Abstract
Syphilis has plagued mankind for centuries and is currently resurgent in the Western hemisphere. While there has been a significant reduction of tertiary disease, and recognition of facilitative interactions with HIV infection, the natural history of syphilis has remained largely unchanged; thus, new strategies are required to more effectively combat this pathogen. The immunopathologic features of experimental syphilis in the rabbit; the course, stages, and pathology of human syphilis; and a comparison of human syphilis with leprosy suggest that the clinical course of syphilis and its tissue manifestations are determined by the balance between delayed type hypersensitivity (DTH) and humoral immunity to the causative agent, Treponema pallidum. A strong DTH response is associated with clearance of the infecting organisms in a well-developed chancre, whereas a cytotoxic T-cell response or strong humoral antibody response is associated with prolonged infection and progression to tertiary disease. Many of the protean symptoms/appearances of secondary and tertiary human syphilis are manifestations of immune reactions that fail to clear the organism, due to a lack of recruitment and more importantly, activation of macrophages by sensitized CD4 T-cells. The Bacillus Calmette Guerin (BCG) vaccination can enhance DTH and has been shown to produce a low, but measurable beneficial effect in the prevention of leprosy, a disease that shows a disease spectrum with characteristics in common with syphilis. In the prevention of syphilis, a potential vaccine protective against syphilis should be designed to augment the DTH response.
Keywords: Syphilis, delayed type hypersensitivity, humoral immunity, primary, secondary and tertiary syphilis, immunohistochemistry
INTRODUCTION
“He who knows syphilis, knows medicine.”
Sir William Osler
Syphilis remains a global public health problem; the World Health Organization (WHO) estimates an incidence of 12 million new cases each year, with more than 90% of the cases occurring in the developing world (1). For Western civilization, syphilis has been a plague since its arrival in Europe in the 15th century. Although it appeared to decline in the late 1990s due to public health measures and safe-sex practices of high-risk groups, the incidence of syphilis started to rebound from a low of 2.1 cases per 100,000 to 3.3 cases per 100,000 in 2007 (2). This increase in North America and Western Europe has occurred almost exclusively in men, many of whom have sex with men and/or are co-infected with human immunodeficiency virus (HIV) (1–4). HIV and syphilis each facilitate infection by the other, and aggravate one another’s clinical course (3). While syphilis is well-known infection to all healthcare providers, its diagnosis, therapy and control still remain a challenge, particularly for those with little experience with the disease.
The particular manifestations of Treponema pallidum infection depend upon time, site, and the immune status of the infected individual. Time (duration of infection) relates to the designation of the stages of syphilis as primary, secondary, and tertiary disease (5–8). These clinical stages, in turn, reflect the interaction of the infectious agent with the host, and the effects of the immune response on the infection. Site refers to whether the lesions are located in the skin or mucous membranes (e.g., mouth), or internally. Since the growth of T. pallidum is dependent upon temperature (the internal temperature of the body is too high for optimal growth), the external surfaces are the major combat zones, in which immune effector mechanisms try to defeat large numbers of rapidly proliferating organisms. In contrast, the internal organs feature evidence of the immune response (lymphadenopathy or splenomegaly) during primary and secondary stages of infection; chronic, smoldering inflammation during the tertiary stage (granulomas) is reflective of an inadequate immune response to persistent infection. In addition, the tertiary stage has characteristic lesions due to nerve damage. The host’s immune status is reflected in the course and pathology of syphilis in its various stages. Of particular importance is the strength of delayed type hypersensitivity (DTH), which is mediated by CD4+ cells. Humoral antibody or CD8+ cytotoxic T-cells (TCTL) are relatively ineffective in clearing syphilitic infections, or in controlling progression of lesions; secondary and tertiary disease ensue if the DTH response is insufficiently effective. Table 1 lists the types of immune reactions to T. pallidum infection. Throughout this review, DTH will refer to a beneficial cell mediated immune host response characterized by an expanded population of antigen specific T cells that produce cytokines locally, activating and recruiting additional lymphocytes and macrophages (9). Macrophages accumulate at the site of DTH and become activated through the CD4 Th1 cell-cytokine-macrophage interactions protecting against infection by destroying and clearing the organism. However, high, persistent localized antigenic challenge can lead to excessive and/or chronic inflammatory response producing immunopathology in the form of granulomatous inflammation, tissue destruction, and the formation of secondary lymphoid organs (e.g. lymphoid follicles and plasma cell infiltrates) (9, 10). In this setting, granulomas are believed to form as a result of the persistence of non-degradable (non-replicative) infectious antigen.
Table 1.
Potential immune responses to infection with Treponema pallidum.
| Immune effector mechanism (Sell classification (9)) |
Gell and Coombs classification |
Mediators | Examples | Role in Syphilis |
|---|---|---|---|---|
| Atopic/anaphylactic (HIR) | Type I | IgE, mast cells | Urticaria, Hives, Asthma | Unknown |
| Neutralization (HIR) | IgG | Endotoxin neutralization: diptheria, tetanus, cholera, etc. | Unknown | |
| Cytotoxic/cytolytic (HIR) | Type II | IgM, IgG, complement, (agglutination, lysis opsonization) | Transfusion reactions, Goodpasture syndrome, Pemphigus | Unknown |
| Immune Complex (Arthus) reaction (HIR) | Type III | IgG, IgA, and/or IgM antibody-antigen complexes, neutrophils, complement | Cutaneous leukocytoclastic vasculitis, Arthus reaction, serum sickness, glomerulonephritis. | Acute inflammatory response |
| Cellular cytotoxic | CD8 T-cells, natural killer cells | Lichen planus, graft-versus-host disease | Not clear (T. pallidum is not, per se, an intracellular pathogen) | |
| Delayed Type Hypersensitivity (DTH) | Type IV | CD4 T-cells, cytokines, Activated macrophages | Destruction of infected macrophages in tuberculosis and leprosy; positive tuberculin test | Activated macrophages destroy T. pallidum |
| Granulomatous reactions* | Macrophages, lymphocytes | Foreign body granuloma, isolation of organisms in granulomas of leprosy, tuberculosis, syphilis | Possible reaction to persistent T. pallidum cell wall antigens |
DTH: delayed type hypersensitivity reaction. HIR: humoral (antibody) immune response.
Granulomas can result from non-immune stimuli as well as from an immune reaction inactivated by antibody or sensitized lymphocytes.
The occurrence of syphilis in patients with depressed immunity produces modifications of the pathology of the disease (7, 8, 11). In immunosuppressed individuals, massive numbers of T. pallidum may be found in internal organs, with little or no inflammation, which supports the theory that high persistent, systemic antigen leads T cell tolerance and T cell independent induction of B cell responses (antibody) (10). The activation of latent syphilis is often seen during the onset of acquired immune deficiency syndrome (AIDS)-related immunosuppression, indicating that the loss of immunity at this time permits outgrowth of the existing infection. The diagnosis in AIDS patients can be complicated by the fact that, because of the suppression of the immune response, seroconversion does not occur.
Our understanding of the progression of the early stages of syphilis infection has been considerably enhanced by studies in experimental animals, in particular, the rabbit (8, 12, 13). From studies in the rabbit, we have been able to deduce that the primary chancre of syphilis is a DTH reaction that is extremely effective in clearing infectious organisms from the site of infection. Although true secondary lesions have not been induced in the rabbit, disseminated skin lesions produced by intravascular inoculation can be elicited. These lesions are not like the secondary skin lesions of humans, but they are nevertheless DTH reactions, and this DTH response is effective in eventually clearing the large numbers of organisms present. Late lesions (tertiary syphilis) are not seen in the rabbit; in humans, late lesions, termed gummas, are granulomatous reactions to long-term smoldering infection with T. pallidum and/or its residual antigens, in patients unable to mount a completely effective DTH response.
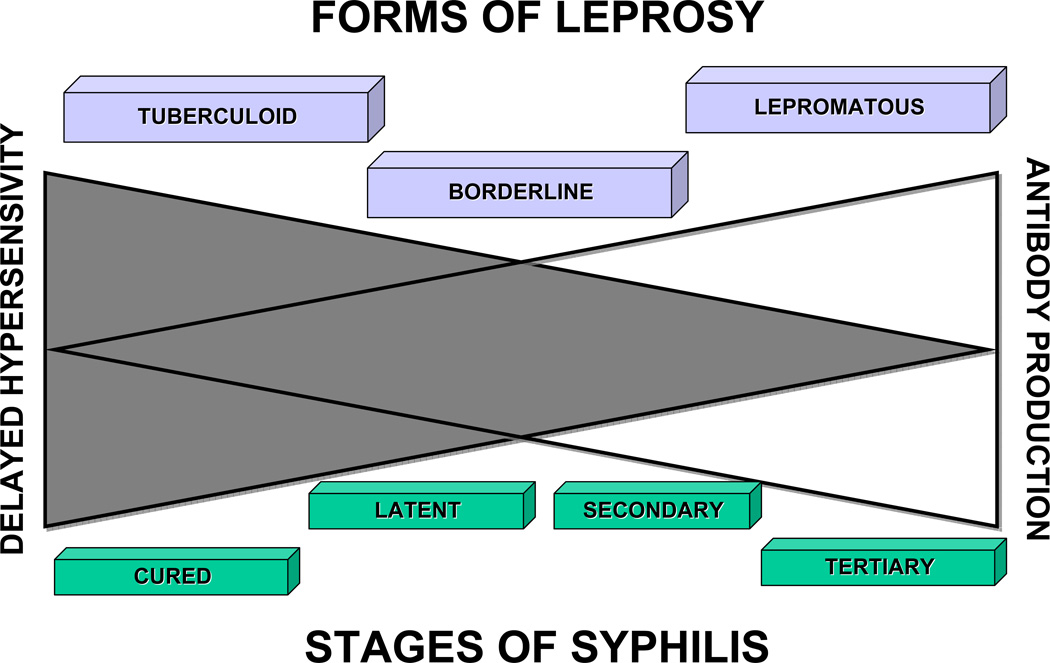
In this article, the pathogenesis of syphilis and the effect of the immune response of the host on the course of the disease, as inferred from an analysis of the cutaneous and other organ pathology, will be critically reviewed. The hypothesis will be presented that patients whose immune systems "cure" themselves of a syphilis infection after the primary stage, and those with prolonged latency, are able to eliminate or suppress the progression of the infection because of effective DTH; in contrast, those who develop tertiary disease may have good antibody production and/or high levels of T-cell mediated cytotoxicity, but they are unable to control the infection because of a relative lack of DTH. This concept will be supported by a comparison of the stages of syphilis with the various forms of leprosy; that analogy illustrates the relative roles of DTH and antibody production in the pathogenesis and progression of these infectious diseases. The topics that will be covered are as follows: 1) a brief historical perspective of the diagnosis and pathology of human syphilis; 2) a description of the experimental lesions in rabbits; 3) a review of the lesions of the major organ systems at varied stages of adult syphilitic infection in humans; 4) the pathology of syphilis in immunosuppressed persons, including AIDS patients; 5) a comparison of the stages of syphilis with the various forms of leprosy; and 6) conclusions.
AN HISTORICAL PERSPECTIVE OF THE PATHOLOGY OF SYPHILIS
Etiology
Early causation analysis
The pathogenesis of syphilis was not at all clear until the identification by Schaudinn and Hoffmann of the causative agent "Spirochaeta pallidum" in 1905 (14). Prior to that time, according to Osler (15): "The story of the search for the cause of syphilis is a tale to make the Judicious grieve. One hundred and twenty-five causes of syphilis… have been established during the last twenty-five years". Fritz Schaudinn, a parasitologist, was able to discern a transparent, delicate, spiral, filamented organism in unfixed tissue sections. The species name "pallida" (pale) was used because of the extraordinary difficulty in staining the organism. Because of these characteristics, many previous attempts to identify a causative agent for the lesions of syphilis were not successful. The use of the silver based stain of Levaditi (16) greatly increased the ability to detect the organism, by then known as Treponema pallidum, in tissues. In 1906, T. pallidum was identified in aortic lesions (17), and it was the American pathologist James Homer Wright (for whom the pathology laboratory at the Massachusetts General Hospital is named) who first identified T. pallidum in gummas (granulomas of tertiary syphilis: typically less cellular than classic granulomas), using the silver-staining procedure, in 1910. Until T. pallidum was definitively shown to be present in tertiary lesions, the tertiary manifestations of syphilis were not clearly associated with T. pallidum infection.
Laboratory Testing
A major breakthrough in the clinical understanding of syphilis was contingent upon the development of critical techniques that permitted the diagnosis of syphilis to be confirmed by laboratory testing. The use of dark-field microscopy to identify organisms in fluids and scrapings taken from lesions was first described in 1906 by Reichert, in Vienna (18). The first reliable serological test for syphilis was developed by Wassermann, in the same year (19). (For a review of serological tests used in syphilis, see references (20–23).) Prior to that date, the diagnosis of syphilis depended upon the gross appearance of the lesions, a demonstrable history of contact, and an "ensemble" of clinical observations based on the acumen developed by outstanding clinicians such as John Hunter, Philippe Ricord and his student, J. Alfred Fournier (18). Their observations were complicated by the fact that co-infections of gonorrhea with syphilis frequently occur; the overlap of symptoms set back differentiation of these two diseases for many years (24). Detailed clinical observations on prison inmates intentionally infected by Ricord finally led to separation of these two sexually transmitted diseases (see review by Rollet (25)). However, the capability of achieving a definitive diagnosis by dark-field microscopic examination or by serology was the critical breakthrough to a clinical understanding of the disease; see figure 1. (For detailed descriptions of the clinical diagnosis and treatment of syphilis prior to the use of penicillin, see references (15, 16, 26)).
Figure 1. The sensitivity of dark field microscopy and Wasserman test in early syphilis.
Chart illustrating the proportion of positive results obtained with the dark-field examination of the chancre and the serologic (blood) Wassermann reaction in the early weeks of syphilis infection (Adapted from reference (18), page 593). More specific serologic tests for syphilis may be negative for up to 10 days after formation of the chancre. The occurrence of seronegative syphilis during active infection has important implications for diagnosis, not only of the patients, but also for seronegative mothers giving birth to newborns with congenital syphilis.
Since it is not possible to culture Treponema pallidum on artificial media, the mainstay of diagnosis of syphilis has been the identification of spirochetes in smears from primary and secondary lesions, by dark-field or fluorescent microscopy, or, more commonly, though by serologic testing for antibodies as surrogate markers of T. pallidum infection (20, 27). The traditional approach to the serodiagnosis of syphilis is a two-step process: screening is first done by tests not specific for treponemes such as the rapid plasma reagin (RPR) test followed by a confirmatory test for those specimens found positive in the screen test, with a treponeme specific test such as the T. pallidum particle agglutination assay (TPPA) (8). Because all of the above tests must be performed in specialty clinics and/or laboratories, they are not available at primary care facilities where syphilis is most likely to be encountered, particularly in resource poor regions of the world. In addition, serologic testing is compromised by a high rate of false positive results, and difficulty in differentiating treated from untreated infections. Recently, simple, rapid point-of-care treponemal tests that do not require electricity or special equipment have become available; they have sensitivity and specificity similar to those of TPPA (www.who.int/std.diagnostics). It is anticipated that these novel, simplified tests will result in better diagnosis and treatment of infected patients, decreased spread of syphilis, and better prevention of mother-to-child transmission. The ideal diagnostic syphilis test is one that that can inexpensively out perform the traditional testing combination of non-treponemal and treponemal tests, and allow quantitative testing, so that the serological response to therapy can be monitored (8).
Clinically, the diagnosis of syphilis (“the great masquerader” (15)) remains challenging. The disease is often missed or miss-identified due to its protean manifestations and could happen because of a lack of completeness of clinical history, and/or inexperience of the treating physician. While the serologic testing of syphilis is the gold standard for diagnosis, serologic testing can give a false negative result in early primary syphilis, in some cases of secondary syphilis, in coinfection with HIV, in other immunosuppressive conditions, in neurosyphilis, and in congenital syphilis (20, 23, 28–33). Therefore, the testing of syphilis in tissue and other bodily fluids offers another important method to diagnose cases of syphilis that escaped screening, or in cases where syphilis was not considered in the clinical differential diagnosis. Silver staining (Levaditi, Warthin-Starry, Steiner, or Dieterle stains), direct immunofluorescence, and immunohistochemistry are methods that can directly detect spirochetes in formalin fixed, paraffin embedded tissue, particularly when organisms are numerous, as is the case in chancres and early lesions of secondary syphilis (28, 34–53). As syphilis progresses to latent and tertiary stages, spirochetes become scarce, so these methods fail, due to the detection limit being exceeded. In blood, fluid, and tissue samples from the later stages of syphilis, polymerase chain reactions (PCR) has been anticipated to be a highly sensitive and specific method to detect T. pallidium (41, 54–66). However, for late-stage disease samples, the sensitivity of PCR (DNA) based methods is lower than immunohistochemistry (protein) techniques (≤36% vs. ≤7%), indicating that while antigens are preserved, T, pallidum specific DNA is degraded. This phenomenon has been described in treated Whipple’s disease, where PCR is typically negative for Tropheryma whippelii DNA once antibiotic therapy has been initiated, yet organisms are still abundant by histology and immunohistochemistry (67, 68). Table 2 reviews the sensitivity of the various methods for detection of syphilis, according to disease stage.
Table 2.
Sensitivity and Specificity of diagnostic tests for syphilis. Serologic tests are the most sensitive.
| % Sensitivity at given stage of infection |
% Specificity | References | |||||
|---|---|---|---|---|---|---|---|
| Primary | Secondary | Latent | Tertiary | ||||
| Serology, non-treponemal | |||||||
| VDRL | 78 (74–87) | 100 | 95 (88–100) | 71 (37–94) | 98 (86–99) | (20) | |
| RPR | 86 (77–100) | 100 | 98 (95–100) | 73 | 98 (93–99) | (20) | |
| USR | 80 (72–88) | 100 | 95 (88–100) | 99 | (20) | ||
| RST | 82 (77–86) | 100 | 95 (88–100) | 97 | (20) | ||
| TRUST | 85 (77–86) | 100 | 98 (95–100) | 99 (98–99) | (20) | ||
| Serology, treponemal | |||||||
| FTA-ABS | 84 (70–100) | 100 | 100 | 96 | 97 (94–100) | (20) | |
| FTA-ABS double staining | 80 (69–90) | 100 | 100 | 98 (97–100) | (20) | ||
| MHA-TP | 76 (69–90) | 100 | 97 (97–100) | 94 | 99 (98–100) | (20) | |
| PCR (PBMC/Serum) | 46 (25–64) | 86 | 66 (62–71) | 100 | (59, 62, 63) | ||
| RT-PCR (whole blood) | 28 (10–53 CI) | 36 (19–55 CI) | 0 | 100 | (60) | ||
| Tissue (Skin/mucosa/exudates) | |||||||
| Dark Field microscopy | 84 (71–100) | 60 (25–100) | 98 (97–100) | (27, 28, 35, 40, 46, 47, 50, 52, 54) | |||
| Silver stain histochemistry* | 86 (50–100) | 40 (0–92) | 4 (0–11) | (34, 36, 38, 40, 41, 44, 47–49, 56) | |||
| Direct immunofluorescence | 90 (80–100) | 70 (68–71) | (35, 36, 50, 52, 53) | ||||
| Immunohistochemistry | 100 | 87 (58–100) | 36 (11–60) | 100** | (28, 35, 38, 40–42, 44, 46, 51) | ||
| PCR (tissue) | 100 | 67 (42–100) | 7 (0–14) | (39–41, 56, 59) | |||
| PCR (lesional smear) | 94 (91–96) | 80 | 98 (96–100) | (54, 58, 61) | |||
| RT-PCR (lesional smear) | 80 (44–97 CI) | 20 (0.5–72 CI) | 0*** | (60) | |||
Dieterle, Steiner, or Warthin-Starry stains.
cross reactivity for Borrelia species (JAC, Personal observation; Dr. Cartun. Treponema pallidum cross-reacts with burgdorferi (Lyme disease). Written Correspondence 2004 to Biocare Medical, LLC, Concord CA regarding CP135 Treponema pallidum polyclonal antibody)
unpublished results- 0/5 gummas were negative by RT-PCR for syphilis; however, 3/5 (60%)of these specimens were positive by immunohistochemistry (J. A. Carlson & D. Wroblewski, Wadsworth Center, Dep't of Health, NY).
CI: 95% confidence intervals. FTA-ABS: fluorescent treponemal antibody absorption test. IHC-TP: immunohistochemistry on formalin fixed, paraffin embedded tissue with antibodies specifc for T. pallidium. MHA-TP: Microhemagglutination assay Treponema pallidum. PBMC: peripheral blood mononuclear cells. PCR: Polymerase chain reaction. RPR: rapid plasma reagin test. RST: regain screen test. RT-PCR: real time polmerase chain reaction. TRUST: toluidine red unheated serum test. USR: unheated serum regain test. VDRL: Venereal disease research laboratory test.
THE PATHOLOGY OF EXPERIMENTAL SYPHILIS IN THE RABBIT
External lesions
Primary chancre
Although the experimental transmission of syphilis to a number of animals, including primates, has been attempted, only the rabbit develops primary lesions that are essentially identical to those seen in humans ((69–71); for a comprehensive review of this subject see (5)). For that reason, the lesions seen in experimental animals other than the rabbit will not be considered in the present review. Human syphilis was first transmitted to the anterior chamber of the eye of rabbits in 1906 by Bertarelli (72), and later Brown and Pierce carried out extensive studies of lesions in the testicle (73) and scrotum (74, 75), as well as in other organs, including the skin (76, 77), eye (78), and regional lymphatics (79). These areas permit rapid growth of the organisms, where as growth is inhibited in the internal organs of the rabbit due to their higher-temperature environments. For optimal lesion development in the skin or testicles, ambient temperatures below 21°C are required (70). The development of the skin lesion, on gross and microscopic scales, closely follows that of the primary human chancre (see figure 2). By means of immunohistologic labeling for lymphocytes and organisms (80, 81), as well as electron microscopy (82), it has been demonstrated that the fully developed chancre is essentially a DTH reaction to the infecting T. pallidum organisms, a reaction that produces rapid destruction and clearing of the organisms by phagocytosis, and destruction by macrophages (5).
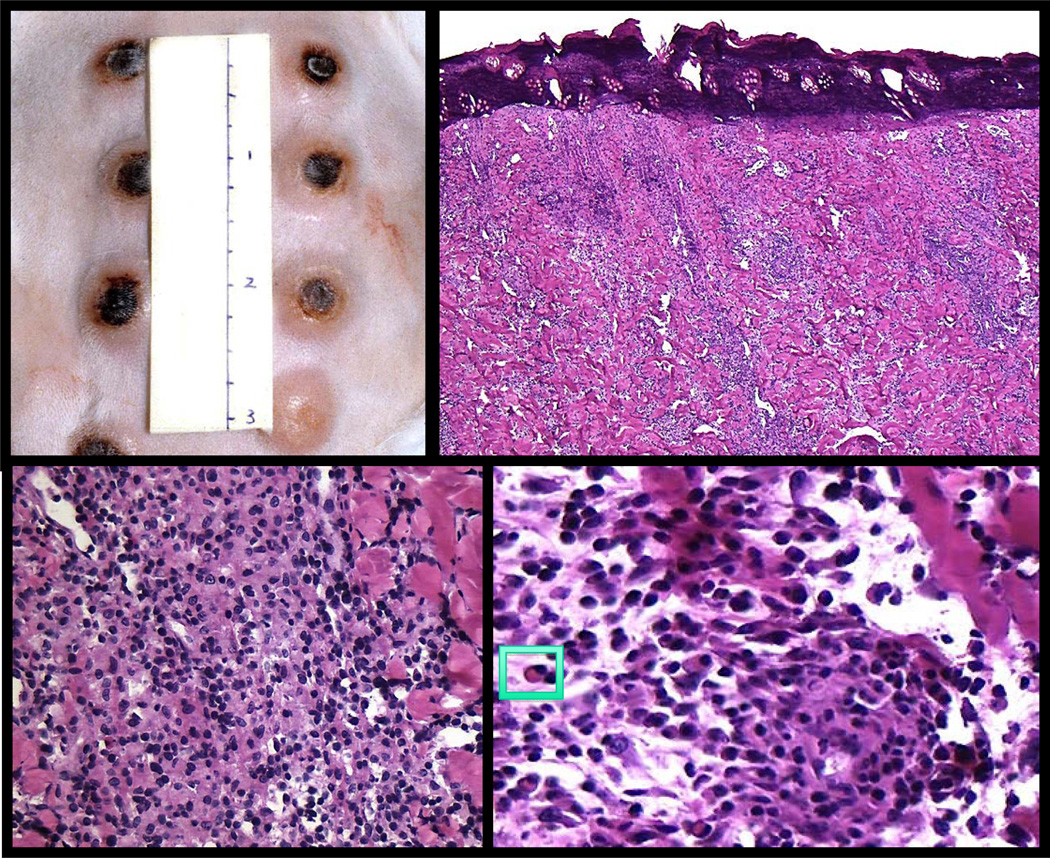
Figure 2. Experimental syphilis infection in the rabbit resembles a human chancre.
Top left panel shows multiple rabbit chancres at the peak of inflammation. Histologically, both the rabbit and human primary chancres have raised, firm erythematous margins with central necrosis (top right panel). Healing occurs within a few weeks in both leaving a small, depressed scar. The inflammatory infiltrate shows a dense, diffuse pattern (bottom left panel) and consists predominately of lymphocytes, macrophages and plasma cells (insert, bottom right panel).
Gross lesions
Injection of viable, infectious T. pallidum into the shaved skin of the rabbit results in development of a firm, raised, oval lesion that develops central necrosis, with sloughing of the necrotic area, followed by complete healing; a small, depressed scarred area is left. The central eschar is more prominent than in human lesions, but it eventually sloughs off to reveal a deep, clean, ulcerated area similar to the classical Hunterian chancre of humans. In the rabbit testis, the sequence of events is essentially identical, except that the inflammation takes place within the testis, and superficial necrosis is not prominent.
Stages of infection
Three stages of experimental infection have been emphasized by Collart and colleagues (69): inductive, reactive, and latent. The duration of each stage depends upon the number of organisms injected (83). For a relatively high number of viable T. pallidum (2×107), the lesions may run the entire disease course in 3 – 4 wks. For smaller numbers of organisms (103), complete resolution of the lesion can take up to 3 mos. For the purpose of description of the evolution of the lesion, the events following inoculation of the larger dose in the testes will now be described. During the inductive phase, there is a rapid increase in the number of structurally intact T. pallidum in the interstitial tissue in the testes or dermis, until by 10–12 days the tissue is filled with organisms. The organisms are at first located in perivascular areas, but they then disseminate throughout the extracellular matrix of the connective tissue. During the first few days there may be a slight perivascular polymorphonuclear leukocyte infiltration (84), and phagocytosis of organisms by these cells has been reported (85). However, it is not clear that latter is a response to viable organisms; it could instead represent a non-specific reaction to non-viable organisms in the inoculums. Over the first 10–12 days, there is an increasing perivascular infiltrate of T-lymphocytes that extends more and more progressively, and eventually becomes prominent in the interstitial tissue of the testes, at the base of the epidermis, and surrounding hair follicles of the skin. By day 10, the interstitial tissue is full of organisms. The cellular infiltrate and appearance of the organisms change abruptly at 13–14 days after infection, signaling the beginning of the reactive stage. Now, large numbers of macrophages appear in the lesions, and the numbers of structurally intact organisms in the interstitial tissue decreases rapidly. The decrease in intact organisms is accompanied the appearance of clumped, immunolabeled T. pallidum antigens in macrophages (80); see figure 3. By electron microscopy, phagocytosis and disarticulation of the organisms within phagolysosomes can be demonstrated (82). While the organisms disappear from the interstitial tissue, they can still be demonstrated in hair follicles, in erector pili muscles and within nerve fibers (84). Rarely, some organisms can be seen inside epithelial and mesenchymal cells (86–90), as well as in cutaneous nerves (84, 91, 92) and nerve cells (93). Their presence inside cells may serve to prolong their ability to evade immune attack (i.e., immunoprotective niche (94)). The presence of organisms within nerves suggests that T. pallidum is able to migrate into the regional ganglia or CNS by passage up the fibers (84, 91–93), leading to loss of sensory function (tabes dorsalis) or to the meningitis seen in human tertiary syphilis (see below). Later, the inflammation becomes concentrated at these sites of intracellular infection, as the organisms are eliminated by the activated macrophages. The marked inflammation during the reactive phase is associated with swelling and proliferation of endothelial cells, and is most like caused by inflammatory mediators such as interleukin-1 and interferon-γ, produced by activated macrophages. This inflammation contributes to the necrosis of the overlying epithelium, and to eventual ulcer formation. During the early latent stage, there is healing of the inflamed tissue. The inflammatory infiltrate is replaced by fibroblasts, followed by scarring in the interstitial tissue of the testis or the dermis of the skin. At the margins of the ulcer, there is acanthosis and hyperplasia of the epithelium, which eventually grows over the granulation tissue formed at the base of the ulcer. Figure 4 summarizes the course of inflammation during the primary infection in rabbit skin. Notable features are the relative absence of polymorphonuclear infiltrate, except in necrotic areas, and the lack of leukoclastic (neutrophilic small vessel) vasculitis. However, polymorphonuclear cells appear in greater numbers in some rabbits, and may play a role in destruction of organisms (95).
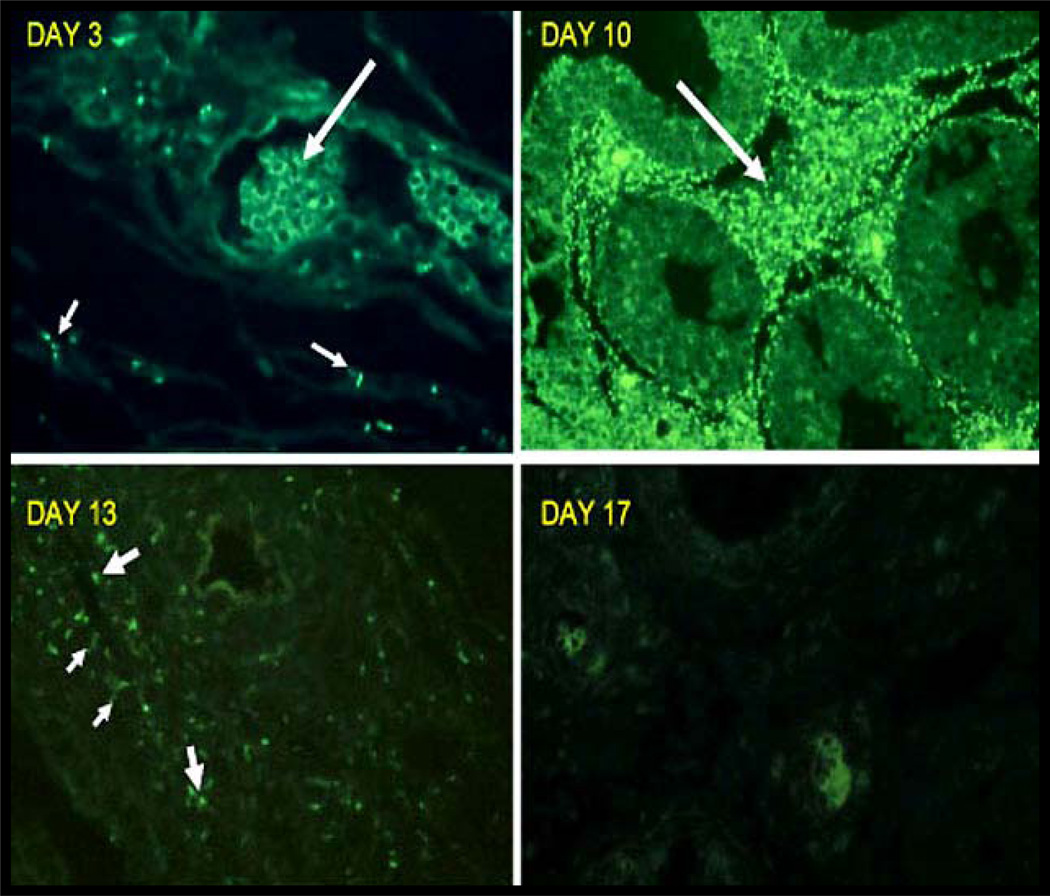
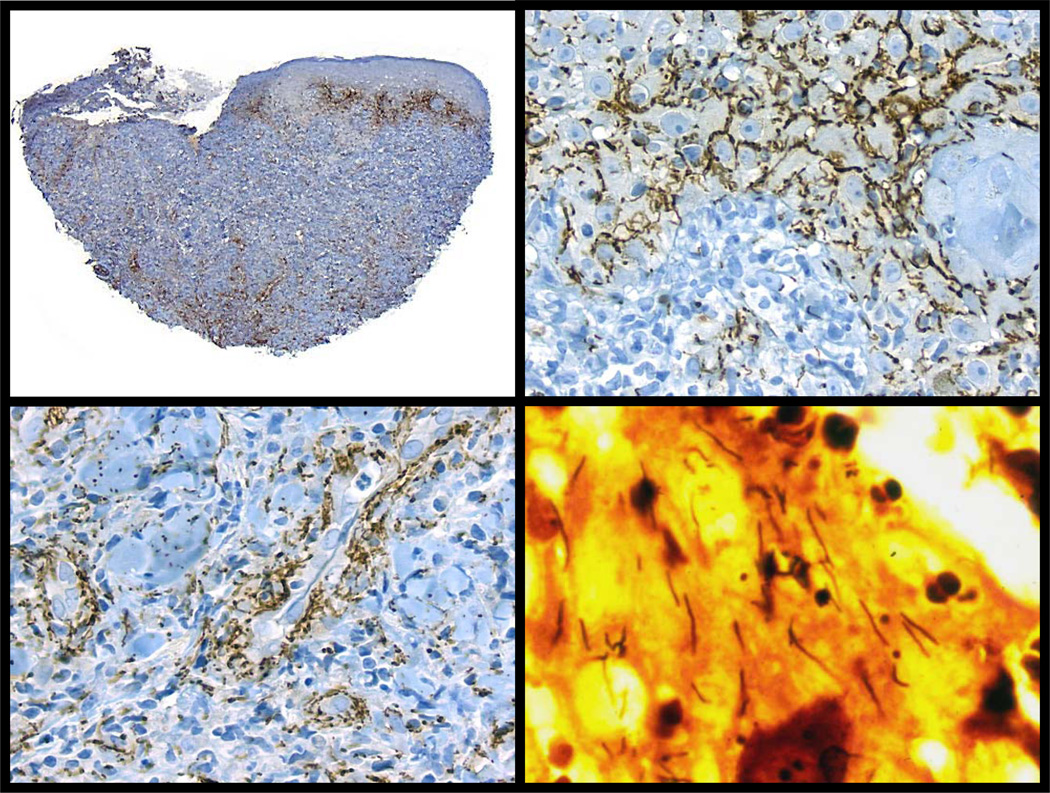
Figure 3. The growth, spread and eventual destruction of T. pallidum in the rabbit testis as demonstrated by direct immunofluorescent examination.
Day 3) The small arrows point to a few T. pallidum in interstitial tissue of testes. The large arrow points to fluorescent red cells in a blood vessel. (400× magnification). Day 10) The large arrow points to fluorescent T. pallidum in the interstitial tissue. The non-fluorescent structures are seminiferous tubules. (200× magnification). Day 13) The smaller arrows point to a few faintly staining spiral T-pallidum. The larger arrows point to fluorescent fragments of T. pallidum in macrophages within testicular lesion (400× magnification). Day 17) Only red cells are fluorescent and no organisms can be identified in multiple sections of the testes. (400x magnification).
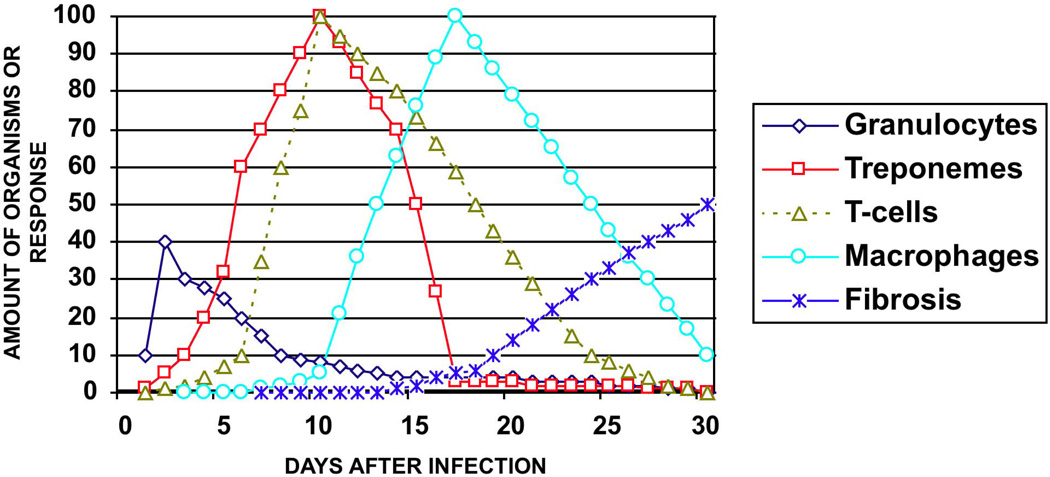
Figure 4. The natural history of a syphilitic rabbit chancre.
This chart illustrates the sequential events in the evolution of the lesions of experimental syphilis. Polymorphonuclear cells are seen in the first few days after inoculation of organisms and then decline. However, in some studies polymorphonuclear cells are seen in higher numbers later during development of the lesion. T. pallidum may been found in very small numbers within 1 day after injection, then increase rapidly so that by day 10–11 the dermis is filled with organisms. T-cells appear within the first few days and increase to a peak about 10 days after infection. Intact organisms co-exist with T-cells during the first 11 days. After 10 days macrophages increase rapidly and by day 13 fragments of digested organisms may be found within macrophages. Organisms are then rapidly cleared from the tissue, so that by 21 days few, if any, may be found. The lesion then heals with regional fibrosis.
Secondary lesions
It is unlikely that true secondary lesions of syphilis have been produced in the rabbit. However, through intravenous injection of shaved rabbits, more prolonged lesions in multiple sites on the shaved skin have been produced (70, 96), and some strains of T. pallidum injected intradermally, at low doses (103), produce lesions away from the site of intradermal injection, about 50 days after inoculation. In the former case, these persistent lesions are more likely to be multiple primary lesions than true secondary lesions. In the second case of late onset, the microscopic characteristics of the lesions are unknown, and their appearance does not correlate with levels of circulating immune complexes (96). When protracted or late onset lesions persist in the presence of increasing antibody titers or production of immune complexes, manifestations of immune complex (Arthus type) reactions (e.g. palpable purpura/leukocytoclastic vasculitis) would be expected. However, this does not appear to be the case, either in experimental disease in the rabbit model, or in human secondary lesions (see below). The multiple cutaneous lesions of the rabbit appear to represent DTH reactions similar to the primary chancre.
Internal lesions
Lymphoid organs
The draining lymph nodes and spleen of T. pallidum infected rabbits demonstrate rapid hyperplasia of T-cell zones (diffuse cortex), followed by persistent hyperplasia of T-cell and B-cell zones, and an increase in medullary plasma cells (97, 98). Although a rapid systemic dissemination of organisms occurs throughout the body of the rabbit (70, 81, 99), little or no evidence is seen of proliferation of organisms, or inflammation in internal organs. Organisms can be identified by transfer of disease or by immunofluorescence in draining lymph nodes, within a few hours of intradermal or intratesticular injection. Absent, however, is any evident increase in numbers of organisms in internal organs, in contrast to what is seen in the external tissues; also absent is any acute or chronic inflammation. Likely, high internal body temperature of the rabbit does not permit growth of the organisms, so that the amount of antigen is insufficient to initiate DTH reactions in these tissues (10). In the latent period that follows active infection, organisms persist in internal organs (70), but there is no evidence of subsequent inflammation that is seen in human tertiary syphilis.
Lymphadenopathy and splenomegaly are caused by hyperplasia as a result of induction of a specific immune response (97). There is a rapid, marked, and lasting increase in the peri-arteriolar T-cell zone of the spleen and the diffuse cortex of the lymph nodes. Large productive germinal centers with production of immunoglobulin positive plasma cells appear later, adjacent to the germinal centers (97, 100).
DTH and clearing of infection
In the rabbit models of syphilis described above, the clearing of organisms by phagocytosis, the morphology of the lesions seen, and the rapid hyperplasia of the T-cell zones of lymphoid organs are most consistent with the idea that the clearing response is the result of mediation of DTH reactions by sensitized T-cells. Hyperplasia of the diffuse cortex of the lymph nodes is associated with DTH; hyperplasia of follicles is associated with antibody production (101). T-cell proliferative responses to T. pallidum antigens appear in the lymph nodes and spleen of rabbits within a few days after infection, whereas serum antibody is not seen until 10 days to 2 weeks (80). Both cellular and humoral responses are maintained for many months after infection (98), suggesting continuing latent infection. In fact, rabbits with latent infection maintain high levels of T-cell reactivity to T. pallidum antigens and show a bias toward production of Th-1 type cytokines (i.e., cellular mediated immunity) over Th-2 cytokines (i.e., non-cellular immunity) (102). Although circulating antibody can play a role in resistance to reinfection in chancre-immune rabbits (103), there is little or no evidence of antibody- or immune complex-mediated tissue lesions. A mixed perivenular, polymorphonuclear-mononuclear infiltrate without fibrinoid necrosis is seen in the dermis following re-infection of chancre-immune rabbits (84). A similar lesion is seen in some patients with early secondary cutaneous syphilis with urticarial lesions (104), and in the rabbit model of disseminated syphilis (105). These results strongly suggest that the primary immune clearance mechanism for proliferating T. pallidum in tissue lesions is DTH. Thus, it follows that vaccine approaches to the prevention of syphilis should be directed towards the development of induction of DTH, rather than antibody production. DTH induction may be possible with the use of selected vectors such as Bacillus Calmette Guerin (BCG) (12) or selected epitopes that elicit T-cell responses. Support of BCG’s efficacy in producing a DTH reaction is demonstrated by the results of BCG vaccination for the prevention of leprosy. Case control studies in India (106) and Brazil (107) and meta-analysis of 29 studies examining BCG vaccination and leprosy (108) have provided clear evidence that BCG has a measureable, albeit low-level, protective effect against leprosy. Other potential effective antigens include the TprK protein, which elicits an opsonic antibody and some protection against infection (109, 110). However, another study indicated that TprK is periplasmic, is not expressed on the surface, and does not elicit protective immunity (111). It is generally accepted that antigens must be present on the outer surface of T. pallidum in order for them to be used to elicit protective immunity (112). Indeed, antibodies to outer membrane proteins can mediate re-infection immunity in rabbits (113, 114). However, to establish this type of immunity, recovery from active infection with T. pallidum is required (70, 71, 84, 100).
One of the proposed causes for persistence of syphilis infection, despite adequate immune response during the primary and secondary stages, is the alteration of cell surface antigens, whereby allowing a few T. pallidum to escape opsonization and macrophage-mediated clearance, and thus avoid recognition by the host immune system (115). However, there is no evidence this mechanism is operative in vivo (there exists a continued ability of immune-labeling to detect organisms in secondary lesions), and the surface changes described may be the by-product of processing of the spirochetes for examination. As T. pallida disseminate widely and in large numbers, the spirochetes may find safe harbor, an “immunoprotective niche” (94, 116), which allows them to persist. Moreover, the amount, context of presentation, and duration of (infectious) antigen dictates the immune response (10). High, persistent and systemic antigen exposure leads to T-cell tolerance and T-cell independent B-cell (humoral) response, i.e., absent DTH response, dominant antibody response, and latent infection. In contrast, high, persistent, and localized in peripheral tissue antigen leads to immunopathology, i.e., the gumma of tertiary syphilis. In addition, during the induction of an immune response, DTH actually appears before circulating antibody is detectable (117). Thus, immune-complex (IC) lesions only appear after development of the primary lesion and most likely play little role in clearing of the infectious organisms. However, IC formation could be active in prevention of re-infection. Indeed, if early syphilis is effectively treated, then re-infection can occur. However, if treatment is delayed until full development and involution of the primary lesion, then re-infection does not occur. Thus, development of a protective immune reaction requires completion of induction and resolution of a DTH response (117).
THE PATHOLOGY OF HUMAN SYPHILIS
Primary human syphilis
The chancre
The primary cutaneous lesion of syphilis, the chancre, begins as a small macule, enlarges to a papule, which can range in diameter from 0.5 to 1.0 cm, and then progresses to a painless erosion or ulcer, and eventually heals after a course of 3 to 8 weeks (15, 18, 26, 118, 119). The classical Hunterian chancre is solitary, round or oval, has sharp indurated margins and a firm button-like cartilaginous base with a convex eroded surface and a raw-ham color; it shows a thin serous discharge and a hemorrhagic border without pus. It is not undermined (18); see figure 5. However, only about half of affected individuals have the classic Hunterian chancre. Others have non-indurated ulcers or chancroid-like lesions (120), and there may be co-infections with other organisms, such as gonorrhea or chancroid, thereby complicating the gross appearance of the lesion. The lesion classically appears on the penis or vagina. If transmission occurs through a mucous membrane, such as the lip, mouth (20% of cases) or tonsils, the appearance may be modified by bacterial infection, but the same general characteristics are present. In recent years, more lesions of the anus have become more common among men who have sex with men. Lesions can also occur on the fingers, breasts or any surface exposed to an active lesion on the partner. The location of the lesion determines the differential diagnosis. Thus, a chancre of the tonsil can be misdiagnosed as diphtheria, Vincent's angina, or lymphoma; chancres of the finger can be misdiagnosed as simple infection (furuncle), felon, tuberculosis, squamous cell carcinoma, tularemia, sporotrichosis, rat-bite fever, anthrax, foreign body reaction, etc. Dentists should be aware of the occasional manifestation of primary syphilis as an oral ulcerations (121). Primary syphilis can also be transmitted by needle inoculation or transfusion (needle transmission may be increasing in the case of drug users); in that case, a primary chancre does not develop. Historically, absence of a chancre has been called "Syphilis d'emblée" (18).
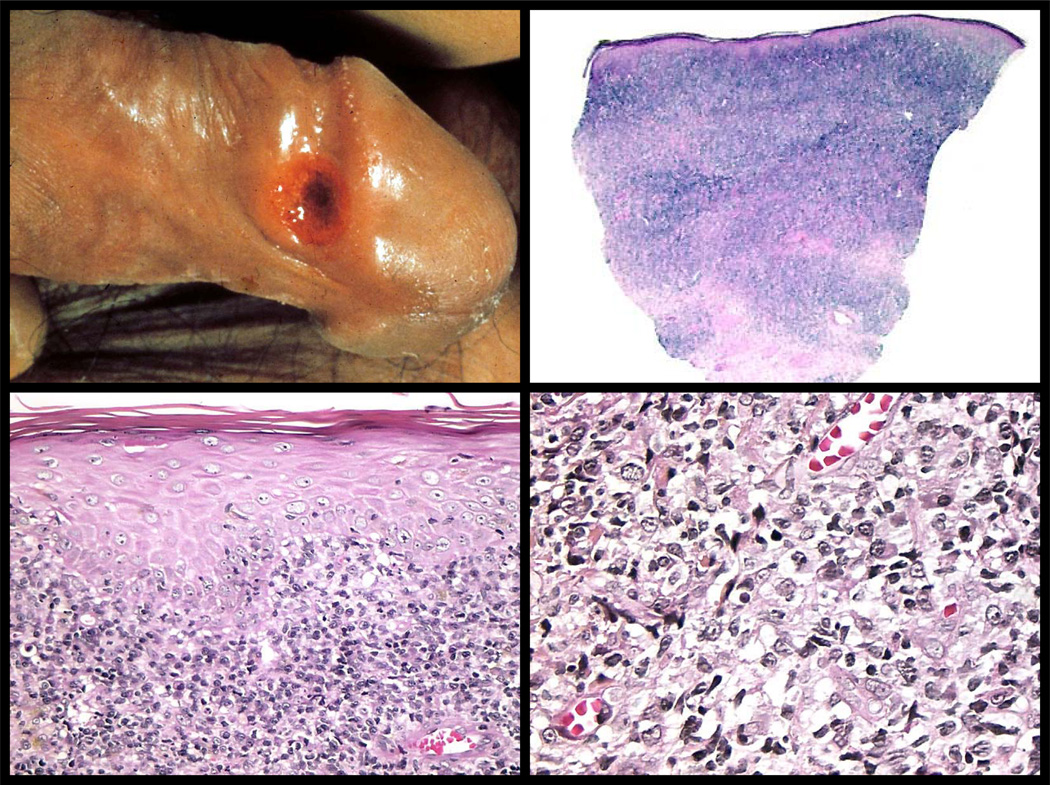
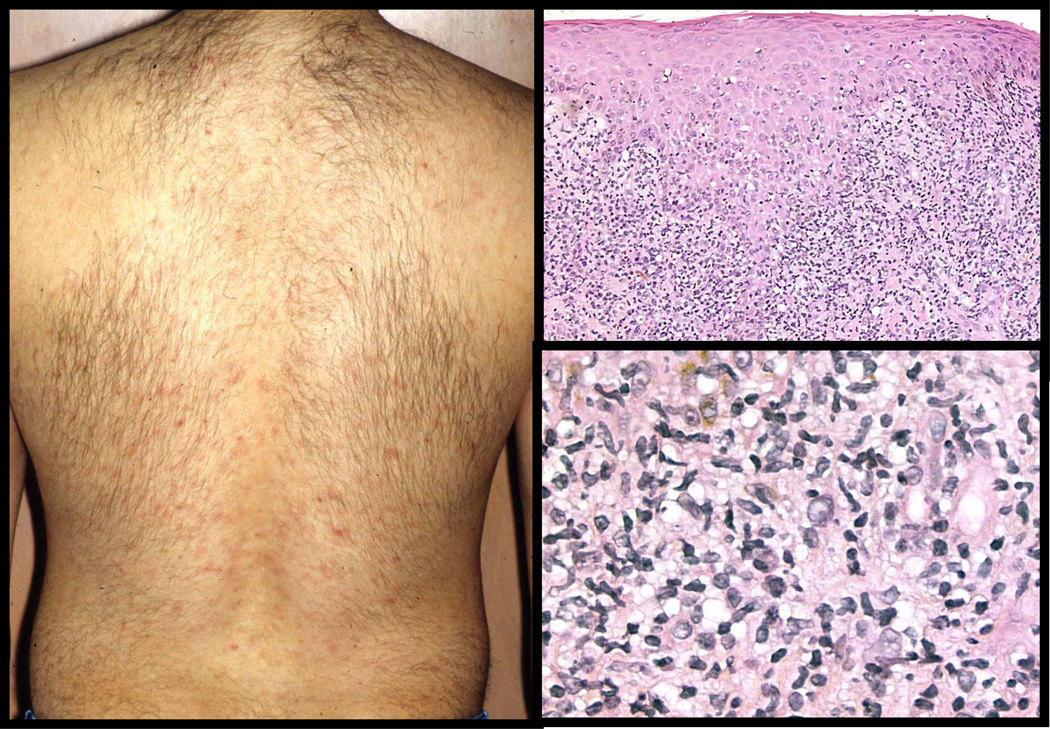
Figure 5. Primary syphilis- the chancre.
The top left panel shows a painless, eroded, button-like papule of a chancre of primary syphilis; the site of primary inoculation. (Clinical photo courtesy of Dr. Ronald Rapini, Houston, Texas). Histologically, chancres are characterized by a diffuse, dense inflammatory infiltrate (top right panel) that consists of lymphocytes, plasma cells, and macrophages (bottom panels). Neutrophils can often be found and are typically associated with an erosion and ulceration.
Diagnosis
Seroconversion is of critical importance in making the diagnosis of syphilis, in addition to useful clinical data such as presence of lymphadenopathy, history of sexual contact, and, of course, demonstration of organisms by dark field examination of fluid from the base of the lesion. However, serology is positive in only 50% of individuals with primary chancres at initial presentation. Most patients will seroconvert within 2–3 weeks, so that repeat serology can be a crucial, if dark-field examination is not available. Automated tests for antibodies (122), Western blotting (123), enzyme immunoassays (21, 124) using recombinant antigens, and detection of T. pallidum DNA by polymerase chain reactions and specific probes for T. pallidum DNA can also aid in accurate diagnosis (55, 57, 59, 60).
Microscopic characteristics
Histologically, the early chancre in the macule and papule form shows swelling and proliferation of endothelial cells, and a perivascular mononuclear infiltrate; organisms may be identified by silver stains or immunolabeling techniques mainly around the involved vessels or lower (basal and lower spinous) layer of the epidermis (28, 42, 119); see figure 6. Later, with occlusion of the vessels and increasing mononuclear cell infiltration, the epidermal surface becomes eroded and eventually sloughs, leaving an ulcer. The epidermis has acanthosis at the margin of the lesion and thins toward the center of the lesion, where inflammatory cells infiltrate it. Underlying the eroded area is a dense infiltrate of lymphocytes and macrophages, with varying numbers of plasma cells in the dermis; see figure 5. At the margins of the lesion, the inflammation becomes less dense and consists of perivascular cuffing with mononuclear cells. Vascular endothelium shows swelling and proliferation, but there is no evidence of active vasculitis (i.e., no leukoclastic vasculitis/immune complex mediated vasculitis). The chancre heals with minimal scarring as the organisms are cleared.
Figure 6. Detection of syphilis by immunohistochemistry and silver stain.
Immunohistochemistry for spirochetes is an effective means to identify primary and secondary syphilis. In this lesion of secondary syphilis, note the abundance of organisms (top left panel), which have a predilection for the epidermis (top right panel) or the deep perivascular region (bottom left panel). Warthin-Starry stain (lower right panel) shows numerous spirochetes from a lesion of condyloma lata; however, note the abundant false positive staining, which can make these preparations difficult to interpret, particularly in organism poor lesions.
Systemic primary syphilis
During the development of primary syphilis, there are systemic changes similar to that seen in the experimental skin and testis rabbit models. Dissemination of organisms from the site of infection via the lymphatics to the blood occurs within a few hours. The early immune response is characterized by marked lymphadenopathy and splenomegaly, due to the hyperplasia associated with induction of specific immunity. Thus, one of the diagnostic characteristics of the primary chancre of syphilis is lymphadenopathy in the draining lymph nodes (15, 18, 26). During this early period of immune induction, organisms most likely find their way to many internal organs, setting the stage for latency and tertiary disease. Although there is preferential hyperplasia of the diffuse cortex, by the time lymph nodes are biopsied, in human syphilis, there is usually marked hyperplasia of both diffuse and nodular cortex. A perivascular mononuclear cell vasculitis may be seen in various organs, and is associated with a proliferative endarteritis similar to that seen in chronic renal allograft rejection (125); see figure 7.
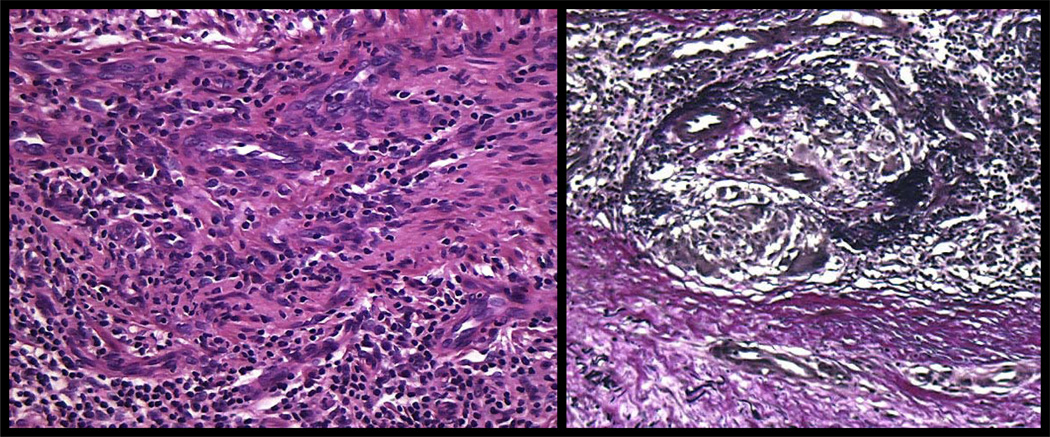
Figure 7. Syphilitic endarteritis obliterans.
Proliferation of swollen, vacuolated endothelial cells accompanied by vessel wall thickening is a frequent finding in cutaneous lesions of syphilis. The inflammatory host response directed at inter-endothelial (see figures 6 and 11) and intra-endothelial spirochetes (90)), likely induces intimal proliferation with subsequent luminal narrowing and ischemia (left panel). Elastic tissue stain demonstrates disruption of an internal elastic lamina and replacement of the intima by fibrous tissue and small vessels (right panel).
Secondary human syphilis
Secondary cutaneous syphilis
Although secondary syphilis is classically considered to appear after healing of the chancre of primary syphilis, or 8 weeks after the initial appearance of the chancre, there is no actual sharp demarcation between primary and secondary cutaneous syphilis. Serologic tests for syphilis are almost always positive in secondary syphilis. The predominance of the skin lesions in secondary syphilis is indicated by the following percentages of various manifestations in a large series of patients on admission: skin lesions 81.1%; throat and mouth 36.3%; genital lesions 19.9%; central nervous system 9.9%; alopecia 7.1%; eye lesions 4%; and visceral lesions 0.2% (18).
Clinical morphology
Lesions of secondary syphilis vary from urticarial to macular to maculopapular to papular to pustular to nodular (34, 48, 49, 119, 126–129); see figures 8–9. Hair loss can also occur (moth-eaten alopecia/alopecia syphilitica), typically affecting the scalp, but also the eyebrows or total body hair loss. Secondary syphilis has been termed the “great masquerader”, because its skin lesions show such diverse clinical and/or histologic morphologies, mimicking alopecia areata (130), bullous pemphigoid (131), cutaneous lymphoid hyperplasia (pseudolymphoma) (132–135), erythema multiforme (126, 136), granuloma annulare (43, 128, 137, 138), histiocytoma (34), leprosy (128, 139, 140), lichen planus (34, 126, 135, 141, 142), lupus erythematosus (43, 128, 143), mycosis fungoides (126, 144–146), pemphigus vulgaris (147), pityriasis lichenoides et varioliformis acuta (PLEVA) (34, 126, 135), pruritic (eczematous) dermatoses (126, 128, 148), psoriasis (34, 128, 149, 150), pustular psoriasis (34, 128), sarcoidosis (34, 135, 151–155), small vessel vasculitis (156), suppurative folliculitis (157), superficial thrombophlebitis (158), Sweet’s syndrome (128, 159), tinea imbricata and erythema annulare centrifugum (34, 160), and urticaria (104). Ulcerative nodular presentations are rare and can occur secondary to follicular pustules (157, 161), or an obliterative endarteritis, known as lues maligna (162, 163). Lues maligna appears to be more common when HIV co-infection is present (162–166).
Figure 8. Lichenoid Secondary Syphilis.
A patient presenting with myriad macules and flat-topped papules (left panel) that are formed by a band-like mixed lymphocytic, histiocytic, and plasma cell rich inflammatory infiltrate accompanied by epidermal hyperplasia (right panels). Lymphocyte exocytosis is frequently seen; numerous spirochetes will be found in these regions by silver stains or immunohistochemistry (see figure 6).
Figure 9. Pustular Secondary Syphilis.
So called rupial syphilis refers to crusted papular and pustular lesions (top left panel). This patient’s histology shows a pustular psoriasis-like pattern with spongiform pustule formation and psoriasiform epidermal hyperplasia (top right and bottom left panels). The presence of plasma cells in the dermal infiltrate is a clue to syphilis (bottom right panel).
The mucous patch/plaque, mucosal secondary syphilis, is the homologue of the skin lesion. The typical mucous patch can occur in the mouth, or on the tongue, lips, or genitalia. It is slightly raised, moderately indurated, and has a smooth, annular, central erosion covered by a pearly or grayish delicate membrane. This lesion usually contains large numbers of viable organisms. Secondary cutaneous lesions in the genital or anal area frequently become very hyperplastic, producing the characteristic lesion that is termed condyloma latum. Condyloma lata are vegetative proliferations of the epidermis; they begin as flat or slightly raised papules, and progress to a button-like raised form with a smooth surface. The flatness of the lesions serves to differentiate the condyloma of syphilis (condyloma latum) from the more papillary and filiform warty masses of condyloma acuminatum, a lesion caused by low risk human papillomaviruses, e.g., HPV 6 or HPV 11 (167).
Histology
The histologic features of secondary syphilis are quite variable and show both epidermal and/or dermal changes with varying densities of inflammatory cells such as plasma cells and neutrophils (34, 47–49, 119, 126–129); see figures 8–9. Jeerapaet and Ackerman (34) listed the constellation of histologic findings in secondary syphilis, ordered by decreasing frequency as: widely dilated blood vessels (96%), large endothelial cells (96%), epidermal hyperplasia (85%), an inflammatory infiltrate that obscures the dermo-epidermal junction (85%), plasma cells present (85%), papillary dermal melanophages (82%), superficial and deep infiltrate (82%), edema or thickening of the papillary dermis (78%), focal parakeratosis (63%), extravasated red blood cells (63%), necrotic keratinocytes (56%), neutrophils in the dermis (44%), scale crust (41%), neutrophils scattered in the epidermis (37%), spongiform pustules (26%), superficial infiltrate only (18%), eosinophils present (18%), thinned epidermis (15%), neutrophils in eccrine ducts (11%), and ulceration (4%). In general, the tissue inflammatory reaction pattern is either lichenoid (lichen planus-like) and/or psoriasiform (psoriasis-like) (34, 119, 126). The lichenoid form features a band-like lymphocytic infiltrate in close apposition to the epidermis, a finding in common with lichen planus, lichenoid drug eruptions, and collagen vascular diseases (168). Acanthosis leads to club-shaped elongation of the rete ridges to give a psoriasiform appearance; hyperkeratosis and parakeratosis associated with a mononuclear infiltrate in the upper dermis, and disruption of the dermal-epidermal junction, may resemble lichen planus. Organisms can be identified in both the epidermis and dermis (35, 36, 42, 47). The lesions of both psoriasis and lichen planus have dermal mononuclear infiltrates, but the degree and extent of inflammation are usually much more extensive in lesions of secondary syphilis, extending into the deep dermis, and with a predominantly perivascular location (47). In multiple reviews of secondary syphilis, polymorphonuclear and eosinophilic infiltrates are found in less than half of cases (34, 126, 128); most of the inflammatory mononuclear cells are lymphocytes, macrophages, and plasma cells (36). Compared to primary syphilis, which shows a predominantly CD4 (helper) lymphocytic phenotype, secondary syphilis shows a predominantly CD8 (cytotoxic) phenotype (36); the immunophenotype could be related to the inability of these CD8 cytotoxic T-cells to clear the infection (168).
Vascular changes are limited to endothelial swelling and proliferation associated with perivascular mononuclear cell accumulation. A pertinent observation is that virulent T. pallidum is able to activate cultured endothelial cells in vitro (169). Spirochete membrane lipoproteins may act to up-regulate ICAM-1 expression in endothelial cells with subsequent binding of lymphocytes. Up-regulation of ICAM-1, VCAM-1, and E-selectin is a property recently attributed to the 47-kDa antigen of T. pallidum (170); up-regulation of these cytokines as well as direct fibrin deposition may contribute to the vascular inflammation found in secondary syphilis. Although macrophages and plasma cells predominate in secondary lesions (119), plasma cells can be absent or very sparse in 25% of lesions, and are seen to a variable degree around vessels in the remaining 75% of lesions, with no particular predominance in relationship to the other cell types (43, 126). However, when present, plasma cells are useful diagnostically, in that they help to differentiate syphilis from lichen planus, psoriasis, and many other skin lesions (119); in addition, plasma cell infiltrates highlight an active humoral immune response in secondary syphilis. The variation in the cellular composition of the inflammatory infiltrate most likely reflects the duration and progression of the lesion, with lymphocytes predominating during the first several days, followed by increasing macrophage participation, and later formation of granulomas.
Evolution of lesions
The key factor determining the duration of the lesions is the ability of the inflammatory cells to clear the site of infecting organisms. Although this clearance appears to be accomplished with great efficiency in the primary lesion, the immune reaction seems to be less able to clear secondary lesions. This could be due to a number of reasons, including 1) the relatively large numbers of organisms present both in the lesion and at other sites throughout the body, 2) operation of an immune effector mechanism that is ineffectual (shift towards humoral immune response denoted by plasma cell infiltrates), or 3) mechanisms that begin to damp the cellular immune response before all of the organisms are eliminated, leading to prolonged, smoldering inflammation. With inadequate treatment, recurrence of lesions that resemble either the primary chancre or secondary lesions occurs in about 15% of patients. Recurrence of a mild lesion at the site of the primary chancre is “termed ‘chancre in situ’ or ‘monorecidive syphilis’". In some cases, a primary chancre-like lesion appears distant from the site of the original lesion and is called "chancriform solitary papule". Usually such lesions do not erode, but have histological characteristics similar to those of the primary lesion. Recurrent secondary lesions look and behave like the original secondary lesions. In addition to the skin and mucous membranes, sites of similar lesions include the eye, bone, nervous system, or other organ systems. These secondary lesions occurring at distant and non-stereotypical sites are all examples of the failure of the immune system to eliminate the infecting organism, resulting in recurrent or persisting inflammation; such lesions are prevented by adequate treatment with antibiotics. During antibiotic-induced clearance of secondary skin lesions, the lesions acquire the characteristics of a DTH reaction, with little or no evidence of humoral antibody directed involvement.
The epidermal proliferative reaction and the dermal infiltrative change suggest that an interplay of inflammatory mediators in the progression of the lesions. T. pallidum directly up-regulates expression of adhesion molecules on endothelial cells (169, 170). Activated T-cells or macrophages can produce factors, such as matrix metallproteinases, that stimulate keratinocyte proliferation or enhance epidermal infiltration by inflammatory cells. Activated keratinocytes can produce factors, such as interleukin (IL)-1, IL-6, and colony stimulating factors that can act to increase T-cell proliferation. Keratinocytes can also produce factors that inhibit T-cells.
Secondary systemic syphilis
Signs and symptoms
Although many patients with secondary syphilis do not show systemic symptoms (Fournier estimated that 50% of women and 75% of men with secondary skin lesions had no systemic symptoms (17)), the remainder of patients display a variety of complaints and lesions that are suggestive of systemic infection. Unproven, but probable, is that most of this population goes on to exhibit tertiary disease. The systemic symptoms and lesions are usually not distinctive; they can include sore throat, malaise, headache, fever, weight loss, nausea, arthrititis, periostitis, myalgia, hepatitis, nephritis and various neurologic signs and symptoms (15, 18, 26). The generalized nature of these signs and symptoms reflects the constitutional effects of systemic inflammation. The localized symptoms most likely indicate focal inflammation in the internal organs affected, whose pathologic findings resemble the cutaneous lesions of secondary syphilis, i.e., DTH reactions to organisms that are present at a local site within the tissue.
Lymphoid organ histopathology
In addition to the persistent lymphoid hyperplasia, the sinusoids of the lymph nodes, particularly those draining infected skin, are filled with enlarged, palely staining macrophages (26). These most likely are re-circulating reactive macrophages that have traversed through affected organs. In secondary syphilis, there may be depletion of diffuse cortex, with marked follicular hyperplasia, which signals a diminished DTH response and increased humoral/antibody response (171). If DTH is the important protective response to T. pallidum infection, follicular hyperplasia could be reflecting activation of the "wrong" component of the immune system (12, 101).
Tertiary human syphilis
Signs and Symptoms
Late syphilis occurs in about one in three untreated, infected individuals (172), but it is completely preventable by treatment with penicillin. The skin is the organ most often affected (70%). The onset of the tertiary form, after a patient has developed secondary syphilis is years (3–7 years) in immunocompetent patients, but it is more rapid in HIV co-infection, occurring within months (173, 174). The classic lesion, the gumma, probably forms as a result of an ineffective DTH reaction; in the form of chronic granulomatous inflammation, presumably at sites of persistent infection. In addition, there is destruction of tissue secondary to loss of sensory nerve function; for example, the chronic osteomyelitis of the knee (Charcot's joint) results from repeated trauma as a result of loss in pain sensation. As in primary and secondary syphilis, disease can be superficial (skin and mucous membranes), or internal (cardiovascular, skeleton and central nervous system). Benign tertiary syphilis can occur any time after the secondary stage has resolved, with precocious lesions occurring within 2 years, and late syphilids (any cutaneous lesion of syphilis) occurring between 2 and 30 years. Tertiary skin lesions can be divided into two basic types: nodules and persistent gummatous ulcers. These two basic manifestations can yield diverse clinical morphologies that again can mimic other diseases: erythemato-violaceous annular scaling plaques similar to sarcoidosis and psoriasis, granuloma annulare-like lesions, juxta-articular nodules, pressure ulcers, pseudochancre redux (penile ulcer), discoid lupus erythematosus-like lesions, necrobiosis-like lesions, and pyoderma gangrenosum-like lesions (53, 152, 175–191); see figure 10. These diverse lesions reflect involvement of the subcutis and/or the dermis by gummas, granulomatous nodules, or psoriasiform and granulomatous inflammation. Since all of these manifestations exhibit granulomatous inflammation (aggregations of epithelioid macrophages), tertiary syphilis must be differentiated from inflammatory and infectious disorders such as sarcoidosis, cutaneous tuberculosis (lupus vulgaris), atypical mycobacteria infections, deep fungal infections, leishmaniasis, leprosy, and palisading granulomatous dermatoses like granuloma annulare (184, 185, 191). Spontaneous regression is rare; therefore, recurrences in the skin become increasingly more destructive with time. In most cases, however, these lesions heal rapidly with penicillin treatment. This diagnostic response to treatment, when considered with a report of production of gummas at inoculation sites in 2 volunteers with treated syphilis, supports the theory that gummas develop at the site of reactivation of endogenous foci of treponemes in previously sensitized individuals who are infected or inadequately treated, or at sites where viable, sequestered treponemes exist (192, 193). See figure 11.
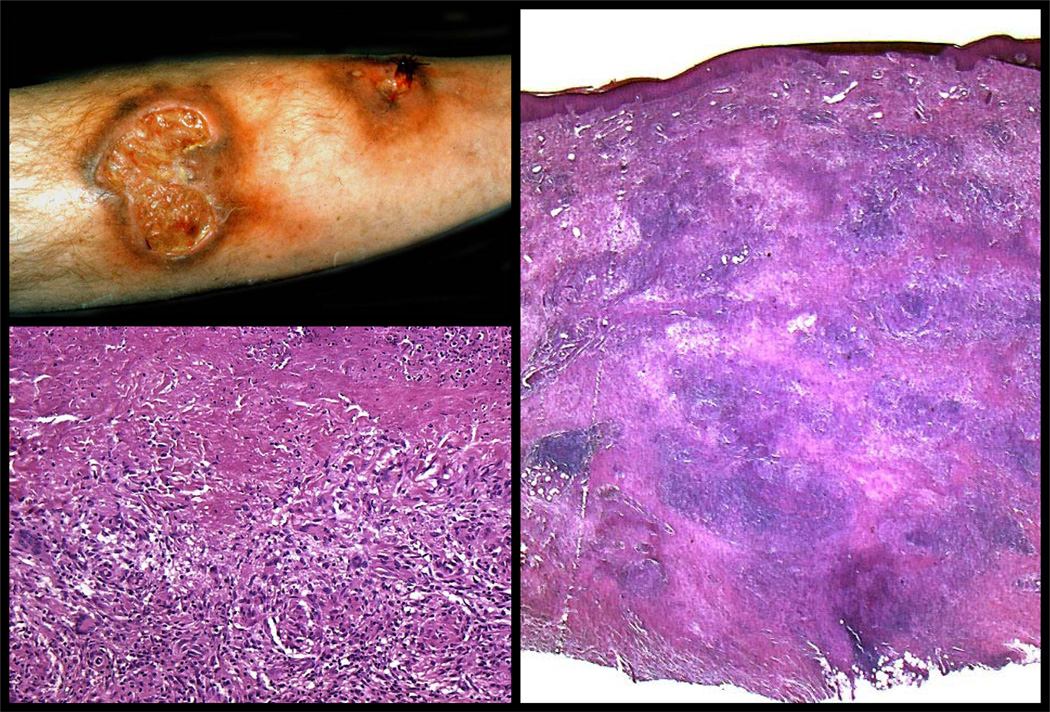
Figure 10. Tertiary cutaneous syphilis- the gumma.
Cutaneous gummata of tertiary syphilis are dermal or subcutaneous nodules, which have a gummy or rubbery consistency. They can occur singly or form grouped, annular or serpingionous lesions that have a prediction for scalp, face, chest, legs, and sites of trauma. They are painless and frequently ulcerated. (Clinical photograph courtesy of the Department of Dermatology, University of Iowa). Histologically, gummata show a central, geographic region of coagulative necrosis surrounded by macrophages and multinucleated giant cells. (Right and bottom left panels). Unlike tuberculosis where the caseous necrosis obliterates all structures, gummata retain some of the structural characteristics of normal tissues. Eventually, gummata undergo fibrosis resulting in a hyalinized or sclerotic central zone of fibrous tissue that is denoted clinically by a depressed, irregular scar or a round fibrous nodule.
Figure 11. Tertiary syphilis- a DTH reaction to viable organisms and/or antigens from non-viable spirochetes?
The granulomatous inflammation of tertiary syphilis has been interpreted as an allergic (DTH) reaction to treponemal antigen, which persists despite the absence of viable spirochetes (193). In support of this is an ultrastructural study that has demonstrated severe degradation of spirochetes in late lesions (257), and the infrequent detection of treponemal antigens or DNA in tertiary syphilis (41, 53, 56). This example of a gumma with abundant positive granular (destroyed treponemal cell wall) supports the contention that non-viable treponemal antigen is promoting the immune response. However, note that rare spirochetes are found in the media of a vessel wall, a possible, protective niche that allows for escape from immune surveillance. (Immunohistochemisty for T. pallidium; no counter stain).
The gumma
A gumma is a form of granuloma (194–197). A granuloma is formed when the cause of the inflammation (foreign body or infectious agent) is not easily removed or inactivated by macrophages (9). Local persistence of foreign material, or infectious or non-infectious antigens results in a collection of macrophages. The macrophages within the gumma exhibit an epithelioid appearance and contain incompletely digested intracellular debris. Some macrophages are multinuclear cells derived from macrophages that have undergone nuclear, but not cytoplasmic, division. These macrophages are often arranged around a central necrotic zone, essentially walling off the necrotic tissue. The central area can contain viable organisms (especially in the case of tuberculosis) that are separated from the surrounding tissue. The gumma can progress from largely cellular in nature (mononuclear or epithelioid) to healed (scar) if the organisms can be eliminated, but it can continue to enlarge, break down (necrose), and ulcerate if the organisms and/or their antigens persist. Surrounding the epithelioid cells are varying numbers of lymphocytes, plasma cells, macrophages, along with fibroblasts and connective tissue scarring, depending on the stage of the progression of the lesion. The gumma is the most characteristic lesion of tertiary syphilis. In general, it is less cellular than the classic tuberculoid granuloma and tends to have broad, irregular acellular zones with preserved outlines of residual structures (ghost cells), rather than circumscribed region of caseous necrosis that is surrounded by epithelioid histiocytes. In addition, gummas are accompanied by fewer lymphocytes and more plasma cells than are commonly seen in tuberculoid granulomas. The presence of necrosis and plasma cells differentiates gummas from sarcoidosis. The gumma phenotype is consistent with a relative lack of cellular immunity. Of note, plasma cells can be few in some lesions of tertiary syphilis similar to secondary syphilis (181).
Skin and mucous membranes
Late external lesions of syphilis represent either residual effects of the healing of secondary lesions or late "syphilids" (any cutaneous or mucosal lesion of tertiary syphilis) (18, 26, 70, 198). Gummas are generally solitary, start as deep swellings that eventually ulcerate. Microscopically, gummas show large areas of necrosis surrounded by lymphocytes, macrophages, multinucleated giant cells, plasma cells and fibroblasts. Arteries are infiltrated by inflammatory cells and often show endarteritis obliterans. Silver stains are generally negative for organisms (41, 56, 186). The residua of the secondary lesions seen in latent syphilis include atrophy (a depressed scarred area), hyperpigmentation due to post inflammatory pigment incontinence, or depigmentation secondary to destruction of melanocytes during the active inflammatory stage. Syphilitic leukoderma is a term applied to the depigmented areas, which are most frequently seen on the neck and shoulders (18); they typically appear during the healing stage of secondary syphilis (199). Treponemes have been identified in leukoderma syphiliticum by electron microscopy (200), and in cutaneous gummas by direct immunofluorescence (53), immunohistochemistry (41)(JA Carlson unpublished data), and PCR (41, 56) (see table 2). In addition to the infectious and inflammatory granulomatous disorders listed above, the differential diagnosis should include non-melanoma skin cancers, for late syphilids that show heaped margins around a central ulcer (due to pseudoepitheliomatous epidermal hyperplasia), or for those late syphilids occurring around oroficial sites. Indeed, basal cell and squamous cell carcinomas have been reported to arise in longstanding or healed gumma (201, 202), underscoring the role of chronic inflammation and scarring in the progression to cancer.
Internal organs
Since syphilis infection can involve any organ of the body, lesions of chronic or tertiary syphilis produce a huge variety of symptoms and findings (26). In pathologic terms, as stated above, the lesions of tertiary syphilis of internal organs can be divided into three general categories: 1) mononuclear cell reactions similar to those of secondary syphilis of the skin; 2) gummas and 3) destructive or degenerative changes secondary to loss of vascular or nerve supply. It is neither desirable nor possible in a review of this length to attempt to describe in detail the extensive tissue lesions seen in late syphilis. Lesions of tertiary syphilis of the vascular system include localized gummas in vessel walls or myocardium and secondary lesions such as aortic aneurysm, coronary occlusion, and valvular insufficiency (203). Mononuclear inflammation is seen in the vasa-vasorum, the small blood vessels that supply the walls of larger arteries. This inflammation can progress to extensive damage of the arterial wall, and then to aneurysm formation and rupture, a relative common cause of death in inadequately treated tertiary syphilis. An apocryphal myth exists regarding the great English surgeon, John Hunter, who is said to have intentionally infected himself with the pus from a recently hanged convict in an attempt to study the pathogenesis of syphilis (24); he subsequently died later from a ruptured aortic aneurysm during a tirade against a hospital administrator. In truth, there is no evidence that Hunter inoculated himself, but rather, he acquired syphilis in a more natural, common, and banal fashion (204). He performed the inoculation experiment in 1767; however, he probably used somebody else for it, referred to in his article simply as "the patient." The above tale that Hunter inoculated himself was spread later, probably as an attempt at cover-up of an unethical experiment (204). In either story, the lethal effects of tertiary syphilis are highlighted. Inflammation of the aortic valves is also prominent in vascular syphilis; it causes separation and contraction of the valve leaflets, leading to aortic insufficiency and heart failure. Inflammation of the wall of the aorta produces a peculiar heaping up and thickening of the endothelium described grossly as "tree barking" (26). Inflammation at the base of the aorta can lead to narrowing of the coronary arteries ostia, and involvement of the coronaries accelerates the accumulation of atherosclerotic plaques. These lesions can cause myocardial infarction. Isolated gummas can occur in the heart itself, and small gummata are seen in vessel walls, particularly in the advential vessels, associated with more diffuse inflammation. Obliterative endarteritis is seen associated with other secondary and tertiary lesions and can be mediated by immune complex deposition. Similar lesions of obliterative endarteritis can be viewed as a consequence of vasculitis or vaso-occlusive disorders (125); see figure 7.
Neurological lesions include gummas, meningo-vascular inflammation (205), inflammation of the cerebral vessels, and general paresis (dementia paralytica). Meningeal reaction in late syphilis can be asymptomatic, but it is associated with inflammatory cells in the CNS and a positive VDRL test (206, 207). PCR detection of T. pallidum DNA in the CSF is a more sensitive test modality. The meningitis of syphilis features thickened meninges and lymphocytic perivascular infiltrate around small vessels. In parenchymal syphilis, diffuse, proliferative inflammatory changes occur in the cerebral cortex. Before the modern era, large gummas were not uncommon, and were usually connected in their periphery to the meninges (18, 26). The manifestations of neurosyphilis appear to be the result, at least partially, from direct effects of T. pallidum in the tissues; the organisms accumulate in large numbers, because of either an inability of the immune response to reach the site of infection, or a lack of the appropriate immune response to control the infection.
Tabes dorsalis (locomotor ataxia) features a loss of sensation associated with demyelination of the dorsal roots and posterior spinal column. The demyelination is secondary to damage to the neurons in the dorsal root ganglia. Ataxia is a common manifestation, and severe sensory denervation can lead to damage to weight-bearing joints, such as Charcot's joint, a degenerative syphilitic arthropathy of the knee caused by repeated trauma. The damage arises because of lack of pain sensation, resulting in inflammation in the dorsal roots and ganglia, and lead to secondary degeneration of ascending neurites from the damaged dorsal roots. As described above, organisms can be seen in the cutaneous nerves of primary lesions, and may be able to migrate from these fibers to local ganglia (84, 91–93).
The presentation of neurosyphilis has changed during the last 40 years (206–209). Patients no longer present with classical symptoms of tabes dorsalis, general paresis, or meningovascular syphilis, but instead tend to present atypically with seizures; ophthalmic symptoms such as poor vision; strokes; confusion; or personality changes (209). In many cases, the infection is detected by incidental findings during a medical examination conducted for other reasons. Positive serum or CSF tests using FTA-ABS is the most important diagnostic modality; non-treponemal serologic tests are not sensitive enough for diagnosis (207). In addition, viable T. pallidum can be demonstrated in the CSF of 30% of patients with early, untreated syphilis (210).
Renal lesions
Although relatively infrequent, renal disease associated with syphilis has been recognized for over 120 years (211). Ten percent of patients with syphilis have kidney inflammation, nephritis. Up to 50% of patients with early syphilis can be demonstrated to have circulating immune complexes containing T. pallidum antigens (212, 213). Deposits consistent with immunoglobulins have been found, and case reports of glomerulonephritis in which treponemal antigen (214) and antibody to T. pallidum (215) have been demonstrated by elution from the involved renal tissue have been described. Walker et al (215) demonstrated both specific antibody and antigen in the renal tissue of a syphilitic patient who had rapidly progressive glomerulonephritis. As is true for cases with other syphilitic lesions, syphilitic renal disease responds to penicillin treatment (216). Acute presentation of nephritis can occur during the Jarisch-Herxheimer reaction that accompanies the massive destruction of T. pallidum during penicillin treatment of early syphilis (217). This phenomenon provides evidence of a role for immune-complex mediated lesions in syphilis; however, it remains poorly understood as to why there is not more evidence for acute vasculitis in secondary and tertiary syphilitic lesions. Perhaps the slow production of small amounts of immune complexes during tertiary disease generates a gradually progressive endarteritis obliterans or glomerulonephritis, without the occurrence of an acute, leukocytoclastic (necrotizing) phase.
Lymphoid organs
The histologic changes of late syphilis in the lymph nodes feature capsular and pericapsular fibrosis, follicular hyperplasia, replacement of the diffuse cortex by the accumulation of histiocytes and giant cells, sheets of plasma cells in the interfollicular areas, and endarteritis (18, 26). Gummas can also be present in lymph nodes. These pathologic changes most likely reflect inflammatory reactions of the granulomatous type to infection. However, the attempt at a clearing DTH response appears to not to be fully effective, as evidenced by the relative depletion of lymphocytes in the diffuse cortex, and the predominance of a humoral antibody response (reflected in the intense follicular hyperplasia, and sheets of plasma cells that are sometimes seen). These observations provide morphological evidence that immune deviation, the selection of antibody production (follicular hyperplasia) over DTH (cortical hyperplasia) can occur in tertiary syphilis.
SYPHILIS AND IMMUNOSUPPRESSION
Transplant recipients
Corticosteroid induced immunosuppression of rabbits infected with T. pallidum allows prolonged increase in numbers of organisms, because the cellular immune response is inhibited (218). When the cortisone is discontinued, there is a rapid "rebound" of the immune response and clearing of the organisms in the animal’s lesions. In humans, the role of the immune response in the pathogenesis of syphilis can be better understood if we study infections in immunosuppressed adults.
Two major groups of patients are available for observations: transplant recipients treated with immunosuppressive drugs and patients with AIDS. Immunosuppressed transplant patients can develop acute syphilitic hepatitis (219, 220). There is a mild portal lymphocytic infiltrate and organisms can be identified in the periportal areas by special stains (219). The hepatitis resolves quickly after penicillin therapy, suggesting that penicillin therapy is effective even in the absence of a fully competent immune defense. In general, acute hepatitis is a very uncommon manifestation of syphilis (221), but it can occur in patients with secondary syphilis, and gumma and fibrosis of the liver is one of the manifestations of tertiary syphilis (26). Apparently, chemical immunosuppression can inhibit the appearance of the typical primary or secondary lesions (which are manifestations of DTH), and can allow massive numbers of organisms to be produced in internal organs.
Syphilis and AIDS
Not only do individuals with AIDS have a higher incidence of syphilis than people without AIDS, but also the course of syphilis is accelerated (3, 30, 33, 210, 222–225), severe, termed Lues maligna, (226), and shows unusual secondary manifestations that include multiple and severe cutaneous ulceration (227) and mycosis fungoides-like lesions (145). Tertiary neurologic (228, 229), optic (230, 231), and otic (232) symptoms, as well as granulomas (233), appear more rapidly after secondary disease in AIDS patients than in non-AIDS patients. In addition, transmission of AIDS can be facilitated by syphilis infection (234, 235). In general, genital ulcer disease caused by T. pallidum is increased in HIV patients (234, 236). Genital ulcers can serve either as sites to receive HIV infection, or as sites of infected cells that transmit HIV infection (225). Blister fluids induced by injection of the 17- and 47 kDa lipoproteins of T. pallidum are highly enriched in CCR5 chemokine receptor-positive mononuclear cells; these cells probably could facilitate the acquisition and transmission of M-trophic strains of HIV (237). Interestingly, lymph nodes from syphilitic patients with HIV display marked follicular and interfollicular hyperplasia, with prominent vascular proliferation, plasma cells, immunoblasts, macrophages, prominent interfollicular lymphoplasmacytic cells, and occasional neutrophils; these findings are consistent with a B-cell response (238). In addition, activation of latent syphilis in HIV-infected individuals appears to coincide with the development of immune depression. Thus, latent syphilis is held in check in HIV-infected individuals who have not yet developed immune defects, but lesions of tertiary syphilis appear when the cellular immune response is depressed. This supports the hypothesis that intact cellular immunity is able to maintain latency, and depression of cellular immunity results in expression of tertiary syphilis. In addition, antibody production can be affected, as serologic tests for syphilis can be negative in AIDS patients, even though T. pallidum is demonstrable in the tissues. Higher doses of penicillin are required to treat syphilis effectively in HIV-infected individuals (210, 225), suggesting a role for immunity in successful penicillin treatment. Although penicillin directly kills T. pallidum, the efficacy of treatment is influenced by the ability of the host’s immune response to clear organisms from the tissue.
T. pallidum superinfection of rabbits latently infected with HIV can not only produce positive tests for HIV infection, but can also delay healing of cutaneous syphilis (239). Rabbits inoculated with HIV-infected human cell lines appear normal and have neither serum antibodies nor incorporation of HIV DNA sequences in their DNA for 6 months after infection. However, after cutaneous inoculation with T. pallidum, two of four HIV injected rabbits had markedly prolonged healing of the consequent chancres. In addition, HIV DNA sequences were detected in the DNA from the peripheral blood white cells of all four rabbits after, but not before, T. pallidum challenge. HIV DNA was also seen in macrophages and lymphocytes in the skin of the syphilitic lesions, but not in non-lesional skin. However, productive infection of rabbits with HIV has yet to be demonstrated, even when HIV-infected rabbits are super-infected with T. pallidum or another infectious agent, such as Vaccinia, Mycobacterium avium, herpes simplex virus, Candida albicans, Mycoplasma incognitos or malignant catarrhal fever virus (240). In vitro (241) and in vivo (240) studies showed that HIV DNA is incorporated into host DNA rabbit macrophages, and that activation of these cells can lead to expression of early (gag), but not late, HIV gene products.
IMMUNE DEVIATION: A COMPARISON OF SYPHILIS AND LEPROSY
Immune deviation
Immune deviation, or split tolerance, is classically defined as the dominance of one immune response mechanism over another for a specific antigen; this condition has been implicated in the tendency for certain individuals to develop IgE (allergy-related) antibodies, rather than IgG antibodies (242). In addition, for reasons that are unclear but may be genetically determined, some individuals tend to mount strong cellular immune responses, but weak antibody responses to certain antigens, whereas other individuals will show the opposite pattern. The type of immune response is mainly determined by differential activation of the two major classes of T-helper cells in the immune response: Th1 and Th2. Th1 cells are important for induction of immune responses associated with increased cell reactivity; they include complement fixing antibodies (opsonins), TDTH (DTH), and TCTL (T-cell mediated cytotoxicity). Th2 cells are involved in non-complement fixing antibody production (IgA, IgE, etc) (9, 243). If DTH mediated by CD4+ TDTH –cells is protective, and if complement-fixing antibody or cytotoxicity mediated by CD8+ TCTL is not, then the immune protective response to syphilis must involve further selection of the different functions of Th1 cells. This focusing of the immune response may be controlled by how the antigen is presented to immune system, its dosage, and its duration (10, 12). In any case, selection of DTH relative to other immune effector mechanisms is critical, for mounting of a protective immune response in syphilis.
Leprosy
The immune response and leprosy
The dependence of the clinical course of leprosy upon the immune reaction of the patient illustrates the importance of DTH as a protective response (244–248). Leprosy can be divided into three groups: tuberculoid, borderline and lepromatous (244). In tuberculoid leprosy, a solitary plaque with hypopigmentation and hypoesthesia is evident; on biopsy, it shows well-formed tuberculoid granulomatous inflammation and rare acid-fast bacilli. Positive reactions to DTH skin challenge tests are preserved, and there is predominant hyperplasia of the diffuse cortex (T-cell zone) of the lymph nodes. The level of antibodies is low. In lepromatous leprosy, numerous nodules and plaques are evident that consist of sheets of foamy macrophages in the dermis; these sheets contain large numbers of viable bacilli, and microcolonies called globi. Reactions to DTH skin tests are diminished or lost, and there is marked follicular hyperplasia in the lymph nodes, with little or no diffuse cortex. The levels of antibodies are high, and some patients can develop immune complexes and vasculitis known as erythema nodosum leprosum (type 2 leprosy reaction- an enhanced Th-2 type/humoral reaction (244)). Lepromatous leprosy patients can also suffer type 1 (Th-1) reversal reactions, in which cell-mediated immunity is up-regulated; indeed, the up-regulation of Th-1/cell-mediated responses is borne out by the beneficial effects of BCG vaccination, which has its greatest effect on patients with lepromatous leprosy; patients who are the ones most likely to transmit M. leprae (106). Borderline leprosy has intermediate findings between these above two described polar forms. The prognosis in tuberculoid leprosy is good, and the response to chemotherapy is excellent. In borderline leprosy, a good response to therapy is associated with a conversion to the tuberculoid form. The prognosis in lepromatous leprosy and the response to chemotherapy are both poor. The above example of the forms of leprosy illustrates the role of DTH in controlling the infection, and the lack of protective response provided by humoral antibodies. This correlation of immune response with disease manifestations and response to therapy is also considered valid for immunity to Candida albicans (249, 250). Depressed DTH is also associated with chronic mucocutaneous candidiasis, a condition in which the infected individual is unable to clear candidial infections (249, 250). As was indicated earlier, we now propose that the clinical stages of syphilis embody a comparable progression of disease manifestations with progressive loss of a DTH response.
Comparison of syphilis and leprosy
Figure 12 shows a diagram illustrating the relationship between the degree of cellular and humoral immune response, and the stage of disease for syphilis and clinical form of leprosy. In this model, the primary chancre of syphilis is considered to be a DTH reaction that essentially clears the site of infectious organisms (12). A cellular response must be dominant, and it must be composed mainly of CD4+ TDTH cells, rather than CD8+ T-cytotoxic cells. If the DTH immune mechanism is dominant, the infection will be cured. If the DTH immune mechanism is not able to clear the infection, replication of the organisms at multiple sites will give rise to secondary reactions, and tertiary disease. In fact, cases of secondary syphilis mimicking leprosy have been reported (139, 140, 154, 185). In addition, the lymph nodes in both secondary syphilis and leprosy can show depletion of diffuse cortex associated with marked follicular hyperplasia (47, 171, 251). Like leprosy, secondary syphilis also shows a form of split tolerance, or T-cell depletion, in which production of antibody is increased, and DTH is decreased (12, 171, 245–247). Because of the large number of organisms present in secondary syphilis, it may take weeks to clear the lesions and many organisms will remain in immuno-protected niches (94). No further lesion development will occur and latent infection will be maintained if DTH remains strong. However, if the DTH immune response declines, organisms will increase and lesions of tertiary syphilis will appear, even in the face of high antibody titers in the serum or in the cerebral spinal fluid. In the experimental skin and testis syphilis models in the rabbit, DTH is able to clear dermal or testicular infection and lesions of secondary and tertiary syphilis do not occur (80, 82). Penicillin treatment of secondary or tertiary syphilis is able to reduce the number of organisms, so that the low level cellular immunity present is able to re-establish control of the infection. However, if suppression of DTH is induced by AIDS or other induced immunosuppression (252), latency can be terminated more rapidly, and can be succeeded by a systemic infection that requires treatment with high doses of penicillin. In tertiary syphilis, studies of Th1 and Th2 cytokine production by lymphocytes demonstrated low Th1 cytokine production (cellular immunity) and high Th2 cytokine production (non-complement, fixing antibody response) (253). The authors propose that this cytokine imbalance is one of the reasons why T. pallidum multiplication and development of lesions occur in tertiary syphilis (253).
Figure 12. A comparison of the stages of syphilis and leprosy and their relationship to the balance of humoral and cell-mediated immunity.
The overlapping triangles indicate the relative strength of delayed hypersensitivity and antibody production. The cross-hatched triangle indicates delayed hypersensitivity; the open triangle, antibody production. High levels of delayed hypersensitivity (DTH) are associated with cure; weak DTH is associated with progressive disease; balanced DTH and antibody production with borderline leprosy and latent syphilis. Progression of syphilis to the tertiary stage is most likely more related to depressed T-cell immunity, with or without high levels of antibody production.
CONCLUSIONS
Sir William Osler (15) is largely credited with the statement that syphilis is the "Great Masquerader" because it mimics the signs and symptoms of so many other diseases (254). In 1897, Osler advised "know syphilis in all its manifestations and relations, and all other things clinical will be added unto you". However, as pointed out by Jackson (255), Osler most likely borrowed this concept from Jonathan Hutchinson, who, in 1879, gave a presentation entitled "An Address on Syphilis as an Imitator" (255). The myriad clinical manifestations of syphilis reflect the fact that the disease is a systemic infection of many organs, and each infected individual responds with diverse manifestations that reflect varied host immune responses. Overall, the pathogenesis of syphilis in experimental rabbits; in normal adult humans; and in immunosuppressed individuals supports the hypothesis that the course of syphilis is determined by the type and intensity of the immune response.
The tendency to generate one form of specific immunity at the expense of another is known as immune deviation (12, 256). In many non-bacterial infectious diseases, including viral and mycobacterial infections, DTH responses have been shown to be responsible for curing infections, whereas humoral antibody responses are associated with prolonged, progressive infection. The most notable among such infections are leprosy and chronic mucocutaneous candidiasis. In both of them, a strong DTH response is associated with cure; other immune effector mechanisms are not successful. Nonetheless, the other mechanisms, such as humoral antibody production are a major defense against many bacterial infections, and CD8+ TCTL are effective against intracellular infections, in particular, by viruses (9).
This review has proposed that the course and type lesions of syphilis infection are determined largely by the strength of the DTH response. The DTH response is able to clear tissues of infection, whereas humoral antibody responses, including the complement system, and CD8+ T-cell cytotoxicity response are not. Thus, individuals with dominant DTH responses are able either to cure an infection after the primary stage, or to enter prolonged latency. Individuals, who respond primarily by antibody production or a CD8+ T-cell response (particularly after the primary stage), tend to develop secondary and tertiary disease. Rabbits inoculated experimentally, whom mount a strong DTH response, do not develop secondary or tertiary disease, but instead enter prolonged latency. The latent stage of syphilis can be activated to tertiary disease through depression of DTH such as occurs associated with HIV infection. In syphilis, some patients have a DTH response adequate to resolve the primary chancre or clear secondary lesions, but the level of DTH decreases with aging (or as a result of immunosuppression) thereby allowing the development of tertiary disease. Thus, analysis of the pathologic lesions of syphilis suggests that the course and progression of the disease are determined by the strength of the DTH immune response. Methods that augment DTH reactions should be of benefit in the prevention and treatment of syphilis. Proof of this concept is seen in the effects of BCG vaccination, which has a protective effect against leprosy in endemic areas (108).
Acknowledgments
The research by the authors reported in this paper was supported by NIH grants AI 14262 and 19810
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hook EW, 3rd, Peeling RW. Syphilis control--a continuing challenge. N Engl J Med. 2004 Jul 8;351(2):122–124. doi: 10.1056/NEJMp048126. [DOI] [PubMed] [Google Scholar]
- 2.Eaton M. Syphilis and HIV: Old and New Foes Aligned Against Us. Curr Infect Dis Rep. 2009 Mar;11(2):157–162. doi: 10.1007/s11908-009-0023-5. [DOI] [PubMed] [Google Scholar]
- 3.Karp G, Schlaeffer F, Jotkowitz A, Riesenberg K. Syphilis and HIV co-infection. Eur J Intern Med. 2009 Jan;20(1):9–13. doi: 10.1016/j.ejim.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Velicko I, Arneborn M, Blaxhult A. Syphilis epidemiology in Sweden: re-emergence since 2000 primarily due to spread among men who have sex with men. Euro Surveill. 2008 Dec;11(13):50. doi: 10.2807/ese.13.50.19063-en. [DOI] [PubMed] [Google Scholar]
- 5.Sell S, Norris SJ. The biology, pathology, and immunology of syphilis. Int Rev Exp Pathol. 1983;24:203–276. [PubMed] [Google Scholar]
- 6.Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatologic clinics. 2008 Apr;26(2):191–202. doi: 10.1016/j.det.2007.12.001. v. [DOI] [PubMed] [Google Scholar]
- 7.Kent ME, Romanelli F. Reexamining syphilis: an update on epidemiology, clinical manifestations, and management. Ann Pharmacother. 2008 Feb;42(2):226–236. doi: 10.1345/aph.1K086. [DOI] [PubMed] [Google Scholar]
- 8.Peeling RW, Hook EW., 3rd The pathogenesis of syphilis: the Great Mimicker, revisited. J Pathol. 2006 Jan;208(2):224–232. doi: 10.1002/path.1903. [DOI] [PubMed] [Google Scholar]
- 9.Sell S. Immunology, immunopathology and immunity. Washinton, DC: ASM Press; 2001. [Google Scholar]
- 10.Zinkernagel RM. Antiinfection immunity and autoimmunity. Ann N Y Acad Sci. 2002 Apr;958:3–6. doi: 10.1111/j.1749-6632.2002.tb02942.x. [DOI] [PubMed] [Google Scholar]
- 11.Pialoux G, Vimont S, Moulignier A, Buteux M, Abraham B, Bonnard P. Effect of HIV infection on the course of syphilis. AIDS Rev. 2008 Apr-Jun;10(2):85–92. [PubMed] [Google Scholar]
- 12.Sell S, Hsu PL. Delayed hypersensitivity, immune deviation, antigen processing and T-cell subset selection in syphilis pathogenesis and vaccine design. Immunol Today. 1993 Dec;14(12):576–582. doi: 10.1016/0167-5699(93)90196-R. [DOI] [PubMed] [Google Scholar]
- 13.Leader BT, Godornes C, VanVoorhis WC, Lukehart SA. CD4+ lymphocytes and gamma interferon predominate in local immune responses in early experimental syphilis. Infect Immun. 2007 Jun;75(6):3021–3026. doi: 10.1128/IAI.01973-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schaudinn F, Hoffmann E. Vorlaufiger Bericht uber das Vorkommen von Spirochaeten in syphilitischen Krankheitsproduckten und bei Papillomen. Arbeiten aus dem K Gesundheitsamte. 1905;22:527–534. [PubMed] [Google Scholar]
- 15.Osler W, Churchman J. Syphilis. In: Osler W, McCrae T, editors. Modern Medicine. Philadelphia: Lea and Febiger; 1914. pp. 144–215. [Google Scholar]
- 16.Levaditi C. Sur la coloration du Spirochaete pallida Schaudinn dans les coupes. C R Seanc So Biol. 1905;59:326–338. [Google Scholar]
- 17.Reuter K. Spirochaete pallida in der Aortenwand bei hellerscher Aortitiism. Munch Med Wochenschr. 1906;53:778. [Google Scholar]
- 18.Stokes JH. Modern Clinical Syphology. W. B. Saunders Co; 1934. [Google Scholar]
- 19.Wasserman AV, Neisser A, Bruck C. Eine serodiagnostische Reaction bei Syphilis. Dtsch Med Wochenschr. 1906;32:745–746. [Google Scholar]
- 20.Larsen SA, Steiner BM, Rudolph AH. Laboratory diagnosis and interpretation of tests for syphilis. Clin Microbiol Rev. 1995 Jan;8(1):1–21. doi: 10.1128/cmr.8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egglestone SI, Turner AJ. Serological diagnosis of syphilis. PHLS Syphilis Serology Working Group. Commun Dis Public Health. 2000 Sep;3(3):158–162. [PubMed] [Google Scholar]
- 22.Young H. Syphilis. Serology. Dermatologic clinics. 1998 Oct;16(4):691–698. doi: 10.1016/s0733-8635(05)70034-6. [DOI] [PubMed] [Google Scholar]
- 23.Manavi K, Young H, McMillan A. The sensitivity of syphilis assays in detecting different stages of early syphilis. International journal of STD & AIDS. 2006 Nov;17(11):768–771. doi: 10.1258/095646206778691185. [DOI] [PubMed] [Google Scholar]
- 24.Qvist G. John Hunter's alleged syphilis. Ann R Coll Surg Engl. 1977 May;59(3):206–209. [PMC free article] [PubMed] [Google Scholar]
- 25.Rollet J. Traite des maladies veneriennes. Paris: 1865. [Google Scholar]
- 26.MacCallum EG. A text-book of pathology. W. B. Saunders Co; 1924. [Google Scholar]
- 27.Romanowski B, Forsey E, Prasad E, Lukehart S, Tam M, Hook EW., 3rd Detection of Treponema pallidum by a fluorescent monoclonal antibody test. Sex Transm Dis. 1987 Jul-Sep;14(3):156–159. doi: 10.1097/00007435-198707000-00007. [DOI] [PubMed] [Google Scholar]
- 28.Phelps RG, Knispel J, Tu ES, Cernainu G, Saruk M. Immunoperoxidase technique for detecting spirochetes in tissue sections: comparison with other methods. Int J Dermatol. 2000 Aug;39(8):609–613. doi: 10.1046/j.1365-4362.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 29.Johnson PD, Graves SR, Stewart L, Warren R, Dwyer B, Lucas CR. Specific syphilis serological tests may become negative in HIV infection. AIDS. 1991 Apr;5(4):419–423. doi: 10.1097/00002030-199104000-00010. [DOI] [PubMed] [Google Scholar]
- 30.Johns DR, Tierney M, Felsenstein D. Alteration in the natural history of neurosyphilis by concurrent infection with the human immunodeficiency virus. N Engl J Med. 1987 Jun 18;316(25):1569–1572. doi: 10.1056/NEJM198706183162503. [DOI] [PubMed] [Google Scholar]
- 31.Erbelding EJ, Vlahov D, Nelson KE, Rompalo AM, Cohn S, Sanchez P, et al. Syphilis serology in human immunodeficiency virus infection: evidence for false-negative fluorescent treponemal testing. J Infect Dis. 1997 Nov;176(5):1397–1400. doi: 10.1086/517330. [DOI] [PubMed] [Google Scholar]
- 32.Tikjob G, Russel M, Petersen CS, Gerstoft J, Kobayasi T. Seronegative secondary syphilis in a patient with AIDS: identification of Treponema pallidum in biopsy specimen. Journal of the American Academy of Dermatology. 1991 Mar;24(3):506–508. doi: 10.1016/s0190-9622(08)80082-5. [DOI] [PubMed] [Google Scholar]
- 33.Hicks CB, Benson PM, Lupton GP, Tramont EC. Seronegative secondary syphilis in a patient infected with the human immunodeficiency virus (HIV) with Kaposi sarcoma. A diagnostic dilemma. Ann Intern Med. 1987 Oct;107(4):492–495. doi: 10.7326/0003-4819-107-4-492. [DOI] [PubMed] [Google Scholar]
- 34.Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973 Mar;107(3):373–377. [PubMed] [Google Scholar]
- 35.Lee WS, Lee MG, Chung KY, Lee JB. Detection of Treponema pallidum in tissue: a comparative study of the avidin-biotin-peroxidase complex, indirect immunoperoxidase, FTA-ABS complement techniques and the darkfield method. Yonsei medical journal. 1991 Dec;32(4):335–341. doi: 10.3349/ymj.1991.32.4.335. [DOI] [PubMed] [Google Scholar]
- 36.Engelkens HJ, ten Kate FJ, Judanarso J, Vuzevski VD, van Lier JB, Godschalk JC, et al. The localisation of treponemes and characterisation of the inflammatory infiltrate in skin biopsies from patients with primary or secondary syphilis, or early infectious yaws. Genitourinary medicine. 1993 Apr;69(2):102–107. doi: 10.1136/sti.69.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McBroom RL, Styles AR, Chiu MJ, Clegg C, Cockerell CJ, Radolf JD. Secondary syphilis in persons infected with and not infected with HIV-1: a comparative immunohistologic study. Am J Dermatopathol. 1999 Oct;21(5):432–441. doi: 10.1097/00000372-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 38.Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. Journal of cutaneous pathology. 2004 Oct;31(9):595–599. doi: 10.1111/j.0303-6987.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 39.Wenhai L, Jianzhong Z, Cao Y. Detection of Treponema pallidum in skin lesions of secondary syphilis and characterization of the inflammatory infiltrate. Dermatology (Basel, Switzerland) 2004;208(2):94–97. doi: 10.1159/000076479. [DOI] [PubMed] [Google Scholar]
- 40.Buffet M, Grange PA, Gerhardt P, Carlotti A, Calvez V, Bianchi A, et al. Diagnosing Treponema pallidum in secondary syphilis by PCR and immunohistochemistry. The Journal of investigative dermatology. 2007 Oct;127(10):2345–2350. doi: 10.1038/sj.jid.5700888. [DOI] [PubMed] [Google Scholar]
- 41.Behrhof W, Springer E, Brauninger W, Kirkpatrick CJ, Weber A. PCR testing for Treponema pallidum in paraffin-embedded skin biopsy specimens: test design and impact on the diagnosis of syphilis. Journal of clinical pathology. 2008 Mar;61(3):390–395. doi: 10.1136/jcp.2007.046714. [DOI] [PubMed] [Google Scholar]
- 42.Quatresooz P, Pierard GE. Skin Homing of Treponema pallidum in Early Syphilis: An Immunohistochemical Study. Appl Immunohistochem Mol Morphol. 2008 Sep 16; doi: 10.1097/PAI.0b013e3181788186. [DOI] [PubMed] [Google Scholar]
- 43.McCalmont T, Macerenco R. Syphilis: it's around and ready for (Mis)Diagnosis. J Cutan Pathol. 2008;35(1):95. [abstract]. [Google Scholar]
- 44.Martin-Ezquerra G, Fernandez-Casado A, Barco D, Jucgla A, Juanpere-Rodero N, Manresa JM, et al. Treponema pallidum distribution patterns in mucocutaneous lesions of primary and secondary syphilis: an immunohistochemical and ultrastructural study. Hum Pathol. 2009 May;40(5):624–630. doi: 10.1016/j.humpath.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 45.Quatresooz P, Pierard GE. Skin homing of Treponema pallidum in early syphilis: an immunohistochemical study. Appl Immunohistochem Mol Morphol. 2009 Jan;17(1):47–50. doi: 10.1097/PAI.0b013e3181788186. [DOI] [PubMed] [Google Scholar]
- 46.Venturini M, Sala R, Semenza D, Santoro A, Facchetti F, Calzavara-Pinton P. Reflectance confocal microscopy for the in vivo detection of Treponema pallidum in skin lesions of secondary syphilis. Journal of the American Academy of Dermatology. 2009 Apr;60(4):639–642. doi: 10.1016/j.jaad.2008.11.901. [DOI] [PubMed] [Google Scholar]
- 47.Engelkens HJ, ten Kate FJ, Vuzevski VD, van der Sluis JJ, Stolz E. Primary and secondary syphilis: a histopathological study. International journal of STD & AIDS. 1991 Jul-Aug;2(4):280–284. doi: 10.1177/095646249100200411. [DOI] [PubMed] [Google Scholar]
- 48.Pandhi RK, Singh N, Ramam M. Secondary syphilis: a clinicopathologic study. Int J Dermatol. 1995 Apr;34(4):240–243. doi: 10.1111/j.1365-4362.1995.tb01588.x. [DOI] [PubMed] [Google Scholar]
- 49.Jordaan HF, Louw M. The moth-eaten alopecia of secondary syphilis. A histopathological study of 12 patients. Am J Dermatopathol. 1995 Apr;17(2):158–162. doi: 10.1097/00000372-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Daniels KC, Ferneyhough HS. Specific direct fluorescent antibody detection of Treponema pallidum. Health Lab Sci. 1977 Jul;14(3):164–171. [PubMed] [Google Scholar]
- 51.Beckett JH, Bigbee JW. Immunoperoxidase localization of treponema pallidum: its use in formaldehyde-fixed and paraffin-embedded tissue sections. Arch Pathol Lab Med. 1979 Mar;103(3):135–138. [PubMed] [Google Scholar]
- 52.Hook EW, 3rd, Roddy RE, Lukehart SA, Hom J, Holmes KK, Tam MR. Detection of Treponema pallidum in lesion exudate with a pathogen-specific monoclonal antibody. J Clin Microbiol. 1985 Aug;22(2):241–244. doi: 10.1128/jcm.22.2.241-244.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Handsfield HH, Lukehart SA, Sell S, Norris SJ, Holmes KK. Demonstration of Treponema pallidum in a cutaneous gumma by indirect immunofluorescence. Arch Dermatol. 1983 Aug;119(8):677–680. [PubMed] [Google Scholar]
- 54.Orle KA, Gates CA, Martin DH, Body BA, Weiss JB. Simultaneous PCR detection of Haemophilus ducreyi, Treponema pallidum, and herpes simplex virus types 1 and 2 from genital ulcers. J Clin Microbiol. 1996 Jan;34(1):49–54. doi: 10.1128/jcm.34.1.49-54.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Norris SJ, Sell S. Role of polymerase chain reaction in the diagnosis of gastric syphilis. Hum Pathol. 1996 Aug;27(8):749–750. doi: 10.1016/s0046-8177(96)90443-8. [DOI] [PubMed] [Google Scholar]
- 56.Zoechling N, Schluepen EM, Soyer HP, Kerl H, Volkenandt M. Molecular detection of Treponema pallidum in secondary and tertiary syphilis. Br J Dermatol. 1997 May;136(5):683–686. [PubMed] [Google Scholar]
- 57.Kouznetsov AV, Prinz JC. Molecular diagnosis of syphilis: the Schaudinn-Hoffmann lymph-node biopsy. Lancet. 2002 Aug 3;360(9330):388–989. doi: 10.1016/S0140-6736(02)09598-3. [DOI] [PubMed] [Google Scholar]
- 58.Palmer HM, Higgins SP, Herring AJ, Kingston MA. Use of PCR in the diagnosis of early syphilis in the United Kingdom. Sex Transm Infect. 2003 Dec;79(6):479–483. doi: 10.1136/sti.79.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kouznetsov AV, Weisenseel P, Trommler P, Multhaup S, Prinz JC. Detection of the 47-kilodalton membrane immunogen gene of Treponema pallidum in various tissue sources of patients with syphilis. Diagn Microbiol Infect Dis. 2005 Feb;51(2):143–145. doi: 10.1016/j.diagmicrobio.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 60.Gayet-Ageron A, Ninet B, Toutous-Trellu L, Lautenschlager S, Furrer H, Piguet V, et al. Assessment of a real-time PCR test to diagnose syphilis from diverse biological samples. Sex Transm Infect. 2009 Jan 20; doi: 10.1136/sti.2008.034314. [DOI] [PubMed] [Google Scholar]
- 61.Liu H, Rodes B, Chen CY, Steiner B. New tests for syphilis: rational design of a PCR method for detection of Treponema pallidum in clinical specimens using unique regions of the DNA polymerase I gene. J Clin Microbiol. 2001 May;39(5):1941–1946. doi: 10.1128/JCM.39.5.1941-1946.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marfin AA, Liu H, Sutton MY, Steiner B, Pillay A, Markowitz LE. Amplification of the DNA polymerase I gene of Treponema pallidum from whole blood of persons with syphilis. Diagn Microbiol Infect Dis. 2001 Aug;40(4):163–166. doi: 10.1016/s0732-8893(01)00275-9. [DOI] [PubMed] [Google Scholar]
- 63.Pietravalle M, Pimpinelli F, Maini A, Capoluongo E, Felici C, D'Auria L, et al. Diagnostic relevance of polymerase chain reaction technology for T. pallidum in subjects with syphilis in different phases of infection. New Microbiol. 1999 Apr;22(2):99–104. [PubMed] [Google Scholar]
- 64.Burstain JM, Grimprel E, Lukehart SA, Norgard MV, Radolf JD. Sensitive detection of Treponema pallidum by using the polymerase chain reaction. J Clin Microbiol. 1991 Jan;29(1):62–69. doi: 10.1128/jcm.29.1.62-69.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Noordhoek GT, Wolters EC, de Jonge ME, van Embden JD. Detection by polymerase chain reaction of Treponema pallidum DNA in cerebrospinal fluid from neurosyphilis patients before and after antibiotic treatment. J Clin Microbiol. 1991 Sep;29(9):1976–1984. doi: 10.1128/jcm.29.9.1976-1984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grimprel E, Sanchez PJ, Wendel GD, Burstain JM, McCracken GH, Jr, Radolf JD, et al. Use of polymerase chain reaction and rabbit infectivity testing to detect Treponema pallidum in amniotic fluid, fetal and neonatal sera, and cerebrospinal fluid. J Clin Microbiol. 1991 Aug;29(8):1711–1718. doi: 10.1128/jcm.29.8.1711-1718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramzan NN, Loftus E, Jr, Burgart LJ, Rooney M, Batts KP, Wiesner RH, et al. Diagnosis and monitoring of Whipple disease by polymerase chain reaction. Annals of internal medicine. 1997 Apr 1;126(7):520–527. doi: 10.7326/0003-4819-126-7-199704010-00004. [DOI] [PubMed] [Google Scholar]
- 68.Schaller J, Carlson JA. Erythema nodosum-like lesions in treated Whipple's disease: signs of immune reconstitution inflammatory syndrome. Journal of the American Academy of Dermatology. 2009 Feb;60(2):277–288. doi: 10.1016/j.jaad.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 69.Collart P, Franceschini P, Durel P. Experimental rabbit syphilis. The British journal of venereal diseases. 1971 Dec;47(6):389–400. doi: 10.1136/sti.47.6.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner TB, Hollander DH. Biology of the treponematoses based on studies carried out at the International Treponematosis Laboratory Center of the Johns Hopkins University under the auspices of the World Health Organization. Monogr Ser World Health Organ. 1957;(35):3–266. [PubMed] [Google Scholar]
- 71.Chesney AM. Immunity in syphilis. Medicine. 1926;5:463–447. [Google Scholar]
- 72.Bartarelli E. Sulla transmissione della sifilide al coniglio. Riv Ig San Pubbl. 1906;17:646–660. [Google Scholar]
- 73.Brown WH, Pierce LP. Experimental syphilis in the rabbit. I. Primary infection in the testicle. J Exp Med. 1920;31:475–498. doi: 10.1084/jem.31.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown WH LP, L.P P. Experimental syphilis in the rabbit. II Primary infection in the scdotum. Part 1. Reaction to infection. J Exp Med. 1920;31:709–727. doi: 10.1084/jem.31.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brown W, Pierce L. Experimental syphilis in the rabbit. II Primary infection in the scrotum. Part 2 Scrotal lesions and the character of the scrotal infection. J Exp Med. 1920;31:729–748. doi: 10.1084/jem.31.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brown W, Pierce L. Experimental syphilis the rabbit IV. Cutaneous syphilis. Part 1 Affections of the skin and appendages. J Exp Med. 1920;32:445–471. doi: 10.1084/jem.32.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown W, Pierce L. Experimental syphilis in the rabbit IV. Cutaneous syphilis. Part 2. Clinical aspects of cutaneous syphilis. J Exp Med. 1920;32:473–495. doi: 10.1084/jem.32.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brown W, Pierce L. Experimental syphilis in the rabbit. VII. Affections of the eye. J Exp Med. 1921;34:167–181. doi: 10.1084/jem.34.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown W, Pierce L. Experimental syphilis in the rabbit. III. Local dissemination, local recurrence, and involvement of the regional lymphatics. J Exp Med. 1920;31:749–764. doi: 10.1084/jem.31.6.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lukehart SA, Baker-Zander SA, Lloyd RM, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. II. Nature of cellular infiltration and Treponema pallidum distribution in testicular lesions. J Immunol. 1980 Jan;124(1):461–467. [PubMed] [Google Scholar]
- 81.Sell S, Gamboa D, Baker-Zander SA, Lukehart SA, Miller JN. Host response to Treponema pallidum in intradermally-infected rabbits: evidence for persistence of infection at local and distant sites. The Journal of investigative dermatology. 1980 Dec;75(6):470–475. doi: 10.1111/1523-1747.ep12524230. [DOI] [PubMed] [Google Scholar]
- 82.Sell S, Baker-Zander S, Powell HC. Experimental syphilitic orchitis in rabbits: ultrastructural appearance of Treponema pallidum during phagocytosis and dissolution by macrophages in vivo. Lab Invest. 1982 Apr;46(4):355–364. [PubMed] [Google Scholar]
- 83.Izzat NN, Knox JM, Werth JA, Dacres WG. Evolution of syphilitic chancres with virulent Treponema pallidum in the rabbit. The British journal of venereal diseases. 1971 Apr;47(2):67–72. doi: 10.1136/sti.47.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sell S, Salman J, Norris SJ. Reinfection of chancre-immune rabbits with Treponema pallidum. I. Light and immunofluorescence studies. Am J Pathol. 1985 Feb;118(2):248–255. [PMC free article] [PubMed] [Google Scholar]
- 85.Musher DM, Hague-Park M, Gyorkey F, Anderson DC, Baughn RE. The interaction between Treponema pallidum and human polymorphonuclear leukocytes. J Infect Dis. 1983 Jan;147(1):77–86. doi: 10.1093/infdis/147.1.77. [DOI] [PubMed] [Google Scholar]
- 86.Sykes JA, Miller JN, Kalan AJ. Treponema pallidum within cells of a primary chancre from a human female. The British journal of venereal diseases. 1974 Feb;50(1):40–44. doi: 10.1136/sti.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Metz J, Metz G. [Electron microscopical demonstration of Treponema pallidum in primary and secondary syphilitic skin lesions] Arch Dermatol Forsch. 1972;243(3):241–254. [PubMed] [Google Scholar]
- 88.Lauderdale V, Goldman JN. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. The British journal of venereal diseases. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sykes JA, Miller JN. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971 Sep;4(3):307–314. doi: 10.1128/iai.4.3.307-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Azar HA, Pham TD, Kurban AK. An electron microscopic study of a syphilitic chancre. Engulfment of Treponema pallidum by plasma cells. Arch Pathol. 1970 Aug;90(2):143–150. [PubMed] [Google Scholar]
- 91.Ovcinnikov N, Deletorskij V. Treponema pallidum in peripheral nerves of rabbit syphiloma. Vest N Dermatol Venerol. 1975;5:38–40. [PubMed] [Google Scholar]
- 92.Ovcinnikov NM, Delektorskij VV. Treponema pallidum in nerve fibres. The British journal of venereal diseases. 1975 Feb;51(1):10–18. doi: 10.1136/sti.51.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sell S, Salman J. Demonstration of Treponema pallidum in axons of cutaneous nerves in experimental chancres of rabbits. Sex Transm Dis. 1992 Jan-Feb;19(1):1–6. doi: 10.1097/00007435-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 94.Medici MA. The immunoprotective niche--a new pathogenic mechanism for syphilis, the systemic mycoses and other infectious diseases. J Theor Biol. 1972 Sep;36(3):617–625. doi: 10.1016/0022-5193(72)90012-4. [DOI] [PubMed] [Google Scholar]
- 95.Borenstein LA, Ganz T, Sell S, Lehrer RI, Miller JN. Contribution of rabbit leukocyte defensins to the host response in experimental syphilis. Infect Immun. 1991 Apr;59(4):1368–1377. doi: 10.1128/iai.59.4.1368-1377.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strugnell RA, Drummond L, Faine S. Secondary lesions in rabbits experimentally infected with Treponema pallidum. Genitourinary medicine. 1986 Feb;62(1):4–8. doi: 10.1136/sti.62.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baker-Zander S, Sell S. A histopathologic and immunologic study of the course of syphilis in the experimentally infected rabbit. Demonstration of long-lasting cellular immunity. Am J Pathol. 1980 Nov;101(2):387–414. [PMC free article] [PubMed] [Google Scholar]
- 98.Sell S, Baker-Zander SA, Lloyd RM. T-cell hyperplasia of lymphoid tissues of rabbits infected with Treponema pallidum: evidence for a vigorous immune response. Sex Transm Dis. 1980 Apr-Jun;7(2):74–84. doi: 10.1097/00007435-198004000-00009. [DOI] [PubMed] [Google Scholar]
- 99.Magnusson B. [Berger's flocculation reaction in the routine diagnosis of syphilis.] Nord Med. 1950 Mar 3;43(9):371–373. [PubMed] [Google Scholar]
- 100.Magnusson H, Rosenau B. The rate development and degree of acquired immunity in experimental syphilis. Am J Syph. 1948;32:418–436. [PubMed] [Google Scholar]
- 101.Turk J, Oort J. Germinal center activity in relation to delayed hypersensitivity. In: Cottier H, Odortchenko N, Schindler R, Congdon C, editors. Germinal Centers in Immune Responses. New York: Springer; 1967. [Google Scholar]
- 102.Arroll TW, Centurion-Lara A, Lukehart SA, Van Voorhis WC. T-Cell responses to Treponema pallidum subsp. pallidum antigens during the course of experimental syphilis infection. Infect Immun. 1999 Sep;67(9):4757–4763. doi: 10.1128/iai.67.9.4757-4763.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bishop NH, Miller JM. Humoral immune mechanisms in acquired syphilis. In: Schell R, Musher D, editors. Pathogenesis and immunology of Treponemal infection. New York: Marcel Dekker; 1983. pp. 241–269. [Google Scholar]
- 104.McNeely MC, Jorizzo JL, Solomon AR, Jr, Smith EB, Cavallo T, Sanchez RL. Cutaneous secondary syphilis: preliminary immunohistopathologic support for a role for immune complexes in lesion pathogenesis. Journal of the American Academy of Dermatology. 1986 Apr;14(4):564–571. doi: 10.1016/s0190-9622(86)70070-4. [DOI] [PubMed] [Google Scholar]
- 105.Jorizzo JL, McNeely MC, Baughn RE, Cavallo T, Solomon AR, Smith EB. Rabbit model of disseminated syphilis: immunoblot and immunohistologic evidence for a role of specific immune complexes in lesion pathogenesis. Journal of cutaneous pathology. 1988 Jun;15(3):150–160. doi: 10.1111/j.1600-0560.1988.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 106.Zodpey SP, Ambadekar NN, Thakur A. Effectiveness of Bacillus Calmette Guerin (BCG) vaccination in the prevention of leprosy: a population-based case-control study in Yavatmal District, India. Public Health. 2005 Mar;119(3):209–216. doi: 10.1016/j.puhe.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 107.Cunha SS, Rodrigues LC, Pedrosa V, Dourado IM, Barreto ML, Pereira SM. Neonatal BCG protection against leprosy: a study in Manaus, Brazilian Amazon. Lepr Rev. 2004 Dec;75(4):357–366. [PubMed] [Google Scholar]
- 108.Zodpey SP. Protective effect of bacillus Calmette Guerin (BCG) vaccine in the prevention of leprosy: a meta-analysis. Indian J Dermatol Venereol Leprol. 2007 Mar-Apr;73(2):86–93. doi: 10.4103/0378-6323.31891. [DOI] [PubMed] [Google Scholar]
- 109.Centurion-Lara A, Castro C, Barrett L, Cameron C, Mostowfi M, Van Voorhis WC, et al. Treponema pallidum major sheath protein homologue Tpr K is a target of opsonic antibody and the protective immune response. J Exp Med. 1999 Feb 15;189(4):647–656. doi: 10.1084/jem.189.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morgan CA, Molini BJ, Lukehart SA, Van Voorhis WC. Segregation of B and T cell epitopes of Treponema pallidum repeat protein K to variable and conserved regions during experimental syphilis infection. J Immunol. 2002 Jul 15;169(2):952–957. doi: 10.4049/jimmunol.169.2.952. [DOI] [PubMed] [Google Scholar]
- 111.Hazlett KR, Sellati TJ, Nguyen TT, Cox DL, Clawson ML, Caimano MJ, et al. The TprK protein of Treponema pallidum is periplasmic and is not a target of opsonic antibody or protective immunity. J Exp Med. 2001 May 7;193(9):1015–1026. doi: 10.1084/jem.193.9.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cameron CE, Lukehart SA, Castro C, Molini B, Godornes C, Van Voorhis WC. Opsonic potential, protective capacity, and sequence conservation of the Treponema pallidum subspecies pallidum Tp92. J Infect Dis. 2000 Apr;181(4):1401–1413. doi: 10.1086/315399. [DOI] [PubMed] [Google Scholar]
- 113.Blanco DR, Champion CI, Lewinski MA, Shang ES, Simkins SG, Miller JN, et al. Immunization with Treponema pallidum outer membrane vesicles induces high-titer complement-dependent treponemicidal activity and aggregation of T. pallidum rare outer membrane proteins (TROMPs) J Immunol. 1999 Sep 1;163(5):2741–2746. [PubMed] [Google Scholar]
- 114.Lewinski MA, Miller JN, Lovett MA, Blanco DR. Correlation of immunity in experimental syphilis with serum-mediated aggregation of Treponema pallidum rare outer membrane proteins. Infect Immun. 1999 Jul;67(7):3631–3636. doi: 10.1128/iai.67.7.3631-3636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lukehart SA. Scientific monogamy: thirty years dancing with the same bug: 2007 Thomas Parran Award Lecture. Sex Transm Dis. 2008 Jan;35(1):2–7. doi: 10.1097/OLQ.0b013e318162c4f2. [DOI] [PubMed] [Google Scholar]
- 116.Liang FT, Brown EL, Wang T, Iozzo RV, Fikrig E. Protective niche for Borrelia burgdorferi to evade humoral immunity. Am J Pathol. 2004 Sep;165(3):977–985. doi: 10.1016/S0002-9440(10)63359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sell S, Weigle W. The relationship between delayed hypersensitivity and circulating antibody induced by protein antigens in guinea pigs. J Immunol. 1959;83:257–263. [PubMed] [Google Scholar]
- 118.Lee V, Kinghorn G. Syphilis: an update. Clin Med. 2008 Jun;8(3):330–333. doi: 10.7861/clinmedicine.8-3-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ackerman AB, Chongchitnant N, Sanchez J, Guo Y, Bennin B, Reichel, et al. Syphilis. Histologic diagnosis of of inflammatory skin disease: an algorithmic method based on pattern analysis. 2nd ed. Baltimore: Williams & Wilkins; 1997. pp. 744–751. [Google Scholar]
- 120.Chapel TA. The variability of syphilitic chancres. Sex Transm Dis. 1978 Apr-Jun;5(2):68–70. doi: 10.1097/00007435-197804000-00009. [DOI] [PubMed] [Google Scholar]
- 121.Alam F, Argiriadou AS, Hodgson TA, Kumar N, Porter SR. Primary syphilis remains a cause of oral ulceration. Br Dent J. 2000 Oct 14;189(7):352–354. doi: 10.1038/sj.bdj.4800767. [DOI] [PubMed] [Google Scholar]
- 122.Hagedorn HJ, Kraminer-Hagedorn A, De Bosschere K, Hulstaert F, Pottel H, Zrein M. Evaluation of INNO-LIA syphilis assay as a confirmatory test for syphilis. J Clin Microbiol. 2002 Mar;40(3):973–978. doi: 10.1128/JCM.40.3.973-978.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sambri V, Marangoni A, Eyer C, Reichhuber C, Soutschek E, Negosanti M, et al. Western immunoblotting with five Treponema pallidum recombinant antigens for serologic diagnosis of syphilis. Clin Diagn Lab Immunol. 2001 May;8(3):534–539. doi: 10.1128/CDLI.8.3.534-539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gutierrez J, Vergara MJ, Soto MJ, Piedrola G, Maroto M. Clinical utility of a competitive ELISA to detect antibodies against Treponema pallidum. J Clin Lab Anal. 2000;14(2):83–86. doi: 10.1002/(SICI)1098-2825(2000)14:2<83::AID-JCLA8>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Carlson JA, Ng BT, Chen KR. Cutaneous Vasculitis Update: Diagnostic Criteria, Classification, Epidemiology, Etiology, Pathogenesis, Evaluation and Prognosis. Am J Dermatopathol. 2005 Dec;27(6):504–528. doi: 10.1097/01.dad.0000181109.54532.c5. [DOI] [PubMed] [Google Scholar]
- 126.Abell E, Marks R, Jones EW. Secondary syphilis: a clinico-pathological review. Br J Dermatol. 1975 Jul;93(1):53–61. doi: 10.1111/j.1365-2133.1975.tb06476.x. [DOI] [PubMed] [Google Scholar]
- 127.Alessi E, Innocenti M, Ragusa G. Secondary syphilis. Clinical morphology and histopathology. Am J Dermatopathol. 1983 Feb;5(1):11–17. [PubMed] [Google Scholar]
- 128.Jordaan HF. Secondary syphilis. A clinicopathological study. Am J Dermatopathol. 1988 Oct;10(5):399–409. [PubMed] [Google Scholar]
- 129.Puavilai S, Charuwichitratana S, Polnikorn N, Sakuntabhai A, Timpatanapong P. Clinical and histopathological features of secondary syphilis. J Med Assoc Thai. 1993 Feb;76(2):85–92. [PubMed] [Google Scholar]
- 130.Lee JY, Hsu ML. Alopecia syphilitica, a simulator of alopecia areata: histopathology and differential diagnosis. Journal of cutaneous pathology. 1991 Apr;18(2):87–92. doi: 10.1111/j.1600-0560.1991.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 131.Lawrence P, Saxe N. Bullous secondary syphilis. Clin Exp Dermatol. 1992 Jan;17(1):44–46. doi: 10.1111/j.1365-2230.1992.tb02533.x. [DOI] [PubMed] [Google Scholar]
- 132.McComb ME, Telang GH, Vonderheid EC. Secondary syphilis presenting as pseudolymphoma of the skin. Journal of the American Academy of Dermatology. 2003 Aug;49(2 Suppl):S174–S176. doi: 10.1067/mjd.2003.329. Case Reports. [DOI] [PubMed] [Google Scholar]
- 133.Graham WR, Jr, Duvic M. Nodular secondary syphilis. Arch Dermatol. 1982 Mar;118(3):205–206. [PubMed] [Google Scholar]
- 134.Hodak E, David M, Rothem A, Bialowance M, Sandbank M. Nodular secondary syphilis mimicking cutaneous lymphoreticular process. Journal of the American Academy of Dermatology. 1987 Nov;17(5 Pt 2):914–917. doi: 10.1016/s0190-9622(87)70280-1. [DOI] [PubMed] [Google Scholar]
- 135.Lopez Estebranz JL, Gil Martin R, Iglesias Diez L. Sifilis secundaria; estudio clinico-histologico de 50 casos. Acata Dermosifiliogr. 1995;86:649–656. [Google Scholar]
- 136.Wu CC, Tsai CN, Wong WR, Hong HS, Chuang YH. Early congenital syphilis and erythema multiforme-like bullous targetoid lesions in a 1-day-old newborn: detection of Treponema pallidum genomic DNA from the targetoid plaque using nested polymerase chain reaction. Journal of the American Academy of Dermatology. 2006 Aug;55(2 Suppl):S11–S15. doi: 10.1016/j.jaad.2005.11.1062. [DOI] [PubMed] [Google Scholar]
- 137.Green KM, Heilman E. Secondary syphilis presenting as a palisading granuloma. Journal of the American Academy of Dermatology. 1985 May;12(5 Pt 2):957–960. doi: 10.1016/s0190-9622(85)70122-3. [DOI] [PubMed] [Google Scholar]
- 138.Jain HC, Fisher BK. Annular syphilid mimicking granuloma annulare. Int J Dermatol. 1988 Jun;27(5):340–341. doi: 10.1111/j.1365-4362.1988.tb02366.x. [DOI] [PubMed] [Google Scholar]
- 139.Sehgal VN, Lal S, Jain S, Bhattacharya SN, Thappa DM, Logani KB. Secondary syphilis mimicking borderline (BL) leprosy. J Dermatol. 1993 Feb;20(2):102–104. doi: 10.1111/j.1346-8138.1993.tb03839.x. [DOI] [PubMed] [Google Scholar]
- 140.Fonseca E, Garcia-Silva J, del Pozo J, Yebra MT, Cuevas J, Contreras F. Syphilis in an HIV infected patient misdiagnosed as leprosy. Journal of cutaneous pathology. 1999 Jan;26(1):51–54. doi: 10.1111/j.1600-0560.1999.tb01791.x. [DOI] [PubMed] [Google Scholar]
- 141.Lochner JC, Pomeranz JR. Lichenoid secondary syphilis. Arch Dermatol. 1974 Jan;109(1):81–83. [PubMed] [Google Scholar]
- 142.Carbia SG, Lagodin C, Abbruzzese M, Sevinsky L, Casco R, Casas J, et al. Lichenoid secondary syphilis. Int J Dermatol. 1999 Jan;38(1):53–55. doi: 10.1046/j.1365-4362.1999.00634.x. [DOI] [PubMed] [Google Scholar]
- 143.Shatley MJ, Walker BL, McMurray RW. Lues and lupus: syphilis mimicking systemic lupus erythematosus (SLE) Lupus. 2001;10(4):299–303. doi: 10.1191/096120301680417002. [DOI] [PubMed] [Google Scholar]
- 144.Levin DL, Greenberg MH, Hasegawa J, Roenigk HH., Jr. Secondary syphilis mimicking mycosis fungoides. Journal of the American Academy of Dermatology. 1980 Jul;3(1):92–94. doi: 10.1016/s0190-9622(80)80229-5. [DOI] [PubMed] [Google Scholar]
- 145.Liotta EA, Turiansky GW, Berberian BJ, Sulica VI, Tomaszewski MM. Unusual presentation of secondary syphilis in 2 HIV-1 positive patients. Cutis. 2000 Nov;66(5):383–386. 9. [PubMed] [Google Scholar]
- 146.Cochran RE, Thomson J, Fleming KA, Strong AM. Histology simulating reticulosis in secondary syphilis. Br J Dermatol. 1976 Sep;95(3):251–254. doi: 10.1111/j.1365-2133.1976.tb07011.x. [DOI] [PubMed] [Google Scholar]
- 147.Mignogna MD, Fortuna G, Leuci S, Mignogna C, Delfino M. Secondary syphilis mimicking pemphigus vulgaris. J Eur Acad Dermatol Venereol. 2009 Apr;23(4):479–480. doi: 10.1111/j.1468-3083.2008.02926.x. [DOI] [PubMed] [Google Scholar]
- 148.Cole GW, Amon RB, Russell PS. Secondary syphilis presenting as a pruritic dermatosis. Arch Dermatol. 1977 Apr;113(4):489–490. [PubMed] [Google Scholar]
- 149.Talanin NY, Shelley ED, Kapkaev RA, Kang MK, Skvortzova OA. Koebner reaction in psoriasis due to secondary syphilis. Cutis. 1994 Nov;54(5):332–334. [PubMed] [Google Scholar]
- 150.Neumann HA, Berretty PJ, Faber WR. Granulomatous secondary syphilis coinciding with PUVA treatment in a patient with psoriasis: an apparent failure of PUVA therapy. Acta dermato-venereologica. 1981;61(1):82–85. [PubMed] [Google Scholar]
- 151.Kahn LB, Gordon W. Sarcoid-like granulomas in secondary syphilis. A clinical and histopathologic study of five cases. Arch Pathol. 1971 Nov;92(5):334–337. [PubMed] [Google Scholar]
- 152.Perry HO, Lofgren RK. Secondary and tertiary syphilis presenting as sarcoidal reactions of the skin. Cutis. 1984 Sep;34(3):253–255. 7–8. [PubMed] [Google Scholar]
- 153.Sapra S, Weatherhead L. Extensive nodular secondary syphilis. Arch Dermatol. 1989 Dec;125(12):1666–1669. [PubMed] [Google Scholar]
- 154.Papini M, Bettacchi A, Guiducci A. Nodular secondary syphilis. Br J Dermatol. 1998 Apr;138(4):704–705. doi: 10.1046/j.1365-2133.1998.02191.x. [DOI] [PubMed] [Google Scholar]
- 155.Lantis LR, Petrozzi JW, Hurley HJ. Sarcoid granuloma in secondary syphilis. Arch Dermatol. 1969 Jun;99(6):748–752. [PubMed] [Google Scholar]
- 156.Chao YC, Chen CH, Chen YK, Chou CT. A large ulcer and cutaneous small-vessel vasculitis associated with syphilis infection. Scand J Rheumatol. 2006 Mar-Apr;35(2):147–151. doi: 10.1080/03009740500381229. [DOI] [PubMed] [Google Scholar]
- 157.Mikhail GR, Chapel TA. Follicular papulopustular syphilid. Arch Dermatol. 1969 Oct;100(4):471–473. [PubMed] [Google Scholar]
- 158.Jordaan HF. Widespread superficial thrombophlebitis as a manifestation of secondary syphilis--a new sign. A report of 2 cases. S Afr Med J. 1986 Oct 11;70(8):493–494. [PubMed] [Google Scholar]
- 159.Jordaan HF, Cilliers J. Secondary syphilis mimicking Sweet's syndrome. Br J Dermatol. 1986 Oct;115(4):495–496. doi: 10.1111/j.1365-2133.1986.tb06244.x. [DOI] [PubMed] [Google Scholar]
- 160.Cotterman C, Eckert L, Ackerman L. Syphilis mimicking tinea imbricata and erythema annulare centrifugum in an immunocompromised patient. Journal of the American Academy of Dermatology. 2009 Jul;61(1):165–167. doi: 10.1016/j.jaad.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 161.Blanes M, Peiro FM, Benito C, Belinchon I. Generalized ulcerated nodules in a patient with human immunodeficiency virus infection--quiz case. Arch Dermatol. 2009 Jul;145(7):829–834. doi: 10.1001/archdermatol.2009.142-a. [DOI] [PubMed] [Google Scholar]
- 162.Fernandez-Guarino M, Aldanondo Fernandez de la Mora I, Gonzalez Garcia C, Harto Castano A, Moreno Izquierdo R, Jaen Olasolo P. [Malignant syphilis in patients with human immunodeficiency virus (HIV)] Actas Dermosifiliogr. 2006 Jul-Aug;97(6):400–403. doi: 10.1016/s0001-7310(06)73428-7. [DOI] [PubMed] [Google Scholar]
- 163.Fisher DA, Chang LW, Tuffanelli DL. Lues maligna. Presentation of a case and a review of the literature. Arch Dermatol. 1969 Jan;99(1):70–73. doi: 10.1001/archderm.99.1.70. [DOI] [PubMed] [Google Scholar]
- 164.Merkert R. Generalized ulcers. Noduloulcerative syphilis (malignant syphilis, lues maligna) Arch Dermatol. 1997 Aug;133(8):1027–1028. 30–31. doi: 10.1001/archderm.1997.03890440111016. [DOI] [PubMed] [Google Scholar]
- 165.Morar N, Ramdial PK, Naidoo DK, Dlova NC, Aboobaker J. Lues maligna. Br J Dermatol. 1999 Jun;140(6):1175–1177. [PubMed] [Google Scholar]
- 166.Sands M, Markus A. Lues maligna, or ulceronodular syphilis, in a man infected with human immunodeficiency virus: case report and review. Clin Infect Dis. 1995 Feb;20(2):387–390. doi: 10.1093/clinids/20.2.387. [DOI] [PubMed] [Google Scholar]
- 167.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nature reviews. 2002 May;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 168.Magro CM, Crowson AN. Lichenoid and granulomatous dermatitis. Int J Dermatol. 2000 Feb;39(2):126–133. doi: 10.1046/j.1365-4362.2000.00868.x. [DOI] [PubMed] [Google Scholar]
- 169.Riley BS, Oppenheimer-Marks N, Hansen EJ, Radolf JD, Norgard MV. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992 Mar;165(3):484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- 170.Lee KH, Choi HJ, Lee MG, Lee JB. Virulent Treponema pallidum 47 kDa antigen regulates the expression of cell adhesion molecules and binding of T-lymphocytes to cultured human dermal microvascular endothelial cells. Yonsei medical journal. 2000 Oct;41(5):623–633. doi: 10.3349/ymj.2000.41.5.623. [DOI] [PubMed] [Google Scholar]
- 171.Turner DR. Lymphadenopathy in secondary syphilis. J Pathol. 1971 Jul;104(3) x. [PubMed] [Google Scholar]
- 172.Gjestland T. The Oslo study of untreated syphilis; an epidemiologic investigation of the natural course of the syphilitic infection based upon a re-study of the Boeck-Bruusgaard material. Acta Derm Venereol Suppl (Stockh) 1955;35(Suppl 34):3–368. doi: 10.2340/00015555343368. Annex I-LVI. [DOI] [PubMed] [Google Scholar]
- 173.Gregory N, Sanchez M, Buchness MR. The spectrum of syphilis in patients with human immunodeficiency virus infection. Journal of the American Academy of Dermatology. 1990 Jun;22(6 Pt 1):1061–1067. doi: 10.1016/0190-9622(90)70153-9. [DOI] [PubMed] [Google Scholar]
- 174.Bari MM, Shulkin DJ, Abell E. Ulcerative syphilis in acquired immunodeficiency syndrome: a case of precocious tertiary syphilis in a patient infected with human immunodeficiency virus. Journal of the American Academy of Dermatology. 1989 Dec;21(6):1310–1312. doi: 10.1016/s0190-9622(89)80316-0. [DOI] [PubMed] [Google Scholar]
- 175.Frohlich [Juxtaarticular nodes in tertiary syphilis.] Z Haut Geschlechtskr. 1951 Jun 15;10(12) li. [PubMed] [Google Scholar]
- 176.Bloom D. A case for diagnosis: tertiary syphilis? Necrobiosis lipoidica? AMA Arch Derm Syphilol. 1951 Feb;63(2):268–269. [PubMed] [Google Scholar]
- 177.Szeskin J. [Polymorphous cutaneous tertiary syphilis with tuberoserpinginous, squamous, grouped tuberous and tuberous with squamous lesions.] Actas Dermosifiliogr. 1956 Mar;47(6):509–510. [PubMed] [Google Scholar]
- 178.Pembroke AC, Michell PA, McKee PH. Nodulo-squamous tertiary syphilide. Clin Exp Dermatol. 1980 Sep;5(3):361–364. doi: 10.1111/j.1365-2230.1980.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 179.Pareek SS. Unusual location of syphilitic alopecia: a case report. Sex Transm Dis. 1982 Jan-Mar;9(1):43–44. doi: 10.1097/00007435-198201000-00010. [DOI] [PubMed] [Google Scholar]
- 180.Matsuda-John SS, McElgunn PS, Ellis CN. Nodular late syphilis. Journal of the American Academy of Dermatology. 1983 Aug;9(2):269–272. doi: 10.1016/s0190-9622(83)70137-4. [DOI] [PubMed] [Google Scholar]
- 181.Tanabe JL, Huntley AC. Granulomatous tertiary syphilis. Journal of the American Academy of Dermatology. 1986 Aug;15(2 Pt 2):341–344. doi: 10.1016/s0190-9622(86)70173-4. [DOI] [PubMed] [Google Scholar]
- 182.Chung G, Kantor GR, Whipple S. Tertiary syphilis of the face. Journal of the American Academy of Dermatology. 1991 May;24(5 Pt 2):832–835. doi: 10.1016/0190-9622(91)70126-m. [DOI] [PubMed] [Google Scholar]
- 183.Drobacheff C, Moulin T, Van Landuyt H, Merle C, Vigan M, Laurent R. [Cutaneous tertiary syphilis with neurological symptoms] Ann Dermatol Venereol. 1994;121(1):34–36. [PubMed] [Google Scholar]
- 184.Sekkat A, Sedrati O, Derdabi D. [Cutaneomucous tertiary syphilis] Ann Dermatol Venereol. 1994;121(2):146–151. [PubMed] [Google Scholar]
- 185.Sule RR, Deshpande SG, Dharmadhikari NJ, Joshi VR. Late cutaneous syphilis. Cutis. 1997 Mar;59(3):135–137. [PubMed] [Google Scholar]
- 186.Varela P, Alves R, Velho G, Santos C, Massa A, Sanches M. Two recent cases of tertiary syphilis. Eur J Dermatol. 1999 Jun;9(4):300–302. [PubMed] [Google Scholar]
- 187.Wu SJ, Nguyen EQ, Nielsen TA, Pellegrini AE. Nodular tertiary syphilis mimicking granuloma annulare. Journal of the American Academy of Dermatology. 2000 Feb;42(2 Pt 2):378–380. doi: 10.1016/s0190-9622(00)90117-8. [DOI] [PubMed] [Google Scholar]
- 188.Rocha N, Horta M, Sanches M, Lima O, Massa A. Syphilitic gumma--cutaneous tertiary syphilis. J Eur Acad Dermatol Venereol. 2004 Jul;18(4):517–518. doi: 10.1111/j.1468-3083.2004.00960.x. [DOI] [PubMed] [Google Scholar]
- 189.Wollina U, Koch A, Abdel-Naser MB, Schonlebe J. Pressure ulcer-like presacral gummata in a patient with tertiary syphilis. Int Woun J. 2005 Mar;2(1):74–76. doi: 10.1111/j.1742-4801.2005.00076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pereira TM, Fernandes JC, Vieira AP, Basto AS. Tertiary syphilis. Int J Dermatol. 2007 Nov;46(11):1192–1195. doi: 10.1111/j.1365-4632.2007.03438.x. [DOI] [PubMed] [Google Scholar]
- 191.Hervier B, Wastiaux H, Freour T, Masseau A, Corvec S, Armingeat T, et al. [Sarcoidosis-like granulomatosis revealing a tertiary syphilis.] Rev Med Interne. 2009 Feb 25; doi: 10.1016/j.revmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 192.Olansky S. Late benign syphilis. Med Clin North Am. 1964;48:653. [Google Scholar]
- 193.Sanchez MR, Luger AFH. Syphilis. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. Dermaology in general medicine. New York: McGraw-Hill; 1993. pp. 2703–243. [Google Scholar]
- 194.Turk GL, Heesom N. Delayed Hypersensitivity. Res Monogr Immunol. 1980;1:1–259. [Google Scholar]
- 195.Spector WG, Heesom N. The production of granulomata by antigen-antibody complexes. J Pathol. 1969 May;98(1):31–39. doi: 10.1002/path.1710980105. [DOI] [PubMed] [Google Scholar]
- 196.Adams DO. The granulomatous inflammatory response. A review. Am J Pathol. 1976 Jul;84(1):164–192. [PMC free article] [PubMed] [Google Scholar]
- 197.Epstein WL. Granulomatous hypersensitivity. Prog Allergy. 1967;11:36–88. [PubMed] [Google Scholar]
- 198.Weedon D. Skin Pathology. Edinburgh: Churchill Livingstone; 2002. Spirochetal infections; pp. 649–657. [Google Scholar]
- 199.Poulsen A, Secher L, Kobayasi T, Weismann K. Treponema pallidum in leukoderma syphiliticum demonstrated by electron microscopy. Acta dermato-venereologica. 1988;68(2):102–106. [PubMed] [Google Scholar]
- 200.Poulsen A, Kobayasi T, Secher L, Weismann K. Treponema pallidum in human chancre tissue: an electron microscopic survey. Acta dermato-venereologica. 1986;66(5):423–430. [PubMed] [Google Scholar]
- 201.Cruickshank AH, McConnell EM, Miller DG. Malignancy in Scars, Chronic Ulcers, and Sinuses. Journal of clinical pathology. 1963 Nov;16:573–580. doi: 10.1136/jcp.16.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Narouz N, Wade AA, Allan PS. Can basal cell carcinoma develop on the site of a healed gumma? International journal of STD & AIDS. 1999 Sep;10(9):623–625. doi: 10.1258/0956462991914672. [DOI] [PubMed] [Google Scholar]
- 203.Tavora F, Burke A. Review of isolated ascending aortitis: differential diagnosis, including syphilitic, Takayasu's and giant cell aortitis. Pathology. 2006 Aug;38(4):302–308. doi: 10.1080/00313020600820898. [DOI] [PubMed] [Google Scholar]
- 204.Weyers W. The Abuse of Man. New York: Ardor Scribendi; 2003. [Google Scholar]
- 205.Davis RL, Robertson DM. Textbook of neuropathology. Baltimore: Williams and Wilkins; 1985. [Google Scholar]
- 206.Kofman O. The changing pattern of neurosyphilis. Can Med Assoc J. 1956 May 15;74(10):807–812. [PMC free article] [PubMed] [Google Scholar]
- 207.Timmermans M, Carr J. Neurosyphilis in the modern era. J Neurol Neurosurg Psychiatry. 2004 Dec;75(12):1727–1730. doi: 10.1136/jnnp.2004.031922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Marra CM. Update on neurosyphilis. Curr Infect Dis Rep. 2009 Mar;11(2):127–134. doi: 10.1007/s11908-009-0019-1. [DOI] [PubMed] [Google Scholar]
- 209.Hooshmand H, Escobar MR, Kopf SW. Neurosyphilis. A study of 241 patients. JAMA. 1972 Feb;219(6):726–729. doi: 10.1001/jama.219.6.726. [DOI] [PubMed] [Google Scholar]
- 210.Lukehart SA, Hook EW, 3rd, Baker-Zander SA, Collier AC, Critchlow CW, Handsfield HH. Invasion of the central nervous system by Treponema pallidum: implications for diagnosis and treatment. Ann Intern Med. 1988 Dec 1;109(11):855–862. doi: 10.7326/0003-4819-109-11-855. [DOI] [PubMed] [Google Scholar]
- 211.Bradley S. On syphylitic dropsy. Br Med J. 1871;1:116–117. doi: 10.1136/bmj.1.527.116-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Engel S, Diezel W. Persistent serum immune complexes in syphilis. The British journal of venereal diseases. 1980 Aug;56(4):221–222. doi: 10.1136/sti.56.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Solling J, Solling K, Jakobsen KU, From E. Circulating immune complexes in syphilis. Acta dermato-venereologica. 1978;58(3):263–267. [PubMed] [Google Scholar]
- 214.Tourville DR, Byrd LH, Kim DU, Zajd D, Lee I, Reichman LB, et al. Treponemal antigen in immunopathogenesis of syphilitic glomerulonephritis. Am J Pathol. 1976 Mar;82(3):479–492. [PMC free article] [PubMed] [Google Scholar]
- 215.Walker PD, Deeves EC, Sahba G, Wallin JD, O'Neill WM., Jr. Rapidly progressive glomerulonephritis in a patient with syphilis. Identification of antitreponemal antibody and treponemal antigen in renal tissue. Am J Med. 1984 Jun;76(6):1106–1112. doi: 10.1016/0002-9343(84)90866-0. [DOI] [PubMed] [Google Scholar]
- 216.Barr JJ, Cole H, Driver J, Leas R, Miller M, Strauss L. Acute syphilitic nephrosis treated with penicillin. JAMA. 1946;131:741–743. [PubMed] [Google Scholar]
- 217.Scott V, Clark E. Syphilitic nephrosis as a manifestation of renal Herxheimer reaction following penicillin therapy for syphilis. Am J Syph Gonorrhea Vener Dis. 1946;30:463–467. [PubMed] [Google Scholar]
- 218.Lukehart SA, Baker-Zander SA, Lloyd RM, Sell S. Effect of cortisone administration on host-parasite relationships in early experimental syphilis. J Immunol. 1981 Oct;127(4):1361–1368. [PubMed] [Google Scholar]
- 219.Johnson PC, Norris SJ, Miller GP, Scott LD, Sell S, Kahan BD, et al. Early syphilitic hepatitis after renal transplantation. J Infect Dis. 1988 Jul;158(1):236–238. doi: 10.1093/infdis/158.1.236. [DOI] [PubMed] [Google Scholar]
- 220.Petersen LR, Mead RH, Perlroth MG. Unusual manifestations of secondary syphilis occurring after orthotopic liver transplantation. Am J Med. 1983 Jul;75(1):166–170. doi: 10.1016/0002-9343(83)91183-x. [DOI] [PubMed] [Google Scholar]
- 221.Sherlock S. The liver in secondary (early) syphilis. N Engl J Med. 1971 Jun 24;284(25):1437–1438. doi: 10.1056/NEJM197106242842513. [DOI] [PubMed] [Google Scholar]
- 222.Ditzen AK, Braker K, Zoellner KH, Teichmann D. The syphilis-HIV interdependency. International journal of STD & AIDS. 2005 Sep;16(9):642–643. doi: 10.1258/0956462054944381. [DOI] [PubMed] [Google Scholar]
- 223.Auvert B, Buve A, Ferry B, Carael M, Morison L, Lagarde E, et al. Ecological and individual level analysis of risk factors for HIV infection in four urban populations in sub-Saharan Africa with different levels of HIV infection. AIDS. 2001 Aug;15(Suppl 4):S15–S30. doi: 10.1097/00002030-200108004-00003. [DOI] [PubMed] [Google Scholar]
- 224.Czelusta A, Yen-Moore A, Van der Straten M, Carrasco D, Tyring SK. An overview of sexually transmitted diseases. Part III. Sexually transmitted diseases in HIV-infected patients. Journal of the American Academy of Dermatology. 2000 Sep;43(3):409–432. doi: 10.1067/mjd.2000.105158. quiz 33-6. [DOI] [PubMed] [Google Scholar]
- 225.Hook EW., 3rd Syphilis and HIV infection. J Infect Dis. 1989 Sep;160(3):530–534. doi: 10.1093/infdis/160.3.530. [DOI] [PubMed] [Google Scholar]
- 226.Don PC, Rubinstein R, Christie S. Malignant syphilis (lues maligna) and concurrent infection with HIV. Int J Dermatol. 1995 Jun;34(6):403–407. doi: 10.1111/j.1365-4362.1995.tb04441.x. [DOI] [PubMed] [Google Scholar]
- 227.Tucker SC, Yates VM, Thambar IV. Unusual skin ulceration in an HIV-positive patient who had cutaneous syphilis and neurosyphilis. Br J Dermatol. 1997 Jun;136(6):946–948. [PubMed] [Google Scholar]
- 228.Breustedt W, Krause H, Leonhard T, Porstmann T, Volk D. [Syphilis in HIV infection. 2 cases of neurosyphilis] Dermatol Monatsschr. 1989;175(4):232–241. [PubMed] [Google Scholar]
- 229.Calderon W, Douville H, Nigro M, Bono G, Muratori S. Concomitant syphilitic and HIV infection. A case report. Acta Neurol (Napoli) 1990 Apr;12(2):132–137. [PubMed] [Google Scholar]
- 230.Thami GP, Kaur S, Gupta R, Kanwar AJ, Sood S. Syphilitic panuveitis and asymptomatic neurosyphilis: a marker of HIV infection. International journal of STD & AIDS. 2001 Nov;12(11):754–756. doi: 10.1258/0956462011924146. [DOI] [PubMed] [Google Scholar]
- 231.McLeish WM, Pulido JS, Holland S, Culbertson WW, Winward K. The ocular manifestations of syphilis in the human immunodeficiency virus type 1-infected host. Ophthalmology. 1990 Feb;97(2):196–203. doi: 10.1016/s0161-6420(90)32605-2. [DOI] [PubMed] [Google Scholar]
- 232.Smith ME, Canalis RF. Otologic manifestations of AIDS: the otosyphilis connection. Laryngoscope. 1989 Apr;99(4):365–372. doi: 10.1288/00005537-198904000-00001. [DOI] [PubMed] [Google Scholar]
- 233.Dawson S, Evans BA, Lawrence AG. Benign tertiary syphilis and HIV infection. AIDS. 1988 Aug;2(4):315–316. doi: 10.1097/00002030-198808000-00012. [DOI] [PubMed] [Google Scholar]
- 234.Tobian AA, Quinn TC. Herpes simplex virus type 2 and syphilis infections with HIV: an evolving synergy in transmission and prevention. Curr Opin HIV AIDS. 2009 Jul;4(4):294–299. doi: 10.1097/COH.0b013e32832c1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Reynolds SJ, Risbud AR, Shepherd ME, Rompalo AM, Ghate MV, Godbole SV, et al. High rates of syphilis among STI patients are contributing to the spread of HIV-1 in India. Sex Transm Infect. 2006 Apr;82(2):121–126. doi: 10.1136/sti.2005.015040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Rompalo AM, Lawlor J, Seaman P, Quinn TC, Zenilman JM, Hook EW., 3rd Modification of syphilitic genital ulcer manifestations by coexistent HIV infection. Sex Transm Dis. 2001 Aug;28(8):448–454. doi: 10.1097/00007435-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 237.Sellati TJ, Waldrop SL, Salazar JC, Bergstresser PR, Picker LJ, Radolf JD. The cutaneous response in humans to Treponema pallidum lipoprotein analogues involves cellular elements of both innate and adaptive immunity. J Immunol. 2001 Mar 15;166(6):4131–4140. doi: 10.4049/jimmunol.166.6.4131. [DOI] [PubMed] [Google Scholar]
- 238.Farhi DC, Wells SJ, Siegel RJ. Syphilitic lymphadenopathy. Histology and human immunodeficiency virus status. Am J Clin Pathol. 1999 Sep;112(3):330–334. doi: 10.1093/ajcp/112.3.330. [DOI] [PubMed] [Google Scholar]
- 239.Tseng CK, Hughes MA, Hsu PL, Mahoney S, Duvic M, Sell S. Syphilis superinfection activates expression of human immunodeficiency virus I in latently infected rabbits. Am J Pathol. 1991 May;138(5):1149–1164. [PMC free article] [PubMed] [Google Scholar]
- 240.Sell S, Tseng CK. Multiple superinfections fail to activate defective human immunodeficiency virus-1 (HIV-1) infection of rabbits. J Acquir Immune Defic Syndr Hum Retrovirol. 1995 Jul 1;9(3):211–226. [PubMed] [Google Scholar]
- 241.Tseng CK, Leibowitz J, Sell S. Defective infection of rabbit peripheral blood monocyte cultures with human immunodeficiency virus type 1. AIDS Res Hum Retroviruses. 1994 Mar;10(3):285–293. doi: 10.1089/aid.1994.10.285. [DOI] [PubMed] [Google Scholar]
- 242.Ishizaka K. Regulation of IgE synthesis. Annu Rev Immunol. 1984;2:159–182. doi: 10.1146/annurev.iy.02.040184.001111. [DOI] [PubMed] [Google Scholar]
- 243.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996 Oct 31;383(6603):787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 244.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006 Apr;19(2):338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Turk JL, Bryceson AD. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- 246.Godal T. Immunological aspects of leprosy--present status. Prog Allergy. 1978;25:211–242. [PubMed] [Google Scholar]
- 247.Skinsnes OK. Immuno-pathology of leprosy: the century in review. Pathology, pathogenesis, and the development of classification. Int J Lepr Other Mycobact Dis. 1973 Jul-Sep;41(3):329–360. [PubMed] [Google Scholar]
- 248.Panayi GS. Does rheumatoid arthritis have a clinicopathological spectrum similar to that of leprosy? Ann Rheum Dis. 1982 Feb;41(1):102–103. doi: 10.1136/ard.41.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rogers TJ, Balish E. Immunity to Candida albicans. Microbiol Rev. 1980 Dec;44(4):660–682. doi: 10.1128/mr.44.4.660-682.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Lilic D. New perspectives on the immunology of chronic mucocutaneous candidiasis. Curr Opin Infect Dis. 2002 Apr;15(2):143–147. doi: 10.1097/00001432-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 251.Hartsock RJ, Halling LW, King FM. Luetic lymphadenitis: a clinical and histologic study of 20 cases. Am J Clin Pathol. 1970 Mar;53(3):304–314. doi: 10.1093/ajcp/53.3.304. [DOI] [PubMed] [Google Scholar]
- 252.Rosenberg ZF, Fauci AS. Immunopathogenesis of HIV infection. FASEB J. 1991 Jul;5(10):2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- 253.Podwinska J, Lusiak M, Zaba R, Bowszyc J. The pattern and level of cytokines secreted by Th1 and Th2 lymphocytes of syphilitic patients correlate to the progression of the disease. FEMS immunology and medical microbiology. 2000 May;28(1):1–14. doi: 10.1111/j.1574-695X.2000.tb01451.x. [DOI] [PubMed] [Google Scholar]
- 254.Meyerhoff E. Letter: The great imitator. Can Med Assoc J. 1976 Mar 20;114(6):503. [PMC free article] [PubMed] [Google Scholar]
- 255.Jackson R. Jonathan Hutchinson on syphilis. Sex Transm Dis. 1980 Apr-Jun;7(2):90–96. doi: 10.1097/00007435-198004000-00012. [DOI] [PubMed] [Google Scholar]
- 256.Stein-Streilein J, Watte C. Cross talk among cells promoting anterior chamber-associated immune deviation. Chem Immunol Allergy. 2007;92:115–130. doi: 10.1159/000099262. [DOI] [PubMed] [Google Scholar]
- 257.Poulsen A, Kobayasi T, Secher L, Weismann K. Treponema pallidum in macular and papular secondary syphilitic skin eruptions. Acta dermato-venereologica. 1986;66(3):251–258. [PubMed] [Google Scholar]