Abstract
Background
The effect of the depth of sedation on the function of the autonomic nervous system is not well known.
Objectives
To describe the effect of level of sedation on heart rate variability as a marker of the function of the autonomic nervous system in patients receiving mechanical ventilation.
Methods
This pilot study was part of a larger study in which sedation level was measured continuously for up to 24 hours. The sample consisted of 14 patients receiving mechanical ventilation. The R-R interval was measured continuously via electrocardiography. Sedation level was determined by using the Patient State Index and was categorized as deep (<60) or light (≥60). Continuous heart rate data of 5 to 10 minutes for each sedation level for each patient were analyzed.
Results
Parasympathetic activity as indicated by root mean square of successive difference of the R-R interval, the high-frequency component, and the percentage of differences of successive N-N intervals (intervals due to normal sinus depolarization) that differed more than 50 milliseconds was significantly lower for deep sedation than for light sedation. The markers indicating sympathetic activity, including the low-frequency component and the ratio of the low-frequency component to the high-frequency component, did not differ significantly between the 2 levels of sedation. Most patients were receiving benzodiazepines.
Conclusions
Deep sedation may be associated with depression of parasympathetic function in patients receiving mechanical ventilation. Use of benzodiazepines most likely contributed to this finding.
Maintaining physiological stability is a primary goal of many therapies in the intensive care unit (ICU), including sedation. Critical illness disturbs normal processes, reducing the ability to adapt to physiological changes. The autonomic nervous system has an important role in maintaining physiological stability. In critically ill patients, autonomic function is blunted by pathological conditions such as multiple organ dysfunction syndrome (MODS) and sepsis.1 Changes in autonomic functions may be one factor associated with adverse outcomes in critical illness. For example, in patients with myocardial infarction, increases in sympathetic nervous activity and decreases in parasympathetic nervous activity have been associated with sudden cardiac death.2 Therefore, a more complete understanding of changes in the function of the autonomic nervous system is important.3
Analysis of heart rate variability (HRV) is used to assess autonomic nervous function in both research and clinical settings. Heart rate and rhythm are affected by modulation of sympathetic and parasympathetic tones. The R-R interval varies even in healthy persons, and it has a cyclic pattern determined by both sympathetic and parasympathetic activity. Each cyclic pattern of sympathetic and parasympathetic activity has individual characteristics.
In HRV analysis, use of the P wave on electrocardiograms (ECGs) would be ideal for assessing autonomic function, because the P wave directly reflects sinus node depolarization. However, because the P wave is too small to be precisely and continuously detected without artifact, the R wave is conventionally used in HRV analysis. All of the R-R intervals used should be generated from normal sinus node depolarization; those associated with dysrhythmias such as premature supraventricular contractions and premature ventricular contractions should be excluded from the analysis. The interval due to normal sinus depolarization is therefore called the N-N interval.
Several methods of HRV analysis are available. Two of these, time domain and frequency domain analysis, are well known and widely used.3 Time domain analysis is the simplest analysis. HRV includes fast fluctuation mediated by parasympathetic nervous activity and slow fluctuation mediated by both parasympathetic and sympathetic nervous activities. Differences in successive N-N intervals indicate fast fluctuation, and differences in all N-N intervals from the mean N-N interval indicate slow fluctuation. In time domain analysis, the square root of mean differences in successive N-N intervals squared is called RMSSD (root mean square of successive difference of R-R interval) and reflects activity of the sympathetic nervous system.3 Similarly, the percentage of intervals in which the difference from successive intervals is longer than 50 milliseconds is called pNN50, indicating fast N-N interval change, and similar to RMSSD, reflects activity of the parasympathetic nervous system3 (Table 1).
Table 1.
Variables used and their significancea
| Variable | Unit of measure | Description | Significance |
|---|---|---|---|
| RMSSD | Milliseconds | Square root of the mean of the sum of the squares of differences between adjacent N-N intervals | Approximate correspondence with HF component |
| pNN50 | Percent | Percentage of difference between adjacent N-N intervals >50 ms | Approximate correspondence with HF component |
| HF norm | Normalized units | HF power in normalized units | Parasympathetic activity |
| LF norm | Normalized units | LF power in normalized units | Sympathetic activity |
| LF:HF ratio | Ratio of LF to HF | Sympathovagal balance |
Frequency domain analysis is performed by using (1) power spectral analysis, a nonparametric method that includes fast Fourier transformation, or (2) parametric methods.4 In frequency domain analysis, components of different frequencies as well as the total area of all spectral components are obtained. Spectral components contain ultra low-frequency, very low-frequency, low-frequency (LF), and high-frequency (HF) components. HF and LF components are often expressed in normalized units (nu), which represent the relative value of each power component in proportion to the total power minus the very low-frequency component.3 Using normalized units in HF and LF can minimize the effects of the changes in total power on the values of the LF and HF components.3 Thus, expression in normalized units is recommended, especially when changes in HF and LF components are compared in the same patient.3 HF is thought to reflect parasympathetic nervous activity, whereas LF contains components of both sympathetic and parasympathetic nervous activity.5 LF is a marker of sympathetic modulation, especially when LF is expressed in normalized units.3 The LF:HF ratio reflects sympathetic-parasympathetic nervous balance or sympathetic modulations3 (Table 1). Short-time recordings of 5 minutes are generally used for calculation of RMSSD and pNN50 and for frequency domain analysis.3
Changes in HRV may be associated with cardiac complications in patients with cardiac disease. Some investigators have suggested that reduced HF is a predictive marker for ventricular tachycar-dia6–8 in specific patients, such as patients with coronary disease7 or hypertrophic cardiomyopathy,6 although the data are not consistent. Huikuri et al9 suggested that reduced LF, indicating sympathetic nerve activity, is associated with sustained ventricular tachycardia or cardiac arrest and that HF is not. However, these inconsistent findings may be due to variations in the method of HRV analysis, underlying disease, and types of arrhythmia. Although no evidence of which component (HF or LF) has the strongest association with fatal arrhythmia is conclusive, alterations in autonomic nervous balance are clearly associated with fatal arrhythmia in specific patients.
HRV has been investigated in a variety of patho-physiological conditions in ICU patients,3 and the value of HRV analysis as a diagnostic tool has been recognized. In critical care and postsurgical settings, patients’ outcomes, including mortality in patients with MODS10 and postoperative length of stay,11 are associated with changes in HRV. Also, pathophysio-logical conditions such as MODS,10,12 sepsis,13 and brain death14 are associated with HRV. Compared with values in healthy persons, LF was reduced in patients with MODS,10 and the LF:HF ratio was lower (<1.0) in patients with sepsis.13 These findings suggest that HRV analysis may have diagnostic value in critical care. However, HRV is affected not only by pathophysiological conditions but also by ICU treatments and events such as sedation,15 mechanical ventilation, and stressful stimulation,16,17 which may affect HRV variables and interfere with the results of HRV analysis. Therefore, a more comprehensive understanding of HRV and factors that affect it is essential.
Sedation is a common intervention to provide optimal comfort for critically ill patients receiving mechanical ventilation.18–20 However, sedation may disturb normal autonomic function.21 Although some researchers have investigated the effect of sedative agents on HRV, only a few have attempted to clarify the relationship between the depth of sedation and HRV. Haberthür et al22 found a correlation between the level of sedation (score on the Ramsay Sedation Scale) and HRV in midazolam-sedated patients receiving mechanical ventilation. Unfortunately, the details of the complete HRV analysis were not described. Kanaya et al23 investigated the relationship between HRV and the depth of sedation induced by propofol. They used processed electroencephalography (bispec-tral index) to assess depth of sedation objectively. However, their sample consisted of patients undergoing oral surgery who were breathing spontaneously, not ICU patients who were receiving mechanical ventilation. Generalization of the results of Kanaya et al23 to critically ill patients receiving ventilatory support may not be possible because mechanical ventilation may also affect HRV.10 In summary, little is known about the effect of the depth of sedation on HRV in critically ill patients receiving mechanical ventilation.
If the depth of sedation is associated with changes in autonomic function, management of the depth of sedation may be important in preventing complications related to changes in the autonomic nervous system. The purpose of this pilot study was to describe the effect of sedation depth on autonomic function in ICU patients receiving mechanical ventilation.
Methods
The pilot study reported here was an analysis of data recorded as part of a larger prospective study24 in which sedation level was measured continuously for up to 24 hours in patients in the medical-respiratory and coronary ICUs at Virginia Commonwealth University Health System, Richmond, Virginia. The larger study was an observational study and was approved by the appropriate institutional review board. All patients in the study or their legally authorized representatives provided written informed consent.
Sample
Patients were eligible for the study if they were more than 18 years old, had been endotracheally intubated and receiving mechanical ventilation for more than 2 hours and were estimated to require intubation for at least 8 additional hours, had no scalp or skull abnormalities (from forehead application of electrodes for the monitor used to determine depth of sedation), and were not receiving neuro-muscular blocking agents. Patients were monitored continuously for up to 24 hours for the larger study; selected sections of these data were used in the analysis in the pilot study (see data extraction). Patients who had insufficient data for analysis in this study or who had an irregular heart rate3 were excluded, because HRV analysis should be completed only during normal sinus rhythm, as described earlier. A total of 32 patients were enrolled in the original study. Of these, 18 were excluded from the pilot study; 16 had insufficient data, 1 had an unacceptable irregular heart rate, and 1 had an electronically damaged data file. Thus, data on 14 patients were analyzed in the pilot study.
Measurements
Heart Rate Variability
A Criticare Systems Scholar II monitor system (Criticare Systems, Inc, Waukesha, Wisconsin) was used to measure heart rate continuously. The monitor provides digital readouts of various physiological measures and includes a waveform display. For recording these data, the monitor was linked through a serial connection to a notebook computer, where the numerical values of each parameter were logged, time-stamped, and recorded at regular 1-second intervals. The monitor provided an amplified analog output voltage corresponding to the ECG waveform signal. The ECG signal was sampled and stored at a rate of 1000 samples per second by a 12-bit resolution notebook computer–based data acquisition card (model DAQ 6024E, National Instruments, Austin, Texas). The sampled ECG data were used to determine the peak-to-peak (R-R) interval and HRV. HRV data were retrieved from digitization of the sampled analog ECG signal from the Scholar II analog output. The R-R interval between successive R waves was used to determine the instantaneous R-R interval for computation of HRV. After data cleaning, time and frequency domain analyses were performed by using commercial software (Log-a-Rhythm, version 3.2.1, Nian-Crae, Inc, Somerset, New Jersey). Frequency domain analysis was performed by using the fast Fourier transform method in this software. All artifacts and ectopic beats were removed by using the software. Spectral analysis of HRV shows 2 major components: LF (0.04 to <0.15 Hz) and HF (0.15–0.40 Hz). Values for these 2 components were expressed as normalized units.
Objective Assessment of Sedation
Depth of sedation was assessed by using the Patient State Analyzer (PSA-4000, Physiometrix, North Billerica, Massachusetts) with forehead electrodes. Depth of consciousness and frontal and posterior lead electroencephalography are used to calculate the Patient State Index (PSI) from 4 electroencephalographic waveforms. The PSI formula is constructed by using stepwise discriminate analysis based on multivariate combinations of electroencephalographic variables sensitive to changes in the level of consciousness. PSI values range from 0 (deeply anesthetized) to 100 (awake). The PSI is well correlated with Bispectral Index25 and with scores on the Ramsay Sedation Scale26 and the Richmond Sedation-Agitation Scale.27 The PSA-4000 continually documents and adjusts the PSI by accounting for forehead electromyelographic activity in the algorithm, thus providing a measure of sedation.28
Data Extraction
PSI data were summarized as 15-second means and then categorized into sedation levels. A PSI value less than 60 was defined as deep sedation; a PSI greater than or equal to 60 was defined as light sedation. The 24-hour data for each patient were examined to identify at least 5 minutes of R-R interval data during both light and deep sedation; the first set of data found was selected for analysis. Up to 10 minutes of data was used. If a sample of more than 5 minutes of continuous R-R data during both light and deep sedation could not be found in a patient’s data, the patient’s data were excluded from the study. As described earlier, 16 patients were excluded from the study because of insufficient data. Of these 16 patients, 9 did not have the minimum number of heart rate measurements required, 6 did not have a period of deep sedation longer than 5 minutes, and 1 did not have a period of light sedation longer than 5 minutes. In addition, in order to evaluate the effect of circadian rhythm on HRV,29 the initial time of analysis in each serial data set was divided into 4 groups (midnight to before 6 AM, 6 AM to before noon, noon to before 6 PM, and 6 PM to before midnight) and compared.
Statistical Analysis
The Kolmogorov-Smirnov test was used to verify normal distribution. In nonnormal distributed data, data were expressed as median and 25th and 75th percentiles. Normally distributed data were expressed as means and standard deviations. For comparisons of continuous variables between 2 sedation levels, paired t tests were used for normally distributed variables and the Wilcoxon test for non-normally distributed variables. The data for categorical variables were analyzed by using the Fisher exact test. The level for significance was set at .05. All data were analyzed by using commercial statistical software (JMP 6.0.2, SAS Institute, Cary, North Carolina, and SPSS for Windows 10.0.7J, SPSS Inc, Chicago, Illinois).
Results
Demographic Data
Demographic data for the 14 subjects are shown in Table 2. The most common admission diagnosis was acute respiratory failure, and the mean score on the Acute Physiology and Chronic Health Evaluation II was 28.4 (SD, 6.6). Mean age was 45 years (SD, 16), and the sample was predominantly (79%) women. Mean length of ICU stay at the time of data collection was 3.6 days (SD, 2.3). A total of 12 of the 14 patients were given sedative agents; 7 received midazolam, and 5 received lorazepam. Propofol was used in only 2 of 14 patients. Fentanyl was given to all patients for analgesia during mechanical ventilation; 2 of the 14 were also given morphine or hydromorphone. Median HRV recording time for analysis was 10 minutes (25th, 75th percentiles, 9.75, 10.00). The median HRV recording time for deep sedation did not differ from that for light sedation level: 10.0 minutes (25th, 75th percentiles, 9.7, 10.00) and 10.0 minutes (25th, 75th percentiles, 9.9, 10.00), respectively (P = .50). Most data used in the analysis were collected during the afternoon (Table 3, P=.56).
Table 2.
Characteristics of the sample (n =14)a
| Patient No. | Age, y | Sex | Intensive care unit length of stay, d | Score on Acute Physiology and Chronic Health Evaluation II | Sedative used | Analgesic agent used |
|---|---|---|---|---|---|---|
| 1 | 65 | Female | 4 | 37 | Midazolam | Fentanyl |
| 2 | 37 | Female | 2 | 25 | Propofol | Fentanyl |
| 3 | 69 | Female | 9 | 35 | None | Fentanyl |
| 4 | 57 | Female | 3 | 20 | Midazolam | Fentanyl |
| 5 | 41 | Female | 5 | 17 | Midazolam/dilaudid | Fentanyl/morphine |
| 6 | 35 | Female | 0 | 31 | Midazolam | Fentanyl |
| 7 | 28 | Female | 7 | 19 | None | Fentanyl/hydromorphone |
| 8 | 22 | Male | 5 | 32 | Lorazepam/midazolam | Fentanyl |
| 9 | 38 | Female | 3 | 23 | Lorazepam/midazolam | Fentanyl |
| 10 | 56 | Male | 2 | 30 | Lorazepam | Fentanyl |
| 11 | 56 | Male | 2 | 32 | Lorazepam | Fentanyl |
| 12 | 42 | Female | 3 | 28 | Propofol | Fentanyl |
| 13 | 60 | Female | 2 | 36 | Lorazepam | Fentanyl |
| 14 | 19 | Female | 3 | 33 | Midazolam | Fentanyl |
Patient 6 was admitted because of sepsis; all other patients were admitted because of acute respiratory failure.
Table 3.
Time of onset of data collection for 2 sedation levelsa
| Time | Level of sedation | |
|---|---|---|
| Deep | Light | |
| Midnight to 5 AM | 0 (0) | 1 (4) |
| 6 AM to 11 AM | 4 (29) | 2 (14) |
| Noon to 5 PM | 6 (43) | 8 (57) |
| 6 PM to 11 PM | 4 (29) | 3 (21) |
All values are number of patients (%).
Patient State Index: <60 for deep sedation and ≥60 for light sedation.
PSI and Heart Rate
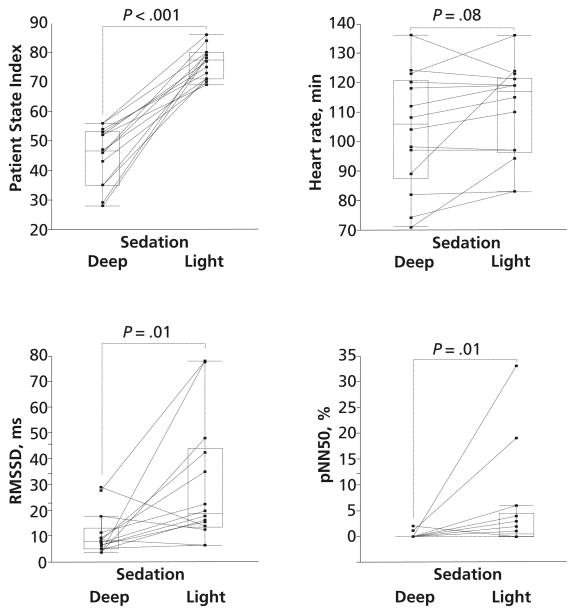
The relationships between PSI and heart rate for deep and light sedation are shown in Figure 1. Median values for PSI were 47 (25th, 75th percentiles, 35, 53) for deep sedation and 78 (25th, 75th percentiles, 71, 80) for light sedation. As expected, PSI differed significantly between deep and light sedation. Differences in heart rate between deep and light sedation were not significant: medians were 106/min (25th, 75th percentiles, 67, 123) for deep sedation and 117/min (25th, 75th percentiles, 96, 121) for light sedation (P = .08).
Figure 1.
Patient State Index, heart rate, square root of the mean of the sum of the squares of differences between adjacent N-N intervals (RMSSD), and percentage of difference between adjacent N-N intervals >50 ms (pNN50) between deep and light sedation. The horizontal lines inside the boxes represent the medians. The boxes encompass the 25th through the 75th percentile, and the error bars indicate the outermost data points other than outliers.
HRV: Time Domain Analysis
RMSSDs and pNN50s, which indicate parasympathetic nerve activity, are shown in Figure 1. Both RMSSD and pNN50 were significantly (both P = .01) lower for deep sedation than for light sedation. Median RMSSD was 7.89 (25th, 75th percentiles, 5.27, 12.9) for deep sedation and 18.7 (25th, 75th percentiles, 13.6, 43.9) for light sedation. Median pNN50 was 0% (25th, 75th percentiles, 0, 0) for deep sedation and 0.5% (25th, 75th percentiles, 0, 4.5) for light sedation.
HRV: Frequency Domain Analysis
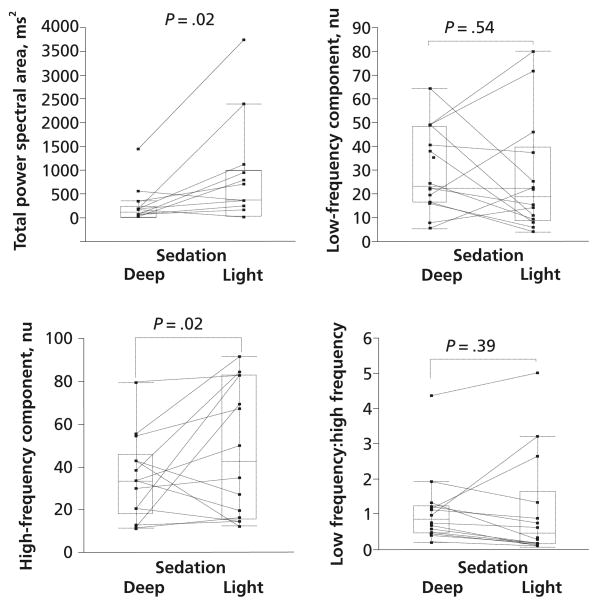
The results of frequency domain analysis are shown in Figure 2. Total power area was significantly (P= .02) lower for deep sedation than for light sedation: medians were 123.4 ms (25th, 75th percentiles, 29.0, 245.8) and 369.1 ms (25th, 75th percentiles, 42.3, 990.1), respectively. LF, a measure of sympathetic nervous activity, did not differ significantly between deep and light sedation: medians were 23.5 (25th, 75th percentiles, 16.7, 48.7) and 18.7 (25th, 75th percentiles, 9.1, 39.8), respectively. HF, a measure of parasympathetic nervous activity, was significantly (P= .02) lower for deep sedation than for light sedation: median values were 33.4 nu (25th, 75th percentiles, 18.4, 45.9) and 42.5 nu (25th, 75th percentiles, 15.6, 82.8), respectively. The LF:HF ratio, a measure of sympathovagal balance, did not differ significantly between deep and light sedation: medians were 0.86 (25th, 75th percentiles, 0.47,1.24) and 0.48 (25th, 75th percentiles, 0.17, 1.65), respectively.
Figure 2.
Total power spectral area, low-frequency component expressed in normalized units (nu), high-frequency component expressed in nu, and low frequency:high frequency ratio between deep and light sedation. The horizontal lines inside the boxes represent the medians. The boxes encompass the 25th through the 75th percentile, and the error bars indicate the outermost data points other than outliers.
Discussion
We think this study is the first one on the relationship between HRV and an objective assessment of the depth of sedation in ICU patients receiving mechanical ventilation. On the basis of age, severity of illness, and admitting diagnosis, the sample was representative of typical medical ICU patients who receive mechanical ventilation. The main findings are that deep sedation was associated with depressed parasympathetic nervous activity and that measures of sympathetic nervous activity and heart rate did not change with changes in the depth of sedation. It is not surprising that reduced parasympathetic activity did not necessarily lead to increases in heart rate. Changes in heart rate do not always accompany changes in HRV in patients during anesthesia.23 Kanaya et al23 speculated that the ability of the end organ to respond appropriately to neural regulation is influenced by anesthetics and contributes to a lack of correlation between heart rate and the results of HRV analysis.
Effect of Sedative/Analgesic Agents
In our study, several measures of HRV (HF, RMSSD, and pNN50) were reduced in deep sedation. These indexes are markers for the level of parasympathetic nervous activity.30 Several researchers31–33 have suggested that midazolam, a benzodiazepine, depresses parasympathetic nervous activity. Galletly et al33 found that the percentage of the HF component, which indicates parasympathetic nervous activity, was decreased by midazo-lam in healthy volunteers. Similarly, in spontaneously breathing patients undergoing dental implantation, Win et al32 found that midazolam decreased the percentage of the HF component but did not change the LF:HF ratio and stated that midazolam depressed parasympathetic nervous activity without sympathetic nervous activation. Furthermore, in patients who underwent a gastroscopy, Agelink et al31 found that midazolam decreased HF and increased the LF:HF ratio and concluded that midazolam could reduce parasympathetic nervous activity. Another benzodiazepine, lorazepam, also depressed parasympathetic nervous activity.31 These findings are consistent with our findings that HF decreased during deep sedation, suggesting depressed parasympathetic nervous activity.
In contrast to the benzodiazepines, propofol induces cardiovascular depression by depressing sympathetic nervous activity.34,35 Ebert34 found that propofol reduced sympathetic activity, as reflected by the serum level of norepinephrine, and that the results of microneurography correlated with the level of sedation. Although midazolam, propofol, and lorazepam were all used for sedation in our study, the most commonly used agent was midazo-lam. Therefore, HRV may have been affected by midazolam and lorazepam, but we cannot make any firm conclusions about sedative drugs because we did not conduct a controlled study.
Fentanyl was used in all patients in our study. Fentanyl has vagomimetic properties,36 increasing vagal tone, but few data on the effect of fentanyl on HRV are available. Because all of our patients received fentanyl, the drug should have activated the parasympathetic nervous system. However, our results indicate that parasympathetic nervous activity decreased during deep sedation. We speculate that the effect of fentanyl may have been too small to overcome the parasympathetic function depressed by benzodiazepines.
Effects of Respiratory States
Respiratory status also affects HRV,37–39 because deep sedation depresses the ventilatory response to PaCO2.40,41 In our study, respiratory rate possibly was decreased and PaCO2 was increased during deep sedation because deep sedation generally depresses the ventilatory response to PaCO240,41; however, we did not obtain data from blood gas analysis. Pöyhönen et al39 showed that an increase in PaCO2 did not change HRV (LF:HF ratio, normalized HF, and LF); thus, a change in PaCO2 probably did not affect our results. However, in anesthetized patients with constant PaCO2, decreases in respiratory rate can further decrease HF, which is a marker of parasympathetic nervous activity.39 Therefore, the decrease in respiratory rate during deep sedation in our patients might be associated with the decrease in HF, but we did not obtain actual respiratory rate data.
Effect of Critical Illness and Circadian Rhythm
Autonomic dysfunction is influenced by non-cardiogenic pathological conditions such as sepsis and MODS.1,10,21 Korach et al13 reported that a reduced LF:HF ratio, indicating low sympathetic tone, was a characteristic of patients with sepsis. In patients with MODS, Schmidt et al10 found that LF and the LF:HF ratio were lower than normal values and suggested that autonomic function was blunted by MODS. Other than in patients with sepsis and MODS, a progressive decrease in LF correlated with severity of illness as determined by scores on the Acute Physiology and Chronic Health Evaluation II in ICU patients.42 These investigations suggest that patients’ status may influence HRV variables, but the notion is not applicable in our study because of the design; our patients were compared with themselves.
HRV is influenced by circadian rhythm. During the night, sympathetic nervous activity decreases and parasympathetic nervous activity increases.43 However, circadian rhythm most likely did not affect our findings, because most data were collected during a similar time of day.
Clinical Implications
Our findings indicate that deep sedation is associated with depressed parasympathetic nervous activity in patients who are receiving mechanical ventilation. Currently, HRV is not routinely measured in the ICU; however, such measurements might be useful in some situations.
In our study, parasympathetic nervous system activity decreased during deep sedation. As described in the “Background” section, depressed parasympathetic nervous system activity during deep sedation may be associated with cardiac complications in patients with cardiac disease, although none of our patients had such complications. Investigation of the processes by which depressed parasympathetic nervous system activity during deep sedation results in cardiac complications is therefore warranted. In addition, investigation of the occurrence of fatal arrhythmias during deep and light sedation is needed.
Depression of the parasympathetic nervous system may affect blood pressure by reducing the ability to adapt rapidly to stressful conditions. This depressed parasympathetic nervous system could be a cause of unstable hemodynamic status during deep sedation.
With a greater understanding of HRV and its effects, continuous HRV monitoring might be useful as an index for the depth of sedation and comfort for ICU patients. Although data establishing HRV as a marker of the depth of sedation22 or agitation44 in ICU patients are limited, investigation of the effects of specific sedative agents on HRV is continuing. Because changes in HRV are influenced by sedative agents, pathophysiological conditions, other drugs, and posture,45 further study of these influences on HRV is needed. In the future, HRV may also be used to detect response to acute stress, such as surgical procedures, and would be especially helpful in patients receiving muscle relaxants. Stressful procedures such as endotracheal intubation16 and surgical incisions17 increased LF, indicating sympathetic tone activation. Eventually, HRV analysis may be helpful in pain assessment in non-communicative patients.46 Further study is needed to clarify practical applications of HRV analysis, including the assessment of the results of patients’ stressful experiences.
Use of HRV analysis in routine clinical practice requires the development of simple methods and devices to measure HRV. First, however, the many factors that potentially affect HRV must be clarified.
Limitations
Our study had some limitations. First, it was a secondary analysis of data from a larger study. Some conditions such as underlying illness may affect HRV3; however, those effects on our findings were probably relatively small. Nevertheless, some short-time changeable factors such as respiratory rate, including mechanical ventilation settings, and body posture that possibly affect HRV could have been confounding factors in our study. And some drugs other than sedatives that our patients used might have affected our findings.
In addition, we could not conduct subgroup analysis for different sedatives because of the small sample size. Taking into account these limitations, we emphasize that our findings are not conclusive, and we cannot provide clinical recommendations. Confirmation of our findings is needed.
Acknowledgments
We thank Dr Nobuko Okubo for critical appraisal of the manuscript. This research was done at Virginia Commonwealth University Health System.
Footnotes
To purchase electronic or print reprints, contact The InnoVision Group, 101 Columbia, Aliso Viejo, CA 92656. Phone, (800) 809-2273 or (949) 362-2050 (ext 532); fax, (949) 362-2049; reprints@aacn.org.
eLetters
Now that you’ve read the article, create or contribute to an online discussion on this topic. Visit www.ajcconline.org and click “Respond to This Article” in either the full-text or PDF view of the article.
FINANCIAL DISCLOSURES
This study was supported by Physiometrix Inc, North Billerica, Massachusetts, and grant F31-NR009623 from the National Institute of Nursing Research to Karen Mellott.
References
- 1.Schmidt HB, Werdan K, Müller-Werdan U. Autonomic dysfunction in the ICU patient. Curr Opin Crit Care. 2001;7(5):314–322. doi: 10.1097/00075198-200110000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Malliani A, Lombardi F, Pagani M, Cerutti S. Power spectral analysis of cardiovascular variability in patients at risk for sudden cardiac death. J Cardiovasc Electrophysiol. 1994;5(3):274–286. doi: 10.1111/j.1540-8167.1994.tb01164.x. [DOI] [PubMed] [Google Scholar]
- 3.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17(3):354–381. [PubMed] [Google Scholar]
- 4.Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly. 2004;134(35–36):514–522. doi: 10.4414/smw.2004.10321. [DOI] [PubMed] [Google Scholar]
- 5.Pomeranz B, Macaulay RJ, Caudill MA, et al. Assessment of autonomic function in humans by heart rate spectral analysis. Am J Physiol. 1985;248(pt 1):H151–H153. doi: 10.1152/ajpheart.1985.248.1.H151. [DOI] [PubMed] [Google Scholar]
- 6.Uemura S, Tomoda Y, Fujimoto S, et al. Heart rate variability and ventricular arrhythmia in clinically stable patients with hypertrophic cardiomyopathy. Jpn Circ J. 1997;61(10):819–826. doi: 10.1253/jcj.61.819. [DOI] [PubMed] [Google Scholar]
- 7.Pruvot E, Thonet G, Vesin JM, et al. Heart rate dynamics at the onset of ventricular tachyarrhythmias as retrieved from implantable cardioverter-defibrillators in patients with coronary artery disease. Circulation. 2000;101(20):2398–2404. doi: 10.1161/01.cir.101.20.2398. [DOI] [PubMed] [Google Scholar]
- 8.Fei L, Statters DJ, Hnatkova K, Poloniecki J, Malik M, Camm AJ. Change of autonomic influence on the heart immediately before the onset of spontaneous idiopathic ventricular tachycardia. J Am Coll Cardiol. 1994;24(6):1515–1522. doi: 10.1016/0735-1097(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 9.Huikuri HV, Koistinen MJ, Yli-Mäyry S, et al. Impaired low-frequency oscillations of heart rate in patients with prior acute myocardial infarction and life-threatening arrhyth-mias. Am J Cardiol. 1995;76(1):56–60. doi: 10.1016/s0002-9149(99)80801-7. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt H, Müller-Werdan U, Hoffmann T, et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit Care Med. 2005;33(9):1994–2002. doi: 10.1097/01.ccm.0000178181.91250.99. [DOI] [PubMed] [Google Scholar]
- 11.Stein PK, Schmieg RE, Jr, El-Fouly A, Domitrovich PP, Buch-man TG. Association between heart rate variability recorded on postoperative day 1 and length of stay in abdominal aortic surgery patients. Crit Care Med. 2001;29:1738–1743. doi: 10.1097/00003246-200109000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Pontet J, Contreras P, Curbelo A, et al. Heart rate variability as early marker of multiple organ dysfunction syndrome in septic patients. J Crit Care. 2003;18(3):156–163. doi: 10.1016/j.jcrc.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Korach M, Sharshar T, Jarrin I, et al. Cardiac variability in critically ill adults: influence of sepsis. Crit Care Med. 2001;29(7):1380–1385. doi: 10.1097/00003246-200107000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Baillard C, Vivien B, Mansier P, et al. Brain death assessment using instant spectral analysis of heart rate variability. Crit Care Med. 2002;30(2):306–310. doi: 10.1097/00003246-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 15.O’Flaherty D. Heart rate variability and anaesthesia. Eur J Anaesthesiol. 1993;10:419–432. [PubMed] [Google Scholar]
- 16.Nishiyama T, Misawa K, Yokoyama T, Hanaoka K. Effects of combining midazolam and barbiturate on the response to tracheal intubation: changes in autonomic nervous system. J Clin Anesth. 2002;14(5):344–348. doi: 10.1016/s0952-8180(02)00370-7. [DOI] [PubMed] [Google Scholar]
- 17.Latson TW, O’Flaherty D. Effects of surgical stimulation on autonomic reflex function: assessment by changes in heart rate variability. Br J Anaesth. 1993;70(3):301–305. doi: 10.1093/bja/70.3.301. [DOI] [PubMed] [Google Scholar]
- 18.Kress JP, Hall JB. Sedation in the mechanically ventilated patient. Crit Care Med. 2006;34:2541–2546. doi: 10.1097/01.CCM.0000239117.39890.E3. [DOI] [PubMed] [Google Scholar]
- 19.Hogarth DK, Hall J. Management of sedation in mechanically ventilated patients. Curr Opin Crit Care. 2004;10:40–46. doi: 10.1097/00075198-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Sessler CN, Grap MJ, Brophy GM. Multidisciplinary management of sedation and analgesia in critical care. Semin Respir Crit Care Med. 2001;22:211–226. doi: 10.1055/s-2001-13834. [DOI] [PubMed] [Google Scholar]
- 21.Buchman TG, Stein PK, Goldstein B. Heart rate variability in critical illness and critical care. Curr Opin Crit Care. 2002;8:311–315. doi: 10.1097/00075198-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Haberthür C, Lehmann F, Ritz R. Assessment of depth of midazolam sedation using objective parameters. Intensive Care Med. 1996;22(12):1385–1390. doi: 10.1007/BF01709555. [DOI] [PubMed] [Google Scholar]
- 23.Kanaya N, Hirata N, Kurosawa S, Nakayama M, Namiki A. Differential effects of propofol and sevoflurane on heart rate variability. Anesthesiology. 2003;98:34–40. doi: 10.1097/00000542-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Grap MJ, Sessler CN, Best AM, et al. Physiologic stability and patients’ comfort during varying levels of sedation in adults receiving mechanical ventilation [abstract] Am J Crit Care. 2006;15(3):334. [Google Scholar]
- 25.Chen X, Tang J, White PF, et al. A comparison of Patient State Index and Bispectral Index values during the perioperative period. Anesth Analg. 2002;95(6):1669–1674. doi: 10.1097/00000539-200212000-00036. [DOI] [PubMed] [Google Scholar]
- 26.Schneider G, Heglmeier S, Schneider J, Tempel G, Kochs EF. Patient State Index (PSI) measures depth of sedation in intensive care patients. Intensive Care Med. 2004;30:213–216. doi: 10.1007/s00134-003-2092-5. [DOI] [PubMed] [Google Scholar]
- 27.Sessler CN, Kollef M, Hamilton A, Grap MJ. Comparison of depth of sedation measured by PSA 4000 and Richmond Agitation-Sedation Scale (RASS) [abstract] Chest. 2005;128:151S. [Google Scholar]
- 28.Jacobsohn E, De Wet C, Tymkew H, et al. Use of the Patient State Index (PSI) to assist in the diagnosis of perioperative neurological injury and brain death. J Clin Monit Comput. 2005;19(3):219–222. doi: 10.1007/s10877-005-3546-9. [DOI] [PubMed] [Google Scholar]
- 29.Bilan A, Witczak A, Palusinski R, Myslinski W, Hanzlik J. Circadian rhythm of spectral indices of heart rate variability in healthy subjects. J Electrocardiol. 2005;38:239–243. doi: 10.1016/j.jelectrocard.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Lombardi F. Clinical implications of present physiological understanding of HRV components. Card Electrophysiol Rev. 2002;6:245–249. doi: 10.1023/a:1016329008921. [DOI] [PubMed] [Google Scholar]
- 31.Agelink MW, Majewski TB, Andrich J, Mueck-Weymann M. Short-term effects of intravenous benzodiazepines on autonomic neurocardiac regulation in humans: a comparison between midazolam, diazepam, and lorazepam. Crit Care Med. 2002;30:997–1006. doi: 10.1097/00003246-200205000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Win NN, Fukayama H, Kohase H, Umino M. The different effects of intravenous propofol and midazolam sedation on hemodynamic and heart rate variability. Anesth Analg. 2005;101:97–102. doi: 10.1213/01.ANE.0000156204.89879.5C. [DOI] [PubMed] [Google Scholar]
- 33.Galletly DC, Williams TB, Robinson BJ. Periodic cardiovascular and ventilatory activity during midazolam sedation. Br J Anaesth. 1996;76:503–507. doi: 10.1093/bja/76.4.503. [DOI] [PubMed] [Google Scholar]
- 34.Ebert TJ. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesi-ology. 2005;103:20–24. doi: 10.1097/00000542-200507000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Robinson BJ, Ebert TJ, O’Brien TJ, Colinco MD, Muzi M. Mechanisms whereby propofol mediates peripheral vasodi-lation in humans: sympathoinhibition or direct vascular relaxation? Anesthesiology. 1997;86:64–72. doi: 10.1097/00000542-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Bowdle TA. Adverse effects of opioid agonists and agonist-antagonists in anaesthesia. Drug Saf. 1998;19(3):173–189. doi: 10.2165/00002018-199819030-00002. [DOI] [PubMed] [Google Scholar]
- 37.Eckberg DL. Human sinus arrhythmia as an index of vagal cardiac outflow. J Appl Physiol. 1983;54:961–966. doi: 10.1152/jappl.1983.54.4.961. [DOI] [PubMed] [Google Scholar]
- 38.Brown TE, Beightol LA, Koh J, Eckberg DL. Important influence of respiration on human R-R interval power spectra is largely ignored. J Appl Physiol. 1993;75:2310–2317. doi: 10.1152/jappl.1993.75.5.2310. [DOI] [PubMed] [Google Scholar]
- 39.Pöyhönen M, Syväoja S, Hartikainen J, Ruokonen E, Takala J. The effect of carbon dioxide, respiratory rate and tidal volume on human heart rate variability. Acta Anaesthesiol Scand. 2004;48:93–101. doi: 10.1111/j.1399-6576.2004.00272.x. [DOI] [PubMed] [Google Scholar]
- 40.Forster A, Gardaz JP, Suter PM, Gemperle M. Respiratory depression by midazolam and diazepam. Anesthesiology. 1980;53:494–497. doi: 10.1097/00000542-198012000-00010. [DOI] [PubMed] [Google Scholar]
- 41.Lumb BL. Nunn’s Applied Respiratory Physiology. 5. Boston, MA: Butterworth-Heinemann; 2000. Anaesthesia; pp. 420–459. [Google Scholar]
- 42.Yien HW, Hseu SS, Lee LC, et al. Spectral analysis of systemic arterial pressure and heart rate signals as a prognostic tool for the prediction of patient outcome in the intensive care unit. Crit Care Med. 1997;25:258–266. doi: 10.1097/00003246-199702000-00011. [DOI] [PubMed] [Google Scholar]
- 43.Furlan R, Guzzetti S, Crivellaro W, et al. Continuous 24-hour assessment of the neural regulation of systemic arterial pressure and RR variabilities in ambulant subjects. Circulation. 1990;81(2):537–547. doi: 10.1161/01.cir.81.2.537. [DOI] [PubMed] [Google Scholar]
- 44.Chase JG, Agogue F, Starfinger C, et al. Quantifying agitation in sedated ICU patients using digital imaging. Comput Methods Programs Biomed. 2004;76(2):131–141. doi: 10.1016/j.cmpb.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Chen GY, Kuo CD. The effect of the lateral decubitus position on vagal tone. Anaesthesia. 1997;52:653–657. doi: 10.1111/j.1365-2044.1997.114-az0106.x. [DOI] [PubMed] [Google Scholar]
- 46.Li D, Puntillo K. What is the current evidence on pain and sedation assessment in nonresponsive patients in the intensive care unit? Crit Care Nurse. 2004;24(5):68, 70, 72–73. [PubMed] [Google Scholar]