Abstract
The study presented here is a laboratory pilot study using diluted car exhaust from a single vehicle to assess differences in toxicological response between primary emissions and secondary products resulting from atmospheric photochemical reactions of gas phase compounds with O3, OH and other radicals. Sprague-Dawley rats were exposed for five hours to either filtered room air (Sham) or one of two different atmospheres: 1. Diluted Car Exhaust (P) + Mt. Saint Helens Ash (MSHA); 2. P+MSHA+SOA (Secondary Organic Aerosol, formed during simulated photochemical aging of diluted exhaust). Primary and secondary gases were removed using a non-selective diffusion denuder. Continuous respiratory data was collected during the exposure, and broncho-alveolar lavage (BAL) and complete blood counts (CBC) were performed 24 hours after exposure. ANOVA models were used to assess the exposure effect and to compare those effects across different exposure types. Total average exposures were 363±66 μg/m3 P+MSHA and 212±95 μg/m3 P+MSHA+SOA. For both exposures, we observed decreases in breathing rate, tidal and minute volumes (TV, MV) and peak and median flows (PIF, PEF and EF50) along with increases in breathing cycle times (Ti, Te) compared to sham. These results indicate that the animals are changing their breathing pattern with these test atmospheres. Exposure to P+MSHA+SOA produced significant increases in Total Cells, Macrophages and Neutrophils in the BAL and in-vivo chemiluminescence of the lung. There were no significant differences in CBC parameters. Our data suggest that simulated atmospheric photochemistry, producing SOA in the P+MSHA+SOA exposures, enhanced the toxicity of vehicular emissions.
INTRODUCTION
Air pollution is comprised of a complex mixture of emissions from multiple sources. The primary gases and particles emitted by air pollution sources undergo chemical reactions in the atmosphere to produce secondary pollutants, which may make the particles in the ambient air potentially more harmful. Many epidemiologic and toxicological studies have shown that high levels of ambient particulate air pollution are associated with adverse health effects including cardiopulmonary mortality and morbidity. (Brook et al., 2010, Dockery and Pope, 1994, Dockery et al., 1993, Godleski et al., 2000). Among the different sources contributing to air pollution (power plants, motor vehicles, home heating, industrial plants and natural sources), mobile source emissions have been linked to increases in cardiovascular morbidity and mortality (Brook et al., 2010, Laden et al., 2000, Mills et al., 2007, Stupfel, 1976, Lippmann and Chen, 2009).
There is a critical need to understand the role of both primary and secondary pollutants derived from specific sources. We have developed an approach to study the Toxicological Effects of Realistic Emission Source Aerosols (TERESA) and have already used this approach to study toxicity of primary and secondary emissions of coal-fired power plants (Diaz et al., 2011, Godleski et al., 2011a, Godleski et al., 2011c, Kang et al., 2011, Lemos et al., 2011, Wellenius et al., 2011). For the present study we adapted the technologies and approaches developed for the TERESA power plant study (Godleski et al., 2011c, Ruiz et al., 2007, Ruiz et al., 2006) to investigate the health effects of vehicular emission sources using rats.
The study presented here is a laboratory pilot study using diluted car exhaust from a single vehicle. The goal of this study was to develop the capabilities to apply the TERESA approach to vehicular emissions and to determine whether simulated atmospheric photochemistry alters the toxicological response to exposure. Because modern gasoline vehicle exhaust contains virtually no primary particle mass, we added a toxicologically inert seed particle to vehicle exhaust. We then examined the toxicological response to primary emissions plus carrier particles with and without irradiation by simulated sunlight. Irradiation resulted in formation of secondary products. This system has been shown to produce substantial amounts of ozone (O3) and secondary organic aerosol (SOA) (Papapostolou et al., 2011a). The toxicological findings from these aerosols are reported here.
Materials and Methods
Exposure Types
As indicated above, two types of exposure atmospheres were generated. The first represents primary gasoline vehicle exhaust, and the second represents photochemically oxidized gasoline vehicle exhaust. Because modern gasoline vehicle exhaust contains virtually no primary particle mass, we added seed particles. We then irradiated the mixture with simulated sunlight, resulting in formation of secondary products, mostly organic, which condensed onto the seed particles.
Mt St Helens Ash (MSHA) (density, ρp = 2.6 g/cm3) was used as a seed particle because it is both chemically and toxicologically inert (Martin et al., 1986, Raub et al., 1985, Savage et al., 2003, Wiester et al., 1985). The seed aerosol was added to decrease the time required to form a stable accumulation mode size distribution, and to allow exposures to both types of atmospheres (primary and secondary) to be conducted with mass concentrations on the same order of magnitude.
The two types of exposure atmospheres studied are thus primary exhaust (P+MSHA) and photochemically oxidized exhaust, containing secondary aerosol (predominantly organic) formed under irradiation with simulated sunlight (P+MSHA+SOA).
Exposure Generation System
The main features of the exposure system have been explained in detail in a previous publication (Papapostolou et al., 2011a). Briefly, tailpipe emissions from a single vehicle running outside the lab with the throttle slightly open were diluted with ambient air to achieve a consistent carbon monoxide (CO) concentration of 5 ppm (in the car emissions) and then piped to a reaction chamber. The chamber was operated in with continuous airflow at a mean residence time of 50 min. CO was maintained at 5ppm because this concentration was within the range measured in the ventilation stack of a traffic tunnel in which we planned to conduct a future fleet emissions study. Following stabilization of the CO concentration in the reaction chamber, addition of the seed aerosol was started. The MSHA was generated (from an aqueous dispersion) with a High-output Extended Aerosol Respiratory Therapy nebulizer (Westmed Inc, Tucson, AZ). The output of the nebulizer was diluted with particle-free dry air in a 4 L vessel. The aerosol was then mixed with the diluted car exhaust in the chamber. When a steady-state baseline seed aerosol mass concentration was reached, then P+MSHA animal exposures were started; alternatively, if the intended exposure was P+MSHA+SOA, the lamps would be turned on to initiate the photochemical reactions.
Irradiation was provided using 48 UVA-340 fluorescent lamps to best represent the ground-level solar spectrum between 295-365 nm (Carter et al., 1995). Photochemical oxidation of the diluted car exhaust followed the expected pattern of conversion of nitric oxide (NO) to nitrogen dioxide (NO2), accumulation of O3, and formation of SOA in an initial burst of ultrafine particles that evolved into the fine particle size range (Papapostolou et al., 2011a). Once a stable P+MSHA+SOA mass concentration was measured downstream of the chamber, animal exposures were started.
For both exposure types, primary and secondary gases were removed downstream of the photochemical chamber, prior to the point of animal exposure, using a non-selective diffusion denuder (Papapostolou et al., 2011b, Ruiz et al., 2006).
Exposure Assessment
Particle size distribution and concentration were monitored using a Scanning Mobility Particle Sizer (SMPS Model 3934, TSI Inc., Shoreview, MN) coupled with a Condensation Particle Counter (CPC Model 3022A, TSI Inc.) and an Aerodynamic Particle Sizer (APS Model 3321, TSI Inc.). Particle instruments were connected between the denuder outlet and the animal exposure chambers with short pieces of tubing to minimize particle losses. CO was continuously monitored downstream of the chamber (or upstream of the denuder) by infrared absorption (Model 48 Analyzer, Thermo Scientific, Franklin, MA).
Animals
Male Sprague Dawley (SD-CD) rats 250-350g were obtained from Taconic Farms (Rensselaer, NY). Animals were housed and managed according to NIH guidelines for the care and use of laboratory animals. Upon arrival, animals were randomly assigned a unique identification number, which determined the exposure date and exposure group (Aerosol or Filtered Air) for the animal.
Experimental design
Two different exposures were evaluated: P+MSHA and P+MSHA+SOA (described above); both were compared to control (sham) exposures with filtered indoor air.
Each exposure atmosphere consisted of 4 consecutive days of exposures; each day a different set of animals was exposed and later used for specific endpoint analysis. For any given day of exposures, 5 SD-CD rats were exposed to aerosol and 5 to filtered room air in individual exposure chambers for 5 hours. These chambers also served as whole body plethysmographs for assessment of breathing patterns throughout the exposure. At the end of the exposure two animals from each group underwent in vivo chemiluminescence measurement of the heart and lung; these results were reported by Papapostolou et al. (2011). The remaining animals in each group were returned to the housing units and underwent bronchoalveolar lavage (BAL) and blood analysis 24 hours after exposure.
Breathing Pattern Analyses
Animals were exposed in clear polycarbonate chambers to allow observation of the animals during the exposure. The animals usually took 10 to 20 minutes to adapt to these exposure chambers. After acclimation, animals typically slept during most of the exposure.
During the exposures, continuous respiratory data were collected using a whole body plethysmography system (Buxco Electronics Inc., Wilmington, NC). Flow through each chamber was maintained at 1.5LPM. Buxco airflow transducers (TRD5700) were connected to the chambers and to a reference chamber that compensated for changes in pressure in the system. Each transducer was calibrated to its respective chamber using a 1.5 LPM flow at the beginning of each week. Before each exposure, a daily check of the accuracy of these calibrations was performed. Continuous breathing cycle data collected from each animal were reduced to 10 minute averages of the following parameters: Frequency (f), Tidal Volume (TV), Inspiratory Time (Ti), Expiratory Time (Te), Enhanced Pause (Penh), Accumulated Volume (AV), Minute Volume (MV), Peak of Inspiratory Flow (PIF), Peak of Expiratory Flow (PEF), Relaxation Time (RT), End Inspiratory Pause (EIP), End Expiratory Pause (EEP), Delta Inspiratory – Expiratory Flow (dV), Expiratory Flow at 50% (EF50) and Pause (PAU). Units of measure for respiratory parameters are times in seconds, volumes in mL, flows in mL/sec, and frequency is given in breaths/min. A rejection algorithm was automatically included in the breath-by-breath analysis to eliminate noise resulting from animal movement within the chamber. This algorithm uses the default values for rats described in the Buxco Research Systems, Inc. biosystem XA user manual, and includes the following parameters: minimum Ti=0.03sec, maximum Ti=10sec, maximum TV=0.05 mL/sec and a volume balance of 50%.
Complete Blood Count (CBC)
Rats were euthanized with an intraperitoneal overdose of Pentobarbital Sodium (Fatal Plus, Vortech Pharmaceuticals Dearborn, Michigan). The thorax was opened and 2-3mL of blood was obtained via cardiac puncture for CBC. Blood was collected in 2mL Vacutainer tubes with EDTA, refrigerated, and shipped overnight to IDEXX pre-clinical research laboratories in North Grafton, MA for analysis. After collection of blood, the dissection was continued to conduct bronchoalveolar lavage.
Broncho-Alveolar Lavage (BAL)
An incision along the sternum was used to access the thoracic cavity. The trachea was mobilized by blunt dissection and an incision made to introduce a 14G catheter, which was sutured in place. The catheter was connected to a 10mL glass syringe. Six consecutive 5mL washes with endotoxin-free Dulbecco’s phosphate-buffered saline (PBS) were carried out. The first wash was centrifuged and the supernatant was collected and frozen with dry ice and later used for β-N-Acetyl Glucuronidase and total protein determinations (Godleski et al., 2011b). Cell re-suspension of the first wash plus the other five washes was used for cell count, viability, and cell type determinations (Saldiva et al., 2002). Viability and total cell counts were determined by hemocytometer counts of small aliquots of the re-suspended BAL diluted in trypan blue solution. Cell type was determined from modified Wright-Giemsa-stained cytocentrifuge preparations; 200 cells were counted per sample.
Statistical Analysis
We considered 14 respiratory outcomes (f, TV, AV, MV, Ti, Te, PIF, PEF, RT, EIP, EEP, DV, EF50, Penh), BAL outcomes (Total cell count, viability, and differential counts (macrophages, neutrophils, lymphocytes, etc.) , and 16 CBC outcomes (platelets count, white blood cells, red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin count, and percentile and absolute values for: segmented neutrophils, lymphocytes, monocytes, and eosinophils. For respiratory outcomes, the daily average of continuously measured outcomes were used for ANOVA modeling. Prior to modeling, skewed variables were log-transformed.
For each outcome, ANOVA models were used to assess an exposure effect on the mean and to compare the exposure effects across different scenarios. First, we pooled the data across both exposure types to assess the overall exposure effect. Then we assessed how exposure effects differed between P+MSHA+SOA and P+MSHA exposures.
Results
Exposure
Detailed results from characterization of the exposure generation system have been published in a different paper (Papapostolou et al., 2011a). Table 1 shows particle data collected during animal exposures on different exposure days in the two different exposure types. The mean and standard deviations of particle mass and number concentrations measured during P+MSHA and P+MSHA+SOA exposures are presented. During P+MSHA experiments, the PM concentration averaged 363±66 μg/m3. During this exposure, the particle mass was almost entirely MSHA seed aerosol, because gasoline exhaust contains virtually no primary particles. During P+MSHA+SOA experiments, the PM consisted of MSHA and secondary aerosol, predominantly SOA, that formed in the photochemical chamber and then condensed onto existing seed aerosol surfaces. The P+MSHA+SOA exposure averaged 212±95 μg/m3, where the seed aerosol contributed on average 142 μg/m3 and the SOA mass yield averaged approximately 70 μg/m3. The particle number concentration remained fairly stable during the 5-hr period of animal exposures for either exposure type, with a very low variability (about 5% on average).
Table 1.
Particle mass and number during “Lights OFF” and “Lights ON” animal exposures experiments
| Exposure | Exposure Day |
PN (#/cm3) | PM (μg/m3) | Seed PM (μg/m3) |
SOA (μg/m3) |
|---|---|---|---|---|---|
| (mean ± stdev) | |||||
| P+MSHA | 1 | 5564±282 | 264.4±12.1 | Same as PM | 0.0 |
| 2 | 6163±546 | 404.5±49.3 | |||
| 3 | 6621±576 | 389.7±12.3 | |||
| 4 | 6776±396 | 393.4±21.4 | |||
| Average | 6281±545 | 363.0±66.0 | |||
| P+MSHA+SOA | 1 | 3711±503 | 81.7±6.9 | 60.2±1.4 | 21.5±5.5 |
| 2 | 7722±237 | 209.4±34.6 | 132.2±4.1 | 77.2±30.5 | |
| 3 | 7096±381 | 248.2±20.8 | 156.0±2.8 | 92.2±18.0 | |
| 4 | 6897±274 | 306.5±20.5 | 218.6±5.1 | 87.9±15.4 | |
| Average | 6357±349 | 211.5±95.3 | 141.8±65.4 | 69.7±32.7 | |
Table 2 shows the projected vehicular volatile organic compounds (VOCs), specifically benzene, toluene, ethylbenzene, and xylene (BTEX) concentrations at the point of animal exposures. These concentrations were estimated from the average CO concentrations during the exposure period, using the average ratio of these compounds to the exhaust CO concentrations measured in previously reported experiments (Papapostolou et al., 2011a). For both exposure atmosphere scenarios (P+MSHA and P+MSHA+SOA), the BTEX concentrations were corrected for the denuder’s removal efficiencies for each compound (Papapostolou et al., 2011b). For P+MSHA+SOA exposures, the BTEX concentrations were also corrected for the average fraction of each compound that reacted in the photochemical chamber with the lights on (Papapostolou et al., 2011a). Table 2 reports both the 5-hr average CO concentrations measured in the reaction chamber during the animal exposure experiments as well as the concentrations at the point of exposure, downstream of the denuder.
Table 2.
Projected vehicular BTEX VOCs concentrations at the point of animal exposures: Projected vehicular BTEX VOCs concentrations at the point of animal exposures. These concentrations were estimated from the average CO concentrations during the exposure period, using the ratio of these compounds to the exhaust CO concentrations measured in previously reported experiments (Papapostolou et al., 2011a). For the three exposure atmosphere scenarios (P+MSHA, P+MSHA+SOA and P+MSHA+SG), the upstream VOCs concentrations were corrected for the denuder’s individual VOCs removal efficiencies (Papapostolou et al., 2011b). Also, in the case of the P+MSHA+SOA and P+MSHA+SG scenarios, the VOCs concentrations were also corrected for the average amount of each of the VOCs reacted in the photochemical chamber with the lights on (Papapostolou et al., 2011a). The CO concentrations reported in the table are calculated values from the 5-hr average concentrations measured downstream of the chamber during the animal exposures experiments.
| P+MSHA | P+MSHA+SOA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exposure Day | 1 | 2 | 3 | 4 | Avg ±SD | 1 | 2 | 3 | 4 | Avg ±SD |
| CO (ppm) | 0.6 | 0.5 | 0.56 | 0.42 | 0.52 ±0.078 | 0.31 | 0.74 | 0.9 | 0.9 | 0.71 ±0.28 |
| Benzene (ppb) | 2.7 | 2.3 | 2.3 | 1.9 | 2.3 ±0.33 | 1.4 | 3.3 | 4 | 4 | 3.2 ±1.23 |
| Toluene (ppb) | 2.7 | 2.3 | 2.3 | 1.9 | 2.3 ±0.33 | 1.3 | 3.2 | 3.9 | 3.9 | 3.1 ±1.23 |
| Ethylbenzene (ppb) | 0.6 | 0.5 | 0.5 | 0.4 | 0.5 ±0.08 | 0.3 | 0.7 | 0.9 | 0.9 | 0.7 ±0.28 |
| m/p Xylene (ppb) | 2 | 1.7 | 1.7 | 1.4 | 1.7 ±0.24 | 0.9 | 2.1 | 2.6 | 2.6 | 2.1 ±0.80 |
| o-Xylene (ppb) | 0.7 | 0.6 | 0.6 | 0.5 | 0.6 ±0.08 | 0.3 | 0.8 | 0.9 | 0.9 | 0.7 ±0.29 |
Breathing Pattern
All respiratory data were analyzed as 10-min averages over the 5-hour exposure time to determine (1) whether there was a difference between the exposed and control group for each exposure type, and (2) whether that response (if existing) was different between types (i.e., animals exposed to P+MSHA+SOA and those exposed to P+MSHA). Table 3 shows the breathing pattern changes within the two different scenarios. Overall, we found a similar effect in both magnitude and direction in most of the parameters for both exposure groups. Due to the fact that both groups are similar in magnitude and direction we didn’t find a difference between the 2 exposures, but significant differences were found for the combined exposure groups.
Table 3.
MEANS OF CONTINUOS BREATHING PATTERN VARIABLES
| EXPOSURE | GROUP | f | TV | MV | Ti | Te | PIF | PEF | EIP | EEP | EF50 | Penh |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P+MSHA | Control | 1.56 | 2.49 | 378.00 | 0.17 | 0.31 | 25.90 | 22.24 | 7.79 | 92.55 | 1.26 | 1.18 |
| Exposed | 1.50 | 2.03 | 294.00 | 0.18 | 0.30 | 20.16 | 17.86 | 7.84 | 98.39 | 0.98 | 1.21 | |
| Difference | −0.06 | −0.46 | −84.00 | 0.01 | −0.01 | −5.74 | −4.38 | 0.05 | 5.84 | −0.28 | 0.03 | |
| P+MSHA+SOA | Control | 1.50 | 2.55 | 371.00 | 0.18 | 0.31 | 25.09 | 23.25 | 7.76 | 91.17 | 1.35 | 1.42 |
| Exposed | 1.44 | 2.12 | 289.00 | 0.19 | 0.32 | 19.88 | 17.79 | 7.77 | 101.49 | 0.96 | 1.22 | |
| Difference | −0.06 | −0.43 | −82.00 | 0.01 | 0.01 | −5.21 | −5.46 | 0.01 | 10.32 | −0.39 | −0.20 | |
| p-value* | 0.28 | 0.06 | 0.03 | 0.24 | 0.32 | 0.05 | 0.01 | 0.90 | 0.64 | 0.01 | 0.54 |
Differences from filtered air control exposure for both groups. There are no significant differences between the exposures.
Cellular Response (CBC and Bronchoalveolar Lavage)
There were no significant differences in any of the analyzed CBC parameters, all values were within normal range for the species.
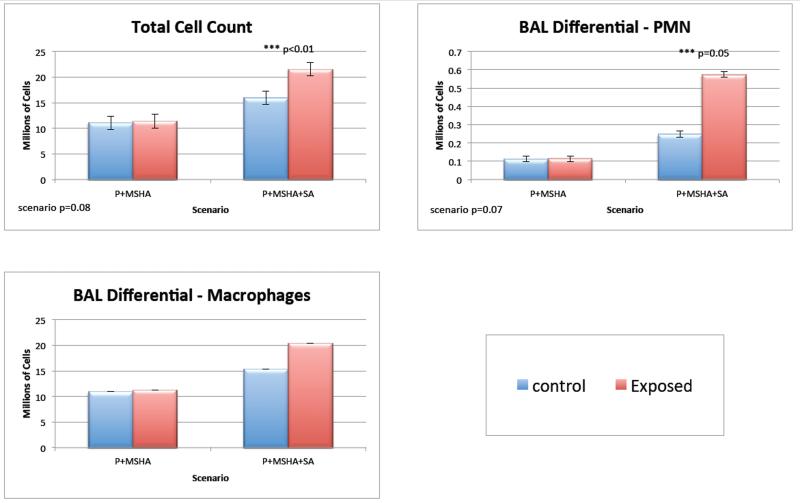
Figure 1 shows the differences for selected parameters in the Broncho Alveolar Lavage (BAL) data. Total cell count was similar for the control group and the P+MSHA group (1.11 and 1.14*106 cells respectively). In contrast, the P+MSHA+SOA exposed group had a 34% increase compared to the controls (1.60 and 2.15*106 cells respectively). This difference was significant (p<0.005), however the P+MSHA+SOA response was not significantly different than P+MSHA comparing the 2 exposures (p=0.08). There was an increase in the macrophages with P+MSHA+SOA exposure, but it was not significant. Differential counts showed a 72% increase in polymorphonuclear (PMN) cells (p=0.05) for P+MSHA+SOA exposure, but there was no change with P+MSHA, when comparing the difference between these two exposures (p=0.07). Increases in BAL total protein were not significant for either one of the exposure groups. BNAG (β-N-Acetyl glucuronidase) was slightly but significantly increased (+0.0005 U/mL p=0.05) for the P+MSHA scenario.
Figure 1.
Changes in Broncho-Alveolar Lavage (BAL) for selected parameters. There is a 34% increase in total cells and 78% increase in PMN’s for the P+MSHA+SOA group compared with controls.
Discussion
Recent reviews (Brook et al., 2010, Lippmann and Chen, 2009) outline the current knowledge about air pollution-generated health effects and emphasize the need for a better understanding of the contributions of different sources to these health effects. This study was a pilot study designed to adapt the TERESA approach for studying source aerosols to vehicular emissions. During this study, we evaluated the toxicity of the primary emissions emitted by the exhaust system of a single vehicle and then compared the toxicity with that of the secondary particles formed from exhaust of the same vehicle in a photochemical reaction chamber that simulated the aging process in the atmosphere.
Our data show that the breathing pattern for both exposed groups (P+MSHA+SOA and P+MSHA) was changed when compared to the control animals, but the changes were similar under the two simulated exposures. In both groups, we observed a decrease in frequency and volumes (TV, MV) and flows (PIF, PEF and EF50) and an increase in times (Ti, Te), suggesting that the animals are reacting to the test atmosphere by slowing their breathing rate. Because this response is seen in both P+MSHA+SOA and P+MSHA, it may indicate a response to MSHA or to the components of the car exhaust not being affected or enhanced by the irradiation of UV light. Nevertheless, our BAL data, as illustrated in Figure 1 shows that there was no change for the exposed P+MSHA group when compared to its control, while there was a statistically significant increase in cellularity with inflammatory cells (Macrophages and PMN) in the P+MSHA+SOA group. Consistent with this inflammatory response, previously published oxidative stress data showed a significant 1.6 fold increase in lung chemiluminescence for the P+MSHA+SOA group (Papapostolou et al., 2011a) and no change for the P+MSHA group. Together, these results suggest that the formation of SOA significantly enhanced the toxicity and the cellular response to the vehicle exhaust.
Exposure to low levels of some of the gases produced during engine gasoline combustion such as NO and CO may have effects that are non-toxic and even therapeutic. Known characteristics of low doses of NO include vasorelaxation, protection against myocardial ischemia reperfusion injury, and improved gas exchange ventilation especially in newborns (Gianetti et al., 2002, Sethi et al., 2008, Ryter et al., 2004). Characteristic therapeutic properties for CO include antioxidative, anti-inflammatory, antiproliferative and antiapoptopic effects (Kim et al., 2006, Morse and Choi, 2008, Sethi et al., 2008, Ryter et al., 2004, Ryter and Otterbein, 2004). For our study, however, NO concentration was below the level of detection in the animal exposure, even in the P+MSHA exposure, where NO is not oxidized to NO2. NO should thus have no role in the observed response. The emissions from the tailpipe of the vehicle were diluted with ambient air to reduce the levels of CO to about 5ppm in the reaction chamber, a point comparable to concentration levels observed at urban highways, (Rogak et al., 1998, Kirchstetter TW et al., 1996, Fraser MP et al., 1998, Pierson WR et al., 1996). The countercurrent denuder removed the residual gases before the chamber output reached the point of animal exposure and animals were predominantly exposed to particle mass. Thus, animal exposure to CO was also below the limit of detection and should not have a role in the observed responses.
The primary particle mass concentration in the diluted gasoline exhaust was very low and most of the emissions are gaseous in the form of NO/NOx and VOCs, this is representative of modern gasoline combustion engine exhaust (Nam et al., 2008, Piock et al., 2011, Schreiber et al., 2007). MSHA has been studied in the past and has shown no effect on breathing pattern during inhalation studies at higher doses than used in this study (Martin et al., 1986, Raub et al., 1985, Wiester et al., 1985). Some mild inflammatory effects have been shown after high dose instillation studies (Martin et al., 1986, Raub et al., 1985, Sanders et al., 1982, Vallyathan et al., 1983), but not after inhalation studies (Savage et al., 2003, Raub et al., 1985). Therefore, the breathing pattern changes produced by P+MSHA exposure are most likely the result of the tailpipe emissions rather than the MSHA. The observed changes in breathing pattern with the exposure to P+MSHA raises the possibility that some gaseous components from the vehicle emissions may be condensing on the seed particles during their residence time in the chamber. It is possible that this coating was enough to generate a change in the breathing pattern of our test animals but did not affect the other analyzed parameters. The respiratory changes were mild, and the rate change might reflect the conscious perception by the animals of being exposed to the aerosols. However, when we compared the BTEX concentrations of our exposure scenarios (Table 2) with previously reported data in the literature (Jia et al., 2008, Pankow et al., 2003, Batterman et al., 2006) we found that our estimates are at the high end of the ambient concentrations for urban areas but below levels measured in parking garages, decreasing the likelihood that animals “smell” or sense the exposure, and consciously react with changes in their breathing pattern.
In this study, we adapted the TERESA methodology that was previously used to study the health effects of primary and secondary particles from coal-fired power plant emissions (Diaz et al., 2011, Godleski et al., 2011a, Godleski et al., 2011c, Lemos et al., 2011). Comparing the results from the two studies, we found a greater effect with the single vehicle exhaust than with the power plants, as measured by BAL and IVCL. The respiratory function change with some scenarios at the power plants was more consistent with a shallow rapid breathing pattern, previously linked to an irritative response and more acidic aerosols (Amdur et al., 1952, Chen et al., 1992, Chen et al., 1991, Nikolov et al., 2008).
When comparing to previous Concentrated Ambient Particles (CAPs) studies (Clarke et al., 1999, Gurgueira et al., 2002, Saldiva et al., 2002, Clarke et al., 2000), the biological response with the aged tailpipe aerosol is similar to the inflammatory changes produced by exposures to CAPs; the changes in breathing pattern, in contrast, are different. Clarke et al. (1999) found an increase in TV and PEF in rats with SO2-induced chronic bronchitis, accompanied by increases in inflammatory cells in the BAL; Clarke et al, 2000 reported an association of increased PMN’s in BAL correlated with concentrations of Black Carbon (BC), Zn, V. Similarly increased inflammatory cells were also reported by Saldiva et al. (2002), but associated with concentrations of Si, V, Pb and SO4. BC is considered an indicator of traffic sources, Zn has been associated as a byproduct of the combustion of diesel fuel, thus suggesting that some of these earlier finding are related in part to the mobile sources component of air pollution.
Increases in chemiluminescence in lung and heart with CAPs exposure have been reported by Gurgueira et al. (2002). In this study the lung increase in IVCL was related to the concentration of Fe, Mn, Cu and Zn. In previous studies (Ghelfi et al., 2008, Gurgueira et al., 2002, Rhoden et al., 2005) rats exposed to 5 hours of CAPs (average of 333 μg/m3) from Boston showed an increase in the IVCL of the lung on the order of 1.7 times. The TERESA power plant data showed a 1.2 ratio for the animals exposed to the most complex scenario (Oxidized and neutralized primary particles + pinene). Exposure to the aged irradiated tailpipe exhaust produced a 1.6-fold increase in the IVCL of the lung, greater than the power plant aerosol, and similar to CAPs.
BAL and respiratory changes suggest a mild inflammatory response resulting from acute exposure in this study; this response was enhanced for P+MSHA+SOA compared to P+MSHA.
Conclusions
Exposure to the emissions of the tailpipe of the single vehicle resulted in a reduction of respiratory frequency, volumes and flows for both P+MSHA and P+MSHA+SOA exposures. Responses to P+MSHA may suggest that the seed particle (MSHA) may pick up a coating of some gas phase compounds in the exhaust during the residence time the chamber. These coated particles, once delivered into the lung, may be able to cause a change in breathing pattern.
The breathing pattern was the only parameter measured that was sensitive to the P+MSHA exposure. For BAL and IVCL, the SOA created by irradiating the tail pipe emissions augmented the inflammatory response of the animals, suggesting that atmospheric photochemical processes enhanced toxicity of exhaust emitted from motor vehicles.
Footnotes
Declaration of Interest: This publication was made possible by the U.S. Environmental Protection Agency Center for Particle Health Effects at the Harvard School of Public Health (grant R827353), the Harvard NIEHS Center for Environmental Health (grant ES00002) and USEPA Clean Air Research Center grant RD 83479801. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the USEPA. Further, USEPA does not endorse the purchase of any commercial products or services mentioned in the publication.
REFERENCES
- AMDUR MO, SCHULZ RZ, DRINKER P. Toxicity of sulfuric acid mist to guinea pigs. A M A Arch Ind Hyg Occup Med. 1952;5:318–29. [PubMed] [Google Scholar]
- BATTERMAN S, HATZIVASILIS G, JIA C. Concentrations and emissions of gasoline and other vapors from residential vehicle garages. Atmospheric Environment. 2006;40:1828–1844. [Google Scholar]
- BROOK RD, RAJAGOPALAN S, POPE CA, 3RD, BROOK JR, BHATNAGAR A, DIEZROUX AV, HOLGUIN F, HONG Y, LUEPKER RV, MITTLEMAN MA, PETERS A, SISCOVICK D, SMITH SC, JR., WHITSEL L, KAUFMAN JD. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–78. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- CARTER WPL, LUO D, MALKINA IL, PIERCE JA. Environmental chamber studies of atmospheric reactivities of volatile organic compounds. Effects of varying chamber and light source. Final Report to National Renewable Energy Laboratory. 1995 [Google Scholar]
- CHEN LC, MILLER PD, AMDUR MO, GORDON T. Airway hyperresponsiveness in guinea pigs exposed to acid-coated ultrafine particles. J Toxicol Environ Health. 1992;35:165–74. doi: 10.1080/15287399209531606. [DOI] [PubMed] [Google Scholar]
- CHEN LC, PEOPLES SM, AMDUR MO. Pulmonary effects of sulfur oxides on the surface of copper oxide aerosol. Am Ind Hyg Assoc J. 1991;52:187–91. doi: 10.1080/15298669191364578. [DOI] [PubMed] [Google Scholar]
- CLARKE RW, CATALANO PJ, KOUTRAKIS P, MURTHY GG, SIOUTAS C, PAULAUSKIS J, COULL B, FERGUSON S, GODLESKI JJ. Urban air particulate inhalation alters pulmonary function and induces pulmonary inflammation in a rodent model of chronic bronchitis. Inhal Toxicol. 1999;11:637–56. doi: 10.1080/089583799196781. [DOI] [PubMed] [Google Scholar]
- CLARKE RW, COULL B, REINISCH U, CATALANO P, KILLINGSWORTH CR, KOUTRAKIS P, KAVOURAS I, MURTHY GG, LAWRENCE J, LOVETT E, WOLFSON JM, VERRIER RL, GODLESKI JJ. Inhaled concentrated ambient particles are associated with hematologic and bronchoalveolar lavage changes in canines. Environ Health Perspect. 2000;108:1179–87. doi: 10.1289/ehp.001081179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAZ EA, LEMOS M, COULL B, LONG M, ROHR AC, RUIZ P, GUPTA T, KANG C-M, GODLESKI JJ. Toxicological Evaluation of Realistic Emission Source Aerosols (TERESA): Assessment of Breathing Pattern. Inhal Toxicol. 2011;23:42–59. doi: 10.3109/08958378.2010.578169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOCKERY DW, POPE CA., 3RD Acute respiratory effects of particulate air pollution. Annu Rev Public Health. 1994;15:107–32. doi: 10.1146/annurev.pu.15.050194.000543. [DOI] [PubMed] [Google Scholar]
- DOCKERY DW, POPE CA, 3RD, XU X, SPENGLER JD, WARE JH, FAY ME, FERRIS BG, JR., SPEIZER FE. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- FRASER MP, CASS GR, BRT S. Gas-phase and particlephase organic compounds emitted from motor vehicle traffic in a Los Angeles roadway tunnel. Environ Sci Technol. 1998;32:2051–2060. [Google Scholar]
- GHELFI E, RHODEN CR, WELLENIUS GA, LAWRENCE J, GONZALEZ-FLECHA B. Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicological sciences : an official journal of the Society of Toxicology. 2008;102:328–36. doi: 10.1093/toxsci/kfn005. [DOI] [PubMed] [Google Scholar]
- GIANETTI J, BEVILACQUA S, DE CATERINA R. Inhaled nitric oxide: more than a selective pulmonary vasodilator. European journal of clinical investigation. 2002;32:628–35. doi: 10.1046/j.1365-2362.2002.01049.x. [DOI] [PubMed] [Google Scholar]
- GODLESKI JJ, DIAZ EA, LEMOS M, LONG M, RUIZ P, GUPTA T, KANG C-M, COULL B. Toxicological Evaluation of Realistic Emissions of Source Aerosols (TERESA: assessment of cellular responses. Inhal Toxicol. 2011a;23:60–74. doi: 10.3109/08958378.2010.563804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODLESKI JJ, DIAZ EA, LEMOS M, LONG M, RUIZ P, GUPTA T, KANG CM, COULL B. Toxicological Evaluation of Realistic Emission Source Aerosols (TERESA)-power plant studies: assessment of cellular responses. Inhalation Toxicology. 2011b;23(Suppl 2):60–74. doi: 10.3109/08958378.2010.563804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODLESKI JJ, RORH A, KANG C-M, DIAZ EA, RUIZ P, KOUTRAKIS P. Toxicological Evaluation of Realistic Emission Source Aerosols (TERESA): Introduction and Overview. Inhal Toxicol. 2011c;23:1–10. doi: 10.3109/08958378.2010.568019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GODLESKI JJ, VERRIER RL, KOUTRAKIS P, CATALANO P, COULL B, REINISCH U, LOVETT EG, LAWRENCE J, MURTHY GG, WOLFSON JM, CLARKE RW, NEARING BD, KILLINGSWORTH C. Mechanisms of morbidity and mortality from exposure to ambient air particles. Res Rep Health Eff Inst. 2000:5–88. discussion 89-103. [PubMed] [Google Scholar]
- GURGUEIRA SA, LAWRENCE J, COULL B, MURTHY GG, GONZALEZ-FLECHA B. Rapid increases in the steady-state concentration of reactive oxygen species in the lungs and heart after particulate air pollution inhalation. Environmental health perspectives. 2002;110:749–55. doi: 10.1289/ehp.02110749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIA C, BATTERMAN S, GODWIN C. VOCs in industrial, urban, and suburban neighborhoods, part 1: Indoor and outdoor concentrations, variations, and risk drivers. Atmospheric Environment. 2008;42:2083–2100. [Google Scholar]
- KANG C-M, GUPTA T, RUIZ PA, WOLFSON JM, FERGUSON ST, LAWRENCE J, ROHR AC, GODLESKI JJ, KOUTRAKIS P. Aged particles derived from emissions of coal-fired power plants: the TERESA field results. Inhal Toxicol. 2011 doi: 10.3109/08958371003728040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM HP, RYTER SW, CHOI AM. CO as a cellular signaling molecule. Annual review of pharmacology and toxicology. 2006;46:411–49. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- KIRCHSTETTER TW, SINGER BC, HARLEY RA, KENDALL GR, W. C. Impact of oxygenated gasoline use on California light duty vehicle emissions. Environ Sci Technol. 1996;30:661–670. [Google Scholar]
- LADEN F, NEAS LM, DOCKERY DW, SCHWARTZ J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environmental health perspectives. 2000;108:941–7. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEMOS M, DIAZ EA, GUPTA T, KANG C-M, RUIZ P, COULL B, GONZALEZ-FLECHA B. Cardiac and pulmonary oxidative stress in rats exposed to realistic emissions of source aerosols. Inhalation Toxicology. 2011 doi: 10.3109/08958378.2011.601433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIPPMANN M, CHEN LC. Health effects of concentrated ambient air particulate matter (CAPs) and its components. Critical reviews in toxicology. 2009;39:865–913. doi: 10.3109/10408440903300080. [DOI] [PubMed] [Google Scholar]
- MARTIN TR, WEHNER AP, BUTLER J. Evaluation of physical health effects due to volcanic hazards: the use of experimental systems to estimate the pulmonary toxicity of volcanic ash. American journal of public health. 1986;76:59–65. doi: 10.2105/ajph.76.suppl.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLS NL, TORNQVIST H, GONZALEZ MC, VINK E, ROBINSON SD, SODERBERG S, BOON NA, DONALDSON K, SANDSTROM T, BLOMBERG A, NEWBY DE. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. The New England journal of medicine. 2007;357:1075–82. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- MORSE D, CHOI AM. Inhaled CO in the treatment of acute lung injury. American journal of physiology. Lung cellular and molecular physiology. 2008;294:L642–3. doi: 10.1152/ajplung.00054.2008. [DOI] [PubMed] [Google Scholar]
- NAM E, FULPER C, WARILA J, SOMERS J, MICHAELS H, BALDAUF R, RYKOWSKI R, SCARBO C. Analysis of Particulate Matter Emissions from Light-Duty Gasoline Vehicles in Kansas City. Assessment and Standards Division Ofice of Transportation and Air Quality And Air Pollution Prevention and Control Division Ofice of Research and Development U. S. Environmental Protection Agency. 2008 [Google Scholar]
- NIKOLOV MC, COULL BA, CATALANO PJ, DIAZ E, GODLESKI JJ. Statistical methods to evaluate health effects associated with major sources of air pollution: A case study of breathing patterns during exposure to concentrated Boston air particles. (Series C).Journal of the Royal Statistical Society. 2008:357–378. [Google Scholar]
- PANKOW J, LUO W, BENDER D, ISABELLE L, HOLLINGSWORTH J, CHEN C, ASHER W, ZOGORSKI J. Concentrations and co-occurrence correlations of 88 volatile organic compounds (VOCs) in the ambient air of 13 semi-rural to urban locations in the United States. Atmospheric Environment. 2003;37:5023–5046. [Google Scholar]
- PAPAPOSTOLOU V, LAWRENCE J, DIAZ EA, WOLFSON JM, FERGUSON ST, LONG M, GODLESKI JJ, KOUTRAKIS P. Laboratory Evaluation of a Prototype Photochemical Chamber Designed to Investigate the Health Effects of Fresh and Aged Vehicular Exhaust Emissions. Inhal Toxicol. 2011a;23:495–505. doi: 10.3109/08958378.2011.587034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAPAPOSTOLOU V, LAWRENCE J, FERGUSON ST, WOLFSON JM, KOUTRAKIS P. Development and Evaluation of a Countercurrent Parallel-Plate Membrane Diffusion Denuder for the Removal of Gas-Phase Compounds from Vehicular Emissions. Inhal Toxicol. 2011b doi: 10.3109/08958378.2011.619590. in press. [DOI] [PubMed] [Google Scholar]
- PIERSON WR, GERTLER AW, ROBINSON NF, SAGEBIEL JC, ZELINSKA B, BISHOP GA, STEDMAN DH, ZWEIDINGER RB, WR. R. Realworld automotive emissions—summary of studies in the Fort McHenry and Tuscarora mountain tunnels. Atmos Environ. 1996;30:2233–2256. [Google Scholar]
- PIOCK W, HOFFMAN G, BERNDORFER A, SALEMI P, FUSSHOELLER B. Strategies Towards Meeting Future Particulate Matter Emission Requirements in Homogeneous Gasoline Direct Injection Engines. SAE Technical Paper. 2011 2011-01-121. [Google Scholar]
- RAUB JA, HATCH GE, MERCER RR, GRADY M, HU PC. Inhalation studies of Mt. St. Helens volcanic ash in animals. II. Lung function, biochemistry, and histology. Environmental research. 1985;37:72–83. doi: 10.1016/0013-9351(85)90050-7. [DOI] [PubMed] [Google Scholar]
- RHODEN CR, WELLENIUS GA, GHELFI E, LAWRENCE J, GONZALEZ-FLECHA B. PM-induced cardiac oxidative stress and dysfunction are mediated by autonomic stimulation. Biochimica et biophysica acta. 2005;1725:305–13. doi: 10.1016/j.bbagen.2005.05.025. [DOI] [PubMed] [Google Scholar]
- ROGAK SN, POTT U, DANN T, WANG D. Gaseous emissions from vehicles in a traffic tunnel in Vancouver, British Columbia. Journal of the Air & Waste Management Association. 1998;48:604–15. doi: 10.1080/10473289.1998.10463713. [DOI] [PubMed] [Google Scholar]
- RUIZ PA, GUPTA T, KANG CM, LAWRENCE JE, FERGUSON ST, WOLFSON JM, ROHR AC, KOUTRAKIS P. Development of an exposure system for the toxicological evaluation of particles derived from coal-fired power plants. Inhal Toxicol. 2007;19:607–19. doi: 10.1080/08958370701353148. [DOI] [PubMed] [Google Scholar]
- RUIZ PA, LAWRENCE JE, FERGUSON ST, WOLFSON JM, KOUTRAKIS P. A counter-current parallel-plate membrane denuder for the non-specific removal of trace gases. Environ Sci Technol. 2006;40:5058–63. doi: 10.1021/es060563w. [DOI] [PubMed] [Google Scholar]
- RYTER SW, MORSE D, CHOI AM. Carbon monoxide: to boldly go where NO has gone before. Science’s STKE : signal transduction knowledge environment. 2004:RE6. doi: 10.1126/stke.2302004re6. 2004. [DOI] [PubMed] [Google Scholar]
- RYTER SW, OTTERBEIN LE. Carbon monoxide in biology and medicine. BioEssays : news and reviews in molecular, cellular and developmental biology. 2004;26:270–80. doi: 10.1002/bies.20005. [DOI] [PubMed] [Google Scholar]
- SALDIVA PH, CLARKE RW, COULL BA, STEARNS RC, LAWRENCE J, MURTHY GG, DIAZ E, KOUTRAKIS P, SUH H, TSUDA A, GODLESKI JJ. Lung inflammation induced by concentrated ambient air particles is related to particle composition. American journal of respiratory and critical care medicine. 2002;165:1610–7. doi: 10.1164/rccm.2106102. [DOI] [PubMed] [Google Scholar]
- SANDERS CL, CONKLIN AW, GELMAN RA, ADEE RR, RHOADS K. Pulmonary toxicity of Mount St. Helens volcanic ash. Environmental research. 1982;27:118–35. doi: 10.1016/0013-9351(82)90063-9. [DOI] [PubMed] [Google Scholar]
- SAVAGE ST, LAWRENCE J, KATZ T, STEARNS RC, COULL BA, GODLESKI JJ. Does the Harvard/U.S. Environmental Protection Agency Ambient Particle Concentrator change the toxic potential of particles? Journal of the Air & Waste Management Association. 2003;53:1088–97. doi: 10.1080/10473289.2003.10466267. [DOI] [PubMed] [Google Scholar]
- SCHREIBER D, FORSS A, MOHR M, DIMOPOULOS P. Particle Characterisation of Modern CNG, Gasoline and Diesel Passenger Cars. SAE Technical Paper. 2007 2007-24-0123, 2007. [Google Scholar]
- SETHI JM, CHOI AM, CALHOUN WJ, AMEREDES BT. Non-invasive measurements of exhaled NO and CO associated with methacholine responses in mice. Respiratory research. 2008;9:45. doi: 10.1186/1465-9921-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STUPFEL M. Recent advances in investigations of toxicity of automotive exhaust. Environmental health perspectives. 1976;17:253–85. doi: 10.1289/ehp.7617253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALLYATHAN V, MENTNECH MS, TUCKER JH, GREEN FH. Pulmonary response to Mount St. Helens’ volcanic ash. Environmental research. 1983;30:361–71. doi: 10.1016/0013-9351(83)90221-9. [DOI] [PubMed] [Google Scholar]
- WELLENIUS GA, DIAZ EA, GUPTA T, RUIZ P, LONG M, KANG C-M, COULL B, GODLESKI JJ. Electrocardiographic and Respiratory Responses to Coal-Fired Power Plant Emissions in a Rat Model of Acute Myocardial Infarction: Results from the Toxicological Evaluation of Realistic Emissions of Source Aerosols (TERESA) Study. Inhal Toxicol. 2011 doi: 10.3109/08958378.2010.554461. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WIESTER MJ, SETZER CJ, BARRY BE, MERCER RR, GRADY MA. Inhalation studies of Mt. St. Helens volcanic ash in animals: respiratory mechanics, airway reactivity and deposition. Environmental research. 1985;36:230–40. doi: 10.1016/0013-9351(85)90020-9. [DOI] [PubMed] [Google Scholar]