SUMMARY
Members of the ATP-binding cassette (ABC) transporter family (P-glycoproteins, Half-transporters and Multidrug Resistant Proteins) potentially play a role in the development of anthelmintic resistance. The aim of this study was to investigate the possible involvement of ABC transporters in anthelmintic resistance in the bovine parasite, Cooperia oncophora. Partial sequences of 15 members of the ABC transporter protein family were identified, by mining a transcriptome dataset combined with a degenerate PCR approach. Reverse transcriptase PCR showed that most of the ABC transporters identified were constitutively transcribed throughout the life cycle of C. oncophora. Constitutive differences in gene transcript levels between a susceptible and resistant isolate were only observed for Con-haf-9 and Con-mrp-1 in eggs of the resistant isolate, while no differences were observed in L3 or the adult life stage. Analysis of resistant adult worms, collected from calves 14 days after treatment with either ivermectin or moxidectin, showed a significant 3- to 5-fold increase in the transcript levels of Con-pgp-11 compared to non-exposed worms. Interestingly, a 4-fold transcriptional up-regulation of Con-pgp-11 was also observed in L3 of the resistant isolate, after in vitro exposure to different concentrations of ivermectin, whereas this effect was not observed in exposed L3 of the susceptible isolate. The results suggest that the worms of this particular resistant isolate have acquired the ability to up-regulateCon-pgp-11 upon exposure to macrocyclic lactones. Further work is needed to understand the genetic basis underpinning this process and the functional role of PGP-11.
Keywords: Cooperia oncophora, ivermectin resistance, ABC transporters, PGP, HAF, MRP
INTRODUCTION
Cooperia oncophora is a nematode species parasitizing the small intestines of cattle. In temperate climatic regions, co-infections of C. oncophora species with other gastrointestinal parasites such as Ostertagia ostertagi cause important production losses. The control of these helminth infections relies heavily on the use of broad-spectrum anthelmintics such as the macrocyclic lactones (MLs), benzimidazoles (BZs) and levamisole (LEV). However, the intensive and continuous administration of such products have led to the development of resistance in livestock parasites against all anthelmintic classes (Kaplan, 2004). The majority of the reports on ML-resistance in cattle nematodes involve C. oncophora (Coles et al. 2001; Demeler et al. 2009; Edmonds et al. 2010; El-Abdellati et al. 2010a,b). Although the molecular mechanisms underlying the development of ML-resistance in helminth parasites remain elusive, the members of the ATP-binding cassette (ABC) transporter family, such as P-glycoproteins (PGPs), Half-transporters (HAFs) and Multidrug Resistant Proteins (MRPs), are thought to play an important role, since they are suspected to affect the absorption, distribution and elimination of xenobiotics inside these worms. A large number of ABC transporter genes has been identified in nematodes. In the free-living model nematode, Caenorhabditis elegans, 15 pgp genes, 9 haf genes and 8 mrp genes have been identified (Zhao et al. 2007; Lespine et al. 2008). Pgp expression has been observed in all developmental life stages of C. elegans (Lincke et al. 1993), in particular in the intestinal cells, but also in the pharynx, the excretory cells and the chemosensory AWA neurons in the head (Lincke et al. 1992, 1993; Broeks et al. 1995; Nunes et al. 2005). Functional analyses of mrp-1 and pgp-1 in C. elegans describe a protective role against the heavy metal ions, cadmium and arsenite (Broeks et al. 1996). Cel-pgp-2 has a function in the biogenesis of a lysosome-related fat storage organelle (Schroeder et al. 2007) and Cel-pgp-3 is important in defence against the natural toxins, colchicine and chloroquine (Broeks et al. 1995). In parasitic nematode species, the number of identified ABC transporter genes is still expanding. So far, 9 pgp genes, 1 haf gene and 2 mrp genes have been described in Haemonchus contortus (Lespine et al. 2008; Williamson et al. 2011) and 11 partial pgp sequences were recently identified in Teladorsagia circumcincta (Dicker et al. 2011b). In the human filarial worms, 8 pgp genes, 8 haf genes and 5 mrp genes are reported for Brugia malayi (Ardelli et al. 2010) and, for Onchocerca volvulus, 4 pgp genes and 3 haf genes have been described (Huang and Prichard, 1999; Ardelli et al. 2005, 2006; Bourguinat et al. 2008; Lespine et al. 2012). The extent to which the biological role of ABC transporters is conserved between nematode species is still unclear.
In the first reports that associated ML-resistance in nematodes with ABC transporters, higher pgp expression levels or changes in allelic diversity were documented in resistant H. contortus worms (Blackhall et al. 1998; Xu et al. 1998). More recently, a constitutive up-regulation of Hcon-pgp-2 and Hcon-pgp-9 was observed in a triple-resistant H. contortus isolate compared to a susceptible isolate (Williamson et al. 2011). In T. circumcincta, constitutive differences in gene expression between a susceptible and a triple-resistant isolate were most notable in Tci-pgp-9, which was up-regulated in all life-cycle stages of the resistant isolate. Also, high levels of polymorphisms in the partial Tci-pgp-9 nucleotide sequence were identified between the isolates (Dicker et al. 2011b). The involvement of ABC transporters in the mechanism of ML resistance was recently also shown in C. elegans. In these studies, resistant isolates, either generated by IVM-receptor knock-down (glc-1/avr-14/avr-15 triple mutant) or through step-wise exposure to non-lethal doses of ivermectin (IVM), were in vitro cultured with IVM or moxidectin (MOX). Ardelli and Prichard (2008) observed that IVM and MOX induced similar expression profiles with a marked overexpression of mrp-3, mrp-5, mrp-7 and mrp-8. More recently Yan et al. (2012) described an IVM-induced up-regulation of pgp-1, pgp-2, pgp-4, pgp-12, pgp-14, mrp-1, mrp-2, mrp-4, mrp-5, mrp-6, mrp-7, haf-1, haf-2 and haf-3. Additionally, the role of PGPs, HAFs and MRPs in protecting C. elegans from anthelmintic toxicity was investigated in mutant strains (through deletion mutations or RNAi) exposed to IVM. Cel-mrp-3, Cel-mrp-4 and Cel-mrp-8 may play a role in protecting the worm from paralysis induced by IVM (Ardelli and Prichard, 2008), while knock-down of Cel-mrp-1 and Cel-pgp-2 appeared to have the greatest effects in terms of reduced pharyngeal pumping and/or egg production and motility in response to IVM (Yan et al. 2012).
Despite the fact that resistance is widespread in C. oncophora, no reports have yet been published on the potential role of ABC transporters in the development of anthelmintic resistance in this species. For this reason, the aim of the present study was to identify members of the ABC transporter family in C. oncophora and subsequently to investigate their possible involvement in the resistance mechanism by analysing constitutive and inducible changes in gene transcription levels between a susceptible and an IVM-resistant field isolate.
MATERIALS AND METHODS
Parasite material
The anthelmintic-susceptible C. oncophora isolate (CoIVSus) is a lab-maintained isolate, which has never been exposed to drug treatment (El-Abdellati et al. 2010b, 2011). The IVM-resistant C. oncophora isolate (CoIVR08) was collected from a Belgian farm in 2008 (El-Abdellati et al. 2010b). Both isolates are maintained in the laboratory by regular passages, without treatment, through helminth-free calves. For the collection of L1, C. oncophora eggs were purified from faecal samples (Coles et al. 1992) and incubated at 28 °C in deionized water. Sixteen hours later, the hatched L1 were placed on a Baermann apparatus with tap water and collected the next morning. For L2 and L3, coprocultures were incubated for 72 h or 14 days, respectively, at 25 °C and then placed on a Baermann apparatus. L4 and adult worms were recovered live at necropsy, 8 and 21 days post-infection, respectively. The washings of the small intestines were poured over a 116 μm sieve, worms were retained and then placed on a Baermann apparatus.
In vivo-exposed resistant adult worms were recovered live at necropsy, 14 days after subcutaneous treatment with IVM or MOX (0·2 mg/kg body-weight). The controlled efficacy test revealed a 38% and 31% reduction in worm burden, respectively for IVM and MOX (De Graef et al. 2012).
To obtain in vitro-exposed C. oncophora L3, fresh larvae were harvested from coprocultures and ex-sheathed in 0·5% sodium hypochloride (Demeler et al. 2010). Third stage larvae were then incubated at 28 °C in deionized water or in 10−8M IVM (8·7 ng/ml IVM) or 10−7M IVM (87 ng/ml IVM). A stock solution of 10−2MIVM was first prepared in 100% dimethyl sulphoxide (DMSO), while the final dilutions were made in water. After 24 h, the larvae were transferred onto 28 μmsieves suspended in rows of a 24-well plate. Two hours later, the sieves were carefully lifted out of the rows, the migrated larvae were collected from the wells and washed 3 times with deionized water. Pools of larvae were stored at −80 °C until required.
RNA extraction and cDNA synthesis
Total RNA samples were extracted from all C. oncophora life stages, by grinding the parasites with 0·2 ml glass homogenizers (Wheaton), followed by a Trizol® extraction (Invitrogen). Residual genomic DNA was removed by DNase I treatment (Roche). The RNA quality was verified with the Experion™ RNA StdSens Starter kit (Bio-Rad) and the RNA concentration was determined using a Nanodrop® ND-1000 spectrophotometer (NanoDrop Technologies). cDNA was synthesized from 1 μg total RNA by random priming using the iScript cDNA synthesis kit (Bio-Rad) according to the manufacturer’s recommendations.
Identification of C. oncophora ABC transporter genes
An expressed sequence tag (EST) dataset, generated by 454 sequencing, of C. oncophora worms and available on nematode.net v3.0 (PMID: 22139919) (Martin et al. 2012) was analysed for members of the ABC transporter protein family by sequential TBLASTN searches using the protein sequences of known C. elegans ABC transporter genes. Isotig numbers and sequence information of the identified C. oncophora ABC transporter homologues were saved and gene-specific primers (listed in the Supplementary Table 1, online version only) were designed using the online Primer3 software (http://frodo.wi.mit.edu/primer3/). PCR amplifications were carried out by combining 1 μl of cDNA template (CoIVSus adults) and 2·5 μl each of the forward and reverse primer (10 μM) with the Promega PCR reagents. All reactions were run as follows: 2 min at 95 °C, followed by 35 cycles of 95 °C for 30 s, 60 °C for 30 s and 72 °C for 30 s, followed by a final extension at 72 °C for 10 min and then held at 10 °C. PCR products were visualized on a 1·5% agarose gel and stained with 0·5 μg/ml ethidium bromide. Bands were excised and purified with the Geneclean kit® (MPBio). Purified PCR products were cloned using the pGEM®-T easy vector (Promega) and Escherichia coli DH5α competent cells (Stratagene) according to the manufacturer’s protocols. Plasmid products were sequenced bidirectionally with SP6 and T7 vector primers. The sequences were analysed with DNASTAR software (Lasergene version 8). To assign the C. oncophora ABC transporter genes with the correct nomenclature, the partial protein sequences were blasted against the C. elegans protein database on NEMBASE4 (PMID: 21550347) (Elsworth et al. 2011) and consensus sequences were blasted against the Caenorhabditis taxid, using BLASTx on NCBI (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Degenerate PCR approach
Con-pgp-2, Con-pgp-3, Con-pgp-12 and Con-pgp-16 were obtained, using degenerated primers followed by RACE-PCR (Demeler et al. unpublished data). In addition, Con-pgp-9 was isolated with degenerated primers (fw: 5′-TGCHTTGGACGGTTCTGTK-GAA-3′; rv: 5′-AGWAGTAGGATYTTTGGAT-TYC-3′), based on sequence homology between the C. elegans pgp-9 (GenBank ID: NM_075086) and T. circumcincta pgp-9 (provided by Dr. P. Skuce) sequences. The identified sequences were analysed as described above.
Reverse transcription PCR
To investigate the transcription pattern of the individual ABC transporter genes throughout the life cycle of C. oncophora, reverse transcription PCRs (SuperScript™ One-Step RT-PCR with Platinum® Taq, Invitrogen) was carried out. PCR mixtures had final concentrations of 200 ng RNA template, 0·8 μM of both forward and reverse primer, 1 unit of RT/Platinum® Taq Mix, in a reaction buffer containing 0·2 mM of each dNTP and 1·2 mM MgCl2. PCR conditions were set as follows: 30 min at 50 °C, 2 min at 94 °C, followed by 40 cycles of denaturing (15 s at 94 °C), annealing (30 s at 60 °C) and elongation (12 s at 72 °C), followed by a final elongation step at 72 °C for 10 min, after which the PCR mixtures were kept at 10 °C. Glyceraldehyde-3-phosphate dehydrogenase (Con-gapdh) was included as an internal standard.
Quantitative Real-Time PCR
Quantitative real-time PCRs (qRT-PCRs) were performed to compare the constitutive (without drug exposure) and inducible (after exposure to IVM or MOX) transcriptional changes of ABC transporter genes between CoIVSus and CoIVR08 parasite stages. For each biological sample, at least 2 independent RNA extractions were performed. Total RNA (1 μg) was converted to cDNA and diluted 1/5 or used undiluted for Con-pgp-11, Con-pgp-12, Con-pgp-16 and Con-mrp-7. Real-time PCR reactions were prepared with the SYBR Green Master Mix (Applied Biosystems) using 6·4 μl of H2O, 0·8 μl of each amplification primer (10 μM) and 2 μl of cDNA to give a 20 μl reaction volume. All amplification runs were performed on a StepOnePlus Real-Time PCR System (Applied Biosystems), under the following conditions: 95 °C for 20 s, followed by 40–50 cycles of 95 °C for 5 s, optimal annealing temperature (Supplementary Table 1, online version only) for 20 s and an extension of 72 °C for 12 s. A melting curve analysis was performed at the end of the reaction to ensure specificity of the primers. Each run also included a 5-point dilution series of pooled cDNA and a non-template control. Technical replicates of each sample were performed at least in duplicate within the same run. For each transcript, the mean Ct value of the replicates was calculated and then corrected for the run efficiency. Subsequently, Ct values were transformed in relative quantities (Q) using the delta Ct method: Q=E(min Ct – sample Ct). Where E is the amplification efficiency and min Ct is the lowest Ct value. The relative quantities were then normalized with the normalization factor, obtained by the geNorm software for reference genes Con-gapdh and C. oncophora β-tubulin (Con-tubb) (Vandesompele et al. 2002; Van Zeveren et al. 2007). Transcript levels were statistically analysed using an independent-samples t-test (SPSS Statistics 19). Changes of minimum 2-fold with P<0·05 were regarded as being significant.
RESULTS
ABC transporter genes in C. oncophora and their developmental transcription pattern
Analysis of the C. oncophora transcriptome dataset resulted in the identification of 12 partial sequences encoding ABC transporters ranging in size from 129 bp to 1245 bp (Table 1). Based on the best BLASTp results on the NEMBASE4 server against the C. elegans protein database, the C. oncophora sequences were subsequently assigned the putative correct gene name. Four partial P-glycoprotein sequences were identified with highest homology to pgp-1, pgp-2, pgp-3 and pgp-11, respectively. Furthermore, 5 partial Haf transporters were identified (i.e. haf-2, haf-3, haf-4, haf-7 and haf-9) and 3 partial sequences encoding Mrps (i.e. mrp-1, mrp-4 and mrp-7). An additional 3 C. oncophora pgp sequences were identified by a degenerate PCR approach with homology to C. elegans pgp-9, pgp-12 and pgp-16. Reverse transcriptase PCR showed that all ABC transporter genes identified were constitutively transcribed throughout the life cycle of C. oncophora, except for only a very low transcript level of Con-pgp-1 and no expression of Con-pgp-9 in eggs (Table 1).
Table 1.
Sequence sizes, Accession numbers, blast analysis and reverse-transcription PCR results of the 15 partially identified Cooperia oncophora ABC-transporter genes to determine their correct annotation and to show the transcription pattern throughout the lice cycle of C. oncophora
| Transcriptome databasea |
Degenerate PCR |
Transcription values throughout C. oncophora life cycle |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene name | Isogroup | Sequence size (bp) |
Sequence size (bp) |
Accession number |
2 best Nembase4b BLASTp hits | e-value | Maximum identity |
E | L1 | L2 | L3 | L4 | ♂ | ♀ |
| Con-pgp-1 | 10197 | 129 | HE855848 | CE11932 WBGene00003995 locus:pgp-1 protein_id: CAB01232.1 | 9,E-06 | 91% (22/24) | ||||||||
| CE31624 WBGene00004002 locus:pgp-8 protein_id: CAA94221.2 | 1,E-05 | 60% (26/43) | ||||||||||||
| Con-pgp-2 | 02710 | 612 | 3822c | JX262229 | CE29212 WBGene00003996 locus:pgp-2 protein_id: AAB52482.2 | 0·0 | 69% (870/1258) | |||||||
| CE15714 WBGene00004003 locus:pgp-9 protein_id: CAB07855.1 | 0·0 | 42% (543/1285) | ||||||||||||
| Con-pgp-3 | 11823 | 447 | 735 | JX262228 | CE03818 WBGene00003997 locus:pgp-3 protein_id: CAA91495.1 | 1,E-103 | 73% (178/243) | |||||||
| CE03308 WBGene00003998 locus:pgp-4 protein_id: CAA91463.1 | 1,E-101 | 50% (122/243) | ||||||||||||
| Con-pgp-9 | – | – | 278 | HE855849 | CE15714 WBGene00004003 locus:pgp-9 protein_id: CAB07855.1 | 2,E-34 | 75% (69/91) | |||||||
| CE11932 WBGene00003995 locus:pgp-1 protein_id: CAB01232.1 | 4,E-33 | 72% (66/91) | ||||||||||||
| Con-pgp-11 | 07488 | 735 | – | HE855850 | CE34788 WBGene00004005 locus:pgp-11 protein_id: CAA88940.3 | 2,E-76 | 60% (145/240) | |||||||
| CE03260 WBGene00004006 locus:pgp-12 protein_id: CAA91799.1 | 2,E-73 | 58% (140/240) | ||||||||||||
| Con-pgp-12 | – | – | 350 | JX262226 | CE03260 WBGene00004006 locus:pgp-12 protein_id: CAA91799.1 | 5,E-44 | 68% (80/116) | |||||||
| CE03262 WBGene00004008 locus:pgp-14 protein_id: CAA91801.1 | 3,E-43 | 64% (75/116) | ||||||||||||
| Con-pgp-16d | – | – | 298 | JX262227 | CE03263 WBGene00004009 locus:pgp-15 protein_id: CAA91802.1 | 3,E-29 | 55% (54/97) | |||||||
| CE03261 WBGene00004007 locus:pgp-13 protein_id: CAA91800.1 | 1,E-28 | 56% (55/97) | ||||||||||||
| Con-haf-2 | 03369 | 1248 | – | HE855851 | CE07240 WBGene00001812 locus:haf-2 protein_id: AAC71121.1 | 1,E-174 | 73% (297/402) | |||||||
| CE28355 WBGene00001814 locus:haf-4 protein_id: AAC68724.2 | 1,E-129 | 57% (230/402) | ||||||||||||
| Con-haf-3 | 06613 | 1044 | – | HE855852 | CE16149 WBGene00001813 locus:haf-3 protein_id: CAB09418.1 | 1,E-131 | 71% (235/330) | |||||||
| CE15650 WBGene00001811 locus:haf-1 protein_id: CAB02812.1 | 1,E-104 | 55% (184/331) | ||||||||||||
| Con-haf-4 | 09074 | 480 | – | HE855853 | CE28355 WBGene00001814 locus:haf-4 protein_id: AAC68724.2 | 7,E-54 | 78% (110/141) | |||||||
| CE27353 WBGene00001819 locus:haf-9 protein_id: AAK39394.1 | 3,E-45 | 71% (92/129) | ||||||||||||
| Con-haf-7 | 13744 | 234 | – | HE855854 | CE24404 WBGene00001817 locus:haf-7 protein_id: CAB60586.1 | 3,E-23 | 68% (50/73) | |||||||
| CE07240 WBGene00001812 locus:haf-2 protein_id: AAC71121.1 | 3,E-22 | 61% (47/76) | ||||||||||||
| Con-haf-9 | 11142 | 309 | – | HE855855 | CE27353 WBGene00001819 locus:haf-9 protein id: AAK39394.1 | 7,E-38 | 88% (78/88) | |||||||
| CE28355 WBGene00001814 locus:haf-4 protein_id:AAC68724.2 | 7,E-35 | 80% (71/88) | ||||||||||||
| Con-mrp-1 | 04290 | 216 | – | HE855856 | CE39102 WBGene00003407 locus:mrp-l protein_id:ABA03118.1 | l,E-27 | 78% (55/70) | |||||||
| CE34565 WBGene00003407 locus:mrp-l protein_id:AAP82650.1 | l,E-27 | 78% (55/70) | ||||||||||||
| Con-mrp-4 | 09929 | 414 | – | HE855857 | CE09548 WBGene00003410 locus:mrp-4 protein_id:CAA88549.1 | 3,E-52 | 72% (99/137) | |||||||
| CE26370 WBGene00003408 locus:mrp-2 protein id:AAA83299.2 | 2,E-46 | 64% (88/137) | ||||||||||||
| Con-mrp-1 | 04382 | 480 | – | HE855858 | CE32017 WBGene00003413 locus:mrp-7 protein_id:CAA21622.3 | 8,E-41 | 61% (83/135) | |||||||
| CE26370 WBGene00003408 locus:mrp-2 protein_id:AAA83299.2 | l,E-39 | 56% (76/135) | ||||||||||||
PMID: 22139919 (Martin et al. 2012)
PMID: 21550347 (Elsworth et al. 2011).
Denotes a full-length cDNA sequence.
Annotation based on Caenorhabditis briggsae according to Demeler et al. (2012).
Analysis of constitutive transcriptional differences between susceptible and resistant parasites
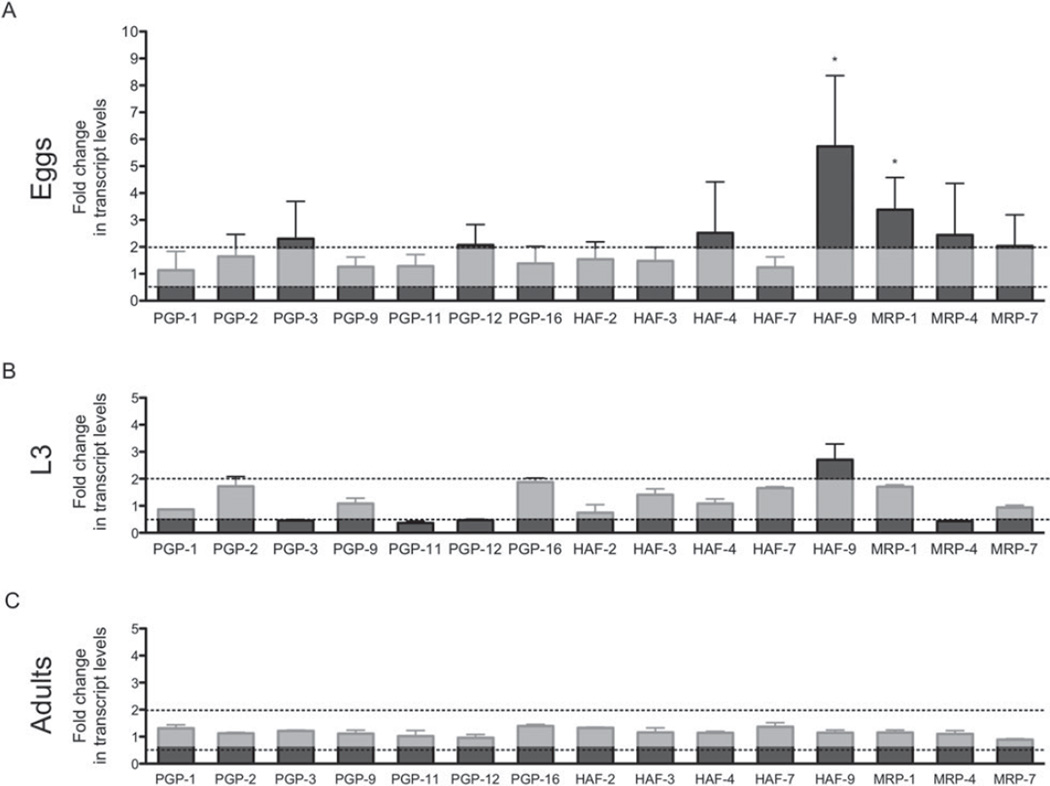
Constitutive differences in transcript levels of the ABC transporter genes between the susceptible and resistant isolates were examined in eggs, L3 and adults worm. The results of the qRT-PCRs are shown in Fig. 1 as the average fold change in mRNA levels compared to non-exposed susceptible eggs, L3 or adult worms. In the C. oncophora egg stage, there was considerable variation in the constitutive transcript levels of ABC transporter genes between biological replicates, but the overall trend showed a higher expression in the eggs of the resistant isolate compared to the susceptible eggs. The up-regulation was only significant in Con-haf-9 (6-fold) and Con-mrp-1 (3-fold). None of the genes analysed showed significant, constitutive differences in transcript levels between susceptible and resistant L3 and adult worms.
Fig. 1.
Fold changes in constitutive mRNA transcript levels of ABC transporter genes in Cooperia oncophora eggs (A) L3 (B) and adult worms (C). The transcript levels in susceptible stages have been set at 1 and the transcript levels±s.d. in CoIVR08 eggs, L3 and adult worms expressed relative to this. Changes of a minimum 2-fold with P<0·05 were regarded as being statistically significant (* P<0·05, ** P<0·01).
Analysis of inducible transcriptional changes in adult worms after in vivo exposure to macrocyclic lactones
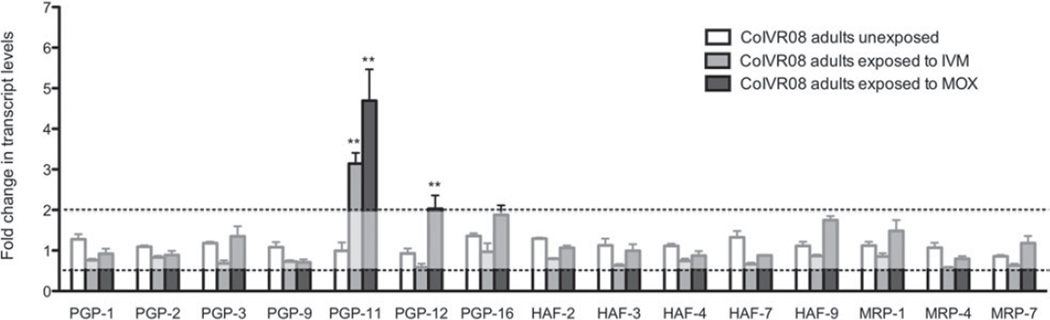
Inducible changes in gene expression levels were investigated in adult worms of the resistant isolate after in vivo exposure to IVM and MOX. The results of the qRT-PCRs are shown in Fig. 2 and presented as the average fold change in mRNA levels compared to non-exposed susceptible worms. The analysis showed that both the IVM and MOX treatment induced a significant up-regulation of Con-pgp-11 transcript levels in the surviving worms, ranging between 3·1 and 4·7-fold increase compared to un-exposed susceptible worms. Apart from Con-pgp-12, which was 2-fold up-regulated in the worms exposed to MOX, none of the other ABC transporter genes investigated showed transcriptional changes exceeding the 2-fold cut-off compared to unexposed susceptible worms.
Fig. 2.
Fold changes in inducible mRNA transcript levels of ABC transporter genes in Cooperia oncophora adult worms. The transcript levels in unexposed susceptible worms have been set at 1 and the transcript levels±s.d. in unexposed CoIVR08, CoIVR08 exposed in vivo to IVM and CoIVR08 exposed in vivo to MOX expressed relative to this. Changes of a minimum 2-fold with P<0·05 were regarded as being significant (* P<0·05, ** P<0·01).
Analysis of inducible transcriptional changes after in vitro exposure of L3 to Ivermectin
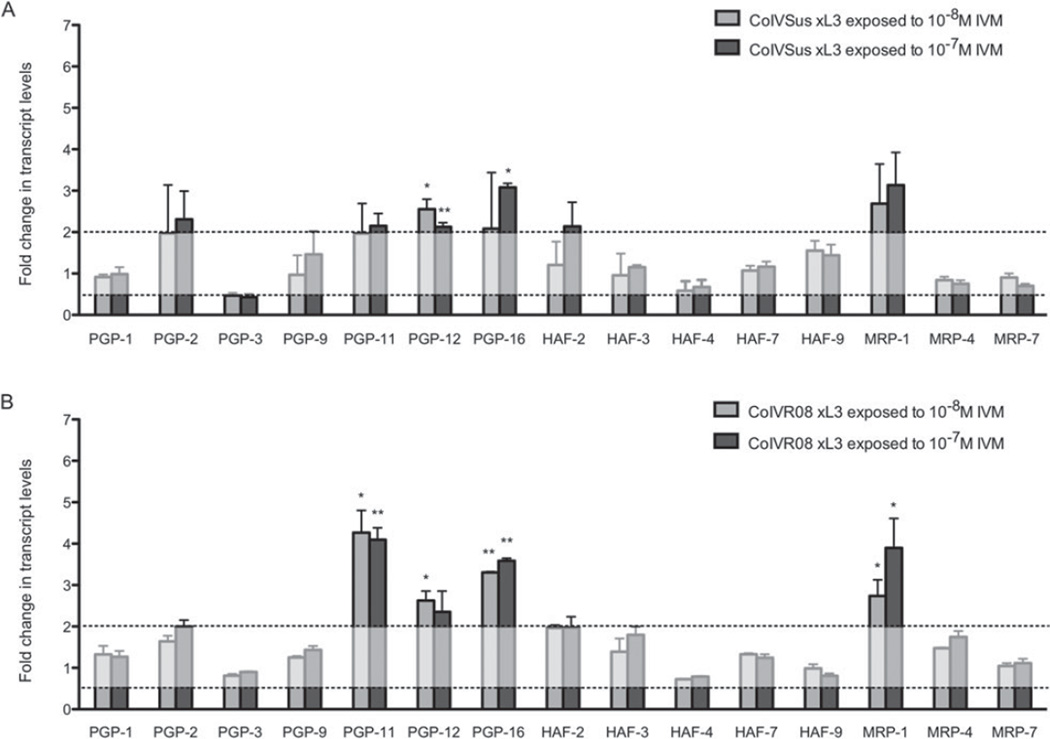
Inducible changes in the transcript levels of the ABC transporter genes were also analysed by exposing L3 stages of the susceptible and resistant isolates to 2 concentrations of IVM in vitro, i.e. 10−8 M and 10−7 M. The results of the qRT-PCR analyses on the worms after exposure are shown in Fig. 3. Significant up-regulations of at least 2-fold were observed for Con-pgp-12 and Con-pgp-16 in both the L3 of the susceptible and resistant isolate after exposure. Furthermore, significantly increased transcript levels of Con-pgp-11 and Con-mrp-1 were induced in resistant L3 and not in susceptible ones (Fig. 3).
Fig. 3.
Fold changes in inducible mRNA transcript levels of ABC transporter genes in Cooperia oncophora L3. The transcript levels in unexposed susceptible L3s have been set at 1 and the transcript levels±s.d. in CoIVSus L3s exposed in vitro to 10−8 M IVM and 10−7 M IVM expressed relative to this (A). The transcript levels in unexposed resistant L3 have been set at 1 and the transcript levels±s.d. in CoIVR08 L3 exposed in vitro to 10−8 M IVM and 10−7 M IVM expressed relative to this (B). Changes of a minimum 2-fold with P<0·05 were regarded as being significant (* P<0·05, ** P<0·01).
DISCUSSION
IVM-resistance has been associated with changes in ABC transporter genes in several parasitic nematodes (Xu et al. 1998; Ardelli et al. 2005, 2006; Ardelli and Prichard, 2007; Prichard and Roulet, 2007; Blackhall et al. 1998, 2008; Bourguinat et al. 2008; Dicker et al. 2011a; Williamson et al. 2011) but, until now, no reports are available on the potential role of ABC transporters in the development of ML-resistance in C. oncophora. In this study, 7 pgp genes, 5 haf genes and 3 mrp genes were identified, either by mining a transcriptome dataset or by a degenerate PCR approach. It is important to note that since most of the identified ABC transporter genes were partial, the currently assigned gene names may still change once more sequence information becomes available. Twelve out of the 15 ABC transporter genes were identified in the transcriptome database (Martin et al. 2012), indicating that these are likely to be the most highly transcribed ABC transporter genes in C. oncophora under normal conditions. Transcripts of Con-pgp-9, Con-pgp-12 and Con-pgp-16 on the other hand were only identified by PCR, suggesting a lower constitutive transcript level. Most of the ABC transporter genes investigated were constitutively transcribed throughout the complete life cycle of C. oncophora, suggesting that they have a basic metabolic function in these worms.
In the present study, differences in constitutive transcript levels were compared between eggs, L3 and adult worms from a susceptible and resistant isolate. No significant differences in the transcription of ABC transporter genes were observed between the L3 and adult parasites of the susceptible and resistant isolates. In the eggs, however, Con-haf-9 and Con-mrp-1 transcript levels were significantly higher in the resistant isolate compared to the susceptible one. However, further information on the constitutive expression of these genes in other nematode species is limited. In laboratory-selected resistant C. elegans worms mrp-1 transcription was highly up-regulated (20 to 36-fold), this was evident even after 3 months without drug exposure (James and Davey, 2009). In H. contortus and O. volvulus, a constitutive up-regulation of pgp-2 transcription has been associated with IVM-resistance, whereas in T. circumcincta pgp-9 was implicated (Blackhall et al. 1998; Prichard and Roulet, 2007; Bourguinat et al. 2008; Dicker et al. 2011b; Williamson et al. 2011). Interestingly, based on the results of this study, neither of these PGPs are likely to be involved in the resistance mechanism of C. oncophora, further suggesting that the resistance mechanism might differ between species.
Although it is often hypothesized that anthelmintic exposure can induce the expression of ABC transporter genes in nematodes, the experimental evidence is still scarce. In C. elegans, resistance was associated with the inducible up-regulation of pgp-1, pgp-2, pgp-4, pgp-12, pgp-14, mrp-1, mrp-2, mrp-3, mrp-4, mrp-5, mrp-6, mrp-7, mrp-8, haf-1, haf-2 and haf-3 after culturing the worms on agar plates with IVM or MOX (Ardelli and Prichard, 2008; James and Davey, 2009; Yan, 2012). In H. contortus, on the other hand, no consistent pattern could be discerned in L3 after exposure to IVM or MOX (Williamson et al. 2011). In this study, the effect of anthelmintic exposure was investigated in vivo 14 days post-treatment, at which time the worms would still be exposed to active drug (Alvinerie et al. 1999; Lifschitz et al. 1999). The most notable change in the surviving worms was observed for pgp-11, in which there was a significant up-regulation after both IVM and MOX treatment. A smaller but significant effect was also observed for pgp-12 induced by MOX treatment, but not by IVM. As far as we are aware, only one other study (Prichard and Roulet, 2007) investigated the expression levels of ABC transporters in worms following in vivo exposure. The authors reported the over-expression of five pgp genes (termed Hc-PgpA, -B, -C, -D and -E) in adult H. contortus worms collected 24 h after the treatment of sheep with IVM, whereas MOX treatment only resulted in the up-regulation of 2 pgp genes (termed Hc-PgpC and -E). Interestingly, according to a phylogenetic analysis, Hc-PgpB showed most homology to the C. elegans pgp-11. Unfortunately, the study did not provide information regarding the levels of up-regulation of the genes analysed.
Although it is debatable to what extent the in vitro assays can mimic in vivo conditions, the inducible up-regulation of pgp-11 was also observed in the L3 of the resistant isolate following in vitro exposure to 2 different concentrations of IVM. Importantly, this up-regulation was not observed in the larvae of the susceptible isolate, suggesting that the resistant worms have acquired the ability to up-regulate pgp-11 upon exposure to MLs. On the other hand, pgp-12, pgp-16 and, to a lesser extent, also mrp-1 were transcriptionally up-regulated in the larvae of both the susceptible and resistant isolate after exposure, suggesting they are part of a more general xenobiotic response. The molecular mechanisms involved in the transcriptional regulation of ABC transporter genes in nematodes are still largely unknown. In mammals, the transcriptional regulation of the multidrug-resistance ABC transporter gene MDR-1 seems to be controlled by the nuclear pregnane X receptor (PXR) and the constitutive androstane receptor (CAR) (Wei et al. 2000; Synold et al. 2001; Maglich et al. 2003). Several members of the nuclear receptor (NR) superfamily have been described in C. elegans, of which the nuclear hormone receptor-8 (NHR-8) seems to be involved in resistance to colchicine and chloroquine (Lindblom and Dodd, 2006; Zhao et al. 2007; Lespine et al. 2012). Since both of these natural toxins are substrates for PGP-3 (Broeks et al. 1995), NHR-8 may play some role in the transcriptional regulation of Cel-pgp-3. However, because of the broad substrate specificity of ABC transporters, it is plausible that a large number of NRs is involved in the transcriptional activation of each ABC transporter gene. Besides transcriptional activation, the regulation of ABC transporters can also occur by post-transcriptional mechanisms such as mRNA stabilization. Recently, in mouse hepatocytes IVM was shown to prolong the half-life of MDR-1a and MDR-1b mRNA, leading to the overexpression of P-glycoprotein through post-transcriptional mRNA stabilization (Ménez et al. 2012).
In summary, the data presented here indicate that resistant C. oncophora worms surviving exposure to IVM and MOX are able to induce pgp-11 transcription, whereas this is not observed in susceptible worms. Whether the up-regulation of this particular P-glycoprotein actually helps to protect the parasites against the toxicity of both MLs is still unclear. Further work is needed to reveal the genetic basis underpinning this inducible up-regulation and to unravel the functional role of PGP-11.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT
J.D.G. is funded by the Institute for the Promotion of Innovation through Science and Technology in Flanders (grant number SBO/81041). This work was in part supported by the National Institutes of Health (M.M., grant number AI081803).
REFERENCES
- Alvinerie M, Sutra JF, Galtier P, Lifschitz A, Virkel G, Sallovitz J, Lanusse C. Persistence of ivermectin in plasma and faeces following administration of a sustained-release bolus to cattle. Research in Veterinary Science. 1999;66:57–61. doi: 10.1053/rvsc.1998.0240. [DOI] [PubMed] [Google Scholar]
- Ardelli BF, Guerriero SB, Prichard RK. Genomic organization and effects of ivermectin selection on Onchocerca volvulus P-glycoprotein. Molecular and Biochemical Parasitology. 2005;143:58–66. doi: 10.1016/j.molbiopara.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ardelli BF, Guerriero SB, Prichard RK. Characterization of a half-size ATP-binding cassette transporter gene which may be a useful marker for ivermectin selection in Onchocerca volvulus . Molecular and Biochemical Parasitology. 2006;145:94–100. doi: 10.1016/j.molbiopara.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Ardelli BF, Prichard R. Effects of ivermectin and moxidectin on the transcription of genes coding for multidrug resistance associated proteins and behaviour in Caenorhabditis elegans . The Journal of Nematology. 2008;40:290–298. [Google Scholar]
- Ardelli BF, Prichard RK. Reduced genetic variation of an Onchocerca volvulus ABC transporter gene following treatment with ivermectin. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101:1223–1232. doi: 10.1016/j.trstmh.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Ardelli BF, Stitt LE, Tompkins JB. Inventory and analysis of ATP-binding cassette (ABC) systems in Brugia malayi . Parasitology. 2010;137:1195–1212. doi: 10.1017/S0031182010000120. [DOI] [PubMed] [Google Scholar]
- Blackhall WJ, Liu HY, Xu M, Prichard RK, Beech RN. Selection at a P-glycoprotein gene in ivermectin- and moxidectin-selected strains of Haemonchus contortus . Molecular and Biochemical Parasitology. 1998;95:193–201. doi: 10.1016/s0166-6851(98)00087-5. [DOI] [PubMed] [Google Scholar]
- Blackhall WJ, Prichard RK, Beech RN. P-glyco-protein selection in strains of Haemonchus contortus resistant to benzimidazoles. Veterinary Parasitology. 2008;152:101–107. doi: 10.1016/j.vetpar.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Bourguinat C, Ardelli BF, Pion SD, Kamgno J, Gardon J, Duke BO, Boussinesq M, Prichard RK. P-glycoprotein-like protein, a possible genetic marker for ivermectin resistance selection in Onchocerca volvulus . Molecular and Biochemical Parasitology. 2008;158:101–111. doi: 10.1016/j.molbiopara.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Broeks A, Gerrard B, Allikmets R, Dean M, Plasterk RH. Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenorhabditis elegans . The EMBO Journal. 1996;15:6132–6143. [PMC free article] [PubMed] [Google Scholar]
- Broeks A, Janssen HW, Calafat J, Plasterk RH. A P-glycoprotein protects Caenorhabditis elegans against natural toxins. The EMBO Journal. 1995;14:1858–1866. doi: 10.1002/j.1460-2075.1995.tb07178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, Waller PJ. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Veterinary Parasitology. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- Coles GC, Watson CL, Anziani OS. Ivermectin-resistant Cooperia in cattle. The Veterinary Record. 2001;148:283–284. [PubMed] [Google Scholar]
- De Graef J, Sarre C, Mills BJ, Mahabir S, Casaert S, De Wilde N, Van Weyenberg M, Geldhof P, Marchiondo A, Vercruysse J, Meeus P, Claerebout E. Assessing resistance against macrocyclic lactones in gastro-intestinal nematodes in cattle using the faecal egg count reduction test and the controlled efficacy test. Veterinary Parasitology. 2012;189:378–382. doi: 10.1016/j.vetpar.2012.04.040. [DOI] [PubMed] [Google Scholar]
- Demeler J, Kuttler U, von Samson-Himmelstjerna G. Adaptation and evaluation of three different in vitro tests for the detection of resistance to anthelmintics in gastro intestinal nematodes of cattle. Veterinary Parasitology. 2010;170:61–70. doi: 10.1016/j.vetpar.2010.01.032. [DOI] [PubMed] [Google Scholar]
- Demeler J, Van Zeveren AM, Kleinschmidt N, Vercruysse J, Hoglund J, Koopmann R, Cabaret J, Claerebout E, Areskog M, von Samson-Himmelstjerna G. Monitoring the efficacy of ivermectin and albendazole against gastro intestinal nematodes of cattle in Northern Europe. Veterinary Parasitology. 2009;160:109–115. doi: 10.1016/j.vetpar.2008.10.030. [DOI] [PubMed] [Google Scholar]
- Dicker AJ, Nath M, Yaga R, Nisbet AJ, Lainson FA, Gilleard JS, Skuce PJ. Teladorsagia circumcincta: the transcriptomic response of a multi-drug-resistant isolate to ivermectin exposure in vitro. Experimental Parasitology. 2011a;127:351–356. doi: 10.1016/j.exppara.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Dicker AJ, Nisbet AJ, Skuce PJ. Gene expression changes in a P-glycoprotein (Tci-pgp-9) putatively associated with ivermectin resistance in Teladorsagia circumcincta . International Journal for Parasitology. 2011b;41:935–942. doi: 10.1016/j.ijpara.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Edmonds MD, Johnson EG, Edmonds JD. Anthelmintic resistance of Ostertagia ostertagi and Cooperia oncophora to macrocyclic lactones in cattle from the western United States. Veterinary Parasitology. 2010;170:224–229. doi: 10.1016/j.vetpar.2010.02.036. [DOI] [PubMed] [Google Scholar]
- El-Abdellati A, Charlier J, Geldhof P, Levecke B, Demeler J, von Samson-Himmelstjerna G, Claerebout E, Vercruysse J. The use of a simplified faecal egg count reduction test for assessing anthelmintic efficacy on Belgian and German cattle farms. Veterinary Parasitology. 2010a;169:352–357. doi: 10.1016/j.vetpar.2010.01.015. [DOI] [PubMed] [Google Scholar]
- El-Abdellati A, De Graef J, Van Zeveren A, Donnan A, Skuce P, Walsh A, Wolstenholme A, Tait A, Vercruysse J, Claerebout E, Geldhof P. Altered avr-14B gene transcription patterns in ivermectin-resistant isolates of the cattle parasites, Cooperia oncophora and Ostertagia ostertagi . International Journal for Parasitology. 2011;41:951–957. doi: 10.1016/j.ijpara.2011.04.003. [DOI] [PubMed] [Google Scholar]
- El-Abdellati A, Geldhof P, Claerebout E, Vercruysse J, Charlier J. Monitoring macrocyclic lactone resistance in Cooperia oncophora on a Belgian cattle farm during four consecutive years. Veterinary Parasitology. 2010b;171:167–171. doi: 10.1016/j.vetpar.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Elsworth B, Wasmuth J, Blaxter M. NEMBASE4: the nematode transcriptome resource. International Journal for Parasitology. 2011;41:881–894. doi: 10.1016/j.ijpara.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Huang YJ, Prichard RK. Identification and stage-specific expression of two putative P-glycoprotein coding genes in Onchocerca volvulus . Molecular and Biochemical Parasitology. 1999;102:273–281. doi: 10.1016/s0166-6851(99)00104-8. [DOI] [PubMed] [Google Scholar]
- James CE, Davey MW. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans . International Journal for Parasitology. 2009;39:213–220. doi: 10.1016/j.ijpara.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Kaplan RM. Drug resistance in nematodes of veterinary importance: a status report. Trends in Parasitology. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Lespine A, Alvinerie M, Vercruysse J, Prichard RK, Geldhof P. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends in Parasitology. 2008;24:293–298. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Lespine A, Ménez C, Bourguinat C, Prichard R. P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: Prospects for reversing transport-dependent anthelmintic resistance. International Journal for Parasitology: Drugs and Drug Resistance. 2012;2:58–75. doi: 10.1016/j.ijpddr.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz A, Virkel G, Imperiale F, Sutra JF, Galtier P, Lanusse C, Alvinerie M. Moxidectin in cattle: correlation between plasma and target tissues disposition. Journal of Veterinary Pharmacology and Therapeutics. 1999;22:266–273. doi: 10.1046/j.1365-2885.1999.00222.x. [DOI] [PubMed] [Google Scholar]
- Lincke CR, Broeks A, The I, Plasterk RH, Borst P. The expression of two P-glycoprotein (pgp) genes in transgenic Caenorhabditis elegans is confined to intestinal cells. The EMBO Journal. 1993;12:1615–1620. doi: 10.1002/j.1460-2075.1993.tb05806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincke CR, The I, van Groenigen M, Borst P. The P-glycoprotein gene family of Caenorhabditis elegans. Cloning and characterization of genomic and complementary DNA sequences. Journal of Molecular Biology. 1992;228:701–711. doi: 10.1016/0022-2836(92)90855-e. [DOI] [PubMed] [Google Scholar]
- Lindblom TH, Dodd AK. Xenobiotic detoxification in the nematode Caenorhabditis elegans . Journal of Experimental Zoology Part A: Comparative Experimental Biology. 2006;305:720–730. doi: 10.1002/jez.a.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, Moore JT. Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. The Journal of Biological Chemistry. 2003;278:17277–17283. doi: 10.1074/jbc.M300138200. [DOI] [PubMed] [Google Scholar]
- Martin J, Abubucker S, Heizer E, Taylor CM, Mitreva M. Nematode.net update 2011: addition of data sets and tools featuring next-generation sequencing data. Nucleic Acids Research. 2012;40(Database issue):D720–D728. doi: 10.1093/nar/gkr1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménez C, Mselli-Lakhal L, Foucaud-Vignault M, Balaguer P, Alvinerie M, Lespine A. Ivermectin induces P-glycoprotein expression and function through mRNA stabilization in murine hepatocyte cell line. Biochemical Pharmacology. 2012;83:269–278. doi: 10.1016/j.bcp.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Nunes F, Wolf M, Hartmann J, Paul RJ. The ABC transporter PGP-2 from Caenorhabditis elegans is expressed in the sensory neuron pair AWA and contributes to lysosome formation and lipid storage within the intestine. Biochemical and Biophysical Research Communications. 2005;338:862–871. doi: 10.1016/j.bbrc.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Prichard RK, Roulet A. ABC transporters and beta-tubulin in macrocyclic lactone resistance: prospects for marker development. Parasitology. 2007;134:1123–1132. doi: 10.1017/S0031182007000091. [DOI] [PubMed] [Google Scholar]
- Schroeder LK, Kremer S, Kramer MJ, Currie E, Kwan E, Watts JL, Lawrenson AL, Hermann GJ. Function of the Caenorhabditis elegans ABC transporter PGP-2 in the biogenesis of a lysosome-related fat storage organelle. Molecular Biology of the Cell. 2007;18:995–1008. doi: 10.1091/mbc.E06-08-0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Naure Medicine. 2001;7:584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- Van Zeveren AM, Visser A, Hoorens PR, Vercruysse J, Claerebout E, Geldhof P. Evaluation of reference genes for quantitative real-time PCR in Ostertagia ostertagi by the coefficient of variation and geNorm approach. Molecular and Biochemical Parasitology. 2007;153:224–227. doi: 10.1016/j.molbiopara.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biology. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature, London. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- Williamson SM, Storey B, Howell S, Harper KM, Kaplan RM, Wolstenholme AJ. Candidate anthelmintic resistance-associated gene expression and sequence polymorphisms in a triple-resistant field isolate of Haemonchus contortus . Molecular and Biochemical Parasitology. 2011;180:99–105. doi: 10.1016/j.molbiopara.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Xu M, Molento M, Blackhall W, Ribeiro P, Beech R, Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Molecular and Biochemical Parasitology. 1998;91:327–335. doi: 10.1016/s0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- Yan R, Urdaneta-Marquez L, Keller K, James CE, Davey MW, Prichard R. The role of several ABC transporter genes in ivermectin resistance in Caenorhabditis elegans . Veterinary Parasitology. 2012;190:519–529. doi: 10.1016/j.vetpar.2012.06.038. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Thomas JH, Chen N, Sheps JA, Baillie DL. Comparative genomics and adaptive selection of the ATP-binding-cassette gene family in caenorhabditis species. Genetics. 2007;175:1407–1418. doi: 10.1534/genetics.106.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.