Abstract
Purpose
To use International Classification of Disease Codes (ICD-9) codes to investigate primary immune deficiency (PID) in New York State.
Methods
We investigated the diagnosis of Primary Immune Deficiency (PID) in New York State (NYS) using the Statewide Planning and Research Cooperative System (SPARCS) database, a comprehensive data reporting system that collects ICD-9 codes for each patient hospitalized in NYS.
Results
From 2000–2004 there were 13,539,358 hospitalizations for 4,777,295 patients; of these, 2,361 patients (0.05 %) were diagnosed with one or more of the ICD-9 codes for PID. Antibody defects were the most common diagnoses made. The PID population had significantly more Caucasians, and fewer African American or Hispanic subjects compared to the general population. Subjects with PID codes were younger, had longer hospitalizations, were less likely to have Medicare and more likely to have Medicaid or Blue Cross insurance. Most hospitalizations were due to respiratory and infectious diseases. Most patients resided in the most populous counties, Kings, New York and Queens, but the distribution of home zip codes was not proportional to county populations.
Conclusions
These data provide useful information on incidence and complications of selected PID diagnoses in one large state.
Keywords: Primary immune deficiency (PID), international classification of disease codes (ICD-9), New York State, severe combined immune deficiency (SCID), Wiskott Aldrich Syndrome (WAS), hospitalizations
Introduction
Primary immune deficiency diseases (PID) result from mutations in one of many genes needed for a functioning immune system [1]. Although the first PIDs were described more than 5 decades ago, mostly in infants, often males with unusual phenotypes and severe disease [2], it is now clear that PIDs affect people of all ages, females and males alike, and have characteristic but not necessarily lethal consequences [1]. These disorders are considered rare, but are collectively more common, each leading characteristic clinical symptoms [1, 3, 4]. Excluding IgA deficiency as the most common but often asymptomatic defect, a textbook estimate of incidence is about 1:10,000 persons [5, 6], but this could be an underestimate, as suggested by a recent household survey [7]. While most patients have recurrent bacterial, viral or fungal infections, systemic symptoms such as fever, weight loss, and failure to thrive, immune defects often lead to other complications, including autoimmune diseases, inflammatory conditions, organ dysfunction, and cancer, especially lymphoma [1, 3, 8, 9]. Perhaps due to different ages of onset and varied disease manifestations, patients are likely to receive care in a number of medical venues while the underlying immune defect remains unrecognized. In the United States, Europe, and England, diagnostic delays of between 6 and 20 years after the characteristic onset of symptoms have been reported [10–15]. While diagnostic delays appear common for patients with PID in general, demographic data on US patient populations show that minorities are perhaps even less likely to be diagnosed with a PID. In reports of hyper IgM syndrome, chronic granulomatous disease, X-linked agammaglobulinemia (XLA), and common variable immune deficiency (CVID), African Americans and patients of Hispanic descent range only between 3 and 8 % [10, 16–19]. We hypothesize this may be due to genetic and/or environmental factors, or barriers to health care access. Although several observational studies have looked at specific PID patient populations, some through the use of national registries [3, 5, 7, 17, 19–30], many questions remain regarding disease incidence, patient demographics, morbidity, and mortality in this population.
In this study we examined data on patients who were given one or more ICD codes used to denote PID during hospitalizations in New York State (NYS) over a five-year period, using a statewide data collection tool that compiles diagnosis and procedure codes for all medical encounters. Our objective was to determine the perceived incidence of these defects, the veracity of coding where possible, the demographics of these subjects, the racial and ethnic groups represented, and the geographic distribution of PIDs in NYS. We also sought to compare the incidence of these defects with that predicted by textbooks, and to determine the number of and primary reasons for hospitalizations, and the sources of payment for these hospitalizations. These data were compared to patients hospitalized in NYS for diagnoses other than PID to determine differences in these populations. We predicted that PID patients might show significant differences in the age of diagnosis, number of hospitalizations, length of stay, racial and ethnic groups, and payment sources as compared to all other hospitalized patients.
Methods
ICD-9 Codes and SPARCS Data
The Statewide Planning and Research Cooperative System (SPARCS) (http://www.health.state.ny.us/statistics/sparcs/) is a comprehensive data reporting system that has continuously collected all diagnosis and procedure codes based on the International Classification of Diseases, Ninth Revision (ICD-9 codes), for each subject hospitalized in NYS since 1979 [31]. These disease codes are universally applied by all care locations and are used primarily for billing purposes. In SPARCS each patient is given a permanent, unique, and anonymous identifier that is included with all codes compiled during each visit to all medical venues in this state.
Immune Disease Related ICD Codes
Using 17 ICD-9 codes that are used to denote the majority of PIDs (Table I) we surveyed the SPARCS database for hospitalized patients given one or more of these ICD-9 codes during the years 2000 to 2004. Although the ICD-9 code 279.00 is a generic root code for most of these, the codes 279 or 279.00 can also be used to characterize subjects who are hypogammaglobulinemic due to non-genetic reasons such as leukemia or lymphoma; thus patients given any of the 51 ICD-9 codes for lymphoma or leukemia were excluded. The codes 279.3 or 279.10 have also been used as a generic code to denote an immune defect due to infection with the human immune deficiency virus (HIV); however, subjects with diagnosis of HIV were excluded in this cohort. The diagnoses of chronic granulomatous disease (CGD), hyper IgE syndrome, and congenital neutropenias, were not examined here as the coding for leukocyte disorders, grouped under IDC-9 codes 288.0 – 288.9, also identifies subjects with low cell numbers due to any cause, including chemotherapy. Data on subjects with ICD-9 codes for ataxia telangiectasia and complement defects, other than single complement deficiency (279.8), were not compiled here.
Table I.
Diagnosis, ICD codes, and numbers of PID patients
| Diagnosis | ICD code |
Patient count |
Percent |
|---|---|---|---|
| Hypogammaglobulinemia unspecified |
279.00 | 490 | 17.96 % |
| Unspecified immune deficiency | 279.3 | 477 | 17.48 % |
| Selective deficiency IgG | 279.03 | 274 | 10.04 % |
| DiGeorge syndrome | 279.11 | 249 | 9.12 % |
| Common variable Immunodeficiency | 279.06 | 242 | 8.87 % |
| Selective IgA Immunodeficiency | 279.01 | 215 | 7.88 % |
| Single Complement Deficiency | 279.8 | 205 | 7.51 % |
| Immunodeficiency with predominant T-cell defect |
279.10 | 169 | 6.19 % |
| Unspecified disorder of immune mechanism |
279.9 | 147 | 5.39 % |
| Combined immunodeficiency (includes SCID) |
279.2 | 143 | 5.24 % |
| Selective IgM Immunodeficiency | 279.02 | 34 | 1.25 % |
| Wiskott-Aldrich syndrome | 279.12 | 30 | 1.10 % |
| Congenital hypogammaglobulinemia (Agammaglobulinemia) |
279.04 | 22 | 0.81 % |
| Transient hypogammaglobulinemia | 279.09 | 17 | 0.62 % |
| Hyper-IgM | 279.05 | 10 | 0.37 % |
| Autoimmune disease not elsewhere classified |
279.4 | 3 | 0.11 % |
| Nezelof’s syndrome | 279.13 | 2 | 0.07 % |
| Total | 2729 | 100.00 % |
Data Extracted
For each patient with one or more of the PID ICD-9 codes, demographics, home zip codes, length of stay, insurance status, and all other ICD-9 codes present over this interval were determined. Codes were identified as primary (reason for hospitalization) or secondary (other conditions present at the same time) code. Only one primary diagnosis is given for each encounter, but up to 13 secondary diagnoses were recorded for each admission. A death rate (for deaths that occurred during hospitalization) was determined for PID subjects and the general hospitalized population. We sorted the ICD-9 diagnosis codes associated with each patient’s admissions by date to determine the stability and variability of the PID diagnoses applied over time. Demographic information for all NYS hospitalized subjects from 2000–2004 was obtained from annual SPARCS reports [31].
Statistical Analysis
Statistical Analysis Software (SAS) (©SAS Institute Inc.) was used to collect data from patients with selected ICD-9 codes. Computer software Microsoft Office Excel (©Microsoft Corporation) was used to extract, analyze and sort the data as well as create tables and graphs. The home zip codes of this population were sorted by county, and the top counties were applied to a map of NYS using Google Images [32]. Demographic data on NYS counties was obtained from the NYS web site [33]. Chi square statistical tests were performed to compare age, demographics, length of stay, and payment source between the PID group and the other hospitalized patients.
Results
Admissions and PID Patients
There were a total of 13,539,358 hospitalizations in NYS over the five-year period, 2000–2004, accounted for by a total of 4,777,295 patients. Using the SPARCS database, we identified 2,361 unique patients who had at least one hospitalization for which one or more of the diagnoses of an immune defect were made, representing 0.05 % of the entire hospitalized population. These subjects had a total of 7,378 admissions over the 5-year period, averaging 3 admissions per patient (range 1 to 76), significantly greater numbers than patients in the general hospital population, who had an average of 1.9 admissions per patient (p<0.001). The average length of stay for PID patients was significantly longer, 8.3 days, than from others in the general hospital population (6 days; p<0.001). Using unique patient identifiers, we found that the largest proportion of these patients were given the ICD-9 code 279.00 (hypogammaglobulinemia; 17.96 %) or other forms of antibody defects. DiGeorge syndrome, usually confirmed by cytogenetics identifying a mutation in chromosome 22q11 [34], was found in 9.12 % of patients. The rarer immune defects, congential agammaglobulinemia and hyper IgM syndromes were diagnosed in 0.81 % and 0.37 % of patients respectively. The ICD-9 codes for the rare but severe defects that are more likely to lead to a hospitalization, Severe Combined Immune Deficiency (SCID) and Wiskott Aldrich syndrome (WAS), were applied in 143 (5.24 %) and 30 (1.10 %) cases, respectively. The ICD code for Nezelof syndrome, no longer in general use, still appeared for 2 patients (0.07 %). As discussed below, because patients may have received different diagnosis codes on different admissions, the number of diagnoses in Table I (2,729) was greater than the unique number of PID patients (2,361).
Demographics of Patients
Subjects given PID codes were ages 0–91, but about 32 % were under age 20, which was significantly younger than the general hospital population, only 17.5 % of whom were under age 20 (p<0.001) (Table II). In the general patient population, 61 % were females and 39 % were males. For PID, the proportions were more equal; females were 54.8 % and 45.3 % were males. The PID population consisted of a significantly greater numbers of Caucasian subjects (p=0.001) and fewer subjects of African American or Hispanic ethnicity, as compared to the general patient population (Table III). Selected minorities (Asian, Native American, Alaskan Native) were also reduced in percent in the PID population. We found that 7.7 % of the PID cohort died during the period of study, only slightly greater than the death rate for the general hospitalized population, at 7.1 %.
Table II.
Ages of patients with PID diagnosis versus the general hospital population
| Age group | PID | General NYS hospital population | ||
|---|---|---|---|---|
| Count | Percent | Count | Percent | |
| 0 to 5 | 462 | 19.57 % | 1657488 | 13.03 % |
| 6 to 14 | 189 | 8.01 % | 249194 | 1.96 % |
| 15 to 19 | 99 | 4.19 % | 318349 | 2.50 % |
| 20 to 44 | 563 | 23.85 % | 3448801 | 27.12 % |
| 45 to 74 | 806 | 34.14 % | 4369750 | 34.36 % |
| 75 to 84 | 177 | 7.50 % | 1738473 | 13.67 % |
| 85+ | 65 | 2.75 % | 936126 | 7.36 % |
| Total | 2361 | 100.0 % | 12,718,181 | 100.0 % |
Table III.
Patient ethnicity and race
| Ethnicity Race | PID diagnosed patients |
General hospital population |
P-Values | ||
|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | ||
| Spanish / Hispanic Origin | |||||
| White | 74 | 3.13 % | 305,809 | 4.04 % | P=0.04 |
| Black or African American |
8 | 0.34 % | 59,624 | 0.79 % | P=0.02 |
| Native American | - | - | 177 | 0.00 % | - |
| Asian | - | - | 1,530 | 0.02 % | - |
| Native Hawaiian | - | - | 19 | 0.00 % | - |
| Other Race | 84 | 3.56 % | 439,322 | 5.80 % | P<0.001 |
| Unknown | 9 | 0.38 % | 43,224 | 0.57 % | P<0.001 |
| Not of Spanish / Hispanic Origin | |||||
| White | 1,351 | 57.22 % | 3,673,154 | 48.50 % | P<0.001 |
| Black or African American |
328 | 13.89 % | 1,106,075 | 14.61 % | P=0.41 |
| Native American or Alaskan Native |
10 | 0.42 % | 29,464 | 0.39 % | P<0.001 |
| Asian | 30 | 1.27 % | 223,504 | 2.95 % | P<0.001 |
| Native Hawaiian | - | - | 308 | 0.00 % | - |
| Other Race | 63 | 2.67 % | 297,769 | 3.93 % | P<0.001 |
| Unknown | 33 | 1.40 % | 163,847 | 2.16 % | P<0.001 |
| Unknown | |||||
| White | 132 | 5.59 % | 380,925 | 5.03 % | P=0.26 |
| Black or African American |
12 | 0.51 % | 78,546 | 1.04 % | P=0.02 |
| Native American | - | - | 909 | 0.01 % | - |
| Asian | 1 | 0.04 % | 5,495 | 0.07 % | P=0.87 |
| Native Hawaiian | - | - | 23 | 0.00 % | - |
| Other Race | 101 | 4.28 % | 376,177 | 4.97 % | P<0.001 |
| Unknown | 125 | 5.29 % | 387,027 | 5.11 % | P<0.001 |
| 2,361 | 100.0 % | 7,572,928 | 100.0 % | ||
Admissions, PIDs Diagnosed and Veracity of Coding
To examine the veracity of the application of ICD coding, we attempted to eliminate subjects with other diseases but for whom the ICD codes in use for PID were applied. While we could remove subjects with HIV or others with hypogammaglobulinemia due to leukemia, lymphoma, or other hematologic malignancies, other subjects with hypogammaglobulinemia due to drugs or other causes could not be identified. ICD 279.04, a code used to denote the congenital agammaglobulinemias such as XLA, was used for 22 patients, 15 males and 7 females, suggesting the application of this code to any subject with very low levels of serum immunoglobulins. Subjects designated by the 279.05 code used for hyper IgM syndromes (10 cases), could have been cases for whom the serum IgM was high for any reason; while the PID diagnosis hyper IgM is predominantly an X-linked disease, 6 males and 4 females were found in this small cohort.
Subjects with SCID and WAS were further examined as these diseases might be more likely to lead to hospitalization and therefore subjects with ICD-9 codes for these might be more readily examined. There were 143 subjects given the ICD-9 code “combined immune deficiency” (279.2, also used for SCID); this included 62 patients over the age of 23, suggesting that subjects found to have other forms of “combined immune defects” might be contained in this category. Of this group, 48 were age 0–5 years, 14 were 5–10, 8 were 11–15, and 11 were 16–23. The rest (62 subjects) were older than this, suggesting that a congenital immune defect was unlikely. However, for the group of 81 patients under age 23 (35 males and 46 females), the procedure code most commonly associated with SCID was Bone Marrow Replacement by Transplant (V4281) in 21 subjects (19 of these between 0 and 8 years and the rest under age 21), suggesting that for this cohort the diagnosis was more likely correct. Of 30 patients who were given the code for WAS (279.12), 3 were female, likely an inappropriate diagnosis given the X-linked nature of this disease. The WAS patients were found in all age ranges; age 0–5 (17), age 6–15 (4), age 16–49 (3), and 50 or older (6). However, 27 of the 30 WAS subjects had been given the ICD-9 procedure code for bone marrow transplant (V4281); additionally the code for thrombocytopenia (287.5,) was applied to 10 subjects, suggesting that the WAS diagnosis was likely to be correct in these cases.
As noted above, while the 2,361 patients had 4,176 admissions during which an immune defect was coded as a diagnosis, this group actually had a total of 7,378 hospitalizations in the studied interval. As patients were not given the PID diagnosis on every admission, we divided the 7,378 admissions into “PID admissions” and “non-PID admissions.” To determine reasons for this, we examined admissions for PID patients in chronological order. For some patients, the diagnosis of PID was not made until several hospitalizations occurred. For others, the diagnosis of PID disappeared after one or more admissions had taken place, suggesting resolution. In a third scenario, the PID diagnosis was not applied for each admission (for example childbirth, trauma, or psychiatric admissions) but only intermittently. However, the application of diagnosis codes was also somewhat arbitrary; for example, one patient had 16 hospitalizations and was given a PID diagnosis on 12 of these admissions. The PID diagnoses applied for this subject included common variable immunodeficiency (279.06), selective immunoglobulin deficiency (279.03), hypogammaglobulinemia (279.00), selective IgM deficiency (279.02) and selective IgA deficiency (279.01) at different times, possibly based on the most prominently reduced immunoglobulin isotype found on each admission.
Medical Complications
To examine the medical complications most commonly associated with the patients diagnosed as having an immune defect, we surveyed the 4,176 admissions which included the PID as a primary or secondary diagnosis (Table IV). Not surprisingly, the majority of these admissions were for respiratory conditions (1,924 admissions), followed by infectious diseases (1,852) (Table V). Table VI further divides these infections into organ locations, the foremost being the respiratory system, skin, or blood. The fewest infections occurred in kidneys or urinary tract.
Table IV.
Admissions of patients with PID as a primary or secondary diagnosis
| PID code and description | Admissions | Percent |
|---|---|---|
| Hypogammaglobulinemia unspecified | 924 | 22.13 % |
| Unspecified immunity deficiency | 531 | 12.72 % |
| Common variable Immunodeficiency | 495 | 11.85 % |
| DiGeorge’s syndrome | 448 | 10.73 % |
| Selective deficiency IgG | 441 | 10.56 % |
| Selective IgA Immunodeficiency | 274 | 6.56 % |
| Combined immunodeficiency (includes SCID) | 247 | 5.91 % |
| Single Complement | 239 | 5.72 % |
| Immunodeficiency with predominant T-cell defect |
197 | 4.72 % |
| Unspecified disorder of immune mechanism | 151 | 3.62 % |
| Wiskott-Aldrich syndrome | 121 | 2.90 % |
| Congenital hypogammaglobulinemia (Agammaglobulinemia) |
36 | 0.86 % |
| Selective IgM Immunodeficiency | 32 | 0.77 % |
| Transient hypogammaglobulinemia | 20 | 0.48 % |
| Hyper-IgM | 18 | 0.43 % |
| Nezelof’s syndrome | 2 | 0.05 % |
| 4,176 | 100.0 % |
Table V.
Other diagnoses on admissions (PID as primary or secondary code only)
| System/Type of condition | Count | Percent |
|---|---|---|
| Respiratory system | 1924 | 20.92 % |
| Infectious and parasitic diseases | 1852 | 20.13 % |
| Circulatory system | 850 | 9.24 % |
| Digestive system | 649 | 7.06 % |
| Immunologic diseases | 594 | 6.46 % |
| Injury and poisoning | 432 | 4.70 % |
| Musculoskeletal system and connective tissue | 328 | 3.57 % |
| Post-operative infections | 283 | 3.08 % |
| Operative and transplant complications, dialysis | 283 | 3.08 % |
| Mental Illness+dependencies | 201 | 2.19 % |
| Congenital anomalies | 201 | 2.19 % |
| Blood and blood-forming organs | 199 | 2.16 % |
| Skin and subcutaneous tissue | 195 | 2.12 % |
| Nutritional and metabolic diseases | 168 | 1.83 % |
| Neoplasm | 143 | 1.55 % |
| Endocrine System | 125 | 1.36 % |
| Complications of pregnancy, childbirth etc. | 120 | 1.30 % |
| Genitourinary system | 113 | 1.23 % |
| Conditions originating in the perinatal period | 107 | 1.16 % |
| Fractures/Concussions/Lacerations | 98 | 1.07 % |
| Fever | 98 | 1.07 % |
| Physical therapy and rehabilitation | 96 | 1.04 % |
| Nervous system and sense organs | 86 | 0.93 % |
| Total | 9199 | 100.00 % |
Table VII.
Infections by location and type
| Location/Type of infection | Count | Percent |
|---|---|---|
| Respiratory | ||
| Pneumonia not specified | 566 | 30.56 % |
| Bronchitis/Bronchiolitis | 85 | 4.59 % |
| Pneumococcal pneumonia | 52 | 2.81 % |
| Sinusitis/Pharyngitis/Tonsillitis | 50 | 2.70 % |
| Pseudomonas pneumonia | 28 | 1.51 % |
| Empyema/ Effusions | 27 | 1.46 % |
| Staph pneumonia | 21 | 1.13 % |
| Other bacterial pneumonia | 20 | 1.08 % |
| Tuberculosis and mycobacterial species | 19 | 1.03 % |
| Laryngitis, tracheitis, croup | 17 | 0.92 % |
| Acute URI | 17 | 0.92 % |
| Otitis/Mastoiditis | 16 | 0.86 % |
| Viral pneumonia | 19 | 1.03 % |
| Fungal pneumonia | 14 | 0.76 % |
| RSV pneumonia | 12 | 0.65 % |
| Lung abscess | 9 | 0.49 % |
| Klebsiella pneumonia | 6 | 0.32 % |
| Gram negative pneumonia | 4 | 0.22 % |
| Legionella pneumonia | 3 | 0.16 % |
| E-coli pneumonia | 2 | 0.11 % |
| Pertussis | 2 | 0.11 % |
| Skin | ||
| Cellulitis | 182 | 9.83 % |
| Viral infections | 42 | 2.27 % |
| Fungal infections | 7 | 0.38 % |
| Others | 4 | 0.22 % |
| Blood | ||
| Bacteremia | 138 | 7.45 % |
| Gastrointestinal | ||
| Bacterial/Protozal/Viral gastroenteritis | 70 | 3.78 % |
| Fungal disease | 8 | 0.43 % |
| Infective not specific | 7 | 0.38 % |
| Dental abscess | 7 | 0.38 % |
| Rectal abscess | 6 | 0.32 % |
| Peritonitis | 3 | 0.16 % |
| Cardiac | ||
| Bacterial endocarditis | 10 | 0.54 % |
| Pericarditis | 2 | 0.11 % |
| Rheumatic fever | 1 | 0.05 % |
| Musculoskeletal | ||
| Osteomyeltis | 28 | 1.51 % |
| Neurologic | ||
| Viral meningitis | 22 | 1.19 % |
| Bacterial meningitis | 12 | 0.65 % |
| Encephalitis | 8 | 0.43 % |
| Intracrainal /Intraspinous abscess | 6 | 0.32 % |
| Fungal | 6 | 0.32 % |
| Renal/ Urologic | ||
| Urinary Tract Infection | 55 | 2.97 % |
| Pyelonephritis | 28 | 1.51 % |
| Genital Infection | 8 | 0.43 % |
| Post-operative | 40 | 2.16 % |
| Other | 163 | 8.80 % |
| Total | 1852 | 100.00 % |
Examining the Use of PID Codes by NYS Counties
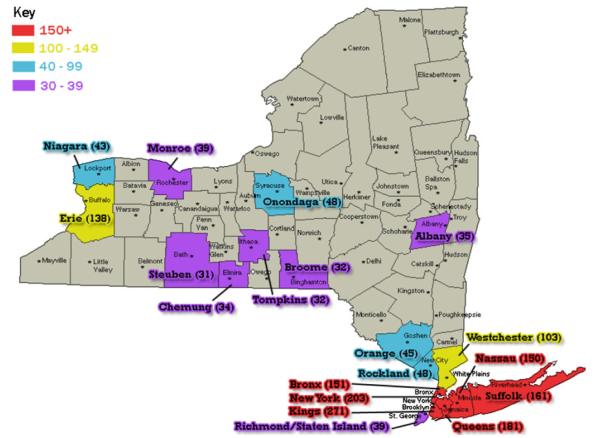
As one might predict, the most populous NYS counties contained the home zip codes of the largest number of patients diagnosed with PID. Kings County with 2.5 million residents had 271 PID patients, New York County (1.6 million residents) had 203 PID patients, and Queens County (2.2 million residents) had 181 PID patients (Fig. 1). Comparing the numbers of patients diagnosed with PIDs to the county populations (for the top 19 of the 62 counties) it appeared that PIDs were not diagnosed proportionally, with a range of 0.005 % to 0.04 % based on county populations. Chemung County had the highest percentage of PID subjects (0.04 %), but it also had the lowest population of these 19 counties.
Fig. 1.
New York State counties that are heavily populated with PID patients. The red colored counties encompass the largest number of PID patients (150 and above). Yellow counties have PID populations from 100–149 people, blue counties 40–99 people, and purple counties 30–39 people
Insurance Coverage
Patients in the general hospital population were more likely to have Medicare (30.2 %) than were PID patients (25.3 %; p<0.001), compatible with the significantly younger age of the PID population. Potentially as a result, significantly larger number of the PID identified patients had Medicaid (21.1 % vs. 19.0 %, p=0.012), HMO policies (18.5 % vs. 15.0 %, p<0.001), or Blue Cross (13.4 % vs. 10.0 %, p<0.001) than other hospitalized subjects.
Discussion
In this study we examined the demographics and associated conditions of subjects given one or more of the current ICD-9 codes used for PID diseases, admitted to NYS hospitals over a 5-year period. While we identified a number of potential problems in the application and mis-application of the PID ICD-9 codes, we found that the PID identified patients were significantly younger than patients in the general hospital population (p<0.001). However, the greatest number of PID patients were still in the 45–74 year age range, perhaps consistent with the observation that many had antibody defects, diagnoses more commonly made in adults [4, 5, 23]. Further investigation of this cohort revealed that these subjects were admitted more often and had longer lengths of stay than non-PID patients. These data suggest that these subjects had a greater severity of illness, paralleling results of a previous study [35]. Despite the ethnic and racial heterogeneity of NYS, patients diagnosed with PID were significantly more likely to be Caucasian than the general patient population. Reasons for this skewing of racial and ethnic makeup are not well understood, but could be due to genetic predisposition, increased utilization of health care by well-insured subjects, and/or lack of access to specialty care where PIDs are more likely to be recognized [9, 35–38]. While we found more PID patients lived in the most populous NYC counties, the home residences of PID subjects were not necessarily proportional to county populations. Again, the reasons for this skewed distribution are not well understood.
For the NYS population of 19.1 million, the commonly estimated 1:10,000 incidence of PID [6] would suggest that 1,900 subjects in NYS would be diagnosed with PID, fewer than identified in our hospitalized patient group. We acknowledge that with current home care treatments, subjects with PID may well not be admitted to the hospital and thus would not be counted in the current study. Based on a random telephone survey, the numbers of subjects in the general population with diagnosed PID appeared higher than expected, possibly by 10-fold.7 For these reasons, we paid specific attention to diagnoses more likely to lead to hospitalizations, such as SCID and WAS. The published incidence of Wiskott-Aldrich Syndrome is 1:250,000 [39]; in our population it was 1:637,000 persons hospitalized during this time frame. Including only SCID-diagnosed subjects under age 23, the incidence of SCID in this cohort would be 1:236,000 persons, also clearly less than the published 1 in 50,000 – 1 in 100,000 [39–41]. More universal diagnosis of SCID, and with it more accurate incidence figures, is likely to emerge from the newborn screening projects now ongoing in a number of states, including NYS.
The main limitations of this study are due to the inherent inaccuracies of ICD coding. ICD-9 codes are used mainly for billing purposes and there are likely to be differences in the application of ICD-9 codes in different medical facilities. The current diagnosis codes for PID would be best applied by persons experienced in immunology; however the location of these physicians is not evenly distributed in NYS. In addition, the ICD-9 codes are imprecise, and have not altered appreciably over the past several decades, although the ICD-11 nomenclature slated for future adoption improves this classification considerably (http://www.who.int/classifications/icd/revision/icd11faq/en/). Other potential flaws include erroneous, miscoded, or missing codes. In particular, subjects with antibody defects were found to have had different codes applied on different hospitalizations; on subsequent admissions, PID codes were not always applied. In contrast, codes for DiGeorge syndrome, Wiskott Aldrich syndrome, and X-linked agammaglobulinemia were more stable; the data attached to these cases is likely to be more reliable. The use of codes has been established in other recent studies: the assessment of rotavirus vaccination acute gastroenteritis [42], methicillin-resistant Staphylococcus aureus infections [43], outcomes of pregnancy in patients with congenital heart disease [44], risk of melanoma in rheumatoid arthritis [45], and drug induced acute liver toxicity [46]. In clinical immunology, ICD codes have been used to demonstrate that the use of intravenous immune globulin was associated with reduced risk of Alzheimer’s disease [47].
Conclusions
Despite inherent drawbacks, including potential misapplication of codes, ICD codes were used here to further define the PID-labeled population in one large state. Further study and refinement of the ICD code data mining process and application to additional large cohorts may yield important information about the PID population as a whole.
Acknowledgments
This work was supported by grants from the National Institutes of Health, AI 101093, AI-467320, AI-48693, NIAID Contract 03–22, and the David S Gottesman Immunology Chair and Baxter Healthcare.
C.C.R. reports previous/current grant funding from the N.I.H, Baxter Pharmaceuticals, and Octapharma as well as previous consulting fees/honoraria from Grifols, Merck, and Baxter.
Abbreviations
- PID
Primary immune deficiency diseases
- NYS
New York State
- SPARCS
Statewide Planning and Research Cooperative System
- XLA
X-linked agammaglobulinemia
- CVID
Common Variable Immune Deficiency
- HIV
Human Immune Deficiency Virus
- CGD
Chronic Granulomatous Disease
- SAS
Statistical Analysis Software
- SCID
Severe Combined Immune Deficiency
- WAS
Wiskott Aldrich Syndrome
Footnotes
Conflicts of Interest E.S.R, P.B., and P.S. report no conflicts of interest.
Contributor Information
Elena S. Resnick, Mount Sinai School of Medicine, Immunology Institute, New York, NY, USA
Priyanka Bhatt, Mount Sinai School of Medicine, Immunology Institute, New York, NY, USA.
Peter Sidi, Mount Sinai School of Medicine, Immunology Institute, New York, NY, USA.
Charlotte Cunningham-Rundles, Mount Sinai School of Medicine, Immunology Institute, New York, NY, USA; Mount-Sinai Medical Center, 1425 Madison Avenue, New-York, NY 10029, USA.
References
- 1.Notarangelo LD, Fischer A, Geha RS, Casanova JL, Chapel H, Conley ME, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124:1161–78. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–8. [PubMed] [Google Scholar]
- 3.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119:1650–7. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bellanti JA, Immunology IV. Clinical applications in health and disease. Saunders; Bethesda: 2012. [DOI] [PubMed] [Google Scholar]
- 5.Joshi AY, Iyer VN, Hagan JB, St Sauver JL, Boyce TG. Incidence and temporal trends of primary immunodeficiency: a population-based cohort study. Mayo Clin Proc. 2009;84:16–22. doi: 10.4065/84.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiehm ER, Ochs H, Winkelstein JA. Immunologic disorders in infants and children. 5th ed Elsevier Saunders; Philadelphia: 2004. [Google Scholar]
- 7.Boyle JM, Buckley RH. Population prevalence of diagnosed primary immunodeficiency diseases in the United States. J Clin Immunol. 2007;27:497–502. doi: 10.1007/s10875-007-9103-1. [DOI] [PubMed] [Google Scholar]
- 8.Samarghitean C, Ortutay C, Vihinen M. Systematic classification of primary immunodeficiencies based on clinical, pathological, and laboratory parameters. J Immunol. 2009;183:7569–75. doi: 10.4049/jimmunol.0901837. [DOI] [PubMed] [Google Scholar]
- 9.Yarmohammadi H, Estrella L, Doucette J, Cunningham-Rundles C. Recognizing primary immune deficiency in clinical practice. Clin Vaccine Immunol. 2006;13:329–32. doi: 10.1128/CVI.13.3.329-332.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92:34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 11.Fasano MB, Sullivan KE, Sarpong SB, Wood RA, Jones SM, Johns CJ, et al. Sarcoidosis and common variable immunodeficiency. Report of 8 cases and review of the literature. Medicine (Baltimore) 1996;75:251–61. doi: 10.1097/00005792-199609000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Mechanic LJ, Dikman S, Cunningham-Rundles C. Granulomatous disease in common variable immunodeficiency. Ann Intern Med. 1997;127:613–7. doi: 10.7326/0003-4819-127-8_part_1-199710150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Thickett KM, Kumararatne DS, Banerjee AK, Dudley R, Stableforth DE. Common variable immune deficiency: respiratory manifestations, pulmonary function and high-resolution CT scan findings. QJM. 2002;95:655–62. doi: 10.1093/qjmed/95.10.655. [DOI] [PubMed] [Google Scholar]
- 14.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 15.Urschel S, Kayikci L, Wintergerst U, Notheis G, Jansson A, Belohradsky BH. Common variable immunodeficiency disorders in children: delayed diagnosis despite typical clinical presentation. J Pediatr. 2009;154:888–94. doi: 10.1016/j.jpeds.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 16.Grimbacher B, Holland SM, Gallin JI, Greenberg F, Hill SC, Malech HL, et al. Hyper-IgE syndrome with recurrent infections–an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 17.Winkelstein JA, Marino MC, Ochs H, Fuleihan R, Scholl PR, Geha R, et al. The X-linked hyper-IgM syndrome: clinical and immunologic features of 79 patients. Medicine (Baltimore) 2003;82:373–84. doi: 10.1097/01.md.0000100046.06009.b0. [DOI] [PubMed] [Google Scholar]
- 18.Johnston DT, Mehaffey G, Thomas J, Young KR, Jr, Wiener H, Li J, et al. Increased frequency of HLA-B44 in recurrent sinopulmonary infections (RESPI) Clin Immunol. 2006;119:346–50. doi: 10.1016/j.clim.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Winkelstein JA, Marino MC, Lederman HM, Jones SM, Sullivan K, Burks AW, et al. X-linked agammaglobulinemia: report on a United States registry of 201 patients. Medicine (Baltimore) 2006;85:193–202. doi: 10.1097/01.md.0000229482.27398.ad. [DOI] [PubMed] [Google Scholar]
- 20.Abuzakouk M, Feighery C. Primary immunodeficiency disorders in the Republic of Ireland: first report of the national registry in children and adults. J Clin Immunol. 2005;25:73–7. doi: 10.1007/s10875-005-0360-9. [DOI] [PubMed] [Google Scholar]
- 21.Al-Herz W. Primary immunodeficiency disorders in Kuwait: first report from Kuwait National Primary Immunodeficiency Registry (2004–2006) J Clin Immunol. 2008;28:186–93. doi: 10.1007/s10875-007-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carneiro-Sampaio MM, Carbonare SB, Rozentraub RB, de Araujo MN, Riberiro MA, Porto MH. Frequency of selective IgA deficiency among Brazilian blood donors and healthy pregnant women. Allergol Immunopathol (Madr) 1989;17:213–6. [PubMed] [Google Scholar]
- 23.Gathmann B, Binder N, Ehl S, Kindle G. The European internet-based patient and research database for primary immunodeficiencies: update 2011. Clin Exp Immunol. 2011;167:479–91. doi: 10.1111/j.1365-2249.2011.04542.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Knerr V, Grimbacher B. Primary immunodeficiency registries. Curr Opin Allergy Clin Immunol. 2007;7:475–80. doi: 10.1097/ACI.0b013e3282f2162c. [DOI] [PubMed] [Google Scholar]
- 25.Leiva LE, Zelazco M, Oleastro M, Carneiro-Sampaio M, Condino-Neto A, Costa-Carvalho BT, et al. Primary immunodeficiency diseases in Latin America: the second report of the LAGID registry. J Clin Immunol. 2007;27:101–8. doi: 10.1007/s10875-006-9052-0. [DOI] [PubMed] [Google Scholar]
- 26.Plebani A, Soresina A, Rondelli R, Amato GM, Azzari C, Cardinale F, et al. Clinical, immunological, and molecular analysis in a large cohort of patients with X-linked agammaglobulinemia: an Italian multicenter study. Clin Immunol. 2002;104:221–30. doi: 10.1006/clim.2002.5241. [DOI] [PubMed] [Google Scholar]
- 27.Rezaei N, Aghamohammadi A, Moin M, Pourpak Z, Movahedi M, Gharagozlou M, et al. Frequency and clinical manifestations of patients with primary immunodeficiency disorders in Iran: update from the Iranian Primary Immunodeficiency Registry. J Clin Immunol. 2006;26:519–32. doi: 10.1007/s10875-006-9047-x. [DOI] [PubMed] [Google Scholar]
- 28.Winkelstein JA, Marino MC, Johnston RB, Jr, Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79:155–69. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Waltenburg R, Kobrynski L, Reyes M, Bowen S, Khoury MJ. Primary immunodeficiency diseases: practice among primary care providers and awareness among the general public, United States, 2008. Genet Med. 2010;12:792–800. doi: 10.1097/GIM.0b013e3181f3e2c9. [DOI] [PubMed] [Google Scholar]
- 30.Kirkpatrick P, Riminton S. Primary immunodeficiency diseases in Australia and New Zealand. J Clin Immunol. 2007;27:517–24. doi: 10.1007/s10875-007-9105-z. [DOI] [PubMed] [Google Scholar]
- 31.Database SPARCS. SPARCS Database. New York: 2009. Available from: http://www.health.state.ny.us/statistics/sparcs/ [Google Scholar]
- 32.Perry NW. Empire State Roads. New York Highways Website. http://www.empirestateroads.com/index.html.
- 33.State & County QuickFacts. 2010 Available from: http://quickfacts.census.gov/qfd/states/36000.html.
- 34.Gennery AR. Immunological aspects of 22q11.2 deletion syndrome. Cell Mol Life Sci. 2012;69:17–27. doi: 10.1007/s00018-011-0842-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham-Rundles C, Sidi P, Estrella L, Doucette J. Identifying undiagnosed primary immunodeficiency diseases in minority subjects by using computer sorting of diagnosis codes. J Allergy Clin Immunol. 2004;113:747–55. doi: 10.1016/j.jaci.2004.01.761. [DOI] [PubMed] [Google Scholar]
- 36.Derose KP, Gresenz CR, Ringel JS. Understanding disparities in health care access–and reducing them–through a focus on public health. Health Aff (Millwood) 2011;30:1844–51. doi: 10.1377/hlthaff.2011.0644. [DOI] [PubMed] [Google Scholar]
- 37.Mehra A, Sidi P, Doucette J, Estrella L, Rouvelas H, Cunningham-Rundles C. Subspecialty evaluation of chronically ill hospitalized patients with suspected immune defects. Ann Allergy Asthma Immunol. 2007;99:143–50. doi: 10.1016/S1081-1206(10)60638-2. [DOI] [PubMed] [Google Scholar]
- 38.Estrella L, Foley ME, Cunningham-Rundles C. X-linked agammaglobulinemia in a 10-year-old child: a case study. J Am Acad Nurse Pract. 2007;19:205–11. doi: 10.1111/j.1745-7599.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 39.Griffith LM, Cowan MJ, Kohn DB, Notarangelo LD, Puck JM, Schultz KR, et al. Allogeneic hematopoietic cell transplantation for primary immune deficiency diseases: current status and critical needs. J Allergy Clin Immunol. 2008;122:1087–96. doi: 10.1016/j.jaci.2008.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan K, Puck JM. Development of population-based newborn screening for severe combined immunodeficiency. J Allergy Clin Immunol. 2005;115:391–8. doi: 10.1016/j.jaci.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 41.Yee A, DeRavin SS, Elliott E, Ziegler JB. Severe combined immunodeficiency: a national surveillance study. Pediatr Allergy Immunol. 2008;19:298–302. doi: 10.1111/j.1399-3038.2007.00646.x. [DOI] [PubMed] [Google Scholar]
- 42.Begue RE, Perrin K. Reduction in gastroenteritis with the use of pentavalent rotavirus vaccine in a primary practice. Pediatrics. 2010;126:e40–5. doi: 10.1542/peds.2009-2069. [DOI] [PubMed] [Google Scholar]
- 43.Sircar KD, Bancroft E, Nguyen DM, Mascola L. Hospitalization of paediatric patients for methicillin-resistant Staphylococcus aureus skin and soft-tissue infection, 1998–2006. Epidemiol Infect. 2010;138:677–82. doi: 10.1017/S095026880999121X. [DOI] [PubMed] [Google Scholar]
- 44.Jain VD, Moghbeli N, Webb G, Srinivas SK, Elovitz MA, Pare E. Pregnancy in women with congenital heart disease: the impact of a systemic right ventricle. Congenit Heart Dis. 2011;6:147–56. doi: 10.1111/j.1747-0803.2011.00497.x. [DOI] [PubMed] [Google Scholar]
- 45.Amari W, Zeringue AL, McDonald JR, Caplan L, Eisen SA, Ranganathan P. Risk of non-melanoma skin cancer in a national cohort of veterans with rheumatoid arthritis. Rheumatology (Oxford) 2011 doi: 10.1093/rheumatology/ker113. [DOI] [PubMed] [Google Scholar]
- 46.Jinjuvadia K, Kwan W, Fontana RJ. Searching for a needle in a haystack: use of ICD-9-CM codes in drug-induced liver injury. Am J Gastroenterol. 2007;102:2437–43. doi: 10.1111/j.1572-0241.2007.01456.x. [DOI] [PubMed] [Google Scholar]
- 47.Fillit H, Hess G, Hill J, Bonnet P, Toso C. IV immunoglobulin is associated with a reduced risk of Alzheimer disease and related disorders. Neurology. 2009;73:180–5. doi: 10.1212/WNL.0b013e3181ae7aaf. [DOI] [PubMed] [Google Scholar]