Abstract
We designed and tested a sampling and analysis system for quantitative measurement of airborne cockroach allergen with sufficient sensitivity for residential exposure assessment. Integrated 1-week airborne particle samples were collected at 10–15 LPM in 19 New York City apartments in which an asthmatic child who was allergic to cockroach allergen resided. Four simultaneous air samples were collected in each home: at heights of 0.3 and 1 m in the child's bedroom and in the kitchen. Extracts of air samples were analyzed by ELISA for the cockroach allergen Bla g2, modified by amplifying the colorimetric signal generated via use of AMPLI-Q detection system (DAKO Corporation, Carpinteria, CA, USA). Settled dust samples were quantified by conventional ELISA. Of the homes where cockroach allergen was detected in settled dust, Bla g2 also was detected in 87% and 93% of air samples in the bedroom and kitchen, respectively. Airborne Bla g2 levels were highly correlated within and between the bedroom and kitchen locations (P < 0.001). Expressed as picogram per cubic meter, the room average geometric mean for Bla g2 concentrations was 1.9 pg/m3 (95% CI 0.63, 4.57) and 3.8 pg/m3 (95% CI 1.35, 9.25) in bedrooms and kitchens, respectively. This method offers an attractive supplement to settled dust sampling for cockroach allergen exposure health studies.
Keywords: Bla g2, Cockroach, Airborne, Sampling, Allergen
Introduction
Mounting evidence suggests that exposure to various environmental allergens, including those from cockroaches and rodents, can be an important risk factor in the development and/or exacerbation of atopic asthma (Chew et al., 2008; Gruchalla et al., 2005; Matsui et al., 2005; Phipatanakul et al., 2000; Rosenstreich et al., 1997). To date, investigators have assessed exposures based primarily on allergen concentrations measured in settled dust (i.e., `dustborne' allergens). Dustborne allergen assessment has been widely adopted because of ease of sample collection and the availability of large dust samples that improve overall analytical sensitivity. Settled dust allergens may also represent surrogates for time-integrated air exposures.
However, uncertain dust settling rates and surface dust collection efficiencies complicate the quantitative relationship between dust and air concentrations. Furthermore, home cleaning prior to field teams arriving can make the measurements not representative of normal living conditions. These parameters likely vary from study to study and make comparisons difficult. As the risks of developing allergen-specific sensitization and subsequent asthma depend to some extent on the quantity of allergens that are inhaled (as well as host factors such as genetic predisposition), measurements of airborne allergen concentrations may represent a more relevant measure of risk, especially if the sampling duration is sufficient to integrate over periodic peaks.
In the past, relatively little effort had been directed at developing sensitive methods for airborne cockroach allergen assessment in residential settings. Airborne cockroach concentrations have been detected in a few studies that employed very high total volume (>300 m3) air samples (Swanson et al., 1989). Detectable levels also were reported in samples collected in 20 New York City apartments at a high flow rate (>180 l/min) over a 24-h duration (Goldstein et al., 1987). Conversely, in studies in low-income housing utilizing low total volume (1–2 m3) air sampling, investigators did not detect cockroach allergens without the use of aggressive sampling techniques (i.e., forced air dust disturbance) (de Blay et al., 1997; Mollet et al., 1997). These samples were collected at a low flow rate of 2 l/min over a 3- to 8-h sampling period.
Recent efforts to isolate airborne cockroach allergens have focused on low-flow, long-term volumetric sampling in homes and in schools (Chew et al., 2005). Other groups have successfully implemented the use of ion-charging devices to collect airborne allergen and have used them to detect airborne cockroach allergens (Custis et al., 2003; Peters et al., 2007). However, this method has two potential detracting features. First, it does not provide quantitative data (i.e., the volumetric data necessary to calculate airborne exposure as a mass concentration in picogram per cubic meter, which is a more relevant metric for evaluating exposure and health risk than mass fraction results reported as nanogram per gram) (Peters et al., 2007). In addition, the ionizers can emit significant levels of ozone in the home, thus potentially exacerbating asthmatic symptoms in the homes of asthmatic study participants (Consumers Union, 2005a).
Until now, there has been no reliable, feasible method for routine, quantitative measurement and analysis of airborne cockroach allergen exposures. The goal of the present study was to develop and evaluate air sampling as an exposure assessment tool for cockroach allergen and to compare airborne cockroach levels to those measured in dust. These objectives were carried out as part of the baseline home measurements in an intervention trial aimed at reducing exposures to cockroach allergens in a cohort of asthmatic children in NYC (Kinney et al., 2002).
Methods
Air sample collection and analysis
Asthmatic children were recruited aged 5–18 years who were allergic to cockroaches and lived in apartments with self-reported cockroach infestation. Subjects were recruited from emergency department logs at the New York Columbia Presbyterian Hospital (Columbia campus). Homes were located in low-income neighborhoods of Northern Manhattan and the South Bronx. Sampling for airborne cockroach allergen (Bla g2) was conducted in 19 apartments of different multi-family buildings. Air samples were collected in conjunction with home site visits scheduled as part of the parent study (Kinney et al., 2002). While many details of apartment layout and occupancy were not collected for this study, most of the homes were 2–3 bedroom apartments in midrise buildings with 4–7 residents. The homes were similar to those of a larger birth cohort investigated by our research team (Chew et al., 2003; Miller et al., 2001). The families were asked to not change their behaviors during the sampling period, and our visits were arranged to fit the families' schedules.
A target sample volume was chosen based on the design goal of collecting a minimum of 5 mg of TSP on each filter. A sample flow rate between 10 and 15 l/min over a 7-day period achieved this goal while also integrating over the natural variations in concentrations that were likely to occur over time. Flows were calibrated with a primary flow Dry Cal DC Lite calibration kit (Bios International Inc, Pompton Plains, NJ, USA). Vacuum was provided by a Linear-Motor-Driven Free Piston vacuum pump (Medo U.S.A. Inc, Hanover Park, IL, USA). Two such pumps were housed in each sampling system. Air samples were collected on pre-weighed polytetrafluoroethylene (PTFE) mesh filters (Quant-Tec-Air Inc, Rochester, MN, USA) housed in standard 37 mm cassettes (SKC Inc, Philadelphia, PA USA). PTFE mesh filters were tested by a Model 8160 Automated Filter Tester (TSI Inc, St Paul, MN, USA) and found to have 97% collection efficiency for 0.3-μm particles at a flow rate of 30 l/min. One sampling system was set up in the kitchen and another in the bedroom. At each home sampling event, four 7-day air samples were collected simultaneously: two in the child's bedroom and two in the kitchen. In each location, one air sample was located at a height of approximately 1 m from the floor to approximate a child's breathing zone exposures and a second sample was located at a height of approximately 0.3 m, to examine whether a vertical gradient existed close to the floor.
The air sampling equipment was designed to be as quiet as possible and tamper resistant. The pumps were housed in an insulated, lockable plastic shell. The assemblage was fitted with an internal timer and counter to verify the time of pump operation. Filter cassettes were operated in open-face mode. The effective sampling probe diameter related to a cassette opening of 37 mm will not bias particle collection for size ranges below 20 μm (Davies, 1968). All sample lines were enclosed within a plastic pipe casing that extended vertically from the side of the pump housing to a height of 1 m, with branches and elbows housing the filter cassettes at 0.3 and 1 m (Figure 1). Filters were pre-weighed and post-weighed (after conditioning in a temperature–humidity-controlled environment for at least 24 h) on a Mettler-Toledo MX Microbalance (Mettler-Toledo International Inc, Columbus, OH, USA). Post-weighed samples were then stored at −20°C until the extraction date. Filters were extracted in 1 ml of phosphate-buffered saline (PBS) with 0.05% Tween 20 (pH 7.4) and shaken at 200 rpm for 1 h at 30°C. Samples were centrifuged at 10 800 g for 1 min, and the supernatant was removed and frozen at −20°C until assayed.
Fig. 1.

Photograph illustrating the features of the sampling apparatus utilized in the study
Cockroach allergen (Bla g2) was assayed by ELISA (Indoor Biotechnologies, Charlottesville, VA, USA), as previously described (Pollart et al., 1991), and modified to include an ELISA detection amplification procedure. The amplification procedure utilized the AMPLIQ detection system (DAKO Corporation, Carpinteria, CA, USA) to amplify the colorimetric signal generated by an assay that uses alkaline phosphatase as the label. The analytical detection limit for the airborne cockroach (CR) method was 0.04 ng/ml of extract (in comparison with earlier literature, this is equivalent to 0.001 Units/ml). A 7-point standard curve was run in duplicate and used on each microtiter plate. Because all samples were extracted into 1 ml of PBS, the limit of detection (LOD) in nanogram/gram for individual samples was computed by dividing 0.04 ng/ml by grams sampled, yielding the median LOD of 7.5 ng/g sampled (interquartile range: 0.48–15.0 ng/g).
Amplification validation, quality control and unknown sample reported values
To validate the amplification procedure, a low-end calibration experiment was performed to establish the LOD for the method as a whole. The procedure uses the variability of low-level analyte responses as an estimate of the standard deviation of the blank signal (Kennedy et al., 1995). For quality control purposes, a field blank was collected and analyzed from each home and routine laboratory blanks were run at a frequency of 10% of the unknown samples. All laboratory standard calibration curves and blanks were reviewed for each plate individually to compare the reported LOD for each plate to the mean optical density signal generated by the blanks.
Four data points were collected for each unknown sample analyzed. Each sample was run in duplicate at full strength and at a 1:2 dilution. Using the instrument-supported software, SoftMax Pro (Molecular Devices Inc, Sunnyvale, CA, USA), values within the linear portion of the standard curve were selected. In some cases, the value of the full strength samples was used, and in other cases, the average of both the full strength and 1:2 dilutions was reported.
Collection and analysis of settled dust
Settled dust was collected from the kitchen and child's bedroom on 70-mm cellulose filters (Whatman International, Maidstone, UK) using a canister vacuum cleaner (Eureka Mighty Mite, Bloomington, IN, USA) and a modified collection nozzle (ALK Inc, Hørsholm, Denmark). In the kitchen, exposed areas of the floor, the top of the refrigerator, and window sills were vacuumed. In the child's bedroom, 2 m2 of the floor surrounding the bed was vacuumed. The vacuuming time for each sample was 4 min. Samples were returned to the laboratory for post-weighing and then stored at −20°C. Dust samples were not sieved. Dust samples were extracted on a platform shaker for 1 h at 30°C in phosphate-buffered solution with 0.05% Tween 20 solution. Cockroach allergen (Bla g2) was assayed by sandwich ELISA, as described earlier, but without the amplification step.
Statistical analysis
Only air sampling data that met data quality criteria were analyzed, including a complete chain of custody, proper filter loading, and adequate sample mass. Eight of 76 possible air sampling events (19 homes × 2 rooms × 2 heights) were voided because of questionable loading or filter damage. Bla g2 measurements below the LOD were reassigned a value of half the LOD.
All variables were tested for normality using the Shapiro–Wilk test. After natural log transformation, all variables satisfied this normality criterion. Correlation coefficients were calculated for airborne measurements collected in different locations and between airborne and dustborne measurements, using the Pearson correlation (2-tailed significance test). Differences between simultaneously measured air samples at 0.3 and 1 m, in the kitchen vs. the bedroom, were tested using the Student's paired t-test. Linear regression analysis was used to further examine the relationship between airborne allergen concentrations and dust concentrations in an attempt to predict airborne allergen concentrations from the dustborne levels.
Results
Airborne Bla g2 and TSP concentrations
Overall, cockroach allergen (Bla g2) was measured above the LOD in 73% and 68% of the kitchen and bedroom air samples, respectively. For homes in which dustborne Bla g2 was detected, the detection rate was higher: 93% for kitchens and 87% for bedrooms. Expressed as nanogram per gram of TSP collected, cockroach allergen (Bla g2) levels in the air of the study homes ranged from below the detection limit to 2500 ng/g of TSP. The geometric mean in the rooms of the homes ranged from 34 ng/g at the bedroom 1-m level to 84 ng/g at the kitchen 0.3-m level. Expressed as picogram per cubic meter of sampled air, Bla g2 concentrations ranged from below the detection limit to 51 pg/m3. The geometric mean in the rooms of the homes ranged from 2.3 pg/m3 at the bedroom 1-m level to 4.0 pg/m3 at the kitchen 0.3-m level (Table 1).
Table 1.
Airborne Bla g2 concentrations for all samples satisfying QA criteria (see methods) expressed as (a) ng/g of total suspended particulates and (b) pg/m3 air sampled
| Percentiles |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Units | Sample location | n | mean | Geom. mean | mina | 25 | 50 | 75 | Max |
| ng/g | Kitchen 0.3 m | 16 | 380 | 84* | 1.6 | 17 | 150 | 620 | 2500 |
| Kitchen 1 m | 14 | 220 | 67 | 1.6 | 26 | 140 | 240 | 1500 | |
| Bedrm 0.3 m | 16 | 160 | 49* | 1.6 | 7.4 | 76 | 230 | 950 | |
| Bedroom 1 m | 15 | 170 | 34 | 1.6 | 3.6 | 62 | 160 | 1100 | |
| pg/m3 | Kitchen 0.3 m | 16 | 13 | 4.0** | 0.2 | 0.7 | 4.9 | 21 | 51 |
| Kitchen 1 m | 14 | 6.1 | 3.0 | 0.2 | 2.0 | 4.9 | 8 | 21 | |
| Bedrm 0.3 m | 16 | 7.8 | 2.5** | 0.2 | 0.3 | 4.2 | 12 | 30 | |
| Bedroom 1 m | 15 | 7.2 | 2.3 | 0.2 | 0.2 | 4.6 | 14 | 29 | |
Significantly different at P < 0.05.
Significantly different at P < 0.10.
One unit = 40 ng of Bla g2.
Min set to 1/2 limit of detection.
The location-specific geometric mean airborne TSP concentrations ranged from 47.2 μg/m3 (95% CI 31.0, 69.1) at the kitchen 0.3-m sample location to 50.5 μg/m3 (95% CI 40.2, 75.7) at the bedroom 1-m location. Individual airborne TSP concentrations ranged from a low of 14.3 μg/m3 to a high of 134.9 μg/m3 (Table 2).
Table 2.
Airborne TSP concentrations for all samples (in μg/m3)
| Percentiles |
||||||||
|---|---|---|---|---|---|---|---|---|
| Sample location | n | mean | Geom. mean | min | 25 | 50 | 75 | max |
| Kitchen 0.3 m | 16 | 56 | 47 | 14 | 27 | 49 | 85 | 117 |
| Kitchen 1 m | 14 | 54 | 45 | 15 | 26 | 47 | 69 | 135 |
| Bedroom 0.3 m | 16 | 57 | 52 | 20 | 38 | 50 | 75 | 115 |
| Bedroom 1 m | 15 | 57 | 51 | 18 | 35 | 55 | 79 | 113 |
Airborne Bla g2 and TSP room air comparisons
As seen in Table 1, Bla g2 concentrations appeared higher nearer to the ground at a height of 0.3 m than at 1 m. However, these differences were not statistically significant. Kitchen air levels also tended to be higher than those measured in the bedroom, and these differences reached the 0.05 significance level for the nanogram per gram metric and the 0.10 significance level for the picogram per cubic meter metric. Comparison of TSP levels showed no significant differences in concentration between the 0.3- and 1-m samples collected within the same room or between rooms of the test apartments (Table 1).
Bla g2 room air and TSP correlations
All airborne Bla g2 variables were correlated significantly between vertical sampling locations (i.e., 0.3 and 1 m) within each room and between the kitchen and bedroom sample locations, whether calculated as picogram per cubic meter (Figure 2a–c) or nanogram per gram (data not shown). Likewise, all TSP variables were significantly correlated (data not shown). However, none of the TSP concentrations were associated with airborne Bla g2 sample concentrations (data not shown).
Fig. 2.
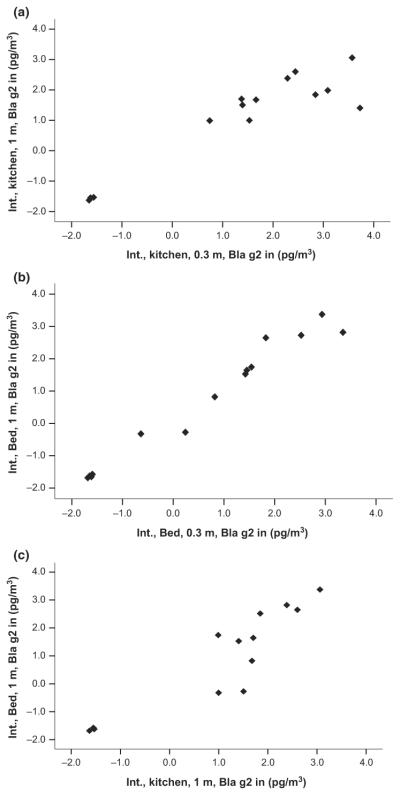
(a) Scatter plot of log-transformed airborne Bla g2 concentrations in picogram per cubic meter for the kitchen 0.3 and 1 m samples (r = 0.92, P < 0.001). Low end data points are below the lower limit of detection (LOD) and were reassigned a value of ½ the detection limit. (b) Scatter plot of log transformed airborne Bla g2 concentrations in picogram per cubic meter for the Bedroom 0.3 and 1 m samples (r = 0.98, P < 0.001). Low end data points are below the lower LOD and were reassigned a value of ½ the detection limit. (c) Scatter plot of log transformed airborne Bla g2 concentrations in picogram per cubic meter for Kitchen 1 m and Bedroom 1 m samples (r = 0.89, P < 0.001). Low end data points are below the lower LOD and were reassigned a value of ½ the detection limit
Bla g2 dust to air comparisons
Airborne Bla g2 concentrations were compared with surface dust Bla g2 samples vacuumed in kitchens and bedrooms (Table 3). Expressed as nanogram per gram, airborne Bla g2 measured in the kitchen at 0.3 m was significantly associated with kitchen dust concentrations expressed as nanogram per gram of dust (r = 0.601, P < 0.05). Associations were significant for both kitchen and bedroom air samples at 0.3 m when dust concentrations were re-expressed in units of nanogram per sample. Expressed as picogram per cubic meter, airborne Bla g2 measured in the kitchen at 0.3 m was significantly associated with the kitchen dust.
Table 3.
Correlations between airborne Bla g2 concentrations and room dust concentrations
| Correlation variables |
Correlations with dust |
||
|---|---|---|---|
| Location | Air sample (units) | Dust in ng/g | Dust in ng/sample |
| Kitchen | 0.3 m (ng/g) | 0.6* | 0.6* |
| 1 m (ng/g) | 0.5** | 0.5** | |
| 0.3 m (ng/m3) | 0.5* | 0.6* | |
| 1 m (ng/m3) | 0.4 | 0.5** | |
| Bedroom | 0.3 m (ng/g) | 0.0 | 0.3 |
| 1 m (ng/g) | −0.1 | 0.3 | |
| 0.3 m (ng/m3) | −0.1 | 0.2 | |
| 1 m (ng/m3) | 0.0 | 0.3 | |
P < 0.05.
P < 0.10.
One unit of Bla g2 = 40 ng of Bla g2.
Regression of the airborne Bla g2 level (in nanogram per gram of TSP) on the kitchen dust Bla g2 concentration (in nanogram per gram) yielded a slope of 0.43 (95% CI 0.08–0.77), R2 = 0.38.
Discussion
In this study, a sensitive and quantitative method for measuring indoor airborne exposures to Bla g2 was developed. The method involved collecting a 7-day integrated TSP sample at a flow rate of approximately 10–15 l/min. We found that Bla g2 allergen was suspended in the air at measurable levels and highly correlated across different locations within homes. The airborne levels of both TSP and Bla g2 varied substantially from home to home, but the within-home variability was small and the between-room concentrations were highly correlated. We also found that the within-home TSP and airborne Bla g2 levels were not associated.
Dustborne sampling vs. airborne sampling for Bla g2
The majority of studies of inner city asthma and cockroach allergens have relied solely on the concentrations of allergens found in the dust of homes (Rosenstreich et al., 1997). While dust samples can be collected relatively quickly in the home and the process does not unduly inconvenience occupants, dustborne sampling is an indirect measure of exposure and a standardized protocol is very difficult to adopt (Lewis and Breysse, 1998; Mansour et al., 2001; Wickens et al., 2004). In addition, the use of dust sampling as an exclusive exposure assessment tool may lead to misinterpretation of the actual allergen burden within study homes (Gold, 2000). Because analysis of allergens in settled dust has limitations, we believe this study provides a supplementary and potentially sounder approach to the assessment of allergen exposure. Airborne sampling has the potential to provide a more direct and relevant measure of inhalation exposure than does dust sampling. Studies using airborne sampling have the potential to broaden our understanding of exposure patterns in homes and to provide more precise and relevant exposure assessments for linkage to health outcomes.
However, several factors have made the development of methods for exposure assessment using airborne allergen sampling difficult. Airborne concentrations of allergens are low and therefore require sensitive assays, large volumes of air, and sufficient mass of suspended particulate to achieve reliable detection. The airborne quantity of allergen is not only dependent on the source of the allergen but on other factors that are difficult to assess including individual activity patterns and levels, patterns of domestic `disturbance' (i.e., vacuuming and/or sweeping), and home ventilation rates. It is believed that these factors might limit the value of fixed area sampling, unless the samplers are located near loci of indoor activity (Platts-Mills et al., 1991). Finally, like dust sampling methods, investigators have not used standardized protocols to collect airborne allergen samples.
For airborne Bla g2 in particular, the prevailing theory is that cockroach allergen, like dust-mite allergen, is associated with large particles (>10 μm) that do not remain airborne after disturbance. It is assumed that airborne Bla g2 cannot be sampled without the use of aggressive sampling techniques (i.e., using strong blowers to re-suspend settled dust) (de Blay et al., 1997; Tovey et al., 1981). For these reasons, few studies have assessed airborne cockroach allergen concentrations.
In this study, we addressed the methodological issues associated with airborne allergen sampling in general and specifically for airborne Bla g2. We show that reliable air sampling for cockroach allergen is possible without the use of aggressive sampling techniques and without scavenging particles, which is a criticism of high volume air sampling methods. We do not dispute the notion that cockroach allergen is associated with large particles. Rather, we hypothesize that our samples are composed, in part, of Bla g2-carrying particles that are of respirable size and that the significance of this airborne fraction may have been overlooked in the past. Finally, in the absence of airborne exposure data, our reported airborne concentrations may have important health implications in asthma sensitization and morbidity.
Airborne Bla g2 distribution and particle size implications
The high correlations we observed for airborne Bla g2 concentrations measured throughout the home suggest that airborne Bla g2 is distributed within homes in a relatively uniform manner. In turn, this suggests that Bla g2 may be associated with smaller particles that have sufficient residence time to remain airborne and spread throughout the home following physical disturbance events. Again, the prevailing theory for cockroach and dust-mite allergen-carrying particles is that both are associated with large particles. This theory is based, in part, on the fact that a peritrophic membrane encases the fecal pellet of both the cockroach and the dust-mite. The membrane binds the allergens of both species, so they cannot be easily suspended (Tovey et al., 1981; Zwick and Popp, 1991). However, the ecology of these species is quite different (i.e., cockroaches are free roaming throughout an apartment, while dust-mites are associated more closely with bedding and other fabric furnishings). Thus, the peritrophic membranes of discarded cockroach fecal pellets are more susceptible to desiccation and/or pulverization as part of normal domestic conditions than the membranes of the dust-mite fecal pellet. These large cockroach fecal pellets can breakdown to produce smaller particles, which can then become suspended and re-suspended in the home (Lehrer and Horner, 1991).
There is supporting evidence that a considerable size fraction of the TSP in inner city homes is of a respirable size and that this fraction contains cockroach allergen. The Baltimore Indoor Environmental Study of Asthma in Kids reported average PM2.5 concentrations that were approximately 30% of their mean PM10 level (McCormack et al., 2008). Most recently, they report mean (±s.d.) indoor PM2.5–10 concentrations of 17.4 ± 21.0 and PM2.5 concentrations of 40.3 ± 35.4 μg/m3 in homes from their cohort (McCormack et al., 2009).
De Lucca et al. used air sampling under low disturbance (i.e., normal living conditions with no dust raising or cleaning activities) and no-disturbance (i.e., overnight while occupants were sleeping) scenarios to identify particles that adsorb and suspend Bla g1. Under low disturbance conditions, 20% of particles eluting cockroach allergen were <10 μm. However, under no-disturbance conditions, the particle size distribution shifted to 40% of the particles <10 μm (De Lucca et al., 1999).
Previous work by de Blay et al. further supports the notion. When they aggressively disturbed and suspended the dustborne reservoirs in their study homes, they found that approximately 7.0% of the total air mass of their samples was composed of particles <5 μm in size and approximately 15% of the suspended particle mass was in the 5- to 10-μm size range. Thus, close to 25% of the mass of airborne particles eluting Bla g2 were below 10 μm (de Blay et al., 1997). It is crucial to point out that the two respirable size fractions were detected only after the homes underwent artificial disturbance, when the mass of these particle fractions was sufficient to achieve the lower LOD limit of their analytical method.
Data from the Healthy Public Housing Initiative (HPHI) in Boston, MA, also demonstrate that a portion of the airborne particle size fraction carrying Bla g2 is smaller than 10 μm while confirming that the detection of airborne Bla g2 is achievable without aggressive sampling (Peters et al., 2007). The air samples in this study were collected using commercially available ion-charging electrostatic precipitators over a period of 2 weeks. This type of collection device has been shown to collect fine particles at a higher rate than large particles (McCormack et al., 2008). In addition, the reported median Bla g2 levels of 60 ng/g in bedroom air samples collected from the HPHI study homes are within the confidence interval of the geometric mean and median bedroom concentrations reported in our study homes.
Towards a Bla g2 inhalation exposure threshold
When expressed as Bla g2 in U/g, which was the conventional units reported in previous dustborne exposure allergen exposure studies, the airborne geometric mean concentration from all 19 locations ranged from approximately 1 U/g to >2 U/g with the highest individual sample concentration reaching 106 U/g. Dustborne concentrations of Bla g2 in the range of 1 to >2 U/g have been reported as a threshold for sensitization in homes of children with asthma (Custovic et al., 1996; Eggleston et al., 1998; Hamilton et al., 1992; Sarpong et al., 1996). These levels are also consistent with literature on dustborne Bla g2 concentrations predicting wheeze in first year of life and sensitization to Bla g2 in school children (Gold et al., 1999; Sporik et al., 1999). Finally, Chew et al. (2008) reported that Bla g2 levels >1 U/g in children's bed and kitchen dust samples were independently associated with cockroach-specific IgE.
Exposure thresholds based on dustborne concentrations are not directly applicable to inhalation exposure and are likely a higher estimate of the biologically effective dose required to elicit molecular immune responses within the lung. In the absence of inhalation thresholds, our data offer a first indication of a relationship between Bla g2 in the dust and Bla g2 in air. This association may provide further insight into the applicability of existing dustborne thresholds and development of future inhalation thresholds. We tested our air to dust associations with a simple linear regression model. The slope was significantly greater than zero, indicating that the airborne Bla g2 concentration increases as the dustborne Bla g2 concentration increases in our homes. We estimate that a concentration 80 ng/g of Bla g2 in kitchen dust will generate a background concentration of approximately 150 ng/g or approximately 6 pg/m3 of Bla g2 in the air of the home. This concentration represents our median kitchen exposure and our 75th percentile bedroom exposure.
The collection rate of our air samples mimics resting breathing volumes and therefore occupant exposure. Based on a daily inhalation rate estimated from daily activities of 15 m3/day and the assumption that the subject will remain in the home for 24 h, the mean daily baseline chronic exposure of our study participants is approximately 45 pg of Bla g2 per day (95%CI 15–300) or 16 ng per year (95%CI 5–108).
Health relevance of chronic airborne Bla g2 exposure
The health relevance of chronic exposure to low concentrations of airborne Bla g2 is unknown. Environmental factors to consider when evaluating the health relevance of airborne Bla g2 exposures include the concentration, size, and residence time of Bla g2-carrying particles in the surface and air compartments of the home. Important human exposure factors include the size of the inhaled Bla g2-carrying particle, the bioavailability of Bla g2 at the lung mucosa, the dose–response effect on the affected cellular components, and individual susceptibility.
In terms of the airborne concentration, when reported as picogram per cubic meter, the airborne Bla g2 levels appear to be very low. However, studies utilizing cockroach extracts have provided evidence that exposure to low levels of cockroach allergen can lead to a measurable immune response. Arruda et al. (1995b) found that cockroach-allergic patients gave positive skin tests to recombinant Bla g4 at concentrations of 10−3 to 10−5 μg/ml and that skin test reactivity was correlated with serum IgE antibody response. Pollart et al. (1991) demonstrated the biologic activity of Bla g2 by performing skin testing on cockroach-allergic patients and found patients gave positive reactions to Bla g2 at 10−1 to 10−4 μg/ml. In vitro studies utilizing airway epithelial cell lines have measured molecular responses to cockroach extracts such as eosinophil release of cytotoxic inflammatory mediators, interleukin-8 expression, and Ca2+ signaling at concentrations as low as 20–300 ng/ml (Bhat et al., 2003; Hong et al., 2004; Sohn et al., 2004).
We interpret the correlation between air concentrations in bedroom and kitchens to suggest that a fraction of both the dustborne and airborne Bla g2 in our homes is associated with particles <10 μm. PM10 and 2 μm particles have been shown to remain suspended for just under 2 and 3 h, respectively, after normal domestic activity. In addition, 2-μm size particles will persist in a home for years if they are not removed by regular vacuuming (Qian et al., 2008). Thus, allergens present on particles <10 μm can become suspended and resuspended into the indoor air compartment of a home in a chronic cycle because of normal domestic activity. Once airborne, these particles can penetrate into the lung where it is available to react with the airway epithelium. Literature utilizing bronchial provocative tests, skin tests, and serum IgE on human subjects and airway epithelial cell lines in vitro has demonstrated a dose-response relationship between direct exposure to cockroach allergen and immunological responses at high and low doses (Arruda et al., 1995b; Bhat et al., 2003; Kang, 1976; Pollart et al., 1991). Our subjects are sensitized and highly allergic to cockroach allergen.
Therefore, from a health perspective, an important but previously undetected burden of airborne Bla g2 exists in homes of children who are allergic to Bla g2 and who have asthma in New York City. This fraction of the allergen reservoir may be composed of relatively fine particles that are suspended and re-suspended on a regular basis by normal domestic activity, thus creating a chronic airborne allergen exposure capable of sensitizing inhabitants to Bla g2. This chronic exposure is independent of other acute exposures that may result from direct contact with contaminated linens and fabrics and/or unintentional disturbance of allergen dust reservoirs.
Clearly, further study of airborne cockroach allergen is warranted to further investigate the actual size of the Bla g2 particles in the air, their relationship to asthma morbidity, and whether other bioactive cockroach proteins (i.e., Bla g4,5,6 and 7) are present in the suspended particulate fraction of homes of asthmatic children. The reported airborne cockroach levels suggest an ongoing burden of inhalation exposure.
Practical Implications.
Until now, cockroach allergen exposures have usually been assessed by collection and analysis of settled dust, on the assumption that airborne cockroach allergen cannot be reliably measured. In this study, a sensitive and quantitative method for measuring indoor airborne exposures to cockroach allergens involving a 7-day integrated total suspended particulate (TSP) sample collected at approximately 10–15 l/min was developed. Investigators are now empowered with an alternative exposure assessment method to supplement their studies and the understanding of allergen aerodynamics in the homes of children with asthma. We report airborne cockroach allergen in apartments, suggesting an ongoing burden of inhalation exposure.
Acknowledgements
This study was supported by NIEHS grant #ESO9142 `Reducing Indoor Allergen Exposures in Northern Manhattan and the South Bronx.' Additional support was provided by the NIEHS Center for Environmental Health in Northern Manhattan (P30 ES09089). We also thank Tom Protus for help in designing and building the field equipment.
References
- Arruda LK, Vailes LD, Hayden ML, Benjamin DC, Chapman MD. Cloning of cockroach allergen, Bla g 4, identifies ligand binding proteins (or calycins) as a cause of IgE antibody responses. J. Biol. Chem. 1995b;270:31196–31201. doi: 10.1074/jbc.270.52.31196. [DOI] [PubMed] [Google Scholar]
- Bhat RK, Page K, Tan A, Hershenson MB. German cockroach extract increases bronchial epithelial cell interleukin-8 expression. Clin. Exp. Allergy. 2003;33:35–42. doi: 10.1046/j.1365-2222.2002.01481.x. [DOI] [PubMed] [Google Scholar]
- de Blay F, Sanchez J, Hedelin G, Perez-Infante A, Veŕot A, Chapman M, Pauli G. Dust and airborne exposure to allergens derived from cockroach in low – cost public housing in Strasbourg (France) J. Allergy Clin. Immunol. 1997;99:107–112. doi: 10.1016/s0091-6749(97)70307-5. [DOI] [PubMed] [Google Scholar]
- Chew GL, Perzanowski MS, Miller RL, Correa JC, Hoepner LA, Jusino CM, Becker MG, Kinney PL. Distribution and determinants of mouse allergen exposure in low-income New York City apartments. Environ. Health Perspect. 2003;111:1348–1351. doi: 10.1289/ehp.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew GL, Correa JC, Perzanowski MS. Mouse and cockroach allergens in the dust and air in northeastern United States inner-city public high schools. Indoor Air. 2005;15:228–234. doi: 10.1111/j.1600-0668.2005.00363.x. [DOI] [PubMed] [Google Scholar]
- Chew GL, Perzanowski MS, Canfield SM, Goldstein IF, Mellins RB, Hoepner A, Ashby-Thompson M, Jacobson JS. Cockroach allergen levels and associations with cockroach-specific IgE. J. Allergy Clin. Immunol. 2008;121:240–245. doi: 10.1016/j.jaci.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Consumers Union New concerns about ionizing air cleaners. Consumer Reports. 2005 May;:22–25. [Google Scholar]
- Custis NJ, Woodfolk JA, Vaughan JW, Platts-Mills TAE. Quantitative measurement of airborne allergens from dust mites, dogs, and cats using an ion-charging device. Clin. Exp. Allergy. 2003;33:986–991. doi: 10.1046/j.1365-2222.2003.01706.x. [DOI] [PubMed] [Google Scholar]
- Custovic R, Green SCO, Taggart A, Smith CAC, Pickering MD, Chapman A, Woodcock X. Domestic allergens in public places II: dog (Can f 1) and cockroach (Bla g 2) allergens in dust and mite, cat, dog and cockroach allergens in the air in public buildings. Clin. Exp. Allergy. 1996;26:1246–1252. [PubMed] [Google Scholar]
- Davies CN. The entry of aerosols into sampling tubes and heads. (ser. 2).Br. J. Appl. Phys. 1968;1:921–932. [Google Scholar]
- De Lucca S, Taylor D, O'Mea T. Measurement and characterization of cockroach allergens detected during normal domestic activity. J. Allergy Clin. Immunol. 1999;104:672–680. doi: 10.1016/s0091-6749(99)70341-6. [DOI] [PubMed] [Google Scholar]
- Eggleston PA, Rosenstreich D, Lynn H, Gergen P, Baker D, Kattan M, Mortimer KM, Mitchell H, Ownby D, Slavin R, Malveaux F. Relationship of indoor allergen exposure to skin test sensitivity in inner-city children with asthma. J. Allergy Clin. Immunol. 1998;102:563–570. doi: 10.1016/s0091-6749(98)70272-6. [DOI] [PubMed] [Google Scholar]
- Gold DR. Environmental tobacco smoke, indoor allergens, and childhood asthma. Environ. Health Perspect. 2000;108(Suppl. 4):643–651. doi: 10.1289/ehp.00108s4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold DR, Burge H, Carey V, Milton DK, Platts-Mills TAE, Weiss ST. Predictors of repeated wheeze in the first year of life: the relative roles of cockroach, birth weight, acute lower respiratory illness, and maternal smoking. Am. J. Respir. Crit. Care Med. 1999;160:227–236. doi: 10.1164/ajrccm.160.1.9807104. [DOI] [PubMed] [Google Scholar]
- Goldstein IF, Reed CE, Swanson MC, Jacobson JS. Aeroallergens in New York inner – city apartments of asthmatics. Advances in Aerobiology, Proceedings 3rd International Conference; Basel. August 6–9, 1986; 1987. pp. 133–138. Experientia Supplementum. [DOI] [PubMed] [Google Scholar]
- Gruchalla RS, Pongracic J, Plaut M, Evans R, III, Visness CM, Walter M, Crain EF, Kattan M, Morgan WJ, Steinbach S, Stout J, Malindzak G, Smartt E, Mitchell H. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J. Allergy Clin. Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Hamilton RG, Chapman MD, Thomas AE, Platts-Mills TAE, Adkinson NF. House dust aeroallergen measurements in clinical practice: a guide to allergen-free home and work environments. Immunol. Allergy Pract. 1992;14:96–112. [Google Scholar]
- Hong J, Lee S, Kim K. German cockroach extract activates protease-activated receptor 2 in human airway epithelial cells. J. Allergy Clin. Immunol. 2004;113:315–319. doi: 10.1016/j.jaci.2003.11.026. [DOI] [PubMed] [Google Scholar]
- Kang B. Study on cockroach antigen as a probable causative agent in bronchial asthma. J. Allergy Clin. Immunol. 1976;58:357–365. doi: 10.1016/0091-6749(76)90115-9. [DOI] [PubMed] [Google Scholar]
- Kennedy ER, Fishbach TJ, Song R, Eller PM, Shulman A. Guidelines for Air Sampling and Analytical Method Development and Evaluation. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health; Cincinnati, OH: 1995. [last accessed on 7 April, 2011]. pp. 65–68. DHHS (NIOSH) Publication No. 95-117. http://www.cdc.gov/niosh/docs/95-117/pdfs/95-117.pdf, [Google Scholar]
- Kinney PL, Northridge ME, Chew GL, Groning E, Joseph E, Correa JC, Prakash S, Goldstein I. On the front lines: an environmental asthma intervention in New York City. Am. J. Public Health. 2002;92:24–26. doi: 10.2105/ajph.92.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer SB, Horner WE. Comparison of cockroach allergenic activity in whole body and fecal extracts. J. Allergy Clin. Immunol. 1991;87:574–580. doi: 10.1016/0091-6749(91)90017-i. [DOI] [PubMed] [Google Scholar]
- Lewis RD, Breysse PN. A comparison of the sampling characteristics of two vacuum surface samplers for the collection of dust mite allergen. Appl. Occup. Environ. Hyg. 1998;13:536–541. [Google Scholar]
- Mansour M, Lanphear BP, Hornung R, Khoury J, Bernstein DI, Menrath W, Decolongon J. A side-by-side comparison of sampling methods for settled, indoor allergens. Environ. Res. 2001;87:37–46. doi: 10.1006/enrs.2001.4284. [DOI] [PubMed] [Google Scholar]
- Matsui E, Simons E, Rand C, Butz A, Buckley T, Breysse P, Eggleston P. Airborne mouse allergen in the homes of inner-city children with asthma. J. Allergy Clin. Immunol. 2005;115:358–363. doi: 10.1016/j.jaci.2004.11.007. [DOI] [PubMed] [Google Scholar]
- McCormack M, Breysse P, Hansel N, Matsui E. Common household activities are associated with elevated particulate matter concentrations in bedrooms of inner-city Baltimore pre-school children. Environ. Res. 2008;106:148–155. doi: 10.1016/j.envres.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack M, Breysse P, Hansel N, Matsui E, Williams D, Curtin-Brosnan J, Eggleston P, Diette G. In-home particle concentrations and childhood asthma morbidity. Environ. Health Perspect. 2009;117:294–298. doi: 10.1289/ehp.11770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Chew GL, Bell CA, Biedermann SA, Aggarwal M, Kinney PL, Tsai WY, Whyatt RM, Perera FP, Ford JG. Prenatal exposure, maternal sensitization, and sensitization in utero to indoor allergens in an inner-city cohort. Am. J. Respir. Crit. Care Med. 2001;164:995–1001. doi: 10.1164/ajrccm.164.6.2011107. [DOI] [PubMed] [Google Scholar]
- Mollet JA, Vailes LD, Avner DB, Perzanowski MS, Arruda LK, Chapman MD, Platts-Mills TAE. Evaluation of German cockroach (Orthoptera:Blattellidae) allergen and seasonal variation in low-income housing. J. Med. Entomol. 1997;34:307–311. doi: 10.1093/jmedent/34.3.307. [DOI] [PubMed] [Google Scholar]
- Peters J, Levy J, Rogers C, Burge H, Spengler JD. Determinants of allergen concentrations in apartments of asthmatic children living in public housing. J. Urban Health. 2007;84:185–197. doi: 10.1007/s11524-006-9146-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phipatanakul W, Eggleston PA, Wright EC, Wood RA. Mouse allergen. II. The relationship of mouse allergen exposure to mouse sensitization and asthma morbidity in inner-city children with asthma. J. Allergy Clin. Immunol. 2000;106:1075–1080. doi: 10.1067/mai.2000.110795. [DOI] [PubMed] [Google Scholar]
- Platts-Mills TAE, Ward GW, Sporik R, Gelber LE, Chapman MD, Heymann PW. Epidemiology of the relationship between exposure to indoor allergens and asthma. Int. Arch. Allergy Appl. Immunol. 1991;94:339–345. doi: 10.1159/000235398. [DOI] [PubMed] [Google Scholar]
- Pollart SM, Mullins DE, Vailes LD, Hayden ML, Platts-Mills TA, Sutherland WM. Identification, quantitation, and purification of cockroach allergens using monoclonal antibodies. J. Allergy Clin. Immunol. 1991;87:511–521. doi: 10.1016/0091-6749(91)90010-l. [DOI] [PubMed] [Google Scholar]
- Qian J, Ferro AR, Fowler KRJ. Estimating the resuspension rate and residence time of indoor particles. J. Air Waste Manag. Assoc. 2008;58:502–516. doi: 10.3155/1047-3289.58.4.502. [DOI] [PubMed] [Google Scholar]
- Rosenstreich DL, Eggleston P, Kattan M, Baker D, Slavin RG, Gergen P, Mitchell H, McNiff-Mortimer K, Lynn H, Ownby D, Malveaux F. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N. Engl. J. Med. 1997;366:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- Sarpong SB, Hamilton RG, Eggleston PA, Adkinson NA. Socioeconomic status and race as risk factors for cockroach allergen exposure and sensitization in children with asthma. J. Allergy Clin. Immunol. 1996;97:1393–1394. doi: 10.1016/s0091-6749(96)70209-9. [DOI] [PubMed] [Google Scholar]
- Sohn M-H, Lee Y-A, Jeong K-Y, Sim S, Kim K-E, Yong T-S, Shin MH. German cockroach extract induces activation of human eosinophils to release cytotoxic inflammatory mediators. Int. Arch. Allergy Immunol. 2004;134:141–149. doi: 10.1159/000078647. [DOI] [PubMed] [Google Scholar]
- Sporik R, Squillace SP, Ingram JM, Rakes G, Honsinger RW, Platts-Mills TAE. Mite, cat, and cockroach exposure, allergen sensitization, and asthma in children: a case–control study of three schools. Thorax. 1999;54:675–680. doi: 10.1136/thx.54.8.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson MC, Campbell AR, Klauck MJ, Reed CE. Correlations between levels of mite and cat allergen in settled and airborne dust. J. Allergy Clin. Immunol. 1989;83:776–783. doi: 10.1016/0091-6749(89)90014-6. [DOI] [PubMed] [Google Scholar]
- Tovey E, Chapman M, Platts-Mills T. Mite faeces are a major source of house dust allergens. Nature. 1981;289:592–593. doi: 10.1038/289592a0. [DOI] [PubMed] [Google Scholar]
- Wickens K, Lane J, Siebers R, Ingham T, Crane J. Comparison of two dust collection methods for reservoir indoor allergens and endotoxin on carpets and mattresses. Indoor Air. 2004;14:217–222. doi: 10.1111/j.1600-0668.2004.00253.x. [DOI] [PubMed] [Google Scholar]
- Zwick H, Popp W. Allergic structures in cockroach hypersensitivity. J. Allergy Clin. Immunol. 1991;87:626–630. doi: 10.1016/0091-6749(91)90380-7. [DOI] [PubMed] [Google Scholar]


