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. Ryanodine receptors and ventricular arrhythmias: emerging trends in mutations, mechanisms and therapies. J Mol Cell Cardiol. 2007;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 21.Goddard CA, Ghais NS, Zhang Y, Williams AJ, Colledge WH, Grace AA, Huang CL. Physiological consequences of the P2328S mutation in the ryanodine receptor (RyR2) gene in genetically modified murine hearts. Acta Physiol (Oxf) 2008;194:123–140. doi: 10.1111/j.1748-1716.2008.01865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marks AR, Priori S, Memmi M, Kontula K, Laitinen PJ. Involvement of the cardiac ryanodine receptor/calcium release channel in catecholaminergic polymorphic ventricular tachycardia. J Cell Physiol. 2002;190:1–6. doi: 10.1002/jcp.10031. [DOI] [PubMed] [Google Scholar]
- 23.Yano M, Yamamoto T, Ikeda Y, Matsuzaki M. Mechanisms of Disease: ryanodine receptor defects in heart failure and fatal arrhythmia. Nat Clin Pract Cardiovasc Med. 2006;3:43–52. doi: 10.1038/ncpcardio0419. [DOI] [PubMed] [Google Scholar]
- 24.Tung CC, Lobo PA, Kimlicka L, Van Petegem F. The amino-terminal disease hotspot of ryanodine receptors forms a cytoplasmic vestibule. Nature. 2010;468:585–588. doi: 10.1038/nature09471. [DOI] [PubMed] [Google Scholar]
- 25.MacLennan DH, Chen SR. Store overload-induced Ca2+ release as a triggering mechanism for CPVT and MH episodes caused by mutations in RYR and CASQ genes. J Physiol. 2009;587:3113–3115. doi: 10.1113/jphysiol.2009.172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keating MT, Sanguinetti MC. Molecular and cellular mechanisms of cardiac arrhythmias. Cell. 2001;104:569–580. doi: 10.1016/s0092-8674(01)00243-4. [DOI] [PubMed] [Google Scholar]
- 27.Marban E. Cardiac channelopathies. Nature. 2002;415:213–218. doi: 10.1038/415213a. [DOI] [PubMed] [Google Scholar]
- 28.Prosser BL, Ward CW, Lederer WJ. Subcellular Ca2+ signaling in the heart: the role of ryanodine receptor sensitivity. J Gen Physiol. 2010;136:135–142. doi: 10.1085/jgp.201010406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L, Meissner G. Regulation of cardiac muscle Ca2+ release channel by sarcoplasmic reticulum lumenal Ca2+ Biophys J. 1998;75:2302–2312. doi: 10.1016/S0006-3495(98)77674-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priori SG, Napolitano C. Cardiac and skeletal muscle disorders caused by mutations in the intracellular Ca2+ release channels. J Clin Invest. 2005;115:2033–2038. doi: 10.1172/JCI25664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yano M, Yamamoto T, Ikemoto N, Matsuzaki M. Abnormal ryanodine receptor function in heart failure. Pharmacol Ther. 2005;107:377–391. doi: 10.1016/j.pharmthera.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Huang F, Shan J, Reiken S, Wehrens XH, Marks AR. Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci U S A. 2006;103:3456–3461. doi: 10.1073/pnas.0511282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Aghdasi B, Dou SJ, Zhang JZ, Liu SQ, Hamilton SL. Functional interactions between cytoplasmic domains of the skeletal muscle Ca2+ release channel. J Biol Chem. 1997;272:25051–25061. doi: 10.1074/jbc.272.40.25051. [DOI] [PubMed] [Google Scholar]
- 35.Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR. Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest. 120:4388, 4398. doi: 10.1172/JCI32726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.