Abstract
Although androgen ablation therapy is effective in treating primary prostate cancers, a significant number of patients develop incurable castration-resistant disease. Recent studies have suggested a potential synergy between vaccination and androgen ablation, yet the enhanced T-cell function is transient. Using a defined tumor antigen model, UV-8101-RE, we found that concomitant castration significantly increased the frequency and function of antigen-specific CD8+ T cells early after the immunization of wild-type mice. However, at a late time point after immunization, effector function was reduced to the same level as noncastrated mice and was accompanied by a concomitant amplification in CD4+CD25+Foxp3+ regulatory T cells (Treg) following immunization. We investigated whether Treg expansion occurred following castration of prostate tumor–bearing mice. In the prostate-specific Pten−/− mouse model of prostate cancer, we observed an accelerated Treg expansion in mice bearing the castration-resistant endogenous prostate tumor, which prevented effector responses to UV-8101-RE. Treg depletion together with castration elicited a strong CD8+ T-cell response to UV-8101-RE in Pten−/− mice and rescued effector function in castrated and immunized wild-type mice. In addition, Treg expansion in Pten−/− mice was prevented by in vivo interleukin (IL)-2 blockade suggesting that increased IL-2 generated by castration and immunization promotes Treg expansion. Our findings therefore suggest that although effector responses are augmented by castration, the concomitant expansion of Tregs is one mechanism responsible for only transient immune potentiation after androgen ablation.
Introduction
Prostate cancer is the most commonly diagnosed malignancy and the second leading cause of cancer-related deaths in males of the Western world (1). Effective treatment of prostate cancer currently involves radical prostatectomy, radiation therapy, and/or androgen ablation (2). Removal of androgen leads to decreased proliferation and programmed cell death in prostate epithelial cells. Castration-resistant tumor and metastatic disease that may arise years later is largely incurable.
Immune therapy may be a promising treatment modality for castration-resistant prostate cancer (3). However, the role of androgen in modulating immune function and the consequence of androgen removal/blockade on adaptive immune responses is not completely understood. Androgen is generally considered as immunosuppressive, and hormone removal increases T-cell function in autoimmune disease models (4, 5). These observations suggest that androgen removal may augment T-cell responses following immunization against prostate tumor antigens, including self-proteins. However, CD8+ T-cell responses to a prostate self-antigen expressed in murine prostate cancer cells were only accentuated if immunization was before castration (6). In contrast, the function of transgenic CD8+ T-cells specific for prostate-specific antigen was enhanced until 4 weeks after castration (7). Furthermore, in a CD4+ transgenic T-cell model, a synergy between androgen deprivation and vaccination alleviated systemic tolerance to a model prostate tumor antigen and increased T-cell proliferation early after castration (8). Thus, the ability of androgen ablation to augment immune responses to prostate tumor antigens may depend on the antigen in question and the strength of the T-cell response.
To determine the mechanism responsible for transiently increased effector function, we first investigated the effect of androgen ablation on CTL responses to a well-defined immunodominant tumor antigen in nontumor-bearing C57BL/6 mice. The ultraviolet light–induced murine sarcoma, UV-8101-RE, expresses a mutant tumor-specific protein that induces a strong CD8+ T-cell response (9). We immunized mice intraprostatically with this antigen and found that concomitant castration enhanced the proportion of antigen-specific CD8+ T cells in the spleen and increased their function early after immunization, which declined by 5 weeks after immunization. However, we also observed an increased proportion of CD4+CD25+Foxp3+ regulatory T cells (Tregs) in the castrated and immunized animals.
We previously showed that functional CD8+ T cells responding to unknown prostate tumor antigens transiently increased following castration of prostate-specific Pten−/− mice (10). In this study, we show that castration of Pten−/− mice also expanded Tregs. An increased frequency of functional CD8+ T cells was only detected when Tregs were depleted before castration and immunization with the model antigen UV-8101-RE. We further show that in vivo blockade of interleukin (IL)-2 prevents Treg expansion. Thus, androgen ablation amplifies both stimulatory and inhibitory arms of the immune system.
Materials and Methods
Animals and cell lines
C57BL/6 mice (8- to 12-week-old; Jackson Laboratory) were maintained under pathogen-free conditions in accordance with Institutional Animal Care and Use Committee guidelines. Prostate-specific Pten−/− mice (12- to 16-week-old) on the syngeneic C57BL/6 background were bred as described previously (10, 11). UV-8101-RE (from Dr. Hans Schreiber, University of Chicago, Chicago, IL) and TRAMP-C1 (from Dr. Owen Witte, UCLA, Los Angeles, California) cell lines were cultured as described previously (10). The authenticity of UV-8101-RE was determined by specific killing of the cell line by α-8101–specific T cells that also recognize the mutant peptide epitope. Expression of SV40 viral DNA sequences and H-2Kb were determined in TRAMP-C1 cells.
Surgery
Mice were injected intraperitoneally (i.p.) with ketamine and xylazine (23.75 mg/mL ketamine + 1.25 mg/mL xylazine; 150 mg/kg). A lower midline incision was made and testes were removed. For immunization, 1 × 106 live UV-8101-RE cells in 20 μL volume, containing bromphenol blue for visualization were injected into both anterior and dorsal lobes of the prostate. All surgical and postsurgical care was in accordance with Wake Forest School of Medicine (WFSM) Winston-Salem, NC, Animal Care and Use Committee guidelines.
ELISPOT assays
Rat anti-mouse IFN-γ antibody (2.5 μg/mL, clone R4-6A2; BD Pharmingen) was used to coat 96-well filtration plates (Millipore). Splenocytes (1 × 106) were stimulated ex vivo with UV-8101-RE cells at 1:1 ratio with irradiated (1,200 rad) syngenic splenocytes as feeder cells for 36 hours in RPMI-1640 medium supplemented with 10% fetal calf serum, 4 mmol/L l-glutamine, and 50 μmol/L 2-mercaptoethanol. After culture, plates were washed and incubated with biotinylated anti-IFN-γ antibody (2.5 μg/mL, clone XMG 1.2; BD Pharmingen). Spots were developed with 3,3′-diaminobenzidine (DAB) substrate (Sigma). The plates were scanned with a plate scanner (Cellular Technology Ltd.) equipped with Image Acquisition 4.5 software, counted using ImageJ software (www.rsbweb.nih.gov/ij) and calculated as number of spot-forming cells per spleen.
Mixed lymphocyte tumor cell cultures and 51Cr release assays
Mixed lymphocyte tumor cell cultures (MLTC) and 51Cr release assays were conducted as described previously (9). The percentage of specific lysis was calculated by the formula: % cytolysis = [(experimental release – spontaneous release)/(maximum release – spontaneous release)] × 100.
Treg depletion
Tregs were depleted by a single i.p. injection of 0.5 mg anti-CD25 antibody (ref. 12; clone PC61; BioXCell), 2 days before castration and/or immunization.
IL-2 neutralization
To neutralize IL-2 function, mice were given 1 mg of anti-IL-2 mAb (clone S4B6; BioXCell) or isotype control i.p. 2 days before castration or sham surgery, as previously described (13).
Peptide production
The mutant p68 RNA helicase peptide (SNFVFAGI) was produced by the WFSM Protein Analysis Core Laboratory essentially as described previously (9). Briefly, the peptide was synthesized on an Applied Biosystems Model 430B automated peptide synthesizer, using the standard Applied Biosystems FastMoc solid-phase peptide synthesis chemistry protocol. The peptide identity and purity were shown by reverse-phase high-performance liquid chromatography, quantitative amino acid analysis, and matrix-assisted laser desorption/ionization—time-of-flight (MALDI-TOF) mass spectrometry.
Flow cytometric analysis
Splenocytes were dissociated and stained with fluorochrome-conjugated antibodies specific for CD4 [clone RM4-5, fluorescein isothiocyanate (FITC)-conjugated], CD8 (clone 53-6.7, FITC-conjugated), and CD62L [clone MEL-14, allophy-cocyanin (APC)-conjugated] from BD Pharmingen; and CD44 [clone IM7, phycoerythrin (PE)-conjugated] and CD25 (clone PC61.5, PE-conjugated) from eBioscience, all used at 1:100 dilution. Tregs were analyzed using the mouse Treg Staining Kit (eBioscience), and manufacturer’s instructions. Cells were analyzed on a FACSCalibur equipped with Cellquest PRO software (BD Biosciences).
Tetramer production
MHC monomer was constructed and purified as described (14), except a plasmid clone expressing H-2Kb was used. The folding, purification, and biotinylation of Kb + peptide complexes were conducted as described (15). To prepare tetramer, 20 μL of monomer (2 mg/mL) was incubated at room temperature with one tenth volume of 128 μL streptavidin-PE (0.5 mg/mL; Jackson ImmunoResearch) or 95 μL streptavidin-PerCP-Cy5.5 (0.2 mg/mL; BD Pharmingen) added in 10-minute intervals and incubated in the dark.
Tetramer enrichment
Tetramer enrichment was conducted as described previously (16). Dissociated splenocytes stained with PE- and PerCP-Cy5.5–conjugated tetramers (4 μg/mL each) were also co-stained with CD8-FITC and CD44-APC. PE tetramer–positive cells were enriched with anti-PE magnetic beads (BD Biosciences) and analyzed on a FACSCalibur. Tetramer-positive cells were defined as PE and PerCP-Cy5.5 double-positive cells when gated on the CD8+CD44+ population. Nearly 100% of the tetramer-positive cells were CD44hi.
Intracellular cytokine staining
MLTC cell suspensions were restimulated with UV-8101-RE cells at the ratio of 1:1 for 4 hours in the presence of 10 μg/mL brefeldin A (Sigma). TRAMP-C1 cells were used as negative control. Following surface CD8 staining, intracellular staining for IFN-γ (cloneXMG1.2; BDPharmingen) was conducted with the Cytofix/Cytoperm Kit (BD Biosciences).
TGF-β ELISA
Blood samples were collected from mice by cardiac puncture and the serum harvested by centrifugation. Total TGF-β levels were determined using a commercial ELISA kit (Promega).
Statistical analysis
Statistical comparisons were conducted using the unpaired Student t test with a 2-sided α level of 0.05. Differences in values at P < 0.05 were considered significant.
Results
Precursor frequency and function of CD8+ T cells responding to a model tumor antigen increased following castration and immunization of nontumor-bearing mice
Previous studies reported that castration induces infiltration of T cells into the prostate in both normal (17) and prostate tumor–bearing mice (10, 17). Using the prostate-specific Pten−/− mouse model of endogenous prostate cancer, we showed that the accumulation of functional T cells in the prostate glands is not long-lasting, evident at 2.5 weeks after castration but diminished at 5 weeks after castration (10).
Antigens shed by dying prostate cancer cells after castration may stimulate CD8+ T-cell responses. In an effort to track effector responses to an antigen located in the prostate, we used a defined tumor model system. The C57BL/6-derived murine sarcoma cell line UV-8101-RE expresses an H-2Kb–restricted immunodominant antigen, which elicits a strong CTL response that rejects the tumor cells (9). To model T-cell interactions to prostate antigens, normal male C57BL/6 mice were immunized with UV-8101-RE cells intraprostatically in both anterior and dorsolateral prostate lobes and were either castrated or sham-treated. Spleens and prostate draining lymph nodes (PDLN) were harvested 2.5 weeks later.
To quantify the number of UV-8101–specific responding CD8+ T cells, we produced H-2Kb tetramers complexed with the mutant peptide. Primary ex vivo analysis of splenocytes detected an increased frequency of tetramer-positive CD8+ T cells in castrated and immunized mice, compared with sham-castrated mice (Fig. 1A). ELISPOT analysis also detected increased IFN-γ–secreting cells in the PDLNs and spleens (Fig. 1B), confirmed by in vitro analysis of cultured splenocytes (Fig. 1C and D). Thus, castration increased the number and functional capacity of CD8+ T cells specific for UV-8101-RE.
Figure 1.
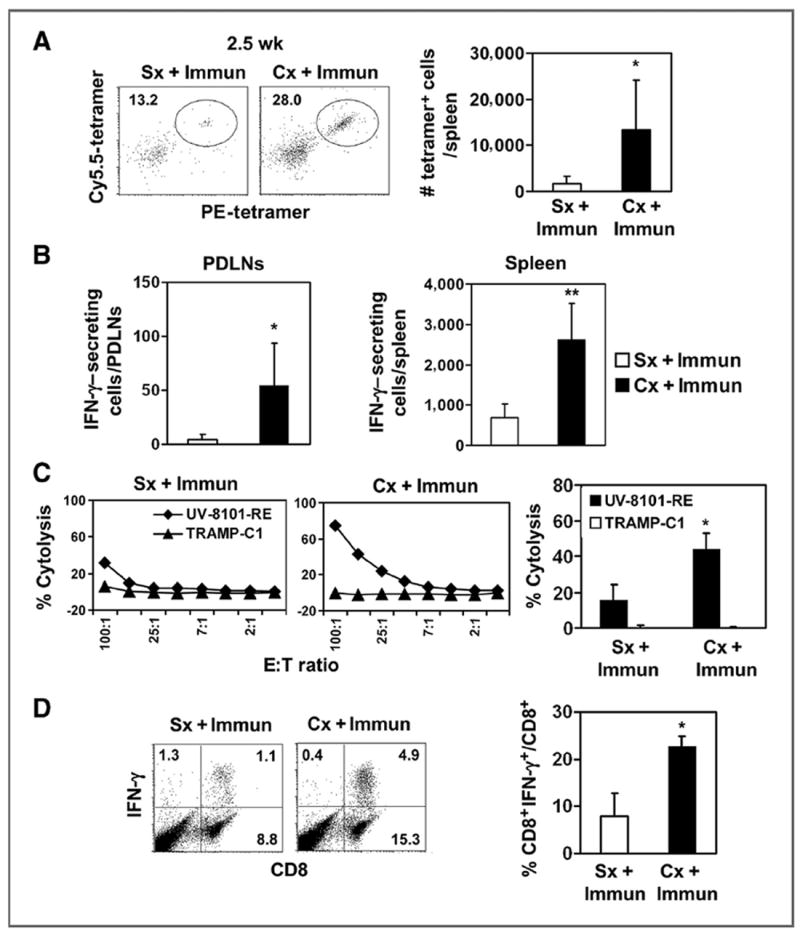
Enhanced antigen-specific effector responses 2.5 weeks after castration and immunization. A, splenocytes were stained with CD8, CD44, PE-tetramer, and Cy5.5-tetramer. Cells enriched with anti-PE beads were analyzed. The representative dot plot (left) shows PE- and Cy5.5-tetramer double-positive cells gated on the CD8+CD44+ population. The number of tetramer-positive cells was quantified (bar graphs; ■, Cx + Immun; □, Sx + Immun). The experiment was repeated once. B, IFN-γ–secreting cells in response to ex vivo UV-8101-RE stimulation were identified using ELISPOT assay with PDLNs (left) and spleen cells (right) from both groups. The average of triplicate wells in 1 representative experiment is shown. C, lytic capacity of MLTC cells, 2.5 weeks after immunization, was determined in a 51Cr release assay. Targets are UV-8101-RE (◆) and TRAMP-C1 (▲). Line graphs show one representative animal from each group and bar graphs show the average of all animals in each group at an effector:target (E:T) ratio of 50:1. D, effector cytokine production was determined by intracellular cytokine staining of MLTC cells. Flow diagram (left) shows representative samples of CD8+ cells that express IFN-γ after ex vivo restimulation. The percentage of CD8+ T cells that express IFN-γ was calculated (right). Data are mean values ± SD. *, P < 0.05; **, P < 0.01 for Sx + immunization (Immun) versus Cx + immunization. One representative experiment with 3 to 5 animals per group is shown. Each experiment was repeated at least once. Cx, castration; Sx, sham treatment.
Enhanced effector function declined 5 weeks after castration and immunization of nontumor-bearing mice
To determine the longevity of effector responses after castration and immunization, we evaluated mice 5 weeks following treatment. The frequency of tetramer-positive cells was similar in castrated or sham-castrated mice (Fig. 2A). Both groups also had similar numbers of IFN-γ–secreting cells in the spleens or PDLNs as determined by ELISPOT analysis (Fig. 2B). MLTCs from mice that were castrated or sham-castrated had low lytic activity (Fig. 2C) and IFN-γ production (Fig. 2D) after ex vivo restimulation at 5 weeks after treatment. Thus, increased function after castration diminished rapidly.
Figure 2.
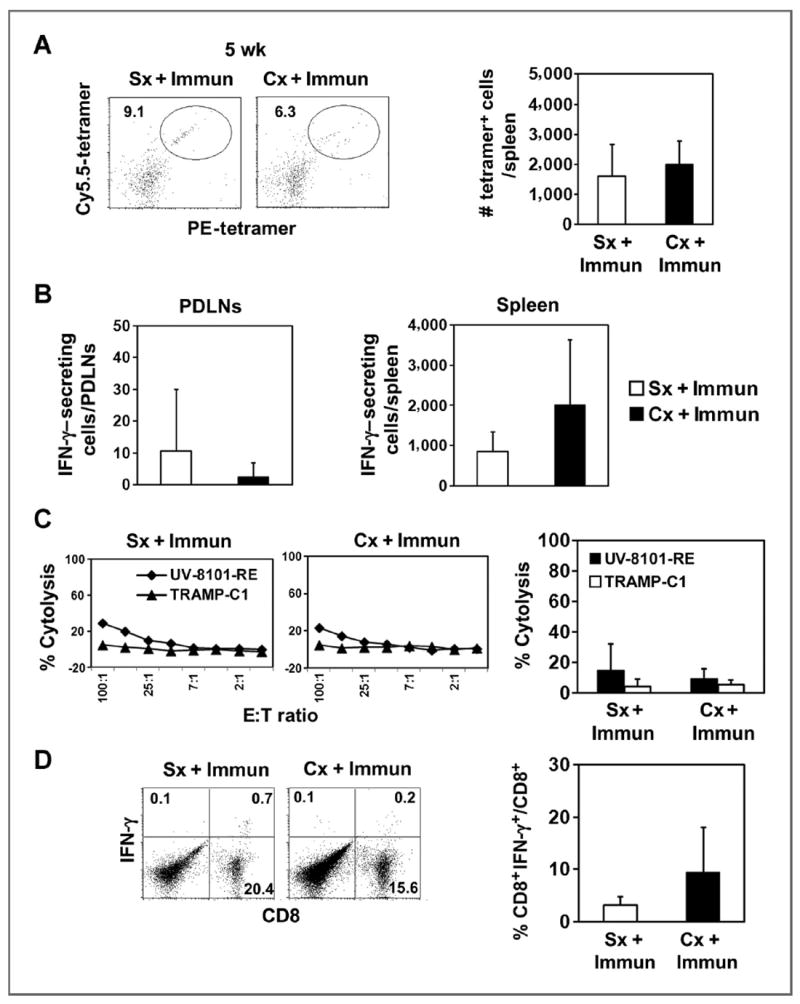
Enhancement of CD8+ effector function by castration was diminished 5 weeks after immunization. A, the representative dot plot (left) shows PE- and Cy5.5-tetramer double-positive cells gated on the CD8+CD44+ population as described in Fig. 1. The number of tetramer-positive cells was quantified and represents the average of 5 animals in one experiment (bar graphs; ■, Cx + Immun; □, Sx + Immun). B, IFN-γ–secreting cells in response to ex vivo UV-8101-RE stimulation were identified using an ELISPOT assay with PDLNs (left) and spleen cells (right) from both groups. The average of triplicate wells in one representative experiment is shown. C, lytic capacity of MLTC cells cultured for 6 days was determined by 51Cr release assay (◆, UV-8101-RE; ▲, TRAMP-C1). Line graphs show one representative animal from each group and the bar graph shows the average of all animals in each group at an E:T ratio of 50:1. D, IFN-γ expression by MLTC cells was assessed by an intracellular cytokine staining assay (left). Percentages of IFN-γ–expressing cells gated on CD8+ T cells were calculated (right). Data are presented as mean values ± SD and one representative experiment of 3 independent experiments with 3 to 5 mice per group is shown. Cx, castration; Immun, immunization; Sx, sham treatment.
Castration increased splenic Tregs following immunization of nontumor-bearing mice
We investigated the mechanism for transient effector function after castration. Systemic Tregs can suppress CD8+ T-cell responses to immunization (18-20). We quantified the proportion and number of Tregs in wild-type animals following immunization and found similar levels at 2.5 weeks following castration and immunization, compared with sham-castrated and immunized mice (Fig. 3A). Interestingly, we detected a modest but significant increase in the percentage of Tregs 5 weeks after castration and immunization (Fig. 3B, representative flow dot plots in Supplementary Fig. S1). The numbers of Tregs were also increased because the spleens were larger after castration (data not shown) as previously reported (5, 21). The frequency and number of Tregs in PDLNs was similar in castrated and intact immunized mice (data not shown). In addition, Tregs expanded only after immunization, as castration alone did not increase Treg proportion or number in the spleen at either time point (Fig. 3C) or in the PDLNs (data not shown), which is consistent with previous reports (5, 22).
Figure 3.
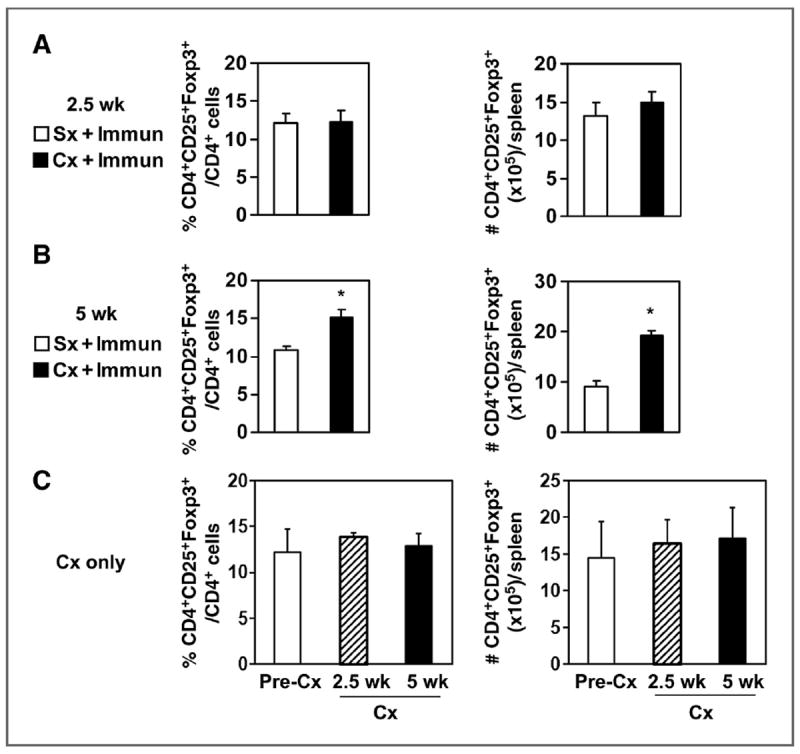
Castration increased splenic CD4+CD25+Foxp3+ Tregs by 5 weeks after immunization of wild-type mice. Splenocytes from mice isolated at 2.5 weeks (A) or 5 weeks after immunization (B), and castration or sham treatment were analyzed by flow cytometry. The number of CD4+CD25+Foxp3+ cells was calculated by multiplying the percentage of CD4+CD25+Foxp3+ cells times the total number of isolated splenocytes and represents the average of 3 animals in the group. *, P < 0.01 versus immunization only group. Data are from one representative experiment of 3 independent repeats. Mice were from the experiments shown in Figs. 1 and 2. C, mice were castrated and analyzed 2.5 weeks or 5 weeks later, as described above. Pooled data of 2 independent experiments are shown with 4 to 5 mice per group in each experiment. Cx, castration; Immun, immunization; Sx, sham treatment.
If immunization was done 5 weeks after castration, lytic capacity (Supplementary Fig. S2A) and effector cytokine production (Supplementary Fig. S2B) of MLTC cells were not enhanced. Thus, reduced CD8+ effector function at 5 weeks after castration may be due to inhibition by increased Tregs.
Castration expanded Tregs in prostate-specific Pten−/− mice
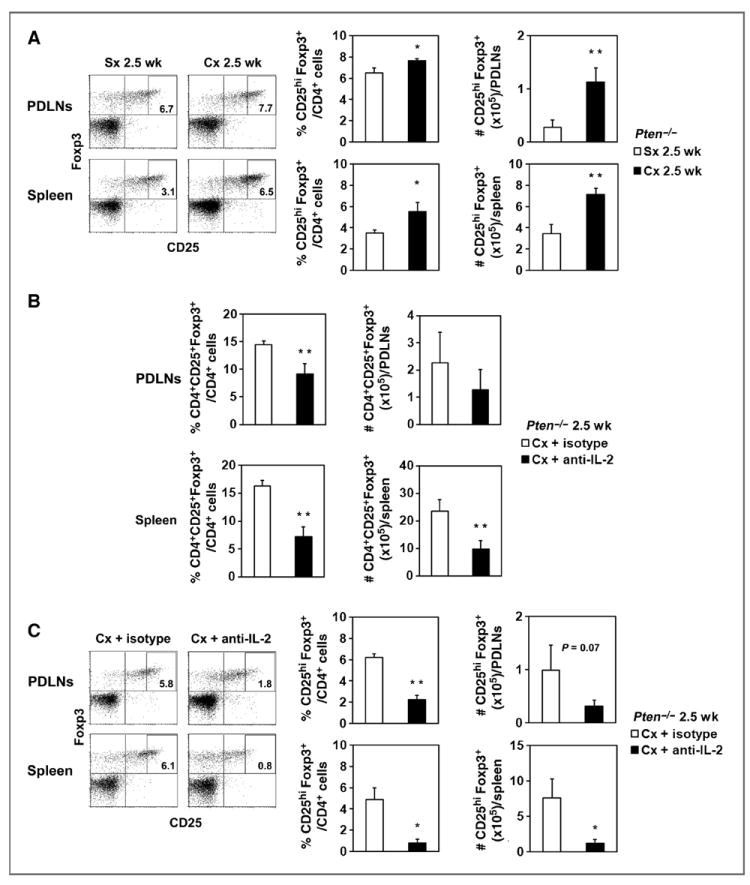
We previously reported that functional granzyme B+CD8+ T cells were increased in cancerous glands of prostate-specific Pten−/− mice 2.5 weeks after castration and declined by 5 weeks after castration (10). To determine whether increased Tregs accounted for this decline, we measured systemic Treg levels in Pten−/− mice. The number of Tregs was significantly increased in PDLNs (Fig. 4A, top), and the number and frequency of Tregs increased in the spleen of castrated Pten−/− mice 2.5 weeks after castration compared with intact or sham-treated mice (Fig. 4A, bottom). Treg numbers remained elevated in the spleens of Pten−/− mice 5 weeks after castration (Fig. 4B, bottom). These data show that Treg expansion is induced by castration in tumor-bearing mice and may downregulate the function of CD8+ T cells activated after castration (10).
Figure 4.
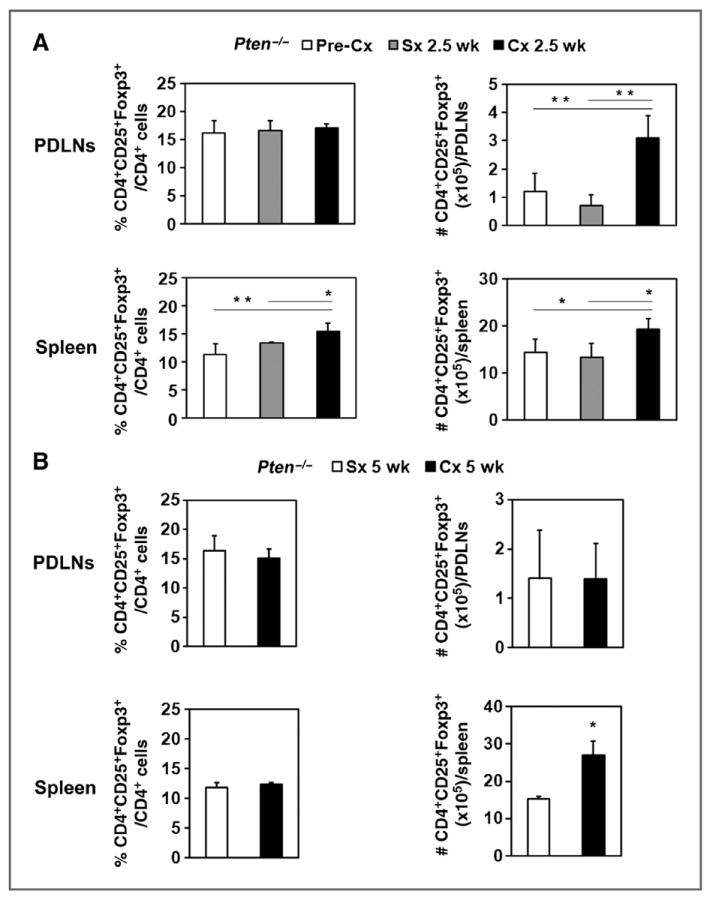
Castration amplified CD4+CD25+Foxp3+ Tregs in the spleen and PDLNs early after castration in prostate tumor–bearing Pten−/− mice. A, PDLNs and spleens were harvested from Pten−/− mice that were either untreated or 2.5 weeks after castration or sham surgery. Cells were analyzed, and the percentage and number of CD4+CD25+Foxp3+ Tregs were quantified as described in Fig. 3. B, PDLN cells and spleens from Pten−/− mice isolated at 5 weeks after castration or sham surgery were analyzed for the percentage and number of CD4+CD25+FoxP3+ Tregs. * P < 0.05; **, P < 0.01 for Cx group versus Sx group or Pre-Cx group. Pooled data of 2 independent experiments are shown with 3 to 5 mice per group in each experiment. Cx, castration; Sx, sham treatment.
CD8+ T-cell effector responses against UV-8101-RE were generated only with concomitant castration and Treg depletion of Pten−/− mice
To determine whether the increased Tregs would prevent generation of effector function to a defined tumor antigen, we castrated and immunized Pten−/− mice intraprostatically with UV-8101-RE cells. A similar number of tetramer-positive cells was detected in the spleens of castrated or sham-treated mice 2.5 weeks after treatment (Fig. 5A), suggesting that castration did not increase the number of responding cells. Tetramer analysis was not conducted on lymph nodes due to insufficient numbers of cells for analysis of individual mice. In addition, ELISPOT analysis detected similar numbers of functional cells in the PDLNs and spleens of both castrated and sham-treated mice (Fig. 5B). Thus, castration alone did not enhance CD8+ T-cell function after immunization, in the presence of the endogenous tumor.
Figure 5.
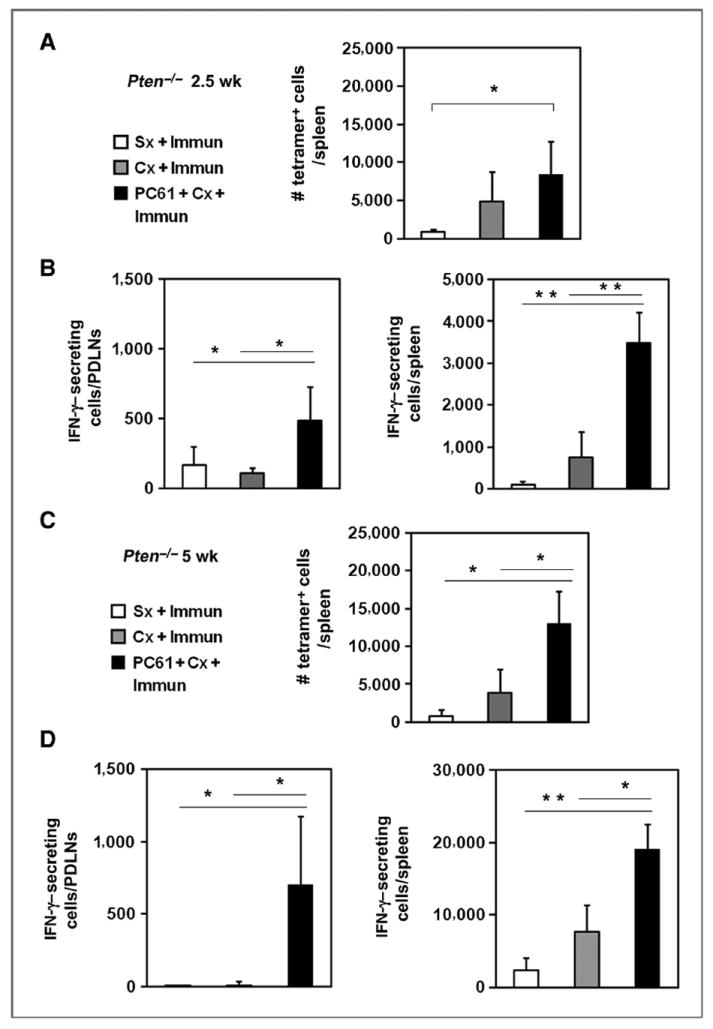
Effector responses to a tumor antigen were detected only after castration with concomitant Treg depletion in prostate tumor–bearing mice. A, Pten−/− mice were analyzed 2.5 weeks after immunization (Immun) and castration (Cx) or sham treatment (Sx). Splenic tetramer-positive cells were enriched by magnetic bead selection followed by flow cytometric analysis. The number of tetramer-positive cells per spleen was calculated (bar graph; □, Sx + Immun;
 , Cx + Immun; ■, Cx + PC61 + Immun). *, P < 0.05 for Cx + PC61 + Immun versus Sx + Immun. B, IFN-γ–secreting splenocytes or PDLN cells in response to ex vivo UV-8101-RE stimulation were quantified using an ELISPOT assay for all 3 groups. C, the number of tetramer-positive cells per spleen 5 weeks after immunization and castration or sham surgery of Pten−/− mice is shown. D, IFN-γ–secreting cells in spleen or PDLNs following ex vivo UV-8101-RE stimulation were quantified using ELISPOT assays for all 3 groups. *, P < 0.05; **, P < 0.001 for Cx + PC61 + Immun group versus Sx + Immun group or Cx + Immun group. Each group contained 3 to 5 animals, and the experiment was repeated once.
, Cx + Immun; ■, Cx + PC61 + Immun). *, P < 0.05 for Cx + PC61 + Immun versus Sx + Immun. B, IFN-γ–secreting splenocytes or PDLN cells in response to ex vivo UV-8101-RE stimulation were quantified using an ELISPOT assay for all 3 groups. C, the number of tetramer-positive cells per spleen 5 weeks after immunization and castration or sham surgery of Pten−/− mice is shown. D, IFN-γ–secreting cells in spleen or PDLNs following ex vivo UV-8101-RE stimulation were quantified using ELISPOT assays for all 3 groups. *, P < 0.05; **, P < 0.001 for Cx + PC61 + Immun group versus Sx + Immun group or Cx + Immun group. Each group contained 3 to 5 animals, and the experiment was repeated once.
Treg depletion together with castration and immunization did not increase the proportion and number of splenic tetramer-positive CD8+ T cells at 2.5 weeks after treatment (Fig. 5A). However, the number of IFN-γ–secreting cells was significantly higher both in the PDLNs and spleen (Fig. 5B). The numbers of tetramer-positive cells (Fig. 5C) and numbers of functional antigen-specific cells (Fig. 5D) remained elevated in the PDLNs and spleens 5 weeks after Treg depletion, showing that enhancement of effector function by Treg depletion is long-lasting.
Treg depletion also enhanced effector responses 5 weeks after castration and immunization of wild-type mice (Supplementary Fig. S3).
Treg expansion in Pten−/− tumor–bearing mice is prevented by in vivo IL-2 blockade
Two key cytokines, TGF-β and IL-2, have been shown to play an important role in the generation and expansion of Tregs. To explore the potential mechanism by which Tregs expand after castration in prostate tumor–bearing mice, we measured the serum level of total TGF-β in Pten−/− mice 2.5 weeks after castration or sham castration. Castration did not increase the concentration of serum TGF-β concentration compared with sham-treated animals, although the level was higher in Pten−/− mice than WT nontumor-bearing mice (Supplementary Fig. S4).
IL-2 is the signature cytokine that expands and maintains Tregs (23, 24). Increased IL-2 levels resulting from effector CD8+ T cells responding to immunizations have been shown to also amplify Tregs (25). Notably, we found an increased population of CD25hi Treg cells in the PDLNs and spleens of castrated mice, suggesting that cell surface expression of CD25 was increased or a population of CD25hi cells was preferentially expanded (Fig. 6A). We were unable to detect IL-2 in the serum of castrated or sham-treated Pten−/− mice (detection limit, 3.1 pg/mL; data not shown).
Figure 6.
Treg expansion after castration in Pten−/− mice was mediated by IL-2. A, dot plots show CD25+Foxp3+ cells gated on CD4+ cells from PDLNs or spleen of one representative mouse in Sx or Cx groups for 2.5 weeks after castration. The rectangles designate the CD25hi population. The percentage and number of CD4+CD25hiFoxp3+ cells were calculated (bar graphs; ■, Cx 2.5 weeks; □, Sx 2.5 weeks). *, P < 0.05; **, P < 0.01 for Cx 2.5 weeks compared with Sx 2.5-week group. B, Pten−/− mice were injected with anti-IL-2 or isotype control monoclonal antibody 2 days before castration. The proportion and number of CD4+CD25+Foxp3+ were quantified in PDLNs and spleens 2.5 weeks after treatment (bar graphs; ■, Cx + anti-IL-2; □, Cx + isotype). **, P < 0.01 for Cx + anti-IL-2 compared with Cx + isotype group. Each group contained 3 mice, and the experiment was repeated once. C, representative dot plots of CD25+Foxp3+ staining gated on CD4+ cells from PDLNs or spleens are shown for one mouse in the Cx + anti-IL-2 or Cx + isotype group. The percentage and number of CD4+CD25hiFoxp3+ cells were quantified (bar graphs; ■, Cx + anti-IL-2; □, Cx + isotype). Mice were from the same experiment as in B. *, P < 0.05; **, P < 0.001 for Cx + anti-IL-2 compared with Cx + isotype group. Cx, castration; Sx, sham treatment.
To determine whether Treg expansion was prevented by IL-2 blockade, we administered IL-2–neutralizing antibody or isotype control 2 days before castration of Pten−/− mice. We analyzed the percentage and number of Tregs in the PDLNs and spleen 2.5 weeks after castration. Indeed, IL-2 neutralization significantly reduced the percentage and number of Tregs in the spleen (Fig. 6B). The Treg proportion in the PDLNs was also decreased, but the total number remained the same as the lymph nodes were enlarged. Finally, IL-2 neutralization reduced CD25hiFoxp3+ cells in the spleen and PDLNs (Fig. 6C, P = 0.07 for numbers in PDLNs), suggesting that Tregs expanded in response to IL-2 generated after castration.
Discussion
Some studies suggest that androgen is immunosuppressive and can increase the proportion of systemic Tregs and thus dampen or ameliorate autoimmune reactions (4, 5, 26). Other reports showed that effector cell function was increased in prostate tumors early after castration and suggested that hormone blockade or surgical castration were immune stimulatory (17, 27). However, immune augmentation must be short-lived, as castration-resistant disease recurs in many patients.
To separate the effect of androgen ablation from tumor-induced immunosuppression, we evaluated CD8+ T-cell responses to a defined antigen in tumor-free mice. The mutant epitope in UV-8101-RE induces strong CD8+ T-cell responses, can be detected with specific probes, and is not expressed in the prostate. Thus, any responses to this epitope were the result of immunization alone and not complicated by shedding of the antigen from dying prostate cells after castration. The increase in CD8+ T-cell precursor frequency and function at 2.5 weeks after castration and immunization of wild-type mice was abrogated by 5 weeks after castration and paralleled by an increase in systemic Tregs. The correlation between Treg expansion and reduced CD8+ T-cell function was supported by the observation that Treg depletion restored CD8+ T-cell function. Administration of anti-CD25 antibody to deplete Tregs can also deplete CD8+ effector T cells (28, 29). Our results strengthen the notion that the timing of Treg depletion is critical to maintaining CD8+ T-cell function.
The increase in systemic Tregs after castration was also observed in prostate-specific Pten−/− mice. Despite the presence of the endogenous castration-resistant prostate tumor, effector T-cell responses to UV-8101-RE were also generated in Pten−/− mice when Tregs were depleted along with castration. The response was maintained at 5 weeks after castration, showing that effector responses generated after castration in the absence of Tregs are long-lived.
Activation of CD8+ T cells in response to immunization or castration of tumor-bearing mice, which is a form of immunization, induces production of IL-2 by activated cells, which in turn expands the pool of precursors by engaging IL-2R on responder cells (30). CD25, the high-affinity receptor for IL-2, is constitutively expressed by Tregs and is crucial for their development and suppressive function (23, 24). Expansion of Tregs by systemic IL-2 following immunization is observed in a variety of models (31-38), showing a direct link between T-cell activation and amplification of inhibitory mechanisms (25). Our results support these observations, as Treg expansion was not observed in castrated and unimmunized wildtype mice.
In Pten−/− mice, Treg expansion after castration was abrogated by neutralization of IL-2, showing that Treg expansion was driven by IL-2. Serum IL-2 was undetectable by ELISA in castrated or sham-castrated Pten−/− mice and suggested that IL-2 generated by effector cells may be quickly consumed by Tregs.
The critical role of TGF-β in generation and function of CD4+CD25+Foxp3+ Tregs has been well established (39-41). Primary prostate cancers produce TGF-β (42-44) and the residual tumor remaining after castration can exert immunosuppressive effects by secreting this cytokine. We detected TGF-β message in the tumors early and late after castration (data not shown), and similar levels of TGF-β were present in the serum of both intact and castrated Pten−/− mice. The increase in systemic Tregs after castration of Pten−/− mice was accelerated compared with wild-type mice, occurring 2.5 weeks after castration. Tumor-produced TGF-β may synergize with IL-2 generated after castration, resulting in early expansion of Tregs (45).
In summary, we have identified a dual effect of androgen ablation on immune function, which amplified both effector and inhibitory arms of the immune system. This dual function was also implied by a recent study of human prostate cancer samples (46). Therapeutic vaccination for treatment of castration-resistant prostate cancers has a small window of opportunity following androgen ablation that may be widened by the addition of Treg depletion to the regimen.
Supplementary Material
A, Splenocytes from mice that were 2.5 weeks or B, 5 weeks after immunization and castration or sham-castration were stained with CD4, CD25 and Foxp3 antibody. Dot plots show CD25 and Foxp3 double-stained cells gated on the CD4+ population from one representative animal for each group.
A, Lytic capacity of MLTC cells from mice 5 weeks after castration and immunization was determined by 51Cr release assay (◆, UV-8101-RE; ▲, TRAMP C-1). Line graphs show one representative animal from each group and bar graphs show the average of all animals in each group at an E:T ratio of 50:1. B, ICS of MLTC cells detected IFNγ production by CD8+ T cells. Dot plots show one representative culture from immunized mice either sham-treated or castrated, and the bar graph shows the average of all cultures with 3-5 mice per group. The experiment was repeated twice.
A, CD4+CD25+Foxp3+ Tregs were depleted systemically two days after PC61 antibody injection. Splenocytes were stained with CD4, CD25 and Foxp3 antibody and dot plots show the population gated on CD4+ lymphocytes. B, PC61 treatment did not eliminate activated CD8+CD25+ T cells. Representative dot plots show splenocytes from mice that were immunized or immunized and castrated stained with CD8 and CD25 antibody. C, Lytic capacity of MLTC cells assessed by 51Cr release assay (◆, UV-8101-RE, ▲, TRAMP C-1). D, The percentage of CD8+ MLTC cells that expressed IFNγ was assessed by flow cytometry in an ICS assay (left panel, representative dot plot; right panel, pooled data from mice in one experiment). *: p<0.05 for Cx + PC61 + Immun group versus Sx + Immun group and Cx + Immun group. One experiment of three independent experiments with 3-5 mice per group is shown.
The serum levels of TGFβ were determined using an enzyme-linked immunosorbent assay (ELISA) for intact WT mice and castrated or sham-castrated Pten-/- mice 2.5 weeks after surgery. *: p<0.001 for WT versus Sx or Cx Pten-/- mice. One representative experiment of two is shown with 3-5 mice per group.
Acknowledgments
The authors thank Dr. George Kulik (WFSM) for providing Ptenloxp/loxP and PB-Cre mice on the C57BL/6 background and Dr. James Rose (WFSM) for use of the gamma counter. They also acknowledge the gift of UV-8101-RE cells from Dr. Hans Schreiber and TRAMP-C1 cells from Dr. Owen Witte. They thank Drs. Rama Yammani and Martha Alexander-Miller (WFSM) for help with ELISPOT experiments; Dr. Alexander-Miller and Dr. Mark Willingham for helpful comments on the manuscript; and the Flow Cytometry Shared Resource, the Protein Analysis Core Laboratory, and the Cell and Viral Vector Core Laboratory, of the Comprehensive Cancer Center at Wake Forest School of Medicine.
Grant Support
The work was supported by American Cancer Society RSG 07-196-01 and WFSM Department of Pathology Research Award (P. Dubey) and 1R01-AI- 068952-01A2 (J. Grayson).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interests were disclosed.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Denmeade SR, Isaacs JT. A history of prostate cancer treatment. Nat Rev Cancer. 2002;2:389–96. doi: 10.1038/nrc801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fijak M, Schneider E, Klug J, Bhushan S, Hackstein H, Schuler G, et al. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011;186:5162–72. doi: 10.4049/jimmunol.1001958. [DOI] [PubMed] [Google Scholar]
- 5.Radojevic K, Arsenovic-Ranin N, Kosec D, Pesic V, Pilipovic I, Perisic M, et al. Neonatal castration affects intrathymic kinetics of T- cell differentiation and the spleen T-cell level. J Endocrinol. 2007;192:669–82. doi: 10.1677/joe.1.07019. [DOI] [PubMed] [Google Scholar]
- 6.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69:571–84. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arredouani MS, Tseng-Rogenski SS, Hollenbeck BK, Escara-Wilke J, Leander KR, Defeo-Jones D, et al. Androgen ablation augments human HLA2.1-restricted T cell responses to PSA self-antigen in transgenic mice. Prostate. 2010;70:1002–11. doi: 10.1002/pros.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell. 2005;7:239–49. doi: 10.1016/j.ccr.2005.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubey P, Hendrickson RC, Meredith SC, Siegel CT, Shabanowitz J, Skipper JC, et al. The immunodominant antigen of an ultraviolet-induced regressor tumor is generated by a somatic point mutation in the DEAD box helicase p68. J Exp Med. 1997;185:695–705. doi: 10.1084/jem.185.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akins EJ, Moore ML, Tang S, Willingham MC, Tooze JA, Dubey P. In situ vaccination combined with androgen ablation and regulatory T-cell depletion reduces castration-resistant tumor burden in prostate-specific pten knockout mice. Cancer Res. 2010;70:3473–82. doi: 10.1158/0008-5472.CAN-09-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 12.Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, et al. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–46. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 14.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 15.Altman JD, Moss PA, Goulder PJ, Barouch DH, McHeyzer-Williams MG, Bell JI, et al. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–6. [PubMed] [Google Scholar]
- 16.Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–69. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden AC, Moser MT, Tri SD, Mercader M, Kuntz SM, Dong H, et al. Augmentation of T cell levels and responses induced by androgen deprivation. J Immunol. 2004;173:6098–108. doi: 10.4049/jimmunol.173.10.6098. [DOI] [PubMed] [Google Scholar]
- 18.Klages K, Mayer CT, Lahl K, Loddenkemper C, Teng MW, Ngiow SF, et al. Selective depletion of Foxp3+ regulatory T cells improves effective therapeutic vaccination against established melanoma. Cancer Res. 2010;70:7788–99. doi: 10.1158/0008-5472.CAN-10-1736. [DOI] [PubMed] [Google Scholar]
- 19.Nicholaou T, Ebert LM, Davis ID, McArthur GA, Jackson H, Dimopoulos N, et al. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin Cancer Res. 2009;15:2166–73. doi: 10.1158/1078-0432.CCR-08-2484. [DOI] [PubMed] [Google Scholar]
- 20.Macatangay BJ, Szajnik ME, Whiteside TL, Riddler SA, Rinaldo CR. Regulatory T cell suppression of Gag-specific CD8 T cell polyfunctional response after therapeutic vaccination of HIV-1-infected patients on ART. PLoS One. 2010;5:e9852. doi: 10.1371/journal.pone.0009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viselli SM, Stanziale S, Shults K, Kovacs WJ, Olsen NJ. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84:337–42. [PMC free article] [PubMed] [Google Scholar]
- 22.Lapierre P, Beland K, Martin C, Alvarez F, Jr, Alvarez F. Forkhead box p3 +regulatory T cell underlies male resistance to experimental type 2 autoimmune hepatitis. Hepatology. 2010;51:1789–98. doi: 10.1002/hep.23536. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3- expressing regulatory T cells. Nat Immunol. 2005;6:1142–51. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 24.Setoguchi R, Hori S, Takahashi T, Sakaguchi S. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–35. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci U S A. 2011;108:7529–34. doi: 10.1073/pnas.1103782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Page ST, Plymate SR, Bremner WJ, Matsumoto AM, Hess DL, Lin DW, et al. Effect of medical castration on CD4+ CD25+ T cells, CD8+ T cell IFN-gamma expression, and NK cells: a physiological role for testosterone and/or its metabolites. Am J Physiol Endocrinol Metab. 2006;290:E856–63. doi: 10.1152/ajpendo.00484.2005. [DOI] [PubMed] [Google Scholar]
- 27.Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A. 2001;98:14565–70. doi: 10.1073/pnas.251140998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curtin JF, Candolfi M, Fakhouri TM, Liu C, Alden A, Edwards M, et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One. 2008;3:e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quezada SA, Peggs KS, Simpson TR, Shen Y, Littman DR, Allison JP. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J Exp Med. 2008;205:2125–38. doi: 10.1084/jem.20080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–65. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weiss L, Letimier FA, Carriere M, Maiella S, Donkova-Petrini V, Targat B, et al. In vivo expansion of naive and activated CD4+CD25+FOXP3+ regulatory T cell populations in interleukin-2-treated HIV patients. Proc Natl Acad Sci U S A. 2010;107:10632–7. doi: 10.1073/pnas.1000027107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berntsen A, Brimnes MK, thor Straten P, Svane IM. Increase of circulating CD4+CD25highFoxp3 +regulatory T cells in patients with metastatic renal cell carcinoma during treatment with dendritic cell vaccination and low-dose interleukin-2. J Immunother. 2010;33:425–34. doi: 10.1097/CJI.0b013e3181cd870f. [DOI] [PubMed] [Google Scholar]
- 33.Brandenburg S, Takahashi T, de la Rosa M, Janke M, Karsten G, Muzzulini T, et al. IL-2 induces in vivo suppression by CD4(+)CD25(+) Foxp3(+) regulatory T cells. Eur J Immunol. 2008;38:1643–53. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 34.Lemoine FM, Cherai M, Giverne C, Dimitri D, Rosenzwajg M, Trebeden-Negre H, et al. Massive expansion of regulatory T-cells following interleukin 2 treatment during a phase I-II dendritic cell-based immunotherapy of metastatic renal cancer. Int J Oncol. 2009;35:569–81. doi: 10.3892/ijo_00000368. [DOI] [PubMed] [Google Scholar]
- 35.Liu CL, Ye P, Yen BC, Miao CH. In vivo expansion of regulatory T cells with IL-2/IL-2 mAb complexes prevents anti-factor VIII immune responses in hemophilia A mice treated with factor VIII plasmidmediated gene therapy. Mol Ther. 2011;19:1511–20. doi: 10.1038/mt.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheffold A, Huhn J, Hofer T. Regulation of CD4+CD25+ regulatory T cell activity: it takes (IL-)two to tango. Eur J Immunol. 2005;35:1336–41. doi: 10.1002/eji.200425887. [DOI] [PubMed] [Google Scholar]
- 37.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigenspecific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci U S A. 2003;100:8886–91. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barthlott T, Moncrieffe H, Veldhoen M, Atkins CJ, Christensen J, O’Garra A, et al. CD25+ CD4+ T cells compete with naive CD4+ T cells for IL-2 and exploit it for the induction of IL-10 production. Int Immunol. 2005;17:279–88. doi: 10.1093/intimm/dxh207. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, et al. Conversion of peripheral CD47+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huber S, Schramm C, Lehr HA, Mann A, Schmitt S, Becker C, et al. Cutting edge: TGF-beta signaling is required for the in vivo expansion and immunosuppressive capacity of regulatory CD4+CD25+T cells. J Immunol. 2004;173:6526–31. doi: 10.4049/jimmunol.173.11.6526. [DOI] [PubMed] [Google Scholar]
- 41.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–7. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eastham JA, Truong LD, Rogers E, Kattan M, Flanders KC, Scardino PT, et al. Transforming growth factor-beta 1: comparative immunohistochemical localization in human primary and metastatic prostate cancer. Lab Invest. 1995;73:628–35. [PubMed] [Google Scholar]
- 43.Jones E, Pu H, Kyprianou N. Targeting TGF-beta in prostate cancer: therapeutic possibilities during tumor progression. Expert Opin Ther Targets. 2009;13:227–34. doi: 10.1517/14728220802705696. [DOI] [PubMed] [Google Scholar]
- 44.Steiner MS, Zhou ZZ, Tonb DC, Barrack ER. Expression of transforming growth factor-beta 1 in prostate cancer. Endocrinology. 1994;135:2240–7. doi: 10.1210/endo.135.5.7956947. [DOI] [PubMed] [Google Scholar]
- 45.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25- cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–27. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 46.Sorrentino C, Musiani P, Pompa P, Cipollone G, Di Carlo E. Androgen deprivation boosts prostatic infiltration of cytotoxic and regulatory T lymphocytes and has no effect on disease-free survival in prostate cancer patients. Clin Cancer Res. 2011;17:1571–81. doi: 10.1158/1078-0432.CCR-10-2804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A, Splenocytes from mice that were 2.5 weeks or B, 5 weeks after immunization and castration or sham-castration were stained with CD4, CD25 and Foxp3 antibody. Dot plots show CD25 and Foxp3 double-stained cells gated on the CD4+ population from one representative animal for each group.
A, Lytic capacity of MLTC cells from mice 5 weeks after castration and immunization was determined by 51Cr release assay (◆, UV-8101-RE; ▲, TRAMP C-1). Line graphs show one representative animal from each group and bar graphs show the average of all animals in each group at an E:T ratio of 50:1. B, ICS of MLTC cells detected IFNγ production by CD8+ T cells. Dot plots show one representative culture from immunized mice either sham-treated or castrated, and the bar graph shows the average of all cultures with 3-5 mice per group. The experiment was repeated twice.
A, CD4+CD25+Foxp3+ Tregs were depleted systemically two days after PC61 antibody injection. Splenocytes were stained with CD4, CD25 and Foxp3 antibody and dot plots show the population gated on CD4+ lymphocytes. B, PC61 treatment did not eliminate activated CD8+CD25+ T cells. Representative dot plots show splenocytes from mice that were immunized or immunized and castrated stained with CD8 and CD25 antibody. C, Lytic capacity of MLTC cells assessed by 51Cr release assay (◆, UV-8101-RE, ▲, TRAMP C-1). D, The percentage of CD8+ MLTC cells that expressed IFNγ was assessed by flow cytometry in an ICS assay (left panel, representative dot plot; right panel, pooled data from mice in one experiment). *: p<0.05 for Cx + PC61 + Immun group versus Sx + Immun group and Cx + Immun group. One experiment of three independent experiments with 3-5 mice per group is shown.
The serum levels of TGFβ were determined using an enzyme-linked immunosorbent assay (ELISA) for intact WT mice and castrated or sham-castrated Pten-/- mice 2.5 weeks after surgery. *: p<0.001 for WT versus Sx or Cx Pten-/- mice. One representative experiment of two is shown with 3-5 mice per group.


