Abstract
DNA damage is a proposed pathogenic factor in neurodegenerative disorders such as Parkinson’s disease. To probe the underpinning mechanism of such neuronal perturbation, we sought to produce an experimental model of DNA damage. We thus first assessed by in situ nick translation and emulsion autoradiography in the mouse brain the effect of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; 4 × 20mg/kg, i.p., every 2 hours), a neurotoxin known to produce a model of Parkinson’s disease, on DNA. Here we show that DNA strand breaks occur in vivo in this mouse model of Parkinson’s disease with kinetics and a topography that parallel the degeneration of substantia nigra neurons, as assessed by FluoroJade-labeling. Previously, nitric oxide synthase (NOS) and cyclooxygenase-2 (Cox-2) were found to modulate MPTP-induced dopaminergic neuronal death. We thus assessed the contribution of these enzymes to DNA damage in mice lacking either neuronal nitric oxide synthase (nNOS), inducible nitric oxide synthase (iNOS), or Cox-2. We found that the lack of Cox-2 and of nNOS, but not of iNOS activity, attenuate MPTP-related DNA damage. We also found that not only nuclear, but mitochondrial DNA as well is a target for the MPTP insult. These results suggest that the loss of genomic integrity can be triggered by the concerted actions of nNOS and Cox-2, and provide further support to the view that DNA damage may contribute to the neurodegenerative process in PD.
Keywords: Cyclooxygenase, DNA, MPTP, neurodegeneration, Nitric Oxide, oxidative damage, PD
Introduction
Compromised genome integrity has emerged as a critical pathogenic event in neurodegenerative diseases [1]. Genetic mutations in molecular pathways responsible for genome maintenance have been linked to accelerated aging phenotypes accompanied by widespread neurodegenerative changes [1]. DNA damage-linked neuronal dysfunction (also called senescence) and death have been invoked in the neurodegenerative processes underlying common sporadic brain disorders such as Parkinson’s disease (PD) [1]. Providing a major impetus to this hypothesis are the demonstrations that enzymes such as cyclin-dependent kinases [2], tumor suppressor protein p53 [3], and poly(ADP-ribose) polymerase-1 (PARP-1) [4,5], whose activation is known to be initiated by DNA damage, were all found to be instrumental in the death of nigrostriatal dopaminergic neurons, the subpopulation of neurons most affected in PD.
Here, we show that DNA strand breaks occur in the substantia nigra pars compacta (SNpc) in the mouse model of PD produced by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) [6]. The occurrence of DNA damage in this model of PD is associated with the activation of PARP and follows the kinetics and topography of degenerating of SNpc neurons. Previously, we have shown that enzymes such as cyclooxygenase-2 (Cox-2), neuronal nitric oxide synthase (nNOS), and inducible NOS (iNOS) contribute to SNpc dopaminergic neurodegeneration in the MPTP model of PD [7-9]. The use of knockout mice, deficient in Cox-2, nNOS, iNOS allowed us to show here that both Cox-2 and nNOS, but not iNOS contribute to the observed DNA damage. Finally, we demonstrate that not only is nuclear DNA, but also mitochondrial DNA that is damaged by the insult, which is interesting in light of the presumed pathogenic role of mitochondrial defects in PD [6]. Although our results are correlative, we hypothesize that the loss of genome integrity documented in the present study may contribute to the degenerative process in this model of PD and perhaps in PD itself.
Materials and methods
Animals and MPTP treatment
For this study, we used 10-week-old male C57/bl mice (Charles River Laboratories, Wilmington, MA), as well as neuronal NOS (nNOS) and inducible nitric oxide synthase (iNOS) knockout mice and their wild-type littermates (from Dawsons’ laboratory at Johns Hopkins University) and Cox-2 knockout mice and their wild-type littermates (from Taconic Farms). All mice (n = 4-8 per group) received four i.p injections of MPTP-HCl (18-20 mg/kg of free base; Sigma-Aldrich, St. Louis, MO) in saline at 2 hr intervals in one day and were sacrificed at selected time points (ranging from 0 to 7 days) after the last injection of MPTP. Control mice received saline only. MPTP handling and safety measures were in accordance with Columbia University and our published guidelines [10,11]. This protocol was in accordance with the NIH guidelines for use of live animals and was approved by the Institutional Animal Care and Use Committees of Columbia University Medical Center and Johns Hopkins University School of Medicine.
Tyrosine hydroxylase immunostaining and counts
At selected time points, 3-4 mice per time point were decapitated and whole brains harvested and processed as before [12]. Coronal sections (10 μm) encompassing the entire midbrain (i.e. Bregma −3.4 mm to Bregma −3.8 mm [13]) were cut on a cryostat and tyrosine hydroxylase (TH) immunostaining was performed using a polyclonal anti-TH antibody (1:1,000; Calbiochem, San Diego, CA) following the procedure reported before [12] with minor modifications. At the end of the TH immunostaining, sections were counterstained with thionin. The numbers of TH-positive cells were counted manually using light microscopy from at least four representative planes of the SNpc for each time point. The total number of TH-positive cells were calculated for each side, averaged for each animal and expressed per section.
DNA strand break by in situ nick translation (ISNT) and emulsion autoradiography
This assay was performed as previously reported [14] using [35S]-dATP and endonuclease-free DNA polymerase I. At selected time points, mice were decapitated and whole brains harvested and immediately frozen in dry-ice-cooled isopentane. As above, midbrain coronal sections (10 μm) were cut on a cryostat, but here they were thaw-mounted onto gelatin coated slides, and stored at −80°C until processed. Briefly, after exposure to formamide and acetylation, tissue sections were incubated with [35S]-labeled dATP, unlabelled nucleotides, and endonuclease-free DNA polymerase I. Adjacent sections incubated in absence of DNA polymerase I were used as negative controls. All sections were coated with Kodak NTB3 emulsion (VWR Scientific, Bridgeport, NJ) and counterstained with hematoxylin and eosin. The criteria for ISNT-labeling included the presence of silver grains over a cell with a visible nucleus and in a number at least five times greater than non-specific labeling. The latter was defined as the number of silver grains in an adjacent, equally-sized background area. The numbers of ISNT-labeled cells were counted following the same procedure as above. Then, for each time point, the numbers of ISNT-labeled cells were normalized by the number of TH-positive cells.
PARP activity and in situ histochemistry
Ventral midbrain, striatum, and cerebellum from mice 0 to 7 days post-MPTP were homogenized, and 20 μg of protein extract from each sample were used to determine PARP activity using a commercial kit (Trevigen, Gaithersburg, MD). The latter is based on the measurement of [32P]-NAD incorporation into nuclear acceptor proteins following the manufacturer’s instructions. Tissue extracts from PARP-1 knockout mice, as well as from wild-type mice treated with the PARP-1 inhibitor 3-aminobenzamide, were used for negative controls. At the end of the incubation period (10 min, 25°C), the reaction was stopped by the addition of ice-cold 20% trichloroacetic acid (TCA). The TCA-insoluble precipitates were washed three times with ice-cold 10% TCA, resuspended in liquid scintillation cocktail, and the incorporation of [32P]-ADP-ribose into TCA-precipitable proteins was quantified by scintillation spectroscopy.
For PARP histochemistry, fresh-frozen cryostat-cut sections (14 μm) collected at the same levels as for ISNT were fixed in ethanol and permeabilized with Triton X-100. This assay is based on the in situ demonstration of biotinyl-ADP ribose incorporation into nuclear acceptor proteins using 6-biotin-17-NAD+ (bio-NAD; Perkin Elmer, Boston, MA). The histochemical method was performed as described previously [15]. Incorporated biotin was detected by avidin:peroxidase-conjugated biotin complex, and color was developed with 3,3′-diaminobenzidine. The sections were counterstained with thionin or subjected to immunostaining using a rat anti-dopamine transporter (DAT) antibody (Chemicon, Temecula, CA), and color was developed with an SG peroxidase substrate kit (Vector Laboratories, Burlingame, CA). The percentages of SNpc PARP+/DAT+ and PARP+/DAT− cells were determined by counting manually ~50 PARP+ cells from at least four representative planes of ventral midbrain per mouse killed at 6 hr after the last MPTP-injection.
FluoroJade histochemistry
To assess the time course of neurodegeneration in the SNpc, mice were anesthetized then perfused with saline and 4% paraformaldehyde at selected time points ranging from 0 to 7 days after the last MPTP injection. Brains were removed, post-fixed overnight in the same fixative and frozen in dry-ice cooled isopentanet. nNOS knockout mice and their wild-type littermates were also used at 10 and 24 hr after the last MPTP injection as above. Cryostat-cut brain sections (30 μm) were mounted onto gelatin-coated slides prior to staining with 0.001% FluoroJade in 0.1% acetic acid as previously described [16]. As above, the total number of FluoroJade -labeled cells were calculated for each side, averaged for each animal, and expressed per section.
ISNT-labeled sections were 3-times thinner than the FluoroJade-labeled sections. In our pilot studies, we defined the relationship between FluoroJade counts and tissue thickness. Five mice were injected with MPTP as detailed above, and killed at 2 days post-MPTP (i.e. peak of FluoroJade-positive SNpc cells). Then, brains were harvested and processed as above for FluoroJade, except that one half of the midbrain was sectioned at 30 μm while the other half was sectioned at 10 μm. The number of FluoroJade-labeled cells per section at 30 μm was 96.9 ± 6.5 and at 10 μm was 34.5 ± 1.8 (mean ± sem). Based on these results, we divided the FluoroJade counts by 2.81 to facilitate the comparison between the ISNT and FluoroJade SNpc cell counts. Then, for each time point, the numbers of FluoroJade-positive cell were normalized by the number of TH-positive cells.
Mitochondrial DNA and Southern blot analysis
Ventral midbrain, striatum, and cerebellum were taken from mice injected with either saline or MPTP at selected time points ranging from 0 to 7 days after the last injection. Tissues were homogenized and treated with proteinase-K overnight at 37°C. After adding 1/4 vol. of 5 M NaCl, high molecular weight DNA was extracted with equal volumes of Sevag’s solution (24:1 chloroform:isoamyl alcohol), followed by precipitation with ammonium acetate and ethanol. Resuspended DNA was digested with XhoI and RNase. Digested samples were precipitated, resuspended in TE buffer and quantified. Samples containing 3 μg of total DNA were heated (20 min, 65°C) then cooled at room temperature. NaOH was then added to a final concentration of 0.1 N, and samples were incubated (15 min, 37°C). Samples were then mixed with alkaline loading dye, loaded onto a horizontal 0.6% alkaline agarose gel, and electrophoresed. DNA was then transferred to Zeta-Probe GT nylon membranes (Bio-Rad Laboratories, Hercules, CA). The membranes were cross-linked and hybridized with a [32P]-labeled mouse mitochondrial DNA-specific PCR-generated probe using primers corresponding to 1924-1953 (forward) and 2505-2473 (reverse) of mouse mtDNA and using TaKaRa LA Taq™ (Takara Bio Inc., Japan). The amplified fragment was gel purified and labeled with [32P]-dATP using the Random Primed DNA Labeling Kit (Roche Diagnostic Corporation). Bands of interest were analyzed and quantified using FluorChem 8800 (Alpha Innotech, San Leandro, CA).
Statistical analysis
All countings were performed by an individual blinded to treatment conditions and time points. All values are expressed as mean ± SEM. Differences among means were analyzed using one- or two-way ANOVA with time, treatment, and/or genotype as the independent factors. When ANOVA showed significant differences, comparisons between means were examined by Newman-Keuls post-hoc testing, except for the instances where all groups were compared against the control group (i.e. saline or time 0); in these cases we used Dunnett post-hoc testing. When only two groups were compared, Student’s T test was used. In all analyses, the null hypothesis was rejected at the 0.05 level.
Results
Time course and topography of MPTP-mediated DNA damage
To demonstrate whether the demise of nigrostriatal dopaminergic neurons is associated with a loss of genome integrity, we first assessed the occurrence of double and single DNA strand breaks in selected brain regions following the administration of the neurotoxin MPTP to mice [6]. DNA strand breaks were assessed at the single cell level by ISNT with radio-labeled nucleotides and emulsion autoradiography [14,17]. As previously reported [12], we found that the regimen of MPTP used here causes ~60% neuronal death in the SNpc by 7 days after the last injection (Table 1); the SNpc is the brain area that houses the cell bodies of the nigrostriatal dopaminergic pathway and is the brain structure the most affected in PD [6]. The selected time points encompassed the entire phase of neurodegeneration that follows this regimen of MPTP injection [18,19]. SNpc TH-positive neuronal counts can be found in Table 1.
Table 1.
SNpc TH-positive neuron counts post-MPTP
| Mean ± sem (n = 4 mice/ time point) |
|
|---|---|
| Saline controls | 151.8 ± 10.9 |
| 0-hr. | 152.0 ± 11.6 |
| 3-hr. | 148.7 ± 12.4 |
| 6-hr. | 132.5 ± 10.6 |
| 8-hr. | 110.0 ± 9.8 |
| 10-hr. | 91.6 ± 11.6 |
| 12-hr. | 78.3 ± 11.2 |
| 1-d. | 62.5 ± 10.4 |
| 2-d. | 39.8 ± 5.4 |
| 4-d. | 45.2 ± 10.2 |
| 7-d. | 44.6 ± 9.4 |
SNpc neuronal counting was performed as outlined in the Materials and methods section.
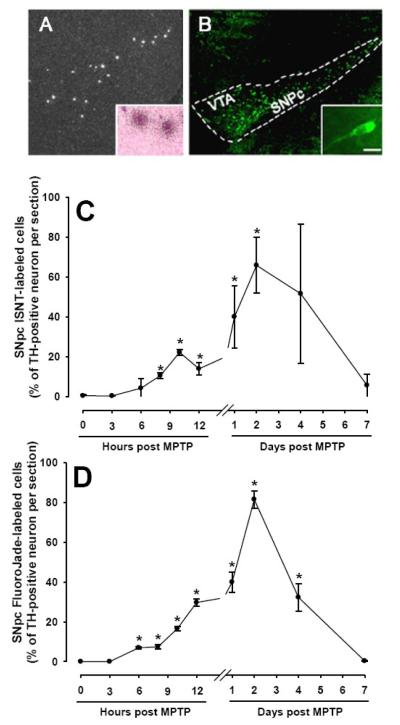
Using ISNT, evidence of DNA damage was found in the SNpc and, to a lesser extent, in other brain regions such as the ventral tegmental area (VTA) of MPTP-injected mice (Fig. 1A). Based on hematoxylin and eosin counterstaining, which provided a light labeling of cellular cytoplasm and nucleic acid materials, we were able to determine that most of the ISNT-positive cells were large and exhibited a neuronal morphology (Fig. 1A inset). Through a time-course study (Fig. 1C), we found that the number of ISNT-positive cells in the SNpc varied significantly (F[9,38] = 4.61, p<0.014) over time following MPTP administration. A few ISNT-positive neurons in the brains of these mice were detected as early as 6 hr post-MPTP (Fig. 1C). Thereafter, their numbers quickly rose, reaching a first peak at 10 hr and an even higher, second peak at ~48 hr post-MPTP (Fig. 1C). By day seven post-MPTP, there was typically ≤1 ISNT-labeled cell per section detected in any of the studied brain regions (Fig. 1C). During the time-course, the greatest number of ISNT-labeled cells was consistently found in the SNpc (Fig. 1A). Roughly half as many ISNT-labeled neurons were detected in other dopaminergic brain regions such as the ventral tegmental area (VTA). Consistent with MPTP relative specificity for ventral midbrain dopaminergic neurons, no more than 3 ISNT-labeled cells per section were detected in non-dopaminergic brain regions (e.g. hippocampus: 2.7 ± 0.1 cell/section at 24 hr post-MPTP, n=5).
Fig. 1.
MPTP causes DNA damage and neuronal degeneration. By 10 hr after the last injections of MPTP, numerous clusters of silver-grain deposits, as evidenced by DNA strand breaks, are seen within the SNpc area and to a lesser extent within the VTA area (A) confined over cells with large nuclei (inset) consistent with them being neurons. Similar findings are found for the cellular FluoroJade-labeling in both ventral midbrain regions (B). The inset in B shows the neuronal morphology of the FluoroJade-labeled cells. Except for the 12 hr time point, the time course of the SNpc ISNT labeling after MPTP injection (C) is very similar to that of the FluoroJade-labeling (D). Data are means ± SEM of 4 to 5 mice per time point after the last injections of MPTP and normalized for the number of TH-positive SNpc neurons (see Table 1 for actual values). *Different from t = 0 (p<0.05, Dunnett post-hoc test). Scale bar = 200 μm (A) and (B) and 20 μm in both insets.
Temporal relationship between MPTP-induced DNA damage and neuronal death
To establish the temporal relationship between DNA damage and neuronal death, a separate set of tissue sections from MPTP-injected mice killed at the same times points as above were stained with FluoroJade, a fluorochrome allowing for the sensitive and reliable histochemical localization of degenerating neurons [16]. Like the number of ISNT-positive neurons, the number of FluoroJade-stained neurons (Fig. 1B and inset) varied in a time-(F[9,38] = 41.61, p<0.001) and region-dependent manner (Fig. 1D). Consistent with the ISNT results, the first FluoroJade-positive cells were also detected by 6 hr post-MPTP, and the largest number of dying neurons was again found in the SNpc (Fig. 1B). Furthermore, the overall time-course of FluoroJade-positive cell numbers was comparable to that of the ISNT-positive cell numbers (Fig. 1C,D). Several FluoroJade-stained neurons were also seen in the VTA (Fig. 1B), while none was seen in any other region of the brain including hippocampus at any of the studied time points. Thus, evidence of cell degeneration, evidenced by FluoroJade, occurs in the brains of MPTP-intoxicated mice with a anatomical distribution and a time-line consistent with the known cell specificity and time-course of degeneration caused by this regimen of MPTP [12].
MPTP-mediated DNA damage is associated with PARP activation
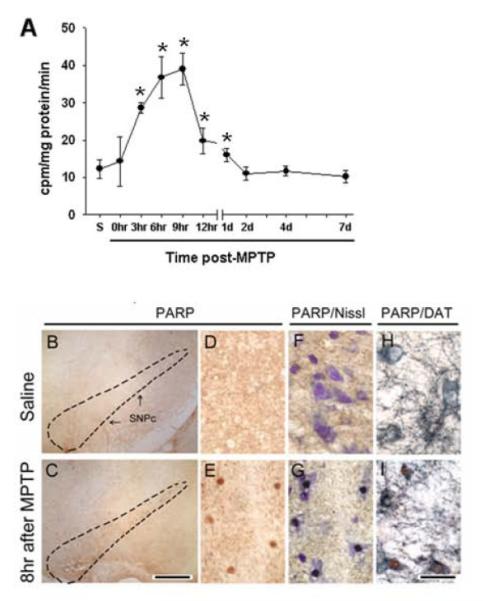
DNA damage activates the DNA-repair and protein-modifying enzyme PARP-1 in many cells including neurons [20]. Again, as mentioned above for the other assays, PARP activity measures in whole tissues from ventral midbrain varied over time (F[9,21] = 9.72, p<0.001) (Fig. 2A). In MPTP-injected mice, ventral midbrain PARP activity began to increase by 3 hr, peaked between 6 and 9 hr (300% increase), and then slowly subsided back to control activity by 2 days after MPTP injections (Fig. 2A). PARP catalytic activity in the cerebellum was low and not significantly altered by MPTP injection (not shown). To determine whether PARP was activated in SNpc dopaminergic neurons, we performed in situ PARP histochemistry combined with immunostaining for DAT at 6 hr after the last injection of saline or MPTP. In saline-injected controls, no PARP histochemical labeling was detected (Fig. 2B,D,F,H). In contrast, this double staining procedure revealed PARP activation in several ventral midbrain cells (Fig. 2C,E), all exhibiting a definite neuronal morphology (Fig. 2G) and nearly all expressing DAT (Fig. 2I): out of ~50 PARP+ SNpc cells per animal, 96 ± 4% were PARP+/DAT+ and 4 ± 2% were PARP+/DAT− (n = 3). No PARP activity or histochemical labeling was detected in ventral midbrains from mice deficient in PARP-1 (data not shown). Thus, after the administration of MPTP, there is a rapid and transient activation of PARP-1, specifically in the ventral midbrain within dopaminergic neurons.
Fig. 2.
MPTP administration activates PARP in dopaminergic neurons. PARP activity rises soon after the last injections of MPTP, and returns to baseline by 2 days after the last injections of MPTP (A). At the level of the ventral midbrain, activation of PARP is seen after MPTP injections (C,E,G,I) in neurons (E, G), which expressed DAT (I) but not after saline injections (B,D,F,H). *Different from t = 0 (p<0.05, Dunnett post-hoc test). Scale bar = 300 μm (B,C), 75 μm (D-I).
MPTP-related oxidative stress implication in DNA damage and neuronal death
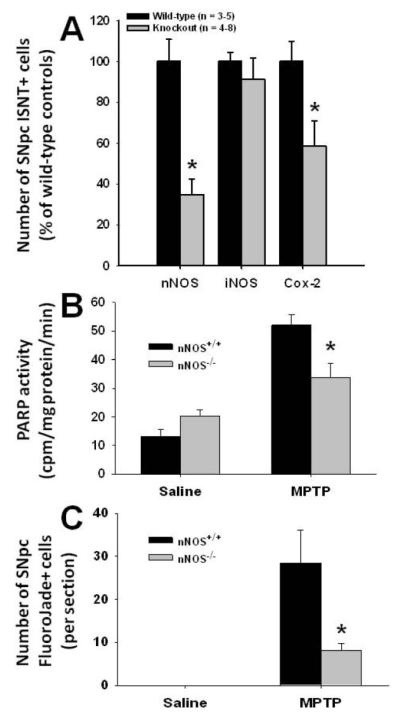
We next sought to determine the underlying mechanism leading to DNA damage in this PD model. Among the previously identified factors involved in MPTP neurotoxicity, we elected to study DNA damage by performing an ISNT assay in MPTP- and saline-injected mice deficient in Cox-2 or in nNOS, the main isoform of NOS in the nervous system. In both Cox-2−/− and nNOS−/− mice, the numbers of neurons positive for ISNT were dramatically smaller than in their wild-type littermates, at 24 hr post-MPTP (Fig. 3A); a similar attenuation was seen at the 10 hr time point (data not shown). In contrast to Cox-2 and nNOS ablation, iNOS deficiency did not significantly decrease MPTP-induced SNpc DNA damage as assessed by ISNT, neither at 10 hr (data not shown) nor at 24 hr post-MPTP (Student’s t-test: t8 = −0.30, P = 0.78) (Fig. 3A).
Fig. 3.
Contribution of NOS isoforms and Cox-2 in MPTP-induced DNA damage. At 24 hr after the last injections of MPTP, nNOS−/− and Cox-2−/−, but not iNOS−/− mice exhibit significantly fewer ISNT-labeled SNpc neurons compared to their respective wild-type littermates (A). Likewise, at 6 hr after the last injections of MPTP, nNOS−/− mice show lower ventral midbrain PARP activity (B) and at 24 hr after the last injections of MPTP, less SNpc FluoroJade-labeled neurons (C) compared to their nNOS+/+ littermates. Values are means ± SEM (n = 3-5). *Significantly different (Newman-Keuls post-hoc test, P<0.01) than wild-type littermates.
Given that the most prominent effect on the number of ISNT-positive cells in the MPTP model was observed for nNOS ablation, we also compared PARP activity and FluoroJade-labeling between nNOS+/+ and nNOS−/− mice at selected time points. Six hr after MPTP administration, nNOS+/+ mice exhibited significantly higher ventral midbrain PARP activity than their nNOS−/− counterparts (Fig. 3B). Furthermore, 24 hr after MPTP administration, nNOS+/+ mice exhibited a significantly higher number of FluoroJade-positive cells than their nNOS−/− counterparts (Fig. 3C). Thus, these results indicate that DNA damage in the SNpc results from an in vivo oxidative insult following MPTP administration, and that neuronal-but apparently not glial-derived NO contributes to the loss of genome integrity in this model of PD.
Mitochondrial DNA is also damaged by MPTP administration
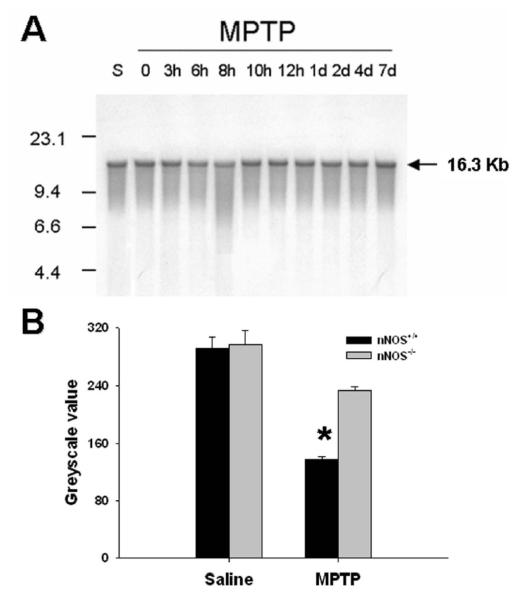
Mitochondrial DNA damage associated with respiratory chain deficiency has been documented within SNpc dopaminergic neurons from PD patients [21]. To test whether mitochondrial DNA is damaged after MPTP administration, the integrity of the entire mitochondrial genome was assessed by Southern blot analysis [22] at different time points post-MPTP injection (Fig. 4A). This experiment revealed evidence of mitochondrial DNA damage (i.e. presence of a tail) primarily in the striatum between 8-10 hr post-MPTP (Fig. 4A). As expected together with the tail saw on the gel, there was also at 8 hr after the last MPTP injection a 63% reduction of intensity of the band corresponding to the intact, full-length 16.3 Kb mitochondrial DNA compared to saline-injected controls (Fig. 4B). Note, that at this time point, striatal dopamine levels and TH activity are already reduced in MPTP-injected mice [23]. At none of the studied time points was damaged mitochondrial DNA detectable in ventral midbrain or cerebellum (data not shown). To determine whether the damage to mitochondrial DNA in the striatum was also provoked by a NO-mediated insult, the same Southern blot analysis was performed in mice deficient in nNOS (Fig. 4B). Compared to the wild-type littermates, nNOS−/− mice showed significantly less (2-way ANOVA, p < 0.05) mitochondrial DNA damage at 8 hr post-MPTP (Fig. 4B).
Fig. 4.
MPTP damages mitochondrial DNA. Southern blot analysis of striatal tissue reveals a tail of higher intensity caused by DNA common deletions and a lower intensity of the intact full-length 16.3 Kb band evidencing intact mitochondrial DNA, at 8 hr after the last injections of MPTP (A). The presented pattern was confirmed in three independent experiments. The reduction of the intact full-length 16.3 Kb band at 8 hr after the last injections of MPTP, is only significant in nNOS+/+ mice, but not in nNOS−/− littermates (B). Values are means ± SEM (n = 3-5). *Significantly lower (Newman-Keuls post-hoc test, P<0.05) than all other groups.
Discussion
The present study demonstrates that DNA damage in the form of strand breaks arises in cell groups located within the ventral midbrain following MPTP administration. Although no double labeling was performed, the positive cells illustrated in Fig. 1 were all located within the anatomical boundaries of the SNpc and the VTA, and were ISNT and FluoroJade positive after the injections of MPTP, a toxin that fails to damage non-dopaminergic elements in the ventral midbrain. These characteristics make it almost certain that the labeled cells are dopaminergic neurons. Although our findings are correlative, the demonstration of genomic and mitochondrial DNA damage by different techniques in an experimental model of PD raises the possibility that these alterations may participate in the neurodegenerative process. We thus speculate that the occurrence of DNA strand breaks in the MPTP model of PD provides further support to the hypothesis that the increased levels of DNA damage markers such as 8-hydroxyguanine and DNA deletions/rearrangements found in post-mortem PD samples may be pathogenically significant [21,24-26]. However, at this point, we cannot exclude with certainty that the observed ISNT-labeling is a consequence rather than a cause of the SNpc neurodegeneration. Arguing against the idea that the occurrence of DNA damage evidenced here by ISNT is a mere, non-specific consequence of dying neurons are the following two observations. First, as indicated in the results section, after MPTP administration, small numbers of cells with a low, but unambiguous ISNT+ signal were consistently counted in the hippocampus, whereas after MPTP administration no FluoroJade+ cells (or any suppressed-silver-stained cells [data not shown]) were detected in this region of the brain. Thus, it is unlikely that the occurrence of the ISNT+ signal is a sheer reflection of the dying process. Incidentally, the fact that some extra-nigrostriatal neurons exhibited DNA damage without dying to the same extent as SNpc neurons (as assessed by FluoroJade labeling) is consistent with the idea that a threshold of DNA damage may have to be reached in order to provoke cellular senescence and death. Second, PARP activation used here as a faithful marker of DNA damage clearly preceded the main wave of neurodegeneration (this finding is discussed below in more detail).
Remarkably, the number of ISNT-positive neurons in the SNpc varied over the studied period in a biphasic fashion. This suggests that MPTP causes genotoxicity in SNpc dopaminergic neurons by more than one mechanism, these mechanisms exerting their respective deleterious effects on DNA in a sequential manner. Consistent with this view and despite the fact that nNOS and Cox-2 mediate dopaminergic neurodegeneration in MPTP mice [7,9] likely by distinct mechanisms, are our observations that the deletion of both enzymes did attenuate DNA damage. Alternatively, it is also possible that SNpc neurons are responding unevenly to the genotoxic effects of MPTP as different neurons may have different arsenals of protection as well as mechanisms of DNA damage surveillance and repair [27]. This may also explain why, at some time points, an unusually large variability in SNpc ISNT+ cell number was consistently observed which cannot be explained by technical reasons given our inclusion of quality control samples in each experiment.
In damaged cells, PARP binds to DNA strand breaks and catalyzes the covalent attachment of ADP-ribosyl polymers, derived from the hydrolysis of NAD+, onto a variety of nuclear proteins including PARP itself [28]. Here, PARP activity was used as a means of confirming the occurrence of DNA damage in the MPTP mouse model of PD, as activity of this enzyme correlates linearly with the number of strand breaks [29,30]. While PARP activity did increase markedly after MPTP administration, which confirmed the occurrence DNA damage in this model of PD, the kinetics of PARP activity after MPTP injection differed from that of ISNT in two striking aspects. First, PARP activity rose prior to any detectable ISNT-labeling, which is consistent with the sensitivity of PARP to minute amounts of DNA damage [28]. Second, PARP activity normalized well before evidence of DNA damage disappeared. The latter discrepancy may find its explanation in the following facts. During PARP-mediated poly(ADP-ribosyl)ation of nuclear proteins, PARP itself becomes poly(ADP-ribosyl)ated, which decreases its affinity for damaged DNA as well as its catalytic activity [31]. Moreover, in vivo, the cellular level of the poly(ADP-ribosyl) polymer relies on the opposing actions of PARP and poly(ADP-ribose) glycohydrolase (PARG), the former synthesizing the polymer and the latter degrading it [28]. The apparent premature normalization of PARP activity may thus be due to the combined effects of the auto-modification of PARP, which inhibits the enzyme, and the activation of PARG, which catabolizes poly(ADP-ribosyl) polymers. Alternatively, it has also been reported that, in some instances, PARP activation may be caused by MAPKs such as ERKs, irrespective of DNA damage [32]. While the possibility of DNA damage-independent of PARP activation remains to be established in in vivo models of human diseases, this idea is certainly worth considering here. Thus, further studies will be required to test these different possibilities.
Given the genotoxic potential of oxidative stress [33], it is tantalizing to consider whether the results obtained here with mice deficient in Cox-2 and nNOS may be linked to damage caused by reactive oxygen and nitrative species (ROS, RNS). Aside from producing extracellular pro-inflammatory prostanoids, Cox-2 can generate ROS during the peroxidase catalysis of prostaglandin G2 conversion to prostaglandin H2 [34]. Upon donation of electrons to Cox, co-substrates such as dopamine become oxidized to dopamine-quinone [35], which is highly reactive with glutathione, amino acids such as cysteinyl, and DNA [36]. In our hands, there was no evidence for a pro-inflammatory role of Cox-2 following MPTP administration to mice [7]. However, there was a marked increase in Cox-2-dependent protein cysteinyl-dopamine content [7], a fingerprint for protein cysteinyl attack by dopamine-quinone [35]. Thus, these data suggest that, Cox-2 does promote oxidative events in MPTP-injected mice. Also relevant for a potential role of oxidation here is our previous demonstration that MPTP, in mice, markedly increases levels of 3-nitrotyrosine in affected brain regions [37]. This post-translation modification probably reflects the alteration of tyrosine residues by peroxynitrite, a reactive species resulting from the combination of superoxide and NO [33]. It is intriguing to note, however, that while ablation of iNOS attenuates neurodegeneration and the nitrative modification of proteins with pathogenic significance for PD such as parkin [8,38], mice deficient in iNOS exhibit the same extent of DNA damage as their wild-type counterparts. NOS activity in tissues such as ventral midbrain derive mainly from nNOS and, to a much lesser extent, from iNOS [8]. It is therefore possible that only a profound reduction in NO bioavailability for peroxynitrite formation, as caused by nullifying nNOS, is required prior to any meaningful abatement of the biochemical reaction that damages DNA. In light of the above discussion, we believe that it is indeed possible for both Cox-2 and nNOS to act in concert to oxidatively damage DNA in the MPTP mouse model.
All neurons possess both a nuclear and a mitochondrial genome; damage to either one may carry dramatic consequences for cell function and survival as discussed below. Because neuronal mitochondria are few in number in the soma [39] and MPTP intoxicates essentially dopaminergic neurons, it is likely that the observed ISNT-labeling in ventral midbrain reflects damage primarily to nuclear DNA in dopaminergic neurons. Supporting this view is our lack of detection of mitochondrial DNA damage in ventral midbrain at all of the studied time points by Southern blot analysis using a specific mitochondrial probe. Conversely, neuronal mitochondria are mainly found in nerve terminals [39], making it not surprising that mitochondrial DNA damage was detected in the striatum, where SNpc dopaminergic neuron nerve terminals are found. Thus, our data suggest that, after MPTP injury and in PD, the neurodegenerative processes may be associated with both nuclear and mitochondrial DNA damage. The striatal mtDNA damage detected by alkaline Southern blot appears to be transient, starting as early as 6 hours and disappearing by 10 hours after MPTP. Since equal amounts of total DNA from tissue homogenates were loaded onto the gel, this would suggest that mtDNA is rapidly repaired after strand breaks are produced. This finding was rather unexpected since nuclear DNA damage had not yet peaked at 10 hours, which raises the possibility that the apparent distinct kinetics of loss of nuclear and mitochondrial DNA integrity may be due to different repair capabilities or to the cellular localization of the insult (i.e. cell body versus terminals). Relevant to the latter aspect, it has been demonstrated that dopaminergic neurodegeneration of the terminals precedes that of the cell bodies following MPTP administration [40].
In conclusion, this work demonstrates that the parkinsonian toxin MPTP causes DNA damage as previously reported in PD autopsy materials. The cascade of deleterious events discussed here, together with previously identified noxious events such as activation of the apoptotic machinery [41], support the potential significance of DNA damage in the MPTP model and in PD as well.
Acknowledgements
The authors wish to thank Mr. Matthew Lucas and Michael Shelley for assistance in preparing this manuscript, as well as Dr. Csaba Szabó for his useful discussion about the PARP data. The authors are supported by NIH/NINDS Grants RO1 NS38586 and NS42269, P50 NS38370, P50 NS38377, P01 NS11766-27A2, P50 NS38367, and U54 ES12078, the US Department of Defense Grant (DAMD 17-99-1-9474 and DAMD 17-03-1), the Parkinson Disease Foundation (New York, USA), the MDA and the Wings Over Wall Street.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J. Aging and genome maintenance: lessons from the mouse? Science. 2003;299:1355–1359. doi: 10.1126/science.1079161. [DOI] [PubMed] [Google Scholar]
- [2].Smith PD, Crocker SJ, Jackson-Lewis V, Jordan-Sciutto KL, Hayley S, Mount MP, O’Hare MJ, Callaghan S, Slack RS, Przedborski S, Anisman H, Park DS. Cyclin-dependent kinase 5 is a mediator of dopaminergic neuron loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. USA. 2003;100:13650–13655. doi: 10.1073/pnas.2232515100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duan W, Zhu X, Ladenheim B, Yu QS, Guo Z, Oyler J, Cutler RG, Cadet JL, Greig NH, Mattson MP. p53 inhibitors preserve dopamine neurons and motor function in experimental parkinsonism. Ann. Neurol. 2002;52:597–606. doi: 10.1002/ana.10350. [DOI] [PubMed] [Google Scholar]
- [4].Cosi C, Colpaert F, Koek W, Degryse A, Marien M. Poly(ADP-ribose) polymerase inhibitors protect against MPTP-induced depletions of striatal dopamine and cortical noradrenaline in C57B1/6 mice. Brain Res. 1996;729:264–269. [PubMed] [Google Scholar]
- [5].Mandir AS, Przedborski S, Jackson-Lewis V, Wang ZQ, Simbulan-Rosenthal M, Smulson ME, Hoffman BE, Guastella DB, Dawson VL, Dawson TM. Poly (ADP-ribose) polymerase activation mediates MPTP-induced parkinsonism. Proc. Natl. Acad. Sci. USA. 1999;96:5774–5779. doi: 10.1073/pnas.96.10.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- [7].Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S, Vila M, Jackson-Lewis V, Przedborski S. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl. Acad. Sci. USA. 2003;100:5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Liberatore G, Jackson-Lewis V, Vukosavic S, Mandir AS, Vila M, McAuliffe WJ, Dawson VL, Dawson TM, Przedborski S. Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 1999;5:1403–1409. doi: 10.1038/70978. [DOI] [PubMed] [Google Scholar]
- [9].Przedborski S, Jackson-Lewis V, Yokoyama R, Shibata T, Dawson VL, Dawson TM. Role of neuronal nitric oxide in MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine)-induced dopaminergic neurotoxicity. Proc. Natl. Acad. Sci. USA. 1996;93:4565–4571. doi: 10.1073/pnas.93.10.4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Przedborski S, Jackson-Lewis V, Naini A, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J. Neurochem. 2001;76:1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- [11].Jackson-Lewis V, Przedborski S. Protocol for the MPTP mouse model of Parkinson’s disease. Nat. Protocols. 2007;2:141–151. doi: 10.1038/nprot.2006.342. [DOI] [PubMed] [Google Scholar]
- [12].Jackson-Lewis V, Jakowec M, Burke RE, Przedborski S. Time course and morphology of dopaminergic neuronal death caused by the neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neurodegeneration. 1995;4:257–269. doi: 10.1016/1055-8330(95)90015-2. [DOI] [PubMed] [Google Scholar]
- [13].Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic Press; New York: 1997. [Google Scholar]
- [14].Bordelon YM, MacKenzie L, Chesselet MF. Morphology and compartmental location of cells exhibiting DNA damage after quinolinic acid injections into rat striatum. J. Comp. Neurol. 1999;412:30–50. doi: 10.1002/(sici)1096-9861(19990913)412:1<38::aid-cne3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [15].Bakondi E, Bai P, Szabo EE, Hunyadi J, Gergely P, Szabo C, Virag L. Detection of poly(ADP-ribose) polymerase activation in oxidatively stressed cells and tissues using biotinylated NAD substrate. J. Histochem. Cytochem. 2002;50:91–98. doi: 10.1177/002215540205000110. [DOI] [PubMed] [Google Scholar]
- [16].Schmued LC, Albertson C, Slikker W., Jr. Fluoro-Jade: a novel fluorochrome for the sensitive and reliable histochemical localization of neuronal degeneration. Brain Res. 1997;751:37–46. doi: 10.1016/s0006-8993(96)01387-x. [DOI] [PubMed] [Google Scholar]
- [17].Iseki S, Mori T. Histochemical detection of DNA strand scissions in mammalian cells by in situ nick translation. Cell Biol. Int. Rep. 1985;9:471–477. doi: 10.1016/0309-1651(85)90155-9. [DOI] [PubMed] [Google Scholar]
- [18].Przedborski S, Vila M. MPTP: A review of its mechanisms of neurotoxicity. Clinical Neurosci. Res. 2001;1:407–418. [Google Scholar]
- [19].Bezard E, Dovero S, Imbert C, Boraud T, Gross CE. Spontaneous long-term compensatory dopaminergic sprouting in MPTP-treated mice. Synapse. 2000;38:363–368. doi: 10.1002/1098-2396(20001201)38:3<363::AID-SYN16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [20].Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends Pharmacol. Sci. 2004;25:259–264. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- [21].Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat. Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- [22].Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL. Enhanced mitochondrial DNA repair and cellular survival after oxidative stress by targeting the human 8-oxoguanine glycosylase repair enzyme to mitochondria. J. Biol. Chem. 2000;275:37518–37523. doi: 10.1074/jbc.M000831200. [DOI] [PubMed] [Google Scholar]
- [23].Ara J, Przedborski S, Naini AB, Jackson-Lewis V, Trifiletti RR, Horwitz J, Ischiropoulos H. Inactivation of tyrosine hydroxylase by nitration following exposure to peroxynitrite and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Proc. Natl. Acad. Sci. USA. 1998;95:7659–7663. doi: 10.1073/pnas.95.13.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B. Oxidative DNA damage in the parkinsonian brain: An apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J. Neurochem. 1997;69:1196–1203. doi: 10.1046/j.1471-4159.1997.69031196.x. [DOI] [PubMed] [Google Scholar]
- [25].Gu G, Reyes PE, Golden GT, Woltjer RL, Hulette C, Montine TJ, Zhang J. Mitochondrial DNA deletions/rearrangements in parkinson disease and related neurodegenerative disorders. J. Neuropathol. Exp. Neurol. 2002;61:634–639. doi: 10.1093/jnen/61.7.634. [DOI] [PubMed] [Google Scholar]
- [26].Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons. Am. J. Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Caldecott KW. DNA single-strand break repair and spinocerebellar ataxia. Cell. 2003;112:7–10. doi: 10.1016/s0092-8674(02)01247-3. [DOI] [PubMed] [Google Scholar]
- [28].D’Amours D, Desnoyers S, D’Silva I, Poirier GG. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 1999;342(Pt 2):249–268. [PMC free article] [PubMed] [Google Scholar]
- [29].Benjamin RC, Gill DM. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J. Biol. Chem. 1980;255:10502–10508. [PubMed] [Google Scholar]
- [30].Ohgushi H, Yoshihara K, Kamiya T. Bovine thymus poly(adenosine diphosphate ribose) polymerase. Physical properties and binding to DNA. J. Biol. Chem. 1980;255:6205–6211. [PubMed] [Google Scholar]
- [31].Zahradka P, Ebisuzaki K. A shuttle mechanism for DNA-protein interactions. The regulation of poly(ADP-ribose) polymerase. Eur. J. Biochem. 1982;127:579–585. [PubMed] [Google Scholar]
- [32].Cohen-Armon M. PARP-1 activation in the ERK signaling pathway. Trends Pharmacol. Sci. 2007;28:556–560. doi: 10.1016/j.tips.2007.08.005. [DOI] [PubMed] [Google Scholar]
- [33].Ischiropoulos H, Beckman JS. Oxidative stress and nitration in neurodegeneration: cause, effect or association? J. Clin. Invest. 2003;111:163–169. doi: 10.1172/JCI17638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mirjany M, Ho L, Pasinetti GM. Role of cyclooxygenase-2 in neuronal cell cycle activity and glutamate-mediated excitotoxicity. J. Pharmacol. Exp. Ther. 2002;301:494–500. doi: 10.1124/jpet.301.2.494. [DOI] [PubMed] [Google Scholar]
- [35].Hastings TG. Enzymatic oxidation of dopamine: the role of prostaglandin H synthase. J. Neurochem. 1995;64:919–924. doi: 10.1046/j.1471-4159.1995.64020919.x. [DOI] [PubMed] [Google Scholar]
- [36].Cavalieri E, Rogan E. Catechol quinones of estrogens in the initiation of breast, prostate, and other human cancers: keynote lecture. Ann. N. Y. Acad. Sci. 2006;1089:286–301. doi: 10.1196/annals.1386.042. [DOI] [PubMed] [Google Scholar]
- [37].Pennathur S, Jackson-Lewis V, Przedborski S, Heinecke JW. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and O,O’-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson’s disease. J. Biol. Chem. 1999;274:34621–34628. doi: 10.1074/jbc.274.49.34621. [DOI] [PubMed] [Google Scholar]
- [38].Chung KK, Thomas B, Li X, Pletnikova O, Troncoso JC, Marsh L, Dawson VL, Dawson TM. S-Nitrosylation of Parkin Regulates Ubiquitination and Compromises Parkin’s Protective Function. Science. 2004;304:1328–1331. doi: 10.1126/science.1093891. [DOI] [PubMed] [Google Scholar]
- [39].Peters A, Palay SL, deF.Webster H. The fine structure of the nervous system. Neurons and their supporting cells. Oxford University Press; New York: 1991. [Google Scholar]
- [40].Herkenham M, Little MD, Bankiewicz K, Yang SC, Markey SP, Johannessen JN. Selective retention of MPP+ within the monoaminergic systems of the primate brain following MPTP administration: an in vivo autoradiographic study. Neuroscience. 1991;40:133–158. doi: 10.1016/0306-4522(91)90180-v. [DOI] [PubMed] [Google Scholar]
- [41].Vila M, Przedborski S. Neurological diseases: Targeting programmed cell death in neurodegenerative diseases. Nat. Rev. Neurosci. 2003;4:365–375. doi: 10.1038/nrn1100. [DOI] [PubMed] [Google Scholar]