Abstract
In a previous study comparing fluconazole and itraconazole administered as antifungal prophylaxis in hematopoietic cell transplant (HCT) recipients, we found that fluconazole administration concurrent with cyclophosphamide (CY)-based conditioning was associated with fewer early toxicities compared to itraconazole. Fluconazole inhibits cytochrome P450 2C9, which is involved with the activation of CY, and so might provide protection from CY-related toxicities.
To investigate this further, we compared CY and CY-metabolite data from patients who received fluconazole (n=56) concurrent with CY-containing conditioning in patients who did not (n=17). The fluconazole group had greater exposure to CY, and lower peak serum concentration of CY-metabolite 4-hydroxycyclophosphamide. In a separate cohort, we examined outcomes in patients randomized to receive either fluconazole (n=152) or placebo (n=147) concurrent with CY-containing conditioning in a prior randomized trial. Patients who received fluconazole experienced less hepatic and renal toxicity, and had lower mortality. No difference in relapsed malignancy was apparent. These data support the hypothesis that fluconazole, when co-administered with CY, decreases CY-related toxicities by inhibiting cytochrome P450 2C9 metabolism.
Introduction
Cyclophosphamide (CY) is an alkylating agent used commonly in myeloablative conditioning regimens for hematopoietic cell transplantation (HCT) 1. It is a pro-drug which undergoes three metabolic pathways: 1) urinary elimination as unchanged CY; 2) detoxification via cytochrome P450 (CYP) 3A4/5 to dechloroethyl-cyclophosphamide (DCCY); and 3) activation via CYP 2A6, 2B6, 3A4, 3A5, 2C9, 2C18 and 2C19 to 4-hydroxycyclophosphamide (HCY). HCY is then converted by β-elimination to toxins acrolein, which is primarily responsible for urothelial toxicity, and phosphoramide mustard (PM), responsible for anti-neoplastic activity. Other metabolites include o-carboxyethyl-phosphoramide mustard (CePM), 4-keto-cyclophosphamide (ketoCY) and hydroxypropyl-phosphoramide mustard (HPPM).
We have previously published early toxicity data from a randomized trial comparing fluconazole with itraconazole as antifungal prophylaxis in HCT recipients; results demonstrated a disequilibrium in CY-metabolites, and renal and hepatic toxicities in patients who received the azole drugs concurrent with CY-containing regimens 2,3. Co-administration of high-dose fluconazole (400 mg daily) with CY was associated with greater exposure to CY and DCCY, while itraconazole (2.5 mg/kg three times daily) was associated with greater exposure to HCY, ketoCY, and (to a lesser extent) CePM 3. In addition, concurrent fluconazole was associated with less renal and hepatic toxicity, and a trend to improved survival at day 20. We hypothesized that, through its inhibition of CYP2C9 metabolism of CY to HCY, high dose (400mg daily) fluconazole co-administration may result in less exposure to HCY metabolites, including toxins responsible for tissue injury. As this implies a potential protective effect of fluconazole administered during CY-containing conditioning, we have investigated this interaction further. Specifically, we examined CY and CY-metabolite data from two cohorts of HCT recipients. One cohort received fluconazole concurrent with CY-based conditioning and another did not. We also re-examined toxicity data from a study of HCT recipients randomized to receive either fluconazole or placebo as antifungal prophylaxis.
Materials and Methods
Study 1. Pharmacokinetics of CY
CY-metabolite data were available from a cohort of 73 allogeneic HCT recipients treated with busulfan (BU)-CY conditioning and variable antifungals, from 2001 to 2005, inclusive. HCTs were performed according to standard institutional practices. Seven days before the infusion of stem cells, CY was infused through a central venous catheter over one to two hours at a dose of 60 mg/kg body weight. On the following day, a second infusion of CY was given, at the same dose. All patients were given anti-seizure prophylaxis with phenytoin. Phenytoin loading dose (10–15 mg/kg) was completed at least six hours before the first dose of BU. Institutional standard practice was to administer fluconazole concomitant with (on or before) conditioning. A subset of patients received alternative regimens due to physician decision or randomization into a trial that began prophylaxis (fluconazole or voriconazole) after conditioning (with receipt of stem cells).
Blood samples were removed from a non-CY infusion port of a central venous access catheter, and placed into tubes containing either p-nitrophenyl hydrazine for analysis of HCY or EDTA (ethylenediaminetetraacetic acid) for other analytes. They were then mixed and centrifuged at the bedside. Plasma was immediately removed, frozen and stored at −80°C until analysis. CY and CY-metabolites (HCY and CEPM) were measured mid-infusion, at the end of the infusion, and at 1, 3, 7, 20, and 24 hours after infusion. Exposure to CY and CY-metabolites was expressed as the area under the curve (AUC; mM/h) derived from the time of the first CY dose to 24 hours after the second CY dose 4. In addition, the peak concentration (Cmax, uM) of CY and HCY were measured after the first and second CY doses. Baseline (day before Bu-CY conditioning) and daily (until HCT day 20) serum creatinine and total bilirubin levels were recorded.
Study 2. Antifungal prophylaxis study
To evaluate the hypothesis that fluconazole decreases the frequency of toxicities due to CY-containing conditioning, clinical outcomes (hepatic and renal toxicities, and survival) were compared in 299 patients who received fluconazole (400 mg daily, orally or intravenously) or placebo concurrent with CY (beginning on the day of CY-containing conditioning), as part of a previously published antifungal prophylaxis trial 5. This study enrolled primarily allogeneic HCT recipients (88%) between 1990 and 1992. HCTs were performed according to standard practice. Conditioning regimens included CY-total body irradiation (TBI) (placebo, n=82; fluconazole, n=97), BU-CY (placebo, n=51; fluconazole, n=39), BU-CY-TBI (placebo, n=14; fluconazole, n=16), p=0.23. All patients receiving BU (n=120) were given anti-seizure prophylaxis with phenytoin. Phenytoin loading dose (10–15 mg/kg) was completed at least six hours before the first dose of BU. Renal and hepatic toxicities, survival and the probability of mortality not related to fungal infection were compared. Renal toxicity was defined as doubling of baseline creatinine concentration (mg/dL) and hepatic toxicity was evaluated as daily total serum bilirubin level (mg/dL).
Both studies were approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. All patients provided informed consent.
Statistical considerations
Continuously valued outcomes were compared using a Wilcoxon rank sum test. Survival estimates at days 20 and 75 were calculated using Kaplan-Meier methodology. Non-fungal mortality was calculated using cumulative incidence estimates. Time from transplant to death without prior fungal infection was the endpoint of interest and fungal infections were treated as competing risk events. Both survival and non-fungal mortality were compared using a log-rank test to day 20 and day 75. All p-values are two-sided.
Results
Study 1
In study 1, we compared AUC-CY, AUC-HCY and AUC-CEPM in patients receiving fluconazole (400 mg daily) concurrent with CY-containing conditioning (group 1, n=56) with those patients who did not (group 2, n=17). In group 2, antifungal prophylaxis included lipid amphotericin at conditioning (n=2), caspofungin at conditioning (n=1), fluconazole at day 0 (n=1), itraconazole at day 1 (n=1), and fluconazole or voriconazole (patients enrolled in a randomized blinded study) at day 0 of HSCT (n=12). Data comparing the AUC for CY, CePM and HCY, and Cmax for CY and HCY are shown in Table 1. The fluconazole treated patients (group 1) had greater AUC-CY (p < 0.0001). While there was no significant difference in AUC-HCY between the two groups, fluconazole recipients had a lower median Cmax-HCY after doses one and two (p=0.0002 and p=0.006, respectively). Data comparing serum creatinine and total bilirubin for the first 20 days following HCT are shown in Table 2. No significant difference in maximum creatinine level or number of patients reaching >2x baseline was observed. There were nonsignificant trends to higher maximum and daily total serum bilirubin levels in the fluconazole group (group 2).
Table 1. Area under the curve (AUC) and peak serum concentration (Cmax) of CY and HCY following first and second doses of CY-containing conditioning for hematopoietic cell transplant (HCT) – Study one.
Group one consists of patients receiving fluconazole concurrent with CY, and group two consists of patients receiving either no drug or a non-azole antifungal agent with CY.
| Drug/metabolite – Median (range) | Group 1, n = 56 | Group 2, n = 17 | P |
|---|---|---|---|
| AUC-CY | 2628 (1375–8086) | 1465 (933–3431) | < 0.0001 |
| AUC-CePM | 489 (215–1165) | 500 (271–738) | 0.686 |
| AUC-HCY | 272 (92–744) | 297 (194–353) | 0.4395 |
| Cmax-CY following dose 1 | 305 (92–744) | 223 (145–302) | < 0.0001 |
| Cmax-CY following dose 2 | 282 (153–1064) | 212 (19–403) | 0.0001 |
| Cmax-HCY following dose 2 | 31 (10–65) | 43 (20–60) | 0.0056 |
AUC indicates area under the curve; CY, cyclophosphamide; CePM, o-carboxyethyl-phosphoramide mustard; HCY, 4-hydroxycyclophosphamide; Cmax, peak serum concentration.
Table 2. Creatinine and bilirubin levels from patients recieving CY-containing conditioning for HCT, measured at baseline (immediately before CY-containing conditioning) and daily for the first 20 days post-HSCT – Study one.
Group one consists of patients receiving fluconazole concurrent with CY, and group two consists of patients receiving either no drug or non-azole antifungal agent with CY.
| Factor | Group 1, n = 56 | Group 2, n = 17 | P |
|---|---|---|---|
| Creatinine (mg/dL) | |||
| Maximum difference from baseline [Median (range)] | 0.20 (0, 2.90) | 0.20 (0.00, 2.10) | 0.62 |
| Number reaching >2x baseline (%) | 9 (16%) | 3 (18%) | 0.28 |
| Bilirubin (mg/dL) | |||
| Maximum level [Median (range)] | 1.65 (0.70, 12.20) | 1.90 (0.90, 33.50) | 0.28 |
| Daily level [Median (range)] | 0.98 (0.47, 5.17) | 1.18 (0.59, 11.57) | 0.18 |
Study 2
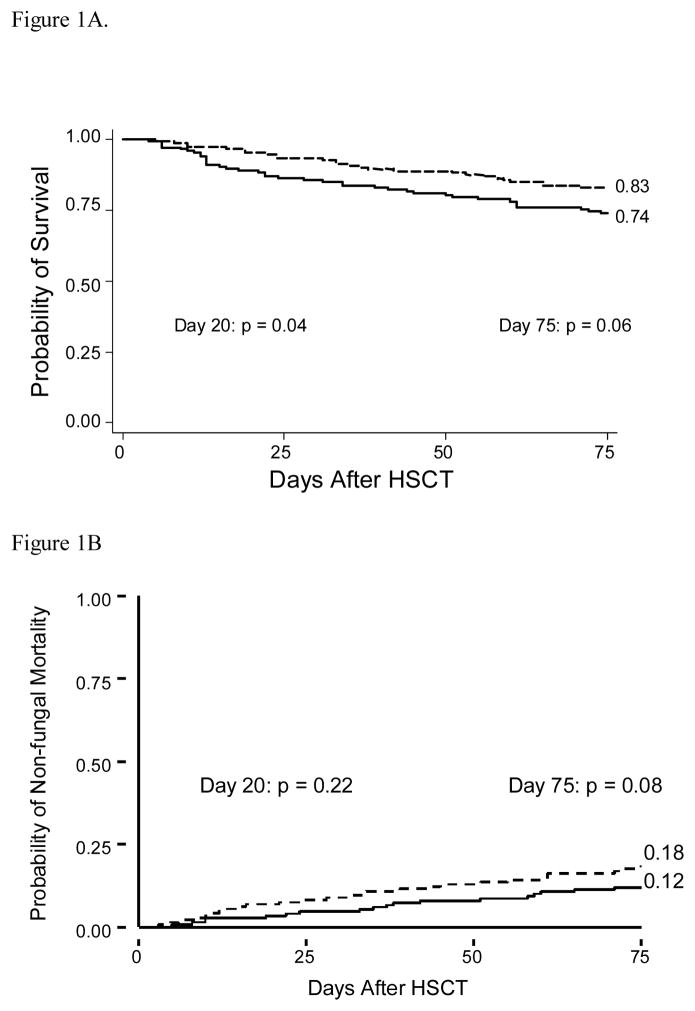
Our prior analysis likely did not include enough patients to observe significant differences in clinical outcomes. We postulated that differences in clinical outcomes may be more reliably measured in patients enrolled in our prior fluconazole vs. placebo trial, which randomized 152 patients to receive fluconazole and 148 patients to receive placebo, with both drugs initiated with conditioning therapy 5. The probability of survival was greater at days 20 and 75 following HCT in patients receiving fluconazole, 95% vs. 89%, p=0.04 and 83% vs. 74%, p=0.06, respectively (figure 1A). There was a trend to lower non-fungal mortality at days 20 and 75 in recipients of fluconazole (figure 1B). There was no difference in the probability of relapse of underlying malignancy at day 110 between patients receiving fluconazole or placebo (p=0.42) 5. Differences in early toxicities were observed in fluconazole recipients (Table 3). Specifically, fewer fluconazole recipients, compared with placebo, experienced a doubling of baseline creatinine (34% vs. 47%, p=0.02) during the first 20 days following HCT. The median daily total serum bilirubin level was also lower in the patients receiving fluconazole [2.0 (range 0.4–27.7) vs. 2.5 (range 0.3–27.8), p=0.05].
Figure 1. Probability of overall survival (1A) and non-fungal mortality (1B) after HSCT – Study two.
Probability of overall survival and non-fungal mortality in patients receiving fluconazole with CY-conditioning and in patients receiving placebo with CY-conditioning.
Key
Placebo ———— Fluconazole --------
Table 3. Creatinine and bilirubin levels from patients receiving fluconazole prophylaxis or placebo concurrent with CY-containing conditioning for HCT – Study two.
Creatinine and bilirubin levels were measured at baseline (immediately before CY-containing conditioning) and daily for the first 20 days post-HCT.
| Factor | Placebo, n=147 | Fluconazole, n=152 | P |
|---|---|---|---|
| Creatinine (mg/dL) | |||
| Maximum difference from baseline [Median (range)] | 0.60 (-0.10, 5.00) | 0.40 (0.00–4.60) | 0.04 |
| Number reaching >2x baseline (%) | 69 (47%) | 51 (34%) | 0.02 |
| Bilirubin (mg/dL) | |||
| Maximum level [Median (range)] | 4.40 (0.50, 56.9) | 3.65 (0.50, 64.50) | 0.09 |
| Daily level [Median (range)] | 2.47 (0.30, 27.84) | 2.03 (0.40, 27.71) | 0.05 |
Discussion
Cyclophosphamide pharmacokinetics data presented here support the hypothesis that high dose fluconazole (400 mg daily), when co-administered with CY-containing conditioning, has an inhibitory effect on CY activation, resulting in greater CY exposure, which may result in fewer conditioning-related toxicities. Toxicity and survival data from two prospective randomized studies (study 2 presented here, and a previously published study 3) indicate an advantage among patients receiving fluconazole concurrent with CY-containing conditioning, compared with placebo (this study) or itraconazole3.
Our finding that recipients of fluconazole concurrent with CY had higher AUC-CY is consistent with the results of a small case-control study which compared plasma clearance of CY in children receiving fluconazole and CY concurrently (cases, n=9) with children receiving CY alone (controls, n=13). In this study, cases had significantly lower plasma clearance of CY and higher AUC-CY 6.
In our study, patients who received fluconazole concurrently with CY had greater AUC-CY and Cmax-CY, and lower Cmax-HCY, with equivalent AUC-HCY. These data suggest that inhibition of CY activation leads to a reduced rate of HCY formation. The comparable AUC-HCY and lower Cmax-HCY suggests prolongation of HCY elimination. Similar findings have been reported from a recent study comparing CY metabolism in wild type and hepatic cytochrome P450 reductase knockout mice. While the knockout mice had reduced CY elimination (thus, greater AUC-CY) and reduced Cmax-HCY, no difference in AUC-HCY between the two groups was seen, as the elimination of HCY was prolonged 7. Interestingly, in the mouse model, myelotoxicity correlated more closely with Cmax-HCY than with AUC-HCY 7. In contrast, much of the published literature reports an association between exposure to CY-metabolites and hepatic toxicity among patients treated with various CY-containing chemotherapy regimens. de Jonge et al found that 1st-course AUC-HCY, but not AUC-PM, predicted the occurrence of veno-occlusive disease (VOD) after multiple courses 8, while Huitema et al report a trend to an association between AUC-HCY and VOD 9. MacDonald et al found no association between AUC-HCY and VOD, but an association between AUC-CEPM and VOD, elevated bilirubin, and non-relapse mortality was observed 4. They hypothesize that the chemically stable CEPM reports on intra-hepatic exposure to acrolein and PM formed from HCY.
Among study one patients, we did not observe a difference in hepatic and renal toxicity during the first 20 days following HCT between those patients who received fluconazole with CY-based conditioning and those who did not. If Cmax-HCY correlates best with toxicity we would expect to see a significant difference; our data demonstrating no difference in AUC-HCY and toxicities argues in favor of the hypothesis that AUC-HCY correlates with (at least hepatic and renal) toxicities following CY administration. It is also possible that there were not enough patients examined to observe meaningful clinical differences. These toxicity data should be interpreted with caution, as patients compared in the two groups were matched only in that they received myeloablative doses of CY and Bu, and received phenytoin.
In contrast, differences in clinical outcomes were measured in patients that received fluconazole or placebo concomitant with CY in our prior randomized trial. As azole antifungal agents are associated with hepatotoxicity (particularly transaminitis) these findings are the converse of what might be expected, strengthening our hypothesis of an interaction between fluconazole and CY activation.
When interpreting our data, one must be mindful of the additional role that phenytoin may play in CY metabolism. As phenytoin is an inducer of CYP450 enzymes, co-administration with CY may exert the opposite effect of fluconazole, by inducing CY activation resulting in greater HCY (and other metabolite) exposure. However, in study one all patients received phenytoin, while in study two approximately one third of patients did not. As the two groups compared in study two were matched for phenytoin exposure, we do not believe that this should introduce significant bias.
In summary, we have demonstrated that at high doses (400mg daily) fluconazole may impact CY activation, exerting a protective effect against CY-related toxicities. However, these drug/metabolite data are limited by the retrospective nature of the study; we cannot conclude that the differences seen are definitely due to a fluconazole-CY interaction. A randomized trial specifically addressing early toxicities as well as therapeutic effects, with concurrent measurements of CY and CY-metabolites, needs to be done to definitively answer this question.
Acknowledgments
Research grants: NIH CA18029
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Jonge ME, Huitema AD, Rodenhuis S, Beijnen JH. Clinical pharmacokinetics of cyclophosphamide. Clin Pharmacokinet. 2005;44:1135–1164. doi: 10.2165/00003088-200544110-00003. [DOI] [PubMed] [Google Scholar]
- 2.Marr KA, Crippa F, Leisenring W, et al. Itraconazole versus fluconazole for prevention of fungal infections in patients receiving allogeneic stem cell transplants. Blood. 2004;103:1527–1533. doi: 10.1182/blood-2003-08-2644. [DOI] [PubMed] [Google Scholar]
- 3.Marr KA, Leisenring W, Crippa F, et al. Cyclophosphamide metabolism is affected by azole antifungals. Blood. 2004;103:1557–1559. doi: 10.1182/blood-2003-07-2512. [DOI] [PubMed] [Google Scholar]
- 4.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–2048. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 5.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation--a prospective, randomized, double-blind study. J Infect Dis. 1995;171:1545–1552. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 6.Yule SM, Walker D, Cole M, et al. The effect of fluconazole on cyclophosphamide metabolism in children. Drug Metab Dispos. 1999;27:417–421. [PubMed] [Google Scholar]
- 7.Pass GJ, Carrie D, Boylan M, et al. Role of hepatic cytochrome p450s in the pharmacokinetics and toxicity of cyclophosphamide: studies with the hepatic cytochrome p450 reductase null mouse. Cancer Res. 2005;65:4211–4217. doi: 10.1158/0008-5472.CAN-04-4103. [DOI] [PubMed] [Google Scholar]
- 8.de Jonge ME, Huitema AD, Beijnen JH, Rodenhuis S. High exposures to bioactivated cyclophosphamide are related to the occurrence of veno-occlusive disease of the liver following high-dose chemotherapy. Br J Cancer. 2006;94:1226–1230. doi: 10.1038/sj.bjc.6603097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huitema AD, Spaander M, Mathjt RA, et al. Relationship between exposure and toxicity in high-dose chemotherapy with cyclophosphamide, thiotepa and carboplatin. Ann Oncol. 2002;13:374–384. doi: 10.1093/annonc/mdf052. [DOI] [PubMed] [Google Scholar]