Abstract
To understand the evolution of a Theory of Mind, we need to understand the selective factors that might have jumpstarted its initial evolution. We argue that a subconscious, reflexive appreciation of others’ intentions, emotions, and perspectives is at the roots of even the most complex forms of Theory of Mind and that these abilities may have evolved because natural selection has favored individuals that are motivated to empathize with others and attend to their social interactions. These skills are adaptive because they are essential to forming strong, enduring social bonds, which in turn enhance reproductive success. We first review evidence from both humans and other animals indicating that reflexive and reflective mental state attributions are inextricably linked and play a crucial role in promoting affiliative social bonds. We next describe results from free-ranging female baboons showing that individuals who show high rates of affiliative behavior form stronger social bonds with other females. These bonds, in turn, are linked to fitness. We then provide data from three different types of social challenges (male immigration, changes in grooming behavior after the death of a close relative, and responses during playback experiments), suggesting that females who manifest high rates of affiliative behavior may also be more motivated to anticipate challenges, react adaptively to setbacks, and respond appropriately to social interactions.
Keywords: personality, primates
Do animals have a Theory of Mind (ToM)? Answers to this question have tended to focus on two properties that might characterize a cognitive process. First, is an animal’s recognition of other individuals’ mental states reflexive and therefore, perhaps immediate and unconscious? Or is it reflective and therefore, more likely to be ruminative and conscious? Second, to what kinds of mental states are animals attentive: more rudimentary psychological states, like another individual’s gaze direction or its intentions, or more complex states, like another individual’s knowledge or beliefs? These distinctions are not easy to draw, even in humans, where reflective, conscious mindreading about others’ knowledge and beliefs is built on and develops gradually from reflexive, unconscious recognition of, for example, another’s direction of gaze (1, 2).
There is considerable evidence that many animals are reflexively attuned to other individuals’ gaze, intentions, and emotions; however, the degree to which they are also reflectively aware of others’ knowledge and beliefs is less clear (3). Problems in assessment arise in part because whenever an animal behaves in ways that suggest an understanding of another’s knowledge, its behavior can often also be explained by simpler mechanisms, including learned contingences. A chimpanzee (Pan troglodytes) who takes food that a rival cannot see might do so because she understands the relation between seeing and knowing or because she has learned the behavioral rule that a rival is motivated to defend food at which he is looking. Although experiments have attempted to distinguish between these explanations (4–7), results have not been easy to interpret. At the very least, they suggest that animals’ understanding of others’ psychological states is quite different and perhaps less subject to conscious reflection than adult humans'. Whatever the explanation, it is clear that attempting to identify precise, definitive benchmarks of mental state attribution in animals has proved to be more elusive and less productive than first hoped.
Here, we take a slightly different approach to the question of mental state attribution in animals and consider the selective factors that might have favored the evolution of a rudimentary ToM. We begin by assuming that a full-blown ToM evolved from more rudimentary, reflexive forms that were themselves adaptive in their own right. As a first step in understanding the evolution of a ToM, therefore, we need to understand the selective factors that might have jumpstarted these rudimentary forms. We argue that a subconscious, reflexive appreciation of others’ intentions, emotions, and perspectives lies at the roots of even the most complex forms of ToM and that these abilities first evolved because natural selection favored individuals that were motivated to attend to other individuals’ social interactions and empathize with them. These skills were favored by selection because they are essential to forming strong, enduring social bonds, which in turn have been shown to enhance reproductive success. We therefore propose that the evolution of a ToM ultimately derives from its role in facilitating the formation of social bonds.
We first review evidence from both humans and other animals indicating that reflexive and reflective mental state attributions are inextricably linked and play a crucial role in promoting affiliative social bonds. Then, using data on wild female baboons (Papio hamadryas ursinus), we suggest that individual variation in the motivation to attend to social interactions and react to social challenges is positively correlated with measures that have previously been shown to be linked to the formation of social bonds and ultimately, enhanced reproductive success.
Reflexive and Reflective Empathy in Animals and Humans
Any attempt to determine whether an animal does or does not understand what another individual knows or thinks is inevitably confounded by the fact that the reflective processes associated with higher levels of ToM are closely linked to—and often hard to distinguish from—the more automatic, reflexive processes that underlie them (8, 9). Although we are consciously aware of the distinction between our own and others’ mental states, we are often unaware of the many cues on which this awareness is based. For example, although higher cortical areas, like the prefrontal cortex, are activated when a human attempts to determine whether another individual can see something, initial responses to gaze direction and goal-directed behavior also activate more primitive areas of the brain, including the superior temporal sulcus (STS) and the amygdala. In both humans and rhesus macaques (Macaca mulatta), the STS is particularly sensitive to the orientation of another individual’s eyes (10, 11).
The same is true of intentional behavior. Although we have conscious access to our reflections about whether someone’s actions are accidental or intentional, many of the neuronal responses that contribute to our eventual decision are more subconscious. In both humans and monkeys, mirror neurons in the inferior parietal lobule are activated when both an individual performs a specific action and he observes someone else perform that action. Significantly, many neurons begin to fire before the other individual actually performs the action, suggesting that these neurons encode not only the specific motor act but also the actor’s intentions (12, 13). Thus, our ability to recognize that gaze has informative content, or to consider whether behavior is intentional, depends crucially on automatic, reflexive neuronal activity of which we are largely unaware.
Similar results emerge in studies of empathy. Reflective, explicit empathy involves the ability to recognize emotional states like grief or fear in others without necessarily experiencing the same emotions oneself (8). However, reflective empathy evokes activity not just in the cortex but also more primitive areas of the brain shared with many animals, including the midbrain, the brainstem, and endocrine systems associated with reactivity, reward, and social attachment (14, 15). Although we can distinguish between our own and others’ emotions, representations of emotions like pain, disgust, and shame in others also activate many of the same areas of the brain that are activated when we experience or imagine the same emotions ourselves (13). Feeling sympathy for or being nice to others is emotionally rewarding in part because it facilitates the release of dopamine, a neurotransmitter associated with personal reward (14). Trust, empathy, and sensitivity to others’ affective states are all facilitated by neuropeptides associated with attachment, maternal behavior, and pair bonding in animals, particularly oxytocin (16, 17). Thus, even the most reflective forms of empathy in humans are derived from and still strongly linked to more rudimentary forms.
Similarly, reflective imitation involves the ability to recognize the goals and intentions of another and to understand that, to achieve the same goal, one must copy that individual’s actions. Human culture depends crucially on this ability, which is also shown to some degree by the great apes (18). Even humans, however, are largely unaware of many of the behaviors in others that they routinely mimic. Like some animals, we have a reflexive, unconscious tendency to mimic the postures, mannerisms, and behavior of individuals with whom we are interacting.
As already noted, in the motor domain the same mirror neurons are activated when an individual performs a movement as when he observes another engaged in that movement. Similarly, both human and nonhuman primates reflexively follow the gaze of others (19), and both human and macaque neonates copy others’ facial expressions (20, 21). The fact that such mimicking is associated with empathy is exemplified by the phenomenon of contagious yawning. It is well known that viewing others yawn can elicit spontaneous yawning in oneself. Even this apparently reflexive response, however, seems to vary according to an individual’s sensitivity to more reflective behavior, including face recognition and understanding of others’ mental states (22). Spontaneous yawning is rare or absent in children with autism spectrum disorder (23, 24). It also occurs at higher frequencies among kin and friends than among strangers, suggesting that contagious yawning is linked to and may also promote affiliation (25). These observations are not limited to humans: chimpanzees are also more likely to yawn in response to the yawns of familiar, as opposed to unfamiliar, individuals (26).
A variety of other observations on what has been termed the chameleon effect (27) supports the view that reflexive mimicry is linked to the formation and maintenance of social bonds and has been favored by evolution because it promotes affiliation (28). Experiments suggest that people unconsciously mimic others when attempting to foster rapport and increase their frequency of mimicry when they are excluded from a group (29, 30). Being imitated increases helpful and affiliative behavior (31) and activates areas in the brain associated with reward processing (32). In contrast, not being imitated increases cortisol levels (33).
Similar observations have been obtained in nonhuman primates. Captive capuchin monkeys (Cebus apella) are more willing to approach and exchange tokens with a human who mimics their actions than one who does not (34). Male chimpanzees’ long-distance pant hoots become more similar acoustically as individuals spend more time together (35, 36), suggesting that call convergence is associated with, and may even promote, social affiliation.
In practice, it is almost impossible to distinguish reflective empathy from more reflexive forms and learned negative associations (9). This problem is not surprising given neurological evidence that the two are closely linked. In an early experiment specifically designed to examine whether one monkey would respond to another’s distress, rhesus macaques were trained to pull chains to obtain a food reward. The apparatus was then rigged so that a monkey in an adjacent cage received a shock each time a particular chain was pulled. Most of the monkeys soon stopped pulling the chain that delivered the shock, even though doing so deprived them of a reward. They were especially likely to avoid the chain if they had previously received shocks themselves (37, 38). Although the monkeys’ responses might at first be interpreted as evidence for reflective empathy, it seems as likely that they became distressed when they saw the other monkey being shocked because it was linked to a negative association for themselves. However, because even the most reflective forms of human empathy also evoke activity in more reflexive, primitive brain systems, these alternative explanations may be impossible to disambiguate.
In a more recent experiment, macaques were given the option of delivering a reward to themselves, another monkey, or no one. Although subjects preferred to reward themselves over others, they nonetheless opted to reward their partner if the alternative was to reward no one. This preference was especially true if the partner was familiar (39). Significantly, the same brain areas that are activated in humans during such exchanges were also activated in monkeys (40), and again—as in humans (41)—the monkeys’ vicarious reinforcement was enhanced if they first inhaled oxytocin (42).
Finally, in another experiment rats were placed in an arena with a cagemate trapped in a translucent tube (43). The free rats quickly learned how to open the tube to liberate their cagemates, and they continued to do so even when given an alternative option to open a tube containing chocolate. (In the latter case, the rat opened both tubes and shared the chocolate.) It is possible that the free rats’ responses may have been provoked in part by their own elevated stress at hearing their cagemates’ alarm calls. However, given neurological evidence that witnessing distress in others activates many of the same brain areas as experiencing distress oneself, this distinction becomes difficult to disambiguate.
In sum, a variety of evidence suggests that reflexive empathy and imitation in both humans and other animals have evolved because they promote affiliation and social bonding. Joint attention and joint action activate areas of the brain associated with the processing of reward, and they are facilitated by the release of oxytocin. Importantly, what seems to be rewarding to animals is not physical contact per se but the specific identity of the social partner. In socially monogamous tamarins (Saguinus oedipus), strongly bonded pairs exhibit higher oxytocin levels than more weakly bonded pairs (17). Among wild chimpanzees, urinary concentrations of oxytocin are higher after individuals groom with a closely bonded partner (both kin and nonkin) than with a less closely bonded partner (44). Evidently, grooming with a close friend or relative is more emotionally rewarding than engaging in the same behavior with a less preferred partner.
If empathy and affiliation have indeed been under strong selective pressure and lie at the roots of ToM, it should be possible to link these behaviors to fitness. Indeed, there is growing evidence that such a link can be made, because empathy and affiliation help individuals to form and maintain social bonds, and these bonds promote fitness.
Strong, enduring social bonds are a distinctive and adaptive feature of many animal societies. Such bonds are not limited to those formed by heterosexual mated pairs but extend to same-sex bonds formed between both kin and nonkin. Correlations between same-sex bonds and measures of health or reproductive success have been documented in rodents, horses, dolphins, chimpanzees, baboons, and humans (45). Strong bonds buffer individuals against stress and disease and perhaps as a result are correlated with longevity and offspring survival.
These observations suggest that natural selection has favored empathy and imitation, because they are part of the cognitive and emotional skills that an individual needs to recognize others’ social relationships, understand their motives and intentions, and keep track of, anticipate, and react adaptively to social events and challenges. We now explore these questions in more detail, focusing on data derived from a long-term study of wild baboons living in the Okavango Delta of Botswana.
Empathy, Social Bonds, and Reproductive Success in Wild Female Baboons
Social Bonds.
Like many other species of Old World monkey, baboons live in large social groups (∼75 individuals) composed of both kin and nonkin. Males emigrate from their natal group at adulthood. Females assume dominance ranks similar to their mothers’, and the female dominance hierarchy typically remains stable for many years (3). Females form strong grooming relationships with a subset of other females, the strongest bonds occurring among close matrilineal kin (46).
Despite the fact that high-ranking females enjoy priority of access to resources such as food and mates, female reproductive success in baboons—like female reproductive success in humans and other animals—is influenced less by a female’s dominance rank than by the strength and stability of her bonds with other females. We evaluated females’ bond strength using two indices of sociality. The first index, the Composite Sociality Index (CSI), measured dyadic bond strength based on females’ rates of approaches, groom presents, grooming initiations, and grooming durations with other females. The second index, the Partner Stability Index (PSI), measured females’ retention of their top three partners across years. Over a 17-y period, offspring survival was significantly positively correlated with the CSI (47), whereas longevity was significantly correlated with a combination of the strength and stability of females’ relationships with their top partners (48). Females also experienced lower stress (as measured by fecal glucocorticoid metabolites) when their grooming network was more focused (49). Thus, the strength and stability of females’ social partners were correlated with several measures of fitness. Interestingly, however, variation in the strength of social bonds was not fully explained by obvious demographic attributes like dominance rank or availability of kin. Although females established their closest bonds with kin, kin varied in the strength of their bonds, and some females without close kin established close bonds with others.
These observations suggest that some individuals are more motivated or skilled than others at establishing and maintaining social bonds and that variation in patterns of affiliation that are correlated with fitness may result in large part from variation in personality styles. We therefore attempted to determine whether different patterns of behavior were more or less associated with social bond strength.
Personality Styles and Social Bond Strength.
We applied exploratory principle component analysis to the behavior of 45 female baboons over a 7-y period (50). To construct the components that were used to identify personality dimensions, we calculated annual rates for several behaviors not considered in previous analyses of sociality. These behaviors included the frequency that females were alone, the rate at which they were friendly to other females, the rate at which they were aggressive to other females (corrected for dominance rank), and the frequency with which they grunted when approaching higher- and lower-ranking females. Among baboons, grunts serve as signals of benign intent and facilitate friendly interactions (51). When females grunt to higher-ranking individuals, they are less likely to receive aggression. Conversely, when females grunt to lower-ranking individuals, those individuals are less likely to show submissive behavior. We were especially interested in the frequency with which females grunted to lower-ranking individuals, because such vocalizations do not benefit the signaler in any obvious way. Instead, they seem to function primarily to alleviate the anxiety of the recipient.
Our analysis identified three relatively stable personality dimensions, each characterized by a distinct suite of behaviors that could not be explained by dominance rank or availability of kin. Females scoring high on the Nice dimension were friendly to all females and often grunted to lower-ranking females, apparently to signal benign intent. Aloof females were aggressive, were less friendly, and grunted primarily to higher-ranking females. Loner females were often alone, were relatively unfriendly, and also grunted most often to higher-ranking females (50). The baboons themselves apparently recognized these differences, because they approached females who scored high on Nice at high rates but approached females scoring high on Aloof and Loner at much lower rates (ref. 50, table 1). Personality designations remained relatively stable over time.
Importantly, the different personality attributes were associated in different ways with measures of fitness. Females who scored high on Nice had strong social bonds (high CSI scores) and stable preferences for their top partners. Females who scored high on Aloof had lower CSI scores overall but very stable preferences with their top partners. In contrast, Loner females had significantly lower CSI scores, less stable partner preferences, and significantly higher glucocorticoid (GC) levels (ref. 50, table 2).
These results suggest that there are costs and benefits associated with particular personality characteristics. For example, selection would seem to act against females scoring high on the Loner dimension, because these individuals were under more stress than others and formed weaker bonds that yielded low CSI scores and low partner stability. This observation begs the obvious question of why any female would adopt the Loner strategy. Loners were not isolated and unfriendly solely because of their subordinate status or lack of kin; although these demographic factors contributed to their scores on this component, their behavior exacerbated them. Moreover, some Loners did have close kin, whereas other females who consistently scored high on Nice did not. If Loners were often the victims of circumstances, what skills or motivation allowed some individuals and not others to overcome these circumstances?
In sum, female baboons varied not only in the strength and stability of their bonds but also in the personality traits associated with these bonds—particularly the ability or motivation to interact with others.
To test whether variation in personality traits was also associated with variation in females’ ability and/or motivation to keep track of, anticipate, and react adaptively to social events, we examined females’ responses to three different types of social challenges. We were interested not in females’ responses to adversity itself—because we expected little individual variation in responses to real, ongoing threats—but their ability to anticipate adversity, respond adaptively to adversity after it had occurred, and keep track of social interactions that had the potential to influence their own relationships. Because previous research had shown that, as a group, most females responded positively to these challenges, we expected that any differences that did emerge would be small.
Personality Styles and Responses to Social Challenges.
Male immigration.
In the Okavango Delta, male immigrants that achieve alpha status often commit infanticide (3). Perhaps as a result, both immigration and instability in the alpha male position cause a significant increase in females’ GC levels. Lactating females are particularly likely to experience elevated GC levels, though during some immigration events females in all reproductive states show significant increases (52–54). These responses are associated with a decrease in sociality among females (54), which may reflect their heightened vigilance and reactivity.
We examined increases in females’ GC levels from 2 wk before to 2 wk after four different immigration events in 2002, 2003, 2004, and 2005. All events involved the takeover of the alpha male position. We tested whether the magnitude of the GC changes of individual females was linked to their personality styles. Importantly, by focusing on GC changes in the 2 wk immediately after the immigration event, we were able to assess females’ anticipation of the threat of infanticide rather than their responses to the actual act.
Consistent with previous results, the majority (75%) of individuals showed an increase in GC levels after immigration. However, some of the variation in females’ GC levels also seemed to be linked to their personality scores. The correlation between percent change in GC levels and Aloof scores was weakly negative (β = −10.15, SE = 10.5, t = −0.962, P > 0.10), as was the correlation for Loner scores (β = −11.24, SE = 11.62, t = −0.968, P > 0.10) (Fig. 1). In contrast, the correlation between Nice scores and change in GC levels was positive, though nonsignificant (β = 5.278, SE = 10.00, t = 0.527, P > 0.10) (Fig. 1). There were no significant effects of reproductive state.
Fig. 1.
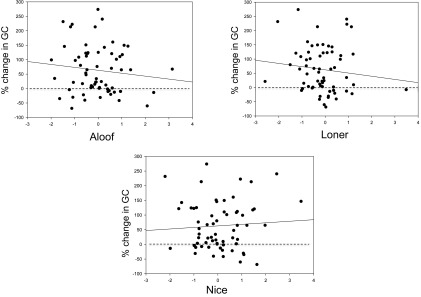
Percent change in females’ GC levels from 2 wk before to 2 wk after the immigration of a potentially infanticidal male. Only immigration events in which an immigrant attained the alpha rank were included in analysis. n = 33 females present for 1–3 events for a total of 64 female-events. Dashed lines indicate no change; solid lines indicate least square regression (statistics and probability values given in the text). The x axis denotes females’ scores on each of the three principle components (Aloof, Loner, and Nice) in the immigration year. Each point represents 1 female-y.
Thus, individuals who scored high on Nice tended to show increases in GC levels in response to male immigration, whereas those who scored high on Aloof and Loner tended to be less responsive.
Changes in grooming behavior after the death of a close relative.
Females also experience elevated GC levels after the death of a close adult female relative, probably in part because the death results in the loss of a regular grooming partner. Previous analyses have shown that, in the 3 mo after this loss, bereaved females increase both grooming rates and the number of female grooming partners (55). These responses may facilitate the repair of females’ social networks through the establishment of new bonds.
To examine individual differences in response to this challenge, we compared the number of each bereaved female’s different grooming partners in the 3 mo after the death of a close female relative with the mean number of grooming partners for unaffected females in the group during the same period (controlling for reproductive state). (This method was chosen to control for variation in sampling rates across time.) Whether females had a higher or lower number of partners than unaffected females seemed to be related to their personality scores. Females scoring high on the Loner component had fewer grooming partners than unaffected females (β = −1.138, SE = 0.866, t = −1.314, P = 0.203). In contrast, correlations between the relative number of grooming partners were positive but nonsignificant for both Aloof (β = 0.366, SE = 0.624, t = 0.586, P = 0.564) and Nice (β = 0.799, SE = 0.509, t = 1.569, P = 0.132) scores (Fig. 2).
Fig. 2.

The relative number of a female’s different grooming partners in the 3 mo after the death of a close relative (mother, adult daughter, or sister) compared with the mean number of grooming partners for all other females in those months (controlled for reproductive state). n = 18 females who lost from one to three close relatives for a total sample of 24 female-y. One outlier was removed. Legend is the same as in Fig. 1.
Thus, females who scored high on the Loner component had fewer grooming partners compared with unaffected females in the ensuing 3 mo, suggesting that they were unsuccessful in rebuilding their social network. This decrease occurred despite the fact that females who scored high on the Loner component tended to show a greater increase in GC levels than other females in the 2 wk after the death of a close relative, particularly when that relative was a mother or adult daughter (rs = 0.771, N = 6, P > 0.10). In contrast, females who scored high on the Aloof and Nice components responded to the death of a close relative by grooming comparatively more females than unaffected individuals.
Variation in the strength of responses during playback experiments.
Playback experiments are designed to test subjects’ knowledge of other individuals’ dominance ranks and kinship as well as their memory of recent social interactions and their participants. Consider reconciliation, for example. Baboons often grunt to their opponents after aggression, and these grunts serve to restore opponents to baseline levels of tolerance (56). In an experiment designed to determine whether reconciliation by kin could serve as a proxy for direct reconciliation, victims were played the grunt of the close relative of a recent opponent. Subjects were significantly more likely to approach their opponent after hearing a grunt from their opponent’s relative (test condition) than after hearing a grunt from a female from a different matriline (control condition) (57). In so doing, subjects showed that they remembered not only the specific nature of a recent interaction and the identity of the participants but also the kinship relations (or close associates) of other females in their group. Thus, by responding more strongly during tests than control trials, subjects showed that they were not only reactive but also appropriately reactive, in the sense that they responded strongly only to relevant stimuli.
For this analysis, we considered variation in females’ responses to playback stimuli in five previously conducted experiments that tested baboons’ memory of recent social interactions and knowledge of other individuals’ relationships (Table S1) (57–61). We used duration of looking toward the speaker in test compared with control trials as our dependent measure, because this response was used in all experiments. Because the strength of subjects’ responses varied across experiments, we ranked each subject’s duration of response in each experiment relative to response duration of other subjects. Thus, a subject who responded more strongly in the test vs. the control condition received a high positive ranking, whereas a subject that responded more strongly in the control condition received a negative ranking.
The correlations between strength of response and Aloof, Loner, and Nice scores were all positive, but only the Nice scores reached statistical significance (Aloof: β = 0.381, SE = 0.580, t = 0.657, P > 0.10; Loner: β = 0.625, SE = 0.634, t = 0.986, P > 0.10; Nice: β = 1.250, SE = 0.566, t = 2.246, P = 0.027) (Fig. 3). Thus, although most females responded more strongly during test than control trials, females who scored high on the Nice component were the most responsive.
Fig. 3.

Variation in females’ responses to playback stimuli in five different experiments (Table S1). Subjects were ranked according to the strength of their response in experimental trials minus control trials. n = 33 females in one to five experiments for a total of 73 subjects. One outlier was removed. Legend is the same as in Fig. 1.
Discussion: Social Challenges.
Previous analyses (50) showed that females scoring high on the Nice component have stronger social bonds with other females. The data presented here suggest that, by three independent measures, these individuals may also be more responsive to social challenges and more motivated to attend to social interactions within their group (Table 1).
Table 1.
Signs of the β-coefficients (regression slopes) of the relation between personality component scores (Nice, Aloof, and Loner) and three social challenges (details in the text)
| Nice | Aloof | Loner | |
| Male immigration | + | − | − |
| Change in grooming partner number after relative’s death | + | + | − |
| Playback experiments | +* | + | + |
P < 0.05.
First, females who score high on Nice may better anticipate threats that have yet to occur. Such females showed a greater anticipatory increase in GC levels at the arrival of a potentially infanticidal immigrant, suggesting that they recognized the threat that such a male represented even before he attacked any infants. Second, females who score high on Nice may respond more adaptively to setbacks that have occurred. Females with high Nice and Aloof scores had more grooming partners than unaffected females after the death of a close relative, suggesting that they were attempting to identify new social partners. In contrast, females who scored high on Loner had fewer partners than other females, suggesting that they made no such effort. Finally, there was some indication that females who scored high on Nice were more appropriately reactive during playback experiments than other females, perhaps because they were generally more attentive to social interactions and events in their group.
It is important to emphasize that all of the observed differences were small. As a group, females responded positively to each of the three challenges, so it is not surprising that individual variation in response strength was subject to a ceiling effect. The attributes associated with females who scored high on the Nice component were not unique to these individuals; rather, such females seemed more consistently to show strong anticipatory and reactive responses to challenges. Clearly, however, this hypothesis will need to be tested in future research.
Several recent studies of birds have investigated the relationship between problem solving ability and fitness. Results suggest that variation in problem solving does not result solely from differences in motivation or reactivity but instead may reflect genuine differences in cognitive ability (62–64). In contrast, we have no evidence that female baboons vary in their cognitive abilities—that, for example, Nice females are, by some measure, more skilled at problem solving. Instead, data suggest that differences in personality styles may be associated with greater responsiveness to social challenges and greater motivation to attend to, recall, and anticipate social interactions. In a somewhat similar study of captive rhesus macaques, males who scored high on a sociability index (defined as the motivation to seek out others) experienced better health. These males also coped better both physiologically and behaviorally during unstable social conditions, and they seemed more motivated than other males to manage unpredictable social circumstances (65). In contrast to males that scored low on the sociability index, they seemed to find social interactions rewarding rather than aversive. Similar differences were observed among Nice and Loner female baboons.
Conclusions
Human behavior during cooperative interactions is often contrasted with behavior of chimpanzees, which in captivity show little evidence of prosocial behavior. Indeed, experiments explicitly designed to compare the behavior of children and chimpanzees suggest that humans are unique not only in their motivation to participate in activities that involve shared goals and joint action but also their concern for the welfare of others (66–68). Most forms of apparent prosociality and empathy in animals can be explained functionally in terms of parental behavior, nepotism, or direct benefit. There is little evidence in animals—even apes—for the kind of reflective empathy that permits an individual to disassociate his own emotions and needs from others’ or be sensitive to another’s welfare independent of his own (69).
However, although cooperation among humans clearly differs from cooperation among animals in many nontrivial ways, more naturalistic studies suggest that the contrasts are not as stark as initially proposed. For example, wild chimpanzees engage in a variety of cooperative, sometimes risky ventures with long-term social partners, including grooming, food sharing, and border patrols (70). These interactions seem to be emotionally rewarding. As already mentioned, both male and female chimpanzees experience elevations in urinary oxytocin after grooming with a preferred partner (44), and males that share meat with others experience a significant drop in testosterone (71). Thus, chimpanzees in the wild regularly engage in joint, cooperative action and seem to derive pleasure and reduced tension from what are, arguably, rather prosocial activities.
We have suggested that a full-blown ToM evolved from a rudimentary form that is reflexively attentive and sensitive to others’ attention, emotions, and intentions. This rudimentary form of shared attention is widespread in many animals, still present in humans, and difficult to disambiguate—behaviorally and neurologically—from humans’ more derived, reflective ToM. We can explain the evolution of rudimentary ToM by noting that it facilitates attentiveness to others’ emotional states and thereby promotes the formation of strong social bonds, which are linked to fitness. Shared attention and sensitivity to others’ emotions may promote bond formation, because they include a subconscious tendency to mimic individuals with whom we are interacting. When two individuals interact, imitation makes the behavior of each partner more predictable to the other, and predictability and control are major modulators of stress (72). We may therefore find individuals who imitate us attractive in part because they are predictable.
In sum, a rudimentary ToM may have evolved because, in social animals, reflexive empathy and joint attention are both emotionally rewarding and adaptive. Individuals who are motivated to attend to others’ behavioral, attentional, and emotional states are more likely to interact positively with others and form stronger social bonds. Strong social bonds, in turn, contribute to reproductive success. Individual variation in the strength of social bonds, stress levels, and responsiveness to social challenges may stem less from variation in cognitive ability than variation in the motivation to attend to and participate in social interactions.
Methods
Data were derived from a long-term study of wild chacma baboons (P. hamadryas ursinus) in the Moremi Game Reserve, Botswana. The group had been observed since 1978. Maternal kinship was known for all individuals. The primary causes of mortality were infanticide and predation (details in ref. 1).
Statistical analyses were conducted using R statistical software (version 2.15; R Foundation for Statistical Computing). For exploratory principle component analysis, we used the principle function in the psych package with the default varimax rotation. To test for a relation between scores on principle components and dependent variables, we used linear mixed models (lmer in R), entering female identification and year as random factors.
Acknowledgments
We thank the Office of the President, Republic of Botswana and the Department of Wildlife and National Parks for permission to work in the Moremi Reserve. We thank J. Beehner, T. Bergman, C. Crockford, A. Engh, M. Heesen, L. Moscovice, R. Wittig, K. Seyfarth, C. Shaw, M. Mokopi, and A. Mokopi for assistance with data collection and J. Silk and two anonymous reviewers for comments. Research was supported by the National Science Foundation, the National Institutes of Health, the Leakey Foundation, the National Geographic Society, and the University of Pennsylvania. Research was approved by the Animal Care and Use Committee of the University of Pennsylvania (Protocol No. 19001).
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VII: The Human Mental Machinery,” held January 10–12, 2013, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vii.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1301223110/-/DCSupplemental.
References
- 1.Onishi KH, Baillargeon R. Do 15-month-old infants understand false beliefs? Science. 2005;308(5719):255–258. doi: 10.1126/science.1107621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apperly IA. What is “theory of mind”? Concepts, cognitive processes and individual differences. Q J Exp Psychol (Hove) 2012;65(5):825–839. doi: 10.1080/17470218.2012.676055. [DOI] [PubMed] [Google Scholar]
- 3.Cheney D, Seyfarth R. Baboon Metaphysics: The Evolution of a Social Mind. Chicago: Univ of Chicago Press; 2007. [Google Scholar]
- 4.Kaminski J, Call J, Tomasello M. Chimpanzees know what others know, but not what they believe. Cognition. 2008;109(2):224–234. doi: 10.1016/j.cognition.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Bugnyar T. Knower-guesser differentiation in ravens: Others’ viewpoints matter. Proc Biol Sci. 2011;278(1705):634–640. doi: 10.1098/rspb.2010.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLean E, Hare B. Bonobos and chimpanzees infer the target of another’s attention. Anim Behav. 2012;83(2):345–353. [Google Scholar]
- 7.Crockford C, Wittig RM, Mundry R, Zuberbühler K. Wild chimpanzees inform ignorant group members of danger. Curr Biol. 2012;22(2):142–146. doi: 10.1016/j.cub.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 8.Hecht EE, Patterson R, Barbey AK. What can other animals tell us about human social cognition? An evolutionary perspective on reflective and reflexive processing. Front Hum Neurosci. 2012;6:224. doi: 10.3389/fnhum.2012.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Waal FB. The antiquity of empathy. Science. 2012;336(6083):874–876. doi: 10.1126/science.1220999. [DOI] [PubMed] [Google Scholar]
- 10.Jellema T, Baker CI, Wicker B, Perrett DI. Neural representation for the perception of the intentionality of actions. Brain Cogn. 2000;44(2):280–302. doi: 10.1006/brcg.2000.1231. [DOI] [PubMed] [Google Scholar]
- 11.Klein JT, Shepherd SV, Platt ML. Social attention and the brain. Curr Biol. 2009;19(20):R958–R962. doi: 10.1016/j.cub.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fogassi L, et al. Parietal lobe: From action organization to intention understanding. Science. 2005;308(5722):662–667. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- 13.Rizzolatti G, Maddalena F-D. In: Handbook of Neuroscience for the Behavioral Sciences. Vol 1. Rizzolatti G, Maddalena F-D, editors. New York: Wiley; 2009. pp. 337–357. [Google Scholar]
- 14.Decety J. The neuroevolution of empathy. Ann N Y Acad Sci. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- 15.Decety J, Jackson PL. The functional architecture of human empathy. Behav Cogn Neurosci Rev. 2004;3(2):71–100. doi: 10.1177/1534582304267187. [DOI] [PubMed] [Google Scholar]
- 16.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 17.Snowdon CT, et al. Variation in oxytocin is related to variation in affiliative behavior in monogamous, pairbonded tamarins. Horm Behav. 2010;58(4):614–618. doi: 10.1016/j.yhbeh.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buttelmann D, Carpenter M, Call J, Tomasello M. Enculturated chimpanzees imitate rationally. Dev Sci. 2007;10(4):F31–F38. doi: 10.1111/j.1467-7687.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd SV, Klein JT, Deaner RO, Platt ML. Mirroring of attention by neurons in macaque parietal cortex. Proc Natl Acad Sci USA. 2009;106(23):9489–9494. doi: 10.1073/pnas.0900419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meltzoff AN, Moore MK. Imitation of facial and manual gestures by human neonates. Science. 1977;198(4312):75–78. doi: 10.1126/science.198.4312.75. [DOI] [PubMed] [Google Scholar]
- 21.Ferrari PF, et al. Neonatal imitation in rhesus macaques. PLoS Biol. 2006;4(9):e302. doi: 10.1371/journal.pbio.0040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Platek SM, Critton SR, Myers TE, Gallup GG. Contagious yawning: The role of self-awareness and mental state attribution. Brain Res Cogn Brain Res. 2003;17(2):223–227. doi: 10.1016/s0926-6410(03)00109-5. [DOI] [PubMed] [Google Scholar]
- 23.Helt MS, Eigsti IM, Snyder PJ, Fein DA. Contagious yawning in autistic and typical development. Child Dev. 2010;81(5):1620–1631. doi: 10.1111/j.1467-8624.2010.01495.x. [DOI] [PubMed] [Google Scholar]
- 24.Senju A, et al. Absence of contagious yawning in children with autism spectrum disorder. Biol Lett. 2007;3(6):706–708. doi: 10.1098/rsbl.2007.0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norscia I, Palagi E. Yawn contagion and empathy in Homo sapiens. PLoS One. 2011;6(12):e28472. doi: 10.1371/journal.pone.0028472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell MW, de Waal FB. Ingroup-outgroup bias in contagious yawning by chimpanzees supports link to empathy. PLoS One. 2011;6(4):e18283. doi: 10.1371/journal.pone.0018283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chartrand TL, Bargh JA. The chameleon effect: The perception-behavior link and social interaction. J Pers Soc Psychol. 1999;76(6):893–910. doi: 10.1037//0022-3514.76.6.893. [DOI] [PubMed] [Google Scholar]
- 28.Lakin J, Jefferis V, Cheng C, Chartrand T. The chameleon effect as social glue: Evidence for the evolutionary significance of nonconscious mimicry. J Nonverbal Behav. 2003;27(2):145–162. [Google Scholar]
- 29.Lakin JL, Chartrand TL. Using nonconscious behavioral mimicry to create affiliation and rapport. Psychol Sci. 2003;14(4):334–339. doi: 10.1111/1467-9280.14481. [DOI] [PubMed] [Google Scholar]
- 30.Lakin JL, Chartrand TL, Arkin RM. I am too just like you: Nonconscious mimicry as an automatic behavioral response to social exclusion. Psychol Sci. 2008;19(8):816–822. doi: 10.1111/j.1467-9280.2008.02162.x. [DOI] [PubMed] [Google Scholar]
- 31.Van Baaren R, Holland R, Kawakami K, van Knippenberg A. Mimicry and prosocial behavior. Psychol Sci. 2004;15(1):71–74. doi: 10.1111/j.0963-7214.2004.01501012.x. [DOI] [PubMed] [Google Scholar]
- 32.Kühn S, et al. Why do I like you when you behave like me? Neural mechanisms mediating positive consequences of observing someone being imitated. Soc Neurosci. 2010;5(4):384–392. doi: 10.1080/17470911003633750. [DOI] [PubMed] [Google Scholar]
- 33.Kouzakova M, van Baaren R, van Knippenberg A. Lack of behavioral imitation in human interactions enhances salivary cortisol levels. Horm Behav. 2010;57(4–5):421–426. doi: 10.1016/j.yhbeh.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 34.Paukner A, Suomi SJ, Visalberghi E, Ferrari PF. Capuchin monkeys display affiliation toward humans who imitate them. Science. 2009;325(5942):880–883. doi: 10.1126/science.1176269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitani JC, Hunley KL, Murdoch ME. Geographic variation in the calls of wild chimpanzees: A reassessment. Am J Primatol. 1999;47(2):133–151. doi: 10.1002/(SICI)1098-2345(1999)47:2<133::AID-AJP4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Crockford C, Herbinger I, Vigilant L, Boesch C. Wild chimpanzees produce group-specific calls: A case for vocal learning? Ethology. 2004;110:221–243. [Google Scholar]
- 37.Masserman JH, Wechkin S, Terris W. “Altruistic” behavior in rhesus monkeys. Am J Psychiatry. 1964;121:584–585. doi: 10.1176/ajp.121.6.584. [DOI] [PubMed] [Google Scholar]
- 38.Wechkin S, Massserman J, Terris T. Shock to a conspecific as an aversive stimulus. Psychon Sci. 1964;1(1):47–48. [Google Scholar]
- 39.Chang SW, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (macaca mulatta) Front Neurosci. 2011;5:27. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang SW, Gariépy J-F, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci. 2013;16(2):243–250. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: Evidence and future directions. Horm Behav. 2012;61(3):410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci USA. 2012;109(3):959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334(6061):1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crockford C, et al. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc Biol Sci. 2013;280(1755):20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seyfarth RM, Cheney DL. The evolutionary origins of friendship. Annu Rev Psychol. 2012;63:153–177. doi: 10.1146/annurev-psych-120710-100337. [DOI] [PubMed] [Google Scholar]
- 46.Silk J, Alberts S, Altmann J, Cheney D, Seyfarth R. Stability of partner choice among female baboons. Anim Behav. 2012;83(6):1511–1518. doi: 10.1016/j.anbehav.2012.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silk JB, et al. The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proc Biol Sci. 2009;276(1670):3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silk JB, et al. Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol. 2010;20(15):1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 49.Crockford C, Wittig RM, Whitten PL, Seyfarth RM, Cheney DL. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus) Horm Behav. 2008;53(1):254–265. doi: 10.1016/j.yhbeh.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Seyfarth RM, Silk JB, Cheney DL. Variation in personality and fitness in wild female baboons. Proc Natl Acad Sci USA. 2012;109(42):16980–16985. doi: 10.1073/pnas.1210780109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheney D, Seyfarth R, Silk J. The role of grunts in reconciling opponents and facilitating interactions among adult female baboons. Anim Behav. 1995;50(1):249–257. [Google Scholar]
- 52.Beehner J, Bergman T, Cheney D, Seyfarth R, Whitten P. The effect of new alpha males on female stress in free-ranging baboons. Anim Behav. 2005;69(5):1211–1221. [Google Scholar]
- 53.Engh A, et al. Female hierarchy instability, male immigration, and infanticide increase glucocorticoid levels in female chacma baboons. Anim Behav. 2006;71(5):1227–1237. [Google Scholar]
- 54.Wittig RM, et al. Focused grooming networks and stress alleviation in wild female baboons. Horm Behav. 2008;54(1):170–177. doi: 10.1016/j.yhbeh.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Engh AL, et al. Behavioural and hormonal responses to predation in female chacma baboons (Papio hamadryas ursinus) Proc Biol Sci. 2006;273(1587):707–712. doi: 10.1098/rspb.2005.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cheney DL, Seyfarth RM. Reconciliatory grunts by dominant female baboons influence victims’ behaviour. Anim Behav. 1997;54(2):409–418. doi: 10.1006/anbe.1996.0438. [DOI] [PubMed] [Google Scholar]
- 57.Wittig RM, Crockford C, Wikberg E, Seyfarth RM, Cheney DL. Kin-mediated reconciliation substitutes for direct reconciliation in female baboons. Proc Biol Sci. 2007;274(1613):1109–1115. doi: 10.1098/rspb.2006.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. Hierarchical classification by rank and kinship in baboons. Science. 2003;302(5648):1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- 59.Engh A, Hoffmeier R, Cheney D, Seyfarth R. Who me? Can baboons infer the target of vocalisations? Anim Behav. 2006;71(2):381–387. [Google Scholar]
- 60.Wittig R, Crockford C, Seyfarth R, Cheney D. Vocal alliances in chacma baboons, Papio hamadryas ursinus. Behav Ecol Sociobiol. 2007;61(6):899–909. [Google Scholar]
- 61.Cheney DL, Moscovice LR, Heesen M, Mundry R, Seyfarth RM. Contingent cooperation between wild female baboons. Proc Natl Acad Sci USA. 2010;107(21):9562–9566. doi: 10.1073/pnas.1001862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keagy J, Savard J-F, Borgia G. Complex relationship between multiple measures of cognitive ability and male mating success in satin bowerbirds, Ptilonorhynchus violaceus. Anim Behav. 2011;81(4):1063–1070. [Google Scholar]
- 63.Cole EF, Morand-Ferron J, Hinks AE, Quinn JL. Cognitive ability influences reproductive life history variation in the wild. Curr Biol. 2012;22(19):1808–1812. doi: 10.1016/j.cub.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 64.Cauchard L, Boogert N, Lefebvre L, Dubois F, Doligez B. Problem-solving performance correlates with reproductive success in a wild bird population. Anim Behav. 2013;85(1):19–26. [Google Scholar]
- 65.Capitanio JP. Individual differences in emotionality: Social temperament and health. Am J Primatol. 2011;73(6):507–515. doi: 10.1002/ajp.20870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tomasello M, Carpenter M, Call J, Behne T, Moll H. Understanding and sharing intentions: The origins of cultural cognition. Behav Brain Sci. 2005;28(5):675–691. doi: 10.1017/S0140525X05000129. [DOI] [PubMed] [Google Scholar]
- 67.Warneken F, Tomasello M. Varieties of altruism in children and chimpanzees. Trends Cogn Sci. 2009;13(9):397–402. doi: 10.1016/j.tics.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 68.Silk JB, House BR. Evolutionary foundations of human prosocial sentiments. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10910–10917. doi: 10.1073/pnas.1100305108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheney DL. Extent and limits of cooperation in animals. Proc Natl Acad Sci USA. 2011;108(Suppl 2):10902–10909. doi: 10.1073/pnas.1100291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mitani J. In: Cooperation in Primates and Humans: Mechanisms and Evolution. Kappeler PM, van Schaik CP, editors. Berlin: Springer; 2006. pp. 101–113. [Google Scholar]
- 71.Sobolewski M, Brown J, Mitani J. Territoriality, tolerance and testosterone in wild chimpanzees. Anim Behav. 2012;84(6):1469–1474. [Google Scholar]
- 72.Sapolsky R. Why Zebras Don’t Get Ulcers. New York: Holt; 2004. [Google Scholar]


