Abstract
Wnt signaling is a fundamental pathway in embryogenesis which is evolutionary conserved from metazoans to humans. Much of our understanding of Wnt signaling events emerged from key developmental studies in drosophila, zebra fish, xenopus, and mice. Considerable data now exists on the role of Wnt signaling beyond these developmental processes and in particular its role in health and disease. The focus of this special issue is on Wnt/β-catenin and its diverse physiological cell signaling pathways in neurodegenerative and neuropsychiatric disorders. This special issue is composed of six reviews and two original articles selected to highlight recent advances in the role of Wnt signaling in CNS embryonic development, in adult brain function, in neurodegenerative conditions such as Alzheimer’s disease, schizophrenia, NeuroAIDS, and in gliomas. The finding that β-catenin can translocate to the nucleus where it binds to TCF/LEF transcription factors to regulate target gene expression was a seminal observation that linked β-catenin/LEF to T cell development and differentiation. We also provide a nostalgic look on recent advances in role of Wnts in T cell development and maturation. These reviews highlight the extensive body of work in these thematic areas as well as identify knowledge gaps, where appropriate. Understanding Wnt function under healthy and diseased conditions may provide a therapeutic resource, albeit it a challenging one, in diseases where dysfunctional and/or diminished Wnt signaling is a prominent player in the disease process.
Keywords: Wnt signaling, β-catenin, Neurodegenerative diseases, T cell differentation, NeuroAIDS
Introduction
Pioneer studies by Christiane Nüsslein-Volhard and Eric Wieschaus in developmental genetics of Drosophila Melanogaster identified a segment polarity gene that when mutated leads to a wingless phenotype of fruit flies. This gene was named Wingless (Wg) and set the course for the evolution of the Wnt signaling field. Nüsslein-Volhard and Eric Wieschaus won the Nobel prize in 1995 in physiology and medicine for their extensive studies in genetics of fruit flies (Nusslein-Volhard and Wieschaus 1980). Wingless homologues were found in a number of species, the most prominent of which is the discovery in 1982 by Nusse and Varmus of the first mammalian homologue ofWingless, termed Intl-1(Nusse and Varmus 1982). Int-1 was identified as the insertion site for the mouse mammary tumor virus (MMTV) on chromosome 15 that disrupted the mammalian homologue of Wg, leading to breast tumorgenesis. The term Wnt evolved to represent an amalgam between wingless and integration (int-1) site. Nineteen Wnts have been discovered thus far in mammals, which are highly conserved.
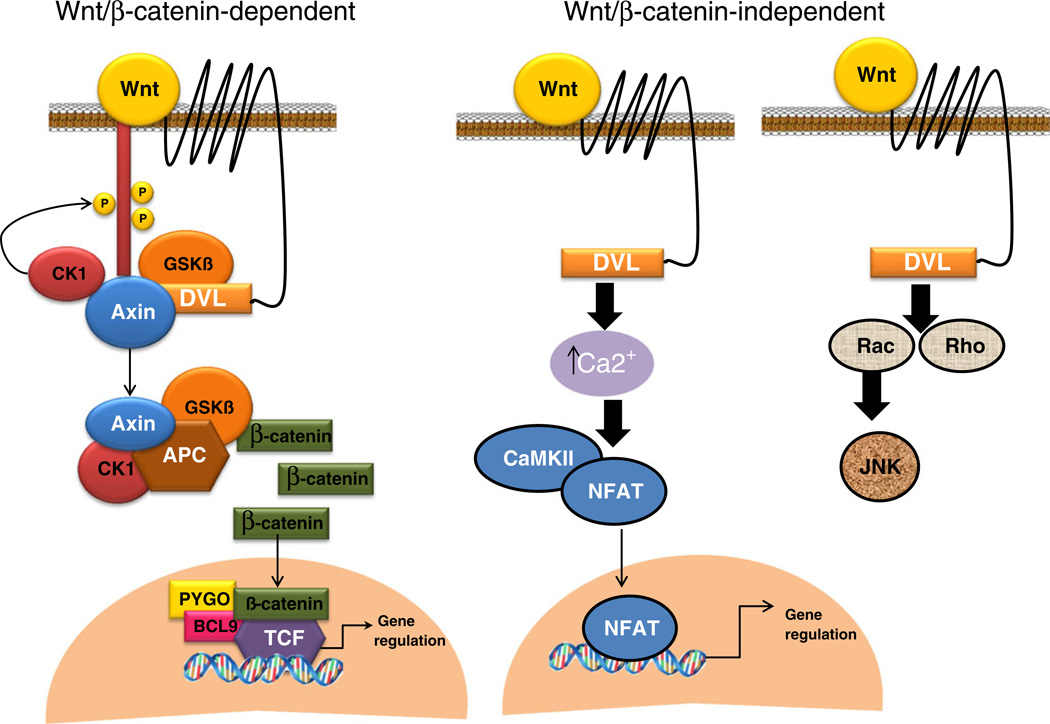
Wnt signaling regulates many cellular processes including cell differentiation, proliferation/senescence, survival/apoptosis, regeneration/”stemness”/, and even wound healing. Its effects are reported across tissues from brain to gastrointestinal track. Developmental biology still fuels much of the progress in details of Wnt signaling events and how they regulate embryonic development. Classically the pathway is defined as canonical (β-catenin dependent) or noncanonical (β-catenin independent). The signal transduction cascade involves binding of 1 of 19 ≈ 300–450aa small secreted glycoproteins (Wnts) to 1 of 10 seven transmembrane frizzled receptors (Fz) to initiate either canonical or noncanonical (e.g., calmodulin/Ca2+ pathway and the planar polarity pathway) signaling (Fig. 1). This traditional classification is now being challenged because the same Wnt can transduce a canonical or noncanonical signal, depending on receptor context (Mikels and Nusse 2006). These findings provide a greater appreciation for versatility of Wnt proteins and the role of receptor context in eliciting canonical or noncanonical signaling. A timeline of significant discoveries in Wnt signaling is shown in Table 1. The time line is not intended to be comprehensive list but represents key findings in the Wnt field.
Fig. 1.
Wnt signaling. A simplified diagram ofWnt β-catenin dependent and independent signaling: a β-catenin-dependent pathway: β-catenin dependent signaling is initiated by binding of Wnts to frizzled seven transmembrane receptors. This binding leads to a series of events culminating in deactivation of a multi-protein β-catenin destruction complex. Subsequently, hypophosphorylated β-catenin translocates to the nucleus where it binds TCF/LEF transcription factors to regulate gene expression. b Wnt β-catenin independent/calcium pathway. Wnts binding to frizzled lead to calcium influx, which activates calmodulin-dependent protein kinase II and NFAT. NFAT binds to its cognate target gene to regulate gene expression. c Wnt β-catenin independent/planar cell polarity pathway: Wnt binding to frizzled leads to Rac/Rho activation and subsequently to activation of kinases such as JNK that modulates cytoskeletal rearrangements. This figure is adapted from (Henderson and Al-Harthi 2011) and (Mulligan and Cheyette 2012)
Table 1.
A time line of seminal discoveries in Wnt signaling field
| Date | Discovery | Reference |
|---|---|---|
| 1980 | Wingless gene discovered in Drosophila | (Nusslein-Volhard and Wieschaus, 1980) |
| 1982 | First Wnt gene in mammals | (Nusse and Varmus 1982) |
| 1989 | β-catenin binding to E-cadherins, its role in cell adhesion | (Ozawa et al. 1989) |
| 1992 | GSK3β identified as a negative regulator of β-catenin | (Siegfried et al. 1992) |
| 1993 | APC binds to β-catenin/start of link to colon cancer | (Rubinfeld et al. 1993) |
| 1995 | β-catenin binds LEF-1 in nucleus, its role as a transcriptional co-activator; Fz bind Wnts | (Behrens et al. 1996) (Bhanot et al. 1996; Yang-Snyder et al. 1996) |
| 1997 | TOPflash construct comes into scene as a powerful tool | (Korinek et al. 1997) |
| 2000 | Crystal structure of β-catenin/TCF binding; LRP is a co-receptor for Wnts | (Graham et al. 2000; Pinson et al. 2000; Tamai et al. 2000) |
| 2003 | ROR & derailed/RYK are additional Wnt receptor | (Oishi et al. 2003; Yoshikawa et al. 2003) |
| 2009 | Discovery of two Wnt inhibitors: IWP targeting Porcupine & IWR targeting Axin | (Chen et al. 2009) |
Although Wnt/β-catenin signaling is typically induced by binding of Wnt glycoproteins to cognate receptor/coreceptor, atypical signaling can occur. Recent evidence indicates that β-catenin is packaged in exosomes, resulting in decreased levels of β-catenin independent of GSK3β activity or proteasomal degradation (Chairoungdua et al. 2010). Additionally, pharmacological inhibitors of GSK3β activity such as synthetic small molecules and lithium chloride (LiCl) can induce β-catenin-dependent signaling in the absence of Wnt ligands. Additional receptors for Wnt ligands such as ROR (Oishi et al. 2003) and Drosophila Derailed receptor (Yoshikawa et al. 2003) have also been identified.
In 2012 alone, there were over 1500 articles on Wnt signaling and many reviews have been recently published on Wnt signaling and it various biologic functions. The 30th anniversary of the discovery of Int-1 was commemorated by a personal perspective of the evolution of the Wnt field through a firsthand encounter by two prominent investigators, Drs. Roel Nusse and Dr. Harold Varmus (Nusse and Varmus 2012). The objective of this special issue is to provide a collection of timely reviews and primary articles to highlight scientific consensus, significant advances, and remaining gaps in our knowledge regarding the role of Wnt signaling in health and disease in the CNS. With the recognition that there are vast areas that one could highlight in this special issue and with the goal of not duplicating recent reviews on the topic, six reviews and two original communications were selected to underscore the overall theme of Wnt/β-catenin and its diverse physiological cell signaling pathways in neurodegenerative and neuropsychiatric disorders. The first two reviews define the role of Wnt signaling in CNS embryonic development and in the adult brain. The remaining reviews highlight the role of Wnt signaling in a number of neurodegenerative conditions including Alzheimer’s disease, schizophrenia, NeuroAIDS, and gliomas. Lastly, a nostalgic look on recent advances in role of Wnts in T cell development and maturation is included as one of the themes in this special issue. The reason for this particular review is because the initial finding of β-catenin/TCF-1 association laid the foundation for nuclear localization of β-catenin and its function as a transcriptional co-regulator (Table 1). This seminal finding established a dual role for β-catenin (a transcriptional co-regulator and binding partner for E-cadherins) that led to explosions in β-catenin regulation of gene expression. The specific topics in this special issue are:
Wnt signaling in vertebrate neural development and function: Drs Kimberly A. Mulligan and Benjamin N.R. Cheyette provide a comprehensive review of Wnt involvement in early events in brain development (Mulligan and Cheyette 2012). Their timely review focuses on the plethora of data documenting the role of Wnts in providing early cues within the embryo to guide anterior-posterior axis specification of the neural plate. Wnts also regulate neuronal tube development, neural stem cells proliferation and differentiation, axon and dendrite growth, and synapse formation.
A role for Wnt/β-catenin signaling in the neural mechanisms of behavior: Drs. Maguschak and Ressler review the role of Wnt/β-catenin signaling in the adult brain (Maguschak and Ressler 2012). Wnt signaling plays a significant role in neuronal synapse formation and remodeling, dendrite growth and arborization, neurotransmission, neuroplasticity, neurogenesis, and neuroprotection. They also provide provocative data to suggest an association between β-catenin/GSK3β axis and several mood disorders including mania and depression. Studies from Dr. Ressler’s laboratory using transgenic mice demonstrated that β-catenin expression is required for memory consolidation in the amygdala (Maguschak and Ressler 2008). Wnt inhibition impairs long-term retention of spatial memory, impairs recognition tasks, and impairs consolidation of fear memory. These events may be linked to rate of β-catenin stabilization of synaptic structures during memory consolidation. The authors provide a provocative model for a mechanism by which Wnt/β-catenin affects memory formation. As such, one would envision that a better understanding of the interface between Wnt signaling and neurocognitive disorders could inform novel strategies for therapeutic interventions.
Wnt signaling: Role in Alzheimer Disease and Schizophrenia. Wnt signaling dysfunction is associated with a number of CNS conditions including autism, schizophrenia, mood disorders, epilepsia, and Alzheimer’s disease. Dr. Nibaldo Inestrosa, Carla Montecinos-Oliva, and Marco Fuenzalida review the role of Wnts in Alzheimer’s disease and schizophrenia (Inestrosa et al. 2012). β-amyloid-mediated neurotoxicity is linked to lower β-catenin level. Lithium, which inhibits GSK3β, leading to β-catenin activation can reverse Aβ-toxicity in small animal models.Wnt 3A can also exert the same neuroprotective effect in animal models of AD. Clinical evidence also exists for reduced β-catenin levels in Alzheimer’s disease patients harboring Presenilin-1 inherited mutations. Lower β-catenin is also linked to increasing level of phosphorylated Tau. The studies highlighted by Inestrosa et all point to emerging evidence between Wnt/β-catenin signaling in Alzheimer’s disease and schizophrenia. It is important to recognize, however that both conditions are vastly complicated and are likely to involve many players/signaling events in addition to Wnt/β-catenin. Nonetheless, understanding the role of Wnt signaling in these clinical conditions will inform novel angels for therapeutic intervention.
Interplay between Wnt/β-catenin signaling and HIV: Virologic and biologic consequences in the CNS. While much of studies in the Wnt signaling field have focused on signaling events that regulate Wnts expression/function/signal transduction pathway and or role in specific diseases such as Alzheimer’s, cancer, gastrointestinal (GI) inflammatory bowel disease, emerging data links an interaction between Wnt/β-catenin signaling and microbial pathogens. Several viruses induce β-catenin, such as Epstein-Barr virus (Shackelford et al. 2003), human papillomavirus, (Rampias et al. 2010) and hepatitis C virus (Liu et al. 2011). β-catenin induction by these viruses is linked to oncogenesis. The Al-Harthi lab has defined a unique interaction between Wnt/β-catenin signaling and HIV in astrocytes, which leads to repressed HIV replication. The perspective by Dr. Al-Harthi summarizes studies from her lab pointing to molecular regulation of HIV replication by β-catenin signaling, HIV mechanism to evade the suppressive action of β-catenin, and the biologic consequences of perturbed β-catenin signal in astrocytes (Al-Harthi 2012). Interestingly, recent studies have identified another microbial interaction with β-catenin signaling. Specifically, type III secretion systemof salmonella is demonstrated to activate β-catenin in the GI track leading to inflammatory events reminiscent of inflammatory bowel disease (Lu et al. 2012). β-catenin inhibits inflammatory responses mediated by commensal bacteria in the GI track (Duan et al. 2007). The interaction between salmonella and β-catenin are outside the scope of this special issue but nonetheless serve to demonstrate a wider interaction between microbial pathogens andWnt/β-catenin signaling thatmay play a role in underlying disease mechanisms.
Wnt/β-catenin signaling in glioma: Glioma is a devastating brain cancer with a fast disease progression and low mortality rate. Drs. K. Zhang, J. Zhang, Han, Pu, and Kang highlight the interaction between Wnt/β-catenin signaling and gliomas (Zhang et al. 2012). β-catenin expression is increased in glioblastoma tissue in comparison to normal brain and is also linked to glioma disease progression. The authors also provide a review of the interplay between Wnt/β-catenin and other signaling pathways, including epidermal growth factor receptor (EGFR)/AKT, Stat 3, and Notch, in gliomas. These signaling interactions provide a complicated network of dysregulated signaling events in gliomas. Constitutive/unregulated β-catenin signaling is linked tomany cancers and is thought to promote increased rate of cell proliferation and invasion. β-catenin signaling has several mechanisms of self regulation, presumably to avoid a sustained over expression signal that leads to malignancy. For example, DKK1, a Wnt inhibitor is an antagonist of β-catenin signaling binding to LRP and sequestering it away from Wnts. DKK1 gene expression is regulated by β-catenin/TCF interaction. Axin, a negative regulator of β-catenin signaling, is also a prominent target gene of β-catenin signaling. There are also a number of secreted frizzled receptors and Wnt inhibitory factors that keep the system in equilibriumand maintain a balance in β-catenin expression. Disrupting this balance can lead to cancers and other abnormalities in multi-organ systems.
β-catenin/TCF-1 pathway in T cell development and differentiation: The discovery of the association between β-catenin and LEF-1 set the stage for the realization that β-catenin is found in the nucleus and has a role as a transcriptional co-regulator. It also highlighted the involvement of β-catenin in T cell development. Sixteen years after this seminal discovery, Dr. Ma, Wang, Fang, and Sun provide a review on the role of β-catenin/LEF in T cell development and differentiation (Ma et al. 2012). β-catenin/LEF is critical for transition of thymocytes from double negative (DN: CD4−CD8−) to double positive (DP: CD4 + CD8+) stage. It is also essential for promoting survival of DP cells through Bcl-XL expression. Growing body of evidence now demonstrate a role for Wnt/β-catenin in T differentiation beyond thymocyte development. Most notable is the involvement of β-catenin/TCF-1 in Th2 (anti-inflammatory) differentiation through induction of GATA-31b. TCF-1 independent of β-catenin also induces Th1 (proinflammatory) differentiation and promotes Th17 (inflammatory) differentiation. Several CNS autoimmune diseases such as multiple sclerosis (MD) are mediated by inflammatory/Th17 cells to induce robust tissue inflammation. β-catenin potentially can be used as a tool to skew Tcell differentiation in favor of anti-inflammatory vs. proinflammatory responses to ameliorate disease pathology. Lastly, there is considerable debate of the absolute requirement for β-catenin in T cell memory formation. Dr. Sun’s group provides relevant data to argue for and against such an association.
The two original manuscripts included reinforce some of the themes in this issue. The article byYun et al. demonstrates a role for Wnt signaling in the pathogenesis of MS-associated chronic pain (Shi et al. 2012a). Dr. Yuan and colleague used a conventional model of MS, Experimental Autoimmune Encephalomyelitis (EAE) in mice and characterized expression of Wnts in the spinal cord dorsal horn. They report that several proteins in Wnt signaling are elevated in the spinal cord dorsal horn of EAE mice. Those are β-catenin, Wnt 3a, Wnt 5a, and ROR2. These findings are interesting and lay the foundation for further studies to decipher cause and effect in elevation of Wnt signaling. Dr. Shi et al. demonstrate that nuclear β-catenin is an essential survival factor for gliomas (Shi et al. 2012b). Particularly, β-catenin accumulates in the nucleus of high grade gliomas to a greater extent than in low grade gliomas. Repression of nuclear β-catenin inhibits proliferation and invasiveness, indicating that nuclear β-catenin accumulation is a prominent feature of malignancy in this model.
A new era in Wnt signaling biology is dawning. Initial studies focused on Wnt signaling in prenatal events. Later studies focused on role of Wnts in tumorogenesis. Significant studies are now targeting role of Wnts in organ homeostasis, in immunity, and organ-specific diseases, including CNS-based diseases. Several tools are available to probe these interactions. The Wnt web page (http://www.stanford.edu/group/nusselab/cgi-bin/wnt/) maintained by the Nusse lab contains significant information on all that is Wnt signaling. Several challenges still remain. It is still unclear what regulates a positiveWnt/β-catenin signal (e.g., proliferation) vs. a negative signal (senescence, cell death). β-catenin partners with TCF/LEF or forkhead transcriptional factors (FOXO), what dictates preferential binding of β-catenin to either transcription factor is not clear?(DeCarolis et al. 2008). It is thought that under oxidative stress, FOXO may compete with TCF/LEF for β-catenin binding (Jin et al. 2008). Association of FOXO with β-catenin has been linked to inhibition of cell proliferation and senescence(DeCarolis et al. 2008). Further, while the role of Wnt signaling in the CNS is highly focused on its effects on neurons, there is paucity of data on the role of Wnt signaling in glia and in immune cells that infiltrate the CNS and drive neurodegenerative processes in diseases such as MS and NeuroAIDS. Lastly, the potential for harnessing Wnt signaling-based therapeutics needs further exploration. Wnt-based therapeutics is challenging. Wnt constitute 19 secreted proteins, 10 frizzled receptors, 3 co-receptors, and hundreds of target genes. Wnt signaling, as mentioned previously, is highly context dependent. Therefore, a blanketed induction or repression of β-catenin, for example, to reach a desired end point for a disease where Wnt is implicated, is likely to have off target responses. For example, a number of small molecules that modulate Wnt signaling have been identified. The concern is that these molecules may have opposing effects depending on cell-type. Our own experience in using small molecules developed for colon epithelial cells indicate that they do not have the same effect in astrocytes (unpublished data). These variable responses underscore how complicated this pathway can be and the need to be cautious in expanding/extrapolating findings from one organ system to another. Most importantly, small molecules should be designed to be highly specific to target cells in question. Nonetheless, manipulation of Wnt signaling provides a great deal of promise in amelioration/reducing diseases where it is a prominent feature of the disease pathology.
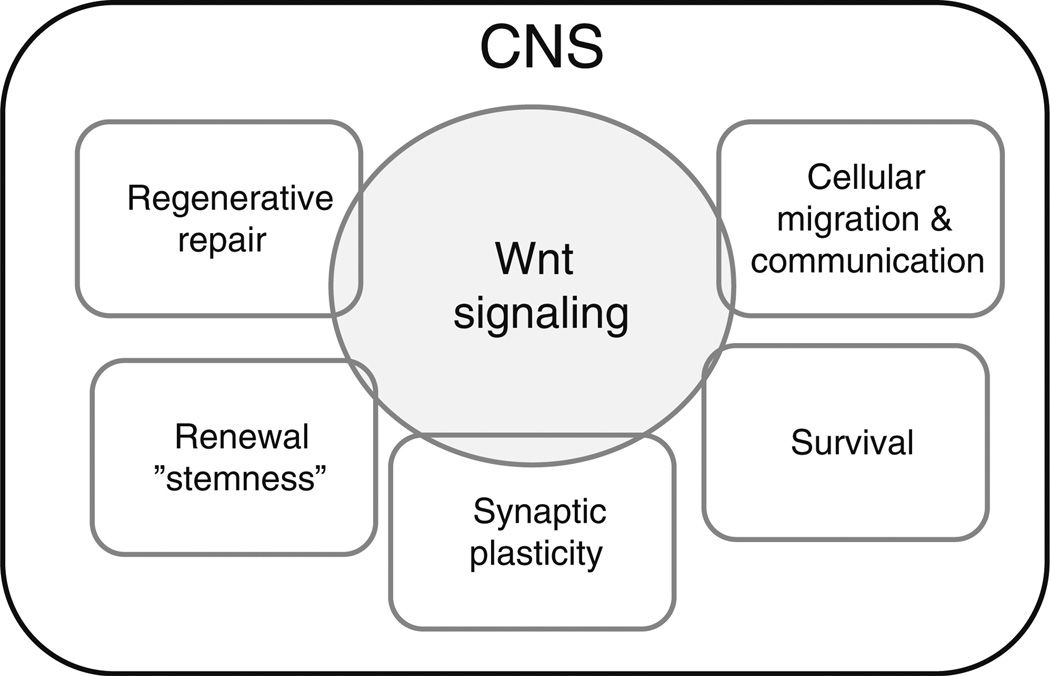
Fig. 2.
Role of Wnt signaling in the adult brain. The diagram highlights several key features of Wnt involvement in homeostatic activities of the adult brain, including events in cellular migration and communication, survival, neurogenesis, synaptic plasticity, and repair
Acknowledgment
This special issue was the brain child of Dr. Howard Gendelman, Editor-in Chief of the Journal of Neuroimmune Pharmacology. I thank him for entrusting me with this jewel and for his continued support throughout this process. I thank my colleagues who contributed to this special issue and provided outstanding reviews that will contribute to a scientific dialogue on the topics. I thank the reviewers who evaluated these articles for their invaluable comments and suggestions to improve the manuscripts. I thank Ms. Robin Taylor for her outstanding administrative assistance as the managing editor for the Journal of Neuroimmune Pharmacology and Mr. Kevin Tomlinson, research administrative assistant, Dept. Immunology/Microbiology, Rush University Medical Center, for his outstanding assistance with graphics. Funding is provided by the National Institutes of Health R01 NS060632, R03 DA 026723, R01 DA 033966, and PO1A1082971.
Biography

Footnotes
Conflict of interest The author has no declaration of conflict of interest
References
- Al-Harthi L. Interplay between Wnt/β-catenin signaling and HIV: Virologic and biologic consequences in the CNS. J NeuroImmune Pharm. 2012 doi: 10.1007/s11481-012-9411-y. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. doi: 10.1038/382225a0. [DOI] [PubMed] [Google Scholar]
- Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Dodge ME, Tang W, Lu J, Ma Z, Fan CW, Wei S, Hao W, Kilgore J, Williams NS, Roth MG, Amatruda JF, Chen C, Lum L. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarolis NA, Wharton KA, Jr, Eisch AJ. Which way does the Wnt blow? Exploring the duality of canonical Wnt signaling on cellular aging. Bioessays. 2008;30:102–106. doi: 10.1002/bies.20709. [DOI] [PubMed] [Google Scholar]
- Duan Y, Liao AP, Kuppireddi S, Ye Z, Ciancio MJ, Sun J. beta-Catenin activity negatively regulates bacteria-induced inflammation. Lab Invest. 2007;87:613–624. doi: 10.1038/labinvest.3700545. [DOI] [PubMed] [Google Scholar]
- Graham TA, Weaver C, Mao F, Kimelman D, Xu W. Crystal structure of a beta-catenin/Tcf complex. Cell. 2000;103:885–896. doi: 10.1016/s0092-8674(00)00192-6. [DOI] [PubMed] [Google Scholar]
- Henderson LJ, Al-Harthi L. Role of beta-catenin/TCF-4 signaling in HIV Replication and Pathogenesis: insights to informing novel Anti-HIV molecular therapeutics. J Neuroimmune Pharmacol. 2011;6:247–259. doi: 10.1007/s11481-011-9266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa N, Montecinos-Oliva C, Marco F. Wnt signaling: Role in Alzheimer Disease and Schizophrenia. J NeuroImmune Pharm. 2012 doi: 10.1007/s11481-012-9417-5. (this issue) [DOI] [PubMed] [Google Scholar]
- Jin T, Fantus IG, Sun J. Wnt and beyond Wnt: multiple mechanisms control the transcriptional property of beta-catenin. Cell Signal. 2008;20:1697–1704. doi: 10.1016/j.cellsig.2008.04.014. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a beta-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Liu J, Ding X, Tang J, Cao Y, Hu P, Zhou F, Shan X, Cai X, Chen Q, Ling N, Zhang B, Bi Y, Chen K, Ren H, Huang A, He TC, Tang N. Enhancement of canonical Wnt/beta-catenin signaling activity by HCV core protein promotes cell growth of hepatocellular carcinoma cells. PLoS One. 2011;6:e27496. doi: 10.1371/journal.pone.0027496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R, Liu X, Wu S, Xia Y, Zhang YG, Petrof EO, Claud EC, Sun J. Consistent activation of the beta-catenin pathway by Salmonella type-three-secretion effector protein AvrA in chronically infected intestine. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00453.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, Wang R, Fang X, Sun Z. b-catein/TCF-1 pathway in T cell development and differentiation. J. Neuro. Immune Pharmacol. 2012 doi: 10.1007/s11481-012-9367-y. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. Beta-catenin is required for memory consolidation. Nat Neurosci. 2008;11:1319–1326. doi: 10.1038/nn.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguschak KA, Ressler KJ. A Role for WNT/beta-Catenin Signaling in the Neural Mechanisms of Behavior. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9350-7. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan KA, Cheyette BN. Wnt Signaling in Vertebrate Neural Development and Function. J Neuroimmune Pharmacol. 2012 doi: 10.1007/s11481-012-9404-x. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R, Varmus HE. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982;31:99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Nusse R, Varmus H. Three decades ofWnts: a personal perspective on how a scientific field developed. EMBO J. 2012;31:2670–2684. doi: 10.1038/emboj.2012.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y. The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells. 2003;8:645–654. doi: 10.1046/j.1365-2443.2003.00662.x. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Brennan J, Monkley S, Avery BJ, Skarnes WC. An LDL-receptor-related protein mediates Wnt signalling in mice. Nature. 2000;407:535–538. doi: 10.1038/35035124. [DOI] [PubMed] [Google Scholar]
- Rampias T, Boutati E, Pectasides E, Sasaki C, Kountourakis P, Weinberger P, Psyrri A. Activation of Wnt signaling pathway by human papillomavirus E6 and E7 oncogenes in HPV16-positive oropharyngeal squamous carcinoma cells. Mol Cancer Res. 2010;8:433–443. doi: 10.1158/1541-7786.MCR-09-0345. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, Munemitsu S, Polakis P. Association of the APC gene product with beta-catenin. Science. 1993;262:1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Shackelford J, Maier C, Pagano JS. Epstein-Barr virus activates beta-catenin in type III latently infected B lymphocyte lines: association with deubiquitinating enzymes. Proc Natl Acad Sci U S A. 2003;100:15572–15576. doi: 10.1073/pnas.2636947100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Gelman BB, Lisinicchia JG, Tang SJ. Chronic-pain-associated astrocytic reaction in the spinal cord dorsal horn of human immunodeficiency virus-infected patients. J Neurosci. 2012a;32:10833–10840. doi: 10.1523/JNEUROSCI.5628-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Qian X, Li L, Zhnag J, Zhu S, Zhu J, Chen L, Zhang K, Han L, Yu S, Pu P, Jiang T, Kang C. Nuclear translocation of b-catenin is essential for glioma cell survival. J NeuroImmune Pharmacol. 2012b doi: 10.1007/s11481-012-9354-3. (this issue) [DOI] [PubMed] [Google Scholar]
- Siegfried E, Chou TB, Perrimon N. wingless signaling acts through zeste-white 3, the Drosophila homolog of glycogen synthase kinase-3, to regulate engrailed and establish cell fate. Cell. 1992;71:1167–1179. doi: 10.1016/s0092-8674(05)80065-0. [DOI] [PubMed] [Google Scholar]
- Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- Yang-Snyder J, Miller JR, Brown JD, Lai CJ, Moon RT. A frizzled homolog functions in a vertebrate Wnt signaling pathway. Curr Biol. 1996;6:1302–1306. doi: 10.1016/s0960-9822(02)70716-1. [DOI] [PubMed] [Google Scholar]
- Yoshikawa S, McKinnon RD, Kokel M, Thomas JB. Wnt-mediated axon guidance via the Drosophila Derailed receptor. Nature. 2003;422:583–588. doi: 10.1038/nature01522. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhang J, Han L, Pu P, Kang C. Wnt/beta-catenin signaling in gliomas. J NeuroImmune Pharmacol. 2012 doi: 10.1007/s11481-012-9359-y. (this issue) [DOI] [PubMed] [Google Scholar]