Summary
The anaphase-promoting complex/cyclosome (APC/C) regulates sister chromatid segregation and the exit from mitosis. Selection of most APC/C substrates is controlled by coactivator subunits (either Cdc20 or Cdh1) that interact with substrate destruction motifs—predominantly the destruction (D) box and KEN box degrons. How coactivators recognize D box degrons and how this is inhibited by APC/C regulatory proteins is not defined at the atomic level. Here, from the crystal structure of S. cerevisiae Cdh1 in complex with its specific inhibitor Acm1, which incorporates D and KEN box pseudosubstrate motifs, we describe the molecular basis for D box recognition. Additional interactions between Acm1 and Cdh1 identify a third protein-binding site on Cdh1 that is likely to confer coactivator-specific protein functions including substrate association. We provide a structural rationalization for D box and KEN box recognition by coactivators and demonstrate that many noncanonical APC/C degrons bind APC/C coactivators at the D box coreceptor.
Highlights
-
•
First structure of a D box motif bound to an APC/C coactivator
-
•
Rationalization of D box and KEN box consensus sequences
-
•
Assignment of some noncanonical APC/C degrons as either degenerate D or KEN boxes
-
•
Binding site on coactivator for binding regulatory proteins and substrates
Introduction
The anaphase-promoting complex/cyclosome (APC/C) is a large cullin-RING E3 ubiquitin ligase responsible for regulating progression through the mitotic phase of the cell cycle and controlling entry into S phase (Barford, 2011; Peters, 2006; Pines, 2011; Thornton and Toczyski, 2006). The temporal regulation of APC/C-mediated ubiquitin-dependent proteolysis of specific cell cycle proteins is primarily determined by its interactions with two coactivator proteins, the paralogs Cdc20 and Cdh1. Cdc20 activates the APC/C early in mitosis and, through the ubiquitination of securin and mitotic cyclins, promotes the metaphase-to-anaphase transition. Cdh1 associates with the APC/C in late mitosis and during G1.
Coactivators function as adaptors to recruit substrates to the APC/C by their capacity to recognize two conserved APC/C degrons present in most APC/C substrates: the nine-residue D (destruction) box (Glotzer et al., 1991; King et al., 1996) and KEN box (Pfleger and Kirschner, 2000). Coactivators are solely responsible for KEN box recognition (Chao et al., 2012); however, the D box degron is engaged by a bipartite coreceptor on the APC/C-coactivator complex generated from both the coactivator and the core APC/C subunit Apc10 (Carroll et al., 2005; Chao et al., 2012; da Fonseca et al., 2011; Hilioti et al., 2001; Kraft et al., 2005). The coactivator’s WD40 β-propeller domain interacts with D box and KEN box degrons (Chao et al., 2012; Kraft et al., 2005), whereas its N-terminal C box stimulates APC/C catalytic activity (Kimata et al., 2008a; Labit et al., 2012).
To a large extent, the processes underlying regulation of APC/C activity are exerted through its coactivators. Phosphorylation controls the association of coactivators with the APC/C, and ubiquitination controls Cdc20 abundance (Foe et al., 2011). Cyclin-dependent kinase (CDK)-mediated phosphorylation of Cdh1 blocks APC/C association, and its dephosphorylation in late mitosis promotes formation of an APC/CCdh1 complex. APC/CCdh1 contributes to Cdc20 ubiquitination, augmenting Cdc20 degradation due to its autoubiquitination (Foe et al., 2011). Cdc20 is also regulated by phosphorylation, with dephosphorylation of specific Cdc20 residues being required for C box-dependent APC/C activation (Labit et al., 2012).
Regulation of APC/C activity is intimately associated with inhibition of substrate recognition by Cdc20 and Cdh1 by means of regulatory proteins that incorporate pseudosubstrate inhibitory motifs to interact with degron recognition sites on coactivators. The mitotic checkpoint complex (MCC), a multimer of Cdc20, Mad2, Mad3/BubR1, and Bub3, is the effector of the spindle assembly checkpoint (Kim and Yu, 2011; Musacchio and Salmon, 2007). A KEN box motif in Mad3/BubR1 engages the KEN box recognition site of Cdc20, blocking KEN box-dependent substrate recognition (Burton and Solomon, 2007; Chao et al., 2012; King et al., 2007; Malureanu et al., 2009; Sczaniecka et al., 2008). In the context of the APC/CMCC complex, D box recognition is prevented through displacement of Cdc20, thereby disrupting formation of the D box coreceptor (Chao et al., 2012; Herzog et al., 2009). The vertebrate protein Emi1 inhibits the APC/C through the combination of its conserved D box and a zinc-binding region, which provide strong APC/C-binding affinity to block substrate binding and antagonizing APC/C E3 ligase activity, respectively (Miller et al., 2006).
The concept of inhibiting APC/C-coactivator complexes through a pseudosubstrate mechanism also applies to Acm1 (APC/C Cdh1 modulator 1), an S. cerevisiae inhibitor of Cdh1 but not Cdc20 (Enquist-Newman et al., 2008). Phosphorylated Acm1 functions with the 14-3-3 proteins Bmh1 and Bmh2, forming stable stoichiometric complexes that inhibit APC/CCdh1 by blocking the interaction of Cdh1 with substrates (Dial et al., 2007; Martinez et al., 2006). Acm1-Bmh1/Bmh2 complexes contribute to nuclear positioning and spindle morphology by preventing Cdh1 localization to the bud neck, thereby inhibiting Cdh1 from interacting with the protein kinase Hsl1 (Martinez et al., 2012). Levels of Acm1 are cell-cycle regulated, with the protein appearing at G1/S and disappearing in late mitosis. Cdc28-dependent phosphorylation of T161 promotes binding of Bmh1 and Bmh2 that contributes to Acm1 stability (Hall et al., 2008; Ostapenko et al., 2008).
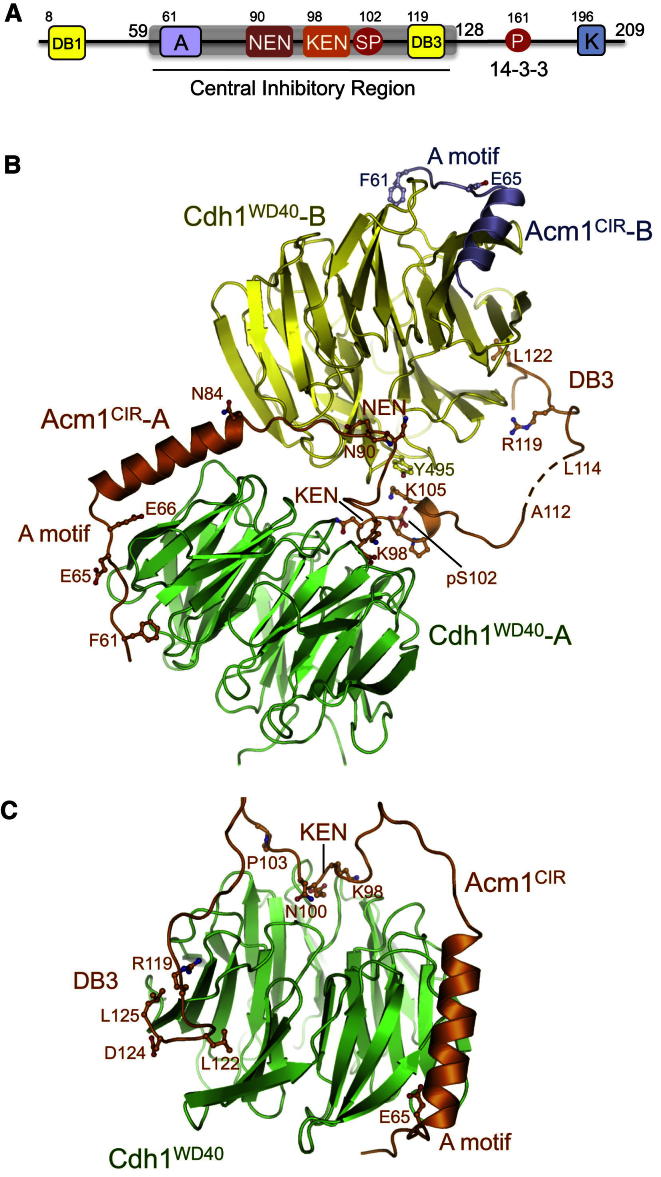
A central inhibitory region (CIR) of Acm1 (residues 59–128) incorporating both a KEN box and a D box (DB3) (Figure 1A) is mainly responsible for the inhibitory properties of Acm1 (Burton et al., 2011; Choi et al., 2008; Enquist-Newman et al., 2008; Ostapenko et al., 2008). These two motifs mediate interactions of Acm1 with the WD40 domain of Cdh1 (Choi et al., 2008; Martinez et al., 2006), indicating that they function as pseudosubstrate motifs, blocking the interaction of substrate with Cdh1. High-affinity Acm1-Cdh1 interactions and optimal APC/CCdh1 inhibition also requires the A motif, a conserved region N-terminal to the KEN motif (Burton et al., 2011; Enquist-Newman et al., 2008) (Figure 1A). Acm1 is degraded by APC/CCdc20 during anaphase in a process that requires the N-terminal D box (DB1), which is highly specific for Cdc20 (Enquist-Newman et al., 2008). Interestingly, Acm1 with disrupted DB3 and KEN boxes becomes a potent substrate of APC/CCdh1 (Burton et al., 2011; Enquist-Newman et al., 2008; Hall et al., 2008).
Figure 1.
Structure of Acm1CIR-Cdh1WD40
(A) Schematic of S. cerevisiae Acm1. A, A motif; DB1, N-terminal D box; NEN, 90N-91E-92N; KEN and DB3, pseudosubstrate KEN and D box (DB3) inhibitory motifs; SP, S102 CDK2 phosphorylation site; P, pT161-14-3-3 binding site; K, K motif.
(B) Acm1CIR-Cdh1WD40 heterotetramer. F113 of Acm1CIR-A was not located in the electron density maps and is represented with a dashed line.
(C) Model for the Acm1CIR-Cdh1WD40 heterodimer. A 13 residue linker was modeled between P103 (KEN) and R119 (DB3) (separated by 15 residues), indicating that it is stereochemically possible for the KEN box and DB3 motif of Acm1 to interact with their respective sites on the same Cdh1 molecule. K123 of the DB3 was omitted for clarity. See also Figure S1.
Regulatory proteins harboring pseudosubstrate motifs bind coactivators with high affinity; thus, they provide a means by which to investigate the molecular details of degron recognition by coactivators. A crystal structure of S. pombe MCC indicated how the KEN motif of Mad3 interacts with and blocks the KEN box recognition site of Cdc20 (Chao et al., 2012). To understand better the mechanisms of D box recognition by coactivators, we have determined the crystal structure of the Cdh1 WD40 domain (Cdh1WD40) in complex with the CIR of Acm1 (Acm1CIR). From the structure, we reveal details of how coactivator interacts with the majority of the D box residues, rationalizing their conservation. We show that mechanisms of KEN box recognition are likely to be universal, and we rationalize noncanonical KEN and D box degrons. Details of how the Acm1 A motif interacts with Cdh1 provide structural evidence that, in addition to D box and KEN box-binding sites, coactivators utilize additional recognition sites to bind regulatory proteins and (potentially) substrates.
Results and Discussion
Acm1 Contacts Cdh1 through Multiple Motifs
The CIR of Acm1 (residues 59–128; Acm1CIR) (Figure 1A) was coexpressed with the WD40 domain of Cdh1 (Cdh1WD40) in insect cells, and the structure of the complex was determined at 2.9 Å resolution (Table 1). This revealed an asymmetric heterotetramer comprising two copies of both Acm1CIR and Cdh1WD40 (Figure 1B). Acm1CIR lacks the 14-3-3 recognition site residue pT161 (Hall et al., 2008); thus, isolation of a stable Acm1CIR-Cdh1WD40 complex without 14-3-3 is consistent with the observation that Acm1–Cdh1 interactions do not require 14-3-3 proteins (Dial et al., 2007; Hall et al., 2008; Martinez et al., 2006).
Table 1.
Data Collection and Refinement Statistics
| Data Collection Statistics | |
|---|---|
| Beamline | DLS IO4-1 |
| Wavelength (Å) | 0.9173 |
| Space group | C2 |
| a (Å) | 198.43 |
| b (Å) | 188.14 |
| c (Å) | 93.44 |
| β (°) | 92.13 |
| Z (molecules per asymmetric unit) | 8 × Cdh1 + 8 × Acm1 |
| Resolution (Å) | 93.38–2.90 (3.06–2.90) |
| Rmerge | 0.102 (0.552) |
| I/σ (I) | 8.0 (1.9) |
| Observed reflections | 206,547 (31,519) |
| Unique reflections | 72,799 (10,826) |
| Completeness (%) | 96.2 (98.0) |
| Multiplicity | 2.8 (2.9) |
| Refinement Statistics | |
| Resolution limits (Å) | 69.27–2.90 |
| Rwork | 0.2243 |
| Rfree | 0.2581 |
| Number of atoms | 21496 |
| Mean B-factors (Å2) | 65.80 |
| Rmsd from Ideal Values | |
| Bond length (Å) | 0.0135 |
| Bond angles (°) | 1.583 |
| Ramachandran Plot Statistics | |
| Preferred (%) | 94.0 |
| Allowed (%) | 4.2 |
| Outliers (%) | 1.8 |
In the Acm1CIR-Cdh1WD40 heterotetramer, a single Acm1CIR subunit (termed Acm1CIR-A) mediates interactions between the two Cdh1WD40 molecules (Cdh1WD40-A and Cdh1WD40-B). The two Cdh1WD40 subunits of the heterotetramer are structurally equivalent, and (as expected) the WD40 domain of Cdh1, a seven-bladed β-propeller, shares an identical conformation to its counterpart in Cdc20 (Chao et al., 2012) (Figure S1 available online). In contrast, the two Acm1CIR subunits of the complex differ radically. The polypeptide chain of Acm1CIR-A is well defined for its entire CIR, whereas the second Acm1 molecule (Acm1CIR-B) is only visualized for its N-terminal A motif and adjacent α helix (residues 60–80) (Figure 1B).
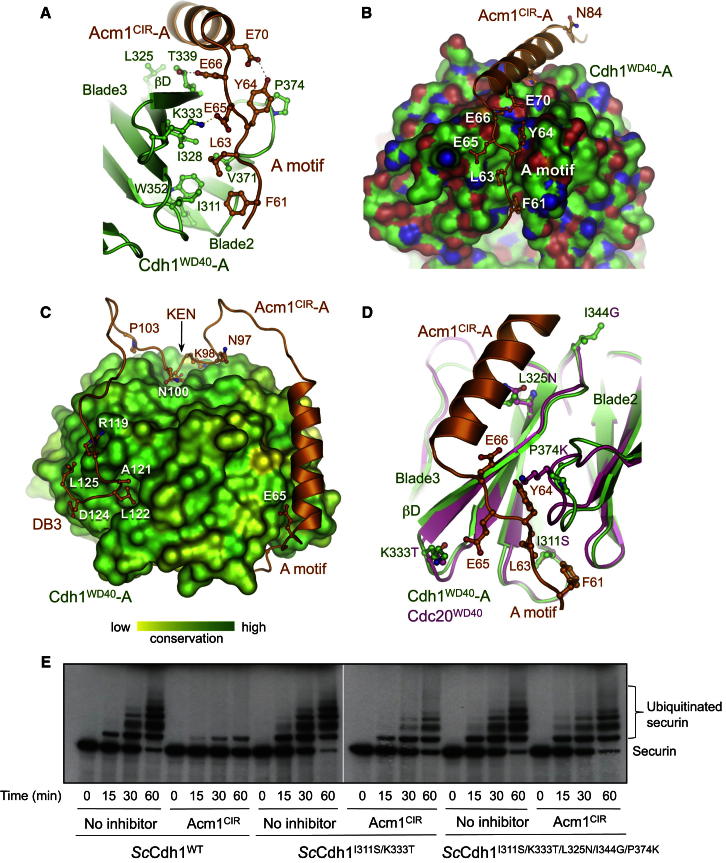
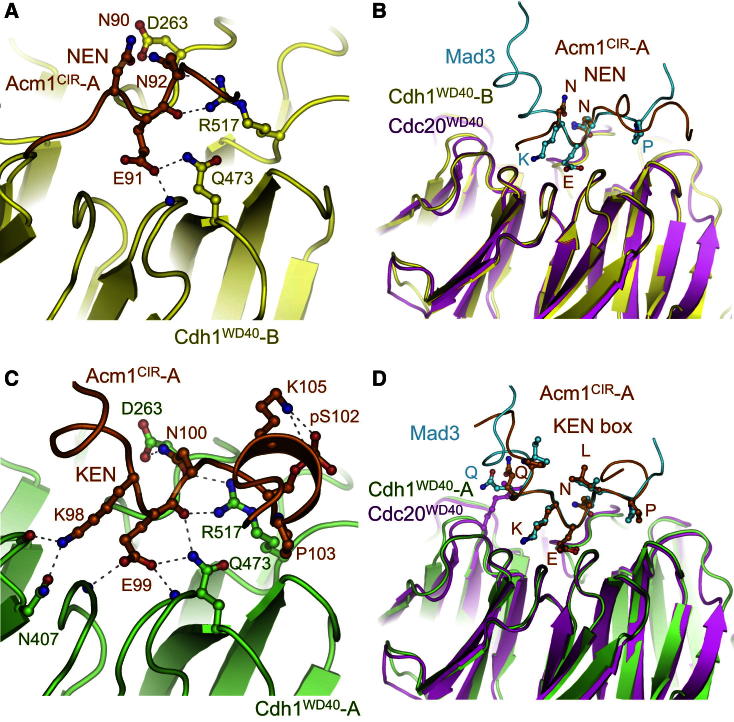
Acm1CIR-A contacts the two Cdh1WD40 molecules through five segments. Its N-terminal A motif (residues 61–70) engages the lower surface of the Cdh1WD40-A toroid, lying in a mainly nonpolar channel between blades 2 and 3 (Figures 1B, 2A, and 2B). The Acm1CIR-A chain then forms an α helix lying parallel to β strand D of blade 3 of Cdh1WD40-A (Figures 1B, 2A, and 2B). Immediately C-terminal to the α helix, Acm1CIR-A contacts Cdh1WD40-B’s KEN box recognition site by means of a highly conserved 90NEN sequence (Enquist-Newman et al., 2008) (Figures 1B and 3A). E91 and N92 of the Acm1 NEN sequence form interactions to Cdh1 that are equivalent to Mad3 KEN box interactions with Cdc20 (Chao et al., 2012) (Figures 3A and 3B).
Figure 2.
The A Motif-Binding Site
(A) Details of A motif-Cdh1WD40 interactions at the interblade groove.
(B) Surface rendition of Cdh1WD40 at the A motif-binding site.
(C) The modeled Acm1CIR-Cdh1WD40 heterodimer with the Cdh1WD40 surface representation colored according to the sequence conservation of Cdc20 and Cdh1. The A motif lies in an interblade channel, which is less well conserved than the D box- and KEN box-binding sites.
(D) Comparison of Cdh1WD40 (green) and Cdc20WD40 (purple) at the A motif-binding site shows why Cdc20 is not inhibited by Acm1. The five residues of Cdh1 that contact the A motif and differ from Cdc20 (I311, L325, K333, I344, and P374) are shown.
(E) Residues of Cdh1 that differ from Cdc20 (shown in C) were replaced with the Cdc20 equivalents, and WT and mutant Cdh1 proteins were tested for their sensitivity to Acm1-mediated inhibition. In vitro APC ubiquitination assays show that the mutant incorporating two key residues (Cdh1I331S/K333T) was poorly inhibited by Acm1 and a mutant incorporating all five residues (Cdh1I331S/K333T/L325N/I344G/P374K) was even less sensitive to Acm1-mediated inhibition.
Figure 3.
The KEN Motif and NEN Sequence Bind to the Conserved KEN Box Site of Coactivators
(A) Details of NEN sequence-Cdh1 interactions.
(B) Comparison of the NEN sequence of Acm1CIR-B bound to Cdh1WD40-B and the KEN motif of S. pombe Mad3 bound to S. pombe Cdc20WD40.
(C) Details of KEN box and CDK phosphorylation site (pS102)-Cdh1 interactions.
(D) Comparison of the KEN motif of Acm1CIR-A bound to Cdh1WD40-A and the KEN motif of S. pombe Mad3 bound to S. pombe Cdc20WD40.
The KEN motif of Acm1CIR-A interacts with the Cdh1WD40-A KEN box receptor (Figures 1B and 3C). Similar to the Mad3 KEN box (Chao et al., 2012), the KEN box in Acm1 is incorporated within an underwound helix (Figures 3C and 3D). P103 of the 98KENXX103P motif contributes to breaking the KEN motif helix. A CDK phosphorylation site overlaps the C terminus of the 98KEN box (102SPAK), with P103 corresponding to P+3 of the KENXXP motif (Figures 1B and 3C). In the crystal structure, electron density showed that S102 is phosphorylated, a modification confirmed by mass spectrometry (data not shown) and consistent with its previous identification in endogenous Acm1 isolated from budding yeast (Hall et al., 2008). By forming a salt bridge with the K105 side chain of the CDK consensus phosphorylation sequence (S/TPXK/R), pS102 stabilizes an incomplete α-helical turn initiated by P103 (Figures 1B and 3C). Participation of pS102 in stabilizing a short α helix resembles the structure of the CDK phosphorylation site in the retinoblastoma protein (Burke et al., 2012), suggesting a potential common structural consequence of CDK phosphorylation.
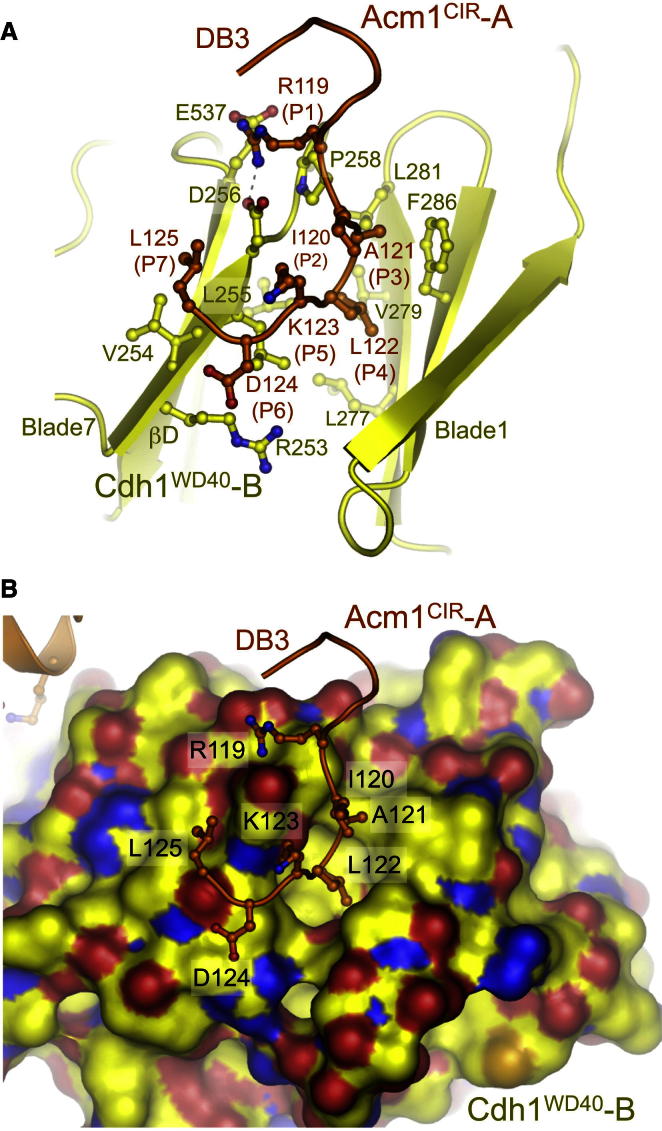
D Box Binds to an Interblade Groove on Cdh1
The region of Acm1CIR-A incorporating the D box motif (DB3) interacts with Cdh1WD40-B at the interface of blades 1 and 7 (Figures 1B and 4). This site is equivalent to that proposed by Chao et al. (2012) as the D box recognition site of Cdc20 in the MCC and, more recently, by (Tian et al., 2012) based on a 2-methyl-2,4-pentandiol (MPD) molecule bound to this site. Seven of the nine consensus D box residues are well defined in the structure, with only the two C-terminal residues not located (Figures 4 and S2). L122 of the D box RXXL motif is anchored in an aliphatic pocket, whereas R119 of the RXXL motif hydrogen bonds with two acidic residues at the top surface of the β-propeller (Figure 4). To anchor L122 within its binding pocket, the D box motif assumes a tight turn between I120(P2) and K123(P5) ([P2] and [P5] refer to the second and fifth positions of the D box, respectively) (Figure 4). The close proximity of these two solvent-exposed residues might exclude bulky amino acids at these positions and explain the predominance of G at P5 of the D box consensus sequence (Glotzer et al., 1991; King et al., 1996) (Figures 5A and S3A).
Figure 4.
D Box Binds to an Interblade Groove in Cdh1
(A) Details of DB3 box-Cdh1 interactions showing the N-terminal seven residues of D box.
(B) Surface rendition of Cdh1 at the D box-binding site. See also Figure S2.
Figure 5.
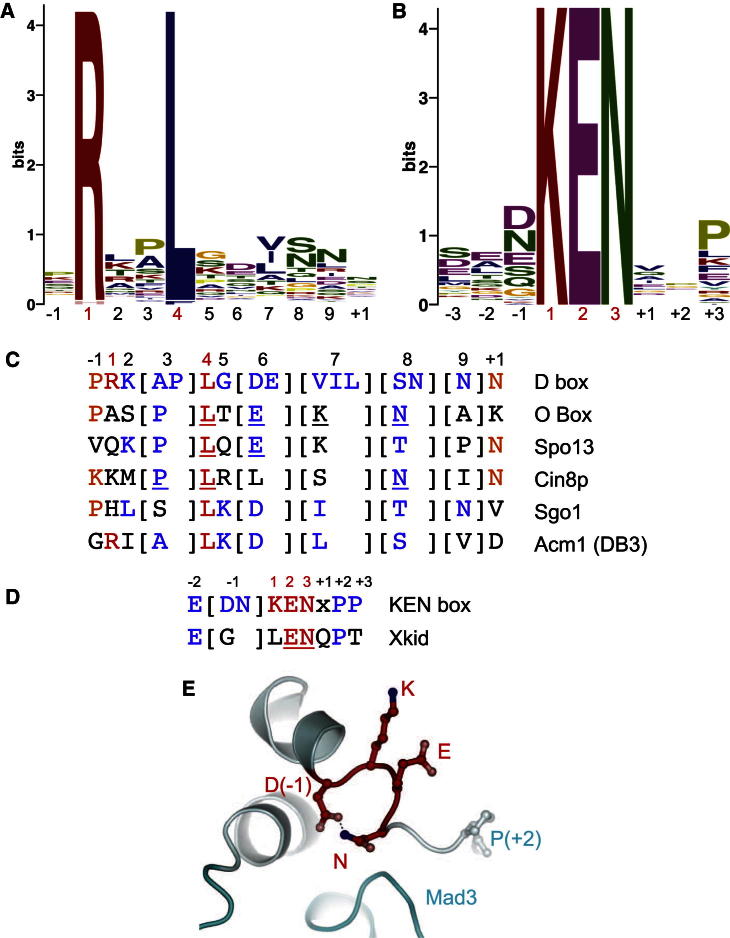
Consensus D Box and KEN Box Motifs and Related Noncanonical APC/C Degrons
(A) Sequence motif of D box derived from 68 APC/C substrates. Sequence motif determined using multiple expectation maximization for motif elicitation (MEME) (Bailey et al., 2009). The D box sequences used are listed in Figure S3A.
(B) Sequence motif of KEN box derived from 46 APC/C substrates. KEN box sequences are listed in Figure S3B.
(C) Comparison of consensus D box with O box degron (Araki et al., 2005), the S. cerevisiae Spo13 degron (Sullivan and Morgan, 2007), the S. cerevisiae Cin8p degron (Hildebrandt and Hoyt, 2001), the D box of human Sgo1 (Karamysheva et al., 2009), and DB3 of Acm1. Underlined residues are essential for APC/C-mediated degradation. Red: D box consensus R and L residues; blue: D box consensus residues; orange: consensus P−1 and P+1 residues.
(D) Comparison of consensus KEN box with the GxEN degron of Xkid (Castro et al., 2003). Red: invariant residues; blue: conserved residues.
(E) Structural rationale for conservation of D or N at P−1 of KEN motif. Side chain of D or N stabilizes the KEN box conformation through a hydrogen bond with the amide side chain of the KEN box N residue. Modeled on the KEN box of Mad3 (Chao et al., 2012).
The methyl group of A121(P3) of the D box is directed toward the coactivator surface to form a hydrophobic contact with the phenyl ring of F286 of Cdh1 (Figure 4). F or Y at this position is conserved in all Cdc20 and Cdh1 homologs (Chao et al., 2012), perhaps explaining the preference for the nonbulky A, P, and S amino acids at this D box position (Figures 5A and S3A). The carboxylate of D124(P6) forms a long hydrogen bond to R253 of Cdh1, rationalizing why D and E are the two most common residues at P6 of the D box (Figures 5A and S3A). Finally, the aliphatic side chain of L125(P7) interacts with a conserved nonpolar patch on Cdh1, explaining the propensity of V, I, and L at this D box position (Figures 5A and S3A). Interestingly, K is the fifth most frequent residue at P7, and modeling shows that its aliphatic moiety would contact the nonpolar surface, whereas its charged amino group would be positioned to contact the acidic cluster of the R(P1)-binding site. Residues P8 and P9 of the Acm1 D box (S and V, respectively) are disordered. S is a preferred residue at P8, whereas N is preferred and V is rare at P9 (Glotzer et al., 1991; King et al., 1996) (Figures 5A and S3A). Thus, the disorder at the C terminus of the D box could result from a nonconsensus residue at P9. Alternatively, the D box coreceptor Apc10 may contribute to the ordering of the D box C terminus.
Disrupting either the L-binding pocket or the R-binding site abrogated the ability of APC/CCdh1 to ubiquitinate securin (a D box-dependent substrate) (Chao et al., 2012), consistent with this model for D box recognition. A cluster of four residues of human Cdh1 was previously implicated in D box recognition (Kraft et al., 2005). Our structure rationalizes two of these residues: L255 (S. cerevisiae numbering) contacts the side chain of the D box L residue, whereas P258 contacts the aliphatic moiety of the D box R (Figure 4A).
The A Motif Contributes to Specificity for Cdh1
Due to the asymmetric heterotetramer, only the A motif and adjacent α helix (residues 61–80) of Acm1CIR-B bind Cdh1WD40-B (Figure 1B). This indicates that the A motif and its immediate flanking regions are sufficient to mediate Acm1-Cdh1 association. Acm1CIR was previously shown to bind Cdh1 independently of the KEN box and DB3 (Burton et al., 2011). E65 and E66 of the A motif contribute to Acm1CIR interactions with Cdh1 (residues 1–128) (Burton et al., 2011), in agreement with findings that residues 59–69 of Acm1 are required for high-affinity Cdh1 binding (Enquist-Newman et al., 2008). The interaction of the A motif with a noncanonical binding site on Cdh1, suggesting the possibility that the A motif could contribute as an APC/CCdh1 degron, is supported by a study showing that APC/CCdh1-mediated ubiquitination of Acm1 (residues 1–128) with mutated DB3 and KEN box motifs, but retaining DB1, is abolished by disrupting the A motif (Figure 4B by Burton et al., 2011).
To explain why Acm1 is not a Cdc20 inhibitor (Enquist-Newman et al., 2008), we analyzed Acm1 contacts to residues specific to Cdh1. We focused on the A motif-binding site of Cdh1, which is less well conserved than the D box- and KEN box-binding sites (Figure 2C). Interactions at this site include a salt bridge linking E65 of Acm1 with K333 of Cdh1 and hydrophobic contacts between F61 and Y64 of Acm1 with I311 and P374 of Cdh1, respectively (Figure 2D). To test the roles of I311 and K333 in conferring Acm1-specific inhibition of Cdh1, we mutated these residues to their counterparts in Cdc20. Although the resulting mutant (Cdh1I311S,K333T) was still able to activate the APC/C to ubiquitinate securin, this activity was now only poorly inhibited by Acm1 (Figure 2E). A second Cdh1 mutant, incorporating additional mutations of Acm1-contacting residues of Cdh1 to their counterparts in Cdc20 (L325N, I344G, and P374K), although still active, was even less sensitive to Acm1-mediated inhibition (Figures 2D and 2E).
These data indicate that the Acm1 A motif contributes to determining Acm1-specificity for Cdh1. However, it is also possible that other regions of Acm1, such as the K box, may also contribute to its specificity for Cdh1 (Figure 1A) (Burton et al., 2011).
Acm1CIR-Cdh1 Heterodimers Mediate APC/C Inhibition
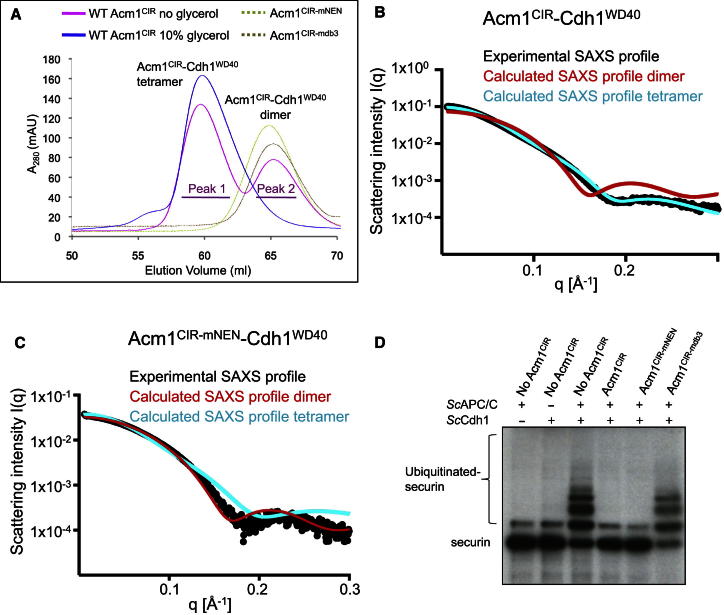
To understand the biological relevance of the Acm1CIR-Cdh1WD40 heterotetramer, we determined the size of Acm1-Cdh1WD40 complexes in solution. Acm1CIR-Cdh1WD40 at ∼1 mg/ml exists as a mixture of heterotetrameric and heterodimeric species as assessed by multiangle light scattering (MALS) (Figures S4A and S4B). Glycerol promotes the conversion of heterodimers into heterotetramers, as shown by size-exclusion chromatography (Figure 6A) and MALS (Figures S4C and S4D). However, similar MALS analysis of a longer fragment of Acm1 (residues 59–209; Acm1ΔN) with a disrupted 14-3-3-binding site (Acm1ΔN-T161A-Cdh1WD40) indicated only a heterodimeric species in glycerol buffer (Figures S4E and S4F). When we coexpressed wild-type (WT) Acm1ΔN with Cdh1WD40 in insect cells, we isolated a complex bound to insect cell 14-3-3 proteins (Figure S4G). The mass of this complex, determined using analytical ultracentrifugation (AUC) at 1.5 mg/ml, is consistent with two Acm1ΔN-Cdh1WD40 heterodimers bound to a 14-3-3 dimer (Figure S4H). To further investigate the structure of Acm1-Cdh1WD40 complexes in solution, we performed small-angle X-ray scattering (SAXS) on Acm1CIR-Cdh1WD40 at a concentration of >10 mg/ml. The experimental SAXS profile closely matches the computed SAXS profile for the Acm1CIR-Cdh1WD40 tetramer (Figure 6B).
Figure 6.
Oligomeric States of Acm1-Cdh1WD40
(A) Size-exclusion chromatography of WT Acm1CIR-Cdh1WD40 without glycerol shows heterotetramers (peak 1) and heterodimers (peak 2). Glycerol (10% v/v) promotes heterotetramers. Associated SDS-PAGE gels are shown in Figures S4B and S4D. Only heterodimers are observed when either the NEN sequence (Acm1CIR-mNEN-Cdh1WD40) or DB3 is disrupted (Acm1CIR-mdb3-Cdh1WD40). The latter two samples were run with 10% (v/v) glycerol. Associated SDS-PAGE gels shown in Figures S4I and S4J.
(B and C) SAXS profiles for Acm1CIR-Cdh1WD40 and Acm1CIR-mNEN-Cdh1WD40 complexes. (B) Experimental SAXS profile (black dots) for Acm1CIR-Cdh1WD40 compared with computed SAXS profiles for the Acm1CIR-Cdh1WD40 tetramer (cyan) and dimer (red). It shows a good fit to the computed SAXS profile of the tetramer (χ2 = 12) but is a poor match to the computed SAXS profile of the dimer (χ2 = 93). The experimental radius of gyration (Rg) for Acm1CIR-Cdh1WD40 is 31 Å, and the calculated radii of gyration for tetramer and dimer models are 28 Å and 20 Å, respectively. The scattering vector q = 4πsin(θ)/λ, where θ is half the scattering angle. (C) Experimental SAXS profile (black dots) of Acm1CIR-mNEN-Cdh1WD40 shows a good fit to the computed SAXS profile of the dimer (χ2 = 3.7) but is a poor match to the computed SAXS profile of the tetramer (χ2 = 9.6). Experimental radius of gyration is 25 Å.
(D) In vitro APC ubiquitination assays with 35S-labeled S. cerevisiae securin and IVT-produced ScCdh1 and WT and mutant Acm1CIR. Acm1CIR-mediated inhibition is abolished by mutation of the DB3 (Acm1CIR-mdb3). In contrast to DB3 mutations, disruption of the NEN sequence (Acm1CIR-mNEN) does not impair Acm1CIR-mediated inhibition of APC/CCdh1 ubiquitination of securin.
To test whether Cdh1-Acm1CIR dimers are sufficient to mediate APC/CCdh1 inhibition, we mutated the NEN sequence of Acm1CIR (Acm1CIR-mNEN), promoting heterodimers as judged by SAXS and size-exclusion chromatography (Figures 6A, 6C, and S4I). The experimental SAXS profile of Acm1CIR-mNEN matches closely to the computed SAXS profile for the modeled Acm1CIR-Cdh1WD40 dimer (Figure 1C). Acm1CIR-mNEN is as effective as WT Acm1CIR at inhibiting APC/CCdh1 (Figure 6D), whereas Acm1CIR-mdb3 (Acm1CIR with a disrupted DB3), which also destabilizes heterotetramers in favor of heterodimers similar to Acm1CIR-mNEN (Figure 6A), is defective as an Acm1 inhibitor (Figures 6D and S4J). Thus, Acm1CIR-Cdh1WD40 heterotetramers are not relevant to Acm1-mediated inhibition of Cdh1. Together, these data indicate that the biologically relevant form of Acm1-Cdh1 is most likely the heterodimer and that the heterotetrameric assembly of Acm1CIR-Cdh1WD40, as observed in the crystal structure, is promoted by high unphysiological protein concentrations and the absence of the Acm1’s C-terminal 81 residues, possibly due to loss of the K motif in Acm1CIR (Figure 1A) (Burton et al., 2011).
Some APC/C Degrons Are Degenerate D and KEN Boxes
These structural data provide a basis for understanding how coactivators recognize D box and KEN box degrons and thus a rationale for D box and KEN box consensus residues. We generated revised D box and KEN box consensus sequences by aligning 68 D box and 46 KEN box motifs, previously published and validated by mutagenesis (Figures 5A, 5B, and S3). This identifies a consensus sequence flanking the KEN residues (Figure 5B) with D and N, the most frequent residues, immediately preceding the KEN residues (position P−1). Model building indicates that either residue could promote and stabilize the KEN box conformation through a side-chain-hydrogen bond to N of the KEN motif (Figure 5E). A consensus P at P+3 and (at lower conservation) at P+2 (Figure 5B) breaks the α helix incorporating the KEN box and directs the polypeptide chain away from the coactivator surface. The similar helical conformations of the KEN box motifs and immediate flanking residues of both Mad3 and Acm1 bound to Cdc20 and Cdh1, respectively (Figure 3D), would suggest that most, if not all, KEN boxes bind coactivators through a similar mechanism.
Our revised D box and KEN box consensus motifs, combined with a structural rationale for conserved residues, prompted us to examine whether nonconsensus APC/C degrons may represent noncanonical D box and KEN box motifs. We note that the consensus D box features a P at P−1 and an N at P+1, thereby extending the D box motif to eleven residues (Figure 5A). The O box identified as an APC/C degron in Orc1 closely matches the D box consensus, except that R(P1) is replaced by A (Figure 5C) (Araki et al., 2005). The O box potentially may bind the D box receptor, consistent with the ability of a D box peptide to interfere with O box recognition by APC/CCdh1 (Araki et al., 2005). Sullivan and Morgan described a novel degron in S. cerevisiae Spo13, a protein that lacks identifiable D and KEN box motifs (Sullivan and Morgan, 2007). However, similar to the O box, the Spo13 motif conforms quite closely to a D box, deviating due to a Q at P1 and a P at P9 (Figure 5C). Both the O box and the Spo13 degron incorporate a K at P7 that could substitute for the canonical R at P1 and interact with acidic residues at the D box R-binding site. Finally, degradation of the spindle motor protein Cin8p depends on a bipartite destruction sequence that comprises a KEN box 22 residues N-terminal to a C-terminal degron that resembles a degenerate D box comprising a conserved and essential L residue (Figure 5C). Cin8p lacks functional canonical D box motifs (Hildebrandt and Hoyt, 2001).
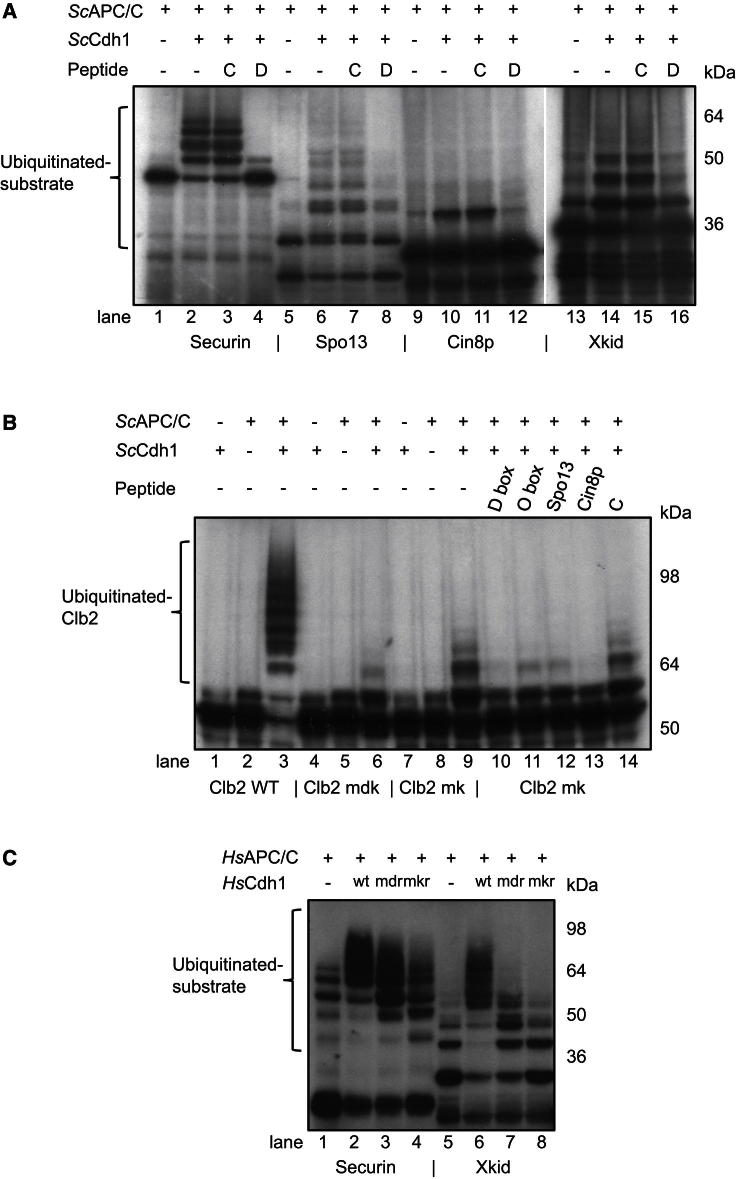
A D box peptide (modeled on Hsl1) inhibits APC/CCdh1-catalyzed ubiquitination of securin, and the same peptide, but not a control peptide, also inhibits ubiquitination of Spo13 and Cin8p (Figure 7A). These results indicate that APC/CCdh1 recognizes all three substrates through its D box coreceptor. To determine whether the Spo13 degron, C-terminal Cin8p degron, and O box are degenerate D boxes, we tested whether peptides incorporating these degrons blocked the ability of APC/CCdh1 to ubiquitinate Clb2. In this assay, we used a KEN box mutant of Clb2 (Clb2mkb) whose ubiquitination is dependent on its recognition by the D box coreceptor. Figure 7B shows that a D box peptide (modeled on Hsl1), but not a control peptide, abrogates Clb2mkb ubiquitination by APC/CCdh1 to a level similar to that of Clb2 with both its D and KEN boxes disrupted (Clb2mdk) (compare lanes 6, 9, 10, and 14). The Cin8p degron peptide strongly reduces Clb2mkb ubiquitination (lane 13), whereas the O box and Spo13 degron peptides less-effectively inhibit Clb2mkb ubiquitination (lanes 11 and 12). These data show that the Cin8p degron is a noncanonical D box degron; interestingly, this degron features a K at P1 of the D box motif. The less-efficient inhibition of Clb2mkb ubiquitination by the O box and Spo13 degron peptides indicates that the O box and Spo13 degron possess a weaker affinity for the D box receptor. These data showing that the Cin8p, O box, and Spo13 degrons interact with the D box receptor, together with the observation that a histidine substitutes for R(P1) in the D box of human shugoshin 1 (Sgo1) (Karamysheva et al., 2009) (Figure 5C), demonstrate that residues other than R can be accommodated at the P1 position of the D box receptor.
Figure 7.
Noncanonical D and KEN Boxes Interact with D and KEN Box Recognition Sites
(A) Ubiquitination of S. cerevisiae securin, S.cerevisiae Spo13, S. cerevisiae Cin8p, and X. laevis Xkid relies on recognition of APC/CCdh1 by the D box coreceptor. Ubiquitination is inhibited by a D box peptide (D) but not by a control peptide (C).
(B) A conventional D box and noncanonical D box peptides (at 1 mM) reduce ubiquitination of 35S-labeled S. cerevisiae Clb2 with a disrupted KEN box (Clb2 mkb) to a level similar to that of Clb2 mdk with disrupted D and KEN boxes in the absence of peptide.
(C) Xkid ubiquitination is dependent on the KEN box recognition site of Cdh1. Ubiquitination is abolished by disrupting either the D box receptor (mdr: HsCdh1V203M) or KEN box receptor (mkr: HsCdh1Q401A/R445L) of Cdh1. WT: wild-type HsCdh1. Human securin is used as a control.
The Acm1CIR-Cdh1WD40 structure shows that the KEN box receptor of Cdh1WD40-B binds a NEN sequence of Acm1, suggesting the possibility that degenerate KEN motifs are recognized as APC/C degrons. Previously, Castro et al. (2003) identified the GxENxP degron in Xkid, which otherwise lacks a canonical KEN box motif. Since the EN and P residues match the consensus KEN motif (Figures 5B and 5D), we considered it possible that the GxENxP motif might bind to the KEN box receptor of the coactivator. In an in vitro ubiquitination assay, we found that glutathione S-transferase (GST) fused to a fragment of Xkid (residues 498–600 incorporating GxENxP) (Castro et al., 2003) was efficiently ubiquitinated by WT Cdh1 but not Cdh1 with a disrupted KEN box receptor (Figure 7C). These data indicate that the GxENxP degron present within residues 498–600 of Xkid requires the KEN box receptor of Cdh1. Figure 7A shows that ubiquitination of the Xkid fragment is also blocked by a D box peptide, in agreement with the findings of Castro et al. (2003) and our data in Figure 7C showing that Cdh1 with a mutant D box receptor ubiquitinates Xkid poorly. Thus, we conclude that efficient Xkid ubiquitination is dependent on both the D and KEN box-binding sites of coactivator.
Conclusions
Our structural analysis of the Acm1CIR-Cdh1WD40 complex reveals the molecular basis for D box recognition by coactivator and demonstrates a mode of KEN box recognition by Cdh1 that is common to Cdc20. We rationalize D box and KEN box consensus sequences and show that noncanonical D box and KEN box motifs are recognized at the D box and KEN box receptors, respectively. The interaction of the Acm1 A motif with Cdh1 reveals a third protein-binding site on Cdh1, a site poorly conserved with Cdc20, and therefore likely to confer coactivator-specific functions. Acm1 inhibits Cdh1 as a heterodimer by sterically blocking D and KEN box recognition. This is similar to how the MCC inhibits KEN box recognition by Cdc20 but differs from the mechanism of D box coreceptor disruption by the MCC (Chao et al., 2012). Since the fission yeast meiotic APC/CCdc20 inhibitor Mes1 has an arrangement of pseudosubstrate KEN and D boxes similar to that of Acm1 (Kimata et al., 2008b), it is likely to exert its effects through a similar mechanism. Furthermore, a meiotic inhibitor of the APC/C in plants, OSD1, containing GxEN and D box motifs separated by 20 residues is also likely to function as a pseudosubstrate inhibitor (Cromer et al., 2012), whereas Emi1 combines both D box-binding and E3 ligase inhibitory mechanisms (Miller et al., 2006).
Experimental Procedures
Preparation of Insect Cell and IVT Expression Vectors
Constructs for Insect Cell Expression
The Saccharomyces cerevisiae Cdh1 WD40 domain (residues 241–550) and Acm1 CIR (residues 59–128) were amplified by PCR and cloned into a modified pFBDM vector (Berger et al., 2004) (Z.Z. and D.B., unpublished data). A double Strep-tag II and a tobacco etch virus (TEV) cleavage site were introduced N-terminal of Cdh1 for affinity purification. A similar approach was used for coexpression of the Cdh1WD40-Acm1 mutants (Acm1CIR-mNEN: Acm1CIR -N90A/E91A/N92A; Acm1CIR-mdb3: Acm1CIR-R119S/L122A-DB3 mutant; Acm1ΔN: Acm1(59–209); Acm1ΔN-T161A: Acm1(59–209)-T161A). Mutagenesis was performed using uracil-specific excision reagent (USER)-based mutagenesis (Bitinaite et al., 2007).
Constructs for IVT-Based Ubiquitination Assays
The S. cerevisiae Cdh1 mutants (ScCdh1I311S/K333T and ScCdh1I311S/K333T/L325N/L344G/P374K), WT Acm1CIR, and mutants (Acm1CIR-mNEN and Acm1CIR-mdb3) were cloned into a pRSET vector. The noncanonical degron substrate S. cerevisiae Spo13 was cloned into pRSET, whereas Xenopus laevis Xkid (residues 498–600) and S. cerevisiae Cin8p (residues 956–1031) were cloned into pRSET with an N-terminal GST tag followed by three K residues. H. sapiens Cdh1 and securin were cloned into pRSET vectors. The KEN box receptor mutants (Q401A and R445L) and D box receptor mutant (V203M) of HsCdh1 (equivalent to Q473A, R517L, and V279M of ScCdh1, respectively; described in Chao et al., 2012) were generated using USER-based mutagenesis (Bitinaite et al., 2007). pRSET vectors for WT S. cerevisiae Cdh1, securin, WT Clb2, and mutant Clb2 (KEN box and combined D box and KEN box) were described by Passmore et al. (2003).
Protein Expression in Insect Cells and Purification
The pFBDM-based construct of the Acm1CIR-Cdh1WD40 complex was used to generate baculovirus that was then amplified to a suitable titer (>1 × 108 plaque-forming units [pfu]/ml). Sf9 cells were grown in Sf-900 II SFM medium (Invitrogen) to a cell density of 2.0 × 106 cells/ml and infected at an moi of 2. The Sf9 cells were harvested after 72 hr, and the cell pellets were frozen at −80°C. To purify the complex, the cell pellets were resuspended and lysed in lysis buffer (50 mM Tris-HCl [pH 8], 300 mM NaCl, 0.4 g/ml DNase, one Protease Inhibitor Cocktail Tablet per 50 ml lysate, 2 mM dithiothreitol (DTT), and 0.5 mM EDTA). The lysate was clarified by centrifugation and subsequently applied to a Strep-Tactin affinity chromatography column (QIAGEN). The bound protein was washed with wash buffer (25 mM Tris-HCl [pH 8], 250 mM NaCl, 2 mM DTT, and 0.5 mM EDTA) and eluted with 2.5 mM desthiobiotin. The N-terminal tag was cleaved using 0.1 mg/ml TEV protease overnight. The complex was further purified by anion-exchange chromatography (RESOURCE Q, GE Healthcare) followed by size-exclusive chromatography (Superdex 75, GE Healthcare) in 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 2 mM DTT, 0.5 mM EDTA, and 10% (v/v) glycerol. The identities of the purified complex subunits were confirmed by mass spectrometry. Acm1CIR-mNEN-Cdh1WD40, Acm1CIR-mdb3-Cdh1WD40, and Acm1ΔN-T161A-Cdh1WD40 were prepared using the same procedure, except that a Superdex 200 column was used for Acm1ΔN-T161A-Cdh1WD40.
Recombinant S. cerevisiae APC/C and recombinant H. sapiens APC/C were prepared as described in Schreiber et al. (2011) and Zhang et al. (2013), respectively.
Crystallization of the Acm1CIR- Cdh1WD40 Complex
Purified Acm1CIR-Cdh1WD40 complex was concentrated to 14 mg/ml and crystallized by sitting-drop vapor diffusion at 4°C in 0.1 M Na cacodylate (pH 6.0), 40% (v/v) MPD, and 3% (w/v) polyethylene glycol (PEG) 8000. Crystals were directly mounted into cryoloops from drops and flash frozen at 100 K for data collection.
Data Collection and Structure Determination
A native data set was collected to 2.9 Å on beamline I04-1 (Diamond Light Source), processed using X-ray detector software (XDS) (Kabsch, 2010), and scaled with Scala (Evans, 2006). Molecular replacement was performed with Phaser (McCoy et al., 2007) using S. pombe Cdc20/Slp1 (Protein Data Bank ID code 4AEZ; Chao et al., 2012) as a search model. Iterative cycles of manual model building and refinement were performed with Coot (Emsley and Cowtan, 2004) and Refmac (Murshudov et al., 2011), respectively. Translation libration screw-motion (TLS) refinement in PHENIX (Adams et al., 2002) was also introduced. The structure was validated with MolProbity (Davis et al., 2007). Data collection and refinement statistics are listed in Table 1. Coordinates were analyzed using COOT (Emsley and Cowtan, 2004), Superpose (Collaborative Computational Project, Number 4, 1994) and PyMol. For analysis and figures, the Acm1CIR-Cdh1WD40 heterotetramer composed of Acm1-A chain I, Acm1-B chain N, Cdh1-A chain A, Cdh1-B chain E was used (Figure S1).
Modeling the Acm1CIR-Cdh1WD40 Dimer
DB3 of Acm1CIR-A-Cdh1WD40-B was superimposed onto Acm1CIR-A-Cdh1WD40-A. The 13 residue linker connecting P103 of the KEN box to R119 of DB3 was modeled using COOT (Emsley and Cowtan, 2004).
SAXS Data Collection and Data Analysis
Acm1CIR-Cdh1WD40 and Acm1CIR-mNEN-Cdh1WD40 complexes were concentrated to approximately 26 mg/ml. SAXS data were collected at the SWING beamline (Synchrotron SOLEIL). The beam size was 400 × 100 μm, the beam energy was 12.4 keV, and the flux was approximately 1012 photons/s. The images were collected using the AVIEV170170 charge-coupled device (CCD) detector, and the SAXS cell to detector distance was 1,892 cm. The samples were loaded in gel filtration buffer using an online high-performance liquid chromatography (HPLC) device and a Shodex KW402.5-4F size-exclusion column. The column and flow cell were maintained at 10°C and the sample holder prior to injection at 4°C. Data were processed using the beamline software FOXTROT, and Guinier analysis was performed using PRIMUS (Konarev et al., 2003). To determine the oligomeric assembly of the Acm1CIR-Cdh1WD40 complex in solution, theoretical SAXS profiles for the heterodimeric and heterotetrameric models of the Acm1CIR-Cdh1WD40 complex were calculated and fitted to the SAXS experimental profiles with the FoXS web server (Schneidman-Duhovny et al., 2010). Radii of gyration (Rg) were determined from the experimental SAXS profile using GNOM (Svergun, 1992), and Rg was calculated using FoXS.
Preparation of the Acm1ΔN-Cdh1WD40-14-3-3 Complex for AUC
Acm1ΔN (residues 59–209) and Cdh1WD40 were coexpressed in insect cells, and the complex was purified as for Cdh1WD40-Acm1CIR but without DTT. The complex was exchanged into AUC buffer (gel filtration buffer without DTT) through size-exclusion chromatography (Superdex 200, GE Healthcare). Eluted fractions were concentrated to 7.5 mg/ml, and flash frozen in liquid nitrogen. AUC runs were performed at a protein concentration of 1.5 mg/ml on a Beckman Coulter XL-I Analytical Ultracentrifuge with interference optics. The run speed was 40,000 rpm at 20°C. We performed 53 scans at 12 min intervals. Sedimentation coefficients and c(M) were determined using the c(s) model within SEDFIT (Schuck, 2000).
Multiple Angle Light Scattering
Purified Acm1CIR-Cdh1WD40 was applied at a concentration of 10 mg/ml to a Superdex 75 analytical gel filtration column (24 ml; GE Healthcare) at a flow rate of 0.5 ml/min in 10 mM Tris-HCl (pH 8.0), 150 mM NaCl, 0.5 mM EDTA, 2 mM DTT, 0.02% (w/v) NaN3 either with or without 10% (v/v) glycerol. Acm1ΔN-T161A-Cdh1WD40 was applied at a concentration of 7 mg/ml to a Superdex 200 analytical gel filtration column (24 ml; GE Healthcare) at a flow rate of 0.5 ml/min in the same buffer with 10% (v/v) glycerol. MALS data were measured at 20°C on the eluate at 658 nm using a DAWN HELEOS light scattering instrument (Eldan) and processed using ASTRA software.
Size-Exclusion Chromatography
Acm1CIR-Cdh1WD40, Acm1CIR-mNEN-Cdh1WD40, and Acm1CIR-mdb3-Cdh1WD40 complexes were loaded at ∼5 mg/ml onto a HiLoad Superdex 75 column in gel filtration buffer at 4°C (except where stated for WT Acm1CIR-Cdh1WD40).
APC/C Ubiquitination Assays
APC/C ubiquitination assays with S. cerevisiae APC/C and S. cerevisiae Cdh1 were adopted and modified from (Passmore et al., 2005) (data in Figures 2E, 6D, 7A, and 7B). 35S-labeled substrates (S. cerevisiae securin [Pds1p], Clb2p [WT, KEN, and D box mutants; described by Passmore et al., 2005], S. cerevisiae Spo13, GST-Cin8p, and X. laevis GST-Xkid) and unlabeled S. cerevisiae WT Cdh1 and mutants (as described above) were prepared in vitro using the TNT T7 Quick Coupled Transcription/Translation System (Promega). Each ubiquitination reaction contains approximately 10 ng of recombinant S. cerevisiae APC/C, 1 μl of 35S-labeled substrate, and 2 μl of Cdh1 in a 10 μl reaction volume with 40 mM Tris-HCl (pH 7.5), 10 mM MgCl2, 0.6 mM DTT, 2.7 mM ATP, 6.6 μg methyl-ubiquitin, 200 ng UBE1, 500 ng S. cerevisiae Ubc4, 200 ng ubiquitin aldehyde (Enzo), and 2 mM LLnL (N-Acetyl-Leu-Leu-Norleu-aldehyde) (Sigma). Reactions were incubated at 22°C for 60 min and analyzed by 10% SDS-PAGE. Gels were fixed and stained with Coomassie blue followed by drying and exposure to BioMax MR Film (Kodak).
APC/C ubiquitination assays with H. sapiens APC/C and H. sapiens Cdh1 were performed using a procedure similar to that for S. cerevisiae APC/C and Cdh1 (Passmore et al., 2005) as described above, except 500 ng UbcH10 was used instead of Ubc4 (data in Figure 7C). 35S-labeled substrates (X. laevis GST-Xkid and H. sapiens securin) and unlabeled H. sapiens Cdh1 and its mutants were prepared in vitro as described above.
APC/C Inhibition Assays
Acm1-Mediated Inhibition with ScAPC/C and ScCdh1
For the Acm1-mediated APC/C inhibition assays, unlabeled WT Acm1CIR and its mutants were prepared in vitro as described above. Acm1CIR was preincubated with in vitro translated (IVT)-generated ScCdh1 or its mutants (1 μl of Cdh1 and 2 μl of Acm1) for 10 min before addition to the reaction mix (10 μl reaction volume). The ubiquitination reactions and analyses were performed as described above.
Peptide-Mediated Inhibition with ScAPC/C and ScCdh1
The following peptides were synthesized by Cambridge Peptides and dissolved in Tris-HCl (pH 7.0) at 10 mM: D box peptide (Hsl1, residues 827–837): Ac-SKRAALSDITNSSD-NH2; O box peptide: Ac-SPASPLTEKNAKSD-NH2; Spo13 peptide: Ac-SVQKPLQEKTPNSD-NH2; Cin8p peptide: Ac-SKKMPLRLSNINSD-NH2; control peptide: Ac-SDSAKSADSASASD-NH2. These peptides correspond to the putative noncanonical D box degrons as defined in Figure 5C (i.e., an eleven residue motif: −1 to +1), flanked by N- and C-terminal serines.
For the peptide-mediated inhibition assays, the ubiquitination reaction was performed as described above, except that peptides were preincubated with ScCdh1 for 10 min at a final concentration of 1 mM.
Acknowledgments
This work was funded by a Cancer Research UK Programme Grant to D.B. and ICR studentships to J.H. and W.C.H.C. We thank the staff at I04 Diamond Light Source (DLS) for help with data collection and J. Pérez of SWING beamline, Synchrotron SOLEIL for help with BioSAXS experiments.
Published: May 23, 2013
Footnotes
Supplemental Information includes four figures and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2013.04.024.
Accession Numbers
Protein coordinates have been deposited in the Protein Data Bank under ID code 4BH6.
Structure factors have been deposited in the Protein Data Bank under ID code R4BH6SF.
Supplemental Information
References
- Adams P.D., Grosse-Kunstleve R.W., Hung L.W., Ioerger T.R., McCoy A.J., Moriarty N.W., Read R.J., Sacchettini J.C., Sauter N.K., Terwilliger T.C. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- Araki M., Yu H., Asano M. A novel motif governs APC-dependent degradation of Drosophila ORC1 in vivo. Genes Dev. 2005;19:2458–2465. doi: 10.1101/gad.1361905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey T.L., Boden M., Buske F.A., Frith M., Grant C.E., Clementi L., Ren J., Li W.W., Noble W.S. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37(Web Server issue):W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barford D. Structural insights into anaphase-promoting complex function and mechanism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:3605–3624. doi: 10.1098/rstb.2011.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger I., Fitzgerald D.J., Richmond T.J. Baculovirus expression system for heterologous multiprotein complexes. Nat. Biotechnol. 2004;22:1583–1587. doi: 10.1038/nbt1036. [DOI] [PubMed] [Google Scholar]
- Bitinaite J., Rubino M., Varma K.H., Schildkraut I., Vaisvila R., Vaiskunaite R. USER friendly DNA engineering and cloning method by uracil excision. Nucleic Acids Res. 2007;35:1992–2002. doi: 10.1093/nar/gkm041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J.R., Hura G.L., Rubin S.M. Structures of inactive retinoblastoma protein reveal multiple mechanisms for cell cycle control. Genes Dev. 2012;26:1156–1166. doi: 10.1101/gad.189837.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.L., Solomon M.J. Mad3p, a pseudosubstrate inhibitor of APCCdc20 in the spindle assembly checkpoint. Genes Dev. 2007;21:655–667. doi: 10.1101/gad.1511107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton J.L., Xiong Y., Solomon M.J. Mechanisms of pseudosubstrate inhibition of the anaphase promoting complex by Acm1. EMBO J. 2011;30:1818–1829. doi: 10.1038/emboj.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll C.W., Enquist-Newman M., Morgan D.O. The APC subunit Doc1 promotes recognition of the substrate destruction box. Curr. Biol. 2005;15:11–18. doi: 10.1016/j.cub.2004.12.066. [DOI] [PubMed] [Google Scholar]
- Castro A., Vigneron S., Bernis C., Labbé J.C., Lorca T. Xkid is degraded in a D-box, KEN-box, and A-box-independent pathway. Mol. Cell. Biol. 2003;23:4126–4138. doi: 10.1128/MCB.23.12.4126-4138.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao W.C., Kulkarni K., Zhang Z., Kong E.H., Barford D. Structure of the mitotic checkpoint complex. Nature. 2012;484:208–213. doi: 10.1038/nature10896. [DOI] [PubMed] [Google Scholar]
- Choi E., Dial J.M., Jeong D.E., Hall M.C. Unique D box and KEN box sequences limit ubiquitination of Acm1 and promote pseudosubstrate inhibition of the anaphase-promoting complex. J. Biol. Chem. 2008;283:23701–23710. doi: 10.1074/jbc.M803695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4 The CCP4 suite: programs for protein crystallography. Acta Crystallogr. D Biol. Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Cromer L., Heyman J., Touati S., Harashima H., Araou E., Girard C., Horlow C., Wassmann K., Schnittger A., De Veylder L., Mercier R. OSD1 promotes meiotic progression via APC/C inhibition and forms a regulatory network with TDM and CYCA1;2/TAM. PLoS Genet. 2012;8:e1002865. doi: 10.1371/journal.pgen.1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Fonseca P.C., Kong E.H., Zhang Z., Schreiber A., Williams M.A., Morris E.P., Barford D. Structures of APC/C(Cdh1) with substrates identify Cdh1 and Apc10 as the D-box co-receptor. Nature. 2011;470:274–278. doi: 10.1038/nature09625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis I.W., Leaver-Fay A., Chen V.B., Block J.N., Kapral G.J., Wang X., Murray L.W., Arendall W.B., 3rd, Snoeyink J., Richardson J.S., Richardson D.C. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35(Web Server issue):W375–W383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dial J.M., Petrotchenko E.V., Borchers C.H. Inhibition of APCCdh1 activity by Cdh1/Acm1/Bmh1 ternary complex formation. J. Biol. Chem. 2007;282:5237–5248. doi: 10.1074/jbc.M606589200. [DOI] [PubMed] [Google Scholar]
- Emsley P., Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Enquist-Newman M., Sullivan M., Morgan D.O. Modulation of the mitotic regulatory network by APC-dependent destruction of the Cdh1 inhibitor Acm1. Mol. Cell. 2008;30:437–446. doi: 10.1016/j.molcel.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 2006;62:72–82. doi: 10.1107/S0907444905036693. [DOI] [PubMed] [Google Scholar]
- Foe I.T., Foster S.A., Cheung S.K., DeLuca S.Z., Morgan D.O., Toczyski D.P. Ubiquitination of Cdc20 by the APC occurs through an intramolecular mechanism. Curr. Biol. 2011;21:1870–1877. doi: 10.1016/j.cub.2011.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzer M., Murray A.W., Kirschner M.W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Hall M.C., Jeong D.E., Henderson J.T., Choi E., Bremmer S.C., Iliuk A.B., Charbonneau H. Cdc28 and Cdc14 control stability of the anaphase-promoting complex inhibitor Acm1. J. Biol. Chem. 2008;283:10396–10407. doi: 10.1074/jbc.M710011200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog F., Primorac I., Dube P., Lenart P., Sander B., Mechtler K., Stark H., Peters J.M. Structure of the anaphase-promoting complex/cyclosome interacting with a mitotic checkpoint complex. Science. 2009;323:1477–1481. doi: 10.1126/science.1163300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrandt E.R., Hoyt M.A. Cell cycle-dependent degradation of the Saccharomyces cerevisiae spindle motor Cin8p requires APC(Cdh1) and a bipartite destruction sequence. Mol. Biol. Cell. 2001;12:3402–3416. doi: 10.1091/mbc.12.11.3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilioti Z., Chung Y.S., Mochizuki Y., Hardy C.F., Cohen-Fix O. The anaphase inhibitor Pds1 binds to the APC/C-associated protein Cdc20 in a destruction box-dependent manner. Curr. Biol. 2001;11:1347–1352. doi: 10.1016/s0960-9822(01)00399-2. [DOI] [PubMed] [Google Scholar]
- Kabsch W. Xds. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamysheva Z., Diaz-Martinez L.A., Crow S.E., Li B., Yu H. Multiple anaphase-promoting complex/cyclosome degrons mediate the degradation of human Sgo1. J. Biol. Chem. 2009;284:1772–1780. doi: 10.1074/jbc.M807083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Yu H. Mutual regulation between the spindle checkpoint and APC/C. Semin. Cell Dev. Biol. 2011;22:551–558. doi: 10.1016/j.semcdb.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimata Y., Baxter J.E., Fry A.M., Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol. Cell. 2008;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Kimata Y., Trickey M., Izawa D., Gannon J., Yamamoto M., Yamano H. A mutual inhibition between APC/C and its substrate Mes1 required for meiotic progression in fission yeast. Dev. Cell. 2008;14:446–454. doi: 10.1016/j.devcel.2007.12.010. [DOI] [PubMed] [Google Scholar]
- King R.W., Glotzer M., Kirschner M.W. Mutagenic analysis of the destruction signal of mitotic cyclins and structural characterization of ubiquitinated intermediates. Mol. Biol. Cell. 1996;7:1343–1357. doi: 10.1091/mbc.7.9.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King E.M., van der Sar S.J., Hardwick K.G. Mad3 KEN boxes mediate both Cdc20 and Mad3 turnover, and are critical for the spindle checkpoint. PLoS ONE. 2007;2:e342. doi: 10.1371/journal.pone.0000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarev P.V., Volkov V.V., Sokolova A.V., Koch M.H.J., Svergun D.I. PRIMUS: a Windows PC-based system for small-angle scattering data analysis. J. Appl. Crystallogr. 2003;36:1277–1282. [Google Scholar]
- Kraft C., Vodermaier H.C., Maurer-Stroh S., Eisenhaber F., Peters J.M. The WD40 propeller domain of Cdh1 functions as a destruction box receptor for APC/C substrates. Mol. Cell. 2005;18:543–553. doi: 10.1016/j.molcel.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Labit H., Fujimitsu K., Bayin N.S., Takaki T., Gannon J., Yamano H. Dephosphorylation of Cdc20 is required for its C-box-dependent activation of the APC/C. EMBO J. 2012;31:3351–3362. doi: 10.1038/emboj.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malureanu L.A., Jeganathan K.B., Hamada M., Wasilewski L., Davenport J., van Deursen J.M. BubR1 N terminus acts as a soluble inhibitor of cyclin B degradation by APC/C(Cdc20) in interphase. Dev. Cell. 2009;16:118–131. doi: 10.1016/j.devcel.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J.S., Jeong D.E., Choi E., Billings B.M., Hall M.C. Acm1 is a negative regulator of the CDH1-dependent anaphase-promoting complex/cyclosome in budding yeast. Mol. Cell. Biol. 2006;26:9162–9176. doi: 10.1128/MCB.00603-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J.S., Hall H., Bartolowits M.D., Hall M.C. Acm1 contributes to nuclear positioning by inhibiting Cdh1-substrate interactions. Cell Cycle. 2012;11:384–394. doi: 10.4161/cc.11.2.18944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Cryst. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J.J., Summers M.K., Hansen D.V., Nachury M.V., Lehman N.L., Loktev A., Jackson P.K. Emi1 stably binds and inhibits the anaphase-promoting complex/cyclosome as a pseudosubstrate inhibitor. Genes Dev. 2006;20:2410–2420. doi: 10.1101/gad.1454006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov G.N., Skubák P., Lebedev A.A., Pannu N.S., Steiner R.A., Nicholls R.A., Winn M.D., Long F., Vagin A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 2011;67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A., Salmon E.D. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Ostapenko D., Burton J.L., Wang R., Solomon M.J. Pseudosubstrate inhibition of the anaphase-promoting complex by Acm1: regulation by proteolysis and Cdc28 phosphorylation. Mol. Cell. Biol. 2008;28:4653–4664. doi: 10.1128/MCB.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore L.A., McCormack E.A., Au S.W., Paul A., Willison K.R., Harper J.W., Barford D. Doc1 mediates the activity of the anaphase-promoting complex by contributing to substrate recognition. EMBO J. 2003;22:786–796. doi: 10.1093/emboj/cdg084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passmore L.A., Barford D., Harper J.W. Purification and assay of the budding yeast anaphase-promoting complex. Methods Enzymol. 2005;398:195–219. doi: 10.1016/S0076-6879(05)98017-8. [DOI] [PubMed] [Google Scholar]
- Peters J.M. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat. Rev. Mol. Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- Pfleger C.M., Kirschner M.W. The KEN box: an APC recognition signal distinct from the D box targeted by Cdh1. Genes Dev. 2000;14:655–665. [PMC free article] [PubMed] [Google Scholar]
- Pines J. Cubism and the cell cycle: the many faces of the APC/C. Nat. Rev. Mol. Cell Biol. 2011;12:427–438. doi: 10.1038/nrm3132. [DOI] [PubMed] [Google Scholar]
- Schneidman-Duhovny D., Hammel M., Sali A. FoXS: a web server for rapid computation and fitting of SAXS profiles. Nucleic Acids Res. 2010;38(Web Server issue):W540–W544. doi: 10.1093/nar/gkq461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber A., Stengel F., Zhang Z., Enchev R.I., Kong E.H., Morris E.P., Robinson C.V., da Fonseca P.C., Barford D. Structural basis for the subunit assembly of the anaphase-promoting complex. Nature. 2011;470:227–232. doi: 10.1038/nature09756. [DOI] [PubMed] [Google Scholar]
- Schuck P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and lamm equation modeling. Biophys. J. 2000;78:1606–1619. doi: 10.1016/S0006-3495(00)76713-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sczaniecka M., Feoktistova A., May K.M., Chen J.S., Blyth J., Gould K.L., Hardwick K.G. The spindle checkpoint functions of Mad3 and Mad2 depend on a Mad3 KEN box-mediated interaction with Cdc20-anaphase-promoting complex (APC/C) J. Biol. Chem. 2008;283:23039–23047. doi: 10.1074/jbc.M803594200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan M., Morgan D.O. A novel destruction sequence targets the meiotic regulator Spo13 for anaphase-promoting complex-dependent degradation in anaphase I. J. Biol. Chem. 2007;282:19710–19715. doi: 10.1074/jbc.M701507200. [DOI] [PubMed] [Google Scholar]
- Svergun D.I. Determination of the regularization parameter in indirect-transform methods using perceptual criteria. J. Appl. Crystallogr. 1992;25:495–503. [Google Scholar]
- Thornton B.R., Toczyski D.P. Precise destruction: an emerging picture of the APC. Genes Dev. 2006;20:3069–3078. doi: 10.1101/gad.1478306. [DOI] [PubMed] [Google Scholar]
- Tian W., Li B., Warrington R., Tomchick D.R., Yu H., Luo X. Structural analysis of human Cdc20 supports multisite degron recognition by APC/C. Proc. Natl. Acad. Sci. USA. 2012;109:18419–18424. doi: 10.1073/pnas.1213438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Yang J., Kong E.H., Chao W.C., Morris E.P., da Fonseca P.C., Barford D. Recombinant expression, reconstitution and structure of human anaphase-promoting complex (APC/C) Biochem. J. 2013;449:365–371. doi: 10.1042/BJ20121374. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.