SUMMARY
Accumulation of 5′-methylthioadenosine (MTA) and S-adenosylhomocysteine (SAH) in bacteria disrupts the S-adenosylmethionine pool to alter biological methylations, synthesis of polyamines and production of quorum sensing molecules. Bacterial metabolism of MTA and SAH depends on MTA/SAH nucleosidase (MTAN), an enzyme not present in humans, and a target for quorum sensing since MTAN activity is essential for synthesis of autoinducer-2 molecules. Crystals of Salmonella enterica MTAN with product and transition state analogues of MTA and SAH explain the pM binding affinity and reveal ‘water-wire’ channel for the catalytic nucleophile. The crystal structure shows an extension of the binding pocket filled with polyethylene glycol. We exploited that discovery by the design and synthesis of new modifications of the currently existing transition state analogues to fill this site. This site was previously unknown in MTANs and reveals powerful inhibitors with solvent access. Novel inhibitors with dissociation constants of 5 to 15 pM are characterized.
INTRODUCTION
5′-Methylthioadenosine nucleosidases (MTANs) hydrolyze S-adenosyl-L-homocysteine (SAH) and S-methyl-5′-thioadenosine (MTA) into S-ribosyl-L-homocysteine (SRH) and S-methyl-5′-thioribose (MTR), respectively (E.C. 3.2.2.9 and E.C. 3.2.2.16) (Duerre, 1962; Shapiro and Mather, 1958). The central molecule in these pathways is S-adenosylmethionine (SAM), the methyl donor for synthesis and macromolecular regulation. Methyl transfer yields SAH as a product. Polyamine synthesis also requires SAM, and MTA is formed as the product. SAM is also used in the synthesis of quorum sensing molecules in bacteria. Quorum sensing molecules act as chemical messengers between bacteria (Evans et al., 2004; Parveen and Cornell, 2011; Withers et al., 2001) Mammals do not express MTA/SAH nucleosidases and, therefore, MTANs have been the targets of antibacterial drug design (De La Haba and Cantoni, 1959; Parsek et al., 1999; Pegg and Williams-Ashman, 1969; Singh et al., 2006). MTAN inhibition causes increased cellular levels of MTA and SAH, product inhibition of polyamine synthases, growth arrest (Raina et al., 1982), and in some species, inhibition of quorum sensing (Gutierrez et al., 2009). In Helicobacter pylori (H. pylori) MTAN is involved in the essential pathway of menaquinone synthesis (Wang et al., 2012a).
MTANs catalyze the hydrolysis of the N-glycosidic bond between N9 of adenine and C1′ of the thioribose, via transition states with SN1 character. Protonation of N7 of substrate and the syn conformation of the ribosidic bond facilitate N9-C1′ bond loss. The ribocation is neutralized by attack of the water nucleophile, thus causing adenine replacement by a nucleophilic water (SN). (Allart et al., 1998; Lee et al., 2003; Lee et al., 2005b; Singh et al., 2005b)
Transition state (TS) analogues are tight-binding inhibitors that mimic specific transition state features (Schramm, 2005a, b). First-generation TS analogues of N-ribosyltransferases resemble early, dissociative TSs while second generation TS analogues resemble fully dissociated ribocation TSs (Evans et al., 2005; Lewandowicz et al., 2003). Some second generation TS analogues inhibited their target enzymes with dissociation constants as low as 10−14 M (Singh et al., 2005a). The TS structures for MTANs from Escherichia coli (E. coli) (Singh et al., 2005b), Neisseria meningitides (N. meningitides) (Singh et al., 2007) and Streptococcus pneumonia (S. pneumonia) (Singh and Schramm, 2007) have been established via kinetic isotope effect (KIE) measurements. These TSs have partial or full ribocation character without participation of the nucleophilic water. E. coli, S. pneumonia, Klebsiella pneumonia (K. pneumonia) and Staphylococcus aureus (S. aureus) MTAN transition states had fully dissociated leaving groups, while N. meningitides and H. pylori were shown to have early SN1 transition states with partial bond order in the N-ribosidic bond (Gutierrez et al., 2007).
Structurally, MTAN belongs to MtnN subfamily of the purine nucleoside phosphorylase (PNP) / uridine phosphorylase (UDP) phosphorylase family. Our 1.36 Å structure of native Salmonella enterica MTAN (SeMTAN) showed a dimeric assembly with one of two active sites occupied by an adenine that copurified with the enzyme. Crystal structures with adenine and known TS analogues revealed an additional 5’-binding pocket. We exploited that feature to synthesize novel inhibitors that bind with Kd values in the low picomolar range.
RESULTS
Tertiary and quaternary structures of S. enterica MTAN
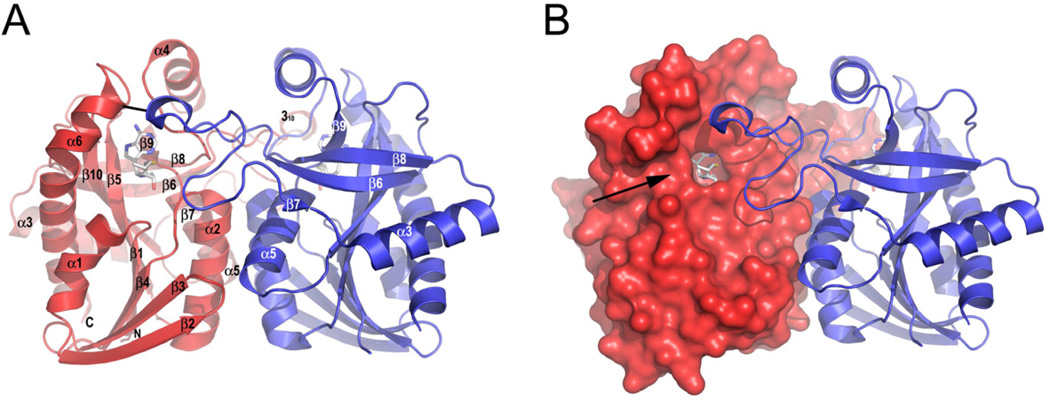
All SeMTAN crystal structures presented here were homodimers consistent with other MTANs (Figure 1A, 1B). The asymmetric units in every case consisted of the SeMTAN homodimer. The SeMTAN monomer core consists of a 3-layer αβα sandwich with central mixed sheet of 10 strands containing cross-over loops. Altogether, there are seven helices around the central β-sheet; helices 1, 3 and 6 are on one side and helices 2, 4, 5 and a small 310-helix are located on the other side (Figure 1A, Figure 2A).
Figure 1.
The structure of Salmonella enterica MTAN (SeMTAN). (A) Quaternary structure of SeMTAN homodimer. The structure is drawn as ribbons, and monomer-A and B are colored in red and blue, respectively. The region between β6 and β7 strands of monomer-B extends to the side of monomer-A (and vice versa) where it forms a part of substrate binding pocket of the neighboring monomer. The black line represents a hydrogen bond not found in E. coli MTAN (see the Discussion). (B) Surface exit of the binding pocket. The monomer-A is shown as a surface representation. The accessible surface area was obtained using the probe radius of 1.4 Å in PyMOL. The arrow points to the exit of the substrate binding pocket. In panels A and B, the transition state analogue, BuT-DADMe-ImmA is bound in the active site of both monomers. The figure was prepared with PyMOL.
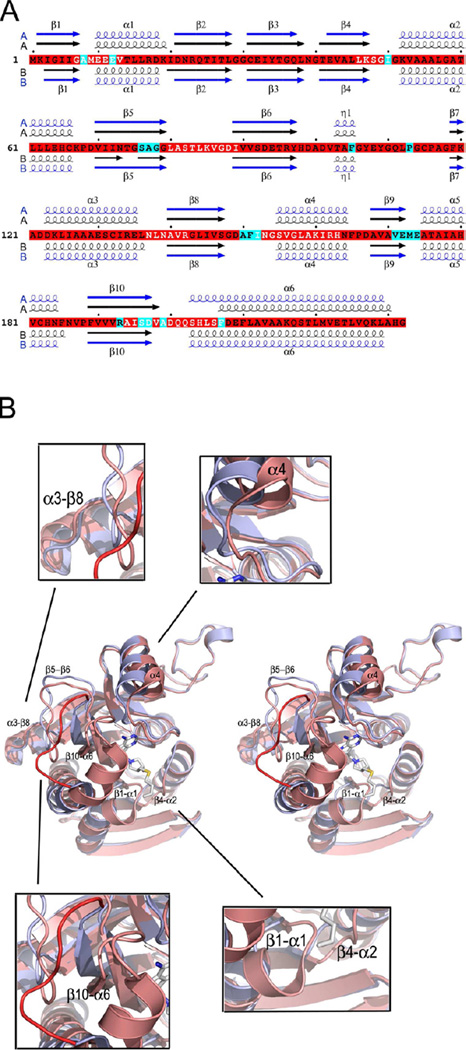
Figure 2.
Changes in the tertiary structure upon inhibitor binding. (A) The sequence of S. enterica MTAN (SeMTAN) and the alignment of secondary structures of inhibitor and adenine bound SeMTAN structures. The secondary structures in black are from Ade-SeMTAN crystal structure where monomer-A was in the open conformation and monomer-B with a bound adenine was in the closed conformation. The secondary structures shown in blue belong to BuT-DADMe-ImmA-SeMTAN structure. In the BuT-DADMe-ImmA-SeMTAN structure, both monomers were in the closed conformation and contained a bound inhibitor, BuT-DADMe-ImmA. The secondary structures of A-monomers and B-monomers are above and below the sequence, respectively. The residues highlighted in cyan are in contact with the inhibitor in the BuT-DADMe-ImmA-SeMTAN crystal structure. (B) Comparison of tertiary structures of the monomers in the open and closed conformation. The structure shown in light blue is the open conformation monomer-A of Ade-SeMTAN. The monomer colored in salmon shows ligand induced closed form. The bound inhibitor is BuT-DADMe-ImmA. The labeled regions move the most upon ligand binding; the same regions are shown with white letters in panel A. The red loop belongs to open conformation monomer and it indicates the site of the most change in the structure. See also Movie S1.
Adenine and polyethylene glycol in the monomer-B of S. enterica MTAN
The crystal structure of apo SeMTAN was determined at 1.36 Å resolution. Monomer-B of the apo SeMTAN dimer was in the ligand-induced closed conformation (Figure 2B, Movie S1) and contained bound adenine (Figure 3A) although adenine was not included in the crystallization trials. Enzyme used in the crystallization trials contained bound adenine by uv spectral analysis, similar to previous reports for E. coli MTAN (Lee et al., 2001). We therefore refer to apo SeMTAN as Ade-SeMTAN. Four hydrogen bonds between adenine and SeMTAN include the main-chain oxygen and nitrogen of Ile152 in hydrogen bonds with N6 and N1 of adenine, respectively. The OD1 and OD2 of Asp197 are in hydrogen bonds to N6 and N7 of adenine, respectively. Non-bonded contacts (< 3.90 Å) between adenine and the protein include Ser76, Ala77, Gly78, Ala150, Phe151, Val171, Glu172, Ser196 and Ala199 (Table S1). Phe151 formed the most intimate contact, but not by an optimal parallel stacking interaction (McGaughey et al., 1998).
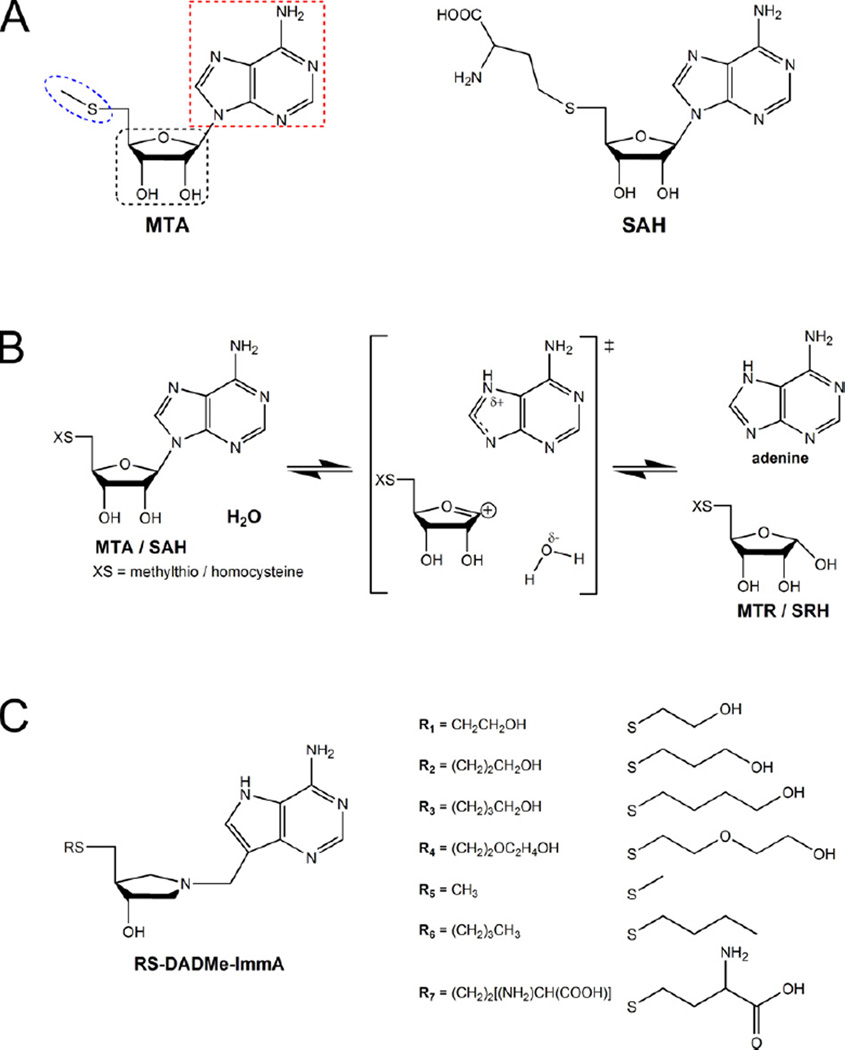
Figure 3.
The substrates and transition state analogues of S. enterica MTAN (SeMTAN). (A) Methylthioadenosine (MTA) and S-adenosylhomocysteine (SAH) are the substrates of MTAN. The substrates and substrate analogues of MTAN can be divided into adenine (red dotted line), ribose (black dotted line) and 5’-alkylthio (blue dotted line) moieties. All these moieties have defined subsites in the active site of MTAN (Lee et al., 2003). (B) The reaction as catalyzed by MTAN. The transition state of MTAN involves a dissociated adenine leaving group and ribocation. In the end of the reaction cycle, the carbocation is neutralized by the activated water molecule producing either methylthioribose (MTR) of S-ribosylhomocysteine (SRH) together with adenine. (C) Transition state analogues. The DADMe-ImmA inhibitors mimic the late dissociative transition state character of MTAN, in which the distance between C1´ of thioribose and N9 of adenine is approximately 3 Å, and they have two chiral centers (3R,4S). The RS-DADMe-ImmA having R1–R4 groups are novel inhibitors designed on the SeMTAN crystal structure in complex with TEG (see also Figure S1). R5 is the transition state mimic of MTA and R7 is the transition state mimic of SAH.
An unknown ligand also occupied the 5′-alkylthio subsite of the binding pocket (Figure S1). Anomalous difference maps gave no peaks for this ligand but the sulfurs of cysteines and methionines of MTAN were located, thus, the molecule in the 5′-alkylthio subsite did not contain sulfur (see the methods section). Triethylene glycol (TEG) fitted the corresponding positive difference map. Bound TEG contacted both monomers of the Ade-SeMTAN homodimer including residues Met9, Ile150, Phe151, Phe207 and Asp208 of monomer-B and Val102 and Phe105 of monomer-A. None of these contacts were hydrogen bonds. Others have also reported a PEG molecule occupying a substrate binding site in E. coli MTAN. But in E. coli MTAN, the site with bound PEG was in an open conformation (Lee et al., 2005b), therefore, PEG binding in E. coli and S. enterica MTANs is different.
Structural changes from the open form to the closed form
Monomer-B of Ade-SeMTAN had a closed site while monomer-A of the same homodimer was in the open conformation. When compared, dramatic changes appeared at the ligand binding site and changes were also induced distant from the active site (Figure 2B, Movie S1). The observed changes were consistent with those described for E. coli and H. pylori MTAN (Lee et al., 2003; Lee et al., 2005b; Ronning et al., 2010). The most prominent reorganization was between β10 and α6, moving toward the bound ligand. The move increased the helical content of α6 to become four residues longer in its N-terminus and to position it closer to the binding site (Figure 2A). The C-terminus of β10 also moved towards the bound ligand. Rearrangement at the β10-α6 region caused concerted motions in nearby regions by causing the β1-α1 loop and also α1 to move. Furthermore, the β4-α2 loop moved the same direction, away from α6. On the other side of α6, the β5-β6 loop moved towards the central region of α6 together with α3, α3-β8 loop and the N-terminus of β8. Near the dimer interface, α4 was pushed towards the neighboring monomer by reorganization of the α6 N-terminus and the loop preceding the α4-helix moved towards the adenine subsite. The closed binding site still has an opening at the surface of the enzyme (Figure 1B).
The ligand-induced structural rearrangements repositioned several important active site residues (Figure 2A). The movement of α1 placed the catalytic glutamate (Glu12) closer to active site where its role is proposed to activate a water molecule for a nucleophilic attack, as suggested from structures with bound transition state analogues. Phe151 and Ile152 of the β8-α4 loop moved to form a stacking interaction and hydrogen bonds with adenine, respectively. Asp197 moved in position to donate a proton to N7 of the adenine. Phe207 of α6 together with Phe151 and Phe105 became part of the 5′-alkylthio subsite. A combination of transition state analysis and crystallographic data with transition state analogues have shown the importance of N7 protonation in forming the transition state and of Asp197 in catalysis. The raised pKa (8.2) enables Asp197 act as a catalytic acid. (Allart et al., 1998; Lee et al., 2005a; Lee et al., 2005b)
When all MTANs in the PDB were compared (Table S2), most show both monomers of the dimer have ligand-induced closed conformations. None of these closed-conformation monomers had only a bound adenine. Only in two structures, both monomers have open conformations (E. coli MTAN, PDB codes 1JYS and 1Z5P) and in one structure, the dimer has an open and a closed monomer (H. pylori MTAN, PDB code 3NM4). In open conformation monomers, ethylene glycol occupied the ribose subsite (H. pylori MTAN, PDB code 3NM4), adenine occupied the adenine subsite (E. coli MTAN, PDB code 1JYS) or glycerol was in the ribose subsite and PEG in the 5′-alkylthio subsite (E. coli MTAN, PDB code 1Z5P). In the open conformation monomer-A of Ade-SeMTAN (this study), a continuous electron-density extended from the 5′-alkylthio subsite to the ribose subsite, a structure resembling a PEG fragment, but no electron-density for adenine was seen. It can be concluded that adenine or PEG alone is not sufficient to change the conformation from the open form into closed form.
Structure-based design from existing transition state analogues
The binding modes of adenine and TEG in the closed form of Ade-SeMTAN suggested novel inhibitors. Structural superposition of Ade-SeMTAN and SeMTAN with a bound butylthio-(3R,4S)-1[9-deazaadenin-9-yl)methyl]-3-hydroxy-4-methylthio-pyrrolidine (BuT-DADMe-ImmA) (PDB code 4F3C, this study) revealed close overlap of the adenines with TEG present in the 5′-alkylthio subsite (Figure S1). It was observed that a group much longer than a butyl group could be accommodated by the 5′-alkylthio subsite in SeMTAN. New inhibitors were designed with DADMe-ImmA cores with oxygen and carbon substituents in elongated 5′-alkylthio groups (Figure 3C). Four new inhibitors were synthesized with 2-hydroxyethylthio (-S-CH2CH2OH), 3-hydroxypropylthio [-S-(CH2)2CH2OH], 4-hydroxybutylthio [-S-(CH2)3CH2OH] or 2-(2-hydroxyethoxy)ethylthio [-S-(CH2)2OC2H4OH] groups attached to the DADMe-ImmA core. An inhibitor having two ethylene glycol (C-C-O) repeats (DiEGT-DADMe-ImmA) was the best inhibitor according to a measured Ki* value of 5 pM (Table 1). DiEGT-DADMe-ImmA was used in co-crystallization experiments, as well.
Table 1.
The slow-onset inhibition constants (Ki*) and the Gibbs free energy values (ΔG). The DADMe-ImmA inhibitors are the mimics of the late dissociative transition state in which the distance between C1’of thioribose and N9 of adenine of the MTA is elongated. All these transition state analogues exhibited slow-onset inhibition and they diffuse out of MTAN very slowly. The top four inhibitors are novel inhibitors particularly designed for this study. The lower four inhibitors were cocrystallized with S. enterica MTAN and the corresponding structures were determined in this study. The ΔΔG values were determined by using methylthio-DADMe-ImmA as a reference.
| Inhibitor | Ki* (pM) | ΔG (kcal mol−1 ) | ΔΔG (kcal mol−1) |
|---|---|---|---|
| 2-Hydroxyethylthio-DADMe-ImmA | 11.0 ± 2.2 | −14.9 ± 0.1 | 0.5 |
| 3-Hydroxypropylthio-DADMe-ImmA | 7.0 ± 2.3 | −15.2 ± 0.2 | 0.2 |
| 4-Hydroxybutylthio-DADMe-ImmA | 14.8 ± 2.7 | −14.8 ± 0.1 | 0.6 |
| 2-(2-hydroxyethoxy)ethylthio-DADMe-ImmA Aka diethyleneglycolthio-DADMe-ImmA | 5.0 ± 0.7 | −15.4 ± 0.1 | 0 |
| Homocysteine-DADMe-ImmA | 69.0 ± 12.1 | −13.9 ± 0.1 | 1.5 |
| Methylthio-DADMe-ImmA | 5.0 ± 0.4 | −15.4 ± 0.1 | 0 |
| Butylthio-DADMe-ImmA | 1.5 ± 0.4 | −16.1 ± 0.2 | −0.7 |
Structures and a water network of S. enterica MTAN with transition state analogues
Crystal structures of SeMTAN in complex with four different inhibitors were determined (Table 2, Table 3). All inhibitors had the same core structure (-DADMe-ImmA) to mimic the late dissociative transition state character, but with different substituents at the 5′-thiol-position (Figure 3C). The groups were methylthio (MT), butylthio (BuT), homocysteine (Homocys) or diethyleneglycolthio (DiEGT). The Homocys-DADMe-ImmA and DiEGT-DADMe-ImmA inhibitors have not been previously reported in any crystal structures.
Table 2.
The data collection and refinement statistics (see also Table S2).
| Adenine (PDB ID 4F1W) |
Methylthio-DADMe Immucillin-A (PDB ID 4F2W) |
Butylthio-DADMe Immucillin-A (PDB ID 4F3C) |
Homocys-DADMe Immucillin-A (PDB ID 4F3K) |
DiEtglycolthio-DADMe Immucillin-A (PDB ID 4F2P) |
|
|---|---|---|---|---|---|
| Data collection statistics | |||||
| Space group | P212121 | P212121 | P21 | P212121 | P212121 |
| Unitt cell parameters a (Å) | 66.05 | 69.41 | 68.05 | 52.49 | 52.41 |
| b (Å) | 68.12 | 82.54 | 46.61 | 70.49 | 70.14 |
| c (Å) | 89.66 | 93.11 | 70.94 | 122.83 | 122.50 |
| β (°) | - | - | 114.78 | - | - |
| Temperature (K) | 100 | 100 | 100 | 100 | 100 |
| Wavelength (Å) | 1.075 | 1.075 | 1.075 | 1.075 | 1.075 |
| Resolution (Å) | 54.24-1.36 (1.43-1.36) | 61.77-2.00 (2.11-2.00) | 64.41-1.93 (2.03-1.93) | 61.41-1.85 (1.95-1.85) | 61.25-1.64 (1.73-1.64) |
| Rpim (%) | 3.5 (30.7) | 8.1 (14.1) | 4.0 (14.8) | 7.3 (42.0) | 3.7 (20.0) |
| Completeness (%) | 100 (99.9) | 99.0 (96.8) | 99.7 (99.4) | 99.8 (99.6) | 98.7 (97.2) |
| Mn(I/sd) | 12.9 (2.6) | 6.7 (4.1) | 11.7 (4.2) | 9.5 (3.2) | 10.9 (2.9) |
| Unique reflections | 87459 (12605) | 36435 (5119) | 30569 (4402) | 39679 (5700) | 55300 (7822) |
| Redundancy | 11.5 (11.2) | 6.7 (6.8) | 4.0 (3.9) | 5.3 (5.1) | 7.6 (7.6) |
| Mosaicity (°) | 0.6 | 1.1 | 0.6 | 0.4 | 0.6 |
| B-factor from Wilson plot (Å2) | 13 | 16 | 20 | 18 | 19 |
| Refinement statistics | |||||
| Resolution (Å) | 41.95-1.36 | 46.18-2.00 | 37.46-1.93 | 39.93-1.85 | 39.85-1.64 |
| Total number of reflections | 87381 | 36380 | 30557 | 39608 | 55244 |
| Working set: number of reflections | 83002 | 34563 | 29019 | 37617 | 52433 |
| Rfactor(%) | 11.29 | 17.72 | 18.55 | 17.76 | 16.33 |
| Test set: number of reflections | 4379 | 1817 | 1538 | 1991 | 2811 |
| Rfree (%) | 14.60 | 21.60 | 23.27 | 21.48 | 19.35 |
| Protein atoms | 3664 (A,B) | 3497 (A,B) | 3463 (A,B) | 3445 (A,B) | 3560 (A,B) |
| Water atoms | 389 | 271 | 164 | 247 | 340 |
| Inhibitor atoms | - | 40 | 46 | 52 | 75 |
| Adenine atoms | 10 | - | - | - | - |
| Ethylene glycol atoms | 28 | 24 | 12 | 8 | 4 |
| Diethylene glycol atoms | 7 | - | - | - | - |
| Triethylene glycol atoms | 30 | - | - | - | - |
| Tetraethylene glycol atoms | 39 | - | - | - | - |
| Acetate atoms | - | - | - | - | 4 |
| Glycerol atoms | - | - | - | - | 18 |
| Chloride atoms | - | 2 | - | - | - |
| Geometry statistics | |||||
| Rmsd (bond distance) (Å) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Rmsd (bond angle) (°) | 1.44 | 1.45 | 1.39 | 1.38 | 1.48 |
| Rmsd B | |||||
| Main chain bonded atoms (Å2) | 1.02 | 0.66 | 1.09 | 0.91 | 0.96 |
| Side chain bonded atoms (Å2) | 2.52 | 1.19 | 1.41 | 1.54 | 1.68 |
| Average B | |||||
| Main chain atoms (Å2) | 12.22 | 15.00 | 25.55 | 20.28 | 22.25 |
| Side chain atoms (Å2) | 17.18 | 18.27 | 29.01 | 24.28 | 26.33 |
| Water atoms (Å2) | 32.30 | 26.78 | 32.17 | 30.61 | 34.75 |
| Inhibitor atoms (Å2) | - | 10.58 | 17.95 | 17.44 | 18.35 |
| Adenine (Å2) | 12.42 | - | - | - | - |
| Ethylene glycol atoms (Å2) | 40.16 | 39.68 | 36.05 | 39.44 | 36.30 |
| Diethylene glycol atoms (Å2) | 38.69 | - | - | - | - |
| Triethylene glycol atoms (Å2) | 37.28 | - | - | - | - |
| Tetraethylene glycol atoms (Å2) | 39.46 | - | - | - | - |
| Acetate atoms (Å2) | - | - | - | - | 36.75 |
| Glycerol atoms (Å2) | - | - | - | - | 46.56 |
| Chloride atoms (Å2) | - | 20.35 | - | - | - |
| Ramachandran plot | |||||
| Most favored region (%) | 92.7 | 91.2 | 90.8 | 92.1 | 91.8 |
| Additionally allowed regions (%) | 7.3 | 8.8 | 8.9 | 7.7 | 8.0 |
| Generously allowed regions (%) | 0 | 0 | 0.3 | 0.2 | 0.2 |
| Disallowed regions (%)* | 0 | 0 | 0 | 0 | 0 |
Table 3.
Crystallization conditions. In this study, five different binary complexes of S. enterica MTAN were crystallized and the structures determined.
| PDB code | Ligand bound in the active sites |
Crystallization conditions | Cryo protectant |
|---|---|---|---|
| 4F1W | Adenine, PEG | 0.1 M imidazole, pH 8.0, 1 M (NH4)2HPO4 | 35 % PEG 400, 15 min |
| 4F2W | MT-DADMe-ImmA | 0.1 M tris, pH 7.0, 35 % 2-methyl-2,4-pentanediol, 0.2 M NaCl | 39 % 2-methyl-2,4-pentanediol, 11 % PEG 400, 5 min |
| 4F3C | BuT-DADMe-ImmA | 20 % PEG 3350, 0.2 M lithium acetate | 40 % glycerol, 30 min |
| 4F3K | Homocys-DADMe-ImmA | 0.1 M MES, pH 6.5, 12 % PEG 20000 | 47 % glycerol, 30 min |
| 4F2P | DiEGT-DADMe-ImmA | 0.1 M HEPES, pH 6.5, 27 % PEG 3350, 0.2 M sodium acetate | 39 % glycerol, 30 min |
In all structures, both active sites had bound inhibitor and were in the closed conformation. Superposition of all eight inhibitor-bound monomers revealed common binding modes (Figure 4, Table S1). The 9-deazaadenine groups of DADMe-ImmAs were located in the adenine subsite with the same hydrogen bonds and contacts for adenine in Ade-SeMTAN. The O and N of Ile152 were hydrogen bonded to N1 and N6 of 9-deazaadenine, respectively. The OD1(Asp197) formed a hydrogen bond with N6 of 9-deazaadenine and OD2(Asp197) was in turn hydrogen bonded to N7 of 9-deazaadenine. Binding of the 4-methylthiopyrrolidine moieties of MT-DADMe-ImmA, BuT-DADMe-ImmA, Homocys-DADMe-ImmA and DiEGT-DADMe-ImmA was also the same. There was one more direct hydrogen bond between the bound inhibitors and SeMTAN that was seen in the other four inhibitor-SeMTAN crystal structures reported here. The additional hydrogen bond was between OE1(Glu174) and O3′ of pyrrolidine (Figure 4). Major differences in the binding modes of the four inhibitors were seen in the 5′-alkylthio binding site and in the water network near the catalytic water molecule (Figure 4).
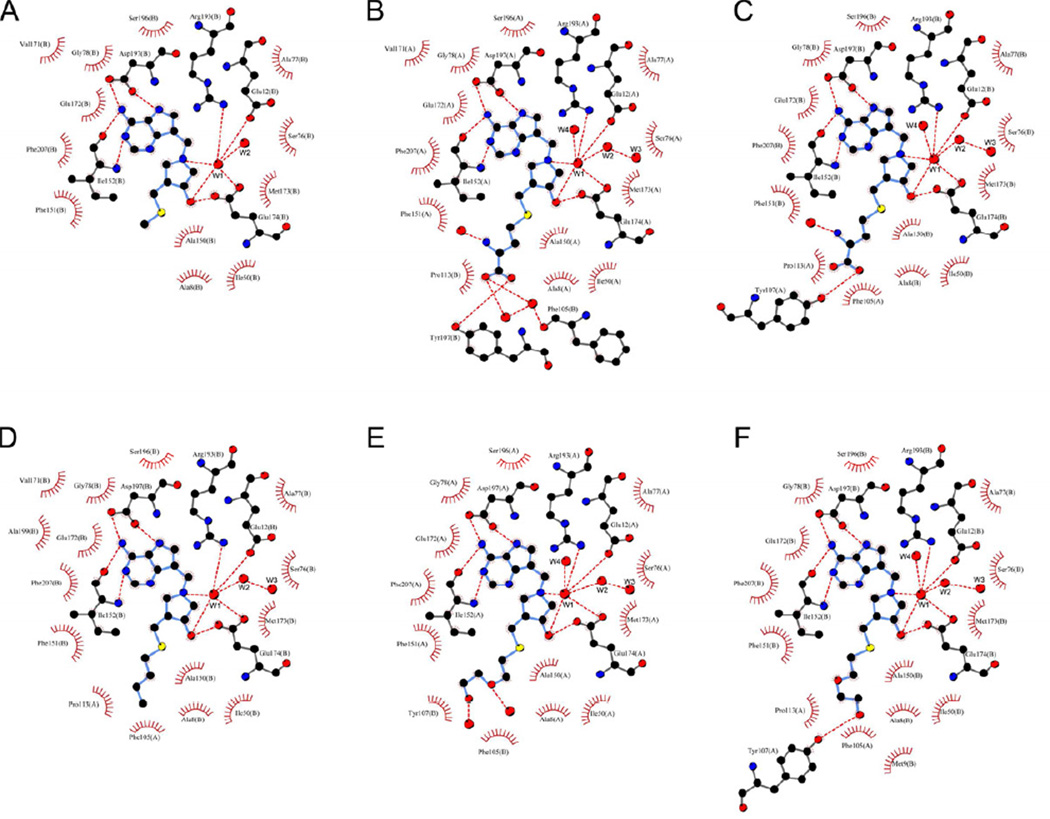
Figure 4.
Schematic presentation of active sites from binary structures of S. enterica MTAN (SeMTAN). The structures were determined in the presence of MT-DADMe-ImmA (A), Homoc-DADMe-ImmA (B,C), BuT-DADMe-ImmA (D) and DiEGT-DADMe-ImmA (E,F). The direct and water mediated hydrogen bonds are shown as red dotted lines, determined using a maximum donor-acceptor distance of 3.35 Å. All the other residues are involved in the hydrophobic contacts (see also Table S1). The carbons, oxygens, nitrogens and sulfurs are colored in black, red, blue and yellow, respectively. The waters are shown as red balls. The W1 water is the active site nucleophilic water. The short water trail consists of W1, W2 and W3 waters. The 5´-alkylthio moieties of Homoc-DADMe-ImmA (B,C) and DiEGT-DADMe-ImmA (E,F) have different binding modes and hydrogen bonds in the binding pockets of SeMTAN homodimer. The figure was prepared with LigPlot (Laskowski and Swindells, 2011; Wallace et al., 1995).
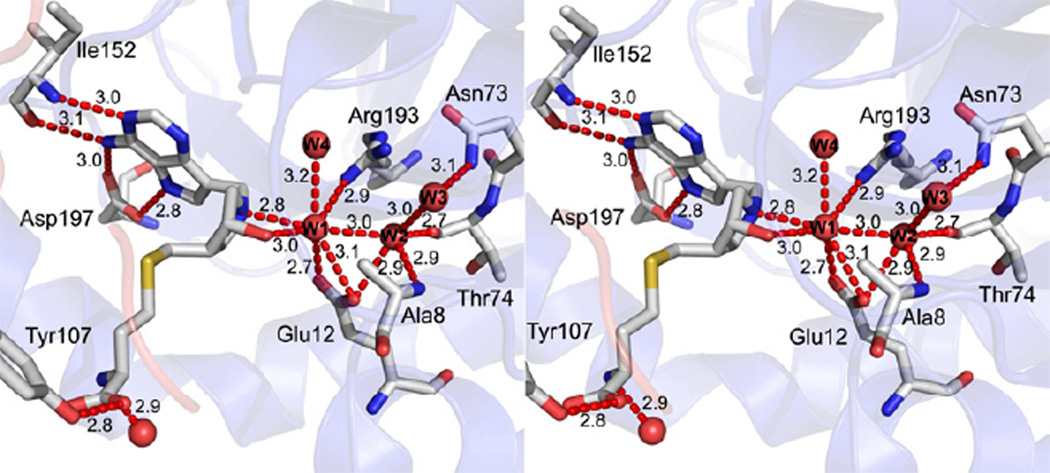
All inhibitor-SeMTAN structures showed a conserved trail of three or two waters (Figure 4, Figure 5). The first is the catalytic water, hydrogen bonded to OE1(Glu12), OE2(Glu12), OE2(Glu174), NH1(Arg193), N1′ of pyrrolidine, O3′ of pyrrolidine and the 2nd water of the water trail. The 2nd water of the trail had hydrogen bonds with the 1st and the 3rd water molecules of the water trail, and with N(Ala8), OE1(Glu12), O(Thr74), NH1(Arg193) and NH2(Arg193). The 3rd water was hydrogen bonded only to N(Ala8) and ND2(N73). Two waters were resolved with MT-DADMe-ImmA-SeMTAN and in monomer-A of BuT-DADMe-ImmA-SeMTAN. Three waters were resolved in monomer-B of BuT-DADMe-ImmA-SeMTAN and in both monomers of Homocys-DADMe-ImmA-SeMTAN and DiEGT-DADMe-ImmA-SeMTAN. A fourth molecule (W4) in the active sites of Homocys-DADMe-ImmA-SeMTAN and DiEGT-DADMe-ImmA-SeMTAN was not part of the water trail but was hydrogen bonded to OE1(Glu172), N(Met173), N(Glu174), OE2(Glu174), NH1(Arg193) and NH2(Arg193). The trail of three waters (W1-W3) was also present in the closed conformation monomer of Ade-SeMTAN structure. Alignment of the crystal structure of E. coli MTAN with a bound MTA (PDB code 1Z5O) with Homocys-DADMe-ImmA-SeMTAN or DiEGT-DADMe-ImmA-SeMTAN structures showed that the water W4 was approximately 1.8 Å away from the binding site of the O2´ of ribose of MTA. Therefore, the W4 water replaces the ribosyl O2´. The active site of E. coli MTAN with bound products methylthioribose (MTR) and adenine (PDB code 1Z5N) the catalytic water converted to the covalent α-C1-hydroxyl group following its nucleophilic reaction to form bound 5-methylthio-α-D-ribose (MTR) (Figure 3B).
Figure 5.
The active site of monomer-B of Homoc-DADMe-ImmA-SeMTAN. The figure shows the hydrogen bonds (red dotted lines) between a bound inhibitor (homocysteine-DADMe-ImmA) and SeMTAN. A short water trail with the associated hydrogen bonding network is included. Tyr107 is part of the neighboring monomer-A. Waters are shown as red spheres and W1-W4 waters are discussed in the text. W1 is the catalytic water molecule. The carbons, oxygens, nitrogens and sulfurs are colored in grey, red, blue and yellow, respectively. The hydrogen bonding distances are shown in angstroms (Å). The figure was prepared with PyMOL.
Accommodation in the 5′-alkylthio subsite of S. enterica MTAN
The 5′-alkylthio subsite with bound inhibitors can accommodate molecules of approximately 7 to 9 Å wide. The 5′-alkylthio subsite is formed by Met9(B), Ile50(B), Val102(A), Phe105(A), Pro113(A) and Phe207(B) of both monomer-A and monomer-B.
The 5′-methylthio group of MT-DADMe-ImmA occupied the 5′-alkylthio subsite of the corresponding crystal structure and was completely buried by the residues of the binding pocket of the same monomer (Figure 4A). Only one residue (Ile50) was in contact with the methyl group. Of the four inhibitor bound crystal structures in this study, MT-DADMe-ImmA was the only inhibitor that adopted the same conformation in both active sites of SeMTAN homodimers.
The 5′-tail of BuT-DADMe-ImmA took full advantage of the 5′-alkylthio binding pocket. The methyl end of the butylthio group was located at the exit of the binding pocket and the butyl group was surrounded by hydrophobic residues: Ile50(A), Val102(B), Phe105(B), Pro113(B) and Phe207(A) (Figure 4D). In contrast to the MT-DADMe-ImmA-SeMTAN, residues contributing to butylthio binding are primarily from the neighboring monomer. Bound BuT-DADMe-ImmA differs in subunits A and B since the methyltip of the butylthio group faced to different directions.
Homocys-DADMe-ImmA is a transition state mimic of SAH, a MTAN substrate (Figure 3). No MTAN structures have been reported with Homocys-DADMe-ImmA. Similar to BuT-DADMe-ImmA, the homocysteine group had distinct conformations in the active sites of SeMTAN (Figure 4B and 4C). Commonly, the homocysteine group was surrounded by Ile50(A), Phe105(B), Tyr107(B), Pro113(B) and Phe207(A). The carboxylate terminus extended beyond the binding pocket and was bound on the surface of the enzyme. This allowed an additional hydrogen bond between OXT of the homocysteine carboxylate group and the OH of Tyr107 in the neighboring monomer. Moreover, the amino of homocysteine formed a hydrogen bond with a water molecule on the surface of SeMTAN near the exit of the 5′-alkylthio subsite and pointed towards the CE2/CD2 side of phenyl ring of Phe105 (3.6 Å), but not towards the energetically favored center of the phenyl ring (Levitt and Perutz, 1988). In the second active site, the CNH-COOH group of homocysteine was rotated around the CB-CA bond by approximately 25 degrees to position the NH of homocysteine > 4 Å from the phenyl ring of Phe105 and the carboxylate oxygen was hydrogen bonded to O(Phe105) via a water molecule (Figure 4B).
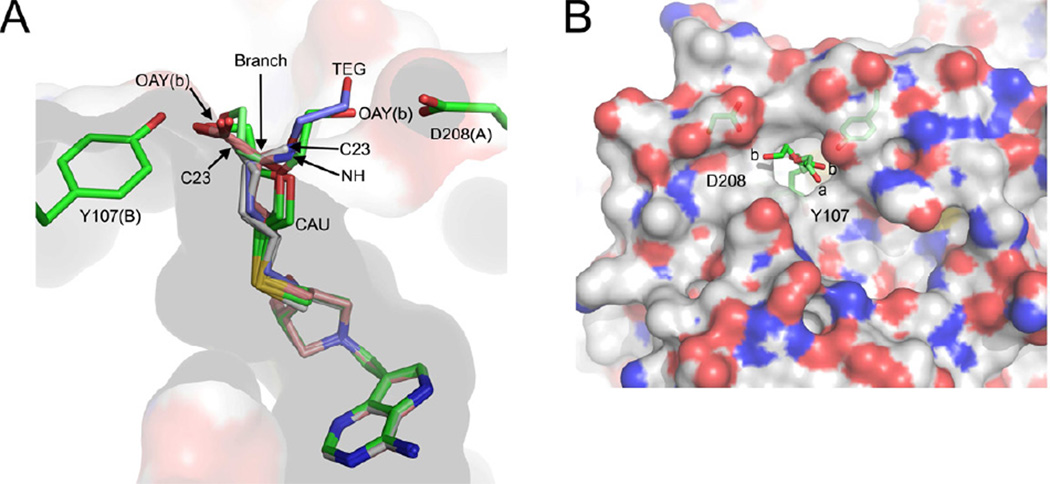
In monomer-A, the DiEGT moiety of DiEGT-DADMe-ImmA had a single conformation, facing Tyr107 of the neighboring subunit. Binding of the DiEGT group was accommodated by Ile50(A), Phe105(B), Tyr107(B) and Phe207(A) but no direct hydrogen bonds were formed between the DiEGT moiety and SeMTAN. The second ethylene glycol repeat of DiEGT extended out of the 5′-alkylthio subsite and was bound on the surface of the enzyme similar to Homocys-DADMe-ImmA-SeMTAN. Both oxygen atoms of DiEGT formed hydrogen bonds with water molecules (Figure 4E). In monomer-B, DiEGT-DADMe-ImmA had two binding modes. In one, the DiEGT group was similar in binding as seen in monomer-A, however, the oxygen at the terminal end of the DiEGT (OAY) formed a hydrogen bond with the OH(Tyr107) (Figure 4F). In the same subunit, the other conformation of the DiEGT group was facing the opposite direction, towards Asp208(B) (Figure 6B). The conformer facing Asp208 was no longer in contact with Tyr107(A) and Pro113(A) of the neighboring monomer. Instead, additional hydrogen bonds were formed between the oxygen atom (OAY) in the end of the DiEGT moiety with OD1 of Asp208 and a water mediated hydrogen bond with OE2 of Glu11.
Figure 6.
Binding modes of transition state analogues with S. enterica MTAN (SeMTAN). (A) The binding pocket of monomer-A. The eight monomers of four SeMTAN dimers in complex with DADMe-ImmA based inhibitors as well as the adenine and triethylene glycol (TEG) bound monomer were superpositioned. The carbons of BuT-DADMe-ImmA, Homocys-DADMe-ImmA and DiEGT-DADMe-ImmA are colored in grey, salmon and green, respectively. The carbons of TEG are shown in light blue. MT-DADMe-ImmA is also included but is not visible in the figure. The longer inhibitors either face the Asp208 of the same monomer or Tyr107 of monomer-B; the site where the inhibitor molecule rotates either towards D208 or Y107 is indicated by an arrow and named branch point. The DiEGT-DADMe-ImmA of monomer-B of DiEGT-DADMe-ImmA-SeMTAN structure has two conformations where the oxygen [(OAY(b)] of DiEGT moiety is hydrogen bonded to Asp208 or Tyr107 (b stands for monomer-B). The C23 indicates the binding site of the carbon atom at the tip of the butylthio moiety of BuT-DADMe-ImmA. NH is the nitrogen atom of homocysteine of Homoc-DADMe-ImmA, and CAU is the carbon atom of the first ethyleneglycol repeat of diethyleneglycolthio moiety of DiEGT-DADMe-ImmA. (B) The two binding pocket extensions on the surface of SeMTAN in complex with DiEGT-DADMe-ImmA. The carbons, oxygens, nitrogens and sulfurs of the accessible surface area are shown in grey, red, blue and yellow, respectively. The binding modes of DiEGT moieties of monomer-A (a) and monomer-B (b) are shown. The oxygen labeled b is labeled as OAY(b) in panel A. The D208 and Y107 indicate putative binding site extensions that could be utilized by longer 5´-alkylthio substituted inhibitors. The figures were prepared with PyMOL
Surface extensions of the 5′-alkylthio subsite
The two major binding modes for inhibitors of SeMTAN have the 5′-thio-substituted groups positioned towards Tyr107 of the neighboring monomer or directed to Asp208 of the same monomer (Figure 6A). The 5′-alkylthio subsite can be extended into additional subsite extensions characterized by deep grooves on the protein surface (Figure 6B). Extension-Y107 originates from Tyr107 and extension-D208 is next to Asp208, and the branch point (Figure 6A) for these extensions is near the exit of the 5′-alkylthio subsite. The surface grooves interact in distinct ways with inhibitors extending beyond the enzyme surface. Thus, carbon (C23) of butylthio in monomer-A of BuT-DADMe-ImmA-SeMTAN was in extension-D208, and in monomer-B, the same carbon was in extension-Y107. Homocys-DADMe-ImmA-SeMTAN had the CA carbon of homocysteine at the branch point and positioned the amino and carboxylate groups into extension-D208 and extension-Y107, respectively. The TEG bound in monomer-B of Ade-SeMTAN traversed the branch point and occupied the extension-D208 site, placing the oxygen atom of TEG in the same place as C23 of butylthio and N of homocysteine in the corresponding inhibitors (Figure 6A). In contrast, the DiEG moiety of DiEGT-DADMe-ImmA has a distinct orientation indicating that the 5′-alkylthio channel is wide enough to permit distinct conformations of the ligand (Figure 6A). Emerging from the surface, the DiEG moiety occupied either the extension-Y107 (monomer-A) or extension-D208 sites. In monomer-B the diethyleneglycolthio group occupied both enxtension-Y107 and extension-D208 (Figure 6B).
Changes in the surface area of S. enterica MTAN dimers
The surface areas of SeMTAN vary substantially depending on the active site ligand (Table 4). In the case of an Ade-SeMTAN dimer, the accessible surface area was 17394 Å2. If the monomers of SeMTAN both adopted the open conformation, the surface area of that dimer would be approximately 18168 Å2, thus binding of one adenine decreased the surface area by approximately 4 % (Table 4). BuT-DADMe-ImmA binding decreased the surface area by 9 % compared to both monomers in the open conformation. With BuT-DADMe-ImmA bound to both monomers, their sizes were equivalent. But with MT-DADMe-ImmA or DiEGT-DADMe-ImmA at both dimer sites, the surface areas of the monomers differed (Table 4). Therefore, the size of a monomer and dimer depend on the size and the nature of the bound inhibitor.
Table 4.
The surface areas and the changes in the surface areas upon ligand binding. The accessible surface areas were calculated without including any hetero atoms and using the same residue range. The Apo homodimer was made from the open conformation monomer of Ade-SeMTAN crystal structure. When the surface areas of liganded dimers were compared to the artificially created Apo dimer, it was noticed that having BuT-DADMe-ImmA in both active sites causes the dimer shrink
| Bound ligand | Surface of monomer-A (Å2) |
Surface of monomer-B (Å2) |
Interface area (Å2) |
Surface of dimer (Å2) |
Decrease in the surface area (%) |
|---|---|---|---|---|---|
| Apo1 | 10368 | 10367 | 2567 | 18168 | - |
| Adenine2 | 10368 | 9721 | 2694 | 17394 | 4.3 |
| MT-DADMe-ImmA3 | 9870 | 9927 | 2863 | 16934 | 6.8 |
| Homocys-DADMe-ImmA3 | 9814 | 9914 | 2810 | 16917 | 6.9 |
| DiEGT-DADMe-ImmA3 | 9715 | 9952 | 2796 | 16871 | 7.1 |
| BuT-DADMe-ImmA3 | 9667 | 9681 | 2813 | 16534 | 9.0 |
Both monomers are in the open conformation.
One monomer is in the open conformation and the other one is in the closed conformation.
Both monomers are in the closed conformation.
DISCUSSION
Both S. enterica and E. coli MTANs copurified with a bound adenine. In E. coli MTAN, adenine was bound in both active sites while SeMTAN contained an adenine only in one active site. The monomers of E. coli MTAN were in the open conformation but SeMTAN has the monomer with bound adenine in a closed conformation. SeMTAN also showed catalytic cooperativity between the homodimer active sites. When only the first catalytic site of SeMTAN was inhibited, the second active site showed reduced catalytic activity (Thomas et al., 2012). Thus, adenine binding to SeMTAN supports negative cooperativity. In contrast, the active sites of E. coli MTAN homodimer are catalytically independent (Thomas et al., 2012). At the amino acid sequence level SeMTAN and E. coli MTAN differ in only ten amino acids but their catalytic and structural differences are significant (Thomas et al., 2012). With bound ligands, major rearrangements occur in the active sites of MTAN. The β8-α4 and β10-α6 loops move the most, and these loops contain important residues for ligand binding and catalysis. The catalytic site geometry needs to be stabilized for a sufficient time for reaction to occur. When compared with EcMTAN (bound MT-DADMe-ImmA, PDB code 1Y6Q), SeMTAN has an additional hydrogen bond at the catalytic site between His204 of β10-α6 loop and Ala104 of the neighboring monomer (Figure 1A). The β10-α6 loop moves to open and close the active site. As product release is rate-limiting in most N-ribosyl transferases, it has been proposed that this hydrogen bond participates in the rate-limiting kinetic step of SeMTAN (Thomas et al., 2012). This difference may explain the kcat values of 20 and 2.3 reported for SeMTAN and EcMTAN, respectively and likewise, the differences in dissociation constants of 2 and 5 pM for MT-DADMe-ImmA (Thomas et al., 2012).
The water channel in SeMTAN has the clear role of providing the nucleophilic water and a path for its replacement in each catalytic cycle. Water trails also serve as “proton wires” in the active site of enzymes and could assist in the ionization of the attacking water (Meyer, 1992). Water is not a strong nucleophile and needs to be activated prior to nucleophilic attack. The structure permits speculation that the deprotonation of the catalytic water is accomplished by the combined action of Glu12 and the water trail. A recent report of the E. coli MTAN in complex with BuT-DADMe-ImmA shows that one water, presumed to be the nucleophilic water, survives the vacuum of nanoelectrospray quadrupole time-of-flight mass spectrometer analysis, thus, waters two and three are less tightly bound (Wang et al., 2012b). The position of the active site trail of waters is conserved in the crystal structures of S. enterica, E. coli, S. pneumonia, S. aureus, V. cholerae and N. meningitides MTANs. H. pylori MTAN is an exception in the group as the Asn73 of SeMTAN that is in hydrogen bonds to the last water of the water trail is replaced by a Phe75 in H. pylori MTAN. The side-chain of the Phe75 prevents the binding of the 2nd and 3rd waters of the trail and H. pylori MTAN has only the catalytic water molecule.
MTANs bind most 5′-thio-substituded DADMe-ImmA inhibitors with the E. coli MTAN showing the highest affinity for these analogues (Singh et al., 2005a). Specificity and thermodynamic studies with 5′-thio-substituded DADMe-ImmA inhibitors binding to the SeMTAN revealed favorable enthalpic and entropic properties (Thomas et al., 2012). As the size of the protein decreases with inhibitors bound, unfavorable entropic components of binding are implied. However, these must be overcome by favorable entropic contributions and we propose these to come from displacing waters in the 5’-alkylthio binding pocket of SeMTAN while maintaining motion of the inhibitor and surrounding amino acids in this hydrophobic pocket capable of accommodating a wide range of ligands. BuT-DADMe-ImmA binds the most tightly with a dissociation constant of 1.5 pM. MT-DADMe-ImmA gave a dissociation constant of 5 pM, approximately three times weaker binding. BuT-DADMe-ImmA has three more carbon atoms in the tail region compared to MT-DADMe-ImmA. Additional carbons that in the 5′-alkylthio subsite make additional hydrophobic contacts and contribute to higher affinity of BuT-DADMe-ImmA. When the ΔG values of MT-DADMe-ImmA and BuT-DADMe-ImmA were compared, the ΔΔG value was −0.7 kcal mol−1 for BuT-DADMe-ImmA.
Our discovery of the elongated 5′-alkylthio channel in SeMTAN led to the design and chemical synthesis of four new inhibitors to explore this channel. No previous data of any MTANs have shown the existence or utilization of these binding site extensions. As the PEG fragment suggested an oxygen would be tolerated, we replaced the methylthio- group of MT-DADMe-ImmA with 2-hydroxyethylthio-, 3-hydroxypropylthio-, 4-hydrobutanol- and DiEG- groups. Dissociation constants for the new inhibitors were 5 to 15 pM, similar to MT-DADMe-ImmA despite their ability to more completely fill the 5’-alkylthio channel. An isosteric comparison of BuT-DADMe-ImmA and 3-hydroxypropylthio-DADMe-ImmA reveals a four-fold penalty for placing a hydroxyl group instead of a methyl group in the hydrophobic 5′-alkylthio subsite. The ΔΔG of BuT-DADMe-ImmA compared to 3-hydroxypropylthio-DADMe-ImmA was −0.9 kcal mol−1. One of the oxygen ethers of the ethylene glycol repeat of DiEGT-DADMe-ImmA is also located in the hydrophobic environment of 5’-alkylthio subsite. This unfavorable interaction is overcome by the favorable interactions at the extension-Y107 and extension-D208 sites. The Homocys-DADMe-ImmA with a dissociation constant of 69 pM binds more weakly than MT-DADMe-ImmA, DiEGT-DADMe-ImmA, and BuT-DADMe-ImmA. The ΔG of Homocys-DADMe-ImmA binding was also 1.5–2.2 kcal mol−1 higher when compared to three previously mentioned inhibitors. Even though the carboxylate oxygen at the 5′-end Homocys-DADMe-ImmA is hydrogen bonded to a OH(Tyr107), the charge delocalization on the two oxygen atoms of the carboxylate group could weaker this interaction. Despite our comparison of relative binding affinity, the reader will appreciate that any inhibitor with pM affinity is rare and requires the capture an array of favorable interactions at the adenine, ribocation, 5′-alkylthio and water binding sites. These are readily apparent from the structures of SeMTAN presented here. The discovery of picomolar inhibitors extending from catalytic site into the solvent has enabling implications for chemical biology approaches. The structure predicts it will be possible to design click-chemistry inhibitors extending into the solvent for the exploration of MTAN protein-protein interactions.
EXPERIMENTAL PROCEDURES
Purification and crystallization of SeMTAN
Recombinant SeMTAN was produced and purified as described (Thomas et al., 2012). After purification, SeMTAN was dialyzed into 100 mM HEPES, pH 7.0 and stored in −80°C. Crystallization screenings used sitting drop vapor diffusion (Jancarik and Kim, 1991). Crystallization drops used 0.5 µl of enzyme solution with 0.5 µl of well solutions, and the drops were kept at room temperature. Crystals of SeMTAN in complex with an inhibitor, were obtained in using the same protocol as for the Ade-SeMTAN crystals except prior to crystallization trials, recombinant SeMTAN (11 to 14 mg/ml) was combined 2 to 3-fold molar excess inhibitor solution. The enzyme was incubated with the inhibitor on ice for 15 min (Table 3). Attempts to soak inhibitors into apo SeMTAN were unsuccessful.
Data collection and processing
Crystals were soaked in the cryoprotectant (cryo) solution (Table 3) and flash-cooled in liquid nitrogen. The cryo solutions were prepared freshly and, in addition to mother liquor, contained the relevant inhibitor. All the data were collected at beamline X29A at Brookhaven National Laboratory, New York. Images were processed using iMOSFLM (Leslie and Powell, 2007) and intensities were scaled and merged with SCALA (Evans, 2006) of the CCP4 program suite (Collaborative Computational Project, 1994). The data collection and processing statistics are provided in Table 2.
Structure determination, model building and refinement
The crystal structure of Ade-SeMTAN was determined by molecular replacement using E. coli MTA/SAH nucleosidase (PDB code 3O4V) as the search model and MOLREP (Vagin and Teplyakov, 1997) program of CCP4 program suite. Molecular replacement gave one good solution and rigid body refinement followed by restrained refinement in REFMAC5 (Murshudov et al., 1997) using isotropic B-factors, gave a model with an R-factor of 28.5 % and an Rfree of 30.8 %. Subsequently, the model was built and refined in COOT (Emsley et al., 2010) and REFMAC5, respectively. The monomer-A of the apo SeMTAN dimer was modeled with an open conformation and the monomer-B of the same dimer with a bound adenine and polyethylene glycol had adopted the closed conformation.
Data from SeMTAN with BuT-DADMe-ImmA were collected and treated the same way. The closed conformation monomer-B of Ade-SeMTAN was used as the search model in the molecular replacement calculations. The MOLREP routine was able to find both monomers of the dimer after two rounds of rotational and translational searches. Refined SeMTAN dimer with BuT-DADMe-ImmA had a bound inhibitor molecule in both active sites and both monomers were in the closed conformation. For the SeMTAN crystal structures with MT-DADMe-ImmA, Homocys-DADMe-ImmA or DiEGT-DADMe-ImmA, the structure of the SeMTAN dimer with BuT-DADMe-ImmA was used as the search model for determining these crystal structures. All the crystal structures in this study were built in COOT and refined in REFMAC5. After adding water molecules, the corresponding inhibitors and other hetero molecules such as adenine, glycerol, ethylene glycol, acetate and/or chloride were identified in Fo-Fc maps at 3 σ level and included in the refinement cycles (Table 2).
Under conditions of crystallography, we used saturating concentrations of these high affinity inhibitors, and both active sites were filled. Adenine, on the other hand, is much weaker binder (Cornell et al., 1996). Once one active site contains a bound adenine, the affinity of adenine for the second active site of SeMTAN homodimer is reduced extensively due to negative cooperativity (Thomas et al., 2012) and, therefore, adenine was seen only in one active site of SeMTAN.
Identification of the unknown molecule bound in the active site of monomer-B of Ade-SeMTAN
In the Ade-SeMTAN crystal structure, monomer-B had an adenine bound in the adenine subsite of the active site. In addition, a continuous Fo-Fc electron-density map at 3 σ was located at the 5’-alkylthio subsite and extended away from the bound adenine towards the enzyme. The data set for Ade-SeMTAN was collected to 1.36 Å and was highly redundant, permitting calculation of an anomalous difference map to detect atoms heavier than oxygen. No peaks were located at the 5’-alkylthio subsite. Peak heights at the side-chains of cysteines and methionines were readily resolved. Thus, the ligand at the 5’-alkylthio subsite did not contain sulfur atoms. The shape of the Fo-Fc map at the 5’-alkylthio subsite conformed to a bound polyethylene glycol molecule. After a triethylene glycol was added into this density and refined, no negative difference map density was observed supporting the assignment as a fragment of polyethylene glycol.
Inhibitor synthesis and inhibition constants
The MT-DADMe-ImmA, BuT-DADMe-ImmA and Homocys-DADMe-ImmA (Figure 3C) were synthetized as described (Singh et al., 2005a). The DiEGT-DADMe-ImmA, OH-EtT-DADMe-ImmA, OH-PrT-DADMe-ImmA and OH-BuT-DADMe-ImmA were synthesized as described in the Supplemental Information. The slow-onset inhibition constant (Ki*) values of all the inhibitors were obtained using the protocol as described by Thomas and coworkers (Thomas et al., 2012). This value is the equilibrium dissociation constant following slow-onset inhibition has equilibrated. Slow onset inhibition constants were measured in these instances since there was a delay before the onset of inhibition by these compounds. It has been well established that this delay is due to slow conformational changes in enzyme systems into a transition state configuration which is capable of tight binding with transition state analogues (Merkler et al., 1990). The ΔG values were generated from Ki* values using equation: ΔG = RT ln Ki*. To obtain ΔΔG values, methylthio-DADMe-ImmA was chosen as a reference point.
Structure validation and analyses
The final structures were analyzed with MolProbity (Chen et al., 2010; Davis et al., 2007). The protein interfaces and surfaces were obtained using the ‘Protein interfaces, surfaces and assemblies service PISA’ at the European Bioinformatics Institute (Krissinel, 2010; Krissinel and Henrick, 2007). Hetero atoms were excluded from the calculations and residues 1–230 were analyzed. The ligand-protein contacts were produced with LigPlot (Laskowski and Swindells, 2011; Wallace et al., 1995). The models and the corresponding library files of MT-DADMe-ImmA, Homocys-DADMe-ImmA and DiEGT-DADMe-ImmA were produced with PRODRG2 server (Schuttelkopf and van Aalten, 2004) for the RAFMAC5. The coordinates and structure factors have been deposited at the RCSB PDB as entries (Table 2) 4F1W (RCSB072350) for Ade-SeMTAN, 4F2W (RCSB072386) for MT-DADMe-ImmA-SeMTAN, 4F3C (RCSB072402) for BuT-DADMe-ImmA-SeMTAN, 4F3K (RCSB072410) for Homocys-DADMe-ImmA-SeMTAN, and 4F2P (RCSB072379) for DiEGT-DADMe-ImmA-SeMTAN.
Supplementary Material
HIGHLIGHTS.
The 1.36 Å structure of S. e. MTAN reveals nucleophile and inhibitor mechanisms.
New inhibitors were designed and synthesized based on the crystallographic data.
Picomolar Kd’s and structures confirm the inhibitor design strategy.
Additional subsites were identified that could be utilized in inhibitor design.
ACKNOWLEDGEMENTS
This work was supported by GM41916, The New Zealand Foundation for Research, Science and Technology and by a fellowship from the Sigrid Jusélius Foundation (to A.M.H.). Structural data were measured at the beamline X29A at the Case Center for Synchrotron Biosciences (CCSB) located at the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratories (BNL), New York. This publication was made possible by the Center for Synchrotron Biosciences grant, P30-EB-009998, from the National Institute of Biomedical Imaging and Bioengineering (NIBIB). Use of the National Synchrotron Light Source, Brookhaven National Laboratory, was supported by the U.S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-98CH10886. Salmonella enterica MTAN is a community nominated target for the NYSGRC which is supported by NIH grant U54 GM094662 (to S.C.A., PI). We acknowledge Drs. Yury Patskovsky and Jeffrey Bonanno for assistance in data collection, data processing and structural validation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allart B, Gatel M, Guillerm D, Guillerm G. The catalytic mechanism of adenosylhomocysteine/methylthioadenosine nucleosidase from Escherichia coli--chemical evidence for a transition state with a substantial oxocarbenium character. European journal of biochemistry / FEBS. 1998;256:155–162. doi: 10.1046/j.1432-1327.1998.2560155.x. [DOI] [PubMed] [Google Scholar]
- Chen VB, Arendall WB, 3rd, Headd JJ, Keedy DA, Immormino RM, Kapral GJ, Murray LW, Richardson JS, Richardson DC. MolProbity: all-atom structure validation for macromolecular crystallography. Acta crystallographica Section D, Biological crystallography. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project N. The CCP4 suite: programs for protein crystallography. Acta crystallographica Section D, Biological crystallography. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Cornell KA, Swarts WE, Barry RD, Riscoe MK. Characterization of recombinant Eschericha coli 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase: analysis of enzymatic activity and substrate specificity. Biochemical and biophysical research communications. 1996;228:724–732. doi: 10.1006/bbrc.1996.1723. [DOI] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, Snoeyink J, 3rd, Richardson JS, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic acids research. 2007;35:W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Haba G, Cantoni GL. The enzymatic synthesis of S-adenosyl-L-homocysteine from adenosine and homocysteine. The Journal of biological chemistry. 1959;234:603–608. [PubMed] [Google Scholar]
- Duerre JA. A hydrolytic nucleosidase acting on S-adenosylhomocysteine and on 5'-methylthioadenosine. The Journal of biological chemistry. 1962;237:3737–3741. [Google Scholar]
- Emsley P, Lohkamp B, Scott WG, Cowtan K. Features and development of Coot. Acta crystallographica Section D, Biological crystallography. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GB, Furneaux RH, Lenz DH, Painter GF, Schramm VL, Singh V, Tyler PC. Second generation transition state analogue inhibitors of human 5’-methylthioadenosine phosphorylase. Journal of medicinal chemistry. 2005;48:4679–4689. doi: 10.1021/jm050269z. [DOI] [PubMed] [Google Scholar]
- Evans GB, Furneaux RH, Schramm VL, Singh V, Tyler PC. Targeting the polyamine pathway with transition-state analogue inhibitors of 5'-methylthioadenosine phosphorylase. Journal of medicinal chemistry. 2004;47:3275–3281. doi: 10.1021/jm0306475. [DOI] [PubMed] [Google Scholar]
- Evans P. Scaling and assessment of data quality. Acta crystallographica Section D, Biological crystallography. 2006;62:72–82. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Crowder T, Rinaldo-Matthis A, Ho MC, Almo SC, Schramm VL. Transition state analogs of 5'-methylthioadenosine nucleosidase disrupt quorum sensing. Nature chemical biology. 2009;5:251–257. doi: 10.1038/nchembio.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez JA, Luo M, Singh V, Li L, Brown RL, Norris GE, Evans GB, Furneaux RH, Tyler PC, Painter GF, et al. Picomolar inhibitors as transition-state probes of 5'-methylthioadenosine nucleosidases. ACS chemical biology. 2007;2:725–734. doi: 10.1021/cb700166z. [DOI] [PubMed] [Google Scholar]
- Jancarik J, Kim SH. Sparse-Matrix Sampling - a Screening Method for Crystallization of Proteins. J Appl Crystallogr. 1991;24:409–411. [Google Scholar]
- Krissinel E. Crystal Contacts as Nature's Docking Solutions. J Comput Chem. 2010;31:133–143. doi: 10.1002/jcc.21303. [DOI] [PubMed] [Google Scholar]
- Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. Journal of molecular biology. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. Journal of chemical information and modeling. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Lee JE, Cornell KA, Riscoe MK, Howell PL. Structure of E. coli 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase reveals similarity to the purine nucleoside phosphorylases. Structure. 2001;9:941–953. doi: 10.1016/s0969-2126(01)00656-6. [DOI] [PubMed] [Google Scholar]
- Lee JE, Cornell KA, Riscoe MK, Howell PL. Structure of Escherichia coli 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase inhibitor complexes provide insight into the conformational changes required for substrate binding and catalysis. The Journal of biological chemistry. 2003;278:8761–8770. doi: 10.1074/jbc.M210836200. [DOI] [PubMed] [Google Scholar]
- Lee JE, Luong W, Huang DJ, Cornell KA, Riscoe MK, Howell PL. Mutational analysis of a nucleosidase involved in quorum-sensing autoinducer-2 biosynthesis. Biochemistry. 2005a;44:11049–11057. doi: 10.1021/bi050493q. [DOI] [PubMed] [Google Scholar]
- Lee JE, Smith GD, Horvatin C, Huang DJ, Cornell KA, Riscoe MK, Howell PL. Structural snapshots of MTA/AdoHcy nucleosidase along the reaction coordinate provide insights into enzyme and nucleoside flexibility during catalysis. Journal of molecular biology. 2005b;352:559–574. doi: 10.1016/j.jmb.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Leslie AGW, Powell HR. Processing Diffraction Data with Mosflm. In: Read RJS, L J, editors. Evolving Methods for Macromolecular Crystallography. NATO Science Series: Springer Netherlands; 2007. pp. 41–51. [Google Scholar]
- Lewandowicz A, Tyler PC, Evans GB, Furneaux RH, Schramm VL. Achieving the ultimate physiological goal in transition state analogue inhibitors for purine nucleoside phosphorylase. The Journal of biological chemistry. 2003;278:31465–31468. doi: 10.1074/jbc.C300259200. [DOI] [PubMed] [Google Scholar]
- Levitt M, Perutz MF. Aromatic rings act as hydrogen bond acceptors. Journal of molecular biology. 1988;201:751–754. doi: 10.1016/0022-2836(88)90471-8. [DOI] [PubMed] [Google Scholar]
- McGaughey GB, Gagne M, Rappe AK. pi-Stacking interactions. Alive and well in proteins. The Journal of biological chemistry. 1998;273:15458–15463. doi: 10.1074/jbc.273.25.15458. [DOI] [PubMed] [Google Scholar]
- Merkler DJ, Brenowitz M, Schramm VL. The rate constant describing slow-onset inhibition of yeast AMP deaminase by coformycin analogues is independent of inhibitor structure. Biochemistry. 1990;29:8358–8364. doi: 10.1021/bi00488a023. [DOI] [PubMed] [Google Scholar]
- Meyer E. Internal water molecules and H-bonding in biological macromolecules: a review of structural features with functional implications. Protein science : a publication of the Protein Society. 1992;1:1543–1562. doi: 10.1002/pro.5560011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta crystallographica Section D, Biological crystallography. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Parsek MR, Val DL, Hanzelka BL, Cronan JE, Jr., Greenberg EP. Acyl homoserine-lactone quorum-sensing signal generation. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4360–4365. doi: 10.1073/pnas.96.8.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parveen N, Cornell KA. Methylthioadenosine/S-adenosylhomocysteine nucleosidase, a critical enzyme for bacterial metabolism. Molecular microbiology. 2011;79:7–20. doi: 10.1111/j.1365-2958.2010.07455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg AE, Williams-Ashman HG. Phosphate-stimulated brdown of 5'-methylthioadenosine by rat ventral prostate. The Biochemical journal. 1969;115:241–247. doi: 10.1042/bj1150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raina A, Tuomi K, Pajula RL. Inhibition of the synthesis of polyamines and macromolecules by 5'-methylthioadenosine and 5'-alkylthiotubercidins in BHK21 cells. The Biochemical journal. 1982;204:697–703. doi: 10.1042/bj2040697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronning DR, Iacopelli NM, Mishra V. Enzyme-ligand interactions that drive active site rearrangements in the Helicobacter pylori 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase. Protein science : a publication of the Protein Society. 2010;19:2498–2510. doi: 10.1002/pro.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm VL. Enzymatic transition states and transition state analogues. Current opinion in structural biology. 2005a;15:604–613. doi: 10.1016/j.sbi.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Schramm VL. Enzymatic transition states: thermodynamics, dynamics and analogue design. Archives of biochemistry and biophysics. 2005b;433:13–26. doi: 10.1016/j.abb.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Schuttelkopf AW, van Aalten DM. PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta crystallographica Section D, Biological crystallography. 2004;60:1355–1363. doi: 10.1107/S0907444904011679. [DOI] [PubMed] [Google Scholar]
- Shapiro SK, Mather AN. The enzymatic decomposition of S-adenosyl-L-methionine. The Journal of biological chemistry. 1958;233:631–633. [PubMed] [Google Scholar]
- Singh V, Evans GB, Lenz DH, Mason JM, Clinch K, Mee S, Painter GF, Tyler PC, Furneaux RH, Lee JE, et al. Femtomolar transition state analogue inhibitors of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli. J Biol Chem. 2005a;280:18265–18273. doi: 10.1074/jbc.M414472200. [DOI] [PubMed] [Google Scholar]
- Singh V, Lee JE, Nunez S, Howell PL, Schramm VL. Transition state structure of 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase from Escherichia coli and its similarity to transition state analogues. Biochemistry. 2005b;44:11647–11659. doi: 10.1021/bi050863a. [DOI] [PubMed] [Google Scholar]
- Singh V, Luo M, Brown RL, Norris GE, Schramm VL. Transition-state structure of neisseria meningitides 5'-methylthioadenosine/S-adenosylhomocysteine nucleosidase. Journal of the American Chemical Society. 2007;129:13831–13833. doi: 10.1021/ja0754204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Schramm VL. Transition-state analysis of S. pneumoniae 5'-methylthioadenosine nucleosidase. Journal of the American Chemical Society. 2007;129:2783–2795. doi: 10.1021/ja065082r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V, Shi W, Almo SC, Evans GB, Furneaux RH, Tyler PC, Painter GF, Lenz DH, Mee S, Zheng R, et al. Structure and inhibition of a quorum sensing target from Streptococcus pneumoniae. Biochemistry. 2006;45:12929–12941. doi: 10.1021/bi061184i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K, Haapalainen AM, Evans GB, Burgos ES, Tyler PC, Gulab SA, Guan R, Schramm VL. "Femtomolar Transition State Analogues Bind to MTANs with Favorable Enthalpy and Entropy". Biochemistry. 2012 doi: 10.1021/bi3009938. [DOI] [PubMed] [Google Scholar]
- Vagin A, Teplyakov A. MOLREP: an automated program for molecular replacement. J Appl Crystallogr. 1997;30:1022–1025. [Google Scholar]
- Wallace AC, Laskowski RA, Thornton JM. LIGPLOT: a program to generate schematic diagrams of protein-ligand interactions. Protein engineering. 1995;8:127–134. doi: 10.1093/protein/8.2.127. [DOI] [PubMed] [Google Scholar]
- Wang S, Haapalainen AM, Yan F, Du Q, Tyler PC, Evans GB, Rinaldo-Matthis A, Brown RL, Norris GE, Almo SC, et al. A Picomolar Transition State Analogue Inhibitor of MTAN as a Specific Antibiotic for Helicobacter pylori. Biochemistry. 2012a;51:6892–6894. doi: 10.1021/bi3009664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lim J, Thomas K, Yan F, Angeletti RH, Schramm VL. A complex of methylthioadenosine/S-adenosylhomocysteine nucleosidase, transition state analogue, and nucleophilic water identified by mass spectrometry. Journal of the American Chemical Society. 2012b;134:1468–1470. doi: 10.1021/ja211176q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers H, Swift S, Williams P. Quorum sensing as an integral component of gene regulatory networks in Gram-negative bacteria. Current opinion in microbiology. 2001;4:186–193. doi: 10.1016/s1369-5274(00)00187-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.