Abstract
The purpose of this review is to highlight the potential role of exercise in promoting neuroplasticity and repair in Parkinson’s disease (PD). Exercise interventions in individuals with PD incorporate goal-based motor skill training in order to engage cognitive circuitry important in motor learning. Using this exercise approach, physical therapy facilitates learning through instruction and feedback (reinforcement), and encouragement to perform beyond self-perceived capability. Individuals with PD become more cognitively engaged with the practice and learning of movements and skills that were previously automatic and unconscious. Studies that have incorporated both goal-based training and aerobic exercise have supported the potential for improving both cognitive and automatic components of motor control. Utilizing animal models, basic research is beginning to reveal exercise-induced effects on neuroplasticity. Since neuroplasticity occurs at the level of circuits and synaptic connections, we examine the effects of exercise from this perspective.
Introduction and the Motor Problem in Parkinson’s disease
Parkinson’s disease (PD) is a progressive neurodegenerative disorder that is characterized by the loss of dopamine (DA) due to the degeneration of substantia nigra pars compacta (SNpc) dopaminergic neurons. Characteristic features of PD include motor (bradykinesia, rigidity, tremor, gait dysfunction, and postural instability) and cognitive impairment (frontal lobe executive dysfunction), as well as mood disorders. In healthy individuals, motor performance is dependent on the interaction between unconscious (automatic) and volitional (cognitive) control of movement 1, 2. Conversely, in PD, the early and preferential loss of DA in the dorsal basal ganglia leads to diminished automatic and increased cognitive (frontal cortex) control of motor movements. Consequently, individuals with PD must handle and sustain a larger cognitive load to execute either motor or cognitive tasks2, 3. DA replacement therapy alleviates some motor features of PD, but with less beneficial effects observed on cognitive function 4. In the last decade there is mounting evidence for the role of exercise in improving motor performance that may include facilitating both the cognitive and automatic control of movement.
Epidemiological studies have supported a link between strenuous exercise and reduced risk for PD 5, 6. Additionally, a number of studies and published reviews on exercise in normal aging and in PD provide the background that supports the benefits of exercise, physical activity, and environmental enrichment 7–9. While the field of exercise and PD remains an area of ongoing research, the overall purpose of this review is to draw attention to published studies in humans and animal models of PD that may support the beneficial effects of exercise through neuroplastic mechanisms. First we introduce the concept that exercise, through goal-directed and aerobic training may enhance neuroplasticity important for driving motor and cognitive behavioral improvement in PD. Second, we report findings from animal studies demonstrating the neuroprotective and neurorestorative capacity of intensive exercise. Finally we present data on the potential role of exercise in overall brain health that may influence the structural (connectivity) and physiological properties of brain function. Neuroplasticity is a process by which the brain encodes experiences and learns new behaviors and is defined as the modification of existing neural networks by adding or modifying synapses in response to changes in behavior or environment, that encompasses exercise 10. Neuroplasticity includes a wide spectrum of structural and physiological mechanisms including synaptogenesis, neurogenesis, neuronal sprouting and potentiating synaptic strength, all of which can lead to the strengthening, repair, and/or formation of neuronal circuitry.11 Importantly, exercise-induced benefits on brain health (blood flow, trophic factors, immune system) may help to create the optimal environmental milieu required for neuroplasticity to occur in the injured brain. This review highlights exercise approaches used to drive behavioral improvement in individuals with PD and findings in animal studies that support the potential for targeting neuroplasticity.
Exercise and Parkinson’s disease
Goal-Based Exercise
Exercise is a general term to describe a physical activity that is planned, structured, and repetitive for the purpose of conditioning any part of the body. A major interest in utilizing exercise for neuro-rehabilitation in PD has been that it incorporates many aspects of practice important for goal directed motor skill learning. These elements include repetition, intensity, and challenge which together with skill training lead to improvement in motor performance. Since prefrontal cognitive circuits are critically involved in early phases of motor learning, another important component of exercise in PD is cognitive engagement. Cognitive engagement may be facilitated by (i) feedback (e.g. verbal or proprioceptive), (ii) cueing (i.e. attention), (ii) dual tasking (i.e. attention), and (iv) motivation. The following sections will present studies that have incorporated these concepts. These studies have utilized a variety of different exercise modalities that include but are not limited to treadmill training 12, 13, amplitude training,14, 15 Tai Chi,16, 17 tango dancing,18, 19 boxing20 and forced cycling.21, 22
Gait impairments that involve reductions in speed and step length as well as increased stride length variability are common in PD and impact quality of life 23. Treadmill exercise is commonly used for improving gait capacity since it can be easily adjusted for speed (and gradient) thus increasing intensity and challenge of gait practice. Studies, using treadmill exercise (with or without body weight support typically used for the purpose of maintaining safety) have shown that through exercise practice individuals with mild to moderate stages of PD can improve gait performance, including velocity, stride length, cadence, postural stability, gait rhythmicity, and joint excursion 24, 25. While the majority of treadmill studies have reported these benefits, a few studies have shown that despite a similar period of practice, no significant improvement in gait capacity occurred. One possible explanation for this discrepancy in gait outcomes may be due to differences in the amount of feedback and cognitive engagement during practice 26, 27. Severity of disease, which may affect cognition, may also be a confounder. While the challenge of repetitively controlling dynamic balance in conjunction with the proprioceptive feedback from the moving treadmill may be helpful for learning, verbal feedback and/or cues that draw attention and facilitate cognitive engagement to the motor task practice may be what underlies the treadmill training benefits observed in most PD studies. Both immediate (upon completion of training 4 to 12 weeks) and long-term retention (lasting several months) of gait improvements have been reported following cessation of treadmill exercise 24, 28, 29. Interestingly benefits of treadmill training, a largely lower extremity task, have also been shown to transfer to improvements in the Unified Parkinson’s Disease Rating Scale (UPDRS) motor score. For example, the benefits of increased movement amplitude and speed in gait (stride length and velocity) appear to transfer to increased amplitude and speed of finger and foot tapping 28, 29. One possible explanation for this phenomenon is the following. During gait practice, characteristics of the motor behavior involved in gait training, such as speed and amplitude, and reinforced through cognitive engagement may serve to guide the learning of a general schema for simple motor performance (e.g. finger tapping). Brain regions, including but not limited to the hippocampus, that remain relatively unaffected in PD may contribute to these aspects of learning. Another possible explanation may be related to neuroprotection and/or increased dopaminergic availability that occur with cognitive challenge. Conversely, exercise that is unduly stressful may dampen this effect through similar but contrasting mechanisms. Finally, exercise effects on circuitry involved in cognitive and automatic components of motor movements may be involved in these more general exercise effects (see below).
Exercise and motor training have also been used to improve balance, since balance impairments lead to high morbidity in PD 30, 31. Some exercise modalities targeting both gait and balance, also incorporate aspects of goal skill training while increasing cognitive engagement. For example, in amplitude training individuals with PD are asked to focus on generating large amplitude movements involving the whole body during the practice of a skill. This form of exercise, which incorporates a significant amount of verbal feedback and attention strategies, results in improvements in movement speed as well as amplitude that appear to be analogous to results observed with treadmill 14. Similarly, Tai Chi focuses on dynamic postural control via weight shifting to control center of gravity during maximal movements 32. Findings from these studies show that, following 24 weeks of twice weekly sessions, Tai Chi leads to improved stride length and maximum excursion as well as reduced falls compared to resistance training or stretching. These benefits were retained for at least 2 months 17. Other forms of exercise approaches that combine skill practice with cognitive engagement include dance, such as the Argentinian Tango, and Boxing. Dance employs cognitive engagement through coordinating with a partner in addition to the cueing and increased attention provided by the music and rhythm 33. Earhardt and colleagues showed that by the end of 12 months of Tango dancing individuals with PD had improved balance, walking, and dual tasking capability 34. Finally, studies using boxing have also shown improvement in balance and gait in individuals with PD. Boxing incorporates dynamic balance activities with multidirectional movements comparable to exercise regimens that specifically target balance practice 20.
Despite a wide spectrum of exercise modalities these studies share common elements including goal-based practice for the acquisition of a skill (gait and dynamic balance) in a supervised environment to facilitate learning through feedback (reinforcement). Feedback serves several purposes including (i) challenging patients beyond self-selected levels of perceived capability, (ii) maintaining motivation, and (iii) facilitating the engagement of individuals to become cognitively aware of movements that were previously automatic and unconscious. Other factors that could impact exercise effects on motor skill learning include age, cognition, and disease severity 35.
While a number of exercise studies have typically utilized instructors or physical therapists to facilitate goal-directed learning through cognitive engagement, other forms of feedback and attention strategies can also be included in the exercise regimen including virtual reality, electronic gaming (Wii for example), dual task practice and auditory and visual cueing 36, 37. The fact that individuals with PD show retention of task benefits (especially gait and balance) after a period of time without training is consistent with motor learning and underlying neuroplasticity 38. As stated earlier an additional benefit of these various forms of goal-based practice may include transfer from one learned behavior to another (i.e. trained behavior to a related untrained behavior).
Goal-Based plus Aerobic Exercise
A predominant feature of PD is the loss of automatic control of motor movements such as balance and gait. Automaticity is defined as the ability to perform a skilled movement without conscious attention or executive control 39 (see Box 1). Early depletion of DA, within caudal regions of the basal ganglia (dorsal striatum in rodents), results in impaired automatic circuitry. Specifically DA depletion results in increased inhibitory drive of the indirect pathway in the striatal-thalamic-cortical circuit due to reduced DA D2 receptor (DA-D2R) activation. In the classic PD model this increased inhibitory tone induces motor impairments, including bradykinesia 40. However, accumulating evidence suggest that neuroplasticity within this corticostriatal circuit is also impaired under conditions of DA denervation 41–44, which may give rise to an aberrant learning that further impairs automatic motor behavior 45, 46. While a small number of studies have demonstrated that individuals with PD can acquire some degree of automaticity after simple motor skill practice,3, 47 the loss and restoration of automaticity in PD in general remains a difficult problem to solve.
BOX 1. The Development of Automatic Movements (Automaticity).
The basal ganglia contribute to cognitive and automatic components of motor skill performance. The basal ganglia and its cortical connections also play an essential role in procedural motor learning, including the acquisition and retention of automaticity (For review see 2, 127). Motor learning is defined as a practice related change or improvement in motor performance. The initial phase of motor skill learning involves the activation of circuits involved in reward based and goal directed learning. This circuit includes connections between the rostral, also called the associative regions of the basal ganglia (dorsal medial striatum in rodents) with the prefrontal cortex. This early phase of learning involves DA, and the DA-D1 and D2 receptors. Extended training of a motor skill involves a shift from goal directed to habitual (stimulus response) based learning. This latter phase of learning leads to decreasing activation of circuits in the prefrontal-rostral basal ganglia and increasing activation of circuits in the caudal, also called the sensorimotor regions of the basal ganglia (dorsal lateral striatum in rodents) with the sensorimotor cortex. Dopamine and the DA-D2R are important in these latter aspects of learning. DA depletion predominant in the caudal basal ganglia of individuals with PD, leads to aberrant habitual learning and loss of automatic motor control.
Exercise studies employing both components of intensive and challenging goal-based practice in combination with aerobic training have provided some evidence of restored neuroplasticity in the striatal-thalamic-cortical-motor circuit responsible for automatic motor control. Utilizing body weight supported treadmill training. Fisher and colleagues demonstrated that individuals with early stage PD were capable of engaging in gait training at faster speeds than their self-selected pace while maintaining observationally normal execution of movement 12. Over an 8-week period (24 sessions total) patients were asked to make corrections in posture, arm swing, and stride length, as treadmill speeds were gradually increased thus challenging problem solving operations and increasing attentional demands. Patients were also instructed to reach and maintain a metabolic equivalent (MET) of greater than 3.0 METS and/or 75% of an age-adjusted maximum heart rate (AAMHR). Along with improved gait and balance parameters, an exercise related decrease in cortico-motor excitability through an increase in cortical silent period duration (CSP) using Transcranial Magnetic Stimulation (TMS) was shown 12. In addition, using positron emission tomography (PET)-imaging and [18F]fallypride, a DA-D2/D3R receptor, Fisher and colleagues reported that 8 weeks of treadmill exercise was accompanied by an increase in DA-D2R binding potential within the dorsal striatum of individuals with early stage PD 48. Taken together changes in cortico-motor excitability with increased DA-D2 receptor availability may be associated with mitigating inappropriate inhibitory drive of the automatic circuit. Further evidence for exercise effects on neuroplasticity and circuitry involved in automatic motor control is observed in studies utilizing forced cycling 49. Using a stationary tandem bicycle Alberts and colleagues “forced” individuals with PD to achieve pedaling rates that were 30% greater than their preferred rate thus combining aspects of cognitive engagement with aerobic training.21, 22 This led to central changes as evidenced by improved automatic manual dexterity as well as increased connectivity between cortico-subcortical regions involved in automatic control using functional magnetic resonance imaging (fMRI).
Taken together these data support that exercise paradigms incorporating both goal-directed practice and aerobic training may work synergistically to facilitate neuroplasticity necessary for overcoming aberrant circuitry within the basal ganglia. Dual task practice without aerobic exercise provides insight into the role of cognitive motor training without an exercise component. Though studies point to preserved motor skill learning in individuals with early stage PD, fMRI studies demonstrate that the acquisition and learning of dual task training in PD is limited and occurs principally through compensatory cortical circuits. This is in contrast to healthy subjects where dual task training leads to activation of subcortical basal ganglia pathways involved in automatic motor control 3. Additionally cognitive impairments common in early stage PD may hamper other aspects of motor skill learning, including the development of context dependency. Context dependency occurs after motor skill learning in PD. This is demonstrated through diminished performance of a newly acquired motor skill either when the augmented cues used to learn the task are removed or the environmental or practice (random versus blocked) conditions are altered 50, 51. The beneficial role of exercise, and specifically incorporating aerobic training, may be to facilitate neuroplasticity and improve motor learning. This may occur through enhanced blood flow and alterations in the brain environment that are important for restoring physiological and structural function (see below). Consistent with this notion, studies in animals have shown that brain changes seen in exercise are distinct from those observed in learning 52. Only a few studies have examined the effects of aerobic training alone that incorporate limited or no aspects of skill learning 13, 22, 49. While preliminary, these studies appear to demonstrate only modest gains in motor skill performance. In general, taken together these findings support several concepts. First that while motor learning may be limited or impaired in PD, exercise may improve both goal directed motor skill practice and ultimately through repair of the basal ganglia and its connections, automaticity. Second that the acquisition and recovery of a lost motor skill in PD through exercise requires cognitive engagement and goal-directed practice. While a few exercise studies have begun to help establish the benefits of both goal directed and aerobic training, future research is clearly needed to further establish how different exercise modalities either alone or in combination may contribute to restoration of behavioral function and automaticity in PD 53, 54.
The loss of DA in the basal ganglia not only impacts automatic behavior but also impairs cognitive (executive) functions55, 56, particularly mental flexibility and set shifting related to alterations in fronto-striatal connectivity 57–59. Cognition is affected early and progresses with disease severity and involves a number of neurotransmitter systems including dopaminergic, serotoninergic, noradrenergic, and cholinergic 60, 61. In addition to improving motor performance, aerobic exercise may improve cognitive function in PD as well. It is well established that exercise leads to cognitive improvement in normal aging and Alzheimer’s disease 62–64. In these studies, fMRI data suggests that aerobic exercise may generate more efficient neuronal activity in the prefrontal regions similarly affected in PD 65, 66. Tanaka and colleagues showed that following a 6-month aerobic exercise program, individuals with PD showed improved executive function 67. Similarly, Cruise and colleagues showed cognitive improvement in individuals with PD, including working memory and verbal fluency with aerobic training 68–70. While promising, these findings have been demonstrated in individuals with minimal to moderate disease severity that are able to follow the training protocol. With greater disease severity and increased disruption of corticostriatal circuitry, cognitive impairment progresses into dementia 60. A major gap in our knowledge is to determine if the benefits of exercise can still be evident in patients with dementia and later stages of PD.
Exercise Studies in Animal Models of PD
Animal models provide an important tool to investigate the mechanisms by which exercise induces neuroplasticity in the mammalian brain. Two commonly used models of DA-depletion include the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned mouse and the 6-hydroxydopamine (6-OHDA)-lesioned rat models.71 Both toxins lead to the destruction of nigrostriatal DA neurons and the subsequent depletion of DA in the dorsal striatum. Exercise has been shown to improve motor performance in these models, including parkinsonian features 72–77. These models have utility in investigating the underlying molecular mechanisms involved in exercise-induced neuroplasticity in both neuroprotection and neurorestoration studies. While genetic models of familial forms of PD are now available it has not yet been established if exercise can provide protection from age-related decline in DA neurotransmission. It is anticipated that much of what we learn in toxin models will be applicable to many of the current genetic models. In the following sections we draw from the basic research literature to show how animal models provide insights and create a framework that can guide translational studies in humans with disease.
Effects of Exercise on Neuroprotection
Studies examining the potential neuroprotective effects of exercise have primarily utilized MPTP or 6-OHDA toxin models of PD. These models have been historically used to examine mechanisms of dopaminergic cell death and therapies that may slow this process. For the purpose of investigating neuroprotective effects, forced or voluntary exercise is introduced before, during, or immediately after toxin administration. These studies have reported improvement in motor function, along with the preservation of DA neurons and the restoration of dopaminergic terminals, using tyrosine hydroxylase (TH) immunostaining, within the striatum. In these toxin models, neuroprotection has been principally attributed to an exercise-induced increase of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) or glial-derived neurotrophic factor (GDNF) 78–81. An alternative mechanism for neuroprotection of these models may be though the exercise induced down regulation of DAT, the primary uptake system for 6-OHDA and MPTP 73, 75. Importantly, other factors that may influence neuroprotective effects of exercise include the relative time frame of exercise initiation to toxin administration, as well as the extent (severe versus mild) of the toxin-induced injury. For example exercise initiated one week post-toxin administration fails to protect from cell death 82. Additionally, exercise administered to an animal with a mild toxin-induced injury also fails to demonstrate neuroprotection, despite evidence of behavioral recovery. An alternative suggested process for exercise-induced recovery of behavioral function that does not involve neuroprotection is neurorestoration 75.
Effects of Exercise on Neurorestoration
In contrast to neuroprotection, neurorestoration is defined as the brain response to exercise initiated well after toxin induced cell death has been completed. These studies have shown that exercise can increase post-lesion DA neurotransmission by enhancing the vesicular release of DA and increasing synaptic occupancy and decreasing DA clearance through reduced DAT expression. In addition, exercise may alter DA receptor expression. Specifically, intensive treadmill exercise in the MPTP mouse model reverses the reduction of DA-D2R in the dorsal striatum normally occurring following lesion. Restoration of DA-D2R, in combination with increase DA release are known to be critically important in the later phase of motor learning when automaticity is developed 83. Thus, exercise induced increase in DA neurotransmission along with increased DA-D2R expression observed within the dorsal striatum of the MPTP mouse model may contribute to neuroplastic mechanisms involved in exercise-induced improvement of motor behavior and restoration of automaticity.
Exercise may modulate glutamatergic neurotransmission as well. Glutamate and its receptors are known to contribute to neuroplasticity and synaptic strengthening during the learning process. DA depletion in the striatum induces hyper-excitability in the indirect pathway in response to alterations in glutamatergic expression (receptors and neurotransmitter release) and underlies critical aspects of motor impairment in PD 84. Studies in the MPTP mouse model have demonstrated that intensive exercise can restore aspects of glutamate receptor expression, including the glutamate receptor subtype AMPA 85. Alterations in AMPA receptor and its subunits have been reported in many neurological disease states and are considered a viable target for drug therapy 86. In addition to its receptors, exercise can also alter the storage and release of glutamate in presynaptic terminals that may also improve circuit function and diminish the increased inhibitory drive of the DA depleted striatum 41–44. While additional effects of exercise on cortical and striatal function are likely to be involved, taken together these studies support that exercise through its effects on neurotransmitters and their receptors may help to restore neurophysiological properties of synapses within the injured striatum needed for normal motor learning and behavior.
Effects of Exercise on Dendritic Spines
DA-depletion in the striatum leads to the loss of dendritic spines on striatal medium spiny neurons (MSNs) in both animal models87–89 and in PD90. These morphological changes reflect the loss of synapses and hence reduction in neurotransmission not only in PD but a wide spectrum of brain disorders including Alzheimer’s disease and Fragile-X syndrome 91. Spine loss occurs predominantly on DA-D2R-containing MSNs of the indirect pathway, reflecting the dysfunction in neurotransmission in this circuitry 92, 93.
While an important question in exercise yet to be fully addressed in PD and its animal models, studies in healthy rodents subjected to different exercise paradigms have demonstrated experience-dependent increases in dendritic spine density in a number of regions including the hippocampus and cerebellum 70, 94. One hypothesis to be explored in models of PD is that exercise can reverse dendritic spine loss in DA-D2R containing striatal neurons.
The Effects of Exercise on Brain Health
While exercise may have very targeted effects on specific basal ganglia circuits such as those highlighted in corticostriatal neurotransmission through glutamate and its modulation by DA, the effects of exercise also have more global effects on factors that influence general brain health. These include (i) blood flow through vascularization and angiogenesis, (ii) activation of beneficial affects of the immune system, (iii) induction of neurotrophic factors, and (iv) neurogenesis 95.
Exercise and Blood Flow
Exercise increases blood flow in the healthy brain in a wide range of animal species undergoing various exercise regimens 96, 97. Thus exercise may facilitate neuroplasticity by influencing the vasculature of the central nervous system (CNS) through angiogenesis and altered blood brain barrier (BBB) permeability. The delivery of peripheral signaling molecules originating from muscle or adipose tissue including insulin, angiogenic factors such as vascular endothelial growth factor (VEGF), hypoxia mediated factors such as hypoxia-induced factor 1 (HIF-1), leptin, and neurotrophic factors including BDNF98 can be promoted.
While there are currently no studies supporting the effects of exercise on the cerebral vasculature in animal models of PD, studies in healthy rodents have shown that exercise can alter hippocampal cerebral blood flow and elevate hippocampal, striatal, and substantia nigra levels of VEGF,99–101 a mediator of angiogenesis, cell growth, and neuroprotection. Long-term exercise elicits change in regional blood perfusion of underlying motor circuits that may contribute to changes in brain connectivity related to synaptogenesis but also enhance synaptic function 102. Interestingly, rats that have undergone aerobic exercise have an increase in the density of capillaries in the cerebral motor regions, without an increase in the number of synapses, but display improved cortical related behaviors52. Conversely, rats learning new motor skills have a greater number of synapses per neuron within the motor cortex, without an increase in capillary density.96, 103, 104 This relationship between blood flow, synaptic function, and synaptogenesis underscores the complexity of mechanisms that exercise may utilize to promote brain circuitry and its function in the DA-depleted brain.
Exercise and the Immune System
The vast majority of our understanding of exercise and its effects on the immune system is derived from studies in healthy individuals, including athletes.105 In general, studies support an exercise-induced beneficial effect of the immune system in the CNS.106–109 Currently there are very few studies exploring the relationship between exercise and the immune system in individuals with PD. Yet it is well recognized that there is a strong immune component in PD 110. Reports have shown that exercise (cycling) can increase plasma levels of the anti-inflammatory cytokine IL-10 in individuals with PD along with improved motor performance.111, 112 In addition, the cytokine IL-6, while generally considered a pro-inflammatory marker in PD that correlates with functional impairment (decreased walking speed), may in the context of exercise, play an anti-inflammatory role. Specifically, IL-6, which originates in skeletal muscle, has been shown with exercise to elicit an anti-inflammatory response that includes elevated expression of a number of factors including IL-10 and IL-1RA, and as well as inhibition of factors like tumor necrosis factor alpha (TNF-alpha).113–115
Another recently identified role of exercise on the function of the immune system may be through the modulation of cells of the myeloid lineage including monocytes, macrophages and CNS resident microglia.105, 116, 117 These cells generate a vast repertoire of soluble factors including cytokines, chemokines, and growth factors. The large number of CNS resident microglia and perivascular macrophages form an integrated network in close proximity with neurons suggesting that these cells interact with numerous CNS cell types and circuits.118 An important series of questions involving the role of exercise and the immune systems in PD include determining if pro-inflammatory stereotypic response to injury and CNS inflammation respond to distinct stimuli including exercise thus reversing their deleterious effects.119 For example, can classically activated myeloid cells, termed M1-type cells, which are thought to contribute to the pathology of PD, be converted through exercise into M2-type myeloid cells that secrete cytokines thought to have beneficial consequences that enhance neuroplasticity. The fact that exercise has been shown to induce a conversion of M1-type to M2-type myeloid cells in adipose tissue macrophages coupled with inhibition of M1-type macrophage infiltration support this hypothesis.120 Since peripheral macrophages have been shown to infiltrate the brain from the periphery, it is intriguing to speculate whether activated peripheral macrophages can infiltrate the CNS, and promote beneficial effects such as BDNF expression and other chemo-attractants to enhance neuroplasticity and repair at sites of injury and disease 121.
Exercise and Neurogenesis
It is well established that the adult mammalian brain including humans displays a high degree of neurogenesis, the birth of newborn cells. However, neurogenesis is limited by both age and to a very limited number of anatomical sites including the regions adjacent to the lateral ventricle and hippocampus {Feliciano, 2013 #43937}. Exercise and environmental enrichment in normal rodents have been shown to have a number of important influences on neurogenesis including increasing (i) the rate of newborn cell numbers, (ii) the fraction that differentiate into neurons, and (iii) the proportion that incorporate into neuronal circuits 122. Currently, there are few reports in the literature directly addressing the interactions of neurogenesis, exercise, and DA-depletion in animal models. The fact that exercise increases neurogenesis in the hippocampus and subventricular zone does not necessarily translate into the potential role of exercise in increasing neuron numbers in important basal ganglia circuitry within the striatum, cortex, or thalamus 123. While decreased gliosis is observed in these regions with exercise there are no reports supporting elevated neurogenesis in the basal ganglia with exercise. The fact that exercise have been shown to enhance the survival and integration of transplanted cells in animal models of PD reflects the importance of experience in influencing cell integration into circuits potentially meaningful for functional motor behavior 124.
In conclusion, animal models have played a major role in allowing us to better understand the underlying mechanisms of exercise and its effects on restoring motor behavior in the DA-depleted brain. The majority of findings are beginning to highlight the importance of focusing on the synapse as the critical therapeutic target. Exercise can restore important circuits in motor behavior by modulating DA and glutamate neurotransmission as well as influencing general brain health.
Overall Conclusions and Impact on Clinical Care
Over the last decade, a primary focus of neuro-rehabilitation has been to alleviate the motor deficits of PD through exercise 10, 125. The general idea is that exercise incorporating goal based motor skill learning improves motor skill performance in PD and that this may be enhanced through cognitive engagement. More importantly, studies suggest that combining goal based with aerobic training the possibility for improving automatic with cognitive motor control may be possible and thus reduce the attentional demands of consciously processing behaviors such as walking.126 Aerobic exercise may contribute to more general improvement in brain health and repair, through the recruitment of the immune system and/or increasing blood flow and trophic factor signaling as examples. These aerobic exercise benefits are likely to impact connectivity through priming the brain environment conducive for promoting synaptic neuroplasticity leading to altered circuitry. This review raises another fundamental question, which is can an individual learn themselves out of PD? Based on published studies, and current understanding of the detrimental effects of DA loss on brain circuitry, the most obvious response is no. However, exercise studies may be pointing towards potential and important neuroplastic mechanisms that through restoring some degree of basal ganglia circuitry provide a window of opportunity to improve motor learning and behavioral performance.
Based on published studies in both animals and individuals with PD, exercise has been shown to be important in improving motor function in PD and to facilitate neuroplasticity. Future research will continue to add to exercise related mechanisms of neuroplasticity. Thus, exercise should be considered an essential treatment for PD, particularly in individuals with mild to moderate disease. Ongoing research is required, however, to address large gaps in knowledge. Specifically studies with non-invasive neuroimaging are still needed to discern the relative contribution of either goal based or aerobic exercise alone or in combination and their effect on brain function, connectivity and motor behavior. In addition, the important role of exercise in individuals with more advanced disease and with more severe cognitive impairment is needed. By elucidating the precise exercise-induced mechanisms of neuroplasticity, we can begin to better understand its role in disease modification and identify novel therapeutic targets including pharmacological approaches to supplement exercise for improved treatment and potentially a cure in PD.
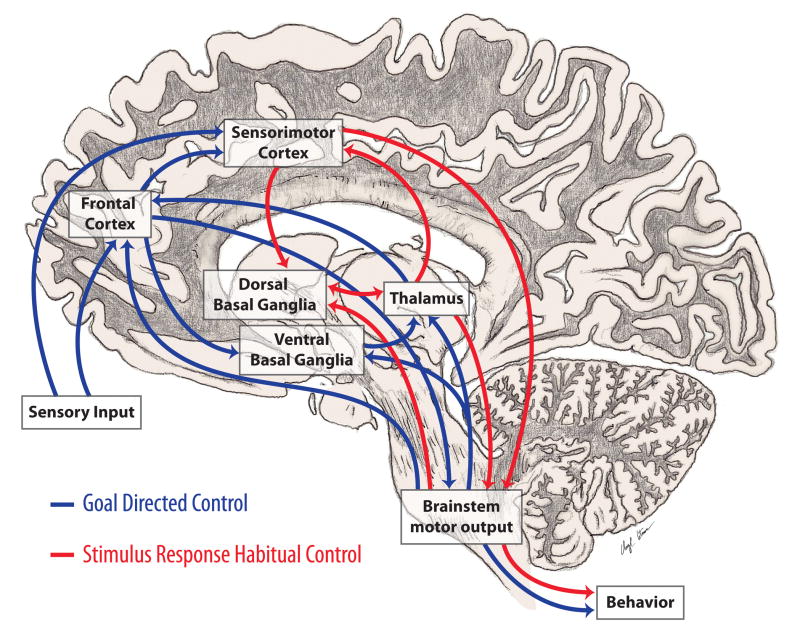
Figure 1. Cognitive and Automatic Motor Control.
Motor control incorporates multiple cortical and subcortical structures. Most important are the connections between the basal ganglia and cortex that are involved in cognitive and automatic aspects of motor control. In PD, loss of DA in the caudal basal ganglia leads to impaired automatic movements involving circuits important in stimulus based habitual learning (red arrows) and over-reliance on cognitive components of motor control and circuits involved in reward based learning (blue arrows).
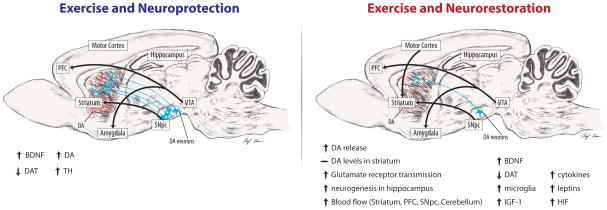
Figure 2. Exercise and Neuroprotection and Neurorestoration in Rodent Models of PD.
The figure highlights some reported benefits of the effects of exercise in rodent PD neurotoxin models. The left panel indicates exercise effects when exercise is delivered either before or during the period of toxin-induced (6-OHDA, or MPTP) dopaminergic cell death. Intensive exercise promotes elevation of neurotrophic factors, such as BDNF, and protects from toxin-induced striatal DA depletion and cell loss of SNpc neurons. These findings are consistent with epidemiological data reporting the effect of intensive exercise in lowering the risk for PD. The right panel indicates exercise effects when exercise is administered days to weeks after toxin-induced dopaminergic cell death. Studies suggest that intensive exercise may strengthen motor (dorsal basal ganglia) circuits and behavioral performance through mechanisms that include improved DA and glutamate neurotransmission and global brain health. These data are consistent with the potential role of exercise in modifying the course of PD.
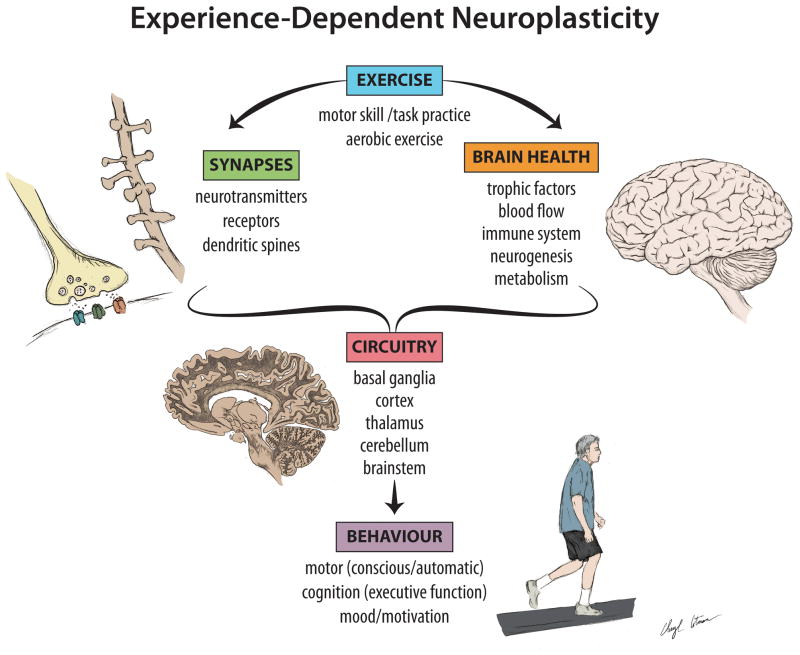
Figure 3. Exercise and Neuroplasticity in PD.
Clinical and basic research studies support the effects of exercise on neuroplasticity in PD. Neuroplasticity is a process by which the brain encodes experiences and learns new behaviors and is defined as the modification of existing neural networks by adding or modifying synapses. Evidence is accumulating that both goal directed and aerobic exercise may strengthen and improve motor circuitry through mechanisms that include but are not limited to alterations in DA and glutamate neurotransmission, as well as structural modifications of synapses. In addition, exercise may promote neuroprotection of substantia nigra neurons and their existing connections. Finally, exercise-induced alterations in blood flow and general brain health may promote conditions for neuroplasticity important for facilitating motor skill learning, including cognitive and automatic motor control and overall behavioral performance. While more studies are clearly needed, taken together these findings are supportive of a disease modifying effect of exercise in PD.
Acknowledgments
Findings described from authors work were supported by the NIH, US Army NETRP, Zumberge Foundation, George and MaryLou Boone, USC-CTSI, Provost Collaboration Fund at USC, and Team Parkinson (Los Angeles). We would like to thank Carolee Winstein for her thoughtful discussions and insights. We would like to thank other members of our research group for their thoughtful discussions including Carolee Winstein, Brett Lund, Eve Kelland, Natalie Kintz, William Toy, Daniel Stefanko, Brian Leyshon, Daniel Holschneider, Wendy Gilmore, and Ruth Wood. Special thank you to Cheryl Cotman for designing the illustrations.
Footnotes
Contributors
All authors contributed to conception and design, acquisition of data, or analysis and interpretation of data discussed in this paper; drafted or revised the paper; and approved the final version to be published.
Conflicts of interest
All authors declare that there are no conflicts of interest to report.
Search strategy and selection criteria
References for this review were identified by searches of PubMed using the search terms “exercise” linked to “Parkinson’s”, “neuroplasticity”, “environmental enrichment”, “dopamine”, “glutamate”, “synaptogenesis”, “striatum and physiology”, “ basal ganglia”, “physical activity”. We mainly selected publications in the past 15 years but we did not exclude commonly referenced and highly regarded older publications. We also searched the reference list of articles identified and selected those we judged relevant.
References
- 1.Mazzoni P, Wexler NS. Parallel explicit and implicit control of reaching. PLoS ONE. 2009;4:e7557. doi: 10.1371/journal.pone.0007557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redgrave P, Rodriguez M, Smith Y, et al. Goal-directed and habitual control in the basal ganglia: implications for Parkinson’s disease. Nat Rev Neurosci. 2010;11(11):760–72. doi: 10.1038/nrn2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu T, Hallett M. Neural correlates of dual task performance in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;422(3):164–8. doi: 10.1136/jnnp.2007.126599. [DOI] [PubMed] [Google Scholar]
- 4.Schapira AH, Agid Y, Barone P, et al. Perspectives on recent advances in the understanding and treatment of Parkinson’s disease. Eur J Neurol. 2009;16(10):1090–9. doi: 10.1111/j.1468-1331.2009.02793.x. [DOI] [PubMed] [Google Scholar]
- 5.Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75(4):341–8. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64(4):664–9. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 7.Cotman CW, Berchtold NC. Physical activity and the maintenance of cognition: learning from animal models. Alzheimers Dement. 2007;3(2 Suppl):S30–7. doi: 10.1016/j.jalz.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Berchtold NC, Castello N, Cotman CW. Exercise and time-dependent benefits to learning and memory. Neuroscience. 2010;167(3):588–97. doi: 10.1016/j.neuroscience.2010.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petzinger GM, Fisher BE, Van Leeuwen JE, et al. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson’s disease. Mov Disord. 2010;25 (Suppl 1):S141–5. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51(1):S225–39. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 11.Sudhof TC, Malenka RC. Understanding synapses: past, present, and future. Neuron. 2008;60 (3):469–76. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson’s disease. Arch Phys Med Rehabil. 2008;89(7):1221–9. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schenkman M, Hall DA, Baron AE, Schwartz RS, Mettler P, Kohrt WM. Exercise for People in Early- or Mid-Stage Parkinson Disease: A 16-Month Randomized Controlled Trial. Phys Ther. 2012;92 (11):1395–410. doi: 10.2522/ptj.20110472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Farley BG, Koshland GF. Training BIG to move faster: the application of the speed-amplitude relation as a rehabilitation strategy for people with Parkinson’s disease. Exp Brain Res. 2005;167(3):462–7. doi: 10.1007/s00221-005-0179-7. [DOI] [PubMed] [Google Scholar]
- 15.Fox CM, Ramig LO, Ciucci MR, Sapir S, McFarland DH, Farley BG. The science and practice of LSVT/LOUD: neural plasticity-principled approach to treating individuals with Parkinson disease and other neurological disorders. Semin Speech Lang. 2006;27(4):283–99. doi: 10.1055/s-2006-955118. [DOI] [PubMed] [Google Scholar]
- 16.Corcos DM, Comella CL, Goetz CG. Tai chi for patients with Parkinson’s disease. N Engl J Med. 2012;366(18):1737–8. doi: 10.1056/NEJMc1202921. [DOI] [PubMed] [Google Scholar]
- 17.Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. N Engl J Med. 2012;366(6):511–9. doi: 10.1056/NEJMoa1107911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hackney ME, Earhart GM. Effects of dance on balance and gait in severe Parkinson disease: A case study. Disabil Rehabil. 2010;32(8):679–84. doi: 10.3109/09638280903247905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackney ME, Earhart GM. Effects of dance on movement control in Parkinson’s disease: a comparison of Argentine tango and American ballroom. J Rehabil Med. 2009;41(6):475–81. doi: 10.2340/16501977-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Combs SA, Diehl MD, Staples WH, et al. Boxing Training for Patients With Parkinson Disease: A Case Series. Phys Ther. 2010;91(1):132–42. doi: 10.2522/ptj.20100142. [DOI] [PubMed] [Google Scholar]
- 21.Alberts JL, Linder SM, Penko AL, Lowe MJ, Phillips M. It is not about the bike, it is about the pedaling: forced exercise and Parkinson’s disease. Exerc Sport Sci Rev. 2011;39(4):177–86. doi: 10.1097/JES.0b013e31822cc71a. [DOI] [PubMed] [Google Scholar]
- 22.Ridgel AL, Vitek JL, Alberts JL. Forced, not voluntary, exercise improves motor function in Parkinson’s disease patients. Neurorehabil Neural Repair. 2009;23(6):600–8. doi: 10.1177/1545968308328726. [DOI] [PubMed] [Google Scholar]
- 23.Frenkel-Toledo S, Giladi N, Peretz C, Herman T, Gruendlinger L, Hausdorff JM. Effect of gait speed on gait rhythmicity in Parkinson’s disease: variability of stride time and swing time respond differently. J Neuroengineering Rehabil. 2005;2:23. doi: 10.1186/1743-0003-2-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herman T, Giladi N, Gruendlinger L, Hausdorff JM. Six weeks of intensive treadmill training improves gait and quality of life in patients with Parkinson’s disease: a pilot study. Arch Phys Med Rehabil. 2007;88(9):1154–8. doi: 10.1016/j.apmr.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Herman T, Giladi N, Hausdorff JM. Treadmill training for the treatment of gait disturbances in people with Parkinson’s disease: a mini-review. J Neural Transm. 2009;116(3):307–18. doi: 10.1007/s00702-008-0139-z. [DOI] [PubMed] [Google Scholar]
- 26.Skidmore FM, Patterson SL, Shulman LM, Sorkin JD, Macko RF. Pilot safety and feasibility study of treadmill aerobic exercise in Parkinson disease with gait impairment. J Rehabil Res Dev. 2008;45(1):117–24. doi: 10.1682/jrrd.2006.10.0130. [DOI] [PubMed] [Google Scholar]
- 27.Canning CG, Allen NE, Dean CM, Goh L, Fung VS. Home-based treadmill training for individuals with Parkinson’s disease: a randomized controlled pilot trial. Clin Rehabil. 2012;26(9):817–26. doi: 10.1177/0269215511432652. [DOI] [PubMed] [Google Scholar]
- 28.Miyai I, Fujimoto Y, Yamamoto H, et al. Long-term effect of body weight-supported treadmill training in Parkinson’s disease: a randomized controlled trial. Arch Phys Med Rehabil. 2002;83(10):1370–3. doi: 10.1053/apmr.2002.34603. [DOI] [PubMed] [Google Scholar]
- 29.Frazzitta G, Bertotti G, Riboldazzi G, et al. Effectiveness of intensive inpatient rehabilitation treatment on disease progression in Parkinsonian patients: a randomized controlled trial with 1-year follow-up. Neurorehabil Neural Repair. 2012;26(2):144–50. doi: 10.1177/1545968311416990. [DOI] [PubMed] [Google Scholar]
- 30.Allen NE, Canning CG, Sherrington C, et al. The effects of an exercise program on fall risk factors in people with Parkinson’s disease: a randomized controlled trial. Mov Disord. 2010;25(9):1217–25. doi: 10.1002/mds.23082. [DOI] [PubMed] [Google Scholar]
- 31.Allen NE, Sherrington C, Paul SS, Canning CG. Balance and falls in Parkinson’s disease: A meta-analysis of the effect of exercise and motor training. Mov Disord. 2011 doi: 10.1002/mds.23790. [DOI] [PubMed] [Google Scholar]
- 32.Hackney ME, Kantorovich S, Levin R, Earhart GM. Effects of tango on functional mobility in Parkinson’s disease: a preliminary study. J Neurol Phys Ther. 2007;31(4):173–9. doi: 10.1097/NPT.0b013e31815ce78b. [DOI] [PubMed] [Google Scholar]
- 33.Foster ER, Golden L, Duncan RP, Earhart GM. Community-based argentine tango dance program is associated with increased activity participation among individuals with Parkinson’s disease. Arch Phys Med Rehabil. 2013;94(2):240–9. doi: 10.1016/j.apmr.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan RP, Earhart GM. Randomized controlled trial of community-based dancing to modify disease progression in Parkinson disease. Neurorehabil Neural Repair. 2012;26(2):132–43. doi: 10.1177/1545968311421614. [DOI] [PubMed] [Google Scholar]
- 35.Nieuwboer A, De Weerdt W, Dom R, Bogaerts K. Prediction of outcome of physiotherapy in advanced Parkinson’s disease. Clin Rehabil. 2002;16(8):886–93. doi: 10.1191/0269215502cr573oa. [DOI] [PubMed] [Google Scholar]
- 36.de Bruin N, Doan JB, Turnbull G, et al. Walking with music is a safe and viable tool for gait training in Parkinson’s disease: the effect of a 13-week feasibility study on single and dual task walking. Parkinsons Dis. 2010;2010:483530. doi: 10.4061/2010/483530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.dos Santos Mendes FA, Pompeu JE, Modenesi Lobo A, et al. Motor learning, retention and transfer after virtual-reality-based training in Parkinson’s disease--effect of motor and cognitive demands of games: a longitudinal, controlled clinical study. Physiotherapy. 2012;98(3):217–23. doi: 10.1016/j.physio.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 38.Rochester L, Baker K, Hetherington V, et al. Evidence for motor learning in Parkinson’s disease: acquisition, automaticity and retention of cued gait performance after training with external rhythmical cues. Brain Res. 2010;1319:103–11. doi: 10.1016/j.brainres.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Cameron IG, Watanabe M, Pari G, Munoz DP. Executive impairment in Parkinson’s disease: Response automaticity and task switching. Neuropsychologia. 2010;48(7):1948–57. doi: 10.1016/j.neuropsychologia.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 40.DeLong M, Wichmann T. Changing views of basal ganglia circuits and circuit disorders. Clin EEG Neurosci. 2010;41(2):61–7. doi: 10.1177/155005941004100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Calabresi P, Pisani A, Centonze D, Bernardi G. Synaptic plasticity and physiological interactions between dopamine and glutamate in the striatum. Neurosci Biobehav Rev. 1997;21(4):519–23. doi: 10.1016/s0149-7634(96)00029-2. [DOI] [PubMed] [Google Scholar]
- 42.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321(5890):848–51. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson’s disease models. Nature. 2007;445(7128):643–7. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- 44.Picconi B, Centonze D, Hakansson K, et al. Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nat Neurosci. 2003;6(5):501–6. doi: 10.1038/nn1040. [DOI] [PubMed] [Google Scholar]
- 45.Beeler JA, Cao ZF, Kheirbek MA, et al. Dopamine-dependent motor learning: insight into levodopa’s long-duration response. Ann Neurol. 2010;67(5):639–47. doi: 10.1002/ana.21947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beeler JA, Frazier CR, Zhuang X. Putting desire on a budget: dopamine and energy expenditure, reconciling reward and resources. Front Integr Neurosci. 2012;6:49. doi: 10.3389/fnint.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- 48.Fisher BE, Li Q, Nacca A, et al. Treadmill Exercise Elevates Striatal Dopamine D2 Receptor Binding Potential In Patients with Early Parkinson’s Disease. NeuroReport. 2013 doi: 10.1097/WNR.0b013e328361dc13. In Press. [DOI] [PubMed] [Google Scholar]
- 49.Beall E, Lowe M, Alberts JL, et al. The Effect of Forced-Exercise Therapy for Parkinson’s Disease on Motor Cortex Functional Connectivity. Brain connectivity. 2013 doi: 10.1089/brain.2012.0104. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Verschueren SM, Swinnen SP, Dom R, De Weerdt W. Interlimb coordination in patients with Parkinson’s disease: motor learning deficits and the importance of augmented information feedback. Exp Brain Res. 1997;113(3):497–508. doi: 10.1007/pl00005602. [DOI] [PubMed] [Google Scholar]
- 51.Onla-or S, Winstein CJ. Determining the optimal challenge point for motor skill learning in adults with moderately severe Parkinson’s disease. Neurorehabil Neural Repair. 2008;22(4):385–95. doi: 10.1177/1545968307313508. [DOI] [PubMed] [Google Scholar]
- 52.Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci U S A. 1990;87(14):5568–72. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mirelman A, Maidan I, Herman T, Deutsch JE, Giladi N, Hausdorff JM. Virtual reality for gait training: can it induce motor learning to enhance complex walking and reduce fall risk in patients with Parkinson’s disease? J Gerontol A Biol Sci Med Sci. 2011;66(2):234–40. doi: 10.1093/gerona/glq201. [DOI] [PubMed] [Google Scholar]
- 54.Pompeu JE, Mendes FA, Silva KG, et al. Effect of Nintendo Wii-based motor and cognitive training on activities of daily living in patients with Parkinson’s disease: A randomised clinical trial. Physiotherapy. 2012;98(3):196–204. doi: 10.1016/j.physio.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 55.Owen AM. Cognitive dysfunction in Parkinson’s disease: the role of frontostriatal circuitry. Neuroscientist. 2004;10(6):525–37. doi: 10.1177/1073858404266776. [DOI] [PubMed] [Google Scholar]
- 56.Ekman U, Eriksson J, Forsgren L, Mo SJ, Riklund K, Nyberg L. Functional brain activity and presynaptic dopamine uptake in patients with Parkinson’s disease and mild cognitive impairment: a cross-sectional study. Lancet Neurol. 2012;11(8):679–87. doi: 10.1016/S1474-4422(12)70138-2. [DOI] [PubMed] [Google Scholar]
- 57.Godefroy O, Azouvi P, Robert P, Roussel M, LeGall D, Meulemans T. Dysexecutive syndrome: diagnostic criteria and validation study. Ann Neurol. 2010;68(6):855–64. doi: 10.1002/ana.22117. [DOI] [PubMed] [Google Scholar]
- 58.Yeterian EH, Pandya DN. Prefrontostriatal connections in relation to cortical architectonic organization in rhesus monkeys. J Comp Neurol. 1991;312(1):43–67. doi: 10.1002/cne.903120105. [DOI] [PubMed] [Google Scholar]
- 59.Lewis SJ, Dove A, Robbins TW, Barker RA, Owen AM. Cognitive impairments in early Parkinson’s disease are accompanied by reductions in activity in frontostriatal neural circuitry. J Neurosci. 2003;23(15):6351–6. doi: 10.1523/JNEUROSCI.23-15-06351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ray NJ, Strafella AP. The neurobiology and neural circuitry of cognitive changes in Parkinson’s disease revealed by functional neuroimaging. Mov Disord. 2012;27(2):1484–92. doi: 10.1002/mds.25173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65(8):1239–45. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 62.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9(1):58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 63.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends Cogn Sci. 2007;11(8):342–8. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Voss MW, Heo S, Prakash RS, et al. The influence of aerobic fitness on cerebral white matter integrity and cognitive function in older adults: Results of a one-year exercise intervention. Hum Brain Mapp. 2012 doi: 10.1002/hbm.22119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness: recent findings and future directions. J Mol Neurosci. 2004;24(1):9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- 66.Angevaren M, Aufdemkampe G, Verhaar HJ, Aleman A, Vanhees L. Physical activity and enhanced fitness to improve cognitive function in older people without known cognitive impairment. Cochrane Database Syst Rev. 2008;(3):CD005381. doi: 10.1002/14651858.CD005381.pub3. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka K, Quadros AC, Jr, Santos RF, Stella F, Gobbi LT, Gobbi S. Benefits of physical exercise on executive functions in older people with Parkinson’s disease. Brain Cogn. 2009;69(2):435–41. doi: 10.1016/j.bandc.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 68.Cruise KE, Bucks RS, Loftus AM, Newton RU, Pegoraro R, Thomas MG. Exercise and Parkinson’s: benefits for cognition and quality of life. Acta Neurol Scand. 2011;123(1):13–9. doi: 10.1111/j.1600-0404.2010.01338.x. [DOI] [PubMed] [Google Scholar]
- 69.Eadie BD, Redila VA, Christie BR. Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J Comp Neurol. 2005;486(1):39–47. doi: 10.1002/cne.20493. [DOI] [PubMed] [Google Scholar]
- 70.Stranahan AM, Khalil D, Gould E. Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus. 2007;17(11):1017–22. doi: 10.1002/hipo.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petzinger GM, Jakowec MW. Animal Models of Basal Ganglia Injury and Degeneration and their Application to Parkinson’s Disease Research. In: Ebadi M, Pfeiffer RF, editors. Parkinson’s Disease. BocaRaton, FL: CRC Press; 2005. [Google Scholar]
- 72.Fisher BE, Petzinger GM, Nixon K, et al. Exercise-induced behavioral recovery and neuroplasticity in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse basal ganglia. J Neurosci Res. 2004;77(3):378–90. doi: 10.1002/jnr.20162. [DOI] [PubMed] [Google Scholar]
- 73.Petzinger GM, Walsh JP, Akopian G, et al. Effects of treadmill exercise on dopaminergic transmission in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci. 2007;27(20):5291–300. doi: 10.1523/JNEUROSCI.1069-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119(3):899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 75.O’Dell SJ, Gross NB, Fricks AN, Casiano BD, Nguyen TB, Marshall JF. Running wheel exercise enhances recovery from nigrostriatal dopamine injury without inducing neuroprotection. Neuroscience. 2007;144(3):1141–51. doi: 10.1016/j.neuroscience.2006.10.042. [DOI] [PubMed] [Google Scholar]
- 76.Smith BA, Goldberg NR, Meshul CK. Effects of treadmill exercise on behavioral recovery and neural changes in the substantia nigra and striatum of the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse. Brain Res. 2011;1386:70–80. doi: 10.1016/j.brainres.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pothakos K, Kurz MJ, Lau YS. Restorative effect of endurance exercise on behavioral deficits in the chronic mouse model of Parkinson’s disease with severe neurodegeneration. BMC Neurosci. 2009;10 (1):6. doi: 10.1186/1471-2202-10-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gerecke KM, Jiao Y, Pagala V, Smeyne RJ. Exercise Does Not Protect against MPTP-Induced Neurotoxicity in BDNF Happloinsufficent Mice. PLoS ONE. 2012;7(8):e43250. doi: 10.1371/journal.pone.0043250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85(2):299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 80.Real CC, Ferreira AF, Chaves-Kirsten GP, Torrao AS, Pires RS, Britto LR. BDNF receptor blockade hinders the beneficial effects of exercise in a rat model of Parkinson s disease. Neuroscience. 2013 doi: 10.1016/j.neuroscience.2013.01.060. In Press. [DOI] [PubMed] [Google Scholar]
- 81.Wu SY, Wang TF, Yu L, et al. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25(1):135–46. doi: 10.1016/j.bbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 82.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21(12):4427–35. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yin HH, Mulcare SP, Hilario MR, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12(3):333–41. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends Neurosci. 2007;30(5):211–9. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 85.VanLeeuwen JE, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. J Neurosci Res. 2010;88(3):650–68. doi: 10.1002/jnr.22216. [DOI] [PubMed] [Google Scholar]
- 86.Chang PK, Verbich D, McKinney RA. AMPA receptors as drug targets in neurological disease--advantages, caveats, and future outlook. Eur J Neurosci. 2012;35(12):1908–16. doi: 10.1111/j.1460-9568.2012.08165.x. [DOI] [PubMed] [Google Scholar]
- 87.Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989;503(2):334–8. doi: 10.1016/0006-8993(89)91686-7. [DOI] [PubMed] [Google Scholar]
- 88.Ingham CA, Hood SH, van Maldegem B, Weenink A, Arbuthnott GW. Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Exp Brain Res. 1993;93(1):17–27. doi: 10.1007/BF00227776. [DOI] [PubMed] [Google Scholar]
- 89.Cheng HW, Rafols JA, Goshgarian HG, Anavi Y, Tong J, McNeill TH. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentation: a Golgi study. Exp Neurol. 1997;147(2):287–98. doi: 10.1006/exnr.1997.6618. [DOI] [PubMed] [Google Scholar]
- 90.McNeill TH, Brown SA, Rafols JA, Shoulson I. Atrophy of medium spiny I striatal dendrites in advanced Parkinson’s disease. Brain Res. 1988;455(1):148–52. doi: 10.1016/0006-8993(88)90124-2. [DOI] [PubMed] [Google Scholar]
- 91.van Spronsen M, Hoogenraad CC. Synapse pathology in psychiatric and neurologic disease. Curr Neurol Neurosci Rep. 2010;10(3):207–14. doi: 10.1007/s11910-010-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Day M, Wang Z, Ding J, et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9(2):251–9. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- 93.Neely MD, Schmidt DE, Deutch AY. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience. 2007;149(2):457–64. doi: 10.1016/j.neuroscience.2007.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pysh JJ, Weiss GM. Exercise during development induces an increase in Purkinje cell dendritic tree size. Science. 1979;206(4415):230–2. doi: 10.1126/science.482938. [DOI] [PubMed] [Google Scholar]
- 95.Cotman CW, Berchtoldb NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in Neurosciences. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 96.Swain RA, Harris AB, Wiener EC, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117(4):1037–46. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 97.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol. 2008;104(1):306–14. doi: 10.1152/japplphysiol.00853.2007. [DOI] [PubMed] [Google Scholar]
- 98.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30(9):464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 99.Fabel K, Tam B, Kaufer D, et al. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18(10):2803–12. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 100.Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Curr Neurovasc Res. 2006;3(1):15–23. doi: 10.2174/156720206775541787. [DOI] [PubMed] [Google Scholar]
- 101.Villar-Cheda B, Sousa-Ribeiro D, Rodriguez-Pallares J, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson’s disease. J Cereb Blood Flow Metab. 2009;29(2):230–4. doi: 10.1038/jcbfm.2008.127. [DOI] [PubMed] [Google Scholar]
- 102.Yang J, Sadler TR, Givrad TK, Maarek JM, Holschneider DP. Changes in brain functional activation during resting and locomotor states after unilateral nigrostriatal damage in rats. Neuroimage. 2007;36(3):755–73. doi: 10.1016/j.neuroimage.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kleim JA, Freeman JHJ, Bruneau R, et al. Synapse formation is associated with memory storage in the cerebellum. Proc Natl Acad Sci U S A. 2002;99(20):13228–31. doi: 10.1073/pnas.202483399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12(1):110–9. doi: 10.1038/jcbfm.1992.14. [DOI] [PubMed] [Google Scholar]
- 105.Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63. [PubMed] [Google Scholar]
- 106.Marchetti B, Abbracchio MP. To be or not to be (inflamed)--is that the question in anti-inflammatory drug therapy of neurodegenerative disorders? Trends Pharmacol Sci. 2005;26(10):517–25. doi: 10.1016/j.tips.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 107.Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful? J Cereb Blood Flow Metab. 2003;23(4):385–94. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- 108.Schwartz M, Butovsky O, Bruck W, Hanisch UK. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29(2):68–74. doi: 10.1016/j.tins.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 109.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40(2):133–9. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 110.Huang Y, Halliday GM. Aspects of innate immunity and Parkinson’s disease. Front Pharmacol. 2012;3:33. doi: 10.3389/fphar.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Scalzo P, Kummer A, Cardoso F, Teixeira AL. Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci Lett. 2010;468(1):56–8. doi: 10.1016/j.neulet.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 112.Cadet P, Zhu W, Mantione K, et al. Cyclic exercise induces anti-inflammatory signal molecule increases in the plasma of Parkinson’s patients. Int J Mol Med. 2003;12(4):485–92. [PubMed] [Google Scholar]
- 113.Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- 114.Pedersen BK, Febbraio M. Muscle-derived interleukin-6--a possible link between skeletal muscle, adipose tissue, liver, and brain. Brain Behav Immun. 2005;19(5):371–6. doi: 10.1016/j.bbi.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 115.Steensberg A, Dalsgaard MK, Secher NH, Pedersen BK. Cerebrospinal fluid IL-6, HSP72, and TNF-alpha in exercising humans. Brain Behav Immun. 2006;20(6):585–9. doi: 10.1016/j.bbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 116.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18(5):407–13. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 117.Silveira EM, Rodrigues MF, Krause MS, et al. Acute exercise stimulates macrophage function: possible role of NF-kappaB pathways. Cell Biochem Funct. 2007;25(1):63–73. doi: 10.1002/cbf.1365. [DOI] [PubMed] [Google Scholar]
- 118.Wake H, Moorhouse AJ, Nabekura J. Functions of microglia in the central nervous system - beyond the immune response. Neuron Glia Biol. 2012:1–7. doi: 10.1017/S1740925X12000063. In Press. [DOI] [PubMed] [Google Scholar]
- 119.Tremblay ME, Majewska AK. A role for microglia in synaptic plasticity? Commun Integr Biol. 2011;4 (2):220–2. doi: 10.4161/cib.4.2.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kawanishi N, Yano H, Yokogawa Y, Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc Immunol Rev. 2010;16:105–18. [PubMed] [Google Scholar]
- 121.Vivar C, Potter MC, van Praag H. All About Running: Synaptic Plasticity, Growth Factors and Adult Hippocampal Neurogenesis. Curr Top Behav Neurosci. 2012 doi: 10.1007/7854_2012_220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Abrous DN, Koehl M, Le Moal M. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85(2):523–69. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- 123.Marxreiter F, Regensburger M, Winkler J. Adult neurogenesis in Parkinson’s disease. Cell Mol Life Sci. 2013;70(3):459–73. doi: 10.1007/s00018-012-1062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dobrossy MD, Nikkhah G. Role of experience, training, and plasticity in the functional efficacy of striatal transplants. Prog Brain Res. 2012;200:303–28. doi: 10.1016/B978-0-444-59575-1.00014-4. [DOI] [PubMed] [Google Scholar]
- 125.Fisher B, Sullivan KJ. Activity-dependent factors affecting post-stroke functional outcomes. Topics in Stroke Rehabilitation. 2001;8(3):31–44. doi: 10.1310/B3JD-NML4-V1FB-5YHG. [DOI] [PubMed] [Google Scholar]
- 126.Yogev G, Giladi N, Peretz C, Springer S, Simon ES, Hausdorff JM. Dual tasking, gait rhythmicity, and Parkinson’s disease: which aspects of gait are attention demanding? Eur J Neurosci. 2005;22(5):1248–56. doi: 10.1111/j.1460-9568.2005.04298.x. [DOI] [PubMed] [Google Scholar]
- 127.Ashby FG, Turner BO, Horvitz JC. Cortical and basal ganglia contributions to habit learning and automaticity. Trends Cogn Sci. 2010;14(4):208–15. doi: 10.1016/j.tics.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]