Abstract
One prominent view holds that episodic memory emerged recently in humans and lacks a “(neo)Darwinian evolution” [Tulving E (2002) Annu Rev Psychol 53:1–25]. Here, we review evidence supporting the alternative perspective that episodic memory has a long evolutionary history. We show that fundamental features of episodic memory capacity are present in mammals and birds and that the major brain regions responsible for episodic memory in humans have anatomical and functional homologs in other species. We propose that episodic memory capacity depends on a fundamental neural circuit that is similar across mammalian and avian species, suggesting that protoepisodic memory systems exist across amniotes and, possibly, all vertebrates. The implication is that episodic memory in diverse species may primarily be due to a shared underlying neural ancestry, rather than the result of evolutionary convergence. We also discuss potential advantages that episodic memory may offer, as well as species-specific divergences that have developed on top of the fundamental episodic memory architecture. We conclude by identifying possible time points for the emergence of episodic memory in evolution, to help guide further research in this area.
Keywords: animal models, hippocampus, prefrontal cortex, parahippocampal region, entorhinal cortex
In humans, episodic memory has been defined as the capacity to recall specific experiences, as if one were to “mentally time travel” to reexperience individual events (1, 2). Although a prominent view holds that episodic memory is unique to humans (1, 3), accumulating evidence indicates that birds and rodents can demonstrate a memory capacity that satisfies behavioral criteria for episodic memory (4–6). Does this evidence imply that episodic memory capacity is fundamentally conserved across avian and mammalian species? Or does it suggest “episodic-like” memory capacity evolved separately in a few species and thus is the result of convergent evolution? Notably, these important questions cannot be answered by focusing on behavior alone because it is difficult, perhaps impossible, to distinguish between homologous and analogous memory capacities. Here, our objective is to shed light on the potential evolution of episodic memory. We go beyond previous efforts by integrating the behavioral evidence across species with a comparative analysis of the neurobiology and neural mechanisms underlying episodic memory capacity. We also discuss the potential functions of episodic memory in an evolutionary context, as well as species-specific divergences.
Episodic Memory Capacity Across Species
Episodic memory refers to the memory for specific personal experiences. Although accurate, this definition does not capture the considerable challenge associated with distinguishing episodic memory from other memory capacities. A common mistake is to assume that one-trial learning is a sufficient criterion for episodic memory capacity. This is clearly not the case, as nonepisodic memories can be formed after a single exposure [e.g., conditioned taste aversion or familiarity (4, 7, 8)]. In this section, we consider the main approaches used to define and demonstrate episodic memory capacity across species.
Subjective Measures of Episodic Recall.
Because the concept of episodic memory was first studied in cognitive psychology, one approach is to define it in terms of the subjective experience associated with episodic recall. Specifically, Tulving (1) proposed that episodic recall involves the ability to “mentally time travel” to reexperience specific events, a capacity that requires a sense of self, subjective time, and autonoetic awareness (conscious awareness that the experience occurred in the past). Although this definition may capture the phenomenological aspects associated with episodic memory in humans, it relies entirely on verbal reports of subjective mental experiences. Because this definition of episodic memory precludes its investigation in animals, the hypothesis that this capacity is unique to humans lacks falsifiability. The absence of objective measures for episodic memory is also not conducive to rigorous scientific investigation in human studies. A more productive approach to defining episodic memory is to identify fundamental features that can be measured experimentally.
Receiver Operating Characteristics.
The main objective of the receiver operating characteristics (ROC) approach is to use signal detection analyses to characterize recognition memory performance. More specifically, this method can be used to objectively quantify the relative contributions of episodic recollection versus familiarity in a recognition memory task. Although this approach was originally developed for human studies, it was successfully adapted to rodents and provided strong evidence that rodents have recollective and familiarity processes similar to humans (9). However, considerable effort is required to adjust the experimental parameters (e.g., response biases) for each species. Therefore, although this approach has distinct advantages (for a comprehensive review, see refs. 8 and 10), it is unlikely to become widely used across species.
Memory for “Events in Context.”
The events-in-context approach capitalizes on the fact that, in the episodic memory system, information about specific events is tied to the spatial, temporal, and other situational contexts in which they occurred (2, 11, 12). Based on this operational definition, demonstrations that animals can remember events in context (12–14) provided compelling evidence that core properties of episodic memory are present in nonhumans. This capacity is often termed episodic-like to emphasize that, whereas it does not address the phenomenological aspects associated with episodic memory in humans, it satisfies three key behavioral criteria (4):
i) Content: The individual remembers information about the event (“what”) and its context of occurrence (e.g., “where” or “when” it happened).
ii) Structure: The information about the event and its context is integrated in a single representation.
iii) Flexibility: The memory can be expressed to support adaptive behavior in novel situations.
These criteria have provided a solid theoretical framework for behavioral tests of episodic memory. It is important to note that the criteria are usually satisfied using converging evidence from multiple studies, as it is impractical to address them all in every experiment. Here, we examine the three main approaches used to study the memory for events in context: (i) “what-where-when,” (ii) “what-where,” and (iii) “what-when.” The distinct content requirements of these models provide an opportunity to investigate different aspects of episodic memory capacity.
Memory for what-where-when.
An influential animal model of episodic memory took advantage of the natural caching behavior of scrub jays. In an ingenious paradigm, Clayton and Dickinson (12) demonstrated that scrub jays could remember what food they stored (worms or peanuts), as well as where (the location in the cage) and when (4 h or 124 h ago) it was cached, thus fully satisfying the content criterion. Similar evidence of what-where-when memory has also been reported in other bird species, including other corvids [magpies (15)] and noncorvids [black-capped chickadees (16)]. This approach has also been adapted to many mammalian species, including rats (14, 17–19), mice (20), meadow voles (21), pigs (22), nonhuman primates (23, 24), and humans (25, 26). It is important to note that the structure and flexibility criteria have been much less investigated than the content criterion so it remains to be determined whether all these species will meet all three behavioral criteria. As of now, there is evidence for what-where-when integration (structure criterion) in birds (27), rodents (17), and primates (23). Evidence for the flexibility criterion comes from the demonstration that the what-where-when memory can be updated with new information [birds (28, 29); rodents (14)], and that it can be expressed spontaneously [i.e., without training or in response to an unexpected test; birds (30); rodents (19, 20)]. Although this approach has been momentous, leading to the development of a number of animal models of episodic memory, it also has limitations. In particular, the content criterion is very stringent, requiring memory for what, where, and when. On one hand, this is a positive aspect of the model as it established a very high threshold for the first convincing behavioral demonstration of episodic memory in animals. On the other hand, this criterion may be overly restrictive. In fact, there is no clear evidence that all episodic memories contain all three types of information. Therefore, other forms of memory for events in context should also be considered episodic, such as memories involving a subset of the two (e.g., what-where), other types of contextual information [e.g., internal context (31)], or possibly where-when associations [no “what” component (32)].
Memory for what-where.
This approach focuses on the memory for the spatial context of episodic memory, the ability to remember where specific events occurred. It is important to note that this capacity does not simply correspond to spatial memory (memory for “where”), as it requires animals to remember specific what-where associations (i.e., specific items in specific places). In these paradigms, the “what” component refers to the presentation of a specific item (e.g., odor, object). The “where” component varies depending on the species, typically referring to a specific place in an environment in rodent studies, or to a specific location on a screen (or complex visual scene) in primate studies. Tasks involving item-place associations have been used extensively in rats (e.g., refs. 33–36) and nonhuman primates [e.g., item-scene associations (37)], particularly to study the neural basis of episodic memory. Paradigms relying on spontaneous preference, which require no training, have also been developed (e.g., ref. 38). A more detailed review of what-where approaches, including their use as preclinical tests for assessment of cognitive function in animal models of aging and Alzheimer’s disease, is available elsewhere (39).
Memory for what-when.
This approach requires subjects to remember the temporal context in which specific events occurred, a defining feature of episodic memory (1, 2). There are different forms of memory for when events occurred, including memory for the order of events in a sequence, for how long ago events happened, and for the time of day at which they took place (6, 40–43). The vast majority of studies have focused on memory for the order of events, which reflects the capacity of episodic memory to preserve the “flow of events” as they occurred in experience (1, 2). The typical paradigm involves the presentation of a sequence of items (e.g., odors, objects), followed by a choice between two of the presented items. Memory for order is expressed by selecting (e.g., refs. 13 and 44), or preferentially exploring (e.g., ref. 45), the item that appeared earlier in the sequence. Importantly, information about the spatial context is irrelevant to performance. This basic approach has been used in rodents (13, 44), and similar approaches have been developed in nonhuman primates (46–48) and humans (49–51). Notably, the NIH Toolbox Cognition Battery proposes a what-when paradigm, which requires memory for sequences of pictured events, as the new standard measure for episodic memory capacity in humans (for a review, see refs. 39 and 52).
Section Summary.
The evidence reviewed strongly suggests that core properties of episodic memory are present across mammals, as well as in a number of bird species. Although the ROC method has distinct advantages, the memory for the events-in-context approach is more practical and widely used. Therefore, the latter is more appropriate to examine episodic memory capacity across species and shed light on its evolution. What-where-when paradigms have the strictest behavioral criteria and thus are better suited for determining whether a given species has the capacity for episodic memory. In contrast, paradigms that focus on isolating a specific form of contextual information (e.g., what-where, what-when) are promising for investigating the types of contextual information fundamental to episodic memories, as well as elucidating its critical neurobiological substrate (see below). Although no single definition or approach is likely to capture all features of episodic memory, converging evidence from these operational approaches has greatly furthered our understanding of episodic memory across phylogeny.
Brain Structures Important for Episodic Memory
Studies of neurological patients and functional neuroimaging in humans have shown that episodic memory critically depends on the integrity of the hippocampus (3, 53, 54) but also involves a large network of cortical areas that includes the adjacent parahippocampal region and the prefrontal cortex (55, 56). In this section, we review basic anatomical and functional evidence to determine the extent to which these structures are conserved in mammals and birds.
Hippocampus.
The hippocampus has been identified in many species, including a large breadth of mammals (57, 58), birds (59, 60), reptiles [medial cortex, (61)], and teleost fish [dorsolateral telencephalon (61, 62)]. The neurobiological and functional evidence strongly suggests that the hippocampus is a homologous structure across species.
In mammals, the hippocampus is remarkably conserved across species, including humans, nonhuman primates, pigs, rodents, and bats (57, 58). The cytoarchitecture can be easily identified by the dense layers of folded cell bodies that make up hippocampal subregions, including the subiculum, dentate gyrus, and cornu Ammonis (CA) fields (58, 63, 64) (Fig. 1). Major inputs to the hippocampus originate from the entorhinal cortex and synapse on all subfields. Within the hippocampus, the dentate gyrus projects to CA3 through mossy fiber connections. CA3 projects to itself, through recurrent connections, as well as to CA1, through the Schaffer collaterals. The major outputs of the hippocampus originate from CA1 and the subiculum, and terminate in the entorhinal cortex (for a comprehensive account of the hippocampal circuitry, see ref. 64). Additionally, a major anatomical characteristic of the mammalian hippocampus is a connection with the septum, which is conserved across all mammals. The function of the hippocampus is also well conserved across mammalian species. In fact, the hippocampus is critical for spatial memory in rats (reviewed in ref. 65), nonhuman primates (66), and humans (67). Moreover, neurophysiological studies have identified hippocampal neurons that encode specific places in an environment (place cells) in rodents (68–70), nonhuman primates (71, 72), and humans (73), as well as in bats (74).
Fig. 1.
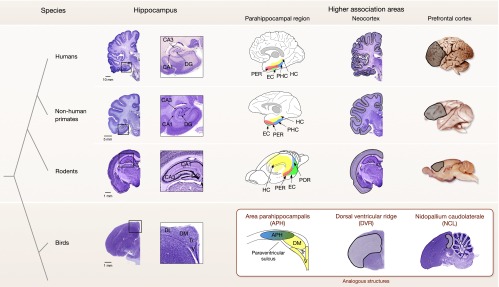
Brain regions important for episodic memory. Anatomical comparison of the hippocampus (avian hippocampus), parahippocampal region (avian area parahippocampalis), associational neocortex (avian dorsal ventricular ridge), and prefrontal cortex (avian nidopallium caudolaterale). The mammalian hippocampus shows distinct subregions, which are less evident in the avian hippocampus. The mammalian parahippocampal region is shown in diagrams (adapted with permission from ref. 81. Copyright Wiley-Liss, Inc.) to highlight the conserved relative spatial locations among species, with similar adjacent locations of area parahippocampalis and hippocampus in birds. Neocortical areas in mammals and associational areas of the dorsal ventricular ridge are outlined. The prefrontal cortex is shown in whole brains in mammals (medial surface in rat) and in a sagittal section in the bird. Human, nonhuman primate (Macaca mulatta) and rodent (Rattus norvegicus) sections were adapted with permission from http://www.brains.rad.msu.edu, and www.brainmuseum.org supported by the US National Science Foundation, and bird (Taeniopygia guttata) sections from http://zebrafinch.brainarchitecture.org. DG, dentate gyrus; DL, dorsolateral region; DM, dorsomedial region; EC, entorhinal cortex, HC, hippocampus; PER, perirhinal cortex; PHC, parahippocampal cortex; POR, postrhinal cortex; Tr, triangular region; V, V-shaped layer.
Birds also have a hippocampus, which arises from the same developmental origin as mammals (59, 60, 75). As in mammals, a hippocampal-septal pathway is a major feature of the avian hippocampus (75, 76). The avian hippocampal subregions are not as visually obvious (Fig. 1) but nonetheless show homologies to those in mammals. Based on anatomical connectivity, Atoji and Wild (60) noted that the dorsomedial area of the hippocampus is similar to the mammalian subiculum and CA regions, whereas the V-shaped layer in the ventromedial portion is similar to the mammalian dentate gyrus. However, a consensus on the exact homologies of hippocampal subregions is lacking (59, 60, 75). Functionally, the avian hippocampus is similar to the mammalian hippocampus. Neurons in the avian hippocampus also show distinct place fields (reviewed in ref. 77), and lesions to the avian hippocampus specifically disrupt spatial memories (78–80). Notably, hippocampal lesions similarly impair spatial memories in turtles and goldfish (61), further evidence that theses functional similarities result from a long neurobiological ancestry.
Parahippocampal Region.
In mammals, the hallmark of cortical–hippocampal connectivity is the existence of associative cortical structures that serve as an interface between the hippocampus and the rest of the neocortex. These associative regions include the entorhinal cortex, perirhinal cortex, and parahippocampal cortex [postrhinal cortex in rodents (81)], which are collectively referred to as the parahippocampal region (Fig. 1). There are two main information processing pathways within the parahippocampal region (Fig. 2A). The “what” pathway, composed of the perirhinal and lateral entorhinal cortex, is important for processing and representing features of specific objects or items. In rodents and primates, this system receives information from all sensory modalities (81–83), is critical for object memory (84–86), and contains neurons that respond to specific objects (47, 87–89). The second pathway processes “where” information and is composed of the parahippocampal/postrhinal cortex and medial entorhinal cortex. This system primarily receives visuospatial information (81, 83). Consistent with a role in processing “where” information, neurons in a subregion of the medial entorhinal cortex fire in a triangular grid pattern as animals explore an environment [grid cells (90)]. Evidence for grid cells has been reported in rodents (90), nonhuman primates (91), and humans (92), as well as in bats (74). Although species differences exist in the information processed by these pathways, the distinct informational segregation is conserved across rats, nonhuman primates, and humans (8, 83, 93).
Fig. 2.
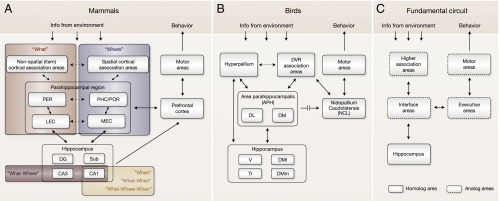
Neural circuits underlying episodic memory capacity in mammalian and avian species. (A) Schematic diagram of neural mechanisms supporting episodic memory encoding and expression in mammals. After information from the environment reaches the neocortex, the processing of “what” and “where” information is divided in parallel streams of cortical association areas. This functional segregation is maintained in the parahippocampal region, where the information is further processed before it reaches the hippocampus. Episodic memories are formed when the hippocampus integrates information about a specific event (what happened) with the context in which it occurred (e.g., where and/or when it happened). Although what-where coding has been shown in regions CA3 and CA1, lesion studies suggest that this type of integration depends specifically on region CA3. Recent evidence suggests that region CA1 provides an internal representation of elapsed time (when), which could support the formation of what-when and what-where-when associations. Episodic recall is thought to occur when the integrated event-in-context representation is reactivated in the hippocampal network, which leads to the reactivation of the associated representations in parahippocampal and neocortical association areas. The process by which the retrieved memories can guide behavior depends on the prefrontal cortex. (B) Comparable circuit in the avian brain. (C) Fundamental circuit hypothesized to support episodic memory across species. Anatomical, behavioral, and physiological evidence demonstrate that this system involves homologous and analogous structures. DG, dentate gyrus; DL, dorsolateral region; DM, dorsomedial region (lateral and medial); DVR, dorsal ventricular ridge; LEC, lateral entorhinal cortex; MEC, medial entorhinal cortex; PER, perirhinal cortex; PHC, parahippocampal cortex; POR, postrhinal cortex; Sub, subiculum; Tr, triangular region; V, V-shaped layer.
In birds, the primary inputs and outputs of the hippocampus originate in the area parahippocampalis (60) (Figs. 1 and 2B). Afferents to area parahippocampalis arise from several locations, including the dorsal ventricular ridge and hyperpallium. Its efferents project back to the same structures and to the V-shaped layer and triangular region of the avian hippocampus. Therefore, the avian hippocampus has access to information from all modalities through the area parahippocampalis (60), much like the mammalian system. However, it is unknown whether the dorsolateral and dorsomedial subregions of area parahippocampalis are involved in segregated informational streams. As in the medial entorhinal cortex in mammals, grid-like cells have been observed near the avian hippocampus although their exact location remains unclear (77).
To summarize, the extent to which the mammalian parahippocampal region and the avian area parahippocampalis are homologous remains to be determined. However, it is clear there are similarities in the circuit organization and functions of these regions across mammals and birds, and especially within mammals.
Prefrontal Cortex.
The size of the prefrontal cortex varies greatly across mammals, especially between primates and rodents (Fig. 1), but there is strong evidence of anatomical and functional correspondence across species (94–96). The prefrontal cortex receives information from most cortical association areas and strongly projects to cortical and subcortical motor regions, suggesting that it plays a key role in the representation and execution of actions (97, 98) (Fig. 2A). The prefrontal cortex is also connected to the hippocampus by a direct pathway from CA1 (99) and indirect connections through the parahippocampal region (81, 82). Importantly, individual prefrontal neurons exhibit delay-related activity in nonhuman primates (reviewed in refs. 97 and 98) and rodents (100), activity that may contribute to working memory, inferential reasoning, and decision-making abilities. These findings are consistent with the view that the prefrontal cortex is the primary executive region of the brain, a structure particularly important for bridging perception, memory, and action (97, 98).
Birds also have an executive region thought to be similar to the mammalian prefrontal cortex, called the nidopallium caudolaterale (101, 102). Importantly, the nidopallium caudolaterale directly projects to motor regions and has indirect access to the hippocampus through the area parahippocampalis (101) (Fig. 2B). Delay-related neuronal activity has also been observed in individual nidopallium caudolaterale neurons (103). However, it is important to note that, despite these similarities to the mammalian prefrontal cortex, the nidopallium caudolaterale is not homologous to its mammalian counterpart [i.e., the similarities are due to convergent evolution (103)].
Section Summary.
In sum, the hippocampus, parahippocampal region, and prefrontal cortex form a neural system that is thought to underlie episodic memory capacities in humans, but this basic neurobiology is not unique to humans. Considerable evidence shows that this circuit is present across mammals and that a comparable circuit exists in the avian brain. Interestingly, regions that are homologous to the hippocampus also exist in reptiles and bony (teleost) fish. Considering the long evolutionary history and structure–function similarities, it seems reasonable to hypothesize that the human episodic memory circuit shares an ancestral protoepisodic memory system with other mammals and possibly birds.
Neural Mechanisms Underlying Episodic Memory
Episodic memory in mammals depends on the hippocampus, the parahippocampal region, and the prefrontal cortex. However, until recently, it was unclear how this network of structures could give rise to episodic memory. In fact, considerable progress has been made in recent years toward understanding the specific contribution of each structure, as well as the nature of their functional relationships. Here, we describe a model, derived primarily from rodent and primates studies, summarizing the neural mechanisms thought to support the encoding and expression of episodic memories in mammals (Fig. 2A).
Processing Information About Events and Elements of Context.
After being processed by sensory receptors and thalamic nuclei, information from the external world reaches primary sensory areas of the neocortex. A hierarchy of association cortical areas then processes this information at increasing levels of complexity and abstraction, culminating in multimodal representations. This information is funneled into the parahippocampal region, which mediates communications between the neocortex and the hippocampus (104).
The processing of “what” (e.g., stimuli, items) and “where” information is generally segregated into parallel streams. This functional segregation is maintained in the parahippocampal region (8, 82, 93): the perirhinal and lateral entorhinal areas play a critical role in item memory (what) whereas the postrhinal and medial entorhinal areas are important for the memory of contextual information (where). In contrast, the neural basis of the memory for “when” is much less understood. Although the hippocampus may play a critical role in processing “when” information under specific conditions (105), this capacity is generally thought to depend on other cortical and subcortical structures [e.g., striatum (106)].
Integrating Event and Context Information.
Before reaching the hippocampus, information about the “what,” “where,” or “when” of individual events is not yet integrated into a single representation and thus does not satisfy the structure criterion for episodic memory. Episodic memory requires the integration of the representation of a single event with its distinctive contextual information, and it is this process that critically depends on the hippocampus (for potential mechanisms, see 107).
What-where integration.
Studies in rodents (33–36) and primates (37) show that the hippocampus plays a critical role in forming specific item–place associations. It is important to note that the spatial layout is already well-learned in these paradigms so deficits after hippocampus lesions cannot be solely attributed to an impairment in processing “where” information. Similarly, the deficits cannot be attributed to a deficiency in processing “what” information, as this capacity is normal in animals with hippocampal damage (13, 33, 86). The integration of what-where information can also be demonstrated in the coding properties of individual hippocampal neurons. A study by Wood et al. (108) showed that different subsets of neurons selectively coded for “what” (e.g., a specific odor) and “where” (e.g., a specific location) information whereas others coded for specific what-where conjunctions (a specific odor in a particular place). More recent studies have shown that the emergence of what-where coding parallels the learning of item–place associations (109, 110). Although lesions studies suggest what-where integration depends on subregion CA3 but not CA1 (34), what-where neural coding has been reported in both subregions with no significant differences reported (108, 109).
What-when integration.
Accumulating evidence suggests that the hippocampus also plays a critical role in forming what-when associations, including memory for the order in which specific events occurred. For instance, in sequence memory paradigms, rats with hippocampal damage were shown to have normal memory for the individual items presented (what) but consistently failed to remember the temporal relationships among events [what-when (13, 44)]. Functional neuroimaging studies have shown that the hippocampus is strongly engaged during performance of similar tasks in humans as well (49–51). Furthermore, recent electrophysiological evidence suggests that a fundamental role of the hippocampus is to provide an internal representation of elapsed time, which could support the formation of what-when memories (47, 111, 112). In fact, recent studies have shown that individual hippocampal neurons exhibit robust timing signals during stimulus-free intervals [“time cells” (111, 113)] and during the presentation of sequences of events (47). In addition, the pattern of activity in hippocampal ensembles has been shown to gradually change over time, a form of population coding that could serve as a timing signal (32, 114). The above lesion and electrophysiological studies provide converging evidence that this capacity primarily depends on subregion CA1 of the hippocampus.
Episodic Recall and Response Selection.
Episodic recall is thought to occur when the integrated event-in-context representation is reactivated, involving a pattern completion process that can be initiated by cueing the hippocampal network with elements of the event or context. This hippocampal reactivation leads to the reactivation of the corresponding representations in the parahippocampal region and other cortical association areas (8, 104). The process by which the retrieved information can guide behavior is thought to critically depend on the prefrontal cortex (97, 98, 115, 116). First, the episode-specific patterns of activity retrieved in the hippocampus are thought to reach the prefrontal cortex, either directly or through the parahippocampal region. The prefrontal cortex then evaluates the retrieved information and plans the appropriate course of action, which is then conveyed to motor regions (97, 98, 115, 116).
Section Summary.
Significant progress has been made in our understanding of the neural circuits underlying episodic memory capacity in mammals. In its essence, the circuit requires higher association areas to process the sensory information (neocortex), interface areas to communicate with the hippocampus (parahippocampal region), the hippocampus to integrate and retrieve information about the episode, and executive areas to produce the appropriate behavior (prefrontal cortex). Although little is known about the neural mechanisms underlying episodic memory in birds, it is important to note that they have a similar circuit that could perform the same fundamental operations. The corresponding system in birds involves a combination of homologous (the hippocampus and, to some degree, the area parahippocampalis) and analogous (dorsal ventricular ridge, nidopallium caudolaterale) structures (Fig. 2B). Therefore, we hypothesize that a fundamental circuit may be shared between species that demonstrate episodic memory abilities (Fig. 2C).
Functions of Episodic Memory Across Species
As we examine the evolution of episodic memory, it is important to consider its potential functions across species. What are its potential contributions to the fitness of an individual? What advantage could it provide? Episodic memory is not necessary for animals to find food, shelter, mates, or to avoid dangerous situations. However, given the dynamic nature of the environment, the ability to remember unique experiences could certainly help animals be more successful. This advantage may be especially beneficial under conditions of limited resources, when incremental gains in the likelihood of success can amount to large effects on long-term survival. As mentioned earlier, our central argument is that fundamental properties of episodic memory, as well as their underlying neural circuits, are shared across mammals and birds. Therefore, some basic functions of episodic memory should be common across species.
Memory-Based Predictions.
The purpose of memory is not to reminisce about the past, but to allow us to think, reason, and plan for the future (117). Along these lines, we propose that the main function of episodic memory is to provide memory-based predictions to support adaptive behavior in the present or immediate future (116). There are two ways in which episodic memory could contribute to this capacity. First, episodic memory is the only memory system to provide spatially and temporally specific information about single experiences. For instance, when faced with a specific need (e.g., a tool), an individual could use episodic memory to make predictions as to how to satisfy this need (e.g., look where the tool was last seen). This unparalleled specificity allows animals to take into account unique events in guiding their behavior and to quickly adapt to changing circumstances. Second, episodic memory could contribute to memory-based predictions by supporting the capacity to make novel inferences. In fact, it has been proposed that a fundamental role of the hippocampus is to integrate episodic and semantic memories into a relational (declarative) memory network (116, 118). Because many of our memories overlap in information content, the network is thought to represent relationships among memories by linking them using their common elements. This network structure could support the flexible expression of inferred relationships between elements that were never experienced together, such as deducing a novel trajectory between two locations or the social hierarchy among a group of individuals. It should be noted that nondeclarative memory abilities also extract regularities from the environment to support the ability to generalize to other situations, but the process requires multiple exposures and lacks flexibility of expression (i.e., is tied to specific cues).
Planning for the Distant Future.
Future planning involves making predictions about the distant future (many hours ahead) to anticipate future needs, an extension of the capacity for memory-based predictions described above. In humans, future planning involves “episodic future thought,” the ability to simulate plausible future events or scenarios [e.g., imagining future activities to determine what to pack for an upcoming trip (119–121)]. Interestingly, there is considerable overlap between the neural circuits involved in retrieving episodic memories and those involved in simulating future events, suggesting that the two capacities are intrinsically linked (119–121). Does this capacity for future planning extend beyond humans? Any attempt to examine future planning in animals must address the Bischof-Köhler hypothesis, which states that only humans can dissociate themselves from their current motivational state and take action for future needs (122). The criteria for demonstrating future planning in animals are as follows: (i) the behavior involved should be a novel action or combination of actions, (ii) the action should be appropriate for the future motivational state, and (iii) the anticipatory action should not have been extensively reinforced (4, 122–124). The first study satisfying all criteria has been conducted in scrub jays. In this study, the birds demonstrated the ability to make provisions for a future need, thus showing that they could dissociate themselves from their current motivational state and spontaneously plan for the next day (123). Accumulating evidence suggests that apes are also capable of future planning, as they can save tools for future use (125) and can override immediate drives in favor of future needs (126, 127). Although it is clear that the behavior of other animals can be future-oriented or based on future consequences [e.g., selecting an item to receive a reward (41)], it remains to be determined whether animals other than humans, apes, and scrub jays are capable of future planning.
Building Social Relationships and Networks.
Episodic memory could be particularly useful for processing and using social information. Although some aspects of social information are static (e.g., who is related to whom?), others can change over time (e.g., who has been cooperative? who has been aggressive?) and thus could depend on the capacity to remember specific experiences. Interestingly, the species in which episodic memory capacity has been most convincingly demonstrated (primates, rodents, and scrub jays) are highly social (128, 129). Recent evidence suggests that humans with episodic memory impairments have social circles that are limited compared with controls, suggesting that episodic memory may be crucial for establishing and/or maintaining social bonds (130). Thus, there may be a relationship between episodic memory capacity and social interactions.
Species-Specific Uses of Episodic Memory.
Although we have so far emphasized the commonalities in episodic memory capacity, there are also clear differences across species. These divergences include unique uses of episodic memory, as well as species-specific attributes. For instance, in humans, episodic memory is thought to be intrinsically tied to other mental capacities such as language, a sense of self, empathy, and theory of mind (1, 56, 131). Although such characteristics were initially used as evidence that episodic memory is unique to humans, according to the present conceptual framework, they represent species-specific attributes (or modules) associated with the expansion of neocortical (particularly prefrontal) areas in humans. Other species-specific uses of episodic memory may include meadow voles predicting when and where a sexually receptive females will be located (21) and hummingbirds keeping track of the location, quality, and renewal rate of different sources of nectar (132). Episodic memory may be of particular importance in hummingbirds because of the enormous energy cost in gathering nectar, which makes repeat visits or poor planning highly detrimental (132).
Section Summary.
Given that several species demonstrate episodic memory capacity, it is reasonable to assume that it offers significant advantages. Some of these benefits could be common across species; others may be species-specific. However, further research is needed before we can understand the specific nature of these advantages or establish that they are causally linked with an increase in fitness.
Conclusions
Episodic memory is the remarkable capacity to remember specific personal experiences. Although it was originally thought that this capacity was particular to humans, the ample evidence reviewed here indicates that core properties of episodic memory are present across mammals, as well as in birds. This cross-species approach to episodic memory research is made possible by the use of operational definitions that can be applied across species, a method we strongly suggest should be used in animal and human studies. The most common approach to investigate episodic memory capacity across species is to determine whether animals can remember events within the context in which they occurred (e.g., memory for what-where-when, what-where, or what-when). Using this conceptual framework, we showed that episodic memory in mammals depends on a functional relationship between the hippocampus, parahippocampal region, neocortical association areas, and prefrontal cortex. Importantly, we described a comparable neural circuit in birds, which includes homologous (hippocampus, and to some degree, parahippocampal region) and analogous (dorsal ventricular ridge association areas, nidopallium caudolaterale “prefrontal” area) structures. Finally, we submit that this fundamental circuit underlies episodic memory capacity across species but that species-specific divergences have also evolved around this central architecture.
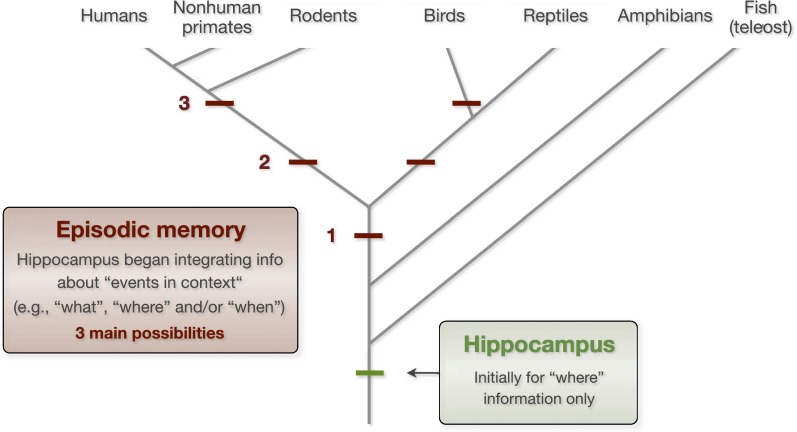
When did episodic memory emerge? Unfortunately, the available evidence cannot support a definitive answer at this time. We speculate that it evolved at a stage when the hippocampus was already present because the hippocampus is known to be a critical substrate. However, we are not implying a one-to-one relationship between the hippocampus and episodic memory (e.g., if the hippocampus is present, the animal has episodic memory capacity). Because the hippocampus is essential for spatial memory across species, ranging from humans to teleost fish, it is likely that its role was limited to the processing of “where” information when it first emerged (Fig. 3). We propose that episodic memory capacity emerged at a later time, when the hippocampus began supporting the integration of information about events in context (e.g., “what”, “where,” and/or “when” information). As the neural architecture of the hippocampus indicates, the content of its associations is determined by its inputs. Thus, the change to supporting episodic memory likely occurred when the hippocampus began receiving highly processed event and contextual information from higher association areas. In light of the cross-species behavioral and neurobiological similarities reviewed here, it is tempting to conclude that episodic memory capacity emerged before mammals and reptiles diverged (possibility 1 in Fig. 3). However, because of the limited data available in nonavian reptiles, the hypothesis that it resulted from convergent evolution (e.g., possibilities 2 and 3 in Fig. 3) cannot be rejected at this time. Addressing this important issue will require converging evidence from anatomical, behavioral, and neurobiological studies in different avian and reptilian species.
Fig. 3.
Possible time points for the emergence of episodic memory in evolution. Initially, the role of hippocampus was likely limited to the processing of spatial information (where). We hypothesize that episodic memory capacity emerged later on, when the hippocampus began supporting the integration of information about events in context (e.g., “what”, “where,” and/or “when” information). The striking behavioral and neurobiological similarities reviewed in this paper suggest that episodic memory capacity emerged before mammals and reptiles diverged (possibility 1). However, additional evidence from birds and reptiles is needed before the alternative hypothesis that episodic memory is the result of convergent evolution (e.g., possibilities 2 and 3) can be safely rejected.
Acknowledgments
This work was supported by NSF CAREER award IOS-1150292, The Feinberg Foundation, and the University of California, Irvine.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VII: The Human Mental Machinery,” held January 10–12, 2013, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vii.
This article is a PNAS Direct Submission.
References
- 1.Tulving E. Episodic memory: From mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- 2.Tulving E. 1972. Episodic and semantic memory. Organization of Memory, eds Tulving E, Donaldson W (Academic, New York), pp 381–402.
- 3.Tulving E, Markowitsch HJ. Episodic and declarative memory: Role of the hippocampus. Hippocampus. 1998;8(3):198–204. doi: 10.1002/(SICI)1098-1063(1998)8:3<198::AID-HIPO2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Clayton NS, Bussey TJ, Dickinson A. Can animals recall the past and plan for the future? Nat Rev Neurosci. 2003;4(8):685–691. doi: 10.1038/nrn1180. [DOI] [PubMed] [Google Scholar]
- 5.Eichenbaum H, Fortin NJ, Ergorul C, Wright SP, Agster KL. Episodic recollection in animals: “If it walks like a duck and quacks like a duck…. Learn Motiv. 2005;36(2):190–207. [Google Scholar]
- 6.Crystal JD. Episodic-like memory in animals. Behav Brain Res. 2010;215(2):235–243. doi: 10.1016/j.bbr.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morris RGM. Episodic-like memory in animals: Psychological criteria, neural mechanisms and the value of episodic-like tasks to investigate animal models of neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci. 2001;356(1413):1453–1465. doi: 10.1098/rstb.2001.0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fortin NJ, Wright SP, Eichenbaum H. Recollection-like memory retrieval in rats is dependent on the hippocampus. Nature. 2004;431(7005):188–191. doi: 10.1038/nature02853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eichenbaum H, Fortin N, Sauvage M, Robitsek RJ, Farovik A. An animal model of amnesia that uses Receiver Operating Characteristics (ROC) analysis to distinguish recollection from familiarity deficits in recognition memory. Neuropsychologia. 2010;48(8):2281–2289. doi: 10.1016/j.neuropsychologia.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishkin M, Suzuki WA, Gadian DG, Vargha-Khadem F. Hierarchical organization of cognitive memory. Philos Trans R Soc Lond B Biol Sci. 1997;352(1360):1461–1467. doi: 10.1098/rstb.1997.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clayton NS, Dickinson A. Episodic-like memory during cache recovery by scrub jays. Nature. 1998;395(6699):272–274. doi: 10.1038/26216. [DOI] [PubMed] [Google Scholar]
- 13.Fortin NJ, Agster KL, Eichenbaum HB. Critical role of the hippocampus in memory for sequences of events. Nat Neurosci. 2002;5(5):458–462. doi: 10.1038/nn834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babb SJ, Crystal JD. Episodic-like memory in the rat. Curr Biol. 2006;16(13):1317–1321. doi: 10.1016/j.cub.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Zinkivskay A, Nazir F, Smulders TV. What-where-when memory in magpies (Pica pica) Anim Cogn. 2009;12(1):119–125. doi: 10.1007/s10071-008-0176-x. [DOI] [PubMed] [Google Scholar]
- 16.Feeney MC, Roberts WA, Sherry DF. Memory for what, where, and when in the black-capped chickadee (Poecile atricapillus) Anim Cogn. 2009;12(6):767–777. doi: 10.1007/s10071-009-0236-x. [DOI] [PubMed] [Google Scholar]
- 17.Ergorul C, Eichenbaum H. The hippocampus and memory for “what,” “where,” and “when”. Learn Mem. 2004;11(4):397–405. doi: 10.1101/lm.73304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eacott MJ, Easton A, Zinkivskay A. Recollection in an episodic-like memory task in the rat. Learn Mem. 2005;12(3):221–223. doi: 10.1101/lm.92505. [DOI] [PubMed] [Google Scholar]
- 19.Kart-Teke E, De Souza Silva MA, Huston JP, Dere E. Wistar rats show episodic-like memory for unique experiences. Neurobiol Learn Mem. 2006;85(2):173–182. doi: 10.1016/j.nlm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Dere E, Huston JP, De Souza Silva MA. Integrated memory for objects, places, and temporal order: Evidence for episodic-like memory in mice. Neurobiol Learn Mem. 2005;84(3):214–221. doi: 10.1016/j.nlm.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Ferkin MH, Combs A, delBarco-Trillo J, Pierce AA, Franklin S. Meadow voles, Microtus pennsylvanicus, have the capacity to recall the “what”, “where”, and “when” of a single past event. Anim Cogn. 2008;11(1):147–159. doi: 10.1007/s10071-007-0101-8. [DOI] [PubMed] [Google Scholar]
- 22.Kouwenberg A-L, Walsh CJ, Morgan BE, Martin GM. Episodic-like memory in crossbred Yucatan minipigs (Sus scrofa) Appl Anim Behav Sci. 2009;117:165–172. [Google Scholar]
- 23.Hoffman ML, Beran MJ, Washburn DA. Memory for “what”, “where”, and “when” information in rhesus monkeys (Macaca mulatta) J Exp Psychol Anim Behav Process. 2009;35(2):143–152. doi: 10.1037/a0013295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin-Ordas G, Haun D, Colmenares F, Call J. Keeping track of time: Evidence for episodic-like memory in great apes. Anim Cogn. 2010;13(2):331–340. doi: 10.1007/s10071-009-0282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland SM, Smulders TV. Do humans use episodic memory to solve a What-Where-When memory task? Anim Cogn. 2011;14(1):95–102. doi: 10.1007/s10071-010-0346-5. [DOI] [PubMed] [Google Scholar]
- 26.Hayne H, Imuta K. Episodic memory in 3- and 4-year-old children. Dev Psychobiol. 2011;53(3):317–322. doi: 10.1002/dev.20527. [DOI] [PubMed] [Google Scholar]
- 27.Clayton NS, Griffiths DP, Emery NJ, Dickinson A. Elements of episodic-like memory in animals. Philos Trans R Soc Lond B Biol Sci. 2001;356(1413):1483–1491. doi: 10.1098/rstb.2001.0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clayton NS, Dickinson A. Memory for the content of caches by scrub jays (Aphelocoma coerulescens) J Exp Psychol Anim Behav Process. 1999;25(1):82–91. [PubMed] [Google Scholar]
- 29.Clayton NS, Yu KS, Dickinson A. Interacting Cache memories: Evidence for flexible memory use by Western Scrub-Jays (Aphelocoma californica) J Exp Psychol Anim Behav Process. 2003;29(1):14–22. [PubMed] [Google Scholar]
- 30.Singer RA, Zentall TR. Pigeons learn to answer the question “where did you just peck?” and can report peck location when unexpectedly asked. Learn Behav. 2007;35(3):184–189. doi: 10.3758/bf03193054. [DOI] [PubMed] [Google Scholar]
- 31.Kennedy PJ, Shapiro ML. Retrieving memories via internal context requires the hippocampus. J Neurosci. 2004;24(31):6979–6985. doi: 10.1523/JNEUROSCI.1388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mankin EA, et al. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci USA. 2012;109(47):19462–19467. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert PE, Kesner RP. Role of the rodent hippocampus in paired-associate learning involving associations between a stimulus and a spatial location. Behav Neurosci. 2002;116(1):63–71. doi: 10.1037//0735-7044.116.1.63. [DOI] [PubMed] [Google Scholar]
- 34.Gilbert PE, Kesner RP. Localization of function within the dorsal hippocampus: The role of the CA3 subregion in paired-associate learning. Behav Neurosci. 2003;117(6):1385–1394. doi: 10.1037/0735-7044.117.6.1385. [DOI] [PubMed] [Google Scholar]
- 35.Day M, Langston RF, Morris RG. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003;424(6945):205–209. doi: 10.1038/nature01769. [DOI] [PubMed] [Google Scholar]
- 36.Rajji T, Chapman D, Eichenbaum H, Greene R. The role of CA3 hippocampal NMDA receptors in paired associate learning. J Neurosci. 2006;26(3):908–915. doi: 10.1523/JNEUROSCI.4194-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaffan D. Scene-specific memory for objects: A model of episodic memory impairment in monkeys with fornix transection. J Cogn Neurosci. 1994;6:305–320. doi: 10.1162/jocn.1994.6.4.305. [DOI] [PubMed] [Google Scholar]
- 38.Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: Evidence of object-location and object-context recognition. Behav Brain Res. 1999;99(2):191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- 39.Snigdha S, et al. A preclinical cognitive test battery to parallel the National Institute of Health Toolbox in humans: Bridging the translational gap. Neurobiol Aging. 2013;34(7):1891–1901. doi: 10.1016/j.neurobiolaging.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Friedman WJ. Memory for the time of past events. Psychol Bull. 1993;113:44–66. [Google Scholar]
- 41.Roberts WA. Are animals stuck in time? Psychol Bull. 2002;128(3):473–489. doi: 10.1037/0033-2909.128.3.473. [DOI] [PubMed] [Google Scholar]
- 42.Eichenbaum H, Fortin N. Episodic memory and the hippocampus: It's about time. Curr Dir Psychol Sci. 2003;12:53–57. [Google Scholar]
- 43.Eacott MJ, Easton A. Episodic memory in animals: Remembering which occasion. Neuropsychologia. 2010;48(8):2273–2280. doi: 10.1016/j.neuropsychologia.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Kesner RP, Gilbert PE, Barua LA. The role of the hippocampus in memory for the temporal order of a sequence of odors. Behav Neurosci. 2002;116(2):286–290. doi: 10.1037//0735-7044.116.2.286. [DOI] [PubMed] [Google Scholar]
- 45.Hannesson DK, Howland JG, Phillips AG. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci. 2004;24(19):4596–4604. doi: 10.1523/JNEUROSCI.5517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petrides M. Impairments on nonspatial self-ordered and externally ordered working memory tasks after lesions of the mid-dorsal part of the lateral frontal cortex in the monkey. J Neurosci. 1995;15(1 Pt 1):359–375. doi: 10.1523/JNEUROSCI.15-01-00359.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Naya Y, Suzuki WA. Integrating what and when across the primate medial temporal lobe. Science. 2011;333(6043):773–776. doi: 10.1126/science.1206773. [DOI] [PubMed] [Google Scholar]
- 48.Templer VL, Hampton RR. Cognitive mechanisms of memory for order in rhesus monkeys (Macaca mulatta) Hippocampus. 2013;23(3):193–201. doi: 10.1002/hipo.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kumaran D, Maguire EA. An unexpected sequence of events: Mismatch detection in the human hippocampus. PLoS Biol. 2006;4(12):e424. doi: 10.1371/journal.pbio.0040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lehn H, et al. A specific role of the human hippocampus in recall of temporal sequences. J Neurosci. 2009;29(11):3475–3484. doi: 10.1523/JNEUROSCI.5370-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ross RS, Brown TI, Stern CE. The retrieval of learned sequences engages the hippocampus: Evidence from fMRI. Hippocampus. 2009;19(9):790–799. doi: 10.1002/hipo.20558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weintraub S, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(Suppl 3):S54–S64. doi: 10.1212/WNL.0b013e3182872ded. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vargha-Khadem F, et al. Differential effects of early hippocampal pathology on episodic and semantic memory. Science. 1997;277(5324):376–380. doi: 10.1126/science.277.5324.376. [DOI] [PubMed] [Google Scholar]
- 54.Eichenbaum H, Fortin NJ. Bridging the gap between brain and behavior: Cognitive and neural mechanisms of episodic memory. J Exp Anal Behav. 2005;84(3):619–629. doi: 10.1901/jeab.2005.80-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11(5):219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 56.Schacter DL, Addis DR, Buckner RL. Remembering the past to imagine the future: The prospective brain. Nat Rev Neurosci. 2007;8(9):657–661. doi: 10.1038/nrn2213. [DOI] [PubMed] [Google Scholar]
- 57.Insausti R. Comparative anatomy of the entorhinal cortex and hippocampus in mammals. Hippocampus. 1993;3(Spec No):19–26. [PubMed] [Google Scholar]
- 58.Manns JR, Eichenbaum H. Evolution of declarative memory. Hippocampus. 2006;16(9):795–808. doi: 10.1002/hipo.20205. [DOI] [PubMed] [Google Scholar]
- 59.Székely AD. The avian hippocampal formation: subdivisions and connectivity. Behav Brain Res. 1999;98(2):219–225. doi: 10.1016/s0166-4328(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 60.Atoji Y, Wild JM. Anatomy of the avian hippocampal formation. Rev Neurosci. 2006;17(1-2):3–15. doi: 10.1515/revneuro.2006.17.1-2.3. [DOI] [PubMed] [Google Scholar]
- 61.Rodríguez F, et al. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J Neurosci. 2002;22(7):2894–2903. doi: 10.1523/JNEUROSCI.22-07-02894.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Broglio C, et al. Hallmarks of a common forebrain vertebrate plan: Specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish. Brain Res Bull. 2005;66(4-6):277–281. doi: 10.1016/j.brainresbull.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 63.Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31(3):571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- 64.van Strien NM, Cappaert NLM, Witter MP. The anatomy of memory: An interactive overview of the parahippocampal-hippocampal network. Nat Rev Neurosci. 2009;10(4):272–282. doi: 10.1038/nrn2614. [DOI] [PubMed] [Google Scholar]
- 65.O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon Press; 1978. [Google Scholar]
- 66.Banta Lavenex P, Lavenex P. Spatial memory and the monkey hippocampus: Not all space is created equal. Hippocampus. 2009;19(1):8–19. doi: 10.1002/hipo.20485. [DOI] [PubMed] [Google Scholar]
- 67.Burgess N, Maguire EA, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 68.O’Keefe J, Dostrovsky J. The hippocampus as a spatial map: Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 69.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261(5124):1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 70.Knierim JJ, Lee I, Hargreaves EL. Hippocampal place cells: Parallel input streams, subregional processing, and implications for episodic memory. Hippocampus. 2006;16(9):755–764. doi: 10.1002/hipo.20203. [DOI] [PubMed] [Google Scholar]
- 71.Nishijo H, Ono T, Eifuku S, Tamura R. The relationship between monkey hippocampus place-related neural activity and action in space. Neurosci Lett. 1997;226(1):57–60. doi: 10.1016/s0304-3940(97)00255-3. [DOI] [PubMed] [Google Scholar]
- 72.Matsumura N, et al. Spatial- and task-dependent neuronal responses during real and virtual translocation in the monkey hippocampal formation. J Neurosci. 1999;19(6):2381–2393. doi: 10.1523/JNEUROSCI.19-06-02381.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ekstrom AD, et al. Cellular networks underlying human spatial navigation. Nature. 2003;425(6954):184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- 74.Yartsev MM, Witter MP, Ulanovsky N. Grid cells without theta oscillations in the entorhinal cortex of bats. Nature. 2011;479(7371):103–107. doi: 10.1038/nature10583. [DOI] [PubMed] [Google Scholar]
- 75.Rattenborg NC, Martinez-Gonzalez D. A bird-brain view of episodic memory. Behav Brain Res. 2011;222(1):236–245. doi: 10.1016/j.bbr.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 76.Atoji Y, Wild JM. Fiber connections of the hippocampal formation and septum and subdivisions of the hippocampal formation in the pigeon as revealed by tract tracing and kainic acid lesions. J Comp Neurol. 2004;475(3):426–461. doi: 10.1002/cne.20186. [DOI] [PubMed] [Google Scholar]
- 77.Bingman VP, Sharp PE. Neuronal implementation of hippocampal-mediated spatial behavior: A comparative evolutionary perspective. Behav Cogn Neurosci Rev. 2006;5(2):80–91. doi: 10.1177/1534582306289578. [DOI] [PubMed] [Google Scholar]
- 78.Colombo M, Cawley S, Broadbent N. The effects of hippocampal and area parahippocampalis lesions in pigeons. II. Concurrent discrimination and spatial memory. Q J Exp Psychol B. 1997;50(2):172–189. doi: 10.1080/713932649. [DOI] [PubMed] [Google Scholar]
- 79.Gagliardo A, Ioalé P, Bingman VP. Homing in pigeons: The role of the hippocampal formation in the representation of landmarks used for navigation. J Neurosci. 1999;19(1):311–315. doi: 10.1523/JNEUROSCI.19-01-00311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hampton RR, Shettleworth SJ. Hippocampal lesions impair memory for location but not color in passerine birds. Behav Neurosci. 1996;110(4):831–835. doi: 10.1037//0735-7044.110.4.831. [DOI] [PubMed] [Google Scholar]
- 81.Furtak SC, Wei S-M, Agster KL, Burwell RD. Functional neuroanatomy of the parahippocampal region in the rat: The perirhinal and postrhinal cortices. Hippocampus. 2007;17(9):709–722. doi: 10.1002/hipo.20314. [DOI] [PubMed] [Google Scholar]
- 82.Lavenex P, Amaral DG. Hippocampal-neocortical interaction: A hierarchy of associativity. Hippocampus. 2000;10(4):420–430. doi: 10.1002/1098-1063(2000)10:4<420::AID-HIPO8>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 83.Suzuki WA, Amaral DG. Functional neuroanatomy of the medial temporal lobe memory system. Cortex. 2004;40(1):220–222. doi: 10.1016/s0010-9452(08)70958-4. [DOI] [PubMed] [Google Scholar]
- 84.Brown MW, Aggleton JP. Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nat Rev Neurosci. 2001;2(1):51–61. doi: 10.1038/35049064. [DOI] [PubMed] [Google Scholar]
- 85.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 86.Feinberg LM, Allen TA, Ly D, Fortin NJ. Recognition memory for social and non-social odors: Differential effects of neurotoxic lesions to the hippocampus and perirhinal cortex. Neurobiol Learn Mem. 2012;97(1):7–16. doi: 10.1016/j.nlm.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 87.Allen TA, Furtak SC, Brown TH. Single-unit responses to 22 kHz ultrasonic vocalizations in rat perirhinal cortex. Behav Brain Res. 2007;182(2):327–336. doi: 10.1016/j.bbr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deshmukh SS, Johnson JL, Knierim JJ. Perirhinal cortex represents nonspatial, but not spatial, information in rats foraging in the presence of objects: Comparison with lateral entorhinal cortex. Hippocampus. 2012;22(10):2045–2058. doi: 10.1002/hipo.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fried I, Cameron KA, Yashar S, Fong R, Morrow JW. Inhibitory and excitatory responses of single neurons in the human medial temporal lobe during recognition of faces and objects. Cereb Cortex. 2002;12(6):575–584. doi: 10.1093/cercor/12.6.575. [DOI] [PubMed] [Google Scholar]
- 90.Fyhn M, Molden S, Witter MP, Moser EI, Moser M-B. Spatial representation in the entorhinal cortex. Science. 2004;305(5688):1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- 91.Killian NJ, Jutras MJ, Buffalo EA. A map of visual space in the primate entorhinal cortex. Nature. 2012;491(7426):761–764. doi: 10.1038/nature11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doeller CF, Barry C, Burgess N. Evidence for grid cells in a human memory network. Nature. 2010;463(7281):657–661. doi: 10.1038/nature08704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burwell RD. The parahippocampal region: Corticocortical connectivity. Ann N Y Acad Sci. 2000;911:25–42. doi: 10.1111/j.1749-6632.2000.tb06717.x. [DOI] [PubMed] [Google Scholar]
- 94.Kesner RP. Neural mediation of memory for time: Role of the hippocampus and medial prefrontal cortex. Psychon Bull Rev. 1998;5:585–596. [Google Scholar]
- 95.Brown VJ, Bowman EM. Rodent models of prefrontal cortical function. Trends Neurosci. 2002;25(7):340–343. doi: 10.1016/s0166-2236(02)02164-1. [DOI] [PubMed] [Google Scholar]
- 96.Uylings HBM, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146(1-2):3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 97.Fuster JM. The prefrontal cortex—an update: Time is of the essence. Neuron. 2001;30(2):319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 98.Goldman-Rakic PS. Regional and cellular fractionation of working memory. Proc Natl Acad Sci USA. 1996;93(24):13473–13480. doi: 10.1073/pnas.93.24.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Verwer RWH, Meijer RJ, Van Uum HFM, Witter MP. Collateral projections from the rat hippocampal formation to the lateral and medial prefrontal cortex. Hippocampus. 1997;7(4):397–402. doi: 10.1002/(SICI)1098-1063(1997)7:4<397::AID-HIPO5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 100.Jung MW, Qin Y, McNaughton BL, Barnes CA. Firing characteristics of deep layer neurons in prefrontal cortex in rats performing spatial working memory tasks. Cereb Cortex. 1998;8(5):437–450. doi: 10.1093/cercor/8.5.437. [DOI] [PubMed] [Google Scholar]
- 101.Güntürkün O. The avian ‘prefrontal cortex’ and cognition. Curr Opin Neurobiol. 2005;15(6):686–693. doi: 10.1016/j.conb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 102.Herold C, et al. The receptor architecture of the pigeons’ nidopallium caudolaterale: An avian analogue to the mammalian prefrontal cortex. Brain Struct Funct. 2011;216(3):239–254. doi: 10.1007/s00429-011-0301-5. [DOI] [PubMed] [Google Scholar]
- 103.Rose J, Colombo M. Neural correlates of executive control in the avian brain. PLoS Biol. 2005;3(6):e190. doi: 10.1371/journal.pbio.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6(6):654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 105.Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behav Neurosci. 1984;98(1):3–22. doi: 10.1037//0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- 106.Buhusi CV, Meck WH. What makes us tick? Functional and neural mechanisms of interval timing. Nat Rev Neurosci. 2005;6(10):755–765. doi: 10.1038/nrn1764. [DOI] [PubMed] [Google Scholar]
- 107.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16(2):130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wood ER, Dudchenko PA, Eichenbaum H. The global record of memory in hippocampal neuronal activity. Nature. 1999;397(6720):613–616. doi: 10.1038/17605. [DOI] [PubMed] [Google Scholar]
- 109.Komorowski RW, Manns JR, Eichenbaum H. Robust conjunctive item-place coding by hippocampal neurons parallels learning what happens where. J Neurosci. 2009;29(31):9918–9929. doi: 10.1523/JNEUROSCI.1378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim J, Delcasso S, Lee I. Neural correlates of object-in-place learning in hippocampus and prefrontal cortex. J Neurosci. 2011;31(47):16991–17006. doi: 10.1523/JNEUROSCI.2859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H. Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron. 2011;71(4):737–749. doi: 10.1016/j.neuron.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shapiro ML. Memory time. Neuron. 2011;71(4):571–573. doi: 10.1016/j.neuron.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 113.Pastalkova E, Itskov V, Amarasingham A, Buzsáki G. Internally generated cell assembly sequences in the rat hippocampus. Science. 2008;321(5894):1322–1327. doi: 10.1126/science.1159775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manns JR, Howard MW, Eichenbaum H. Gradual changes in hippocampal activity support remembering the order of events. Neuron. 2007;56(3):530–540. doi: 10.1016/j.neuron.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ninokura Y, Mushiake H, Tanji J. Representation of the temporal order of visual objects in the primate lateral prefrontal cortex. J Neurophysiol. 2003;89(5):2868–2873. doi: 10.1152/jn.00647.2002. [DOI] [PubMed] [Google Scholar]
- 116.Eichenbaum H, Fortin NJ. The neurobiology of memory based predictions. Philos Trans R Soc Lond B Biol Sci. 2009;364(1521):1183–1191. doi: 10.1098/rstb.2008.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.McGaugh JL. Making lasting memories: Remembering the significant. Proc Natl Acad Sci USA. 2013;110:10402–10407. doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23(2):209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 119.Hassabis D, Kumaran D, Vann SD, Maguire EA. Patients with hippocampal amnesia cannot imagine new experiences. Proc Natl Acad Sci USA. 2007;104(5):1726–1731. doi: 10.1073/pnas.0610561104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Szpunar KK, Watson JM, McDermott KB. Neural substrates of envisioning the future. Proc Natl Acad Sci USA. 2007;104(2):642–647. doi: 10.1073/pnas.0610082104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Addis DR, Wong AT, Schacter DL. Remembering the past and imagining the future: Common and distinct neural substrates during event construction and elaboration. Neuropsychologia. 2007;45(7):1363–1377. doi: 10.1016/j.neuropsychologia.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Suddendorf T, Corballis MC. The evolution of foresight: What is mental time travel, and is it unique to humans? Behav Brain Sci. 2007;30(3):299–313. doi: 10.1017/S0140525X07001975. discussion 313–351. [DOI] [PubMed] [Google Scholar]
- 123.Raby CR, Alexis DM, Dickinson A, Clayton NS. Planning for the future by western scrub-jays. Nature. 2007;445(7130):919–921. doi: 10.1038/nature05575. [DOI] [PubMed] [Google Scholar]
- 124.Shettleworth SJ. Animal behaviour: Planning for breakfast. Nature. 2007;445(7130):825–826. doi: 10.1038/445825a. [DOI] [PubMed] [Google Scholar]
- 125.Mulcahy NJ, Call J. Apes save tools for future use. Science. 2006;312(5776):1038–1040. doi: 10.1126/science.1125456. [DOI] [PubMed] [Google Scholar]
- 126.Naqshbandi M, Roberts WA. Anticipation of future events in squirrel monkeys (Saimiri sciureus) and rats (Rattus norvegicus): Tests of the Bischof-Kohler hypothesis. J Comp Psychol. 2006;120(4):345–357. doi: 10.1037/0735-7036.120.4.34. [DOI] [PubMed] [Google Scholar]
- 127.Osvath M, Osvath H. Chimpanzee (Pan troglodytes) and orangutan (Pongo abelii) forethought: Self-control and pre-experience in the face of future tool use. Anim Cogn. 2008;11(4):661–674. doi: 10.1007/s10071-008-0157-0. [DOI] [PubMed] [Google Scholar]
- 128.Emery NJ, Clayton NS. The mentality of crows: Convergent evolution of intelligence in corvids and apes. Science. 2004;306(5703):1903–1907. doi: 10.1126/science.1098410. [DOI] [PubMed] [Google Scholar]
- 129.Brennan PA, Kendrick KM. Mammalian social odours: Attraction and individual recognition. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2061–2078. doi: 10.1098/rstb.2006.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Davidson PS, Drouin H, Kwan D, Moscovitch M, Rosenbaum RS. Memory as social glue: Close interpersonal relationships in amnesic patients. Front Psychol. 2012;3:531. doi: 10.3389/fpsyg.2012.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Duff MC, Brown-Schmidt S. The hippocampus and the flexible use and processing of language. Front Hum Neurosci. 2012;6:69. doi: 10.3389/fnhum.2012.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.González-Gómez PL, Bozinovic F, Vásquez RA. Elements of episodic-like memory in free-living hummingbirds, energetic consequences. Anim Behav. 2011;81:1257–1262. [Google Scholar]