Abstract
Background:
Relating to Alzheimer’s disease (AD), dependence has been defined as the increased need for assistance due to deterioration in cognition, physical functioning, and behavior. Our objective was to evaluate the association between dependence and measures of functional impairment.
Methods:
Data were compiled by the National Alzheimer’s Coordinating Center. We used multinomial logistic regression to estimate the association between dependence and cognition, physical functioning, and behavior.
Results:
The independent association with dependence was positive. Dependence was most strongly associated with physical functioning. A secondary analysis suggested a strong association of dependence with multiple impairments, as measured by the interaction terms, in more severe patients.
Conclusions:
We find that dependence is simultaneously associated with physical functioning, cognition, and behavior, which support the construct validity of dependence. Dependence might be a more simple measure to explain the multifaceted disease progression of AD and convey the increasing need for care.
Keywords: outcome assessment, dependence, functional impairment, Alzheimer’s disease
Introduction
Alzheimer’s disease (AD) is a multifaceted disease that affects all aspects of a patient’s life. Clinically, AD is characterized by its effect on 3 domains (1) cognition, (2) physical functioning, and (3) behavior. 1 However, patients and caregivers are more concerned with the overall effect of the disease than with its impact on individual domains. A useful measure of AD progression should not only capture the wide-ranging clinical effects of the disease but also communicate this information in a way that is meaningful to multiple audiences.
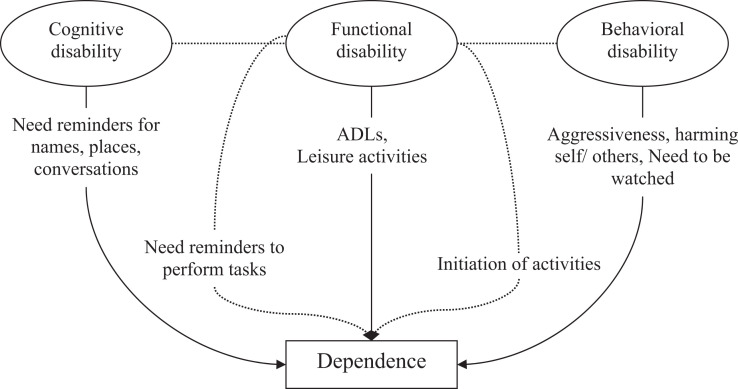
In a recent article, several of us supported dependence as a construct for assessing the impact of AD treatment. 1 Dependence in AD is the level of assistance required by a patient. As part of the conceptual framework of dependence, we suggested “a patient’s level of dependence is logically related (directly and indirectly) to the degree of impairment in the individual domains of cognition, function, and behavior” (Figure 1), and that “dependence in AD can be characterized as the measurable impact of changes in cognition, function, and behavior that result in an increased need for assistance.” We argued that although these domains impact different aspects of AD, they have an interrelated and aggregate effect. Initially, cognitive impairment is subtle and might require patients to seek assistance or reminders of names, places, or conversations. Over time, cognitive deficits might lead to increased reliance on others for keeping appointments, managing finances, or medications. Physical functioning or functional impairment, which is defined as a patient’s inability to perform specific activities, directly translates to dependence on others. When patients with AD can no longer dress themselves, they will be dependent on caregivers to complete parts of their everyday routine. The behavioral sequelae of AD include changes such as apathy, irritability, depression, anxiety, restlessness, agitation, and aggression. For example, wandering often leads to increased personal risk of injury, and the need for management strategies that involve increased dependence on a caregiver. We theorized that for progression in early AD, changes in cognition might be the primary contributor to changes in the level of dependence, and that functional impairment would be minimal in early stages and plays a larger role as the disease progresses.
Figure 1.
Relationship between cognition, function, behavior, and dependence. Solid lines represent direct effects and dotted lines represent indirect effects. Indirect effects are those cognitive or behavioral disabilities expressed as a change of function, for example, needs reminders to perform tasks or initiation of activities.
An important step in developing a health outcome measure is determining the validity of the instrument, that is, the extent to which the outcome measures what it purports to measure. 2 This may include convergent construct validity that measures the degree to which concepts that should be related theoretically are interrelated empirically. 3
In a review of dependence measures including the Independent Living Scale, the Record of Independent Living, the Behavioral Rating Scale for Geriatric Patients, the Nursing Care Dependency Scale, the Dependence Scale, and the Psychogeriatric Dependency Scale, we found correlations with cognition, 4 -6 behavior, 7,8 physical functioning, 9 quality of life, 10 and resource use. 11 However, individual correlations between dependence and other measures of AD progression may be due to joint correlation with a more general underlying measure of disease progression. By estimating the associations simultaneously, the individual associations can be calculated. For example, it is possible that caregivers responding to questions about dependence consider only physical functioning. If this were the case, then univariate analyses between dependence and cognition or behavior might still be significant if these measures are strongly correlated with physical functioning. However, multivariate analyses including all 3 measures will separate each of the associations and provide evidence of their individual relationship with dependence.
The objective of this analysis is to provide further information regarding the convergent construct-related validity of dependence as a health outcome by evaluating its association with functional measures of AD.
Methods
Data
The National Alzheimer’s Coordinating Center’s (NACC) Uniform Data Set (UDS) is a collection of demographic, clinical, and specimen data from Alzheimer’s disease centers (ADCs) funded by the National Institute of Aging. 12 All ADCs enroll and follow patients with a standardized protocol and provide pooled data for research through the NACC. Each ADC recruits differently and is thus not considered a population-based sample. Data analyzed were limited to those patients who were 65 years or older, had mild cognitive impairment (MCI) or dementia, or were cognitively normal. At the time of analysis, data were available from 2005 to 2009 and contained 16 469 patients from 32 different ADCs who had completed initial visits and met our inclusion criteria. Of these, 5512 were diagnosed with possible or probable AD, which is ∼1 in every 1000 patients with AD in the United States. We analyzed the cross-sectional data of the initial visits of the 14 522 patients with complete data. Sensitivity analysis was undertaken to assess the effect of missing data from the Mini-Mental State Examination (MMSE), since it was the variable with most missing data. We imputed MMSE directly from the next available year of data, assuming that the follow-up value would be similar to the missing value. No data collection was undertaken by the authors; all data were provided by the NACC.
Measures
Dependence is captured in the UDS as a 4-level categorical variable. Levels (i)–(iv) indicate increasingly more dependence. 13
Able to live independently
Requires some assistance with complex activities
Requires some assistance with basic activities
Completely dependent
Unknown
Dependence is reported to the intake interviewer or clinician and is obtained by either patient interview or proxy informant report as needed. Although definitions might be different among respondents, this measure is simple to understand and complete. The advantage of the simple nature of this measure is that the respondent’s understanding of dependence is not constrained by the construct of a more complex measure. This allows us to assess the association of the respondent’s conception of dependence with other measures of disability. As far as we know this measure of dependence has not been psychometrically tested, but we consider it to have good face validity as it seems to correspond well with our definition of dependence. Although there are only 4 levels of dependence being measured and it is possible that other measures of dependence will be more sensitive to change, we consider this measure useful for understanding the patient’s characterization of dependence.
To measure cognitive impairment, the MMSE 14 was used. Although the MMSE was developed as a screening instrument, it has been used to measure and show the benefit of treatment in clinical trials and to measure AD progression in longitudinal observational studies. 15 The psychometric properties of the MMSE have been well studied, and it has been found to adequately assess the severity of cognitive impairment and changes over time. 16
The functional activities questionnaire (FAQ) was used to measure physical functioning. The FAQ is used in clinical situations for the assessment of functional deficit for the elderly individuals. The FAQ shows good sensitivity and specificity and interrater reliability. 17 The FAQ contains 10 questions regarding an individual’s ability to perform tasks in the past 4 weeks. When an individual’s scores are added for each of the 10 questions, an individual with full physical functioning will score 0, while the most impaired individual will score between 3 and 30 depending on how many tasks were attempted in the past 4 weeks. To adjust for this difference, we added the score for each task and divided by the number of tasks attempted to give us a range of scores between 0 and 3. Individuals that did not attempt any of the tasks were considered to have a missing score for the FAQ.
To measure the behavior, the neuropsychiatric inventory questionnaire (NPI-Q) was used. The NPI-Q was developed to assess the severity, frequency, and caregiver distress of 12 different neuropsychiatric disturbances common in dementia. 18 The NPI-Q has well-established psychometric properties. 18–21 Only the severity score of the NPI-Q is contained within the UDS.
Statistical Analyses
Dependence was used as the dependent variable (left-hand side variable) and MMSE, FAQ, and NPI-Q (right-hand side variables). All models were controlled for gender, age, age squared, years of education, and location. A dummy variable, location, indicating the ADC from which the data were collected was included to account for the possible differences in the way measures were collected across centers and for possible regional differences. A secondary analysis included second- and third-order interaction terms between FAQ, MMSE, and NPI-Q. It was expected that higher levels of dependence would be more strongly associated with multiple impairments.
We reversed the scale for the MMSE so that an increased score on the reversed scale indicated more severe cognitive disability. This was done so that the coefficient could be interpreted in the same direction as the FAQ and NPI-Q coefficients. Positive coefficients indicate more impairment in cognition, physical functioning, or behavior and are associated with increased dependence.
Since each of the variables of interest is measured on a different scale (FAQ 0-3, MMSE 0-30, and NPI-Q 0-36), we standardized the 3 variables of interest (MMSE, FAQ, and NPI-Q) by subtracting the mean and dividing by the standard deviation (SD).
We used multinomial regression with adjacent comparisons, because it is not restricted by the proportional odds assumption. 22 We limited the analysis to the adjacent comparisons because dependence is an ordinal scale.
Results
The UDS contained 16 469 respondents to the initial visit package that were 65 years or older and had possible or probable AD, MCI, and other dementia or were cognitively normal. The mean age of the patients was 74 years with younger patients more likely to be more independent (Table 1). The average number of years of education was 14.6; more independent individuals had more education. The sample was 57% female. Patients who were more dependent were also more likely to be in a nursing home. Mean scores for the MMSE, FAQ, and NPI-Q were also more severe at higher levels of dependence (Table 2). Cognitively normal and patients with MCI had higher cognition and less functional impairment than probable or possible AD (Appendix A).
Table 1.
Description of the Complete and Full Analysis Populations.
| Complete Population (n = 16 469) | Analyzed Population (n = 14 522) | |
|---|---|---|
| Age, mean (SD) | 74.41 (10.29) | 74.21 (10.13) |
| Years education, mean (SD) | 14.65 (3.58) | 14.67 (3.52) |
| Sex | ||
| Male | 42.52% | 42.92% |
| Female | 57.48% | 57.08% |
| Residence | ||
| Single family residence | 87.19% | 87.93% |
| Retirement community | 5.88% | 5.80% |
| Assisted living/boarding home/adult family home | 2.82% | 2.66% |
| Skilled nursing facility/nursing home | 1.67% | 1.32% |
| Other | 2.05% | 2.08% |
| Unknown | 0.39% | 0.22% |
| Diagnosis | ||
| Cognitively normal | 36.78% | 36.34% |
| MCI | 18.71% | 22.79% |
| Possible AD | 6.12% | 6.03% |
| Probable AD | 27.35% | 27.76% |
| Other dementia | 11.04% | 7.07% |
| Outcome measures | ||
| Mean CDR mean (SD) | 0.63 (0.74) | 0.61 (0.70) |
| Mean MMSEa mean (SD) | 24.68 (6.52) | 24.63 (6.47) |
| Mean MMSEa with follow-up mean (SD) | 24.64 (6.58) | 24.67 (6.47) |
| Mean FAQ mean (SD) | 0.84 (1.03) | 0.81 (1.00) |
| Mean NPI-Q mean (SD) | 3.09 (4.34) | 3.01 (4.25) |
Abbreviations: AD, Alzheimer’s disease; MMSE, minimental state examination; MCI, mild cognitive impairment; CDR, clinical dementia rating scale; FAQ, functional activities questionnaire; NPI-Q, neuropsychiatric inventory questionnaire; SD, standard deviation.
a Normal MMSE scale, not reversed.
Table 2.
Description of the Analyzed Population by Dependence Level.
| Independent, n = 8822 | Needs Assistance With Complex Tasks, n = 3469 | Needs Assistance With Basic Tasks, n = 1675 | Dependent, n = 556 | Unknown, n = 114 | P valuea,b | |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 73.31 (9.95) | 74.93 (9.93) | 76.59 (10.68) | 76.82 (10.61) | 76.32 (10.17) | <.001 |
| Years education, mean (SD) | 15.12 (3.27) | 14.16 (3.66) | 13.75 (3.87) | 13.56 (4.00) | 15.89 (3.57) | <.001 |
| % Female | 59.79% | 51.77% | 54.87% | 53.78% | 49.12% | <.001 |
| % Institutionalizedc | 0.53% | 4.06% | 13.37% | 29.68% | 3.16% | <.001 |
| % Cognitively normal | 57.97% | 3.43% | 2.45% | 0.72% | 23.68% | <.001 |
| % Diagnosed ADd | 11.46% | 65.38% | 72.96% | 73.20% | 27.19% | <.001 |
| Mean CDR mean (SD) | 0.24 (0.31) | 0.85 (0.47) | 1.45 (0.75) | 2.33 (0.80) | 0.75 (0.73) | <.001 |
| Mean MMSE mean (SD) | 27.79 (2.95) | 22.46 (5.38) | 17.72 (7.08) | 9.79 (8.41) | 22.84 (7.17) | <.001 |
| Mean MMSEe with follow up mean (SD) | 27.78 (2.98) | 22.45 (5.41) | 17.71 (7.08) | 9.67 (8.42) | 22.82 (7.13) | <.001 |
| Mean FAQ mean (SD) | 0.21 (0.45) | 1.36 (0.79) | 2.20 (0.76) | 2.75 (0.47) | 1.20 (0.99) | <.001 |
| Mean NPI-Q mean (SD) | 1.51 (2.80) | 4.47 (4.43) | 6.25 (5.36) | 7.95 (5.80) | 5.43 (5.93) | <.001 |
Abbreviations: AD, Alzheimer’s disease; MMSE, Mini-Mental State Examination; CDR, clinical dementia rating scale; FAQ, functional activities questionnaire; NPI-Q, neuropsychiatric inventory questionnaire; SD, standard deviation.
a Proportions were tested using the Pearson chi-square test.
b Means were tested using analysis of variance.
c Institutionalized includes assisted living/boarding home/adult family home, and skilled nursing facility/nursing home.
d Diagnosed AD includes possible and probable AD.
e Normal MMSE scale, not reversed.
There were 14 522 respondents with complete data for all of the variables of interest for which the main analysis was undertaken. Patients who were considered dependent were more likely to be missing data on all measures except the FAQ in which case those considered independent had the highest percentage of missing data (5.22%). The highest percentage of missing data was for those with a response of unknown for their dependence level. The MMSE was missing for 20% of those who were considered dependent. Missing MMSE was due to cognitive/behavior problems in 85% of the cases. To assess the effect of missing data, we substituted follow-up MMSE scores for those that were missing at the initial visit (n = 42). Results from these analyses differed very little from the main findings and did not affect the conclusions (Appendix B).
The results of the main analysis are displayed in Table 3. The table presents the standardized odds ratios (ORs) and P values for each adjacent comparison of dependence. The first row compares “Difficulties with complex tasks” (Dependence = 2) to “Independent” (Dependence = 1). The OR 6.88 suggests that an individual with a FAQ score 1 SD higher is ∼7 times more likely to be classified as having “Difficulties with complex tasks” (Dependence = 2) than to be classified as “Independent” (Dependence = 1). All coefficients were positive and highly significant (<0.001) except in the case of NPI-Q when comparing “Difficulty with basic tasks” to “Difficulty with complex tasks” for which the P value was .051.
Table 3.
Multinomial Logistic Regression of Dependence Level.a
| ORb | P value | |
|---|---|---|
| FAQ (SD = 0.98) | ||
| Difficulty with complex tasks to independence (2-1) | 6.88 | <.001 |
| Difficulty with basic tasks to complex tasks (3-2) | 2.94 | <.001 |
| Dependent to Difficulty with basic tasks (4-3) | 3.27 | <.001 |
| Reversed MMSE (SD = 0.99) | ||
| Difficulty with complex tasks to Independence (2-1) | 2.10 | <.001 |
| Difficulty with basic tasks to complex tasks (3-2) | 1.33 | <.001 |
| Dependent to Difficulty with basic tasks (4-3) | 1.89 | <.001 |
| NPI-Q (SD = 0.98) | ||
| Difficulty with complex tasks to Independence (2-1) | 1.22 | <.001 |
| Difficulty with basic tasks to complex tasks (3-2) | 1.06 | .051 |
| Dependent to Difficulty with basic tasks (4-3) | 1.17 | <.001 |
Abbreviations: ADC, Alzheimer’s disease Center; MMSE, Mini-Mental State Examination; FAQ, functional activities questionnaire; NPI-Q, Neuropsychiatric Inventory questionnaire; OR, odds ratio; SD, standard deviation.
a Complete data analysis of 14 522 respondents. All analyses controlled for age, age squared, sex, years of education, and ADC.
b Standardized coefficients adjust for the difference in scale and are interpreted as the increased likelihood of being at a higher level of dependence for 1 SD increase.
A multinomial model with interaction terms between the coefficients of interest was also undertaken (Appendix C). Some of the main effects had ORs of less than 1. The coefficient 0.62 for “Difficulty with basic tasks to complex tasks” suggests that an individual with higher cognitive impairment but with no difference in physical functioning or behavior is more likely to be less dependent. However, taking into account the interaction terms, if an individual has both higher cognitive and physical functioning impairments, they are ∼3 times more likely to have “Difficulty with basic tasks” compared to only having “Difficulty with basic tasks.” Most coefficients were statistically significant at a P-value of .05 except in the case of more severe patients (Dependence = 4) possibly because of the smaller number of patients in this subgroup. The goodness of fit was tested using Akaike information criteria and Bayesian information criteria, and the model without interaction terms was preferred.
Discussion
Using data from the NACC-UDS and multinomial regression analyses, we demonstrate that measures of physical functioning, cognition, and behavior are associated with respondents’ assessments of dependence. This analysis provides evidence that this measure of dependence correlates with well-known clinical AD measures and supports convergent validity.
A recent study by McLaughlin et al 23 showed that the Dependence Scale was simultaneously correlated with physical functioning, cognition, and behavior as well as economic and quality of life measures in a sample of 196 patients with Alzheimer. Having used a different measure of dependence and standardized our coefficients, our results are not directly comparable but do show correlation in the same direction. The current study has the advantage of a very large sample size, which allows us to investigate interactions between the domains of interest.
The higher ORs at the lowest levels of dependence suggest that dependence is most strongly correlated with FAQ, MMSE, and NPI-Q at lower levels of dependence. The lower ORs at higher levels of dependence suggests that as individuals become more dependent, changes in FAQ, MMSE, or NPI-Q may be less important to assessments of dependence.
It may be that the results of an association study are an artifact of the measures being used. The ORs may be lower at higher dependence because they are less sensitive at higher levels of severity or because of ceiling effects. This would mean that the scales are nonlinear. We do not believe this is the situation as the model with interaction terms shows higher ORs on interaction terms in some instances. If measures were less sensitive or ceiling effects were a problem, then the interaction terms would also be lower in more severe cases.
These results were contrary to previously published supposition 1 that for progression in early AD, changes in cognition might be the primary contributor to changes in the level of dependence, and that functional impairment would be minimal in early stages and play a larger role as the disease progresses. Instead, we found that the FAQ, our measure of functional impairment, was most important in all stages. The model including interaction terms, however, shows that at more severe levels of dependence multiple impairments are the most important as interaction terms are higher than main effects when comparing “Dependent” to “Difficulty with basic tasks.”
Taking into account multiple impairments using interaction terms, the results of the model include some ORs that are less than 1, which are contrary to expectations and difficult to explain. The statistically significant OR of less than 1 for the main effect of MMSE suggests that lower cognition without lower physical functioning or behavior may make patients less dependent. This could be the case from the point of view of the caregiver if at higher levels of severity a decrease in cognition means a patient does not demand as much attention.
Although not statistically significant, the OR less than 1 for NPI-Q may be due to the decline of behavioral challenges in later stages of AD; this might lead to less assistance or possibly less surveillance and therefore less dependence from the point of view of the caregiver.
There are some limitations to this study which include the choice of the dependence measure, the limited number of available covariates, and the mix of patient and caregiver responses. It has been suggested that since dependence occurs on a continuum the ideal measure of dependence should not be characterized by a limited number of discrete levels. 1 The measure of dependence used for this analysis contained 4 levels and only 1 dimension. Similar measures such as the Dependence Scale has been found to have an adequate hierarchical ordering. 24 However, the goal of this study is not to support a particular measure of dependence.
Further analyses could include additional covariates. Other covariates such as medical history, other chronic conditions (depression, cerebrovascular disease, chronic obstructive pulmonary disease, diabetes, or hypertension), or caregivers’ characteristics may also influence dependence and the domains of interest. A subsequent longitudinal analysis might consider how a patient’s dependence changes over time.
This study relies on caregivers’ assessments for those patients unable to respond for themselves; the accuracy of these assessments may depend on caregivers' characteristics. 25 Similarly, it is unknown which evaluations of dependence were reported by the patient and which were reported by the caregiver. Other studies have shown that assessments of a patient’s quality of life differ between patients and caregivers. 25,26 However, with a measure such as dependence for which the outcome is easily observed by the caregiver and on a simple 4-level scale, this is not considered to be a substantial limitation of the study.
This analysis shows that dependence is strongly associated with physical functioning and simultaneously associated with cognition and behavior. This suggests that when patients’ dependence is assessed, it is influenced by the 3 most important domains of AD. These results support the construct-related validity of dependence as a health outcome for AD. The results suggest that dependence could be a useful measure in explaining the impact of AD on economic issues such as the risk of institutionalization and caregiver burden. The results also suggest that dependence captures many of the issues pertinent to caregivers and could be an important tool to help them understand the need for increasing care and responsibility for the patient. Dependence may also be useful to measure the treatment effects as those treatments that improve patients’ independence are likely to be highly valued by both patients and caregivers.
Acknowledgments
The authors are grateful to Leslie Phillips for help in obtaining data from NACC-UDS, David Blough for statistical advice, and Trent McLaughlin for helpful comments on an earlier draft of the manuscript.
Appendix A
Patient’s Characteristics of Complete Case Data by Diagnosis.
| Cognitively Normal (n = 5278) | MCI (n = 3309) | Probable AD (n = 4032) | Possible AD (n = 876) | Other Dementia (n = 1027) | |
|---|---|---|---|---|---|
| Age (SD) | 72.83 (10.39) | 74.44 (9.70) | 76.72 (9.32) | 75.70 (9.87) | 69.39 (10.53) |
| Years education (SD) | 15.41 (3.04) | 14.60 (3.62) | 13.96 (3.74) | 13.89 (9.99) | 14.55 (3.41) |
| Sex | |||||
| Male | 35.16% | 47.36% | 43.41% | 47.83% | 62.41% |
| Female | 64.84% | 52.64% | 56.59% | 52.17% | 37.59% |
| Residence | |||||
| Single family residence | 89.88% | 88.73% | 85.71% | 86.19% | 85.49% |
| Retirement community | 6.84% | 6.50% | 4.64% | 4.68% | 3.70% |
| Assisted living/boarding home/adult family home | 0.68% | 1.75% | 4.99% | 4.79% | 4.77% |
| Skilled nursing facility/nursing home | 0.04% | 0.24% | 2.70% | 3.08% | 4.38% |
| Other | 2.29% | 2.60% | 1.88% | 1.03% | 0.97% |
| Unknown | 0.27% | 0.18% | 0.07% | 0.23% | 0.68% |
| Outcome measures | |||||
| Mean CDR (SD) | 0.11 (0.31) | 0.87 (0.41) | 2.09 (0.87) | 1.87 (0.91) | 2.02 (0.95) |
| Mean MMSEa (SD) | 28.86 (1.51) | 27.16 (2.75) | 19.20 (6.89) | 20.49 (7.21) | 20.11 (7.83) |
| Mean FAQ (SD) | 0.05 (0.20) | 0.37 (0.51) | 1.75 (0.88) | 1.56 (0.95) | 1.79 (0.92) |
| Mean NPI-Q (SD) | 0.84 (1.99) | 2.35 (3.29) | 4.84 (4.70) | 5.14 (5.03) | 7.35 (5.54) |
Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; FAQ, functional activities questionnaire; NPI-Q, Neuropsychiatric Inventory questionnaire; SD, standard deviation. aNormal MMSE scale, not reversed.
Appendix B
Multinomial Logistic Regression of Dependence Level With Additional MMSE.a
| ORb | P value | |
|---|---|---|
| FAQ (SD = 0.98) | ||
| Difficulty with complex tasks to independence (2-1) | 6.87 | <.001 |
| Difficulty with basic tasks to complex tasks (3-2) | 2.94 | <.001 |
| Dependent to difficulty with basic tasks (4-3) | 3.24 | <.001 |
| Reversed MMSE (SD = 0.99) | ||
| Difficulty with complex tasks to independence (2-1) | 2.09 | <.001 |
| Difficulty with basic tasks to complex tasks (3-2) | 1.33 | <.001 |
| Dependent to difficulty with basic tasks (4-3) | 1.88 | <.001 |
| NPI-Q (SD = 0.98) | ||
| Difficulty with complex tasks to independence (2-1) | 1.22 | <.001 |
| Difficulty with basic tasks to complex tasks (3-2) | 1.06 | .039 |
| Dependent to difficulty with basic tasks (4-3) | 1.17 | <.001 |
Abbreviations: ADC, Alzheimer's disease Center; MMSE, minimental state examination; FAQ, functional activities questionnaire; NPI-Q, Neuropsychiatric Inventory questionnaire; OR, odds ratio; SD, standard deviation.
a Complete data analysis of 14 522 respondents. All analyses controlled for age, age squared, sex, years of education, and ADC.
b Standardized coefficients adjust for the difference in scale and are interpreted as the increased likelihood of being at a higher level of dependence for 1 SD increase.
Appendix C
Multinomial Logistic Regression of Dependence Level With Interaction Terms.a
| ORb | P value | ||
|---|---|---|---|
| Main effects | |||
| FAQ (SD = 1.00) | |||
| Difficulty with complex tasks to independence (2-1) | 22.34 | <.001 | |
| Difficulty with basic tasks to complex tasks (3-2) | 1.51 | <.001 | |
| Dependent to difficulty with basic tasks (4-3) | 0.76 | .236 | |
| Reversed MMSE (SD = 6.47) | |||
| Difficulty with complex tasks to independence (2-1) | 5.52 | <.001 | |
| Difficulty with basic tasks to complex tasks (3-2) | 0.62 | .002 | |
| Dependent to difficulty with basic tasks (4-3) | 0.43 | .04 | |
| NPI-Q (SD = 4.25) | |||
| Difficulty with complex tasks to independence (2-1) | 2.04 | <.001 | |
| Difficulty with basic tasks to complex tasks (3-2) | 0.71 | .023 | |
| Dependent to difficulty with basic tasks (4-3) | 0.81 | .632 | |
| Interaction terms | |||
| FAQ × revMMSE (SD = 17.80) | |||
| Difficulty with complex tasks to independence (2-1) | 0.07 | <.001 | |
| Difficulty with basic tasks to complex tasks (3-2) | 3.3 | <.001 | |
| Dependent to difficulty with basic tasks (4-3) | 4.67 | <.001 | |
| NPI-Q × revMMSE (SD = 63.14) | |||
| Difficulty with complex tasks to Independence (2-1) | 0.54 | .001 | |
| Difficulty with basic tasks to complex tasks (3-2) | 1.32 | .268 | |
| Dependent to difficulty with basic tasks (4-3) | 0.52 | .283 | |
| FAQ × NPI-Q (SD = 9.85) | |||
| Difficulty with complex tasks to Independence (2-1) | 0.28 | <.001 | |
| Difficulty with basic tasks to complex tasks (3-2) | 1.86 | <.001 | |
| Dependent to difficulty with basic tasks (4-3) | 1.54 | .276 | |
| Third order (SD = 172.35) | |||
| Difficulty with complex tasks to Independence (2-1) | 3.71 | <.001 | |
| Difficulty with basic tasks to complex tasks (3-2) | 0.58 | .05 | |
| Dependent to difficulty with basic tasks (4-3) | 1.7 | .362 | |
Abbreviations: ADC, Alzheimer’s disease Center; MMSE, minimental state examination; FAQ, functional activities questionnaire; NPI-Q, Neuropsychiatric Inventory questionnaire; OR, odds ratio; SD, standard deviation.
a Complete data analysis of 14 522 respondents. All analyses controlled for age, age squared, sex, years of education, and ADC.
b Standardized coefficients adjust for the difference in scale and are interpreted as the increased likelihood of being at a higher level of dependence for 1 SD increase.
Footnotes
Authors’ Note: This work was completed as a part of the dissertation of Eldon Spackman while a student at the University of Washington.
The authors declared a potential conflict of interest as follows: Eldon Spackman and Sean Sullivan had full access to all the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis. Eldon Spackman has consulted with Genentech, Bayer, and Elan Pharmaceuticals. Sean Sullivan has consulted with Bayer and Elan Pharmaceuticals. Peter Neumann has consulted with Elan Pharmaceuticals. David Veenstra has consulted with Genentech. Srikanth Kadiyala has no conflicts to disclose
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Data collection was supported by National Institute on Aging (NIA) Grant (U01 AG016976) to the Nationl Alzheimer's Coordinating Center.
References
- 1. McLaughlin T, Feldman H, Fillit H, et al. Dependence as a unifying construct in defining Alzheimer's disease severity. Alzheimers Dement. 2010;6(6):482–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Patrick DL, Burke LB, Powers JH, et al. Patient-reported outcomes to support medical product labeling claims: FDA perspective. Value Health. 2007;10(suppl 2):S125–S137. [DOI] [PubMed] [Google Scholar]
- 3. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11(3):193–205. [DOI] [PubMed] [Google Scholar]
- 4. Holtzer R, Wegesin DJ, Albert SM, et al. The rate of cognitive decline and risk of reaching clinical milestones in Alzheimer's disease. Arch Neurol. 2003;60(8):1137–1142. [DOI] [PubMed] [Google Scholar]
- 5. Sarazin M, Stern Y, Berr C, et al. Neuropsychological predictors of dependency in patients with Alzheimer's disease. Neurology. 2005;64(6):1027–1031. [DOI] [PubMed] [Google Scholar]
- 6. Kurtz MM, Moberg PJ, Mozley LH, et al. Cognitive impairment and functional status in elderly institutionalized patients with schizophrenia. Int J Geriatr Psychiatry. 2001;16(6):631–638. [DOI] [PubMed] [Google Scholar]
- 7. Tun SM, Murman DL, Long HL, Colenda CC, von Eye A. Predictive validity of neuropsychiatric subgroups on nursing home placement and survival in patients with Alzheimer's disease. Am J Geriatr Psychiatry. 2007;15(4):314–327. [DOI] [PubMed] [Google Scholar]
- 8. Zhu CW, Leibman C, McLaughlin T, et al. The effects of patient function and dependence on costs of care in Alzheimer's disease. J Am Geriatr Soc. 2008;56(8):1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brickman AM, Riba A, Bell K, et al. Longitudinal assessment of patient dependence in Alzheimer's disease. Arch Neurol. 2002;59(8):1304–1308. [DOI] [PubMed] [Google Scholar]
- 10. Samus QM, Rosenblatt A, Onyike C, et al. Correlates of caregiver-rated quality of life in assisted living: the Maryland Assisted Living study. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):P311–P314. [DOI] [PubMed] [Google Scholar]
- 11. McLaughlin TP, Neumann PJ, Spackman DE, et al. Increasing dependence on others is associated with increased resource use in dementia. Paper presented at: 9th International Conference on ADPD 2009; 2009; Prague, Czech Republic. [Google Scholar]
- 12. National Alzheimer's Coordinating Center. http://www.alz.washington.edu/. Accessed May 20, 2011.
- 13. NACC Uniform Data Set (UDS) Coding Guidebook for Initial Visit Packet; 2008. https://www.alz.washington.edu/NONMEMBER/UDS/DOCS/VER2/ivpguide.pdf. Accessed May 20, 2011.
- 14. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 15. Hensel A, Angermeyer MC, Riedel-Heller SG. Measuring cognitive change in older adults: reliable change indices for the Mini-Mental State Examination. J Neurol Neurosurg Psychiatry. 2007;78(12):1298–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935. [DOI] [PubMed] [Google Scholar]
- 17. Pfeffer RI, Kurosaki TT, Harrah CH, Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323–329. [DOI] [PubMed] [Google Scholar]
- 18. Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44(12):2308–2314. [DOI] [PubMed] [Google Scholar]
- 19. Maidment ID, Fox CG, Boustani M, Rodriguez J, Brown RC, Katona CL. Efficacy of memantine on behavioral and psychological symptoms related to dementia: a systematic meta-analysis. Ann Pharmacother. 2008;42(1):32–38. [DOI] [PubMed] [Google Scholar]
- 20. Rocca P, Marino F, Montemagni C, Perrone D, Bogetto F. Risperidone, olanzapine and quetiapine in the treatment of behavioral and psychological symptoms in patients with Alzheimer's disease: preliminary findings from a naturalistic, retrospective study. Psychiatry Clin Neurosci. 2007;61(6):622–629. [DOI] [PubMed] [Google Scholar]
- 21. Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer's disease: a meta-analysis. JAMA. 2003;289(2):210–216. [DOI] [PubMed] [Google Scholar]
- 22. Brant R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics. 1990;46(4):1171–1178. [PubMed] [Google Scholar]
- 23. McLaughlin T, Buxton M, Mittendorf T, et al. Assessment of potential measures in models of progression in Alzheimer's disease. Neurology. 2010;75(14):1256–1262. [DOI] [PubMed] [Google Scholar]
- 24. Demers L, Oremus M, Perrault A, Champoux N, Wolfson C. Review of outcome measurement instruments in Alzheimer's disease drug trials: psychometric properties of functional and quality of life scales. J Geriatr Psychiatry Neurol. 2000;13(4):170–180. [DOI] [PubMed] [Google Scholar]
- 25. Conde-Sala JL, Garre-Olmo J, Turro-Garriga O, Lopez-Pousa S, Vilalta-Franch J. Factors related to perceived quality of life in patients with Alzheimer's disease: the patient's perception compared with that of caregivers. Int J Geriatr Psychiatry. 2009;24(6):585–594. [DOI] [PubMed] [Google Scholar]
- 26. Sands LP, Ferreira P, Stewart AL, Brod M, Yaffe K. What explains differences between dementia patients' and their caregivers' ratings of patients' quality of life? Am J Geriatr Psychiatry. 2004;12(3):272–280. [PubMed] [Google Scholar]