Abstract
BACKGROUND
Actigraphic data during simulated participant movements were evaluated to differentiate among patient behavior states.
METHODS
Arm and leg actigraphic data were collected on 30 volunteers who simulated 3 behavioral states (calm, restless, agitated) for 10 minutes; counts of observed participant movements (head, torso, extremities) were documented.
RESULTS
The mean age of participants was 34.7 years, and 60% were female. Average movement was significantly different among the states (P < .0001; calm [mean = .48], restless [mean = 2.16], agitated [mean = 3.75]). Mean actigraphic measures were significantly different among states for both arm (P < .0001; calm [mean = 6.8], restless [mean = 28.5], agitated [mean = 52.6]) and leg (P < .0001; calm [mean = 3.5], restless [mean = 18.7], agitated [mean = 37.7]).
CONCLUSION
Distinct levels of behavioral states were successfully simulated. Actigraphic data can provide an objective indicator of patient activity over a variety of behavioral states, and these data may offer a standard for comparison among these states.
Patient movement, especially restlessness and agitation in the critically ill, may result from a variety of physiologic (eg, hypoxemia, ischemia, inadequate pain control) and psychologic conditions (eg, anxiety, fear, disorientation). In extreme forms, patient movement can lead to removal of lifesaving devices and tubes or even harm to patients and healthcare providers. Studies have shown that a majority of intensive care unit (ICU) patients exhibit agitation at some time during their ICU stay, with as many as 71% of patients exhibiting agitated behavior in more than half (58%) of their patient days.1,2 Agitated behavior was described as severe or dangerous in 46% of these patients during 30% of patient-days.1
As a patient safety concern, identification of appropriate methods for measuring agitation has gained much attention.3–5 In a comprehensive review of sedation-agitation scoring systems, De Jonghe et al6 found that although several sedation-agitation tools have been used to measure sedation efficacy in ICU patients, few exhibit satisfactory clinimetric properties. Only a few valid and reliable sedation scales include varying levels of excessive activity or agitated behavior.7–11 There is currently no uniformly accepted “gold standard” for the measurement of agitation.5 Tools currently in use include direct observations and intermittent structured assessments by nurses and other care providers, but these are limited by the experience levels of the assessors and do not provide a continuous measure of activity or agitation.12,13 An appropriate continuous measurement of levels of patient agitation, particularly in the critically ill, is clinically important to optimize titration of sedatives, reduce patient harm, and improve patient outcomes.3,4,14,15
Actigraphy is a method of continuously measuring a person’s physical activity that was originally developed to assess activity levels during sleep.16 Actigraphic devices can detect and record very minor movements (ie, accelerations, linear displacements) during predetermined epochs (periods of time, eg, seconds, minutes, hours) for up to several days; they have been used to track circadian rest-activity cycles17 and to identify states of wakefulness and sleep.18,19 More recently, actigraphy is being evaluated as a measure of restlessness, agitation, or delirium in critically ill patients,20–23 but standardized actigraphic outcomes corresponding to specific types of patient behavior (calm, restless, agitated) that reflect critically ill patients’ movements have not been identified. However, use of actigraphy as a continuous activity monitor may provide objective measurement of patient activity by providing a numeric record of limb movement, which would assist in early identification of excessive random motion that is characteristic of agitation.22 Therefore, the specific aim of this pilot study was to obtain, in a laboratory setting, actigraphic outcome data derived from a variety of simulated behavioral states reflective of those found in the critical ill (eg, calm, restless, and agitated), by using adult volunteers.
MATERIALS AND METHODS
Setting and sample
The study was conducted at the Virginia Commonwealth University School of Nursing in the Clinical Learning Center. A convenience sample of 30 participants was obtained from volunteers, 18 years of age or older, responding to flyers posted throughout both Virginia Commonwealth University campuses. Exclusion criteria included neuromuscular disorders that could result in abnormal movement (eg, Parkinson’s disease), decreased levels of movement (eg, paralysis), severe sensory limitations, or an inability to speak English. Self-reported demographic information was collected from the study participants (age, weight, height, and gender).
Measures
Participant behavioral states were observed by study investigators while 3 actigraphic devices were also recording their movements.
Actigraphy
The actigraph is a small electronic device that can be strapped to the wrist or ankle, and is capable of continuously sensing and recording minimal movements or activity (ie, accelerations, linear displacements) during predetermined epochs (periods of time).16 In studies comparing polysomnography results with wrist actigraphy, significant agreement of sleep–wake cycles between wrist actigraphy and polysomnography has been demonstrated.16 More recently, wrist actigraphy was shown to be an objective indicator of changes in depth of anesthesia or sedation during surgery and recovery.24 To evaluate the use of actigraphy in the critically ill, Grap et al20 compared actigraphic measurements with observed patient activity, subjective sedation-agitation scale scores (Richmond Agitation Sedation Scale [RASS] and Comfort Scale), heart rate, and blood pressure in 20 adult ICU patients over a 2-hour period. They found that wrist actigraphy correlated with the RASS (r = .58), the Comfort Scale (r = .62), and observed patient stimulation and activity events (r = .45), and correlated weakly with systolic, diastolic, and mean arterial pressures. Wrist and ankle actigraphy results were significantly correlated (r = .69; P < .0001), but mean values of each (wrist mean = 418; ankle mean = 147) were significantly different (t = 5.77; P < .0001). Although actigraphic measurements correlated well with observed patient activity, as well as subjective agitation and sedation scales, the patients observed were sedated and few episodes of restless or agitated behavior were observed. It was expected that using simulated behavioral states would provide comprehensive information about a range of patient behaviors categorized as calm, restless, or agitated.
Actigraphs (Basic Motionlogger, Ambulatory Monitoring Inc, Ardsley, NY) were placed on the non-dominant wrist and ankle of each participant. The motionlogger measures long-term gross motor activity and integrates degree and intensity of motion, and contains an accelerometer capable of sensing any motion with minimal resultant force of .01g. Data from the motionlogger (1-second epoch) were downloaded to a computer database file through a serial port connection. Actigraphy measurements were recorded by the motionlogger every second using the proportional integrating measurement (PIM) mode of operation. In PIM mode, a numeric scale of movement activity is provided based on the absolute value of the area under the sensor curve. The PIM data can range from zero, corresponding to no movement when the limb is at rest, to a maximum value of 32,000, corresponding to the most vigorous and extreme movement of the limb.
Participant observation
A team of nurse investigators visually monitored and documented all participant movement during the data-collection period to ensure the simulated behaviors were appropriate for each state. The level of simulated movement per state was verified by 2 expert critical care nurses according to their knowledge of typical movements of calm, restless, and agitated critically ill patients. The investigators recorded the total number of movements observed for each leg, arm, the torso, and the head/neck, for each minute of observation, during each state. The average counts of movements, taken across the 2 investigator observations, were computed.
Procedures
A 10-minute participant behavioral simulation occurred for each of the 3 states (calm, restless, agitated). Each behavioral state was described to the participant and demonstrated by a study member. The calm/sedated state was described as a state experienced when one is resting comfortably or sleeping well (less than 10 movements/minute). The restless state was described as a condition when there is some, but not excessive movement, such as experienced during a restless night’s sleep (~10–20 movements/minute). The agitated state was described as a condition of almost continuous movement or extreme intermittent movement, such as one would see in an agitated patient (>20 movements/minute). The order of the states to be simulated was randomized; the participant selected a card to determine the state assignment at the beginning of first 2 simulated sessions. A rest period of 3 to 5 minutes occurred between each simulated state.
Two study investigators sat unobtrusively near the participant’s hospital bed. Two investigators were used to ensure documentation of all patient movement and to increase the reliability of the data-collection method. At the beginning of each study session, 2 motionloggers were placed on the participant’s nondominant wrist, and 1 motionlogger was placed on the nondominant ankle and secured by the wristband provided with the instrument. Two motionloggers were used on the arm to document their reliability for this purpose. One of the observers synchronized the time of observations with a stopwatch while the participant pressed an event button on one of the wrist motionloggers. This was done at the beginning and end of each state simulation to synchronize the visual observations with the motionlogger data. Once the observation period began, all participant movement was recorded by the 2 investigators throughout the entire observation period (30 minutes). The investigators coached the participants to exhibit the desired simulation of each state according to the information provided by the expert critical care nurses. The purpose of the observation was to ensure adequate differentiation of the 3 separate simulations for each participant.
Statistical analysis
Descriptive statistics were computed to describe the age, weight, height, and gender for the sample of participants. Generalized linear mixed-effects models (GLMMs)25 were used to model and compare actigraphy and total movement across the 3 states (calm, restless, and agitated). GLMMs were chosen because the responses were not normally distributed and there were within-subject correlations that needed to be accounted for because of the repeated-measures nature of the data. The GLMM for investigator-observed movement assumed a Poisson distribution for the response and included fixed effects for location (right leg, left leg, right arm, left arm, torso, head/neck), state (calm, restless, agitated), and the 2-way interaction between location and state. The model included random effects for participants to account for additional variability because of the repeated measures within the participants. Finally, the model included covariates for age, weight, height, and gender, to assess their effect on observed movement. The GLMM for actigraphy was similar to that for movement, except that location only included 2 levels (arm, leg) and there was an additional random watch effect to account for repeated measures within watches. The estimated movement and actigraphy means from the Poisson GLMMs were summarized by location and state. Comparisons in the mean movement and actigraphy between the states were summarized by location and represent the ratio of the means between 2 groups (because Poisson models fit the log of the mean, differences in log means are represented as ratios of means). Confidence intervals for the ratios that do not include 1 indicate statistical significance.
RESULTS
Participants
Participants’ mean age was 34.7 years (standard deviation [SD] = 14.1), mean height was 65.1 inches (SD = 5.4), mean weight was 174.2 pounds (SD = 41.9), and 60% were female. Reliability between the investigator’s observations of movement was high for all locations (Cronbach’s alpha .855–.939). Reliability between the arm 2 watches was high (Cronbach’s alpha = .886).
Actigraphy
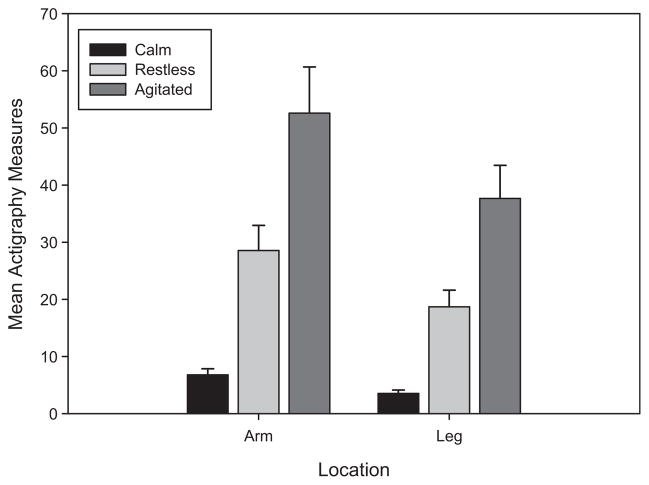
There was evidence of a significant state by location interaction effect on actigraphy measures (F[1,1.6×105] = 46.63, P < .0001). Thus, the differences in actigraphy measures between the states are not the same for the arm and leg locations. There were significant difference in actigraphy measures across the 3 states for both the arm (F[2,1.6×105] = 8846.5, P < .0001) and leg (F[2,1.6×105] = 3361.1, P < .0001) locations. Although actigraphy measures were greater for arm movement than leg movement during all 3 states (all P values < .0001), both arm and leg actigraphy measures increased from the calm to restless to agitated states (all P values < .0001). Actigraphy means were 4.2 to 5.3 times greater for the restless state than for the calm state, 1.8 to 2.0 times greater for the agitated state than for the restless state, and 7.7 to 10.7 times greater for the agitated state than for the calm state. The actigraphy means, standard errors, and 95% confidence intervals for each combination of state and location are summarized in Table I and Fig 1. The ratios of the means between the states are summarized by location in Table II. Age, weight, height, and gender did not have significant effects on actigraphy (all P values > .15).
Table I.
Estimated mean actigraphy measures by state and location
| Location | State | Mean (SE) | 95% CI |
|---|---|---|---|
| Arm | Calm | 6.79 (.48) | (5.87–7.85) |
| Restless | 28.54 (1.98) | (24.74–32.93) | |
| Agitated | 52.59 (3.65) | (45.60–60.66) | |
| Leg | Calm | 3.53 (.27) | (3.03–4.11) |
| Restless | 18.70 (1.98) | (16.18–21.60) | |
| Agitated | 37.65 (2.62) | (32.62–43.45) |
CI, confidence interval; SE, standard error.
Fig. 1.
Estimated mean actigraphy measures and 95% CIs by state and location.
Table II.
Estimated ratios in actigraphy means between states by location
| Location | Comparison | Ratio (SE) | 95% CI |
|---|---|---|---|
| Arm | Restless/calm | 4.204 (.071) | (4.066–4.346) |
| Agitated/restless | 1.843 (.017) | (1.810–1.876) | |
| Agitated/calm | 7.747 (.126) | (7.504–7.997) | |
| Leg | Restless/calm | 5.299 (.173) | (4.970–5.649) |
| Agitated/restless | 2.014 (.032) | (1.952–2.078) | |
| Agitated/calm | 10.671 (.334) | (10.036–11.346) |
CI, confidence interval; SE, standard error.
Movement
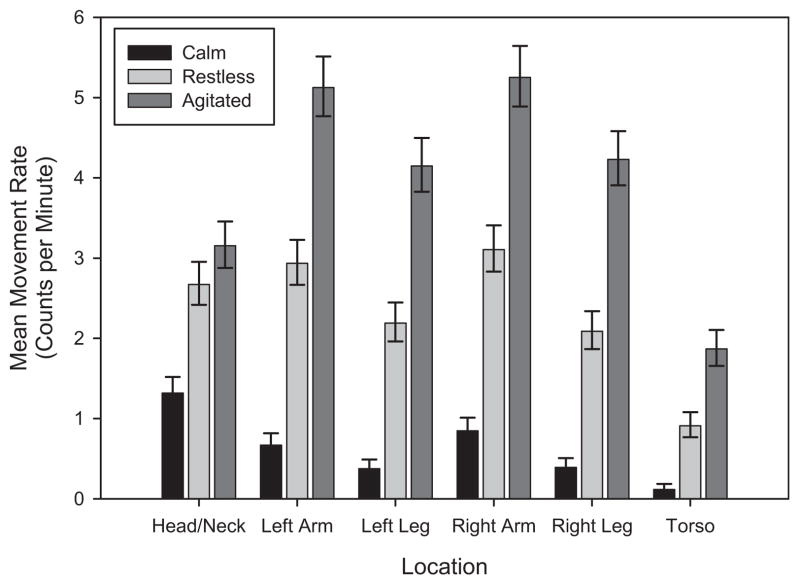
There was evidence of a significant state by location interaction effect on movement counts (F[10,290] = 27.18, P < .0001). Thus, the differences in movement counts among the states are not the same for the 6 locations. There were significant differences in movement counts across the 3 states for both arms (right arm: F[2290] = 296.8, P < .0001; left arm: F[2290] = 293.9, P < .0001), both legs (right leg: F[2290] = 249.7, P < .0001; left leg: F[2290] = 236.9, P < .0001), the torso (F[2290] = 102.9, P < .0001), and the head/neck (F[2290] = 100.1, P < .0001) locations. Movement means (counts per minute) were 2.0 to 7.8 times greater for the restless state than for the calm state, 1.2 to 2.1 times greater for the agitated state than for the restless state, and 2.4 to 16.1 times greater for the agitated state than for the calm state. The means (counts per minute), standard errors, and 95% confidence intervals for each combination of state and location are summarized in Table III and Fig 2. The ratios of the means between the states are summarized by location in Table IV. Female participants had means 1.15 times greater than those of male participants (95% confidence interval [CI] = 1.03, 1.28; P = .0158). Older age (ratio for 5-year increase = 1.02; 95% CI, 1.00–1.04; P = .0146) and greater weight (ratio for 10-pound increase = 1.01; 95% CI, 1.00–1.03; P = .0270) were associated with greater movement, whereas height did not significantly affect movement.
Table III.
Estimated mean movement rates (counts per minute) by state and location
| Location | State | Mean (SE) | 95% CI |
|---|---|---|---|
| Right arm | Calm | .85 (.08) | (.71–1.01) |
| Restless | 3.11 (.15) | (2.82–3.41) | |
| Agitated | 5.25 (.19) | (4.89–5.64) | |
| Left arm | Calm | .67 (.07) | (.55–.82) |
| Restless | 2.93 (.14) | (2.67–3.23) | |
| Agitated | 5.13 (.19) | (4.77–5.51) | |
| Right leg | Calm | .39 (.05) | (.30–.51) |
| Restless | 2.09 (.12) | (1.87–2.34) | |
| Agitated | 4.23 (.17) | (3.91–4.58) | |
| Left leg | Calm | .38 (.05) | (.29–.49) |
| Restless | 2.19 (.12) | (1.96–2.44) | |
| Agitated | 4.15 (.17) | (3.83–4.50) | |
| Torso | Calm | .12 (.03) | (.07–.19) |
| Restless | .91 (.08) | (.77–1.08) | |
| Agitated | 1.87 (.11) | (1.66–2.10) | |
| Head/neck | Calm | 1.32 (.09) | (1.14–1.52) |
| Restless | 2.67 (.14) | (2.42–2.95) | |
| Agitated | 3.15 (.15) | (2.88–3.46) | |
| Average | Calm | .48 (.05) | (.40–.59) |
| Restless | 2.16 (.10) | (1.97–2.36) | |
| Agitated | 3.75 (.13) | (3.50–4.01) |
CI, confidence interval; SE, standard error.
Fig. 2.
Estimated mean movement rates (per minute) and 95% CIs by state and location.
Table IV.
Estimated ratios in movement rates between states by location
| Location | Comparison | Ratio (SE) | 95% CI |
|---|---|---|---|
| Right arm | Restless/calm | 3.66 (.28) | (3.15–4.26) |
| Agitated/restless | 1.69 (.07) | (1.56–1.84) | |
| Agitated/calm | 6.20 (.48) | (5.32–7.21) | |
| Left arm | Restless/calm | 4.38 (.38) | (3.70–5.20) |
| Agitated/restless | 1.75 (.07) | (1.61–1.90) | |
| Agitated/calm | 7.66 (.67) | (6.44–9.10) | |
| Right leg | Restless/calm | 5.34 (.61) | (4.27–6.67) |
| Agitated/restless | 2.03 (.10) | (1.84–2.24) | |
| Agitated/calm | 10.82 (1.26) | (8.61–13.59) | |
| Left leg | Restless/calm | 5.82 (.67) | (4.63–7.31) |
| Agitated/restless | 1.89 (.09) | (1.72–2.09) | |
| Agitated/calm | 11.02 (1.31) | (8.73–13.92) | |
| Torso | Restless/calm | 7.83 (1.65) | (5.18–11.85) |
| Agitated/restless | 2.05 (.16) | (1.77–2.38) | |
| Agitated/calm | 16.07 (3.48) | (10.49–24.61) | |
| Head/neck | Restless/calm | 2.03 (.13) | (1.79–2.30) |
| Agitated/restless | 1.18 (.06) | (1.07–1.30) | |
| Agitated/calm | 2.39 (.15) | (2.12–2.71) | |
| Average | Restless/calm | 4.47 (.30) | (3.91–5.10) |
| Agitated/restless | 1.74 (.04) | (1.66–1.82) | |
| Agitated/calm | 7.76 (.57) | (6.69–8.99) |
CI, confidence interval; SE, standard error.
DISCUSSION
Early identification of increasing restlessness and impending agitation leading to combative behavior in the critically ill is crucial to reduce harm to both patients and their health care providers.26 Nursing response to patient agitation, especially in the mechanically ventilated critically ill patient, often includes increasing the level of sedation. However, over-sedation has been shown to significantly increase the duration of mechanical ventilation and the risk of concomitant complications, as well as ICU length of stay.27 Present methods of sedation-agitation evaluation include intermittent subjective tools that do not provide a continuous assessment of patient movement or indications of increasing restlessness and impending agitation.
Actigraphy detects movement continuously (eg, every second), but has not been tested thoroughly in all patient behavioral states (calm, restless, agitated). In an earlier study,20 we found that actigraphic measurements were associated with sedation scales (RASS, Comfort Scale), but the critically ill participants observed were well sedated and few episodes of restless or agitated behavior were included in the analysis. This study demonstrates that actigraphy measurements via either wrist or leg motionloggers can be used to adequately distinguish among these 3 behavioral states (calm, restless, agitated). Although wrist motionloggers resulted in higher counts than the leg motionlogger, both are useful representations of patient movement and behavioral states. However, use of arm or leg restraints has been shown to limit movement and affect actigraphic data.20,21 As demonstrated in this study, use of a limb that is not restrained (usually a leg) may effectively document the patient’s behavioral state. In addition, both arm and leg actigraphy accurately reflected movement of other parts of the body (torso, head, and other limbs).
In the critical care environment, subjective sedation scales are generally used to determine the patient’s level of agitation and sedation.15,28,29 Useful features of a sedation scale include rigorous multidisciplinary development; ease of administration, recall, and interpretation; well-defined discrete criteria for each level; sufficient sedation levels for effective drug titration; assessment of agitation; demonstration of inter-rater reliability for relevant patient populations; and evidence of validity.30 Although a number of sedation scales have been developed for ICU use, only 4 meet these criteria, have been rigorously tested in different populations, and include a measure of agitation that is graded in severity: the Sedation Agitation Scale,7 Motor Activity Assessment Scale,8 RASS,9 and Adaptation to the Intensive Care Environment instrument.31
Although these evaluation tools provide an intermittent measure of agitation level and can identify patient behavioral state at one moment in time, they do not provide a continuous measure of increasing restlessness that may be provided by a tool such as actigraphy. Thus, subtly increasing restlessness may not be identified early in its progression and may result in dangerous levels of agitation that cause harm to the patient through self-removal of lifesaving lines and tubes, or injury to health care providers from agitated, even combative patients.12,32 In addition, early identification of increasing restlessness may result in titration of more timely and appropriate levels of sedation (ie, at lower doses), rather than use of sedative doses designed to quickly eliminate dangerous, agitated behavior, which may result in over-sedation.
The use of volunteers in this study may limit generalizability of these results because volunteers may not simulate the behavioral states in the same way that they occur in the critically ill. However, the investigators/study trainers were all experienced nurses who have an extensive understanding of the range of behavior states in the critically ill, and the simulated movements for each behavioral state were reviewed by 2 expert critical care nurses. In addition, because agitation is a dangerous condition, especially in the critically ill who are dependent on the maintenance of lifesaving tubes and lines, such behavior is treated immediately, reducing the opportunity to study and accurately measure it in the ICU setting. This study is also limited in the range of behaviors selected for evaluation (calm, restless, agitated), and although we did not include other states such as sleep, the states selected are typically those used in sedation-agitation evaluation tools. Additional studies will be beneficial to evaluate all behavioral states of the critically ill.
Although at present, actigraphy is not designed to provide real-time data about patient movement in the clinical setting, the continued development of this technology may provide a foundation for patient movement/behavioral evaluation methods that are objective and continuous.22,23 Increasing levels of movement identified by actigraphy may herald the onset of increasing restlessness that eventually results in agitation. Real-time automatic tracking and display of actigraphic datacould provide a continuous, objective measure of impending agitation, improving the management of sedative therapy.23 Chase et al33 recently tested an objective agitation measurement of patient motion, using digital video imaging, and classified levels of motion associated with observed patient agitation. Other proposed methods include use of the Bispectral Index.15 These new movement measurement methods may provide a means to consistently and objectively quantify patient restlessness and agitation to improve sedation management and ultimately reduce ICU length of stay and improve patient outcomes.
CONCLUSIONS
Three distinct states common in the critically ill patient were successfully simulated and distinguished statistically. We found that actigraphic data can provide an objective indicator of patient activity over a variety of patient behavioral states (calm, restless, agitated) and motionlogger locations; these data may offer a means of determining and anticipating patient behavioral states in the critically ill patient.
References
- 1.Fraser GL, Prato BS, Riker RR, Berthiaume D, Wilkins ML. Frequency, severity, and treatment of agitation in young versus elderly patients in the ICU. Pharmacotherapy. 2000;20:75–82. doi: 10.1592/phco.20.1.75.34663. [DOI] [PubMed] [Google Scholar]
- 2.Fraser GL, Riker RR. Sedation and analgesia in the critically ill adult. Curr Opin Anaesthesiol. 2007;20:119–23. doi: 10.1097/ACO.0b013e32808255b4. [DOI] [PubMed] [Google Scholar]
- 3.Riker RR, Fraser GL. Sedation in the intensive care unit: refining the models and defining the questions. Crit Care Med. 2002;30:1661–3. doi: 10.1097/00003246-200207000-00049. [DOI] [PubMed] [Google Scholar]
- 4.Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med. 2002;30:119–41. doi: 10.1097/00003246-200201000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Chulay M. Sedation assessment: easier said than done! Crit Care Nurs Clin North Am. 2004;16:359–64. viii. doi: 10.1016/j.ccell.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 6.De Jonghe B, Cook D, Appere-De-Vecchi C, Guyatt G, Meade M, Outin H. Using and understanding sedation scoring systems: a systematic review. Intensive Care Med. 2000;26:275–85. doi: 10.1007/s001340051150. [DOI] [PubMed] [Google Scholar]
- 7.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients. Crit Care Med. 1999;27:1325–9. doi: 10.1097/00003246-199907000-00022. [DOI] [PubMed] [Google Scholar]
- 8.Devlin JW, Boleski G, Mlynarek M, et al. Motor Activity Assessment Scale: a valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med. 1999;27:1271–5. doi: 10.1097/00003246-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166:1338–44. doi: 10.1164/rccm.2107138. [DOI] [PubMed] [Google Scholar]
- 10.Rassin M, Sruyah R, Kahalon A, Naveh R, Nicar I, Silner D. “Between the fixed and the changing”: examining and comparing reliability and validity of 3 sedation-agitation measuring scales. Dimens Crit Care Nurs. 2007;26:76–82. doi: 10.1097/00003465-200703000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Sessler CN, Grap MJ, Ramsay MA. Evaluating and monitoring analgesia and sedation in the intensive care unit. Crit Care. 2008;12(Suppl 3):S2. doi: 10.1186/cc6148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker N, Gillen P. Investigating nurses’ perceptions of their role in managing sedation in intensive care: an exploratory study. Intensive Crit Care Nurs. 2006;22:338–45. doi: 10.1016/j.iccn.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Weir S, O’Neill A. Experiences of intensive care nurses assessing sedation/agitation in critically ill patients. Nurs Crit Care. 2008;13:185–94. doi: 10.1111/j.1478-5153.2008.00282.x. [DOI] [PubMed] [Google Scholar]
- 14.McGaffigan PA. Advancing sedation assessment to promote patient comfort. Crit Care Nurse. 2002;(Suppl):29–36. [PubMed] [Google Scholar]
- 15.Arbour R, Waterhouse J, Seckel MA, Bucher L. Correlation between the Sedation-Agitation Scale and the Bispectral Index in ventilated patients in the intensive care unit. Heart Lung. 2009;38:336–45. doi: 10.1016/j.hrtlng.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin North Am. 2006;12:23–30. viii. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Brown AC, Smolensky MH, D’Alonzo GE, Redman DP. Actigraphy: a means of assessing circadian patterns in human activity. Chronobiol Int. 1990;7:125–33. doi: 10.3109/07420529009056964. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Miyagishi T, Sack RL, Hughes RJ, Blood ML, Lewy AJ. Evaluation of the Actillume wrist actigraphy monitor in the detection of sleeping and waking. Psychiatry Clin Neurosci. 1998;52:160–1. doi: 10.1111/j.1440-1819.1998.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 19.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 20.Grap MJ, Borchers CT, Munro CL, Elswick RK, Jr, Sessler CN. Actigraphy in the critically ill: correlation with activity, agitation, and sedation. Am J Crit Care. 2005;14:52–60. [PubMed] [Google Scholar]
- 21.Lafleur KJ. Will adequate sedation assessment include the use of actigraphy in the future? Am J Crit Care. 2005;14:61–3. [PubMed] [Google Scholar]
- 22.Mistraletti G, Taverna M, Sabbatini G, et al. Actigraphic monitoring in critically ill patients: preliminary results toward an “observation-guided sedation”. J Crit Care. 2009;24:563–7. doi: 10.1016/j.jcrc.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Osse RJ, Tulen JH, Hengeveld MW, Bogers AJ. Screening methods for delirium: early diagnosis by means of objective quantification of motor activity patterns using wrist-actigraphy. Interact Cardiovasc Thorac Surg. 2009;8:344–8. doi: 10.1510/icvts.2008.192278. [DOI] [PubMed] [Google Scholar]
- 24.Weinbroum AA, Ben Abraham R, Ezri T, Zomer J. Wrist actigraphy in anesthesia. J Clin Anesth. 2001;13:455–60. doi: 10.1016/s0952-8180(01)00300-2. [DOI] [PubMed] [Google Scholar]
- 25.Molenbergs G, Verbeke G. Models for discrete longitudinal data. Berlin: Springer; 2006. [Google Scholar]
- 26.Sessler CN, Wilhelm W. Analgesia and sedation in the intensive care unit: an overview of the issues. Crit Care. 2008;12(Suppl 3):S1. doi: 10.1186/cc6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–7. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 28.Rhoney DH, Murry KR. National survey on the use of sedatives and neuromuscular blocking agents in the pediatric intensive care unit. Pediatr Crit Care Med. 2002;3:129–33. doi: 10.1097/00130478-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Rhoney DH, Murry KR. National survey of the use of sedating drugs, neuromuscular blocking agents, and reversal agents in the intensive care unit. J Intensive Care Med. 2003;18:139–45. doi: 10.1177/0885066603251200. [DOI] [PubMed] [Google Scholar]
- 30.Sessler CN. Sedation scales in the ICU. Am J Respir Crit Care Med. 2004;126:1727–30. doi: 10.1378/chest.126.6.1727. [DOI] [PubMed] [Google Scholar]
- 31.De Jonghe B, Cook D, Griffith L, et al. Adaptation to the Intensive Care Environment (ATICE): development and validation of a new sedation assessment instrument. Crit Care Med. 2003;31:2344–54. doi: 10.1097/01.CCM.0000084850.16444.94. [DOI] [PubMed] [Google Scholar]
- 32.Fraser GL, Riker RR, Prato BS, Wilkins ML. The frequency and cost of patient-initiated device removal in the ICU. Pharmacotherapy. 2001;21:1–6. doi: 10.1592/phco.21.1.1.34444. [DOI] [PubMed] [Google Scholar]
- 33.Chase JG, Agogue F, Starfinger C, et al. Quantifying agitation in sedated ICU patients using digital imaging. Comput Methods Programs Biomed. 2004;76:131–41. doi: 10.1016/j.cmpb.2004.03.005. [DOI] [PubMed] [Google Scholar]