Summary
Clathrin and the epithelial-specific clathrin adaptor AP-1B mediate basolateral trafficking in epithelia. However, several epithelia lack AP-1B and mice knocked-out for AP-1B are viable, suggesting the existence of additional mechanisms that control basolateral polarity. Here, we demonstrate a distinct role of the ubiquitous clathrin adaptor AP-1A in basolateral protein sorting. Knock-down of AP-1A causes missorting of basolateral proteins in MDCK cells but only after knock-down of AP-1B, suggesting that AP-1B can compensate for lack of AP-1A. AP-1A localizes predominantly to the TGN and its knock-down promotes spillover of basolateral proteins into common recycling endosomes, the site of function of AP-1B, suggesting complementary roles of both adaptors in basolateral sorting. Yeast two-hybrid assays detect interactions between the basolateral signal of TfR and the medium subunits of both AP-1A and AP-1B. The basolateral sorting function of AP-1A reported here establishes AP-1 as a major regulator of epithelial polarity.
Introduction
Epithelial cells perform key vectorial functions in secretion and absorption that depend on the accurate localization of transporters, channels and receptors to apical and basolateral domains (Philp et al., 2010). Sorting of plasma membrane proteins occurs in both biosynthetic and recycling routes through recognition of apical and basolateral sorting signals by machinery in the trans-Golgi network (TGN) and common recycling endosomes (CRE) (Bryant and Mostov, 2008; Mellman and Nelson, 2008; Rodriguez-Boulan et al., 2005). Whereas apical sorting signals and mechanisms are complex, basolateral sorting requires simple sorting signals, in some cases similar to those used by clathrin-mediated endocytosis (Gonzalez and Rodriguez-Boulan, 2009; Traub, 2009; Weisz and Rodriguez-Boulan, 2009). Like clathrin-mediated endocytosis, basolateral trafficking requires clathrin (Deborde et al., 2008) and clathrin-associated sorting proteins, such as the heterotetrameric adaptor AP-1B (Folsch et al., 1999; Ohno et al., 1999) to coordinate the recruitment of cargo proteins via their sorting signals with the formation of clathrin-coated vesicles. However, many aspects of clathrin-mediated basolateral sorting remain unclear (Mostov et al., 1999), particularly the interactions of basolateral sorting signals with AP-1B, which contrasts with the detailed knowledge available on the interactions of the plasma membrane adaptor AP-2 with endocytic sorting signals (Janvier et al., 2003; Kelly et al., 2008; Owen and Evans, 1998; Traub, 2009).
Independent lines of evidence suggest that clathrin-mediated basolateral sorting requires additional adaptors besides the epithelial-specific AP-1B. Recent work has confirmed that several native epithelia constitutively lack AP-1B, e.g. liver (Ohno et al., 1999), retinal pigment epithelium (Diaz et al., 2009) and kidney proximal tubule (Schreiner et al., 2010). Furthermore, mice depleted of AP-1B through knock-out (KO) of the medium (μ1B) subunit are viable, albeit they develop colon inflammation due to apical mislocalization of cytokine receptors (Takahashi et al., 2011). Thus, epithelia, the majority of the ∼200 estimated cell types in the human body (Alberts, 2002), must have additional, not yet identified, mechanisms controlling basolateral trafficking that allow survival of the organism in the absence of AP-1B . Given the requirement for clathrin in basolateral trafficking (Deborde et al., 2008), these may likely be found among ∼20 clathrin-associated sorting proteins known to coordinate with clathrin various intracellular trafficking processes (Bonifacino and Traub, 2003). Another heterotetrameric adaptor complex, AP-4, has been implicated in basolateral polarity (Simmen et al., 2002) but this adaptor does not interact with clathrin (Bonifacino and Traub, 2003) and its basolateral sorting role has not been confirmed by other studies .
The ubiquitous clathrin-associated heterotetrameric adaptor AP-1A is a strong candidate to play a role in epithelial polarity. AP-1A shares three subunits with AP-1B (γ, β1, σ1), differing only in the possession of a different medium subunit, μ1A instead of μ1B (Ohno et al., 1999). AP-1 KO is embryonic lethal in multicellular organisms, e.g., C. elegans (Lee et al., 1994) and mouse (Zizioli et al., 1999). Whereas γ-adaptin KO blocks mouse development at the morula stage (day 2-3 p.c.) (Zizioli et al., 1999), μ1A KO stops development at the start of epithelial organogenesis (day 13.5 p.c.), indicating that AP-1B can compensate partially for the absence of AP-1A (Meyer et al., 2000). Conversely, the viability of AP-1B KO mice (Takahashi et al., 2011) suggests that AP-1A can compensate for AP-1B function. That AP-1A and AP-1B can compensate for each other asymmetrically during mouse development suggests a role for AP-1A in epithelial polarity, complementary to that of AP-1B.
Here we report a role of AP-1A in basolateral protein sorting at the TGN, complementary to the established basolateral sorting role of AP-1B at recycling endosomes. Together with the reported requirement of AP-1 for dendritic polarization in neurons (Dwyer et al., 2001), these results establish AP-1 as a key regulator of polarized trafficking.
Results
Single and double knock-down of AP-1A and AP-1B in MDCK cells
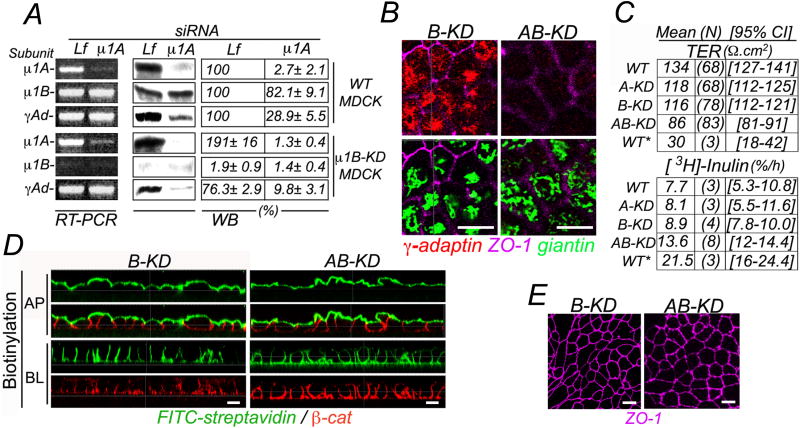
We identified a potent and specific silencing sequence for canine μ1A (μ1A-siRNA) and used it to generate four populations of MDCK cells by AmaxaR electroporation (see Experimental Procedures): WT or Control (wild-type MDCK cells transfected with Luciferase-siRNA), A-KD (WT MDCK cells transfected with μ1A-siRNA), B-KD (μ1B-KD MDCK cells (Gravotta et al., 2007) transfected with Lf-siRNA), and AB-KD (μ1B-KD MDCK cells transfected with μ1A-siRNA). RT-PCR and Western Blot (WB) analysis revealed efficient silencing (> 90%) of μ1A mRNA and protein in A-KD and AB-KD cells. Whereas B-KD cells exhibited significantly increased μ1A, as previously shown (Gravotta et al., 2007), A-KD cells did not show compensatory increase or decrease of μ1B (Fig. 1A). γ-adaptin mRNA levels did not change in A-KD, B-KD or AB-KD cells (Fig. 1A, RT-PCR); in contrast, γ-adaptin protein levels were significantly decreased in B-KD (24%), A-KD (71%) and AB-KD (92%) MDCK cells (Fig. 1A, WB; Fig 1b, immunofluorescence). AB-KD MDCK cells also exhibited a drastic decrease in the level of β-adaptin subunit (Fig. S1), consistent with previous reports indicating that adaptor protein subunits not incorporated into fully assembled adaptors are targeted for degradation (Meyer et al., 2000; Zizioli et al., 1999).
Figure 1. Single and double knock-down of AP-1A and AP-1B in MDCK cells.
MDCK cells, wild type (WT) or permanently depleted of μ1B with shRNA (μ1B-KD) (Gravotta et al., 2007) were transfected by electroporation with control siRNA targeting luciferase (Lf) or μ1A siRNA for three consecutive times (at 3 day-intervals each, see Experimental Procedures) and studied 84 h after the third transfection. (A) RT-PCR (200ng RNA input) and Western blot (WB, 125ug protein/lane); values expressed as % of WT MDCK represent average ± SE.M of three independent experiments. Expression in μ1A siRNA-transfected, relative to Lf siRNA-transfected MDCK cells consistently demonstrated >90% reduction of μ1 A in both WT and B-KD MDCK cells (p < 1.4×10−6 and 1.0×10−9). A-KD cells had no compensatory increase of μ1B (Fig. 1A; p < 0.12), unlike B-KD cells which exhibited increased μ1A levels (Fig. 1A; p < 0.004), as previously shown (Gravotta et al., 2007). RT-PCR analysis did not detect any decrease in γ-adaptin mRNA levels in A-KD, B-KD or AB-KD cells whereas Western blotting detected decreases in protein levels of ∼71 % (p < 2.0×10−4), 25% (p < 0.0012) or 92% (p < 8.3×10−6), respectively, relative to WT MDCK cells. (B) Immunofluorescence microscopy in permeabilized AB-KD MDCK cells confirmed the strong reduction of γ-adaptin in the perinuclear region, highlighted by staining of the Golgi-resident protein giantin. Bar, 12 μm. (C) The trans-epithelial electrical resistance (TER) was slightly decreased, in A-KD (12%, p<1×10−3), and B-KD (14%, p<6×10−5) MDCK cells, and moderately decreased in AB-KD (30%, p<2×10−16) MDCK cells compared to WT MDCK cells, although considerably less than in WT MDCK cells treated with Ca,Mg–free HBSS for 1.5h (WT*) (78%, p=2×10−8). Inulin permeability was not significantly reduced, relative to WT MDCK cells (7.7% leaked to the opposite chamber) in A-KD (8%, p<1) or B-KD (8.9%, p<0.79) but was significantly reduced in AB-KD ( 13.6%, p<2×10−6) MDCK cells. Values shown represent the means of the indicated number of independent measurements (N) and 95% confidence interval (CI). For statistical analysis, see table S1. (D) Domain selective biotinylation of MDCK monolayers grown on polycarbonate filters demonstrate preservation of TJ, as assessed by staining with FITC-streptavidin from the apical or basolateral side (see Experimental Procedures for details). Bar, 12 μm. (E) The morphology of tight junctions was normal in B-KD and AB-KD MDCK cells as revealed by immunostaining of ZO-1. Bar, 12 μm.
Other experiments showed that tight junction (TJ) structure and function were preserved in A-KD, B-KD, and AB-KD MDCK cells (Fig. 1C, E, see table S1, for statistical analysis). FITC-streptavidin decoration of biotinylated monolayers showed selective labeling only of the biotin-exposed domain (Fig. 1D), consistent with the reported impermeability of TJ to negatively charged molecules (Moreno and Diamond, 1974).These experiments validate the various biotin-based polarity assays used in this manuscript to study the roles of AP-1A and AP-1B in the sorting of basolateral membrane proteins.
Double knock-down of AP-1A and AP-1B causes a drastic loss of basolateral PM protein polarity
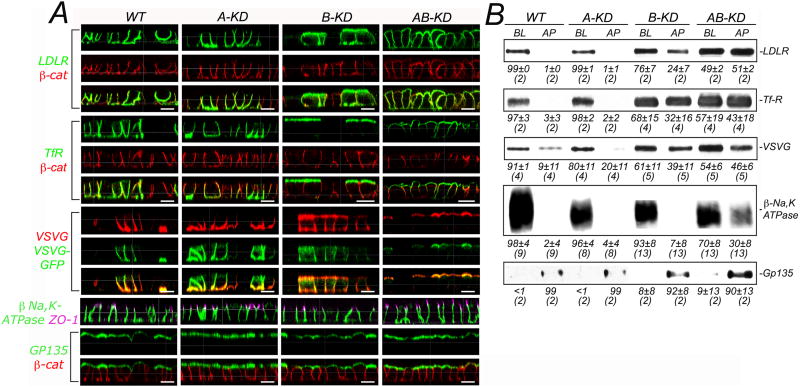
We studied the effect of A-KD, B-KD and AB-KD on the steady-state localization of basolateral and apical markers in polarized MDCK cells using confocal microscopy (Fig. 2A) and quantitative domain-selective biotinylation (Fig. 2B). In agreement with previous studies (Gravotta et al., 2007), we found that B-KD decreased significantly the steady-state basolateral polarity of TfR (68% vs. WT 97%), LDLR (76% vs. WT 99%) and VSVG (61% vs. WT 91%), all expressed via adenoviruses. In contrast, A-KD did not affect or affected only slightly the basolateral polarity of these markers (98%, 99% and 80%, respectively). Strikingly, AB-KD disrupted the steady-state basolateral polarity of these markers to a larger extent than B-KD alone (57%, 49%, 54%, respectively) (Fig. 2A and 2B).
Figure 2. Steady-state distribution of polarity markers in A-KD, B-KD and AB-KD MDCK cells.
A-KD, B-KD and AB-KD MDCK cells, generated as described in the legend to Fig. 1 and plated on polycarbonate filters, were analyzed for the polarized distribution of several endogenous and exogenous PM markers by (A) immunofluorescence or (B) domain-selective biotinylation, streptavidin-agarose retrieval and Western blotting. The distributions of LDL receptor (LDLR), transferrin receptor (TfR) and GFP-tagged vesicular stomatitis virus G protein (VSVG-GFP) were monitored in cell monolayers transduced with the respective adenoviruses 50-54 h after plating and processed for immunofluorescence 26-30 h later (for details, see Experimental Procedures). The remaining markers (NaK-ATPase, β-catenin and gp135) were all endogenous to MDCK cells. (A) Immunofluorescence and confocal microscopy. Cell surface immunolabeling was performed on paraformaldehyde fixed monolayers grown on Transwell chambers, incubated with the respective antibodies to the PM proteins added to both apical and basolateral domains followed by a secondary antibody (shown in green for all proteins except for VSVG-GFP displayed in red). In a second step, cells were permeabilized with saponin to decorate p-catenin or ZO-1 with specific antibodies (shown in red and purple, respectively). Samples were analyzed using a Leica SP2 scanning confocal microscope and images are displayed as x-z sections. Bar, 12 μm (B) Domain-selective biotinylation. Cell surface proteins, subjected to domain-selective biotinylation from the apical (AP) or basolateral (BL) domains and recovered by streptavidin-agarose from cell lysates were analyzed by SDS-PAGE followed by Western blotting. Proteins identified with their respective antibodies, were visualized by chemiluminescence and quantified by NIH-imaging software. Values represent the means ± SD of the number of independent experiments shown between parentheses. Note that B-KD and AB KD (but not A-KD) MDCK cells display loss of polarity of LDLR, TFR and VSVG protein. In contrast, NaK-ATPase polarity is only moderately reduced in AB-KD MDCK cells, whereas the apical PM protein gp135 remains apically polarized under all experimental conditions.
The polarity of two endogenous PM proteins, E-cadherin and β1-integrin, was drastically disrupted by AB-KD but not by A-KD or B-KD (data not shown). Na,K-ATPase, 98% basolateral in WT cells, was not affected by A-KD and B-KD (96% and 93% basolateral, respectively) and was slightly depolarized by AB-KD (70% basolateral). The polarity of the apical PM protein GP135 was not impacted by KD of either one or both adaptors (99% apical in WT and A-KD, 92% apical in B-KD and 90% apical in AB-KD MDCK cells).
The drastic loss of polarity of several basolateral PM proteins in AB-KD cells contrasted with the mild loss of polarity in B-KD cells and the silent phenotype of A-KD cells suggesting that AP-1A has a role in basolateral polarity distinct from that of AP-1B. These results further suggest that AP1A and AP1B sorting roles are asymmetric, with AP-1B capable of compensating for the absence of AP-1A, but not vice-versa.
Double knock-down of μ1A and μ1B-KD causes missorting of newly synthesized TfR and LDR
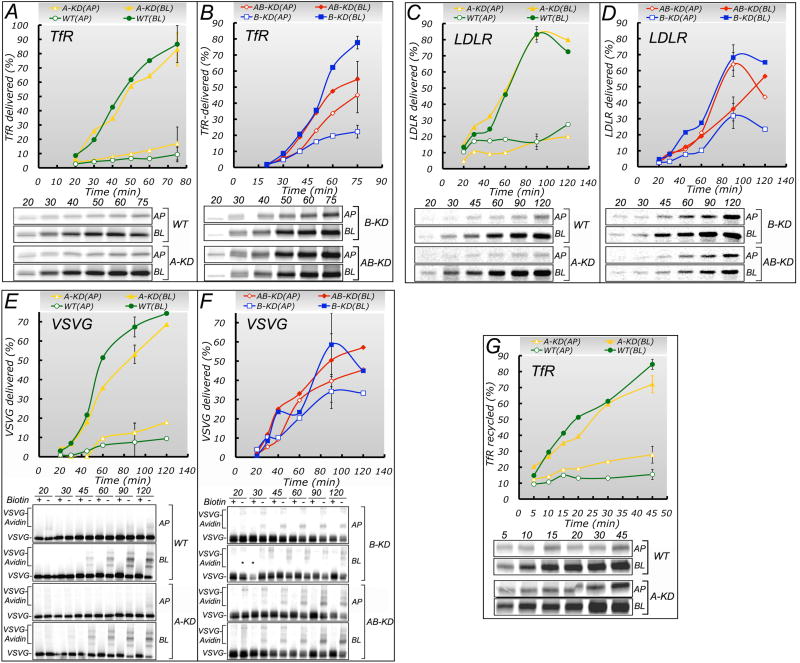
The polarized steady-state distribution of PM proteins reflects the amounts delivered by their biosynthetic and recycling pathways to each PM domain. Previous work has shown that TfR and LDLR move along biosynthetic pathways from TGN to the basolateral PM (Cancino et al., 2007; Gan et al., 2002; Gravotta et al., 2007), whereas VSVG protein utilizes a specialized biosynthetic route through common recycling endosomes (CRE), the site of function of AP-1B (Ang et al., 2004; Cancino et al., 2007; Gravotta et al., 2007). To dissect a possible role of AP-1A in basolateral trafficking, we studied the effect of A-KD, B-KD and AB-KD on the biosynthetic delivery of TfR, LDLR and VSVG protein (Fig. 3, see table S2 for values and statistical analysis). We found that AB-KD MDCK cells dramatically disrupted the polarized biosynthetic delivery of LDLR (WT 79%; AB-KD 36% basolateral) and TfR (WT 91%; AB-KD 52% basolateral), whereas A-KD and B-KD caused a more moderate disruption (LDLR A-KD 79%; B-KD 71 % basolateral) (TfR A-KD 79%; B-KD 74%) (Fig. 3A-D). In contrast with these results, B-KD, but not A-KD, drastically disrupted the biosynthetic route of VSVG protein and AB-KD did not cause additional missorting beyond that caused by AP-1B (Fig. 3E, F, see table S2 for statistical analysis). Taken together, these experiments indicate that both AP-1A and AP-1B are required for accurate biosynthetic sorting of LDLR and TfR, whereas AP-1B, but not AP1A, is required for biosynthetic sorting of VSVG protein.
Figure 3. Effect of AP-1A and AP-1B knock down on the biosynthetic delivery and recycling of basolateral PM proteins.
Biosynthetic (A, F) sorting of several basolateral PM proteins was studied in A-KD, B-KD and AB-KD MDCK cells confluent on polycarbonate filters for 84 h, as detailed in Experimental Procedures. The surface arrival of indicated [35S]-labeled newly synthesized proteins was measured in cells transduced with adenoviruses encoding hTfR, hLDLR or VSVG-GFP, using, ligand capture (TfR) (AB), surface immunoprecipitation (LDLR) (C, D) or surface biotinylation-avidin shift assay (VSVG) (E, F) (see Experimental Procedures for details). Time points showing standard deviation bars represent the average of 4-6 independent experiments; these data and their statistical analysis by ANOVA along with the Bonferroni and Holm corrections for multiple comparisons are shown in table S2. G. The recycling of TfR was studied using a modification of the ligand-capture assay used to measure biosynthetic delivery (see Experimental Procedures) in WT and A-KD MDCK cells. Each time point represents the average of at least two independent experiments. The 45 min time point, showing standard deviation bars, represents the average of 4-6 independent experiments. These data and their statistical analysis by ANOVA along with the Bonferroni and Holm corrections for multiple comparisons are shown in table S2.
AP-1A does not mediate recycling of endocytosed transferrin receptor
We have previously shown that LLC-PK1 cells (which constitutively lack AP-1B) or B-KD MDCK cells missort LDLR or TfR receptor in the recycling route, consistent with the postulated sorting role and localization of AP-1B at CRE (Ang et al., 2004; Cancino et al., 2007; Folsch et al., 2003; Gan et al., 2002; Gravotta et al., 2007). Here, we studied the effect of A-KD on the recycling of TfR, using an adaptation of the ligand capture assay described in Experimental Procedures. A-KD did not disrupt polarized TfR recycling (Fig. 3G and table S2), in striking contrast with B-KD, which caused a profound disruption of TfR recycling, as previously shown (Gravotta et al., 2007).
These results demonstrate that, unlike AP-1B, AP-1A does not mediate recycling of endocytosed proteins. Together with our previous data, these results suggest that AP-1A and AP-1B mediate basolateral protein sorting at different cellular compartments.
AP-1A and AP-1B localize preferentially at TGN and CRE, respectively
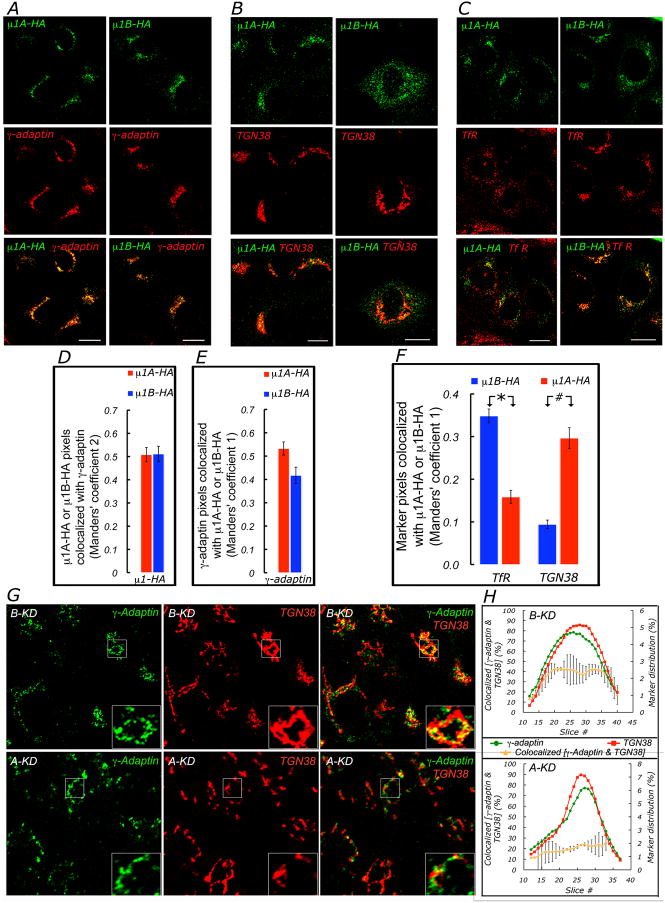
To obtain insight on the site of function of AP-1A, we performed a quantitative analysis of the localization of AP-1A and AP-1B in polarized MDCK cells. Previous data in non polarized cells indicate that AP-1A localizes and functions at the level of the TGN (Doray et al., 2002; Puertollano et al., 2001; Waguri et al., 2003) and studies in subconfluent epithelial cells (LLC-PK1 and FRT cells) suggest that AP-1A localizes to TGN and AP-1B to CRE (Cancino et al., 2007; Folsch et al., 2003; Gan et al., 2002). TGN and CRE have partially overlapping distributions in the perinuclear region, which increases the difficulty of these studies (Cancino et al., 2007; Folsch et al., 2003; Gan et al., 2002). As antibodies capable of detecting endogenous μ1A and μ1B by immunofluorescence in MDCK cells are not available, we transiently transfected hemagglutinin-tagged μ1A (μ1A-HA) or μ1B (μ1B-HA) into WT MDCK cells (Fig. 4). Control experiments indicated that ∼50% of exogenous μ1A-HA and μ1B-HA co-localized with endogenous γ-adaptin, consistent with their efficient integration into the AP-1 complex (Fig. 4A,D). Consistently, a large proportion of the endogenous γ-adaptin colocalized with μ1A-HA or μ1B-HA (Fig. 4A,E).
Figure 4. Co-localization of AP-1A and AP-1B with TGN and recycling endosome markers.
MDCK cells were transfected with cDNAs encoding μ1A-HA or μ1B-HA, cultured as subconfluent monolayers for 72 hours, fixed with paraformaldehyde and permeabilized with saponin and processed for double immunofluorescence and scanning confocal microscopy, as described in Experimental Procedures (A) Monolayers decorated with antibodies to HA and γ-adaptin demonstrated co-localization of μ1A and μ1B with γ-adaptin. Bar, 15 μm. (D,E) Colocalization was analyzed using Manders colocalization coefficients (see supplementary methods). Note the high proportion_(∼ 50%) of both μ1A-HA and μ1B-HA that colocalized with endogenous γ-adaptin, reflecting their incorporation into the AP-1 complex. Manders' coefficient 2: 0.508 ± 0.030 and 0.511 ± 0.033, respectively (n= 30, 33 cells, p > 0.95, for μ1A-HA and μ1B-HA (Student's t-test). (E) Conversely, there was also a high co-localization of γ-adaptin with μ1A-HA or μ1B-HA, Manders' coefficient 1 0.532 ± 0.028 and 0.417 ± 0.035, respectively (n= 30, 33 cells, p > 0.013). (B,C) Cells were immunolabeled with antibodies to influenza HA-tag, TGN38 or Transferrin Receptor (TfR). Cells expressing moderate amounts of μ1A-HA or μ1B-HA displayed a perinuclear distribution similar to that observed for TGN38 and γ-adaptin. TfR distributed in both perinuclear and peripheral endosomes Scale bar= 20um. (f) Values representing mean ± SEM were derived from three independent co-localization experiments of μ1A-HA or μ1B-HA with TGN38- or TfR-labeled compartments. μ1A-HA co-localized more prominently with TGN38 than μ1B-HA(Manders' coefficient 1 = 0.294 ± 0.025 and 0.094 ± 0.010, n=41-40 cells, respectively; [#] p < 3.5 × 10−10, Student's t-test). In contrast, μ1B-HA co-localized more prominently with TfR than μ1A-HA Manders' coefficient 1= 0.348 ± 0.016 and 0.158 ± 0.015, n=69 and 44 cells, respectively; [*] p < 2.8 × 10−13, Student's t-test).(G). A-KD and B-KD MDCK cells, confluent for 84 h, were fixed, immunostained for TGN38 and γ-adaptin, and analyzed by laser scanning confocal microscopy as detailed in Supplementary Information, Experimental Procedures. Shown in the graphs at right are the relative areas expressed as percent values, occupied by TGN38 (red line) and γ-adaptin (green line) in each confocal slice (right ordinate), numbered starting from the top of the monolayer (abscissa), along with the percent colocalization of γ-adaptin with TGN38 (brown line) in A-KD and B-KD. Note that the colocalization of γ-adaptin with TGN38, expressed as the mean ± SD of the fraction of TGN38 area colocalized with γ-adaptin (brown line), is 34 ± 9.8% in B-KD cells and 20 ± 3.4% in A-KD cells (from n ∼ 144-176 cells). The difference was statistically significant (p< 0.0073) (Student's t-test). (Bars,15 μm)
μ1A-HA co-localized more prominently with TGN38 (M1 0.294 ± 0.025) than μ1B-HA (M1 0.094 ± 0.010) (p < 3.5 × 10−10) (Fig. 4B,F). In contrast, μ1B-HA co-localized more prominently with TfR (M1 0.348 ± 0.016) than μ1A-HA (M1 0.158 ± 0.015) (p < 2.8 × 10−13) (Fig. 4E,F).
As a complementary approach, we studied the co-localization of TGN38 with the endogenous γ-adaptin subunit, common to both AP-1A and AP-1B, in B-KD MDCK cells, which express only AP-1A and in A-KD MDCK cells, which express only AP-1B (Fig. 4g). These experiments showed that a substantial and significantly larger proportion of the TGN (labeled with the marker TGN38) co-localized with endogenous γ-adaptin in B-KD than in A-KD MDCK cells (33.7 ± 1.2 % vs. 20.8 ± 3.4 %, respectively; p<0.0073).
In summary, quantitative co-localization experiments in polarized MDCK cells indicate that AP-1A localizes predominantly to the TGN and AP-1B to CRE
AP-1A supports exit of LDLR-GFP from the TGN
We recently used a live-imaging approach to show that chronic knock-down of clathrin heavy chain by siRNA or functional knock-down by acute cross-linking of clathrin light chain, delays selectively the exit of several basolateral cargo proteins from the TGN (Deborde et al., 2008). Here, we used a similar approach to study the role of AP-1 A or AP-1B in the exit of LDLR-GFP from the TGN, defined by the TGN marker sialyltransferase-red fluorescent protein (ST-RFP). The experiments demonstrate that the exit of LDLR-GFP from the TGN is drastically disrupted in AB-KD cells but proceeds at control rates in both A-KD or B-KD cells (Fig. 5, see also movie S1).
Figure 5. Double knock-down of AP-1A and AP-1B blocks TGN exit of LDLR-GFP.
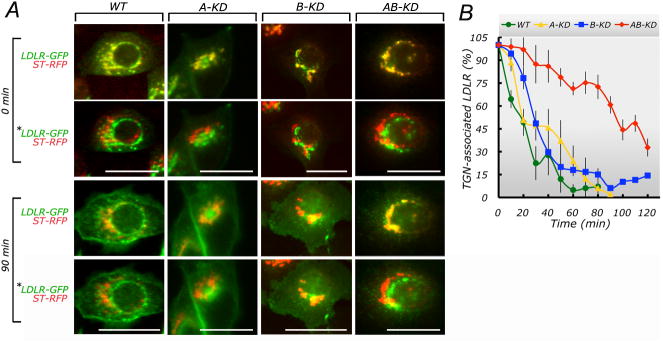
Subconfluent control, A-KD, B-KD or AB-KD MDCK cells, grown on glass coverslips, were microinjected with expression vectors encoding ST-RFP and LDLR-GFP, incubated at 37oC for 1 h to allow synthesis of the protein and at 20oC for 2 h in the presence of 100 ng/ml cycloheximide, to accumulate LDLR-GFP in the TGN. The cells were then live imaged using a 40× objective in the red and green channels (see movie S1 and Experimental Procedures). (A) Fluorescent imaging. Note that in control cells LDLR-GFP co-localizes with ST-RFP at chase time 0 but loses colocalization at 90 min, as LDLR-GFP exits the TGN. A similar result was observed for A-KD and B-KD MDCK cells. In AB-KD cells LDLR-GFP colocalizes to a large extent with ST-RFP at chase times 90 min, indicating that a large fraction of LDLR-GFP displayed a delayed perinuclear exit. Paired images in each panel labeled with an asterisk (*) were generated by pixel-shift of green channel, to better appreciate area of colocalization. Bar, 20 μm. (B) Quantification of TGN exit kinetics. The figure shows the GFP/RFP fluorescence ratios at different chase times. Note that LDLR exit kinetics is not reduced in A-KD or B-KD MDCK cells but is slower in AB-KD MDCK cells. Time courses for LDLR Golgi exit were fit to a simple exponential decay model using the non-linear least squares function in the R software package. The time constant for AB-KD cells was determined to be 75.3 min (95% confidence interval = 64.6 min - 87.7 min; 3 traces). This was significantly slower that the time constants determined for the WT cells (23.9 min, 95% confidence interval = 19.6 min - 28.7 min; 3 traces), the A-KD cells (21.3 min; 95% confidence interval = 17.5 min - 25.5 min; 5 traces), and the B-KD cells (29.9 min; 95% confidence interval = 27.1 min - 32.7 min; 5 traces).
These experiments show that the expression of just AP-1A in MDCK cells is sufficient and necessary for efficient exit of LDLR-GFP from the TGN. This is consistent with previous literature showing that AP-1A facilitates exit from the TGN of a variety of membrane proteins, such as mannose-6-phosphate receptor (Doray et al., 2002; Puertollano et al., 2001; Waguri et al., 2003), VSVG (Chi et al., 2008) and the K-channel Kir 2.1 (Ma et al., 2011). However, our data indicate that AP-1B is also apparently sufficient and necessary for efficient exit of LDLR-GFP from the TGN. This is surprising, as previous evidence indicates that AP-1B mediates basolateral sorting of recycling and newly synthesized proteins at CRE (Ang et al., 2004; Cancino et al., 2007; Folsch et al., 2003; Gan et al., 2002; Gravotta et al., 2007). Our results suggest that AP-1B exhibits a dynamic behavior which allows, in the absence of AP-1A, relocation to the TGN to participate in cargo exit (see Discussion).
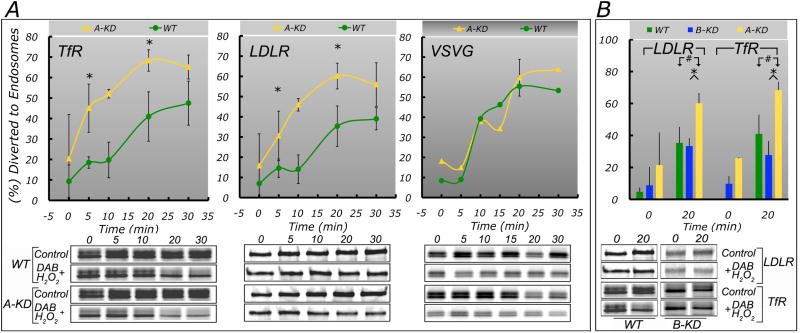
A-KD increases trafficking of basolateral proteins from TGN into CRE
Taken together, the observations reported so far suggest the hypothesis that the silent steady-state phenotype of A-KD MDCK cells results from compensatory diversion of basolateral proteins into CRE and basolateral sorting by AP-1B. To test this hypothesis, we carried out pulse-chase experiments of TfR, LDLR and VSVG in WT, A-KD and B-KD MDCK cells and measured the fraction of radioactively labelled protein transported into CRE using a post-chase “trapping assay” with recycled HRP-Tf (Fig. 6, see Experimental Procedures for detailed protocol). This assay differs from endosomal ablation protocols used by other groups (Ang et al., 2004; Cresawn et al., 2007) in that the crosslinking elicited by HRP/diaminobenzidine (DAB) occurs at the end of -not before- cargo chase into recycling endosomes; this approach may avoid artificial disruption of membrane trafficking dynamics (see discussion by Henry and Sheff, 2008).
Figure 6. AP-1A knock-down enhances trafficking of LDLR and TfR into CRE.
Wild type, A-KD and B-KD MDCK cells, confluent on Transwell chambers for 54 h were transduced either with adenoviruses encoding TfR and LDLR or with adenoviruses encoding TfR and VSVG-GFP for 26-30 h (see Experimental Procedures for details). The cells were then pulsed with [35S]-methionine/cysteine for 15 min, followed by a 2 h chase at 20°C in the presence of horseradish peroxidase-conjugated transferrin (HRP-Tf) to accumulate the radioactively labeled proteins in the TGN and the HRP-Tf in recycling endosomes. Next, the cells were chased at 37°C for the indicated times, in the presence of HRP-Tf, chilled and incubated with DAB/H2O2 to crosslink and trap proteins present in RE loaded with HRP-Tf. Samples were extracted, subjected to immuno-precipitation with antibodies to LDLR, TfR or VSVG protein and analyzed by SDS-PAGE as described in Experimental Procedures. The percent of the radioactively pulse-labeled proteins that became non-extractable upon HRP- and DAB/H2O2- induced-crosslinking was calculated as the difference between control and DAB/H2O2-treated samples and represents the amount of cargo protein diverted into CRE. (A) Values at each time point are average ± SEM from three independent experiments for LDLR an TfR, or from a single experiment for VSVG. Values at 0 and 20 min time points include additional experiments run in triplicates for LDLR and TfR (n=6) and for VSVG (n=7) in A-KD and WT MDCK cells. See table S3 for statistical analysis, (B) Endosomal trafficking in A- and B-KD MDCK cells. Bars represent average ± SEM values at 0 min and 20 min time points for LDLR or TfR in A- or B-KD cells from two independent experiments (n=4). Bar values for LDLR and TfR in A-KD and WT MDCK cells are as described in panel A. Note that the amounts of LDLR and TfR transported into CRE in B-KD or WT MDCK cells were not statistically different from each other. In contrast LDLR and TfR diverted into CRE in A-KD MDCK cells was significantly higher than either B-KD or WT MDCK cells. See table S3 for statistical analysis.
After a 15 min pulse and 2 h incubation at 20°C (to promote accumulation of cargo proteins in the TGN), transfer to 37°C for 20 min resulted in progressive accumulation in CRE of up to ∼30% of TfR and LDLR in WT MDCK (Fig. 6A). Strikingly, in A-KD MDCK, the proportion of LDLR and TfR transported from TGN to CRE doubled (Fig. 6A), whereas in B-KD MDCK it remained the same (Fig. 6B, see values and statistical analysis in Table S3). For VSVG, the amount of protein accumulated in CRE was ∼55% in WT MDCK and did not significantly increase in A-KD or B-KD MDCK cells, in agreement with the postulated obligatory trans-endosomal route of this protein (Ang et al., 2004; Cancino et al., 2007; Gravotta et al., 2007) (see also Fig. 3E,F).
These results indicate that A-KD but not B-KD promote increased trafficking of LDLR and TfR from TGN to CRE, supporting a scenario in which AP-1A controls biosynthetic trafficking of PM proteins along a route independent of CRE.
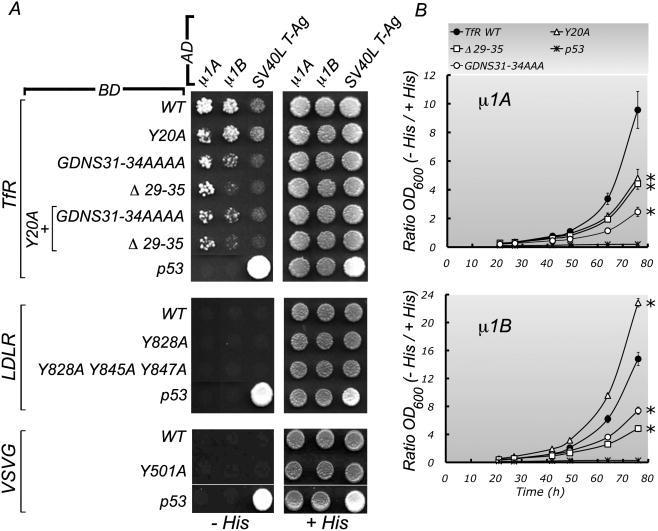
Yeast two-hybrid analysis of the interactions of μ1A and μ1B with basolateral signals
As discussed in Introduction, many basolateral proteins are sorted by signals resembling tyrosine and di-leucine endocytic motifs. One of the PM proteins characterized in this study, VSVG, displays a typical YXXΦ motif as a basolateral sorting signal (Thomas et al., 1993; Thomas and Roth, 1994). In contrast, the two other PM proteins studied here are sorted basolaterally in their biosynthetic and recycling routes by non-conventional basolateral signals distinct from endocytic motifs. For example, TfR utilizes the signal GDNS (Odorizzi and Trowbridge, 1997) whereas LDLR utilizes two basolateral signals, a “weak” proximal basolateral sorting signal (FxNPxY) and a more distal “strong” basolateral signal, GYxY (Matter et al., 1992). To date, no detailed biochemical or structural evidence is available on the interactions of these basolateral sorting signals with AP-1. Hence, we performed Y2H analysis searching for interactions with the medium subunits of this adaptor (Fig. 7). Y2H analysis in plates (Fig. 7A) showed interactions of both μ1A and μ1B with the cytoplasmic domain of TfR, but not with the cytoplasmic domains of LDLR or VSVG. Mutational analysis showed that the interaction with μ1A was sensitive to a substitution in the YXXø motif-based TfR endocytic signal (Y20A) and, partially, to en bloc substitution of the previously described basolateral sorting sequence of TfR (29VDGDNSH) (Dargemont et al., 1993; Odorizzi and Trowbridge, 1997). In contrast, the interaction with μ1B was impaired only by mutation of the 29VDGDNSH sequence (Fig. 7A). Quantitative Y2H analysis of the interaction of TfR tail constructs with μ1A and μ1B in liquid medium (Fig. 7b) yielded results consistent with our interpretation of the experiments carried out in plates.
Figure 7. Yeast two-hybrid analysis of potential interactions between μ1A and μ1B subunits and the cytoplasmic tails of TfR, LDLR and VSVG.
A. Plate assays. The μ1A/μ1B and the cytoplasmic tail constructs were subcloned into Y2H activation domain (AD) and binding domain (BD) vectors, respectively (for details, see Experimental Procedures). Analysis of the TfR cytoplasmic tail indicated that the interaction with u1A is dependent on both the YTRF sequence (amino acids 20-23; consensus motif YXXØ) and the GDNS sequence (amino acids 31-34), while the interaction with μ1B depends exclusively on GDNS. The lack of interaction of the human LDLR constructs with μ1A and μ1B subunits was also observed when the configuration of the assay was inverted by subcloning the LDLR constructs into the AD vector pGAD424 and the μ1A and μ1B constructs into the BD vector pGBT9 (not shown). Interaction of fusion proteins was monitored by activation of HIS3 transcription following plating of AH109 yeast strain double transformants on medium lacking His, Leu and Trp (-His). Plating on medium lacking only Leu and Trp (+His) provided a control for growth and loading of double transformants. B. Liquid medium Y2H assays. Triplicate cultures in +His and -His liquid media of the different double transformants were inoculated at high dilution (OD600 nm ∼0.001) and cultured for up to 76 h. The OD600 were recorded at the indicated intervals on undiluted samples or dilutions that did not exceed OD600 values of ∼0.5. Results were expressed as the OD600 in -His medium at the indicated times divided by the OD600 in +His medium at 21 h, to correct for differences in the density of the initial inocula (the OD600 in +His medium at 21 h was used to avoid values exceeding the range of linearity between OD and number of cells). Growth curves of p53-μ1A and p53- μ1B double transformants were included as negative controls of interactions for μ1A and μ1B, respectively (dashed lines in left and right panels). The data shown are the means +/-SEM of triplicate growth curves for each double transformant. The data points at 64 h and 76 h were analyzed by ANOVA followed by two-tail Dunnett's test. Asterisks at the side of the 76 h data points indicate significant differences at p<0.01 when compared to the TfR WT tail. The same significances were obtained when analyzing the 64 h data points with the exception of the comparison of the TfR WT and Y20A mutant interaction with μ1A, which was significant at p<0.05 instead of p<0.01 (asterisks are not shown for simplicity).
These experiments demonstrate a direct interaction of the basolateral signal of TfR with the medium subunits of AP-1A and AP-1B. The inability to detect interactions of μ1A and μ1B with the basolateral signals of LDLR and VSVG could be due to low binding affinity, the requirement of another subunit of AP-1, or the participation of a linker protein.
Discussion
We have demonstrated that the ubiquitous clathrin adaptor AP-1A plays an important role in basolateral protein trafficking. Our results are consistent with a scenario in which AP-1A operates at the TGN promoting exit of basolateral proteins along a route to the PM that avoids TfR-rich common recycling endosomes. This scenario is supported by the participation of AP1A in the steady-state localization and biosynthetic delivery of basolateral proteins (Fig. 2 and 3), the predominant localization of AP-1A to the TGN in polarized MDCK cells (Fig. 4), its requirement for the exit of LDLR-GFP from TGN in live imaging experiments (Fig. 5), the spillover of basolateral proteins into CRE upon AP-1A KD (Fig. 6) and the ability of μ1 A to interact with basolateral signal of TfR in yeast-two-hybrid experiments (Fig. 7). The role of AP1A in basolateral trafficking demonstrated here is complementary to that reported for AP-1B, which localizes preferentially at CRE, sorting recycling basolateral proteins and newly synthesized proteins that reach CRE after TGN exit, such as VSVG protein (Ang et al., 2004; Cancino et al., 2007; Folsch et al., 2003; Gan et al., 2002; Gravotta et al., 2007; Mostov et al., 1999). Our data support the concept that TGN and endosomes do not operate independently in biosynthetic and recycling routes but, rather, cooperate extensively in polarized trafficking (reviewed by Folsch et al., 2009; Gonzalez and Rodriguez-Boulan, 2009; Weisz and Rodriguez-Boulan, 2009).
Our data suggest that both AP-1A and AP-1B may participate in cargo exit from the TGN as knock-down of both adaptors is required to inhibit this process (Fig. 5). This is consistent with the reported ability of AP-1A to mediate Golgi exit of membrane proteins (Chi et al., 2008; Doray et al., 2002; Ma et al., 2011; Puertollano et al., 2001; Waguri et al., 2003) and with reports indicating that AP-1B can bind phosphoinositides characteristic of both CRE (i.e., PIP-3) via its μ1B subunit (Fields et al., 2010) and TGN (i.e., PI-4P) via its γ1 subunit (Heldwein et al., 2004). It was reported that AP-1B can compensate for lack of AP-1A in retrograde transport of man-6-P receptor (Eskelinen et al., 2002) but not furin (Folsch et al., 2001). Our results cannot discard an apical-basolateral sorting role of AP-1A at endosomes, as there is evidence in the literature supporting a role for this adaptor in retrograde transport of mannose-6-phosphate receptors and Golgi proteins from endosomes to the TGN (Meyer et al., 2001; Robinson et al., 2010; Valdivia et al., 2002) and in anterograde transport of melanogenic enzymes from recycling endosomes to melanosomes in melanocytes (Delevoye et al., 2009; Theos et al., 2005).
We demonstrate here, using Y2H, that GDNS, the non-conventional basolateral signal of TfR interacts with both μ1A and μ1B (Fig. 7). This interaction likely involves a non-canonical binding site in the μ1A and μ1B subunits, different from the hydrophobic pocket used to bind YXXΦ motifs (Owen and Evans, 1998). This is consistent with the proposal that μ1B can bind cargo through conventional and non-conventional sites (Sugimoto et al., 2002). μ1A also interacts with the endocytic signal YTRF of TfR (amino acids 20-23), fitting a YXXø consensus motif. The functional significance of this observation remains unclear at this time. On the other hand, we did not detect interactions of μ1A or μ1B with the well-known basolateral signals of LDLR or VSV G protein. This may reflect either (i) very weak binding that cannot be detected in our Y2H analysis, (ii) a piggy-back mechanism via a third protein; such a mechanism was reported for the interaction of LDLR with AP-1B via its weak basolateral signal FxNPxY (Kang and Folsch, 2011) or (iii) binding to μ1B in conjunction with other subunits of AP-1, as reported for dileucine signals and the γ-σ1 hemicomplex of AP-1(Janvier et al., 2003). We did not observe the interaction between VSVG and μ1B reported by others (Fields et al., 2007). This may reflect a very weak interaction or, alternatively, an indirect interaction through a third protein, e.g., Eps15 (Chi et al., 2008).
The complementary roles of AP-1A and AP-1B in basolateral polarity demonstrated here highlight AP-1 as a major regulator of basolateral trafficking, a function consistent with its absolute requirement for the development of multicellular organisms (Lee et al., 1994; Zizioli et al., 1999). Future work must address the precise sorting roles of AP-1A and AP-1B and the molecular mechanisms underlying the cooperation between TGN and CRE. Understanding these mechanisms requires developing high time and space resolution live imaging assays in fully polarized epithelial cells, as well as comparative analysis of AP-1A and AP-1B interactomes, a task that has already begun (Baust et al., 2006; Fields et al., 2010).
Experimental Procedures
Cloning and siRNA silencing
Canine μ1 A was cloned by RT-PCR with a ProStar Ultra HF RT-PCR kit (Stratagene Cedar Creek, TX), using total RNA obtained from MDCK cells with a RNeasy mini kit (Qiagen, Hilden, Germany) and the following set of custom synthesized primers (Sigma-Genosys The Woodlands, TX): 5′GGCC ATG TCC GCC AGC GCC GTC TAC GTG C 3′ (hμ1A-Sense) and 5′CGCGAGTGACCCAGGCCTCGACCATTAGAGG3′ (hμ1A-Antisense). The μ1A sequence obtained was used to select siRNA candidate sequences through an on line algorithm (http://jura.wi.mit.edu/bioc/siRNAext/home.php). Custom synthesized candidate RNAis (Dharmacon, Lafayette, CO) were tested by RT-PCR after transfection. The potent μ1A-targeted siRNA identified in this screening (GTGCTCATCTGCCGGAATT) and a control Luciferase siRNA (GL2-siRNA) were transfected into MDCK cells by electroporation as previously described (Gravotta et al., 2007). Briefly, trypsinized cells were suspended at 4-5 × 106 cells per 0.125 ml of Nucleofector™ Solution V (Amaxa Inc. USA), supplemented with 160 pmol of siRNA and electroporated (Program “T-23”). Cells were consecutively transfected 3× at 3-days interval. Silencing efficiency was determined by RT-PCR, with inputs of 200 ng of total RNA and by Western blot analysis of total cell lysates (125 μg per track). AP-1A and AP-1B medium subunits were detected by Western blot with two specific antibodies as previously described (Gravotta et al., 2007). Blots were visualized by chemiluminescence (Amersham Pharmacia Biotech, Piscataway, NJ) and quantified with ImageJ software. For experiments, the cells were seeded after the third transfection on Transwell chambers at confluency and used 3-4 days later, as indicated in the corresponding figure legends.
Domain-selective biotinylation and steady-state distribution of membrane proteins
The quantitative steady-state distribution of exogenous (adenovirus transduced) and endogenous membrane proteins was determined by domain-selective surface biotinylation (Sargiacomo et al., 1989) 84 h after seeding MDCK cells at confluency on Transwells, as previously described (Gravotta et al., 2007). Cells were rinsed twice with ice-cold Ca, Mg-HBSS and once with 25 mM triethanolamine-HCl, pH 7.8, 0.25 M sucrose, 0.5 mM Cl2Ca, 1 mM MgCl2 (BB) followed by two successive 20 min incubations at 4°C with 3 mg ml−1 sulfo-NHS-LC-Biotin in BB, added to the apical (0.6 ml) or to the basolateral (0.3 ml) sides. Cells were rinsed twice with BB and incubated at 4°C for 20 min with 40 mM ethanolamine-HCl in BB followed by a quick rinse with Ca,Mg-free HBSS. Cells were lysed in 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 25 mM KCl, 2 mM EDTA, 1% Na-deoxycholate, 0.1% SDS, 1% triton X-100, supplemented with 1 mM PMSF and 15 mg ml−1 Leupeptin/ Pepstatin/ Antipain and 37 μg ml−1 benzamidine-HCl (PIC) for 30 min at 4°C. Biotinylated proteins were retrieved from lysates cleared by centrifugation (18,000 g ×10 min) with streptavidin-Sepharose as described (Sargiacomo et al., 1989) and subjected to Western blot analysis.
Quantitative biochemical surface delivery assays of PM proteins in their biosynthetic and recycling routes
We utilized previously reported surface capture assays to assess the polarized delivery of newly synthesized transferrin receptor (Gravotta et al., 2007) and LDL receptor (Gan et al., 2002). These are surface capture assays that score the amount of cargo protein delivered to the apical or basolateral surfaces as percent of the maximum total surface (apical+basolateral) delivery (100%). For the study of VSVG protein, we introduce here a surface biotinylation-avidin-shift (SBAS) quantitative assay that scores apical and basolateral delivery of the cargo protein as a percent of total cargo protein (surface +intracellular). These assays are briefly described below.
Transferrin receptor
For TfR, we used our recently described surface ligand capture (SLC) assay (Gravotta et al., 2007). Briefly, cells transduced with Ad-TfR were pulse-labeled for 14 min with 0.5 mCi ml−1 of [35S]-Protein labeling mix (EXPRE35S35S; Perkin-Elmer, Waltham, MA) followed by incubation in chase medium (serum-free DMEM supplemented with 0.75% BSA and 100× molar excess of methionine and cysteine) containing 50 μg ml−1 biotin-Tf (B-Tf), added either to the apical or basolateral side. Excess B-Tf was washed with ice-cold Ca,Mg-HBSS 0.5% BSA (Ca,Mg-HBSS-BSA). Cells were lysed in 40 mM Na acetate pH 5.5, 150 mM NaCl, 30 mM KCl, 2.5 mM EDTA, 1.5% Triton X-100, 0.5% BSA (TLB-pH 5.5) supplemented with PIC for 30 min at 4°C. The B-Tf/TfR complex was retrieved from lysates cleared by centrifugation (18,000 g for 10 min) after by a 3-h incubation with avidin-Sepharose followed by centrifugation (18,000 g for 10 min). Samples were processed by SDS-PAGE and analyzed by phosphorImagerR This assay was slightly modified to measure TfR recycling, as follows: [35S]-pulse-labeled cells were initially chased for 3.5 h in chase medium to allow equilibration of newly synthesized TfR within the endosomal compartment, prior to incubation with B-Tf, exactly as detailed above.
LDL receptor
For LDLR, we used a surface immuno-capture (SIC) assay (Gan et al., 2002). Briefly, [35S]-pulse-labeled cells were incubated in chase medium containing 6.5 μg ml−1 rabbit anti-LDLR added either to the apical or basolateral side. Antibody excess was washed with ice-cold Ca,Mg-HBSS-BSA. Cells were lysed with 40 mM Tris-HCl pH 7.6, 150 mM NaCl, 30 mM KCl, 2.5 mM EDTA, 1.5% Triton X-100, 0.5% BSA supplemented with PIC (TLB-pH 7.6) for 30 min at 4°C. The LDLR/anti-LDLR complex present in lysates, cleared by centrifugation (18,000 g ×10 min), was retrieved after 6 h incubation at 4°C with protein A-Sepharose. Samples were processed by SDS-PAGE and analyzed by phosphorImagerR
VSVG
Biosynthetic delivery of this protein to apical and basolateral PM domains was measured using a surface biotinylation avidin shift (SBAS) assay. Cells transduced with an adenovirus encoding VSVG-GFP were pulse-labelled with [35S]-methionine/cysteine, incubated in chase medium and chilled with ice-cold Ca, Mg-HBSS. Cells were subjected to domain-selective surface biotinylation as detailed above and then lysed with TLB-pH 7.6 supplemented with PIC at 4°C for 30 min. Lysates, cleared by centrifugation (18,000 g ×10 min), were subjected to immunoprecipitation with mouse rabbit anti-VSVG antibodies and protein G-Agarose. The immunoprecipitated protein, representing a mixture of biotinylated (surface) plus non-biotinylated cargo-protein (intracellular), was eluted by boiling samples with 40 mM Tris-HCl pH 9.0, 1.5% SDS and 20 mM DTT (170 μl/sample) for 10 min. and divided into two identical 80 μl aliquots: (i) the first aliquot was mixed with 55 μl of avidin-shift-buffer (ASB) (500 μg ml−1 avidin, 35% glycerol and 15 μg ml−1 bromophenol blue); (ii) the second 80 μl aliquot was mixed with 55 μl of avidin-control-buffer (ACB) (3 mM biotin, 500 μg ml−1 avidin, 35% glycerol and 15 μg ml−1 bromophenol blue). Both aliquots were incubated at 60°C for 10 min, loaded side by side on SDS-PAGE and quantify by phosphorImager. In the conditions of this assay only the biotinylated cargo-protein form a tight complex with free avidin in ASB causing a shift away from its normal electrophoretic mobility into a higher molecular mass. On the other incubation with biotin-saturated avidin, ACB does not affect biotinylated and non-biotinylated cargo-protein normal electrophoretic mobility. The proportion of biotinylated protein is determined by the difference cargo-protein determined in ACB and ASB. This assay measures independently the amount of cargo-proteins delivered to the apical or basolateral cell surfaces as a percent of the total amount of cargo protein (total=surface + intracellular). Consequently, the sum of AP and BL values may be less than the total due to the intracellular fraction of the protein.
Quantitative immunofluorescence co-localization of μ1A and μ1B with TGN38 and TfR
The cellular distribution of AP-1A or AP-1B was studied by transient transfection of MDCK cells with HA-tagged μ1 A or μ1B (μ1A-HA, μ1B-HA), originally provided by Heike Folsch and Ira Mellman. MDCK cells, trypsinized from a plate seeded the day before, were suspended in 0.125 ml of Nucleofector™ Solution V (4×106 cells), added to 10 μg of an expression vector encoding μ1A-HA or μ1B-HA, electroporated (Program T-23) before plating them on coverslips (2×104 cells/cm2). Seventy-two hours after transfection cells were fixed for 20min with 3.5%-PFA and permeabilized with 0.075 % saponin and immunolabeled with indicated antibodies as described above. Confocal mages (1040 ×1040) from three independent experiments were collected in a spinning-disc confocal microscope, (Zeiss Axio Observer inverted microscope equipped with a Yokogawa Confocal Scanner Unit CSU-X1 and a Photometrics Cool Snap XQ2 Camera) using a Zeiss Plan APO CHROMAT 63X/1.46-0.60 oil-immersion objective. To quantitate the co-localization of μ1A-HA or μ1B-HA with TGN38, TfR or γ-adaptin -labeled compartments, we utilized the Manders' colocalization coefficients (MCC) 1 and 2 determined with Zeiss co-localization software. MCCs provide a more intuitive measure of colocalization between different cellular markers A and B, than the Pearson correlation coefficient (PCC) as they measure the fraction of A present in B (M1) and vice-versa (M2), independently of signal proportionality. Hence, they are ideal to measure the relative proportion of the two immunolabeled compartments of interest, such as CRE and TGN, occupied by either one of the adaptors.
Endosomal trapping
The experimental design for endosomal trapping was adapted from earlier reports ( Stoorvogel et al., 1988). A-KD, B-KD or WT MDCK cells were co-transduced with Ad-hTfR and Ad-hLDLR or with Ad-hTfR and Ad-VSVG pairs and [35S]-pulse-labeled, followed by a chase at 20°C for 2 h in bicarbonate free medium containing 15 μg ml−1 horseradish peroxidase-conjugated transferrin (HRP-Tf). Cells were incubated at 32°C in chase medium supplemented with HRP-Tf and immediately chilled with ice-cold Ca,Mg-HBSS. Cells were incubated at 4°C with 150 μg ml−1 DAB and 0.030% H2O2 (v/v) in Ca,Mg-HBSS for 1 h (crosslinked sample) or without DAB (control sample). Cells were washed with Ca,Mg-HBSS and lysed with TLB-pH 7.6 supplemented with PIC. Lysates, cleared by centrifugation (18000 g for 10 min), were subjected to sequential immuno-precipitation with specific antibodies (rabbit anti-LDLR, rabbit anti-VSVG or mouse anti-TfR) followed by SDS-PAGE and PhosphorImager analysis. The difference between control and DAB/H2O2 treated-samples, the non-extractable protein after HRP and DAB/H2O2 induced-crosslinking, represents the protein pool diverted into RE.
Statistical Analyses
All statistical analyses were performed in R(R, 2010). All data reported as percentages were logit transformed, and all subsequent statistics performed on the transformed values, with results reported after applying the inverse transform. Consequently, reported 95% confidence intervals are not necessarily symmetric around the mean. Single factor ANOVAs were computed with the aov function. Two-sided pairwise t-tests using a pooled SD were used as post-tests; reported p-values were corrected for multiple hypothesis testing using either Bonferroni or Holm corrections, as indicated in the text.
Supplementary Material
Highlights.
AP-1A sorts cargo proteins from the TGN to the basolateral plasma membrane
Loss of AP-1A causes cargo proteins to spill over into recycling endosomes
AP-1B sorting function at recycling endosomes compensates for AP-1A knock-down
Transferrin receptor basolateral signal binds non-canonical sites in AP-1A and AP-1B
Acknowledgments
We thank Dr. Alfonso Gonzalez for reading and commenting on the manuscript. This work was supported by NIH grants GM034107 and EY08538 to ERB, by funds from Research to Prevent Blindness Foundation, by the Dyson Foundation and by an EMBO Postdoctoral Fellowship to Jose Maria Carvajal-Gonzalez. Drs. Rafael Mattera and Juan Bonifacino were supported by the Intramural Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. We thank B. Lelouvier (NHLBI, NIH) for providing an ImageJ plug-in for automated analysis of confocal images, Drs. Andres Perez-Bay, Ryan Schreiner and Susana Salvarezza, for co-localization experiments and Luciana Gravotta, M.A., for help with statistical analyses. None of the authors have a financial interest related to this work.
Abbreviation List
- PM
plasma membrane
- CRE
common recycling endosomes
- BMP
basolateral membrane protein
- TER
transepithelial electrical resistance
- ST-RFP
sialyl-transferase coupled to red fluorescent protein
- HA-μ1A
hemagglutinin-tagged μ1A
- HA-μB
hemagglutinin-tagged μ1B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alberts B. Molecular biology of the cell. 4th. New York: Garland Science; 2002. [Google Scholar]
- Ang AL, Taguchi T, Francis S, Folsch H, Murrells LJ, Pypaert M, Warren G, Mellman I. Recycling endosomes can serve as intermediates during transport from the Golgi to the plasma membrane of MDCK cells. J Cell Biol. 2004;167:531–543. doi: 10.1083/jcb.200408165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baust T, Czupalla C, Krause E, Bourel-Bonnet L, Hoflack B. Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes. Proc Natl Acad Sci U S A. 2006;103:3159–3164. doi: 10.1073/pnas.0511062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Bryant DM, Mostov KE. From cells to organs: building polarized tissue. Nat Rev Mol Cell Biol. 2008;9:887–901. doi: 10.1038/nrm2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancino J, Torrealba C, Soza A, Yuseff MI, Gravotta D, Henklein P, Rodriguez-Boulan E, Gonzalez A. Antibody to AP1B adaptor blocks biosynthetic and recycling routes of basolateral proteins at recycling endosomes. Mol Biol Cell. 2007;18:4872–4884. doi: 10.1091/mbc.E07-06-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi S, Cao H, Chen J, McNiven MA. Eps15 mediates vesicle trafficking from the trans-Golgi network via an interaction with the clathrin adaptor AP-1. Mol Biol Cell. 2008;19:3564–3575. doi: 10.1091/mbc.E07-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresawn KO, Potter BA, Oztan A, Guerriero CJ, Ihrke G, Goldenring JR, Apodaca G, Weisz OA. Differential involvement of endocytic compartments in the biosynthetic traffic of apical proteins. EMBO J. 2007;26:3737–3748. doi: 10.1038/sj.emboj.7601813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargemont C, Le Bivic A, Rothenberger S, Iacopetta B, Kuhn LC. The internalization signal and the phosphorylation site of transferrin receptor are distinct from the main basolateral sorting information. EMBO J. 1993;12:1713–1721. doi: 10.1002/j.1460-2075.1993.tb05816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, Rodriguez-Boulan E. Clathrin is a key regulator of basolateral polarity. Nature. 2008;452:719–723. doi: 10.1038/nature06828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevoye C, Hurbain I, Tenza D, Sibarita JB, Uzan-Gafsou S, Ohno H, Geerts WJ, Verkleij AJ, Salamero J, Marks MS, et al. AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J Cell Biol. 2009;187:247–264. doi: 10.1083/jcb.200907122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz F, Gravotta D, Deora A, Schreiner R, Schoggins J, Falck-Pedersen E, Rodriguez-Boulan E. Clathrin adaptor AP1B controls adenovirus infectivity of epithelial cells. Proc Natl Acad Sci U S A. 2009;106:11143–11148. doi: 10.1073/pnas.0811227106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray B, Ghosh P, Griffith J, Geuze HJ, Kornfeld S. Cooperation of GGAs and AP-1 in packaging MPRs at the trans-Golgi network. Science. 2002;297:1700–1703. doi: 10.1126/science.1075327. [DOI] [PubMed] [Google Scholar]
- Dunn KW, Kamocka MM, McDonald JH. A practical guide to evaluating colocalization in biological microscopy. Am J Physiol Cell Physiol. 2011;300:C723–742. doi: 10.1152/ajpcell.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31:277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- Eskelinen EL, Meyer C, Ohno H, von Figura K, Schu P. The polarized epithelia-specific mu 1B-adaptin complements mu 1A-deficiency in fibroblasts. EMBO Rep. 2002;3:471–477. doi: 10.1093/embo-reports/kvf092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields IC, King SM, Shteyn E, Kang RS, Folsch H. Phosphatidylinositol 3,4,5-trisphosphate localization in recycling endosomes is necessary for AP-1B-dependent sorting in polarized epithelial cells. Mol Biol Cell. 2010;21:95–105. doi: 10.1091/mbc.E09-01-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields IC, Shteyn E, Pypaert M, Proux-Gillardeaux V, Kang RS, Galli T, Folsch H. v-SNARE cellubrevin is required for basolateral sorting of AP-1B-dependent cargo in polarized epithelial cells. J Cell Biol. 2007;177:477–488. doi: 10.1083/jcb.200610047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H, Mattila PE, Weisz OA. Taking the scenic route: biosynthetic traffic to the plasma membrane in polarized epithelial cells. Traffic. 2009;10:972–981. doi: 10.1111/j.1600-0854.2009.00927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H, Ohno H, Bonifacino JS, Mellman I. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell. 1999;99:189–198. doi: 10.1016/s0092-8674(00)81650-5. [DOI] [PubMed] [Google Scholar]
- Folsch H, Pypaert M, Maday S, Pelletier L, Mellman I. The AP-1A and AP-1B clathrin adaptor complexes define biochemically and functionally distinct membrane domains. J Cell Biol. 2003;163:351–362. doi: 10.1083/jcb.200309020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folsch H, Pypaert M, Schu P, Mellman I. Distribution and function of AP-1 clathrin adaptor complexes in polarized epithelial cells. J Cell Biol. 2001;152:595–606. doi: 10.1083/jcb.152.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Y, McGraw TE, Rodriguez-Boulan E. The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat Cell Biol. 2002;4:605–609. doi: 10.1038/ncb827. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Rodriguez-Boulan E. Clathrin and AP1B: Key roles in basolateral trafficking through trans-endosomal routes. FEBS Lett. 2009 doi: 10.1016/j.febslet.2009.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravotta D, Deora A, Perret E, Oyanadel C, Soza A, Schreiner R, Gonzalez A, Rodriguez-Boulan E. AP1B sorts basolateral proteins in recycling and biosynthetic routes of MDCK cells. Proc Natl Acad Sci U S A. 2007;104:1564–1569. doi: 10.1073/pnas.0610700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldwein EE, Macia E, Wang J, Yin HL, Kirchhausen T, Harrison SC. Crystal structure of the clathrin adaptor protein 1 core. Proc Natl Acad Sci U S A. 2004;101:14108–14113. doi: 10.1073/pnas.0406102101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L, Sheff DR. Rab8 regulates basolateral secretory, but not recycling, traffic at the recycling endosome. Mol Biol Cell. 2008;19:2059–2068. doi: 10.1091/mbc.E07-09-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J Cell Biol. 2003;163:1281–1290. doi: 10.1083/jcb.200307157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang RS, Folsch H. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J Cell Biol. 2011;193:51–60. doi: 10.1083/jcb.201012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BT, McCoy AJ, Spate K, Miller SE, Evans PR, Honing S, Owen DJ. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature. 2008;456:976–979. doi: 10.1038/nature07422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Jongeward GD, Sternberg PW. unc-101, a gene required for many aspects of Caenorhabditis elegans development and behavior, encodes a clathrin-associated protein. Genes Dev. 1994;8:60–73. doi: 10.1101/gad.8.1.60. [DOI] [PubMed] [Google Scholar]
- Ma D, Taneja TK, Hagen BM, Kim BY, Ortega B, Lederer WJ, Welling PA. Golgi Export of the Kir2.1 Channel Is Driven by a Trafficking Signal Located within Its Tertiary Structure. Cell. 2011;145:1102–1115. doi: 10.1016/j.cell.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matter K, Hunziker W, Mellman I. Basolateral sorting of LDL receptor in MDCK cells: the cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Mattera R, Arighi CN, Lodge R, Zerial M, Bonifacino JS. Divalent interaction of the GGAs with the Rabaptin-5-Rabex-5 complex. EMBO J. 2003;22:78–88. doi: 10.1093/emboj/cdg015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I, Nelson WJ. Coordinated protein sorting, targeting and distribution in polarized cells. Nat Rev Mol Cell Biol. 2008;9:833–845. doi: 10.1038/nrm2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Eskelinen EL, Guruprasad MR, von Figura K, Schu P. Mu 1A deficiency induces a profound increase in MPR300/IGF-II receptor internalization rate. J Cell Sci. 2001;114:4469–4476. doi: 10.1242/jcs.114.24.4469. [DOI] [PubMed] [Google Scholar]
- Meyer C, Zizioli D, Lausmann S, Eskelinen EL, Hamann J, Saftig P, von Figura K, Schu P. mu1A-adaptin-deficient mice: lethality, loss of AP-1 binding and rerouting of mannose 6-phosphate receptors. EMBO J. 2000;19:2193–2203. doi: 10.1093/emboj/19.10.2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JH, Diamond JM. Discrimination of monovalent inorganic cations by “tight” junctions of gallbladder epithelium. J Membr Biol. 1974;15:277–318. doi: 10.1007/BF01870092. [DOI] [PubMed] [Google Scholar]
- Mostov K, ter Beest MB, Chapin SJ. Catch the mu1B train to the basolateral surface. Cell. 1999;99:121–122. doi: 10.1016/s0092-8674(00)81643-8. [DOI] [PubMed] [Google Scholar]
- Odorizzi G, Trowbridge IS. Structural requirements for basolateral sorting of the human transferrin receptor in the biosynthetic and endocytic pathways of Madin-Darby canine kidney cells. J Cell Biol. 1997;137:1255–1264. doi: 10.1083/jcb.137.6.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Tomemori T, Nakatsu F, Okazaki Y, Aguilar RC, Foelsch H, Mellman I, Saito T, Shirasawa T, Bonifacino JS. Mu1B, a novel adaptor medium chain expressed in polarized epithelial cells. FEBS Lett. 1999;449:215–220. doi: 10.1016/s0014-5793(99)00432-9. [DOI] [PubMed] [Google Scholar]
- Owen DJ, Evans PR. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science. 1998;282:1327–1332. doi: 10.1126/science.282.5392.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philp N, Shoshani L, Cereijido M, Rodriguez-Boulan E. Epithelial domains. In: Nabi R, editor. Membrane domains. 2010. in press. [Google Scholar]
- Puertollano R, Aguilar RC, Gorshkova I, Crouch RJ, Bonifacino JS. Sorting of mannose 6-phosphate receptors mediated by the GGAs. Science. 2001;292:1712–1716. doi: 10.1126/science.1060750. [DOI] [PubMed] [Google Scholar]
- R Development core team. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2010. http://www.R-project.org. [Google Scholar]
- Robinson MS, Sahlender DA, Foster SD. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev Cell. 2010;18:324–331. doi: 10.1016/j.devcel.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M, Lisanti M, Graeve L, Le Bivic A, Rodriguez-Boulan E. Integral and peripheral protein composition of the apical and basolateral membrane domains in MDCK cells. J Membr Biol. 1989;107:277–286. doi: 10.1007/BF01871942. [DOI] [PubMed] [Google Scholar]
- Schreiner R, Frindt G, Diaz F, Carvajal-Gonzalez JM, Perez Bay AE, Palmer LG, Marshansky V, Brown D, Philp NJ, Rodriguez-Boulan E. The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int. 2010;78:382–388. doi: 10.1038/ki.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen T, Honing S, Icking A, Tikkanen R, Hunziker W. AP-4 binds basolateral signals and participates in basolateral sorting in epithelial MDCK cells. Nat Cell Biol. 2002;4:154–159. doi: 10.1038/ncb745. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Geuze HJ, Griffith JM, Strous GJ. The pathways of endocytosed transferrin and secretory protein are connected in the trans-Golgi reticulum. J Cell Biol. 1988;106:1821–1829. doi: 10.1083/jcb.106.6.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Sugahara M, Folsch H, Koide Y, Nakatsu F, Tanaka N, Nishimura T, Furukawa M, Mullins C, Nakamura N, et al. Differential recognition of tyrosine-based basolateral signals by AP-1B subunit mu1B in polarized epithelial cells. Mol Biol Cell. 2002;13:2374–2382. doi: 10.1091/mbc.E01-10-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D, Hase K, Kimura S, Nakatsu F, Ohmae M, Mandai Y, Sato T, Date Y, Ebisawa M, Kato T, et al. The epithelia-specific membrane trafficking factor AP-1B controls gut immune homeostasis in mice. Gastroenterology. 2011;141:621–632. doi: 10.1053/j.gastro.2011.04.056. [DOI] [PubMed] [Google Scholar]
- Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, Stewart A, Robinson MS, Bennett DC, Cutler DF, et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell. 2005;16:5356–5372. doi: 10.1091/mbc.E05-07-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DC, Brewer CB, Roth MG. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- Thomas DC, Roth MG. The basolateral targeting signal in the cytoplasmic domain of glycoprotein G from vesicular stomatitis virus resembles a variety of intracellular targeting motifs related by primary sequence but having diverse targeting activities. J Biol Chem. 1994;269:15732–15739. [PubMed] [Google Scholar]
- Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10:583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- Valdivia RH, Baggott D, Chuang JS, Schekman RW. The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- Waguri S, Dewitte F, Le Borgne R, Rouille Y, Uchiyama Y, Dubremetz JF, Hoflack B. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol Biol Cell. 2003;14:142–155. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde A, Reaves B, Banting G. Epitope mapping of two isoforms of a trans Golgi network specific integral membrane protein TGN38/41. FEBS Lett. 1992;313:235–238. doi: 10.1016/0014-5793(92)81199-v. [DOI] [PubMed] [Google Scholar]
- Zizioli D, Meyer C, Guhde G, Saftig P, von Figura K, Schu P. Early embryonic death of mice deficient in gamma-adaptin. J Biol Chem. 1999;274:5385–5390. doi: 10.1074/jbc.274.9.5385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.