Abstract
Purpose
Patients with recurrent malignant gliomas treated with stereotactic radiosurgery (SRS) and multiagent systemic therapies were reviewed to determine the effects of patient- and treatment-related factors on survival and toxicity.
Methods and Materials
A retrospective analysis was performed on patients with recurrent malignant gliomas treated with salvage SRS from September 2002 to March 2010. All patients had experienced progression after treatment with temozolomide and radiotherapy. Salvage SRS was typically administered only after multiple post-chemoradiation salvage systemic therapies had failed.
Results
63 patients were treated with SRS for recurrent high-grade glioma; 49 patients had World Health Organization (WHO) Grade 4 disease. Median follow-up was 31 months from primary diagnosis and 7 months from SRS. Median overall survival from primary diagnosis was 41 months for all patients. Median progression-free survival (PFS) and overall survival from SRS (OS-SRS) were 6 and 10 months for all patients, respectively. The 1-year OS-SRS for patients with Grade 4 glioma who received adjuvant (concurrent with or after SRS) bevacizumab was 50% vs. 22% for patients not receiving adjuvant bevacizumab (p = 0.005). Median PFS for patients with a WHO Grade 4 glioma who received adjuvant bevacizumab was 5.2 months vs. 2.1 months for patients who did not receive adjuvant bevacizumab (p = 0.014). Karnofsky performance status (KPS) and age were not significantly different between treatment groups. Treatment-related Grade 3/4 toxicity for patients receiving and not receiving adjuvant BVZ was 10% and 14%, respectively (p = 0.58).On multivariate analysis, the relative risk of death and progression with adjuvant bevacizumab was 0.37 (confidence interval [CI] 0.17–0.82) and 0.45 (CI 0.21–0.97). KPS >70 and age <50 years were significantly associated with improved survival.
Conclusions
The combination of salvage radiosurgery and bevacizumab to treat recurrent malignant gliomas is well tolerated and seems to be associated with improved outcomes. Prospective multiinstitutional studies are required to determine efficacy and long-term toxicity with this approach.
Keywords: Stereotactic radiosurgery, Glioma, Bevacizumab, Vascular endothelial growth factor-A
INTRODUCTION
The diagnosis of malignant glioma carries a poor prognosis, with few long-term survivors. Long-term local control in high-grade gliomas is difficult to achieve because of the infiltrating nature of the disease and its relative resistance to targeted and cytotoxic systemic therapies. Standard treatment for a newly diagnosed malignant glioma includes surgical resection followed by radiation therapy with concurrent temozolomide. This treatment yields a median overall survival (OS) of 12 to 15 months (1). Most malignant gliomas recur locally within a year after the completion of initial treatment (2–4), and recurrent disease is difficult to manage, given the morbidity associated with re-excision (5, 6) or large-volume reirradiation (7) and the limited options for systemic therapy (8–10).
Stereotactic radiosurgery (SRS) offers the potential to obtain local control in recurrent high-grade gliomas with minimal morbidity. Several case series, retrospective studies, and prospective studies have shown the potential efficacy and acceptable toxicity of radiosurgery for this disease (4, 11–15). Although radiation is often thought to destroy tumor vasculature, preclinical studies have demonstrated that radiation can paradoxically stimulate angiogenesis via a hypoxia-inducible-factor-1α—mediated pathway (16). Thus, it might be useful to combine radiation with an agent that inhibits this paradoxic effect.
Bevacizumab is a humanized murine monoclonal antibody that targets vascular endothelial growth factor-A (VEGF) and inhibits angiogenesis (17, 18). This agent has been approved for use by the U.S. Food and Drug Administration for colorectal cancer, non–small-cell lung cancer, renal cell carcinoma, and recently for recurrent high-grade gliomas. Grade 4 gliomas overexpress VEGF, and higher VEGF expression is associated with a poorer prognosis (19, 20). A recent Phase II trial showed efficacy and minimal toxicity for bevacizumab and irinotecan in the treatment of recurrent high-grade gliomas (21, 22). The current study examined the safety and efficacy of salvage SRS and systemic agents, including bevacizumab, in a retrospective series of patients with recurrent malignant gliomas. These patients were heavily pretreated before undergoing SRS, and most patients received additional courses of systemic therapy immediately after SRS.
METHODS AND MATERIALS
Patient selection
Between September 2002 and March 2010, 63 patients with a diagnosis of a recurrent malignant glioma of the brain were treated with salvage SRS at Duke University Medical Center using a linear accelerator–based system. All patients had pathologic results reviewed and confirmed at our institution. Patients were treated at the time of initial diagnosis with a gross or near total resection followed by adjuvant external-beam radiation and temozolomide. Recurrence was confirmed by surgical pathologic analysis and/or neuroimaging, including magnetic resonance imaging (MRI) and/or positron emission tomography. All patients in the study had experienced progression after primary treatment with concurrent temozolomide and external-beam radiotherapy, and salvage systemic therapy had been unsuccessful in nearly all patients before SRS was performed. Most of the patients received additional systemic therapy after SRS was administered. Only patients with World Health Organization (WHO) Grade 3/4 gliomas were included in the analysis. This retrospective study was approved by the Institutional Review Board of Duke University Medical Center.
SRS technique
All radiosurgical procedures were performed with linear-accelerator–based systems. Radiosurgical procedures before March 2008 were performed using a Radionics X-Knife system (Burlington, MA) with a Brown-Roberts-Wells stereotactic head frame for immobilization. Radiosurgical procedures after March 2008 were performed using a Novalis Tx system (Varian, Palo Alto, CA, and BrainLAB, Munich) with a custom U-frame mask for immobilization, a high-definition micromultileaf collimator, and cone-beam computed tomography for image guidance. All patients underwent a simulation computed tomography scan, which was fused to thin-slice, contrast-enhanced T1-weighted MRI. The gross tumor volume was defined based on T1-weighted contrast-enhanced axial MRI images, occasionally with guidance by positron emission tomography. Before March 2008, patients were treated using multiple arcs with conical collimators or six to nine static conformal beams. After March 2008, lesions 3 cm in diameter or smaller were typically treated using four to five dynamic conformal arcs, and larger lesions were treated with intensity-modulated static beams. Doses were prescribed to the isodose line fully encompassing the planning target volume, and single-fraction treatments were limited by the volume-based guidelines established in Radiation Therapy Oncology Group 90-05. Most patients received a short prophylactic course of dexamethasone after SRS.
Systemic therapies and toxicity
A review of patient medical records was performed to determine which systemic therapies each patient received before and after salvage SRS. Systemic therapy administration was directed by the Preston Robert Tisch Brain Tumor Center at Duke University. Many of the patients included in this analysis had previously been treated according to therapeutic protocols approved by the Institutional Review Board. Many of the systemic agents were given in combination. Toxicity was determined based on the Common Terminology Criteria for Adverse Events (version 4). Toxicity was defined as new symptoms or worsening of previous symptoms after salvage radiosurgery was administered. Symptoms occurring after 3 months from salvage radiosurgery that were attributable to disease progression were excluded.
Statistical analysis
Actuarial survival was calculated using the Kaplan-Meier method. Endpoints for analysis included OS from the time of initial (primary) diagnosis, OS from the time of radiosurgery (OS-SRS), and progression-free survival (PFS) from the time of radiosurgery. Patients were censored at the time of the last follow-up visit. Student’s t test was used to compare grade, age, Karnofsky performance status (KPS), number of therapies, time from diagnosis to SRS, and tumor volume between groups. Survival curves were compared using the Wilcoxon (Mann-Whitney U) rank sum test. Multivariate analyses of OS-SRS and PFS in patients with a WHO Grade 4 glioma were performed using a Cox proportional hazards model. Variables used in the analysis included the use of adjuvant bevacizumab, KPS >70, age >50 years, and tumor volume greater than the median (5 cc). Adjuvant bevacizumab was defined as bevacizumab given at the time of SRS or afterward. Statistical calculations were performed using JMP statistical software (version 8, SAS, Cary, NC). The toxicity analysis included all patients who received salvage radiosurgery for a malignant glioma. Survival analysis focused on the subset of patients with a WHO Grade 4 glioma, for consistency with previous reports.
RESULTS
Patient characteristics
Table 1 shows the characteristics of the patients in the study. Of the 63 patients included in the analysis, 45 were men and 18 were women. The median age at the time of radiosurgery was 47 years (range, 19–76 years). Forty-nine patients had a WHO Grade 4 glioma, and 14 patients had a WHO Grade 3 glioma. Six of the 14 patients with a WHO Grade 3 glioma were originally diagnosed with a WHO Grade 1 or 2 glioma, which later dedifferentiated. All of the patients with a WHO Grade 4 glioma had a de novo tumor. The median time from initial diagnosis to salvage SRS was 20 months. The median SRS dose was 15 Gy (range, 12.5–25 Gy). Twelve patients received 25 Gy in five equal fractions, all using the Novalis Tx system, and the remaining patients were treated with a single fraction.
Table 1.
Characteristics of the patients receiving salvage radiosurgery for recurrent Grade 3 or 4 glioma who did or did not receive adjuvant bevacizumab
| Total | −BVZ | +BVZ | |
|---|---|---|---|
| Number of patients | 63 | 21 | 42 |
| WHO grade | |||
| 3 | 14 | 5 | 9 |
| 4 | 49 | 16 | 33 |
| Sex | |||
| F | 18 | 7 | 11 |
| M | 45 | 14 | 31 |
| Median age (y) | 47 | 48 | 47 |
| Median follow–up (mo) | 6.5 | 5.3 | 7.8 |
| Median time from initial diagnosis to salvage SRS (mo) | 19.6 | 19.0 | 20.9 |
| Median KPS at time of SRS | 90 | 90 | 90 |
| Median KPS for WHO Grade 4 | 80 | 80 | 80 |
| Median SRS target volume (cc) | 4.8 | 5.6 | 4.5 |
| Mean number of systemic therapeutic agents before SRS | 3.6 | 3.5 | 3.7 |
| Mean number of systemic therapeutic agents after SRS | 2.9 | 1.1 | 3.8* |
Abbreviations: BVZ = bevacizumab; SRS = stereotactic radiosurgery; WHO = World Health Organization; KPS = Karnofsky performance status; adjuvant BVZ = treatment with BVZ concurrent with radiosurgery or afterward.
p < 0.05.
Salvage SRS was typically administered after multiple courses of salvage systemic therapy had been unsuccessful. The mean number of systemic agents given before salvage SRS and after SRS was 3.6 (range, 1–8) and 3.9 (range, 0–11), respectively. All patients received temozolomide with their initial course of radiation therapy. The most commonly prescribed systemic agents were bevacizumab (n = 51), irinotecan (n = 47), lomustine (CCNU) (n = 24), and etoposide (n = 37). Many of the systemic agents were given in combination. The most common combination was bevacizumab and irinotecan. Of the 51 patients who received bevacizumab, 42 received it during or after SRS. The median KPS at the time of salvage SRS was 80 (range, 50–90). The median KPS of the group receiving adjuvant bevacizumab and of the group not receiving bevacizumab was the same. The median target volume for all patients receiving salvage SRS was 4.8 cc.
The group of patients who received adjuvant bevacizumab after salvage SRS was similar to the group of patients who did not. There was no statistically significant difference between the two groups in WHO grade, age, sex, time from initial diagnosis to salvage SRS, KPS, or volume treated. Patients treated with bevacizumab received a greater number of salvage systemic therapies after SRS than did patients not receiving bevacizumab (p ≤ 0.0001).
Toxicity
Treatment with salvage SRS was relatively well tolerated. Thirty-two percent of patients experienced acute Grade 2 toxicity and 11% of patients experienced Grade 3 toxicity according to the Common Terminology Criteria for Adverse Events (Table 2). One patient died shortly after (2 weeks) receiving salvage SRS without concurrent or post-SRS systemic therapy as a result of fulminant disease throughout the brain. It was unclear for many of the patients whether the toxicities reported were related to radiosurgery, to adjuvant therapies, and/or to progressive disease. The most commonly reported toxicity, which was seen in 1 out of 4 patients treated, was a worsening of preexisting neurologic symptoms. This acute side effect was managed with dexamethasone, and most patients responded favorably. Twenty-one percent of patients were noted to have an increase in the frequency of seizure activity within 3 months of receiving SRS. New-onset seizures were not seen in any of the patients. Radionecrosis was diagnosed by either imaging or repeat biopsy in 10% of patients who received salvage SRS. In most cases, radionecrosis was associated with mild symptoms or was asymptomatic. The rates of toxicity did not differ significantly between patients who did or did not receive adjuvant bevacizumab.
Table 2.
Reported number and rate of toxicity (Common Terminology Criteria for Adverse Events version 4) seen in patients receiving salvage SRS for recurrent WHO Grade 3 or 4 glioma who did or did not receive bevacizumab (BVZ)
| Total | −BVZ | +BVZ | |
|---|---|---|---|
| Number of patients | 63 | 21 | 42 |
| Toxicity (%) | |||
| Grade 2 | 20 (32) | 7 (33) | 13 (31) |
| Grade 3 | 7 (11) | 3 (14) | 4 (10) |
| Grade 4 | 1 (2) | 1 (5) | 0 (0) |
| Radionecrosis | 6 (10) | 4 (19) | 2 (5) |
| Worsening of neurologic symptoms | 16 (25) | 6 (29) | 10 (24) |
| Increase in seizure activity | 13 (21) | 4 (19) | 9 (21) |
| Fatigue | 4 (6) | 0 (0) | 4 (10) |
| Changes in memory | 4 (6) | 1 (5) | 3 (7) |
| Headache | 4 (6) | 2 (10) | 2 (5) |
Abbreviations: SRS = stereotactic radiosurgery; WHO = World Health Organization; BVZ = bevacizumab.
Survival
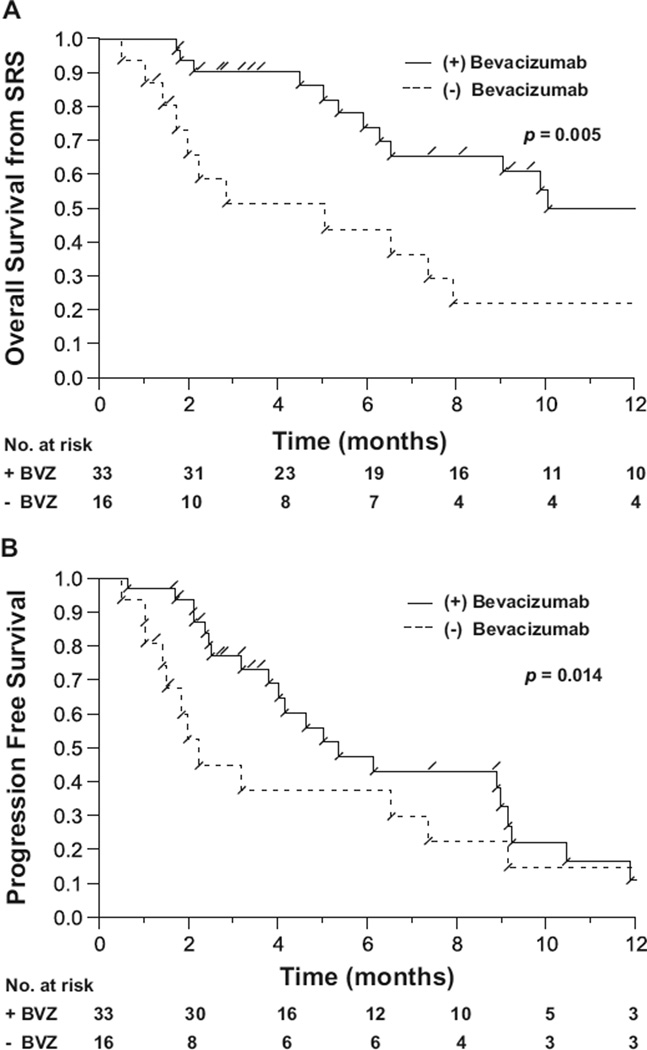
The survival data for patients with a WHO Grade 4 glioma who did or did not receive adjuvant bevacizumab were analyzed. On univariate analysis, there was a statistically significant improvement in OS-SRS in the group of patients who received adjuvant bevacizumab at the time of salvage radiosurgery or shortly thereafter (p = 0.005). The 1-year OS-SRS was 50% in the group of patients receiving adjuvant bevacizumab and 22% in the group not receiving adjuvant bevacizumab (Fig. 1A). The median OS-SRS in patients with a recurrent WHO Grade 4 glioma receiving adjuvant bevacizumab was 11.2 months, compared with 3.9 months in patients not receiving adjuvant bevacizumab. Progression-free survival was also improved in patients receiving adjuvant bevacizumab after SRS, in whom the median PFS was 5.2 months, compared with 2.1 months in patients receiving salvage radiosurgery without adjuvant bevacizumab (p = 0.014) (Fig. 1B). Additionally, OS from the time of primary diagnosis was 47 months for patients treated with adjuvant bevacizumab vs. 25 months for patients treated without adjuvant bevacizumab (p = 0.039).
Fig. 1.
Overall survival (A) and progression-free survival (B) from the time of salvage sterotactic radiosurgery (SRS) for patients with a recurrent World Health Organization Grade 4 glioma who did (+) or did not (−) receive adjuvant bevacizumab (BVZ).
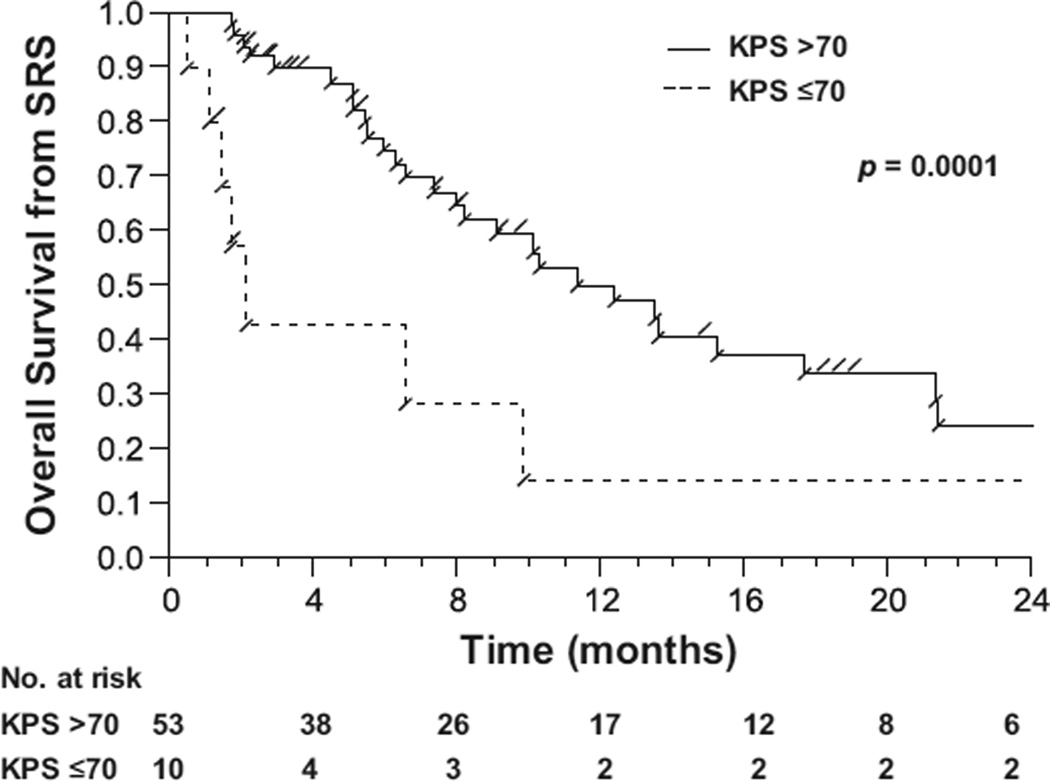
Performance status was a significant predictor of survival. Patients with a KPS >70 had a median OS-SRS of 11.9 months compared with 1.9 months for patients with a KPS <70 (p = 0.0001) (Fig. 2). Patients younger than 50 years old at the time of radiosurgery also had improved OS from the time of SRS. The interval of time from primary diagnosis to salvage SRS did not correlate with OS-SRS. The outcomes in patients treated with the most commonly prescribed systemic agents were analyzed to determine the effect on survival. Besides bevacizumab, irinotecan and CCNU were associated with improved OS survival. However, over 75% of the patients who received irinotecan or CCNU also received bevacizumab.
Fig. 2.
Overall survival from the time of stereotactic radiosurgery (SRS) for patients with a World Health Organization Grade 3 or 4 glioma who had a Karnofsky performance status (KPS) >70 compared with patients with a KPS ≤70.
Table 3 shows the results from a multivariate analysis using a Cox proportional hazards model in patients with WHO Grade 4 glioma treated with salvage SRS. The risks of death and progression were significantly reduced in patients receiving SRS plus adjuvant bevacizumab, with a risk ratio of 0.37 (confidence interval [CI] 0.17–0.82, p = 0.015) and 0.45 (CI 0.21–0.97, p = 0.043), respectively. A KPS >70 was also associated with improved survival from the time of SRS (hazard ratio [HR] 0.24, CI 0.09–0.63, p = 0.005). Age >50 years was associated with an increased risk of death (HR 2.23, CI 1.01–5.10, p = 0.047) and of progression (HR 3.42, CI 1.56–8.07, p = 0.002). Treatment volume was not significantly correlated with survival or progression.
Table 3.
Risk of death and risk of progression after salvage radiosurgery for recurrent Grade 4 glioma with 95% confidence intervals and p values calculated from a multivariable Cox proportional hazards model
| Risk ratio | 95% CI | p | |
|---|---|---|---|
| Risk of death | |||
| Bevacizumab | 0.37 | 0.17–0.82 | 0.015 |
| KPS >70 | 0.24 | 0.09–0.63 | 0.005 |
| Age >50 y | 2.23 | 1.01–5.10 | 0.047 |
| Volume >5 cc | 1.40 | 0.63–3.10 | 0.406 |
| Risk of progression | |||
| Bevacizumab | 0.45 | 0.21–0.97 | 0.043 |
| KPS >70 | 0.59 | 0.25–1.53 | 0.265 |
| Age >50 y | 3.42 | 1.56–8.07 | 0.002 |
| Volume >5 cc | 0.61 | 0.28–1.30 | 0.199 |
Abbreviations: CI = confidence interval; KPS = Karnofsky performance status.
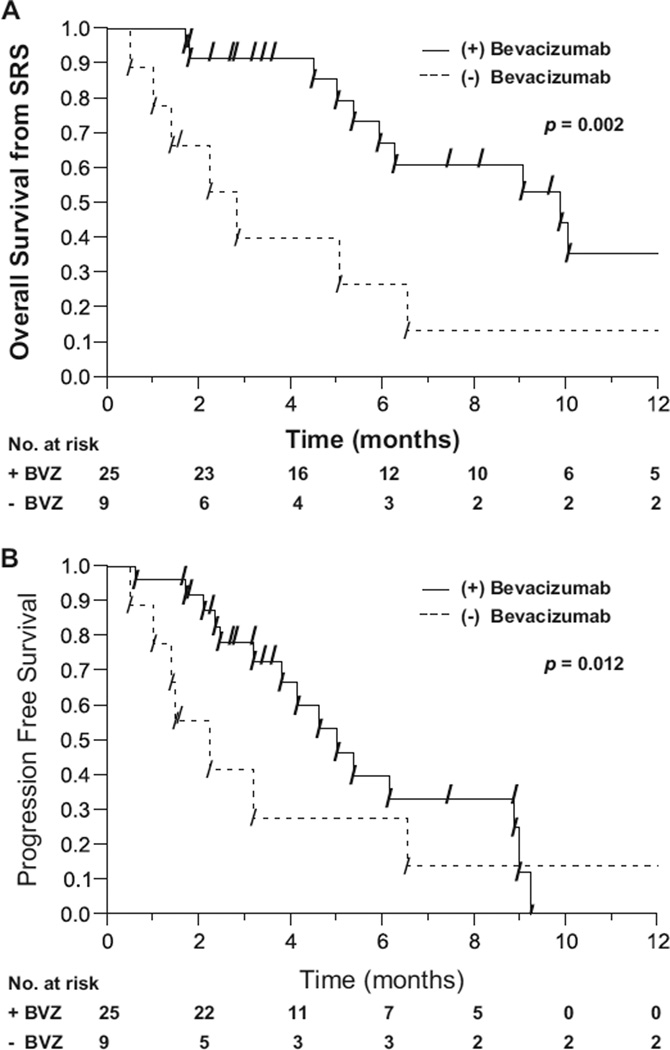
The subset of patients who had experienced progression while receiving bevacizumab before undergoing salvage radiosurgery was analyzed to determine if additional bevacizumab was associated with improved outcomes. Thirty-four patients with a WHO Grade 4 glioma had experienced progression while receiving a treatment regimen containing bevacizumab before undergoing radiosurgery. Of these 34 patients, 25 received additional adjuvant bevacizumab at the time of SRS or shortly thereafter. Nine patients did not receive additional bevacizumab. The patients receiving additional adjuvant bevacizumab had a median OS-SRS of 9.8 months compared with 2.5 months in the group not receiving adjuvant bevacizumab (p = 0.002) (Fig. 3A). Furthermore, the patients receiving adjuvant bevacizumab had a median PFS of 6.3 months vs. 1.4 months in patients not receiving additional bevacizumab (p = 0.012) (Fig. 3B).
Fig. 3.
Overall survival (A) and progression-free survival (B) from stereotactic radiosurgery (SRS) in patients with World Health Organization Grade 4 glioma who had experienced progression while receiving bevacizumab before radiosurgery and who did or did not receive additional bevacizumab at the time of or shortly after salvage radiosurgery.
Nine patients with a recurrent WHO Grade 4 glioma received bevacizumab before salvage radiosurgery only. The median OS from the time of SRS was only 2.5 months in this group of patients, which was significantly worse than in the group of patients receiving bevacizumab after radio-surgery (median survival, 11.2 months; p = 0.01). Survival in patients who received bevacizumab before salvage SRS only was also worse than in patients who received bevacizumab both before and after SRS (median survival, 9.8 months; p = 0.03).
Radiographic findings after radiosurgery
Postradiosurgery MRI scans were available for review on 55 of the 63 patients treated with salvage SRS for a recurrent glioma. The initial post-SRS MRI showed progression in 25 patients, stable disease in 16 patients, and a response in 14 patients. Of the 25 patients who had evidence of progression on the initial post-SRS MRI, 10 were found to have either stable disease or a treatment response on a subsequent scan indicating that the initial MRI demonstrated pseudoprogression. The median interval from the time of SRS to the first post-SRS MRI in patients who showed pseudoprogression was 2 months (range, 2 weeks to 6 months). The incidence of pseudoprogression was 15% in patients who received bevacizumab during or after SRS compared with 25% in patients who did not receive bevacizumab during or after SRS.
DISCUSSION
Most patients treated with surgery and adjuvant chemoradiation for a high-grade glioma of the brain experience recurrence within 1 to 2 years of initial diagnosis. Salvage therapies including chemotherapy and SRS are frequently used but are often unsuccessful. Wong et al. performed an analysis of eight different chemotherapy trials for recurrent glioma and found a median PFS of 10 weeks and OS of 30 weeks (23). In more recent studies, bevacizumab as a single agent and in combination with irinotecan provided a median PFS and OS of 6 and 9 months, respectively (21, 22).
In the current study, the median PFS and OS from the time of SRS were, respectively, 26 weeks and 43 weeks for all patients and 35 weeks and 51 weeks for patients who received adjuvant bevacizumab. The median OS from the time of initial diagnosis was 41 months for all patients and 35 months for patients with a WHO Grade 4 glioma. For reference, the median OS observed in randomized trials of radiation therapy alone and radiation therapy with temozolomide is 9 to 15 months and 10 to 21 months, respectively (1, 24).
Table 4 shows survival data from published reports on stereotactic radiotherapy for recurrent glioblastoma. In the current study, survival from the time of SRS was similar to the results in other series where the median OS and PFS from SRS was 7 to 13 months and 4 to 7 months, respectively. The OS from the time of initial (primary) diagnosis with a malignant glioma was considerably higher in the cohort of patients analyzed in our study than in previous series.
Table 4.
Patient outcomes from published reports on the use of salvage stereotactic radiotherapy for recurrent World Health Organization Grade 4 glioma compared with the results from the current study
| Study | n | Median OS (mo) | Median OS from SRS (mo) | Median PFS from SRS (mo) | Median KPS |
|---|---|---|---|---|---|
| Hall et al. (13) | 26 | 18 | 8 | − | 70 |
| Combs et al. (4) | 32 | 22 | 10 | 5 | 80 |
| Kong et al. (11) | 65 | 23 | 13 | 5 | 100 |
| Biswas et al. (14) | 33 | 17 | 7 | 4 | – |
| Cho et al. (12) | 42 | – | 7 | – | 70 |
| Fogh et al. (15) | 105 | 23 | 11 | – | – |
| Gutin et al. (+BVZ) (34) | 20 | – | 13 | 7 | 90 |
| Current study (+BVZ) | 33 | 47 | 11 | 5 | 80 |
| Current study (−BVZ) | 16 | 25 | 4 | 2 | 80 |
| Current study (all) | 49 | 35 | 9 | 5 | 80 |
Abbreviations: OS = overall survival; PFS = progression-free survival; BVZ = bevacizumab.
In prior studies by Vredenburgh et al. (21) and Desjardins et al. (22), treatment of recurrent gliomas with bevacizumab and irinotecan was found to be well tolerated, with one cerebral hemorrhage and four thromboembolic complications in 35 patients. In the current study, toxicity did not differ between patients who did or did not receive adjuvant bevacizumab with salvage radiosurgery. In many patients, it was difficult to determine if toxicity was related to SRS, adjuvant systemic therapy, and/or disease progression. Overall, SRS was well tolerated, and most side effects could be managed with steroids and/or antiepileptic medications.
There is evidence that bevacizumab works synergistically with chemotherapy and radiation therapy. Anti-VEGF therapy has been shown to normalize tumor vasculature and to decrease interstitial fluid pressure, resulting in increased bioavailability of chemotherapy agents (25, 26). Bevacizumab has also been shown to be cytotoxic to glioma stem cells, which are believed to be more resistant to traditional chemotherapy (27, 28). Radiation therapy can stimulate VEGF production in tumors, which paradoxically leads to increased angiogenesis (29, 30). Furthermore, high-dose SRS has a substantial vascular endothelial cell ablative effect, which may be far more potent than what is observed in conventional radiation therapy (31). Bevacizumab has also been shown to decrease cerebral edema and radio-necrosis after SRS (32, 33).
Appropriate patient selection for salvage SRS seems important to maximize benefits from this treatment modality. In our series, patients with a KPS ≤70 had minimal benefit from salvage radiosurgery. Older patients were also found to have poorer outcomes on multivariate analysis. Bevacizumab has been shown to be well tolerated when used with concurrent radiation therapy in many disease sites. A recently published study from Memorial Sloan-Kettering evaluating the toxicity and efficacy of bevacizumab and hypofractionated stereotactic radiotherapy for recurrent glioma (34) found minimal treatment-associated toxicity. The Memorial Sloan-Kettering study examined hypofractionated stereotactic radiotherapy and bevacizumab as salvage treatment after postradiation recurrence. By contrast, salvage biochemotherapy had been unsuccessful in nearly all of the patients treated in our study (including 39 patients treated with bevacizumab) before they underwent SRS, and SRS was typically given as a last resort after other options had been exhausted.
A major challenge in neurooncology is effectively treating patients with recurrent malignant gliomas in whom bevacizumab has been unsuccessful. Bevacizumab plus irinotecan has been shown to be an effective salvage regimen with good response rates. However, patients typically do not respond to a second bevacizumab-containing regimen. In the current study, patients in whom bevacizumab before radiosurgery had been unsuccessful were found to benefit from additional bevacizumab at the time of salvage radiosurgery or shortly thereafter. A recent study by Torcuator et al. (35) found that bevacizumab plus SRS/fractionated stereotactic radiotherapy was associated with improved survival compared with bevacizumab without SRS/fractionated stereotactic radiotherapy in a population of patients in whom a bevacizumab-containing regimen had previously been unsuccessful. Our findings are consistent with this report and indicate that salvage SRS with bevacizumab may benefit patients with recurrent gliomas who have experienced progression while receiving bevacizumab-based chemotherapy regimens.
Pseudoprogression is characterized by progressive changes on a posttreatment radiographic study that disappear on a subsequent study or are ruled out by a biopsy. Chamberlain et al. reported on pseudoprogression in a series of patients treated with temozolomide and radiation for newly diagnosed glioblastoma (36). In this report, 26 patients had evidence of progression on their initial posttreatment MRI. Fifteen of these patients underwent reoperation, which found no evidence of tumor in 7 of the patients. Another report from Taal et al. found that 15 of 31 patients whose initial posttreatment MRI showed progression were found to have stable disease on a subsequent scan (37). In our study, 10 of the 25 patients whose initial MRI showed evidence of progression were found to have stable disease on a subsequent scan or biopsy. The rate of pseudoprogression was lower in patients who received bevacizumab after SRS. Bevacizumab has been shown to reduce interstitial fluid pressure, edema, and radionecrosis (32, 33). These findings may explain the reduction in the rate of pseudoprogression seen in the group of patients receiving post-SRS bevacizumab.
A prospective study is under way at our institution to explore the effects of bevacizumab and salvage SRS (National Cancer Institute code NCT01017250). In this study, patients with a recurrent malignant glioma receive intravenous bevacizumab (10 mg/kg) the day of salvage SRS. A second bevacizumab dose is administered 2 weeks later. The primary endpoint is neurotoxicity, and secondary endpoints include PFS, OS, quality of life measures, neurocognitive changes, and MRI-based radiographic estimates of mass transfer. A multi-institutional study will ultimately be required to determine the efficacy and long-term toxicity of concurrent bevacizumab and SRS in patients with a recurrent malignant glioma.
Footnotes
Presented at the 51st Annual Meeting of the American Society for Radiation Oncology, Chicago, IL, November 1–5, 2009.
Conflict of interest: none.
REFERENCES
- 1.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study, 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 2.Sneed PK, Gutin PH, Larson DA, et al. Patterns of recurrence of glioblastoma multiforme after external irradiation followed by implant boost. Int J Radiat Oncol Biol Phys. 1994;29:719–727. doi: 10.1016/0360-3016(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 3.Combs SE, Gutwein S, Thilmann C, et al. Stereotactically guided fractionated re-irradiation in recurrent glioblastoma multiforme. J Neurooncol. 2005;74:167–171. doi: 10.1007/s11060-004-2463-y. [DOI] [PubMed] [Google Scholar]
- 4.Combs SE,Widmer V, Thilmann C, et al. Stereotactic radiosurgery: Treatment option for recurrent glioblastoma multiforme. Cancer. 2005;104:2168–2173. doi: 10.1002/cncr.21429. [DOI] [PubMed] [Google Scholar]
- 5.Dirks P, Bernstein M, Muller PJ, et al. The value of reoperation for recurrent glioblastoma. Can J Surg. 1993;36:271–275. [PubMed] [Google Scholar]
- 6.Harsh GR, Levin VA, Gutin PH, et al. Reoperation for recurrent glioblastoma and anaplastic astrocytoma. Neurosurgery. 1987;21:615–621. doi: 10.1227/00006123-198711000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mayer R, Sminia P. Reirradiation tolerance of the human brain. Int J Radiat Oncol Biol Phys. 2008;70:1350–1360. doi: 10.1016/j.ijrobp.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 8.Brandes AA, Fiorentino MV. The role of chemotherapy in recurrent malignant gliomas: An overview. Cancer Invest. 1996;14:551–559. doi: 10.3109/07357909609076900. [DOI] [PubMed] [Google Scholar]
- 9.Rajan B, Ross G, Lim CC, et al. Survival in patients with recurrent glioma as a measure of treatment efficacy: Prognostic factors following nitrosourea chemotherapy. Eur J Cancer. 1994;30A:1809–1815. doi: 10.1016/0959-8049(94)00248-4. [DOI] [PubMed] [Google Scholar]
- 10.Brandes AA, Tosoni A, Amista P, et al. How effective is BCNU in recurrent glioblastoma in the modern era? A phase II trial. Neurology. 2004;63:1281–1284. doi: 10.1212/01.wnl.0000140495.33615.ca. [DOI] [PubMed] [Google Scholar]
- 11.Kong DS, Lee JI, Park K, et al. Efficacy of stereotactic radiosurgery as a salvage treatment for recurrent malignant gliomas. Cancer. 2008;112:2046–2051. doi: 10.1002/cncr.23402. [DOI] [PubMed] [Google Scholar]
- 12.Cho KH, HallWA, Gerbi BJ, et al. Single dose versus fractionated stereotactic radiotherapy for recurrent high-grade gliomas. Int J Radiat Oncol Biol Phys. 1999;45:1133–1141. doi: 10.1016/s0360-3016(99)00336-3. [DOI] [PubMed] [Google Scholar]
- 13.Hall WA, Djalilian HR, Sperduto PW, et al. Stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol. 1995;13:1642–1648. doi: 10.1200/JCO.1995.13.7.1642. [DOI] [PubMed] [Google Scholar]
- 14.Biswas T, Okunieff P, Schell MC, et al. Stereotactic radiosurgery for glioblastoma: Retrospective analysis. Radiation Oncol. 2009;4:11. doi: 10.1186/1748-717X-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fogh SE, Andrews DW, Glass J, et al. Hypofractioned stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010:3048–3053. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 17.Kim KJ, Li B,Winer J, et al. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993;362:841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- 18.Presta LG, Chen H, O’Connor SJ, et al. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997;57:4593–4599. [PubMed] [Google Scholar]
- 19.Salmaggi A, Eoli M, Frigerio S, et al. Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol. 2003;62:297–303. doi: 10.1023/a:1023367223575. [DOI] [PubMed] [Google Scholar]
- 20.Lamszus K, Ulbricht U, Matschke J, et al. Levels of soluble vascular endothelial growth factor (VEGF) receptor 1 in astrocytic tumors and its relation to malignancy, vascularity, and VEGF-A. Clin Cancer Res. 2003;9:1399–1405. [PubMed] [Google Scholar]
- 21.Vredenburgh JJ, Desjardins A, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol. 2007;25:4722–4729. doi: 10.1200/JCO.2007.12.2440. [DOI] [PubMed] [Google Scholar]
- 22.Desjardins A, Reardon DA, Herndon JE, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2008;14:7068–7073. doi: 10.1158/1078-0432.CCR-08-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 24.Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. The Medical Research Council Brain Tumour Working Party. Br J Cancer. 1991;64:769–774. doi: 10.1038/bjc.1991.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 26.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: Role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6:553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bao S, Wu Q, Sathornsumetee S, et al. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006;66:7843–7848. doi: 10.1158/0008-5472.CAN-06-1010. [DOI] [PubMed] [Google Scholar]
- 29.Moeller BJ, Cao Y, Li CY, et al. Radiation activates HIF-1 to regulate vascular radiosensitivity in tumors: Role of reoxygenation, free radicals, and stress granules. Cancer Cell. 2004;5:429–441. doi: 10.1016/s1535-6108(04)00115-1. [DOI] [PubMed] [Google Scholar]
- 30.Heath VL, Bicknell R. Anticancer strategies involving the vasculature. Nat Rev Clin Oncol. 2009;6:395–404. doi: 10.1038/nrclinonc.2009.52. [DOI] [PubMed] [Google Scholar]
- 31.Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240–243. doi: 10.1016/j.semradonc.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez J, Kumar AJ, Conrad CA, et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67:323–326. doi: 10.1016/j.ijrobp.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Gerstner ER, Duda DG, di Tomaso E, et al. VEGF inhibitors in the treatment of cerebral edema in patients with brain cancer. Nat Rev Clin Oncol. 2009;6:229–236. doi: 10.1038/nrclinonc.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutin PH, Iwamoto FM, Beal K, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–163. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torcuator RG, Thind R, Patel M, et al. The role of salvage reirradiation for malignant gliomas that progress on bevacizumab. J Neurooncol. 2010;97:401–407. doi: 10.1007/s11060-009-0034-y. [DOI] [PubMed] [Google Scholar]
- 36.Chamberlain M, Glantz M, Chalmers, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82:81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 37.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoradiation with temozolomide. Cancer. 2008;113:405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]