Abstract
On average, we urban dwellers spend about 90% of our time indoors, and share the intuition that the physical features of the places we live and work in influence how we feel and act. However, there is surprisingly little research on how architecture impacts behavior, much less on how it influences brain function. To begin closing this gap, we conducted a functional magnetic resonance imaging study to examine how systematic variation in contour impacts aesthetic judgments and approach-avoidance decisions, outcome measures of interest to both architects and users of spaces alike. As predicted, participants were more likely to judge spaces as beautiful if they were curvilinear than rectilinear. Neuroanatomically, when contemplating beauty, curvilinear contour activated the anterior cingulate cortex exclusively, a region strongly responsive to the reward properties and emotional salience of objects. Complementing this finding, pleasantness—the valence dimension of the affect circumplex—accounted for nearly 60% of the variance in beauty ratings. Furthermore, activation in a distributed brain network known to underlie the aesthetic evaluation of different types of visual stimuli covaried with beauty ratings. In contrast, contour did not affect approach-avoidance decisions, although curvilinear spaces activated the visual cortex. The results suggest that the well-established effect of contour on aesthetic preference can be extended to architecture. Furthermore, the combination of our behavioral and neural evidence underscores the role of emotion in our preference for curvilinear objects in this domain.
Keywords: neuroaesthetics, design, curvature, habitat theory
On average, Americans spend approximately 90% of their time indoors (1), and there is evidence to suggest that a similar pattern exists worldwide (2). Coupled with our intuition that the physical features of the built environments in which we live and work influence our psychological states, one would expect to find a large empirical literature linking variations in physical features of architecture to psychological states. However, despite some evidence supporting the impact of specific physical architectural features (e.g., building facades and height) on perceptions and preferences (3, 4), there is surprisingly little systematic research on this relationship. One reason for this gap in research could be methodological. Arguably, built environments in their common form do not reduce to a few easily manipulated variables in a laboratory. This limitation partly explains the heavy emphasis on case studies in architecture (5). However, some architects might also be skeptical about the extent to which empirical data gathered by behavioral scientists can be used to optimize the planning, designing, and building of spaces (6). This study represents an attempt to overcome these methodological and principal/philosophical constraints by establishing an empirically driven dialogue between architecture and psychology via neuroscience.
Specifically, we argue that neuroscientific data have an important role to play in bridging the conceptual gap between architecture and psychology by elucidating some of the underlying mechanisms that explain how systematic variations in architectural features lead to behavioral outcomes. This argument is bolstered by current knowledge about the neural underpinnings of basic mental processes that underlie our responses to architecture, including visual perception, spatial navigation, and memory (7). Thus, coupled with a burgeoning literature on neuroaesthetics—the field devoted to the study of neural systems that underlie aesthetic judgments and preference formations (8, 9)—there exists the tantalizing possibility that our intuitions about how we feel and act in built environments can be linked to systematic variations in physical features of those environments. In turn, such evidence could be used to optimize the design of spaces, and possibly improve health (10).
Because this must be considered an exploratory study, an important objective was narrowing the potentially very large number of physical features that could be manipulated within the context of architecture down to a manageable set. For the purposes of the present study, our key variable of interest was the contour of architectural spaces. We selected contour because historically architects have consistently considered it to be an important physical feature in planning, designing, and building spaces (11). Furthermore, the selection of contour was empirically motivated because a number of previous studies have demonstrated that it affects aesthetic judgments. Specifically, early psychological investigations going back almost 100 y examined the effect of contour on feelings (12–14). In the spirit of early empiricists, experimenters manipulated contour using simple stimuli, such as lines or abstract displays composed of curves or angles. The results of these early studies, confirmed later using typography (15), converged to show that curvilinear forms are experienced as softer and more pleasant, whereas angular forms are experienced as harder and more serious.
Modern researchers have extended the focus of those early studies to also include preferences. The results have demonstrated consistently that people typically prefer curvilinear to rectilinear objects, be they geometric forms, household objects, furniture, or car interiors (16–18), and that this effect persists even when controlling for symmetry, prototypicality, and balance (19). Furthermore, much like the earlier studies, contemporary studies have shown that curvature elicits pleasant emotions (16, 18). This finding is important because it suggests that the impact of contour on judgment in the form of greater preference for curvilinear objects might be driven by an affective response to curvature. Interestingly, a similar conclusion was drawn over a century ago by the psychologist Kate Gordon, who stated that “curves are in general felt to be more beautiful than straight lines. They are more graceful and pliable, and avoid the harshness of some straight lines” (20). Note in Gordon’s definition not only the observation of a preference for curvilinear forms, but also their grounding in feelings. We aimed to measure this affective response in architecture using both behavioral and neural methods.
In terms of behavior, we focused on aesthetic judgments and approach-avoidance decisions, the selection of which was based on two reasons. First, both outcomes are of interest to architects and users of spaces alike. Second, from an evolutionary perspective, there is reason to believe that the environmental signals that give rise to aesthetic judgments might be borne out of those that regulate biologically more fundamental behaviors, such as approach-avoidance decisions. This idea is based on what the geologist Jay Appleton called “habitat theory,” according to which the aesthetic satisfaction one derives from contemplating a natural landscape is proportional to the extent to which its physical features signal environmental conditions favorable or unfavorable to survival (21). Similar ideas have been voiced elsewhere (22–24), grounded in the argument that our relationship with our natural environment is influenced by our evolutionary history. As Appleton said eloquently, “Habitat theory postulates that aesthetic pleasure in landscape derives from the observer experiencing an environment favorable to the satisfaction of his biological needs.” He further added that, “The point at which we always seem to run against a brick wall is in understanding more precisely how the actual ingredients of landscape operate on the aesthetic sense” (21).
Essentially, in habitat theory not only do we see a clear link between aesthetic judgments and assessments that are more fundamental to survival, but also a mechanism that describes this relationship.
Extending Appleton’s landscape-based theory to built environments, the architect Grant Hildebrand has proposed that an analogous argument can be put forth regarding our relationships with constructed spaces (25). Specifically, Hildebrand has argued that given our relatively recent shift to built environments, it is likely that features that evolved to regulate our relationships with our natural habitats continue to exert their influence on our interactions with constructed spaces (26, 27). This theory suggests that in the context of constructed spaces one can explore the degree of overlap between observers’ behavioral and neural responses when asked to make aesthetic judgments and approach-avoidance decisions. Furthermore, we believe that contour might be one of the “actual ingredients” (21) that operates on our aesthetic sense and decisions to approach certain built environments and to avoid others.
Aside from contour, we also introduced ceiling height and openness as two control variables into our design. We opted to explicitly control for them within each level of contour because some evidence exists that they can influence cognition and emotion in the context of architecture (28, 29). These aspects were not entered as independent variables of focal interest in the present study because previous empirical evidence linking them specifically to our two outcome measures is absent or limited.
Our study consisted of presenting participants in an functional MRI (fMRI) scanner with photographs of interior spaces that varied in contour (Fig. 1). The study was presented in two runs, administered counterbalanced across participants. In the beauty-judgment run participants were instructed to respond “beautiful” or “not beautiful” upon viewing each stimulus. In the approach-avoidance run participants were instructed to respond “enter” or “exit” upon viewing each stimulus, to indicate whether this was a space they would like to enter or leave. We hypothesized that spaces with curvilinear contours would more likely elicit “beautiful” judgments in the beauty judgment run and “enter” decisions in the approach-avoidance run, than spaces with rectilinear contours. This result would extend earlier findings regarding preferences for curved objects to the domain of architecture, and determine the extent to which aesthetic judgments and approach decisions (as a function of contour) are correlated. In addition, following the completion of fMRI scans, we collected “beauty” and “pleasantness” ratings for all stimuli, enabling us to conduct parametric analyses to further probe the link between brain activation and aesthetic assessment.
Fig. 1.
Examples of stimuli used in the study. The focal aim of the study involved a comparison of contour (i.e., curvilinear vs. rectilinear spaces), although we also controlled for ceiling height (high, low) and openness (open, enclosed) within our two conditions of interest (Methods).
At a neurobiological level, we made dissociable predictions for beauty judgments and approach-avoidance decisions. Regarding the former, a large body of literature in neuroaesthetics has demonstrated that aesthetic judgments activate a distributed neural network (30), including the brain’s reward and affective circuitry (31–36). Indeed, based on the results of the largest meta-analysis of neuroimaging studies of aesthetic appraisal to date, Brown et al. defined a “core circuit for aesthetic processing” (37). Not unlike what has been proposed for the experience of core affect in emotion (38, 39), this circuit includes four structures: orbitofrontal cortex (OFC), basal ganglia, anterior insula, and cingulate cortex. Each structure has a specific role: OFC’s role in reward processing is well established (40). Here, the role underlies the perception of the sensory and reward-based qualities of objects. The anterior insula represents bodily responses in the form of inputs from the interoceptive cortex. The anterior cingulate cortex (ACC), given its strong resting state connectivity with both the OFC and the anterior insula, is proposed to underlie emotional salience monitoring (41). Finally, basal ganglia’s role involves processing hedonic information (42). Because previous behavioral studies have demonstrated that curvature elicits pleasant emotions (16, 18), we hypothesized that compared with viewing rectilinear spaces, viewing curvilinear spaces would activate structures coextensive with the brain’s reward and emotions networks, with specific interest in the regions highlighted in Brown et al.’s meta-analysis of aesthetic appraisal. In turn, we hypothesized that the reverse contrast (i.e., rectilinear-curvilinear) would activate the amygdala. This specific prediction was derived from an earlier fMRI study in which it was shown that viewing rectilinear everyday objects activated the amygdala, suggesting that sharpness might serve as an early warning signal for potential danger (43).
Regarding approach-avoidance decisions, two distinct bodies of evidence informed our predictions. First, the neural systems for approach-avoidance motivations have been shown to be lateralized: approach motivations are lateralized predominantly to the left hemisphere, whereas avoidance emotions are lateralized predominantly to the right hemisphere (44, 45). Furthermore, electrical stimulation of different regions of the brain can unconditionally elicit approach and avoidance behavior (46–49). For example, electrical stimulation of brain regions that receive projections from midbrain dopamine neurons—including the nucleus accumbens as well as mesial prefrontal cortex—elicits approach behavior. In turn, electrical stimulation of the anterior insula and basolateral amygdala elicits avoidance behavior. Aside from this evidence on the motivational bases of approach-avoidance behavior, contemplating approach or avoidance might also activate brain regions implicated in motor imagery or planning of voluntary motor movement, as the person considers entering or exiting the space (50–54). We therefore hypothesized that compared with viewing rectilinear spaces, viewing curvilinear spaces would activate networks associated with approach motivation or regions implicated in motor imagery or execution. In addition, we hypothesized that the reverse contrast (i.e., rectilinear-curvilinear) would activate networks associated with avoidance motivation.
Results
Behavioral.
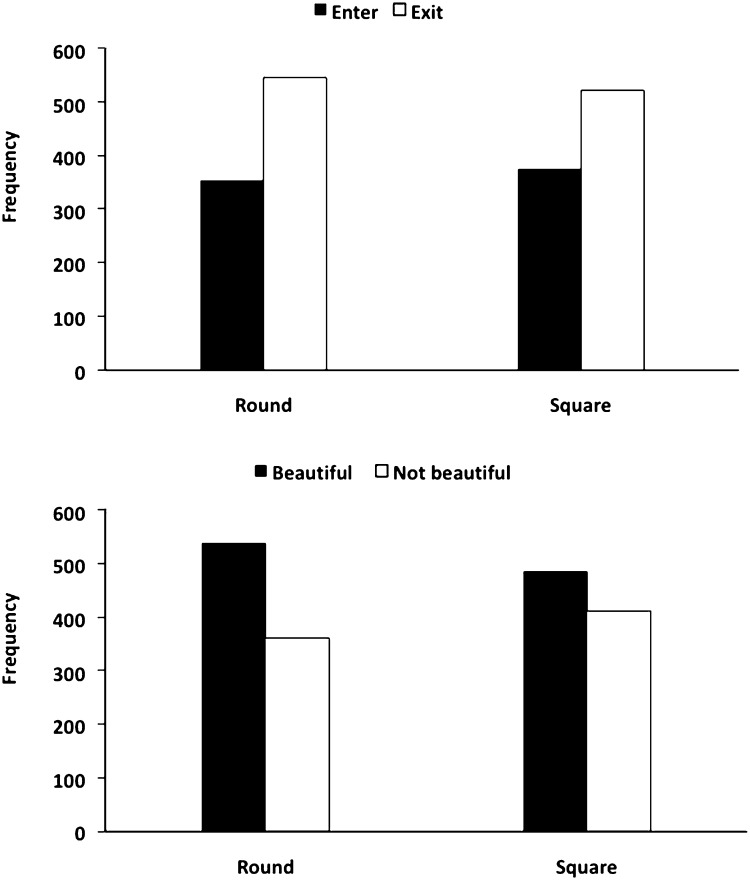
We analyzed the effect of contour on beauty judgments and approach-avoidance decisions made by participants during the scanning session separately. A Wilcoxon Signed Ranks Test demonstrated that contour had a significant effect on beauty judgments, Z = –2.13, P < 0.05. Specifically, participants were more likely to judge spaces as beautiful if they had curvilinear than rectilinear contours (Fig. 2). In contrast, contour had no effect on approach-avoidance decisions, Z = –1.27, P = 0.21 (Fig. 2).
Fig. 2.
Effect of curvilinear and rectilinear spaces on beauty judgments and approach-avoidance decisions. The y axis represents the sum of responses.
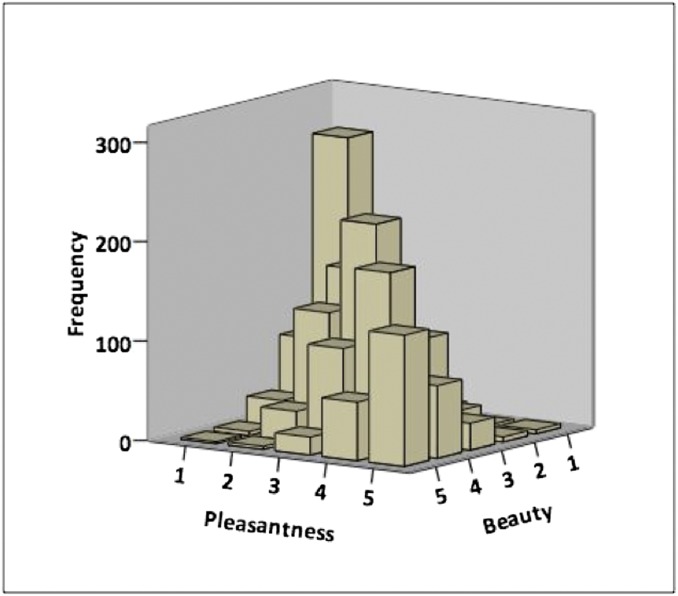
Following the completion of fMRI scanning, participants were presented with all of the stimuli that they had viewed in the scanner once again, and asked to rate each stimulus on pleasantness (using a five-point scale with anchors “very unpleasant” and “very pleasant”) and on beauty (using a five-point scale with anchors “very ugly” and “very beautiful”). Specifically for the stimuli that had been presented in the beauty judgment run, pleasantness ratings (collected outside of the scanner) predicted beauty ratings (collected outside of the scanner), β = 0.73, P < 0.001 (Fig. 3). In fact, pleasantness ratings accounted for 58% of the observed variance in beauty ratings. We then ran a binary logistic regression where we regressed beauty judgments obtained inside the scanner (i.e., “beautiful” or “not beautiful”) onto pleasantness ratings collected outside of the scanner. Pleasantness was once again a significant predictor of beauty judgment, β = –1.30, P < 0.001. Finally, because we obtained pleasantness ratings for all stimuli (and not just those that were presented in the beauty judgment run), we also ran a binary logistic regression where we regressed approach-avoidance decisions obtained inside the scanner (i.e., “enter” or “exit”) onto pleasantness ratings collected outside of the scanner. Pleasantness was a significant predictor of approach-avoidance decisions, β = –1.13, P < 0.001.
Fig. 3.
Pleasantness ratings predict beauty ratings. The y axis represents the sum of responses.
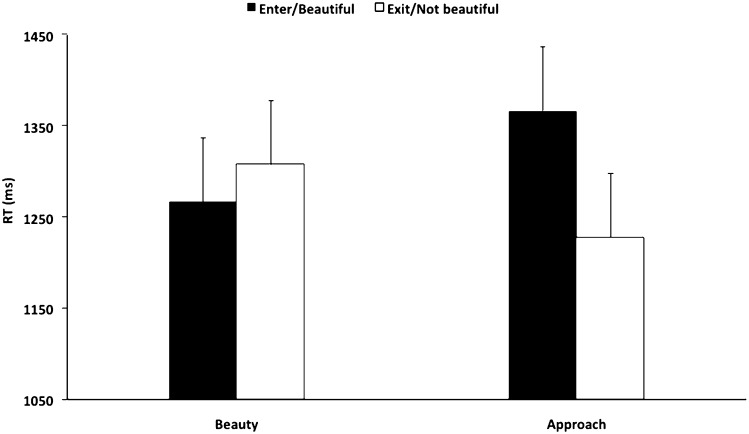
Although we had no a priori prediction about response latency, we nevertheless explored the effect of contour on reaction time involving beauty judgments and approach-avoidance decisions. We conducted this analysis because when rating facial attractiveness, people tend to view more attractive faces for longer periods of time (55, 56). Our results demonstrated that participants viewed spaces that they opted to “enter” for longer periods compared with spaces that they opted to “exit,” t(17) = 2.60, P < 0.05 (Fig. 4). In contrast, there was no difference in reaction time related to judging a space as “beautiful” or “not beautiful,” t(17) = –0.84, P = 0.41. In addition, contour had no effect on reaction time in the context of beauty judgments [t(17) = –0.72, P = 0.48] or approach-avoidance decisions [t(17) = 1.29, P = 0.21].
Fig. 4.
Effect of choice on response latency for beauty judgments and approach-avoidance decisions.
Neural.
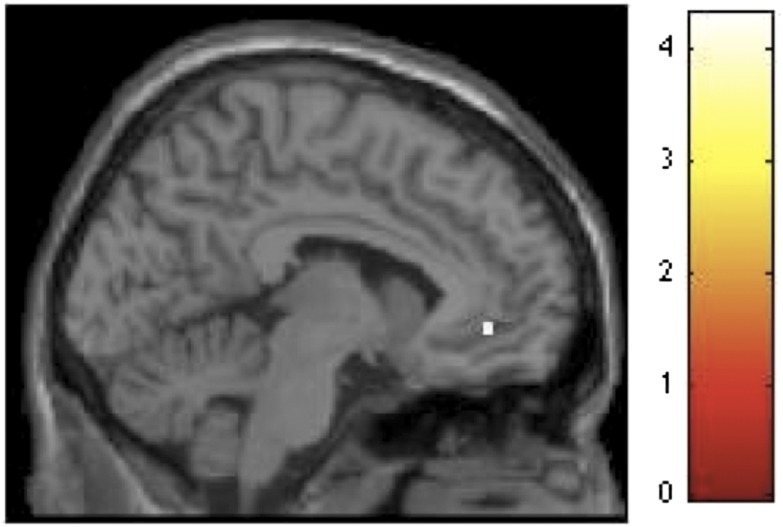
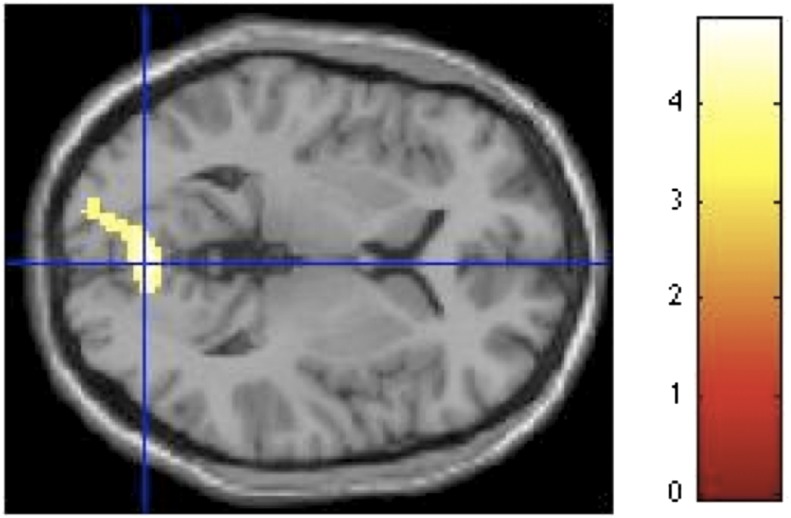
To analyze the fMRI data, we contrasted conditions of interest corresponding to each level of contour by assigning values of 1 and –1 to the regressors of interest, and 0 to all other regressors (Methods). For the beauty judgment run, the contrast of curvilinear-rectilinear spaces revealed significant activation in ACC exclusively (Z = 3.54, x = –6, y = 42, z = –6, k = 11) (Fig. 5). The reverse contrast did not reveal any significant area of activation. To further explore the role of reward and emotion in beauty judgment, we conducted two sets of parametric analyses to investigate the covariation of brain activations in relation to (i) beauty ratings and (ii) pleasantness ratings (both collected outside of the scanner, see above). The first set of analyses involved first-order polynomial expansions exploring linear relationships. The results demonstrated that activation in a distributed network including the frontopolar cortex, superior frontal gyrus, globus pallidus, precuneus, parahippocampus, and the middle occipital gyrus covaried in relation to beauty ratings (Table 1). In addition, activation in precuneus, middle frontal gyrus, and ACC covaried in relation to pleasantness ratings (Table 1). In our second set of analyses we explored second-order polynomial expansions but failed to find any evidence for nonlinear relationships between brain activations and beauty or pleasantness ratings.
Fig. 5.
Curvilinear spaces activate the anterior cingulate cortex in beauty judgments. SPM rendered into standard stereotactic space and superimposed on to sagittal MRI in standard space. Bar represents magnitude of t-score.
Table 1.
Regions activated in the parametric analyses involving postscan beauty and pleasantness ratings collected in relation to the beauty run
| Parameter | Structure | BA | x | y | z | z score | k |
| Beauty | Frontopolar cortex | 10 | −14 | 64 | −2 | 3.68 | 74 |
| Superior frontal gyrus | 6 | −26 | 22 | 60 | 3.68 | 44 | |
| Globus pallidus | – | 16 | −4 | −6 | 3.66 | 99 | |
| Precuneus | 7 | −28 | −74 | 46 | 3.48 | 127 | |
| Parahippocampus | 27 | −26 | −32 | −2 | 3.32 | 35 | |
| Middle occipital gyrus | 19 | −42 | −78 | 14 | 3.31 | 32 | |
| Middle occipital gyrus | 19 | −30 | −86 | 16 | 3.30 | 23 | |
| Pleasantness | Precuneus | 7 | −14 | −68 | 50 | 3.85 | 36 |
| Middle frontal gyrus | 9/46 | 34 | 42 | 10 | 3.77 | 32 | |
| Middle frontal gyrus | 9/46 | −38 | 30 | 14 | 3.35 | 71 | |
| Anterior cingulate cortex | 32 | −18 | 44 | 12 | 3.42 | 17 |
BA, Brodmann area; k, cluster size. The coordinates are reported in MNI space.
For the approach-avoidance run, the contrast of curvilinear-rectilinear contours revealed significant activation in a single cluster (k = 340) in the visual cortex that included left lingual gyrus (Z = 3.83, x = –20, y = –94, z = 8), as well as two regions within the right calcarine (Z = 3.71, x = 2, y = –76, z = –4 and Z = 3.65, x = 10, y = –74, z = –2) (Fig. 6).
Fig. 6.
Curvilinear spaces activate the lingual gyrus and calcarine in approach-avoidance decisions. SPM rendered into standard stereotactic space and superimposed on to transverse MRI in standard space. Bar represents magnitude of t-score.
Finally, to test Appleton’s theory, we conducted a conjunction analysis involving the “beautiful–not beautiful” contrast and the “enter–exit” contrast (Methods). In other words, we examined whether judging a space as beautiful activates the same neural system as deciding to enter a space. This conjunction analysis did not reveal any area of significant activation.
Discussion
Our results demonstrated that participants were more likely to judge curvilinear than rectilinear spaces as beautiful (Fig. 2). In addition, this effect is likely driven by pleasantness, the valence dimension of the affect circumplex (57) (Fig. 4). These results are consistent with evidence from previous studies establishing a preference for curved objects ranging from simple lines to furniture and car interiors (12–20) and the grounding of that preference in affect (16, 18), and extend them to the domain of architecture. Neuroanatomically, our results demonstrated that judging the beauty of curvilinear spaces was associated exclusively with an increase in ACC activity over and above judging the beauty of rectilinear spaces (Fig. 5). As discussed earlier, ACC is part of Brown et al.’s core circuit for aesthetic processing (37), and its activation here is consistent with the wealth of behavioral data that point to the involvement of emotion and reward in preference for curved objects. Lesion and neuroimaging studies have demonstrated the contribution of ACC to reward and emotional processing (58, 59), as have recent functional connectivity studies based on neuroanatomical parcellation, confirming its role in affective processing (60). Along with its rich interconnections with the adjacent OFC (58), the ACC is hypothesized to form a functional network underlying sensory consummatory behavior (61). In combination, our results suggest that judgment of beauty for curvilinear spaces is underpinned by emotion and reward, consistent with the role that emotion is known to play in aesthetic experience (62).
Interestingly, contrary to expectation, we did not observe activation in the amygdala for the reverse contrast (i.e., rectilinear-curvilinear). This finding suggests that in architecture, sharp contour might not serve as an early warning signal for potential danger as it might elsewhere, an observation that would be consistent with the amygdala’s well-established role in fear-conditioning (63, 64). However, a closer examination of the context within which our data were collected and our analytic method might provide additional explanations for the lack of activation observed in the amygdala. In terms of the former, our daily experiences provide us with ample exposure to rectilinear spaces. Arguably, through conditioning, sharp contours might have lost their value as signals for threat within built environments, for example through mere exposure (65). Recently, Leder et al. provided support for the role of context in moderating the effect of contour on preference (66). Specifically, the authors used positive (e.g., cake, chocolate) and negative (e.g., snake, bomb) stimuli to examine if emotional valence modulates preferences for curved objects. The authors found a preference for curved objects if the context was positive, but not if it was negative. A cross-cultural approach would appear to provide one avenue by which the role of past experience as a moderator of amygdala activation in response to architectural stimuli could be investigated.
From a methodological perspective, amygdala activation in response to rectilinear stimuli (43) has been observed with very brief presentation times (85 ms). In contrast, our participants viewed each stimulus for 3,000 ms. It is possible that a longer exposure duration might have triggered additional cognitive processing that served to depress the initial, rapid response in the amygdala frequently observed in relation to fearful stimuli (64). In addition, there is also evidence to suggest that the amygdala exhibits a nonlinear response profile in relation to facial beauty by responding maximally to extremely attractive and unattractive faces, and relatively less so to faces of average attractiveness (67). Insofar as judgment of beauty tracks variations in contour, this finding would suggest that activation in the amygdala could be maximal in relation to maximally curvilinear and maximally rectilinear spaces, although our data do not allow us to examine activation in the amygdala in response to gradations of contour. Future studies in which degree-of-curvature is manipulated systematically could certainly address this possibility.
In addition to the above categorical contrasts involving beauty judgment, we also conducted two parametric analyses involving beauty and pleasantness ratings collected outside of the scanner. The results demonstrated that in the beauty judgment run, brain activation within two distributed networks covaried linearly with beauty and pleasantness ratings (Table 1). Importantly, the activation pattern in relation to beauty ratings consisted of structures known to contribute to aesthetic assessments of visual objects. For example, the frontopolar (BA 10) region has been shown to be activated when subjects are instructed to judge the beauty of geometric patterns (68), consistent with its more general role in evaluative judgments involving one’s thoughts and feelings (69, 70). In addition, activations in the parahippocampus, middle occipital gyrus, precuneus, and superior frontal gyrus have been observed in previous studies involving aesthetic assessments of paintings, sculptures, and scenes (31, 71–74). Interestingly, the structures activated in relation to pleasantness, including the middle frontal gyrus, precuneus, and ACC, have also been shown to be activated for aesthetic assessments of paintings (31, 74). The results from the parametric analyses of beauty and pleasantness ratings suggest that in the context of judging beauty in architecture these two variables activate largely dissociable aspects of the same common network that underlies aesthetic assessment of visual stimuli.
In contrast to its effect on beauty judgments, contour had no effect on approach-avoidance decisions (Fig. 2). There could be a number of reasons for this result. First, the risk associated with judging a space as beautiful is less than the risk associated with the decision to enter that space, however hypothetical. It is therefore possible that the computation underlying approach and avoidance decisions is weighted differently as a function of this hypothetical risk than judgments of beauty. Consistent with this interpretation, whereas a decision to enter a space was associated with significantly higher response latency than a decision to exit a space, there was no difference in reaction time as a function of response in the beauty judgment condition (Fig. 4).
Second, it is also possible that our design might have lacked the degree of fidelity necessary to simulate approach-avoidance decisions that determine behavioral choices in real-life settings. As such, the task would not have fully engaged the decision maker, resulting in a null effect for contour. Methodologically, we opted to use a binary response format for both beauty judgments and approach-avoidance decisions to make comparisons between the two runs possible. As a consequence, our design could not incorporate tasks that, when used in isolation, would appear more ecologically valid for investigating approach-avoidance behavior, such as a visual navigation task.
Finally, the observed behavioral dissociation between beauty judgment and approach-avoidance decisions could also reflect a difference between the impact of contour on “liking” versus “wanting,” well established in the neuroscience of reward (75). In other words, contour may have a genuinely stronger effect on like or dislike for curvilinear spaces than it has on a desire to actually enter or exit these spaces. However, this observed dissociation must be interpreted with some care in light of previous evidence suggesting that in the context of architecture, there may in fact be a close correlation between aesthetic judgments and approach decisions. Specifically, Ritterfeld and Cupchik instructed their participants to rate photographs of interior spaces on semantic, structural, and connotative dimensions. Their results demonstrated that a willingness to live in a space was determined most strongly by the beauty rating assigned to that space (76). Also note that in the present study, pleasantness ratings predicted not only beauty judgments but also approach decisions. Taken together, our results suggest that although contour affected aesthetic judgments and approach-avoidance decisions differently, the two outcome measures might nevertheless be influenced by some of the same underlying mechanisms.
When participants made approach-avoidance decisions, the curvilinear-rectilinear contrast activated the visual cortex (Fig. 6). We did not observe the predicted activations in areas known to be involved in planning voluntary motor movement. Also notable is the bilateral activation observed in the visual cortex. Indeed, 59% of all decisions made in the approach run involved decisions to “exit” spaces (P < 0.001, Binomial Test) (Fig. 2), based on which one would predict relatively greater involvement of the right hemisphere as a reflection of avoidance motivation (44, 45). As alluded to above, the observed pattern could be attributable to the specific task used in the present study, given that it might have not have engaged processes that motivate approach-avoidance sufficiently.
In his now classic book The Experience of Landscape (21), the geologist Jay Appleton defined the problem by asking “What is it that we like about landscape, and why do we like it?” In the book, Appleton attempted to reestablish what he perceived to be the lost link in modern society between preferences for certain landscapes and the latter’s ability to satisfy the biological and survival needs of humans. By extending habitat theory to built environments and focusing on contour, we asked whether curvilinear spaces would affect beauty judgments and approach decisions in similar ways, and whether the neural systems underlying judgments of “beautiful” and decisions to “enter” a space would overlap. Although the evidence presented here suggests that they might not overlap, we have also noted limitations in our design that suggest further experimentation is necessary to more definitively determine the degree of overlap between their neural bases. Of course our observation could simply be a function of context. Specifically, built environments and landscapes might not be comparable in the extent to which they promote an evaluation of their ingredients for biological survival. Based on this argument, manipulating contour in the context of landscapes might impact approach decisions and their neural correlates differently than what was observed for built environments here.
Critical to understanding the role of context in the perceptual analysis of visual scenes of interiors, are studies of how recruitment of specific structures differs between experts in architecture and laypeople in this process. A number of studies have already begun to address this issue. For example, it has been shown that among architects, neural activation in the OFC and subcallosal cingulate gyrus was higher when assessing the aesthetic value of buildings compared with nonarchitects (77), suggesting that expertise moderates the neural representation of value in the reward network. Furthermore, compared with nonarchitecture students, architecture students recruit fewer brain structures for encoding and detecting building stimuli (78), suggesting that their expertise might confer an advantage in terms of neural efficiency in processing domain-specific content. These studies serve to connect studies of expertise in architecture to the broader literature on expertise in empirical aesthetics (79–81). This area would appear to be fertile ground for future research.
Conclusion
Long ago, Le Corbusier opined that “The business of Architecture is to establish emotional relationships by means of raw materials” (11). Le Corbusier was deeply aware of the knowledge that architecture drew from science and engineering toward achieving this goal, mediated as it was by how architectural forms “work physiologically upon our senses.” This awareness suggests that neuroaesthetics lies close to the kernel of modern architecture. Given our increasing propensity to spend time indoors (1), our results suggest that a systematic evaluation of how the physical features of built environments affect human behavior, emotion, and brain function is both timely and within reach. Not only is there the prospect that this interdisciplinary enterprise could lead to the design of more pleasant work and life spaces (7, 10), but these data could also shed light on perhaps a more fundamental question: why it is that we have come to prefer the places that we do.
Methods
Participants.
The participants provided written informed consent under the guidance of the The Universidad de la Laguna REB board–El Comité de Ética de la Investigación y de Bienestar Animal (CEIBA). We recruited 18 (12 females, 6 males) neurologically healthy participants (M = 23.39 y, SD = 4.49) with normal or corrected-to-normal vision. All participants were right handed, as determined by a standard questionnaire (M = 74.72, SD = 19.29) (82).
Materials.
The stimuli for this study consisted of 200 photographs of architectural spaces (Fig. 1). Half of the photographs were used in the beauty judgment run and the other half for the approach-avoidance run. The stimuli were culled from larger architectural image databases available to L.B.F. at the Department of Architecture, Design, and Media Technology in University of Aalborg, Denmark, and to N.R. at The Royal Danish Academy of Fine Arts, Schools of Architecture, Design and Conservation, School of Architecture. Half of the spaces were designated rectilinear and the other half curvilinear. Within each level of contour we also controlled for ceiling height and openness. In other words, within each of the curvilinear and rectilinear sets we included 25 open high-ceiling images, 25 closed high-ceiling images, 25 open low-ceiling images, and 25 closed low-ceiling images. L.B.F. and N.R. reached interrater consensus for the inclusion of each image in the final set. All images were standardized in terms of size and resolution. This procedure was adopted because no available dataset of architectural stimuli existed that provided 100 rectilinear and 100 curvilinear images, balanced for ceiling height and openness. To obtain the stimulus set please contact O.V.
Procedures.
In the course of structural MRI acquisition, participants were familiarized with the task via exposure to trials involving beauty judgments and approach-avoidance decisions. During fMRI scanning the beauty judgment and approach-avoidance runs were administered in counterbalanced order across participants. The task was presented using E-Prime. Each trial within the runs had identical structure: it began with a fixation point “X” presented for 1,000 ms, followed by a stimulus presented for 3,000 ms (during which a response was collected), followed by variable intertrial interval (ITI). The average duration of ITI across all trials was 4,000 ms (selected randomly without replacement from a finite bin varying among 3,000, 4,000, 6,000, and 7,000 ms). Immediately after exiting the fMRI scanner participants rated all stimuli on pleasantness (using a five-point scale with anchors “very unpleasant” and “very pleasant”) and on beauty (using a five-point scale with anchors “very ugly” and “very beautiful”).
fMRI Acquisition.
A 3-Tesla MR scanner with an eight-channel head coil (Signa Excite HD, 16.0 software; General Electric) was used to acquire T1 anatomical volume images (1.0 × 1.0 × 1.0-mm voxels). For functional imaging, T2*-weighted gradient echo spiral-in/out acquisitions were used to produce 35 contiguous 4-mm-thick axial slices [repetition time (TR) = 2,000 ms; echo time (TE) = 21.4 ms; flip angle (FA) = 90°; field of view (FOV) = 260 mm; 64 × 64 matrix; voxel dimensions = 4× 4 × 4.0 mm], positioned to cover the whole brain. The first 10 volumes were discarded to allow for T1 equilibration effects. The number of volumes acquired was 430 (+ 10 dummies).
fMRI Analysis.
Data were analyzed using Statistical Parametric Mapping (SPM8). Head movement was less than 2 mm in all cases. We implemented slice timing to correct for temporal differences between slices within the same volume, using the first slice within each volume as the reference slice. All functional volumes were spatially realigned to the first volume of the first run. A mean image created from realigned volumes was spatially normalized to the Montreal Neurological Institute (MNI) echo planar imaging brain template using nonlinear basis functions. The derived spatial transformation was applied to the realigned T2* volumes, and spatially smoothed with an 8 mm full-width at half-maximum isotropic Gaussian kernel. Time series across each voxel were high-pass filtered with a cutoff of 128 s, using cosine functions to remove section-specific low frequency drifts in the blood-oxygen level-dependent (BOLD) signal. Condition effects at each voxel were estimated according to the general linear model and regionally specific effects compared using linear contrasts. The BOLD signal was modeled as a box-car, convolved with a canonical hemodynamic response function. Each contrast produced a statistical parametric map consisting of voxels where the z-statistic was significant at P < 0.001. We adopted a combination of voxel-level and cluster-size correction to control against false-positives. Specifically, using a random-effects analysis, we reported activations that survived whole-brain voxel-level intensity threshold of P < 0.001, and a minimum cluster size of 10 voxels, uncorrected for multiple comparisons. Previous analyses have demonstrated that this combination adequately controls against false positives for both 2D and 3D volumes (83, 84).
We conducted three sets of analyses. The first analysis was a test of our focal hypothesis, and consisted of comparing curvilinear to rectilinear trials, separately for beauty judgment and approach-avoidance runs. The second analysis geared toward testing Appleton’s theory was based on a conjunction analysis involving the beautiful–not beautiful contrast and the enter–exit contrast. To ensure that (i) both analyses were run based on the same design matrix and (ii) explicitly included our control variables, within each run we created 16 regressors corresponding to a crossing of four variables: contour (rectilinear, curvilinear) × ceiling height (high, low) × openness (open, enclosed) × response (enter/exit or beautiful/not beautiful). Our two focal analyses were conducted by assigning weights of “1” or “–1” to the relevant regressors. Although incorporated into the design, motor response, and ITI were modeled out of the analyses by assigning null weights to their respective regressors. Our third analyses were parametric and involved first-order polynomial expansions exploring linear relationships as well as second-order polynomial expansions exploring nonlinear relationships in relation to beauty and pleasantness ratings (collected outside of the scanner).
In addition to the aforementioned two focal analyses, for the beautiful–not beautiful contrast we also used small volume correction in SPM8 to conduct region-of-interest analyses by creating spheres with a 15-mm radius around the principal activation-likelihood estimation foci extracted in a recent meta-analysis of studies of visual aesthetics (Supplemental table 3 in ref. 37). We were particularly interested in exploring activations in the anterior insula, the amygdala, and specific structures in the basal ganglia. This region-of-interest exploration did not yield additional areas of activation.
Acknowledgments
This work was supported by the following Ministerio de Ciencia a innovación Grant TIN2011-28146, 2011 and Ministerio Industrio, Turismo y Comercio, Avanza Grant TSI-020100-2010-346 under the direction of Jose Luis Gonzalez-Mora; and was supported in part by Servicio de Resonancia Magnética para Investigaciones Biomédicas de la Universidad de La Laguna.
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VII: The Human Mental Machinery,” held January 10–12, 2013, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vii.
This article is a PNAS Direct Submission.
Data deposition: The MRI and behavioral data have been deposited in http://figshare.com.
References
- 1.Klepeis NE, et al. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J Expo Anal Environ Epidemiol. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 2.Ott WR. 1989. Human activity patterns: A review of the literature for estimating time spent indoors, outdoors, and in transit. Proceedings of the Research Planning Conference on Human Activity Patterns, EPA National Exposure Research Laboratory, EPA/600/4-89/004: Las Vegas, NV.
- 3.Stamps AE. Physical determinants of preferences for residential façades. Environ Behav. 1999;31(6):723–751. [Google Scholar]
- 4.Lindal PJ, Hartig T. Architectural variation, building height, and the restorative quality of urban residential streetscapes. J Environ Psychol. 2013;33(1):26–36. [Google Scholar]
- 5.Jones PB, Canniffe E. Modern Architecture Through Case Studies 1945--1990. Oxford: Architectural Press; 2007. [Google Scholar]
- 6.Weber R. Introduction to the special issue: Aesthetics and design!? Empir Stud Arts. 2012;30(1):3–6. [Google Scholar]
- 7.Sternberg EM, Wilson MA. Neuroscience and architecture: Seeking common ground. Cell. 2006;127(2):239–242. doi: 10.1016/j.cell.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Skov M, Vartanian O. Neuroaesthetics. Amityville, NY: Baywood; 2009. [Google Scholar]
- 9.Chatterjee A. Neuroaesthetics: A coming of age story. J Cogn Neurosci. 2011;23(1):53–62. doi: 10.1162/jocn.2010.21457. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein RN. Architectural design and the collaborative research environment. Cell. 2006;127(2):243–246. doi: 10.1016/j.cell.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Le Corbusier . Towards a New Architecture. London, UK: Architectural Press; 1948. [Google Scholar]
- 12.Lundholm H. The affective tone of lines: Experimental researches. Psychol Rev. 1921;28(1):43–60. [Google Scholar]
- 13.Poffenberger AT, Barrows BE. The feeling value of lines. J Appl Psychol. 1924;8(2):187–205. [Google Scholar]
- 14.Hevner K. Experimental studies of the affective value of colors and lines. J Appl Psychol. 1935;19(4):385–398. [Google Scholar]
- 15.Kastl AJ, Child IL. Emotional meaning of four typographical variables. J Appl Psychol. 1968;52(6):440–446. doi: 10.1037/h0026506. [DOI] [PubMed] [Google Scholar]
- 16.Leder H, Carbon C. Dimensions in appreciation of car interior design. Appl Cogn Psychol. 2005;19(5):603–618. [Google Scholar]
- 17.Bar M, Neta M. Humans prefer curved visual objects. Psychol Sci. 2006;17(8):645–648. doi: 10.1111/j.1467-9280.2006.01759.x. [DOI] [PubMed] [Google Scholar]
- 18.Dazkir SS, Read MA. Furniture forms and their influence on our emotional responses toward interior environments. Environ Behav. 2012;44(5):722–734. [Google Scholar]
- 19.Silvia PJ, Barona CM. Do people prefer curved objects? Angularity, expertise, and aesthetic preference. Empir Stud Arts. 2009;27(1):25–42. [Google Scholar]
- 20.Gordon K. Esthetics. New York: Henry Holt; 1909. [Google Scholar]
- 21.Appleton J. The Experience of Landscape. New York, NY: John Wiley and Sons; 1975/1996. [Google Scholar]
- 22.Kellert SR, Wilson EO. The Biophilia Hypothesis. Washington, DC: Island Press; 1993. [Google Scholar]
- 23.Nasar JL. Environmental Aesthetics: Theory, Research, and Applications. Cambridge: Cambridge Univ Press; 1988. [Google Scholar]
- 24.Sagan C, Druyan A. Shadows of Forgotten Ancestors. New York: Ballantine; 1992. [Google Scholar]
- 25.Hildebrand G. Origins of Architectural Pleasure. Berkeley, CA: Univ of California Press; 1999. [Google Scholar]
- 26.Kaplan S. Environmental preference in a knowledge-seking, knowledge-using organism. In: Barkow JH, Cosmides L, Tooby J, editors. The Adapted Mind: Evolutionary Psychology and the Generation of Culture. New York: Oxford Univ Press; 1992. pp. 581–598. [Google Scholar]
- 27.Kaplan S. Aesthetics, affect, and cognition. Environ Behav. 1987;19(1):3–32. [Google Scholar]
- 28.Meyers-Levy J, Zhu R. The influence of ceiling height: The effect of priming on the type of processing that people use. J Consum Res. 2007;34(2):174–186. [Google Scholar]
- 29.Franz G, von der Heyde M, Bülthoff HH. An empirical approach to the experience of architectural space in virtual reality—Exploring relations between features and affective appraisals of rectangular indoor spaces. Autom Construct. 2005;14(2):165–172. [Google Scholar]
- 30.Nadal M, Munar E, Capó MA, Rosselló J, Cela-Conde CJ. Towards a framework for the study of the neural correlates of aesthetic preference. Spat Vis. 2008;21(3–5):379–396. doi: 10.1163/156856808784532653. [DOI] [PubMed] [Google Scholar]
- 31.Vartanian O, Goel V. Neuroanatomical correlates of aesthetic preference for paintings. Neuroreport. 2004;15(5):893–897. doi: 10.1097/00001756-200404090-00032. [DOI] [PubMed] [Google Scholar]
- 32.Kawabata H, Zeki S. Neural correlates of beauty. J Neurophysiol. 2004;91(4):1699–1705. doi: 10.1152/jn.00696.2003. [DOI] [PubMed] [Google Scholar]
- 33.Ishizu T, Zeki S. Toward a brain-based theory of beauty. PLoS ONE. 2011;6(7):e21852. doi: 10.1371/journal.pone.0021852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishai A. Sex, beauty and the orbitofrontal cortex. Int J Psychophysiol. 2007;63(2):181–185. doi: 10.1016/j.ijpsycho.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee A, Thomas A, Smith SE, Aguirre GK. The neural response to facial attractiveness. Neuropsychology. 2009;23(2):135–143. doi: 10.1037/a0014430. [DOI] [PubMed] [Google Scholar]
- 36.Di Dio C, Gallese V. Neuroaesthetics: A review. Curr Opin Neurobiol. 2009;19:1–6. doi: 10.1016/j.conb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Brown S, Gao X, Tisdelle L, Eickhoff SB, Liotti M. Naturalizing aesthetics: Brain areas for aesthetic appraisal across sensory modalities. Neuroimage. 2011;58(1):250–258. doi: 10.1016/j.neuroimage.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrett LF, Mesquita B, Ochsner KN, Gross JJ. The experience of emotion. Annu Rev Psychol. 2007;58:373–403. doi: 10.1146/annurev.psych.58.110405.085709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barrett LF, Wager T. The structure of emotion: Evidence from the neuroimaging of emotion. Curr Dir Psychol Sci. 2006;15(2):79–85. [Google Scholar]
- 40.Kringelbach ML. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat Rev Neurosci. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 41.Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. 2009;30(9):2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: Reward in humans and animals. Psychopharmacology (Berl) 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bar M, Neta M. Visual elements of subjective preference modulate amygdala activation. Neuropsychologia. 2007;45(10):2191–2200. doi: 10.1016/j.neuropsychologia.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rutherford HJV, Lindell AK. Thriving and surviving: Approach and avoidance motivation and lateralization. Emotion Review. 2011;3(3):333–343. [Google Scholar]
- 45.Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cogn Affect Behav Neurosci. 2003;3(3):207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- 46.Knutson B, Greer SM. Anticipatory affect: Neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci. 2008;363(1511):3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Olds ME, Fobes JL. The central basis of motivation: Intracranial self-stimulation studies. Annu Rev Psychol. 1981;32:523–574. doi: 10.1146/annurev.ps.32.020181.002515. [DOI] [PubMed] [Google Scholar]
- 48.Shizgal P. Neural basis of utility estimation. Curr Opin Neurobiol. 1997;7(2):198–208. doi: 10.1016/s0959-4388(97)80008-6. [DOI] [PubMed] [Google Scholar]
- 49.Panksepp J. Affective Neuroscience: The Foundations of Human and Animal Emotions. New York, NY: Oxford Univ Press; 1998. [Google Scholar]
- 50.Hanakawa T, Dimyan MA, Hallett M. Motor planning, imagery, and execution in the distributed motor network: A time-course study with functional MRI. Cereb Cortex. 2008;18(12):2775–2788. doi: 10.1093/cercor/bhn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crammond DJ. Motor imagery: Never in your wildest dream. Trends Neurosci. 1997;20(2):54–57. doi: 10.1016/s0166-2236(96)30019-2. [DOI] [PubMed] [Google Scholar]
- 52.Decety J. The neurophysiological basis of motor imagery. Behav Brain Res. 1996;77(1–2):45–52. doi: 10.1016/0166-4328(95)00225-1. [DOI] [PubMed] [Google Scholar]
- 53.Grush R. The emulation theory of representation: Motor control, imagery, and perception. Behav Brain Sci. 2004;27(3):377–396, discussion 396–442. doi: 10.1017/s0140525x04000093. [DOI] [PubMed] [Google Scholar]
- 54.Deiber MP, et al. Cerebral processes related to visuomotor imagery and generation of simple finger movements studied with positron emission tomography. Neuroimage. 1998;7(2):73–85. doi: 10.1006/nimg.1997.0314. [DOI] [PubMed] [Google Scholar]
- 55.Quinsey VL, Ketsetzis M, Earls C, Karamanoukian A. Viewing time as a measure of sexual interest. Ethol Sociobiol. 1996;17(5):341–354. [Google Scholar]
- 56.Shimojo S, Simion C, Shimojo E, Scheier C. Gaze bias both reflects and influences preference. Nat Neurosci. 2003;6(12):1317–1322. doi: 10.1038/nn1150. [DOI] [PubMed] [Google Scholar]
- 57.Russell JA. Core affect and the psychological construction of emotion. Psychol Rev. 2003;110(1):145–172. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- 58.Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Prog Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 59.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu C, et al. Functional segregation of the human cingulate cortex is confirmed by functional connectivity based neuroanatomical parcellation. Neuroimage. 2011;54(4):2571–2581. doi: 10.1016/j.neuroimage.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 61.Van Hoesen GW, Morecraft RJ, Vogt BA. Connections of the monkey cingulate cortex. In: Vogt BA, Gabriel M, editors. The Neurobiology of the Cingulate Cortex and Limbic Thalamus: A Comprehensive Handbook. Boston, MA: Birkhäuser; 1993. pp. 249–284. [Google Scholar]
- 62.Leder H, Belke B, Oeberst A, Augustin D. A model of aesthetic appreciation and aesthetic judgments. Br J Psychol. 2004;95(Pt 4):489–508. doi: 10.1348/0007126042369811. [DOI] [PubMed] [Google Scholar]
- 63.Phelps EA. Emotion and cognition: Insights from studies of the human amygdala. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 64.LeDoux J. The Emotional Brain: The Mysterious Underpinnings of Emotional Life. New York, NY: Touchstone; 1998. [Google Scholar]
- 65.Marks I, Dar R. Fear reduction by psychotherapies. Recent findings, future directions. Br J Psychiatry. 2000;176:507–511. doi: 10.1192/bjp.176.6.507. [DOI] [PubMed] [Google Scholar]
- 66.Leder H, Tinio PPL, Bar M. Emotional valence modulates the preference for curved objects. Perception. 2011;40(6):649–655. doi: 10.1068/p6845. [DOI] [PubMed] [Google Scholar]
- 67.Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ. Brain systems for assessing facial attractiveness. Neuropsychologia. 2007;45(1):195–206. doi: 10.1016/j.neuropsychologia.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Jacobsen T, Schubotz RI, Höfel L, Cramon DY. Brain correlates of aesthetic judgment of beauty. Neuroimage. 2006;29(1):276–285. doi: 10.1016/j.neuroimage.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 69.Christoff K, Ream JM, Geddes LP, Gabrieli JD. Evaluating self-generated information: Anterior prefrontal contributions to human cognition. Behav Neurosci. 2003;117(6):1161–1168. doi: 10.1037/0735-7044.117.6.1161. [DOI] [PubMed] [Google Scholar]
- 70.Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: An fMRI study. Neuroimage. 2002;15(4):983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
- 71.Fairhall SL, Ishai A. Neural correlates of object indeterminacy in art compositions. Conscious Cogn. 2008;17(3):923–932. doi: 10.1016/j.concog.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 72.Di Dio C, Macaluso E, Rizzolatti G. The golden beauty: Brain response to classical and renaissance sculptures. PLoS ONE. 2007;2(11):e1201. doi: 10.1371/journal.pone.0001201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yue X, Vessel EA, Biederman I. The neural basis of scene preferences. Neuroreport. 2007;18(6):525–529. doi: 10.1097/WNR.0b013e328091c1f9. [DOI] [PubMed] [Google Scholar]
- 74.Vessel EA, Starr GG, Rubin N. The brain on art: Intense aesthetic experience activates the default mode network. Front Hum Neurosci. 2012;6:66. doi: 10.3389/fnhum.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berridge KC. Food reward: Brain substartes of wanting and liking. Neurosci Biobehav Rev. 1995;20(1):1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 76.Ritterfeld U, Cupchik GC. Perceptions of interiors of spaces. J Environ Psychol. 1996;16(4):349–360. [Google Scholar]
- 77.Kirk U, Skov M, Christensen MS, Nygaard N. Brain correlates of aesthetic expertise: A parametric fMRI study. Brain Cogn. 2009;69(2):306–315. doi: 10.1016/j.bandc.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 78.Wiesmann M, Ishai A. Expertise reduces neural cost but does not modulate repetition suppression. Cogn Neurosci. 2011;2(1):57–65. doi: 10.1080/17588928.2010.525628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hekkert P, van Wieringen PCW. The impact of level of expertise on the evaluation of original and altered versions of post-impressionistic paintings. Acta Psychol (Amst) 1996;94(2):117–131. [Google Scholar]
- 80.Müller M, Höfel L, Brattico E, Jacobsen T. Aesthetic judgments of music in experts and laypersons—An ERP study. Int J Psychophysiol. 2010;76(1):40–51. doi: 10.1016/j.ijpsycho.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 81.Vartanian O, Kaufman JC. Psychological and neural responses to art embody viewer and artwork histories. Behav Brain Sci. 2013;36(2):161–162. doi: 10.1017/S0140525X12001823. [DOI] [PubMed] [Google Scholar]
- 82.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 83.Forman SD, et al. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 84.Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: Re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4(4):423–428. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]