Abstract
A neuroethological approach to human and nonhuman primate behavior and cognition predicts biological specializations for social life. Evidence reviewed here indicates that ancestral mechanisms are often duplicated, repurposed, and differentially regulated to support social behavior. Focusing on recent research from nonhuman primates, we describe how the primate brain might implement social functions by coopting and extending preexisting mechanisms that previously supported nonsocial functions. This approach reveals that highly specialized mechanisms have evolved to decipher the immediate social context, and parallel circuits have evolved to translate social perceptual signals and nonsocial perceptual signals into partially integrated social and nonsocial motivational signals, which together inform general-purpose mechanisms that command behavior. Differences in social behavior between species, as well as between individuals within a species, result in part from neuromodulatory regulation of these neural circuits, which itself appears to be under partial genetic control. Ultimately, intraspecific variation in social behavior has differential fitness consequences, providing fundamental building blocks of natural selection. Our review suggests that the neuroethological approach to primate behavior may provide unique insights into human psychopathology.
Keywords: decision, evolution, reward, serotonin, oxytocin
Sensitivity and responsiveness to information about others is critical for human health (1, 2), survival (3), and even financial success (4). To navigate our social worlds, we track the behavior of others and form models of their intentions and emotional states, we actively seek out and exchange information about others, and we flexibly alter our behavior in response to what we know about others. These faculties are so important to human behavior that their disruption constitutes psychopathology (5, 6). These specializations for social behavior reflect a rich evolutionary heritage of adaptation to group life (7–9). Like humans, many nonhuman primates also live in large groups characterized by patterns of social behaviors like grooming, imitative and cooperative foraging, differentiated affiliative relationships, ritualized courtship and mating behavior, and competitive interactions structured by social dominance (10, 11). Not surprisingly, the ability to deftly navigate the social environment has observable consequences for reproductive success in some nonhuman primates (12).
Evolutionary Perspective on Social Behavior
Social behavior places strong and unique demands on the nervous system. Across primate species, group size (a potential proxy of social complexity) is correlated with forebrain volume, after correcting for body size (9). Additional brain tissue beyond that required to maintain a body of a particular size is costly, in both developmental complexity and metabolic demands (7, 13–15). Indeed, social complexity and the elaboration of neural mechanisms to support it are associated with diets high in dependable calorie-rich foods (16–18). Major expansion of the hominine brain during human evolution appears to have coincided with the development of new behaviors that added more calories to the diet, such as eating meat (Homo habilis, ∼2.3 Mya) (19) and cooking (Homo erectus, ∼1.5 Mya) (20).
Social behavior seems likely to depend on homologous neural mechanisms in humans and nonhuman primates (21). Novel behaviors can evolve by connecting, repurposing (i.e., shifted to serve a new function), or elaborating upon ancestral mechanisms that originally served a different function (22), and the evolution of social behaviors seems likely to follow this pattern. A striking example of such elaboration and repurposing is the electrocommunication system of mormyrid fish. These fish have electrosensory receptors that are part of their lateral line system, which originally evolved to aid orienting and the detection of motion (23, 24). In mormyrids, the cerebellum, where sensations from the lateral line system are processed, is greatly enlarged and serves an important role in electrocommunication, a social function absent in the ancestral state (23, 24). The evolution of the neuropeptide oxytocin (OT) is another excellent example of repurposing for social functions. The ancestral anxiolytic (25, 26), approach- and tolerance-enhancing (27–29) roles of OT in early vertebrates may have been coopted to support parental behavior and social bonding in mammals.
In this review, we discuss recent evidence supporting the idea that social behavior can be constructed from the basic building blocks of nonsocial behaviors. In some cases, sociality is supported by general-purpose mechanisms whereas others may require special-purpose mechanisms. By “general purpose,” we mean that a given mechanism is used generally across both social and nonsocial domains whereas, by “special purpose,” we mean that a given mechanism has a privileged role in the social domain. Specialized mechanisms, such as the electrosensory receptor organ of mormyrid fish tuned for species communication and face identification cells in the temporal lobes of primates (30–33) and ungulates (34), are more frequently found near the input stages of social processing (i.e., receiving social information) whereas generalized mechanisms are more common near the output stages of effector control (35). By contrast, a mixture of specialized and generalized mechanisms appear to characterize intermediate computational stages of processing that translate socially specific inputs into motivational signals that guide learning and decision making, ultimately resulting in motor commands that generate behavior (36–38). Our review focuses on recent behavioral, neurobiological, and genetic findings supporting these general principles. Selected examples used in this review to support our claim are summarized in Table 1.
Table 1.
Summary list of selected examples from the current paper on how nonsocial functions are repurposed to serve social functions throughout evolution
| Biological units | Type/region | Nonsocial functions | Social functions |
| Behaviors | Foraging | Reward-seeking, information-seeking (39, 40, 43–45) | Social information-seeking (46–50, 56) |
| Imminent threat response | Reflexive, escape behavior (57) | Gaze aversion (38, 48) | |
| Distant threat response | Cautious exploratory behavior (58) | Social exploration (38, 48) | |
| Neural circuits | Posterior superior sulcus (pSTS) | Multisensory integration, perceiving intention from animacy (80, 82) | Gaze perception, gaze following (81, 83) |
| Lateral intraparietal area (LIP) | Spatial orienting, motor planning (84, 85) | Gaze direction, social value associated with space (35, 86, 87) | |
| Striatum (medial) | Reward and learning (70, 72) | Social image category, reward donation (37, 70) | |
| Orbitofrontal cortex (OFC) | Social image category, received reward during social interactions, social network size (36, 38, 93, 94) | ||
| Anterior cingulate sulcus (ACCs) | Foraging decisions, performance monitoring (41) | Foregone reward during social interactions (36) | |
| Anterior cingulate gyrus (ACCg) | Reward and learning (148) | Shared and donated reward during social interactions, social evaluation, other-regard, mentalizing about others’ states of mind (36, 71, 98–101) | |
| Neuromodulators | Oxytocin/vasopressin | Water regulation, reproduction, anxiolysis (25, 26, 105, 106) | Pair-bonding, parental care, selective aggression, social salience, generosity, trust (27–29, 97, 107–114) |
| HPA axis | Physical stress | Psychosocial stress (social status) (115–117) | |
| HPG axis | Reproduction | Social regulation/control, social opportunity (social status) (118–122) | |
| Serotonin | Cardiac and gastrointestinal functions, mood, memory, reward and learning (133, 134) | Social network integration, social structure, social information processing (124, 141, 142) |
Numbers in parentheses are references cited in the current review.
Parallels Between Social and Nonsocial Behaviors
Many of our behaviors are driven by reinforcement, and we and other animals seek a variety of rewards by foraging. Foraging is one of the most primitive and basic behavioral states, being a feature of essentially all motile, heterotrophic life. It is therefore unsurprising that foraging strategies are under strong selective pressure for maximizing returns on investment. Animals often forage for foods sparsely distributed in locally dense patches (39). As an animal forages in a patch, resources are depleted and the rate of energy intake slows. However, traveling to a new patch may be costly and accompanied by uncertain outcomes, leading to a decision to abandon a patch to maximize its overall rate of consumption. The same principle applies to many everyday decisions made by people. Because resources are often patchily distributed, this model has broad applicability. The optimal solution, known as Charnov’s Marginal Value Theorem, is that a patch should be abandoned when the current rate of consumption falls to the average for the overall environment (39). This model has been remarkably successful at describing the foraging behavior of a wide variety of organisms (40) and recently has been applied to understand neural correlates of foraging decisions (41, 42). In fact, foraging theory has also been applied to problems far afield from its original purpose, including the efficient design of web sites (43) and a description of how computer programmers search for errors in code (44).
Organisms searching for information can be said to be “information foraging” (45). Like foraging for primary rewards, information foraging presents opportunities as well as costs. Costs come in the form of missed opportunities to eat, drink, or sleep because information-seeking behaviors often demand certain postures or behavioral states incompatible with attentive orienting, as well as social costs, such as aggression from conspecifics and missed opportunities to interact with partners. Because social information has reinforcement value (either positive or negative), the basic problems studied by foraging theory may apply to the acquisition of social information. A wealth of behavioral data indicates that both humans and nonhuman primates actively seek social information. Humans and nonhuman primates find social stimuli to be intrinsically rewarding, and certain types of social stimuli are more interesting and reinforcing than others (46–48). For instance, even shortly after birth, human infants look longer at faces than at similar nonface stimuli (49). Likewise, nonhuman primates spend more time looking at pictures of faces directed toward them compared with pictures of faces with averted gaze (50), and direct their gaze more often toward higher-ranking than lower-ranking animals (51). Furthermore, active social interactions such as cooperative transactions (52, 53) or the opportunity to punish a traitor (54), which can be understood using a game theoretic framework (55), can be as motivating as primary rewards in humans. These observations support the hypothesis that the brains of many animals, especially those of primates, have evolved mechanisms that find social information rewarding and worth foraging.
We propose that, because a major function of the brain is to seek resources, it is likely that mechanisms that evolved to support foraging are readily repurposed to solve other, formally similar computational problems. With respect to social behavior, if information about others is a valuable resource, then the biological mechanisms underlying foraging decisions will be used to support social information seeking (56). For example, opportunities and costs associated with social information foraging are likely to engage fundamental biological mechanisms for computing opportunities and costs. Foraging mechanisms seem likely to have become further specialized to cope with the unique demands of interindividual dynamics that arise as a consequence of group living.
Another potential example of similarities between social and nonsocial behaviors arises from the comparison of behavioral responses to predators and social threats. In both cases, an imminent threat evokes fast, reflexive behaviors, such as freezing, defensive aggression, or escape behavior (57). A distant threat, however, elicits cautious exploratory behavior of the threatening object (58). Rhesus macaques, when given the opportunity, will opt to view pictures of dominant monkeys, a potentially threatening social stimulus, over pictures of subordinates (38, 48). Despite this interest, low-status monkeys typically avert their gaze from high-status monkey faces when confronted (48) and look quickly away from dominant male pictures after choosing to see them (48). This behavior is reminiscent of the exploratory behavior of rodents confronted with cat odor (58) and the avoidance behavior in the presence of an actual predator. Indeed, many fundamental behavioral strategies designed for nonsocial settings seem to resonate across behavioral strategies used in social settings.
Neural Circuits Guiding Social Decisions
The neural mechanisms supporting social behaviors are broadly distributed throughout the primate forebrain, overlapping with areas involved in more general-purpose functions (Fig. 1A). Current evidence suggests that most neural circuits involved in social behavior are not dedicated exclusively to “social” functions. Rather, such circuitry is also typically engaged in related nonsocial behaviors, regardless of whether social information is processed in a privileged manner (i.e., special purpose) or not (i.e., general purpose). This evidence supports the hypothesis that the evolution of novel social behaviors has occurred by coopting existing neural hardware for the purpose of interacting with others.
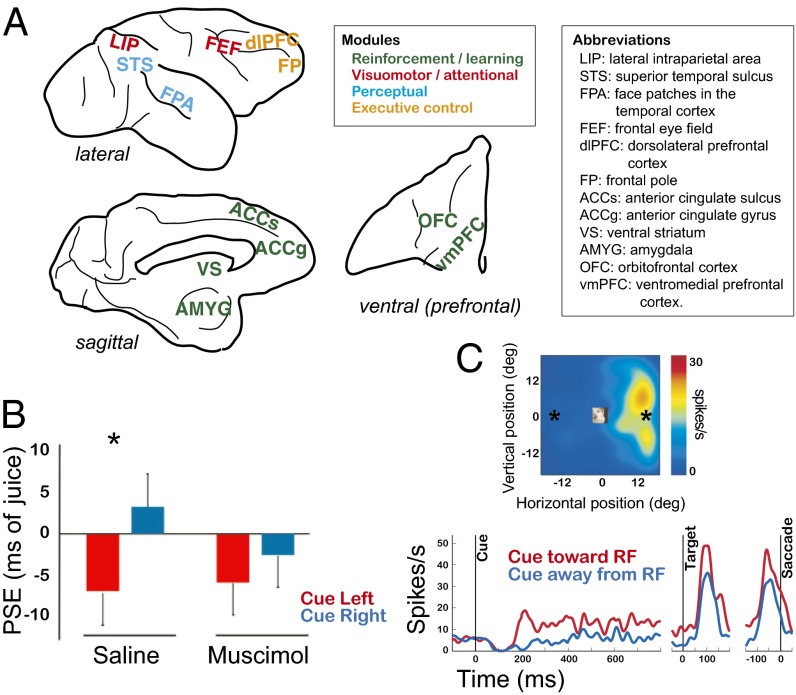
Fig. 1.
Example neural circuits coopted to serve social functions. (A) Representative brain regions in rhesus macaques whose preexisting functions encompass reward, attention, perception, and executive control. (B) Point of subjective equality (PSE), bias for socially-cued target in terms of foregone juice, after saline or muscimol injections in pSTS. Reproduced from (83) with permission from Oxford University Press. (C) LIP neuron showing firing rate enhancement by observed gaze directed toward the receptive field (RF). (Upper) RF map. (Lower) Neuronal activity as a function of time. Reproduced with permission from ref. 86.
Broadly speaking, these circuits can be thought of as organized into input, integrative, and output stages of social processing. The input stage of social processing comprises specialized sensory channels that transduce socially important information, including face-selective (59) and identity-specific cells (60) in primates, pheromone-sensing systems like the vomeronasal organ in rodents (61), and specialized regions for species-specific vocalizations in birds (62) and mammals (63, 64), and language in humans (65). The output stage of social processing comprises socially-specific motor patterns, including highly stereotyped behaviors like allogrooming (66), ritualized play (67), and threat and submission gestures (68). In the integrative stages of social information processing, studies in humans have shown that phenomena such as opprobrium and moral disgust rely in large part on circuits involved in nociception and interoception, particularly those linking the amygdala, periaqueductal gray, insular cortex, and anterior cingulate cortex (ACC) (69). Experiments in both humans and other animals have shown that information about socially relevant stimuli such as attractive faces, bodies, and rewards delivered to others activate regions likewise implicated in nonsocial reward (35, 36, 38, 70–74). These results are consistent with the idea that social processing is largely built upon and extended from other nonsocial computations by these neural circuits.
The demands of dynamic social interactions are likely to have further shaped the functions of neural circuits involved in social behavior (i.e., selection on a mechanism for a specific function). Humans and other primates clearly elaborate upon the aforementioned basic, relatively stereotyped patterns of social behavior. For example, both human and nonhuman primates can covertly attend to a specific location in space without looking at it directly (75, 76), a behavior that seems likely to have evolved to support monitoring of others in social groups (77, 78). Watching another individual shift gaze to an object or location in space typically evokes a gaze shift, as well as a shift in covert attention, in the same direction, in humans and other nonhuman primates (79). This gaze-following response depends upon neural circuits involved in decoding where another individual is looking, and circuits that orient attention and plan gaze shifts. Neurons in the primate superior temporal sulcus (STS) are involved in the integration of converging inputs from multiple sensory modalities (80). A posterior portion of STS (pSTS) seems to have evolved the specialized function of perceiving the gaze of other individuals (81) as well as intention implied from animacy (82). Consistent with its role in gaze perception, inactivating pSTS with muscimol abolishes gaze-following in rhesus macaques (83) (Fig. 1B). Neurons in the primate lateral intraparietal area (LIP), an area important for spatial attention and the oculomotor planning (84, 85), are activated by the mere observation of a monkey looking toward the region of space covered by the neurons’ receptive fields (86) (Fig. 1C). Unlike pSTS, however, inactivating LIP has no specific impact on gaze following (87), consistent with a more generalized role in visuomotor behavior.
As mentioned previously, both human and nonhuman primates are highly motivated by social information. Social information activates key reward areas in humans and nonhuman primates, including the ACC, orbitofrontal cortex (OFC), nucleus accumbens, and caudate nucleus (36–38, 70–74). These observations suggest the possibility that social information and information about primary motivators like food are translated into a common framework or currency that drives both learning and decision making (88). When monkeys choose between fluid rewards and information about others (38, 48), neurons in area LIP simultaneously encode the social value and fluid value associated with a target in space, consistent with a common currency of target/action value (35). By contrast, neurons in the primate striatum, particularly the medial aspect, appear to be more specialized for signaling social information (37). In monkeys choosing between fluid rewards and information about others, similar proportions of neurons (∼30–35%) carried information about fluid outcomes and social image outcomes, but these populations were largely nonoverlapping. Thus, multiple, unique, small ensembles of striatal neurons appear to convey idiosyncratic yet highly specific information about motor responses, contexts, cues, outcomes, or combinations thereof, and this organization extends to social behavior.
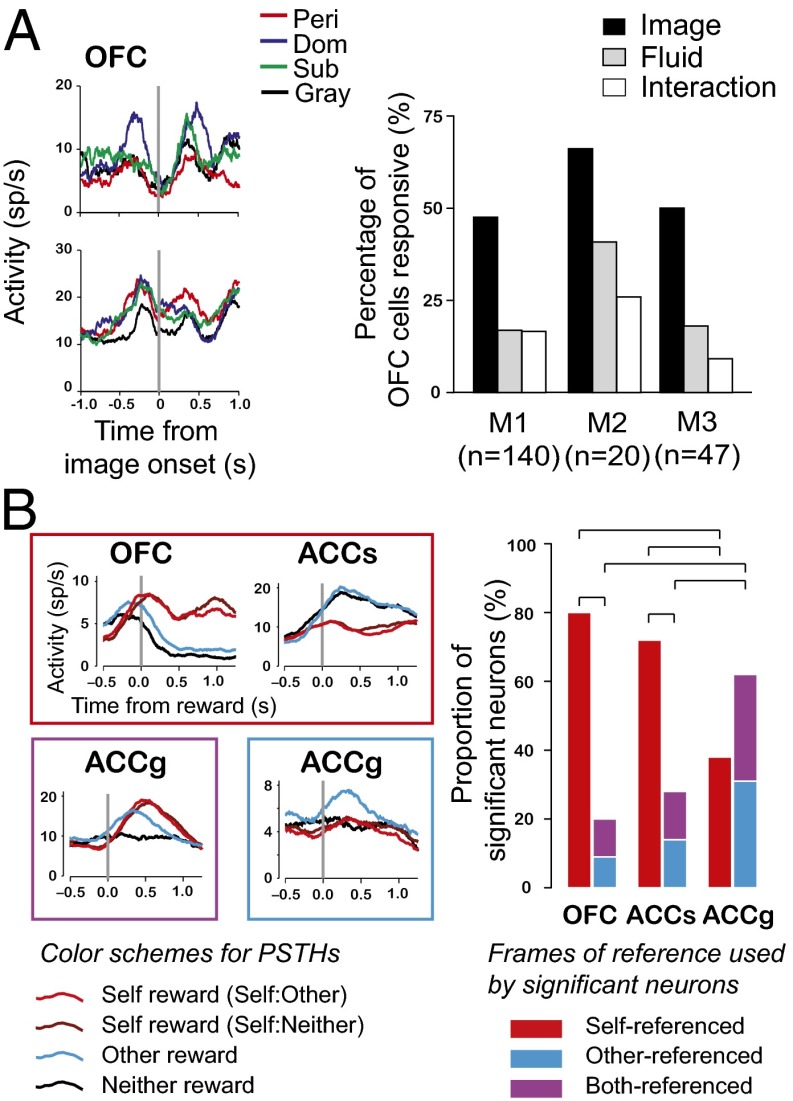
The OFC also encodes the value of rewards like food and money (89). Like the striatum, OFC also contains neurons specialized for social interaction. We found that even when monkeys’ choices were dominated by the value of fluid rewards, the responses of ∼50% of neurons encoded social information, but only ∼20% encoded information about fluid rewards (Fig. 2A) (38). As in striatum, these populations of neurons were largely distinct, but, unlike striatum, they were anatomically intermingled. Notably, individual OFC neurons also signaled categorical information with respect to images of other monkeys (Fig. 2A). On the basis of its connections to gustatory, olfactory, interoceptive, and limbic systems, OFC has been proposed to function as a feeding circuit (90–92). Thus, the observation that more neurons responded to social information than to fluid reward supports the idea that ancestral neural adaptations are repurposed to serve social functions. These findings, along with the observed relationship of OFC size to social network size in humans (93) and group size across primates (94), suggest that OFC is part of a specialized neural circuit that evolved concomitantly with increasing sophistication of social behavior.
Fig. 2.
Reward circuits coopted to serve social functions. (A, Left) Firing rates aligned to social image onset for OFC neurons in a social choice task. (Right) Percentage of OFC neurons with activity significantly modulated by social image category (black bar), fluid amount (gray bar), or their interaction (white bar) for three monkeys (M1–M3). Reproduced from (38) with permission from Elsevier. (B, Left) Firing rates of example neurons from each area, aligned to reward delivery. Box color signifies the category to which these neurons belong in the bar graphs. (Right) Proportion of significant neurons from OFC, ACCs, and ACCg using self, other, and shared frames of reference to encode reward outcomes during a reward-allocation task. Horizontal lines indicate significant differences (P < 0.05, χ2 test). Reproduced from ref. 36 with permission.
Highly specialized neural mechanisms may be required to support complex social interactions that depend on the behavior and intentions of other individuals. This process may require the brain to encode sensory, motor (95), and even reward information in multiple frames of reference (36). We recently investigated how neurons in three frontal cortical areas—anterior cingulate gyrus (ACCg), anterior cingulate sulcus (ACCs), and OFC—encoded reward information while monkeys decided to deliver juice to themselves, to a recipient monkey, or to no one (36). In this social reward-allocation task, monkeys tend to prefer to reward someone over no one, and this prosocial preference is magnified by familiarity and dominance status (96) and significantly modulated by neuropeptide OT (97). We found remarkable specializations in the way neurons in these three areas encoded reward information in this social task. OFC neurons predominantly signaled rewards directly received by the donor monkey, revealing its egocentric encoding scheme; ACCs neurons predominantly signaled rewards foregone by the donor monkey, a process critical for monitoring outcomes and learning; and ACCg neurons signaled rewards delivered to the recipient or mirrored rewards delivered to either the donor or the recipient, indicating specialized functions for other-regarding social behaviors (36) (Fig. 2B). These findings resonate with previous work showing that lesions in ACCg, but not ACCs or OFC, lead to deficits in understanding the meaning of social cues in monkeys (98) and the activation of medial prefrontal and gyral portions of ACC in humans by observing events occurring to others or thinking about others’ states of mind (71, 99–101). Together, these observations suggest that ACCg is a key structure supporting shared experience and social reward and may be specialized in human and nonhuman primates to support complex social interactions.
Neuromodulatory Influences on Social Behavior
Differences between species or between individuals within a species may reflect neuromodulatory influences on the development and function of neural circuits mediating social and nonsocial behaviors. Hormones strongly influence brain development (102, 103) and shape the expression of fundamental behaviors like feeding, fleeing, fighting, and mating (104). Neuropeptides (peptides used by neurons to communicate with one another) set the tone for state-specific neuronal signaling by altering chemical transmission within individual neurons as well as across networks of neurons (104). For example, OT cells in the paraventricular and supraoptic nuclei synchronize their activity to achieve coordinated neurosecretary bursts required for milk ejection during lactation (105).
Neuropeptides involved in these primary functions are often recruited to mediate social behavior. The nonapeptides OT and arginine vasopressin (AVP) nicely illustrate this principle. Both OT and AVP are involved in basic reproductive functions in mammals, including parturition and lactation in females and erection and ejaculation in males (106). Building on pioneering work demonstrating a role for OT in maternal behavior in rats (107), a series of elegant studies in voles has revealed that OT and AVP also regulate social behaviors like pair bonding (108) and selective aggression (109). More recently, it has been shown that exogenous application of OT can promote emotions like trust (110) and encourage generosity (111), in a context-dependent and sometimes idiosyncratic fashion (112).
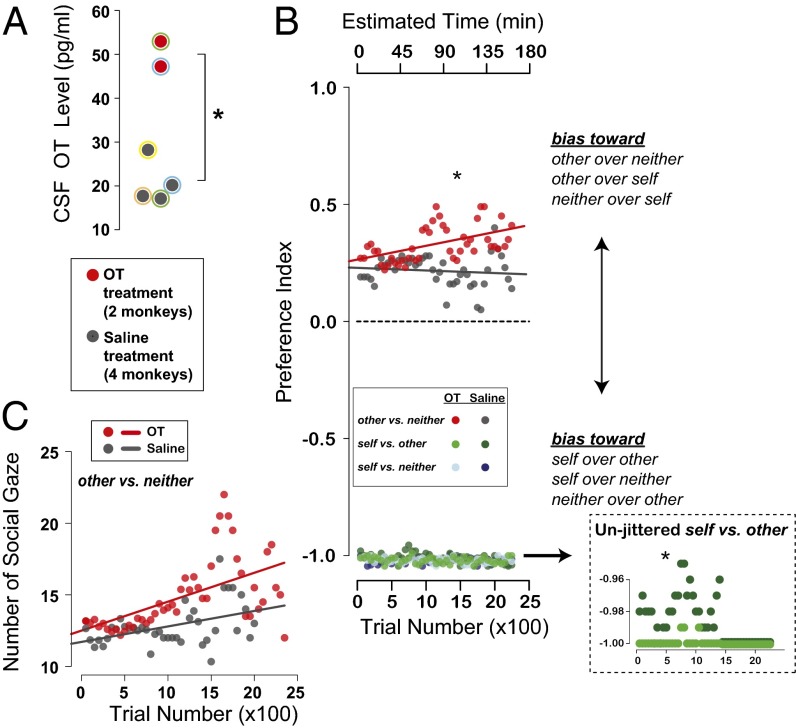
Recently, we demonstrated that OT inhaled via a nebulizer effectively penetrates the central nervous system in rhesus macaques (97) (Fig. 3A), endorsing the potential promise of OT inhalation therapy in individuals with neuropsychiatric disorders marked by social deficits (5). Increasing OT levels in the brain via inhalation also promotes prosocial decisions in monkeys as well as their attention to a social partner (Fig. 3 B and C). Surprisingly, OT also promotes selfish decisions in the same task when there is a perceived cost (97) (Fig. 3B). Furthermore, a recent study in chimpanzees has shown that a rise in OT levels following grooming depends on whether the two animals have strong bonds (113), suggesting that the OT system has been further specialized to process partner-specific affiliative interactions. Together, these observations endorse the idea that neuropeptides like OT, which serves basic sexual and parenting functions, can be coopted to regulate more complex social behaviors in species that live in large, complex groups, like humans and rhesus macaques.
Fig. 3.
Social functions of neuropeptide OT. (A) OT concentration in cerebrospinal fluid (CSF) after inhaling OT (red) or saline (dark gray; *P < 0.05, Welch two-sample t test). Colored outlines on data points indicate animal IDs. (B) Choice preference index for OT (red) and saline (gray) for rewards delivered to: other (recipient) vs. neither, self (actor) vs. other, and self vs. neither in the social reward-allocation task. Data points from self vs. other and self vs. neither are jittered for visibility. Inset shows unjittered data from self vs. other trials. (C) Number of gaze shifts to recipient after reward delivery over the course of each session for other vs. neither choice trials. Reproduced from ref. 97 with permission.
Ultimately, neuropeptides like OT may impact even complex social behavior via a basic set of mechanisms. The anxiolytic effects (25, 26) of OT may have served as a preadaptation for the prolonged interaction necessary for high-intensity parental care in mammals by promoting approach behavior and enhancing tolerance (27–29). These basal functions could then serve as building blocks for more complex social behaviors. Suppressing vigilance and increasing tolerance to nonoffspring may permit extended interactions with others. Ultimately, complex emotions like trust may arise via reduced social apprehension and enhanced tolerance, under the regulatory influence of neuropeptides like OT (114).
Other neuromodulatory systems also contribute to variation in social behavior. For example, the hypothalamic–pituitary–adrenal (HPA) axis has long been associated with social status in primates (115, 116) and may play a critical role in the production of behavior. Yellow-bellied marmots were shown to be more likely to emit alarm calls during periods in which their HPA axis activity (measured by fecal cortisol concentrations) was high compared with periods during which it was low (117). The hypothalamic–pituitary–gonadal (HPG) axis also shapes social behavior in vertebrates. According to the “challenge hypothesis,” males’ androgen levels are modulated according to context-dependent requirements for aggressive behavior (118), and this prediction has been substantiated broadly among vertebrates (119). In rhesus macaques, modulations of testosterone levels in response to social challenge are also dependent on social rank (120). Male social status in African cichlid fish is regulated by gonadotropin-releasing hormone 1, a hormone critical for reproduction, at various levels of neuronal processing (121, 122). These findings resonate with the idea that preexisting signaling pathways, in this case pathways that regulate stress and mating behaviors, are repurposed to shape the development and function of neural circuits mediating social behavior. Through duplication, repurposing, and dynamic regulation of elements, a relatively limited toolkit of basic hormonal mechanisms can be used to generate a wide array of social behavior.
Genetic Regulation of Social Behavior
The influence of genes on social behavior is undeniable because genes shape the neural circuits that produce behavior (123). The adoption of preexisting biological mechanisms for social purposes, and indeed the evolution of social behavior in general, must, therefore, have roots in genetic change, or, in more Darwinian terms, must be based on modification through descent of inherited material. One hint that social behavior influences change in gene pools over time is a handful of studies linking sociality with fitness. In species such as baboons and rhesus macaques, engaging in social interactions is correlated with reproductive output; the offspring of individuals that spend a greater amount of time grooming and associating with others are more likely to survive to 1 y of age (12, 124). This correlation, in female baboons at least, seems to be driven by the quality of social relationships as individuals with the strongest, most enduring social bonds have higher offspring survival (125) and greater longevity (126) than others. These findings suggest that there are adaptive benefits to interacting with others and that social behavior is shaped by natural selection.
However, such findings beg confirmation that social tendencies actually have a genetic basis and ask that we explore the roles of environment and experience in shaping the impact of genes on behavior. Quantitative genetic analysis is a tool that allows researchers to determine the amount of variance in a trait that can be attributed to genes, otherwise known as the amount of additive genetic variance or heritability. Using this technique, dimensions of human personality, including sociability, have been shown to be heritable (127). Similar findings show that the behavioral tendencies of a number of vertebrate species, including some nonhuman primates (128–130), are heritable, thus pointing to a (partly) genetic basis for primate social behavior. Not only are social components of personality heritable, but so too is the extent to which individuals are integrated into their social networks in both humans (131) and rhesus macaques (124). This integration includes social network connections mediated by multiagent relationships, such as friend-of-a-friend relationships. Such indirect social connections might be emergent properties of a social network or reflect meaningful aspects of the way individuals navigate large groups. Nevertheless, humans exploit these connections, and our actions (consciously or not) are influenced by them via reputation, one of the primary mechanisms believed to underlie the evolution of cooperation in humans (132).
Genetic information also shapes the specific proximate mechanisms that underlie the processing of social information and expression of social behavior. An excellent example is the serotonin pathway. Serotonin is involved in a host of peripheral functions, including cardiac and gastrointestinal functions (133). Centrally, serotonin regulates mood, memory, and reward (133, 134). The serotonin pathway is also involved in the expression of social behavior. Genetic studies have tied this neuromodulatory pathway to social behavior in humans and other primates, with variants of two serotonergic genes having been examined in particular depth: a variable insertion in the gene encoding tryptophan hydroxylase (TPH2), the rate limiting enzyme in serotonin synthesis, and the 5HTTLPR (serotonin-transporter-linked polymorphic region) polymorphism within the promoter region of the serotonin transporter gene (SLC6A4, solute carrier family 6 member 4). Both variants have orthologs in humans and rhesus macaques, have been associated with altered development of several brain regions (135, 136), and may influence the intensity and duration of signaling at serotonergic synapses (135, 137).
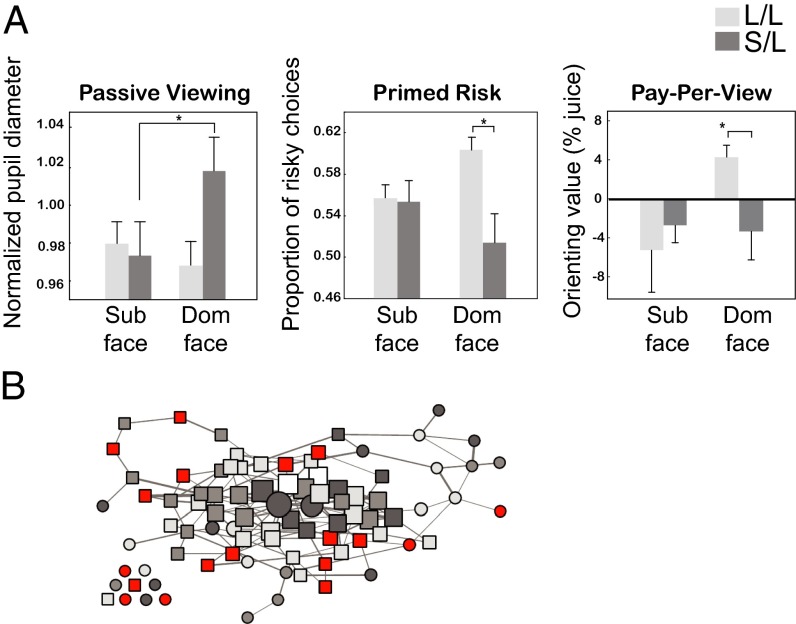
Both TPH2 and SLC6A4 have been associated with social behavior phenotypes and endophenotypes, many of which are likely to have strong ties to serotonin’s central functions, such as the regulation of reward. For example, both genes have been implicated in neuropsychiatric diseases, such as autism and depression (138, 139), which are partly characterized by disruptions in social attention and interaction, and are accompanied by differences in brain response to social stimuli (140). The 5-HTTLPR polymorphism predicts social avoidance in rhesus macaques in response to familiar dominant face images across many contexts. Specifically, rhesus macaques with a copy of the “short” allele spend less time looking at the eyes of other monkeys, show greater pupil dilation—a peripheral index of arousal—when viewing dominant faces, shy away from risk after being primed with dominant faces, and typically avoid dominant faces during a reward-guided decision-making task (141) (Fig. 4A).
Fig. 4.
Genetic variations in the serotonergic system predict social behavior. (A) Monkeys with a “short” copy of the 5-HTTLPR polymorphism (S/L) show increased pupil dilation to a dominant face (Left), suppressed risk following a dominant face flash (Center), and do not forego juice to view a dominant face (Right). (B) Serotonergic gene profiles predict social network position in free-ranging rhesus macaques. Squares, females; circles, males; lines, presence of a grooming interaction between monkeys. Increasing line thickness indicates frequency of interaction. Node size and position reflect social centrality; largest nodes are the most socially central. Monkeys most central in the network were less likely to carry the minor allele for both the 5-HTTLPR or TPH2 length polymorphisms (gray nodes). A was reproduced from ref. 141, and B was reproduced from ref. 124 with permission.
Across the genus Macaca, the 5-HTTLPR polymorphism has been related to social structure, with more despotic species, such as the rhesus macaque, possessing both long and short numbers of repeats, whereas purportedly less despotic macaque species are monomorphic for the long allele (142). This finding may suggest that this polymorphism confers resilience to psycho-socio challenges (143) but may also point to the interplay between serotonin, social behavior, reward, and risk aversion. Heightened social vigilance may confer particular advantages in the competitive situations that occur more frequently in despotic societies (144). The 5-HTTLPR polymorphism is associated with differential activation of a number of brain regions associated with affiliative behavior (e.g., ACC, insular cortex) (145), leading to speculation that serotonergic gene profiles play a role not only in competition, but also in positive social interactions (145).
We recently found preliminary evidence supporting this hypothesis in a study of rhesus macaques living in a free-ranging colony on Cayo Santiago Island, Puerto Rico. An individual monkey’s position in the social (grooming) network was predicted by the interaction between the 5-HTTLPR and TPH2 length polymorphisms. Either mutation alone had no effect on network position, but monkeys with the rare allele of both genes were less well-integrated socially (124) (Fig. 4B). Overall, these results suggest that genetic factors that influence the development and functioning of the serotonin system shape primate social behavior. Serotonin-related genes therefore may be viewed as a valuable example of “candidate genes” that provide tractability to empirical questions about the interaction of genes, neural circuits, and social behavior. These tantalizing findings require further study to understand the specific genetic contributions of this system and other neuromodulatory systems to various aspects of social behavior and cognition.
It is fitting to end a survey of the neuroethology of social behavior on a genetic note, as in doing so we return to the very roots of evolutionary change. Genetic information not only represents a powerful tool to investigate the proximate bases of social behavior, but also allows us to establish direct links between sociality and evolutionary fitness, the ultimate driving force behind natural selection. Genetic information exposes the dynamic contingencies upon which sociality is based, where the interactions between genes that lay the foundations of neural architecture and the social, physical, and biochemical environments in which those genes exist are brought to light, and wherein lie some of the greatest challenges facing future researchers hoping to understand this complex and enigmatic trait.
Concluding Remarks
Social information is clearly valuable—it is worth foraging, often receives privileged attention over other types of information, and is inherently rewarding. The social environment is rife with information and tinged with uncertainty, and as a result much of our mental machinery is applied to reducing the cognitive load of social interaction. Social behaviors impact evolutionary fitness (12, 124), suggesting they are critical for survival and reproduction. Biological mechanisms that primarily functioned to mediate nonsocial behaviors in the ancestral state have been repurposed in some species, like humans and rhesus macaques, to mediate social behavior. Biological mechanisms are rededicated and further modified for social functions at multiple levels of organization, from neurons and circuits, to hormones and genes. It is important to note, however, that social behavior also feeds back upon these mechanisms to shape their structure and function. Manipulations of social network size in rhesus macaques alter cortical thickness and functional coupling across brain areas that support social functions (146). Epigenetics and gene regulation are also essential to guiding changes in neural development and social behavior (116, 121, 147). Epigenetic changes that are related to reinforcement and learning might be particularly powerful and are important directions for future research.
A neuroethological approach to the study of human and nonhuman primate social behavior is powerful in the extent to which it is encompassing and holistic. By presenting the evolution of social behavior through a lens of nonsocial functions, we have provided evolutionarily parsimonious lines of reasoning and evidence, along with tractable avenues for future research. For many human psychopathologies, the interactions between social and nonsocial deficits are poorly understood. Greater comprehension of the general-purpose mechanisms that generate social action and translate social signals may therefore improve disease diagnosis and treatment.
Acknowledgments
We thank Monica L. Carlson for general technical assistance. This work was collectively supported by National Institute of Mental Health Grants K99-MH099093 (to S.W.C.C.), R01-MH095894 (to M.L.P. and S.W.C.C.), R01-MH086712 (to M.L.P., K.K.W., and G.K.A.), R01-MH096875 and R01-MH089484 (to M.L.P. and L.J.N.B.), and F31-MH081443 (to J.T.K.); Department of Defense CDMRP Grant W81XWH-11-1-0584 (to M.L.P. and S.W.C.C.); National Eye Institute Grant R01-EY019303 (to J.M.P.); Cure Autism Now (K.K.W.); The Davis Foundation (K.K.W.); a Duke Institute for Brain Sciences Incubator Award (to M.L.P.); and a Duke Center for Interdisciplinary Decision Sciences Fellowship (to L.J.N.B.).
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “In the Light of Evolution VII: The Human Mental Machinery,” held January 10–12, 2013, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and audio files of most presentations are available on the NAS Web site at www.nasonline.org/evolution_vii.
This article is a PNAS Direct Submission.
References
- 1.Berkman LF. Social support, social networks, social cohesion and health. Soc Work Health Care. 2000;31(2):3–14. doi: 10.1300/J010v31n02_02. [DOI] [PubMed] [Google Scholar]
- 2.Cohen S. Social relationships and health. Am Psychol. 2004;59(8):676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- 3.Barefoot JC, Grønbaek M, Jensen G, Schnohr P, Prescott E. Social network diversity and risks of ischemic heart disease and total mortality: Findings from the Copenhagen City Heart Study. Am J Epidemiol. 2005;161(10):960–967. doi: 10.1093/aje/kwi128. [DOI] [PubMed] [Google Scholar]
- 4.Baron RA, Markman GD. Beyond social capital: The role of entrepreneurs' social competence in their financial success. J Bus Venturing. 2003;18(1):41–60. [Google Scholar]
- 5.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 6.Adolphs R. Cognitive neuroscience of human social behaviour. Nat Rev Neurosci. 2003;4(3):165–178. doi: 10.1038/nrn1056. [DOI] [PubMed] [Google Scholar]
- 7.Allman JM. Evolving Brains. New York: Scientifc American Library; 1999. [Google Scholar]
- 8.Byrne R, Whiten A. Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. Oxford: Oxford Science Publications; 1989. [Google Scholar]
- 9.Dunbar RIM. The social brain hypothesis. Evolutionary Anthropology. 1998;6(5):178–190. [Google Scholar]
- 10.Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT. Primate Societies. Chicago: Univ of Chicago Press; 1987. [Google Scholar]
- 11.Wilson EO. Sociobiology: The New Synthesis. Cambridge, MA: Belknap Press of Harvard Univ Press; 1975. [Google Scholar]
- 12.Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302(5648):1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- 13.Lennie P. The cost of cortical computation. Curr Biol. 2003;13(6):493–497. doi: 10.1016/s0960-9822(03)00135-0. [DOI] [PubMed] [Google Scholar]
- 14.Aiello LC, Wheeler P. The expensive-tissue hypothesis: The brain and the digestive system in human and primate evolution. Curr Anthropol. 1995;36(2):199–221. [Google Scholar]
- 15.Leonard WR, Robertson ML, Snodgrass JJ, Kuzawa CW. Metabolic correlates of hominid brain evolution. Comp Biochem Physiol A Mol Integr Physiol. 2003;136(1):5–15. doi: 10.1016/s1095-6433(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 16.Harding RS. An order of omnivores: Nonhuman primate diets in the wild. In: Harding RS, Teleki G, editors. Omnivorous Primates: Gathering and Hunting in Human Evolution. New York: Columbia Univ Press; 1981. pp. 191–214. [Google Scholar]
- 17.Milton K. Food choice and digestive strategies of two sympatric primate species. Am Nat. 1981;137(4):496–505. [Google Scholar]
- 18.Harvey PH, Clutton-Brock TH, Mace GM. Brain size and ecology in small mammals and primates. Proc Natl Acad Sci USA. 1980;77(7):4387–4389. doi: 10.1073/pnas.77.7.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leakey LS, Tobias PV, Napier JR. A new species of the genus Homo from Olduvai Gorge. Nature. 1964;202(4927):7–9. doi: 10.1038/202007a0. [DOI] [PubMed] [Google Scholar]
- 20.Wrangham R. Catching Fire: How Cooking Made Us Human. Philadelphia: Basic Books; 2009. [Google Scholar]
- 21.Rushworth MF, Mars RB, Sallet J. Are there specialized circuits for social cognition and are they unique to humans? Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2012.11.013. 10.1016/ j.conb.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Katz PS, Harris-Warrick RM. The evolution of neuronal circuits underlying species-specific behavior. Curr Opin Neurobiol. 1999;9(5):628–633. doi: 10.1016/S0959-4388(99)00012-4. [DOI] [PubMed] [Google Scholar]
- 23.Katz PS. Comparative neurophysiology: An electric convergence in fish. Curr Biol. 2006;16(9):R327–R330. doi: 10.1016/j.cub.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 24.Montgomery JC. “Seeing” with nonvisual senses: Mechano- and electrosensory systems of fish. Physiology (Bethesda) 1991;6:73–77. [Google Scholar]
- 25.Neumann ID, Torner L, Wigger A. Brain oxytocin: Differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95(2):567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida M, et al. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29(7):2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kemp AH, Guastella AJ. Oxytocin: Prosocial behavior, social salience, or approach-related behavior? Biol Psychiatry. 2010;67(6):e33–e34; author reply e35. doi: 10.1016/j.biopsych.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Young LJ. The neurobiology of social recognition, approach, and avoidance. Biol Psychiatry. 2002;51(1):18–26. doi: 10.1016/s0006-3223(01)01268-9. [DOI] [PubMed] [Google Scholar]
- 29.Averbeck BB. Oxytocin and the salience of social cues. Proc Natl Acad Sci USA. 2010;107(20):9033–9034. doi: 10.1073/pnas.1004892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perrett DI, Rolls ET, Caan W. Visual neurones responsive to faces in the monkey temporal cortex. Exp Brain Res. 1982;47(3):329–342. doi: 10.1007/BF00239352. [DOI] [PubMed] [Google Scholar]
- 31.Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4(8):2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desimone R. Face-selective cells in the temporal cortex of monkeys. J Cogn Neurosci. 1991;3(1):1–8. doi: 10.1162/jocn.1991.3.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Tsao DY, Freiwald WA, Knutsen TA, Mandeville JB, Tootell RB. Faces and objects in macaque cerebral cortex. Nat Neurosci. 2003;6(9):989–995. doi: 10.1038/nn1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kendrick K. Neurobiological correlates of visual and olfactory recognition in sheep. Behav Processes. 1994;33(1):89–111. doi: 10.1016/0376-6357(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 35.Klein JT, Deaner RO, Platt ML. Neural correlates of social target value in macaque parietal cortex. Curr Biol. 2008;18(6):419–424. doi: 10.1016/j.cub.2008.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang SW, Gariépy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci. 2013;16(2):243–250. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein JT, Platt ML. Social information signaling by neurons in primate striatum. Curr Biol. 2013;23(8):691–696. doi: 10.1016/j.cub.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson KK, Platt ML. Social signals in primate orbitofrontal cortex. Curr Biol. 2012;22(23):2268–2273. doi: 10.1016/j.cub.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Charnov EL. Optimal foraging, the marginal value theorem. Theor Popul Biol. 1976;9(2):129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- 40.Stephens DW, Krebs JR. Foraging Theory. Princeton: Princeton Univ Press; 1986. [Google Scholar]
- 41.Hayden BY, Pearson JM, Platt ML. Neuronal basis of sequential foraging decisions in a patchy environment. Nat Neurosci. 2011;14(7):933–939. doi: 10.1038/nn.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolling N, Behrens TE, Mars RB, Rushworth MF. Neural mechanisms of foraging. Science. 2012;336(6077):95–98. doi: 10.1126/science.1216930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller CS, Remington RW. Modeling information navigation: Implications for information architecture. Hum Comput Interact. 2004;19(3):225–271. [Google Scholar]
- 44.Lawrance J, et al. How programmers debug, revisited: An information foraging theory perspective. IEEE Trans Software Engineering. 2013;39(2):197–215. [Google Scholar]
- 45.Pirolli P. Information Foraging Theory: Adaptive Interaction with Information. Oxford: Oxford Univ Press; 2007. [Google Scholar]
- 46.Emery NJ. The eyes have it: The neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24(6):581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 47.Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proc Biol Sci. 2007;274(1619):1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deaner RO, Khera AV, Platt ML. Monkeys pay per view: Adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15(6):543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 49.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40(1-2):1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 50.Keating CF, Keating EG. Visual scan patterns of rhesus monkeys viewing faces. Perception. 1982;11(2):211–219. doi: 10.1068/p110211. [DOI] [PubMed] [Google Scholar]
- 51.McNelis NL, Boatright-Horowitz SL. Social monitoring in a primate group: The relationship between visual attention and hierarchical ranks. Anim Cogn. 1998;1(1):65–69. [Google Scholar]
- 52.Rilling J, et al. A neural basis for social cooperation. Neuron. 2002;35(2):395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 53.Rand DG, Greene JD, Nowak MA. Spontaneous giving and calculated greed. Nature. 2012;489(7416):427–430. doi: 10.1038/nature11467. [DOI] [PubMed] [Google Scholar]
- 54.de Quervain DJ, et al. The neural basis of altruistic punishment. Science. 2004;305(5688):1254–1258. doi: 10.1126/science.1100735. [DOI] [PubMed] [Google Scholar]
- 55.Lee D. Game theory and neural basis of social decision making. Nat Neurosci. 2008;11(4):404–409. doi: 10.1038/nn2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adams GK, Watson KK, Pearson J, Platt ML. Neuroethology of decision-making. Curr Opin Neurobiol. 2012;22(6):982–989. doi: 10.1016/j.conb.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fanselow MS, Lester LS. A functional behavioristic approach to aversively motivated behavior: Predatory imminence as a determinant of the topography of defensive behavior. In: Bolles RC, Beecher MD, editors. Evolution and Learning. Hillsdale, NJ: Lawrence Erlbaum; 1988. pp. 185–212. [Google Scholar]
- 58.Dielenberg RA, Carrive P, McGregor IS. The cardiovascular and behavioral response to cat odor in rats: unconditioned and conditioned effects. Brain Res. 2001;897(1-2):228–237. doi: 10.1016/s0006-8993(01)02227-2. [DOI] [PubMed] [Google Scholar]
- 59.Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311(5761):670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435(7045):1102–1107. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- 61.Keverne EB. The vomeronasal organ. Science. 1999;286(5440):716–720. doi: 10.1126/science.286.5440.716. [DOI] [PubMed] [Google Scholar]
- 62.Doupe AJ, Konishi M. Song-selective auditory circuits in the vocal control system of the zebra finch. Proc Natl Acad Sci USA. 1991;88(24):11339–11343. doi: 10.1073/pnas.88.24.11339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eliades SJ, Wang X. Neural substrates of vocalization feedback monitoring in primate auditory cortex. Nature. 2008;453(7198):1102–1106. doi: 10.1038/nature06910. [DOI] [PubMed] [Google Scholar]
- 64.Ghazanfar AA, Hauser MD. (2) auditory behaviour of primates: A neuroethological perspective. Curr Opin Neurobiol. 2001;11(6):712–720. doi: 10.1016/s0959-4388(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 65.Damasio AR, Geschwind N. The neural basis of language. Annu Rev Neurosci. 1984;7(1):127–147. doi: 10.1146/annurev.ne.07.030184.001015. [DOI] [PubMed] [Google Scholar]
- 66.Schino G, Scucchi S, Maestripieri D, Turillazzi PG. Allogrooming as a tension-reduction mechanism: A behavioral approach. Am J Primatol. 1988;16(1):43–50. doi: 10.1002/ajp.1350160106. [DOI] [PubMed] [Google Scholar]
- 67.Grant EG, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21(3-4):246–259. [Google Scholar]
- 68.Deag JM. Aggression and submission in monkey societies. Anim Behav. 1977;25(2):465–474. [Google Scholar]
- 69.Moll J, Zahn R, de Oliveira-Souza R, Krueger F, Grafman J. Opinion: The neural basis of human moral cognition. Nat Rev Neurosci. 2005;6(10):799–809. doi: 10.1038/nrn1768. [DOI] [PubMed] [Google Scholar]
- 70.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–294. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 71.Mobbs D, et al. A key role for similarity in vicarious reward. Science. 2009;324(5929):900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moll J, et al. Human fronto-mesolimbic networks guide decisions about charitable donation. Proc Natl Acad Sci USA. 2006;103(42):15623–15628. doi: 10.1073/pnas.0604475103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith DV, et al. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30(7):2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Azzi JC, Sirigu A, Duhamel JR. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc Natl Acad Sci USA. 2012;109(6):2126–2131. doi: 10.1073/pnas.1111715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eriksen CW, Yeh Y-Y. Allocation of attention in the visual field. J Exp Psychol Hum Percept Perform. 1985;11(5):583–597. doi: 10.1037//0096-1523.11.5.583. [DOI] [PubMed] [Google Scholar]
- 76.Herrington TM, Assad JA. Temporal sequence of attentional modulation in the lateral intraparietal area and middle temporal area during rapid covert shifts of attention. J Neurosci. 2010;30(9):3287–3296. doi: 10.1523/JNEUROSCI.6025-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hunnius S. The early development of visual attention and its implications for social and cognitive development. Prog Brain Res. 2007;164:187–209. doi: 10.1016/S0079-6123(07)64010-2. [DOI] [PubMed] [Google Scholar]
- 78.Moore T, Armstrong KM, Fallah M. Visuomotor origins of covert spatial attention. Neuron. 2003;40(4):671–683. doi: 10.1016/s0896-6273(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 79.Shepherd SV. Following gaze: Gaze-following behavior as a window into social cognition. Front Integr Neurosci. 2010;4:5. doi: 10.3389/fnint.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruce C, Desimone R, Gross CG. Visual properties of neurons in a polysensory area in superior temporal sulcus of the macaque. J Neurophysiol. 1981;46(2):369–384. doi: 10.1152/jn.1981.46.2.369. [DOI] [PubMed] [Google Scholar]
- 81.Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3(1):80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- 82.Gao T, Scholl BJ, McCarthy G. Dissociating the detection of intentionality from animacy in the right posterior superior temporal sulcus. J Neurosci. 2012;32(41):14276–14280. doi: 10.1523/JNEUROSCI.0562-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roy A, Shepherd SV, Platt ML. Reversible inactivation of pSTS suppresses social gaze following in the macaque (Macaca mulatta) Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss123. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annu Rev Neurosci. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Snyder LH, Batista AP, Andersen RA. Intention-related activity in the posterior parietal cortex: A review. Vision Res. 2000;40(10-12):1433–1441. doi: 10.1016/s0042-6989(00)00052-3. [DOI] [PubMed] [Google Scholar]
- 86.Shepherd SV, Klein JT, Deaner RO, Platt ML. Mirroring of attention by neurons in macaque parietal cortex. Proc Natl Acad Sci USA. 2009;106(23):9489–9494. doi: 10.1073/pnas.0900419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roy A, Platt ML. 2009. Neuronal mechanisms of gaze following: Insights from a choice task. Society for Neuroscience (Chicago, IL), Program No. 455.5/Z1.
- 88.Levy DJ, Glimcher PW. The root of all value: A neural common currency for choice. Curr Opin Neurobiol. 2012;22(6):1027–1038. doi: 10.1016/j.conb.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Padoa-Schioppa C, Assad JA. Neurons in the orbitofrontal cortex encode economic value. Nature. 2006;441(7090):223–226. doi: 10.1038/nature04676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Carmichael ST, Price JL. Sensory and premotor connections of the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1995;363(4):642–664. doi: 10.1002/cne.903630409. [DOI] [PubMed] [Google Scholar]
- 91.Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363(4):615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- 92.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371(2):179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 93.Lewis PA, Rezaie R, Brown R, Roberts N, Dunbar RI. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57(4):1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dunbar RI. Neocortex size and group size in primates: A test of the hypothesis. J Hum Evol. 1995;28(3):287–296. [Google Scholar]
- 95.Rizzolatti G, Fabbri-Destro M. The mirror system and its role in social cognition. Curr Opin Neurobiol. 2008;18(2):179–184. doi: 10.1016/j.conb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Chang SW, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (macaca mulatta) Front Neurosci. 2011;5:27. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chang SW, Barter JW, Ebitz RB, Watson KK, Platt ML. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci USA. 2012;109(3):959–964. doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313(5791):1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 99.Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16(2):235–239. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 100.Waytz A, Zaki J, Mitchell JP. Response of dorsomedial prefrontal cortex predicts altruistic behavior. J Neurosci. 2012;32(22):7646–7650. doi: 10.1523/JNEUROSCI.6193-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 102.McEwen BS. Physiology and neurobiology of stress and adaptation: Central role of the brain. Physiol Rev. 2007;87(3):873–904. doi: 10.1152/physrev.00041.2006. [DOI] [PubMed] [Google Scholar]
- 103.Keverne EB. Understanding well-being in the evolutionary context of brain development. Philos Trans R Soc Lond B Biol Sci. 2004;359(1449):1349–1358. doi: 10.1098/rstb.2004.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Adkins-Regan E. Hormones and Animal Social Behavior. Princeton: Princeton Univ Press; 2005. [Google Scholar]
- 105.Belin V, Moos F. Paired recordings from supraoptic and paraventricular oxytocin cells in suckled rats: Recruitment and synchronization. J Physiol. 1986;377:369–390. doi: 10.1113/jphysiol.1986.sp016192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322(5903):900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 107.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 108.Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113(5):1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- 109.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 110.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 111.Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS ONE. 2007;2(11):e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends Cogn Sci. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 113.Crockford C, et al. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc Biol Sci. 2013;280(1755):20122765. doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Front Neuroendocrinol. 2009;30(4):548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 115.Abbott DH, et al. Are subordinates always stressed? A comparative analysis of rank differences in cortisol levels among primates. Horm Behav. 2003;43(1):67–82. doi: 10.1016/s0018-506x(02)00037-5. [DOI] [PubMed] [Google Scholar]
- 116.Tung J, et al. Social environment is associated with gene regulatory variation in the rhesus macaque immune system. Proc Natl Acad Sci USA. 2012;109(17):6490–6495. doi: 10.1073/pnas.1202734109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Blumstein DT, Patton ML, Saltzman W. Faecal glucocorticoid metabolites and alarm calling in free-living yellow-bellied marmots. Biol Lett. 2006;2(1):29–32. doi: 10.1098/rsbl.2005.0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wingfield JC, Hegner RE, Dufty AM, Jr, Ball GF. The” challenge hypothesis”: Theoretical implications for patterns of testosterone secretion, mating systems, and breeding strategies. Am Nat. 1990;136(6):829–846. [Google Scholar]
- 119.Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: Meta-analyses of the challenge hypothesis. Anim Behav. 2006;71(2):265–277. [Google Scholar]
- 120.Higham JP, Heistermann M, Maestripieri D. The endocrinology of male rhesus macaque social and reproductive status: A test of the challenge and social stress hypotheses. Behav Ecol Sociobiol. 2013;67(1):19–30. doi: 10.1007/s00265-012-1420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fernald RD. Social control of the brain. Annu Rev Neurosci. 2012;35:133–151. doi: 10.1146/annurev-neuro-062111-150520. [DOI] [PubMed] [Google Scholar]
- 122.Burmeister SS, Jarvis ED, Fernald RD. Rapid behavioral and genomic responses to social opportunity. PLoS Biol. 2005;3(11):e363. doi: 10.1371/journal.pbio.0030363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Plomin R. Behavioral Genetics. 4th Ed. New York: Worth; 2001. [Google Scholar]
- 124.Brent LJN, et al. Genetic origins of social networks in rhesus macaques. Sci Rep. 2013;3:1042. doi: 10.1038/srep01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silk JB, et al. The benefits of social capital: Close social bonds among female baboons enhance offspring survival. Proc Biol Sci. 2009;276(1670):3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Silk JB, et al. Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol. 2010;20(15):1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 127.Johnson AM, Vernon PA, Feiler AR. Behavioral genetic studies of personality: An introduction and review of the results of 50+ years of research. In: Boyle G, Matthews G, Saklofske D, editors. Handbook of Personality Theory and Assessment. London: Sage; 2008. pp. 145–173. [Google Scholar]
- 128.Weiss A, King JE, Figueredo AJ. The heritability of personality factors in chimpanzees (Pan troglodytes) Behav Genet. 2000;30(3):213–221. doi: 10.1023/a:1001966224914. [DOI] [PubMed] [Google Scholar]
- 129.Fairbanks LA, et al. Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol Psychiatry. 2004;55(6):642–647. doi: 10.1016/j.biopsych.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 130.Williamson DE, et al. Heritability of fearful-anxious endophenotypes in infant rhesus macaques: A preliminary report. Biol Psychiatry. 2003;53(4):284–291. doi: 10.1016/s0006-3223(02)01601-3. [DOI] [PubMed] [Google Scholar]
- 131.Fowler JH, Dawes CT, Christakis NA. Model of genetic variation in human social networks. Proc Natl Acad Sci USA. 2009;106(6):1720–1724. doi: 10.1073/pnas.0806746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nowak MA. Five rules for the evolution of cooperation. Science. 2006;314(5805):1560–1563. doi: 10.1126/science.1133755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hayes DJ, Greenshaw AJ. 5-HT receptors and reward-related behaviour: A review. Neuroscience &. Biobehav Rev. 2011;35(6):1419–1449. doi: 10.1016/j.neubiorev.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 134.Sirviö J, Riekkinen P, Jr, Jäkälä P, Riekkinen PJ. Experimental studies on the role of serotonin in cognition. Prog Neurobiol. 1994;43(4-5):363–379. doi: 10.1016/0301-0082(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 135.Hariri AR, Holmes A. Genetics of emotional regulation: The role of the serotonin transporter in neural function. Trends Cogn Sci. 2006;10(4):182–191. doi: 10.1016/j.tics.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 136.Jedema HP, et al. Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol Psychiatry. 2010;15(5):512–522. doi: 10.1038/mp.2009.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chen GL, Novak MA, Hakim S, Xie Z, Miller GM. Tryptophan hydroxylase-2 gene polymorphisms in rhesus monkeys: Association with hypothalamic-pituitary-adrenal axis function and in vitro gene expression. Mol Psychiatry. 2006;11(10):914–928. doi: 10.1038/sj.mp.4001870. [DOI] [PubMed] [Google Scholar]
- 138.Caspi A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- 139.Coon H, et al. Possible association between autism and variants in the brain-expressed tryptophan hydroxylase gene (TPH2) Am J Med Genet B Neuropsychiatr Genet. 2005;135B(1):42–46. doi: 10.1002/ajmg.b.30168. [DOI] [PubMed] [Google Scholar]
- 140.Canli T, Congdon E, Todd Constable R, Lesch KP. Additive effects of serotonin transporter and tryptophan hydroxylase-2 gene variation on neural correlates of affective processing. Biol Psychol. 2008;79(1):118–125. doi: 10.1016/j.biopsycho.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 141.Watson KK, Ghodasra JH, Platt ML. Serotonin transporter genotype modulates social reward and punishment in rhesus macaques. PLoS ONE. 2009;4(1):e4156. doi: 10.1371/journal.pone.0004156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wendland JR, et al. Differential functional variability of serotonin transporter and monoamine oxidase a genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet. 2006;36(2):163–172. doi: 10.1007/s10519-005-9017-8. [DOI] [PubMed] [Google Scholar]
- 143.Suomi SJ. Risk, resilience, and gene x environment interactions in rhesus monkeys. Ann N Y Acad Sci. 2006;1094(1):52–62. doi: 10.1196/annals.1376.006. [DOI] [PubMed] [Google Scholar]
- 144.Homberg JR, Lesch K-P. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011;69(6):513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 145.Canli T, Lesch KP. Long story short: The serotonin transporter in emotion regulation and social cognition. Nat Neurosci. 2007;10(9):1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- 146.Sallet J, et al. Social network size affects neural circuits in macaques. Science. 2011;334(6056):697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- 147.Curley JP, Jensen CL, Mashoodh R, Champagne FA. Social influences on neurobiology and behavior: Epigenetic effects during development. Psychoneuroendocrinology. 2011;36(3):352–371. doi: 10.1016/j.psyneuen.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Amemori K, Graybiel AM. Localized microstimulation of primate pregenual cingulate cortex induces negative decision-making. Nat Neurosci. 2012;15(5):776–785. doi: 10.1038/nn.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]