Abstract
Background
Epigenetic features such as DNA hypomethylation have been associated with conditions related to cardiovascular risk. We evaluated whether lower blood DNA methylation in heavily methylated repetitive sequences predicts the risk of ischemic heart disease and stroke.
Methods
We quantified blood DNA methylation of LINE-1 repetitive elements through PCR-pyrosequencing in 712 elderly individuals from the Boston-area Normative Aging Study. We estimated risk-factor adjusted relative risks (RRs) for ischemic heart disease and stroke at baseline (242 prevalent cases); as well as in incidence (44 new cases; median follow-up, 63 months); and subsequent mortality from ischemic heart disease (86 deaths; median follow-up, 75 months).
Results
Blood LINE-1 hypomethylation was associated with baseline ischemic heart disease (RR=2.1 [95% confidence interval = 1.2 to 4.0] for lowest vs. highest methylation quartile) and for stroke (2.5 [0.9 to 7.5]). Among participants free of baseline disease, individuals with methylation below the median also had higher risk of developing ischemic heart disease (4.0 [1.8 to 8.9]) or stroke (5.7 [0.8 to 39.5]). In the entire cohort, persons with methylation below the median had higher mortality from ischemic heart disease (3.3 [1.3 to 8.4]) and stroke (2.8 [0.6 to 14.3]). Total mortality was also increased (2.0 [1.2 to 3.3]). These results were confirmed in additional regression models using LINE-1 methylation as a continuous variable.
Conclusions
Subjects with prevalent IHD and stroke exhibited lower LINE-1 methylation. In longitudinal analyses, persons with lower LINE-1 methylation were at higher risk for incident ischemic heart disease and stroke, and for total mortality.
Epigenetics is related to stable and heritable patterns of gene expression and genome function that do not involve changes in DNA sequence.1 DNA methylation is a reversible epigenetic mechanism that, in mammals, modifies genome function through the addition of methyl groups to cytosine to form 5-methyl-cytosine (5mC). About 55% of the human genome consists of repetitive elements, including approximately 500,000 Long Interspersed Nucleotide Elements (LINE-1, also indicated as L1), which are heavily methylated.2 Because of their high representation throughout the genome, LINE-1 methylation correlates with global genomic DNA methylation content.2,3 Demethylation of LINE-1 elements increases their activity as retrotransposable sequences, which may induce genomic alterations by insertion and or homologous recombination,4,5 and deregulate gene transcription.6
A global loss of DNA methylation has been related to atherosclerosis7 – a chronic disease of large and medium-sized arteries associated with cardiovascular morbidity and mortality – in a variety of tissues, including smooth-muscle cells,8,9 atherosclerotic lesions,10 and peripheral blood leukocytes.11 Global DNA hypomethylation has been shown to precede the formation of atherosclerosis in Apoe-/- mice,12 and associated with hyperhomocysteinemia and aortic lipid deposition in mutant mice deficient in methylenetetrahydrofolate reductase.13 In human investigations, although the range of variation in healthy subjects is relatively narrow, LINE-1 methylation or global genomic methylation content measured in blood DNA has been found to be lower in people with risk factors for atherosclerotic disease, such as older age,14,15 smoking,16 folate deficiency,17 and hyperhomocysteinemia.11 In a recent investigation, we showed that short-term exposure to air pollution from vehicular traffic (an environmental risk factor for cardiovascular disease) was associated with lower methylation of LINE-1, but not of Alu elements in peripheral blood leukocytes.18
Castro et al.11 have found that higher plasma homocysteine, an established predictor of cardiovascular risk, and atherosclerotic disease were associated with reduced global methylation in blood leukocyte DNA. Using vascular smooth cells in culture, Yi-deng et al.9 observed hypomethylation of LINE-1 and Alu elements under a high homocysteine concentration. Dietary B-complex vitamins, such as folate, vitamin B6 and B12 , participating in the one-carbon metabolic cycle that makes methyl donors available for DNA methylation, have the capacity to lower the circulating levels of homocysteine, prevent DNA methylation loss, and reduce coronary plaque regression.19,20 A recent meta-analysis of randomized controlled trials, however, showed that B-complex vitamins supplementation failed to prevent cardiovascular events.21
Zaina et al.22 have proposed that DNA hypomethylation detected in advanced atherosclerotic plaques might represent a passive phenomenon due to loss of methyl groups in highly-proliferating smooth muscle cells, whereas peripheral blood hypomethylation might reflect hyperproliferation of cell types involved in immune or inflammatory responses. In that respect, plaque formation, which is a clonal process, would parallel the global hypomethylation found both in tumor tissues and blood DNA in patients with cancer, which also is characterized by proliferating cells with clonal origin accompanied by systemic inflammation.7
Taken together these findings suggest that blood DNA hypomethylation represent an easily measurable marker reflecting the presence and the progression of atherosclerosis. Because atherosclerotic lesions often precede the clinical manifestation of ischemic cardiovascular diseases, such as heart disease and stroke, blood DNA hypomethylation might be used to identify persons at risk of cardiovascular events.
In the present study on a population of elderly individuals in the greater Boston area, we evaluated whether lower blood LINE-1 methylation predicted increased risk and mortality from ischemic heart disease and stroke. In particular, we explored the association of lower LINE-1 DNA methylation with (1) prevalence of ischemic heart disease and stroke at baseline; (2) risk of developing ischemic heart disease and stroke among subjects who were free of baseline disease; and (3) risk of death from ischemic heart disease and stroke in the entire study population.
Methods
Study Subjects
The study included 712 elderly men who, as of 1 March 1999, were active participants in the Normative Aging Study, a longitudinal investigation of aging established in 1963 by the U.S. Veterans Administration.23 Participants have had comprehensive clinical examinations at 3- to 5-year intervals, with excellent rates of continued participation (<1% annual attrition for all causes). Examination procedures and methods for collection of clinical information and physical parameters have been described previously.24,25
Starting in March 1999, the Normative Aging Study protocol was updated to include collection of a blood sample for DNA extraction at each of the visits. Of the 805 active participants, 723 (90%) agreed to donate blood samples that were used for DNA methylation analysis. This analysis was unsuccessful on 11 subjects (1%), leaving 712 participants (age 55-92 years; mean = 72.3 years). The examination at which a DNA sample was first obtained was considered the baseline examination. Because study participants are called for examination on a rolling cycle of 3-5 years and some of the subjects donated a blood DNA sample only at the second request, the baseline date ranged between 1 March and 31 October 2007. The study was approved by the Institutional Review Boards of all participating Institutions. All participants gave written informed consent.
Diagnoses of IHD and Stroke
All subjects were evaluated at the baseline examination for preexisting cardiovascular disease (including nonfatal ischemic heart disease or stroke) based on medical records and physician exams from the current or past study visits.26 Hospital records and any other clinical documentation for every report of cardiovascular event were reviewed by a board-certified cardiologist who was unaware of the participant's DNA methylation results. Myocardial infarction was defined by: 1) unequivocal electrocardiographic changes (i.e., pathologic Q waves); or 2) diagnostic increases in serum enzymes (glutamic-oxaloacetic transaminase and lactic dehydrogenase) together with chest discomfort consistent with myocardial infarction. Angina was defined by Framingham Heart Study criteria as recurrent chest discomfort lasting no more than 15 min, distinctly related to exertion or excitement and relieved by rest or nitroglycerin.26 Nonfatal stroke was defined as neurological deficit of sudden or rapid onset that persisted for at least 24h. In total, we identified 212 (30%) participants with ischemic heart disease and 51 (7%) with stroke at baseline. Twenty-one (3%) participants had both baseline ischemic heart disease and stroke, with the remaining 470 (66%) free of ischemic heart disease and stroke at baseline.
Incidence Follow-up
Of the 470 participants free of the baseline diseases, 356 (76%) had follow-up visits by 31 October 2007, during which new occurrences of non-fatal ischemic heart disease or stroke were assessed using the same criteria as at baseline. Thirty-four (7%) of participants who did not have follow-up examinations had died before the date of the next scheduled visit. An additional 33 participants (7%) had a follow-up visit scheduled after 31 October 2007 and were not included in the present analysis. The remaining 47 subjects lost to follow-up (10%) did not report for a scheduled visit because they had moved out of state, were too ill to attend, or were untraceable. Median follow-up was 63 months (min=26, max=98), for 21,225 months of total analysis time-at-risk.
Mortality follow-up
Regular mailings to study participants were used to maintain vital-status information, and death certificates were obtained for decedents. Medical records in each instance of ischemic heart disease or stroke death were reviewed by a board-certified cardiologist to ensure accurate classification of primary causes of death. Mortality follow-up was available for 705 (99%) of the 712 study participants. Median mortality follow-up was 75 months (min=1, max=100), for 50,258 months of total analysis time at risk.
DNA Methylation Analysis of LINE-1 Repetitive Elements
7 ml of whole blood was collected by venous phlebotomy in EDTA tubes. Buffy coat was extracted and stored in cell lyses solution until DNA extraction. All samples were coded and frozen at −20°C. DNA was extracted using the QiAmp DNA blood kits (QIAGEN). LINE-1 methylation analysis was performed on all samples between May and December 2007 using previously published methods.27 Briefly, buffy coat DNA from whole blood was bisulfite-treated using EZ-DNA Methylation-Gold™ Kits (Zymo Research, Orange, CA). Pyrosequencing was performed using previously described primers and conditions2,27 with the following modifications: a 50 μl PCR was carried out in 25 μl GoTaq Green Master mix (Promega, Madison, WI), 1 pmol biotinylated forward primer, 1 pmol reverse primer, 50 ng bisulfite-treated genomic DNA and water. The Pyrosequencing assay is a method that quantitatively assesses the proportion of methylated sites in LINE-1 repetitive elements dispersed throughout the genome. Each sample was tested in two replicates. DNA methylation was expressed as % 5-methylated cytosines (%5mC) over total (methylated + unmethylated) cytosines.
Statistical Analysis
Odds ratios (ORs) and 95% confidence intervals (CIs) for the association between LINE-1 methylation and prevalent ischemic heart disease or stroke at baseline were computed using unconditional logistic models. Cumulative rates for new cardiovascular events or survival were estimated using the Kaplan-Meier method, and association with LINE-1 methylation was estimated using Hazard Ratios (HRs) and 95%CIs from Cox-proportional hazard regression models. We considered the proportional hazards assumption to be valid after inspection of plots of survival estimates by LINE-1 methylation levels. Mortality analysis, which included subjects with and without ischemic heart disease or stroke at baseline, used Cox regression with baseline ischemic heart disease or stroke as stratification variables, allowing a different baseline hazard curve in each stratum.
For all models, we report unadjusted results as well as results adjusted for established predictors of cardiovascular disease and survival. Adjustment variables included age, body mass index, smoking history (ever, pack-years), alcohol drinking (two drinks/day or more), diagnosis of hypertension, diastolic blood pressure, systolic blood pressure, diabetes (physician diagnosis or fasting blood glucose>126mg/dl), serum HDL, serum total cholesterol, statin use, plasma homocysteine, glomerular filtration rate, as well as percent neutrophils and lymphocytes in the differential white blood count. All analyses were performed in Stata 9.0 (Stata Corp., College Station, TX).
Results
Baseline Characteristics of the Study Participant
The baseline characteristics of the study subjects are shown in Table 1. Compared with participants without ischemic heart disease or stroke, individuals with ischemic heart disease or stroke were older, had higher cumulative smoking, higher prevalence of hypertension and diabetes, higher plasma homocysteine, and lower HDL cholesterol and glomerular filtration rate. Participants with ischemic heart disease or stroke had lower diastolic blood pressure, and more frequent use of antihypertensive medications and statins. Participants with ischemic heart disease or stroke had higher percent neutrophils and lower percent lymphocytes in the differential white blood count.
Table 1. Baseline characteristics and risk factorsa of the Normative Aging Study participants according to baseline diagnosis of ischemic heart disease or stroke.
| No Ischemic Heart Disease or Stroke (n = 470) | Ischemic Heart Disease (n = 212) | Stroke (n = 51) | Ischemic Heart Disease or Stroke (n = 242)b | |||||
|---|---|---|---|---|---|---|---|---|
| Age (years) | 71.4 | (6.7) | 73.9 | (6.3) | 76.5 | (6.7) | 74.1 | (6.6) |
| Body Mass Index (kg/m2) | 28.1 | (4.0) | 28.4 | (4.1) | 28.5 | (4.2) | 28.4 | (4.1) |
| Ever smokers; No. (%) | 326 | (70) | 150 | (71) | 30 | (60) | 163 | (67) |
| Cumulative smoking (pack-yr) | 29.3 | (27.4) | 34.9 | (28.4) | 36.0 | (25.8) | 34.9 | (28.1) |
| Alcohol drinkers (>2 drinks/day); No. (%) | 86 | (18) | 40 | (19) | 12 | (24) | 45 | (19) |
| Hypertension; No. (%) | 286 | (61) | 193 | (91) | 42 | (82) | 216 | (89) |
| Antihypertensive treatment; No. (%) | 204 | (43) | 184 | (87) | 38 | (75) | 204 | (84) |
| Blood pressure | ||||||||
| Systolic (mmHg) | 133.3 | (16.7) | 131.6 | (19.0) | 130.1 | (17.9) | 132.0 | (18.6) |
| Diastolic (mmHg) | 79.3 | (8.7) | 75.3 | (9.6) | 74.5 | (10.6) | 75.7 | (9.6) |
| Diabetes; No. (%) | 72 | (15) | 53 | (25) | 14 | (28) | 62 | (26) |
| Statin use; No. (%) | 97 | (21) | 133 | (63) | 21 | (43) | 144 | (60) |
| Serum total cholesterol (mg/dL) | 267.5 | (140.4) | 260.2 | (156.5) | 263.7 | (135.0) | 261.9 | (156.0) |
| Serum HDL (mg/dL) | 51.0 | (13.9) | 45.8 | (10.5) | 46.3 | (12.2) | 45.9 | (10.6) |
| Total homocysteine (nmol/mL) | 10.6 | (4.3) | 11.4 | (3.7) | 12.4 | (3.9) | 11.4 | (3.6) |
| Glomerular filtration rate (mL/min) | 73.9 | (15.5) | 66.2 | (16.8) | 61.6 | (18.3) | 66.1 | (15.5) |
| White blood cell count (n/μL) | 6,545 | (3,695) | 6,742 | (2,660) | 6,655 | (1,523) | 6,710 | (2,533) |
| Neutrophils (%) | 61.6 | (8.8) | 62.8 | (8.9) | 67.0 | (8.8) | 63.3 | (8.8) |
| Lymphocytes (%) | 26.2 | (8.3) | 24.7 | (8.0) | 21.5 | (6.1) | 24.3 | (7.8) |
| Monocytes (%) | 8.7 | (2.1) | 8.7 | (2.0) | 8.4 | (1.6) | 8.7 | (2.0) |
| Eosinophils (%) | 3.2 | (2.1) | 3.5 | (2.1) | 2.9 | (2.2) | 3.4 | (2.2) |
| Basophils (%) | 0.5 | (0.5) | 0.6 | (0.5) | 0.6 | (0.5) | 0.6 | (0.5) |
| C-reactive protein (mg/L)c | 3.5 | (11.6) | 4.4 | (7.0) | 7.0 | (1.8) | 4.6 | (7.0) |
| LINE-1 methylation (%5mC, continuous) | 77.4 | (2.0) | 76.9 | (2.1) | 76.8 | (2.3) | 76.9 | (2.1) |
| LINE-1 methylation (%5mC, by quartiles) | ||||||||
| 86.3-78.8 | 117 | (24.9) | 33 | (15.6) | 8 | (15.7) | 39 | (16.1) |
| 78.7-77.5 | 119 | (25.3) | 49 | (23.1) | 9 | (17.6) | 53 | (21.9) |
| 77.4-76.2 | 117 | (24.9) | 62 | (29.2) | 15 | (29.4) | 71 | (29.3) |
| 76.1-68.1 | 117 | (25.3) | 68 | (32.1) | 19 | (37.3) | 79 | (32.6) |
Mean (SD) unless otherwise indicated.
At baseline, 21 study participants had both ischemic heart disease and stroke. Therefore, the total number of individuals with ischemic heart disease and stroke at baseline is lower than the sum of ischemic heart disease and stroke.
C-reactive protein was measured only in a subset of 431 of the 712 study participants.
Cross-sectional Association at Baseline of LINE-1 Methylation with Ischemic Heart Disease and Stroke
The LINE-1 methylation assay showed high reproducibility in duplicate samples repeated in different batches of Pyrosequencing analysis (intra-class correlation coefficient=0.99). LINE-1 methylation was not associated with the length of storage between sample collection and methylation analysis. LINE-1 methylation at baseline showed a negative association with age (-0.23 %5mC [95% CI = −0.50 to 0.04] per 10-year increase in age), white blood cell (WBC) count (−0.08 %5mC [−0.18 to 0.02] age-adjusted estimate per 2000/μl increase in WBC count,), and percent lymphocytes (−0.29 %5mC [-0.51 to −0.07] age-adjusted estimate per 10% increase in percent lymphocytes), whereas a positive association was found with percent neutrophils (−0.21 %5mC [0.00 to 0.42] age-adjusted estimate per 10% increase in percent neutrophils) (eTable 1, http://links.lww.com). LINE-1 methylation was inversely associated with an existing diagnosis of hypertension at baseline (age-adjusted OR=0.6 [0.3 to 1.0] for subjects in the lowest vs. highest quartile-based category of LINE-1 methylation) (eTable 2, http://links.lww.com). LINE-1 methylation levels were not associated with plasma total homocysteine (0.05 %5mC [−0.11 to 0.22] age-adjusted estimate per 4 nmol/ml increase in total homocysteine) (eTable 1).
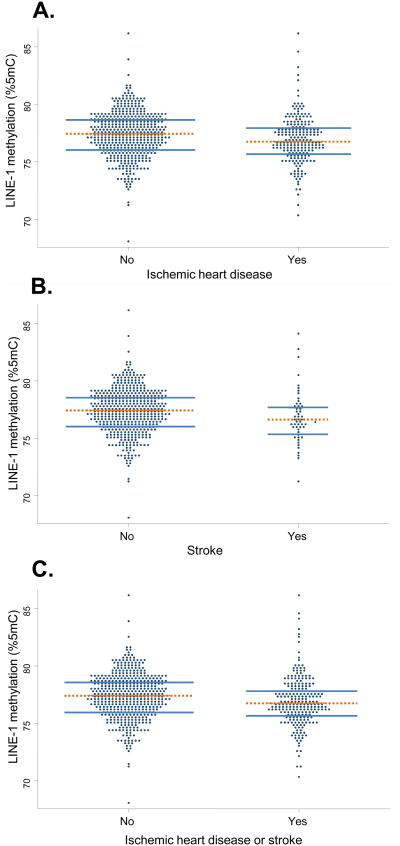
LINE-1 methylation at the baseline examination was lower in subjects with pre-existing ischemic heart disease and stroke (Table 1 and Figure). Using quartile-based categories of LINE-1 methylation, we found that prevalence of ischemic heart disease and stroke at baseline increased in a dose-response fashion with decreasing levels of LINE-1 methylation in blood DNA (Table 1). The results of the unadjusted analyses were similar in multivariable models adjusting for the variables tested in Table 2.
Table 2. Association of LINE-1 methylation and other risk factors with prevalent ischemic heart disease or stroke at the baseline examination—Crude estimates and multivariable models.
| Ischemic Heart Disease | Stroke | Ischemic Heart Disease or Stroke | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| crude | multivariable* | crude | multivariable* | crude | multivariable* | |||||||
| OR | (95%CI) | OR | (95%CI) | OR | (95%CI) | OR | (95%CI) | OR | (95%CI) | OR | (95%CI) | |
| LINE-1 methylation | ||||||||||||
| 86.3-78.8 (%5mC)b | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| 78.7-77.5 (%5mC) | 1.5 | (0.9 to 2.4) | 1.6 | (0.9 to 3.1) | 1.1 | (0.4 to 3.0) | 0.9 | (0.3 to 3.1) | 1.3 | (0.8 to 2.2) | 1.4 | (0.8 to 2.6) |
| 77.4-76.2 (%5mC) | 1.9 | (1.1 to 3.1) | 2.1 | (1.1 to 3.9) | 1.9 | (0.8 to 4.6) | 2.0 | (0.7 to 6.4) | 1.8 | (1.1 to 2.9) | 1.9 | (1.1 to 3.5) |
| 76.1-68.1 (%5mC) | 2.1 | (1.3 to 3.4) | 2.1 | (1.2 to 4.0) | 2.4 | (1.0 to 5.6) | 2.5 | (0.9 to 7.5) | 2.0 | (1.3 to 3.2) | 2.2 | (1.2 to 3.9) |
| Age (continuous, 10 yrs) | 1.4 | (1.0 to 2.2) | 2.3 | (1.2 to 4.6) | 1.6 | (1.1 to 2.3) | ||||||
| BMI (continuous, 5 kg/m2) | 1.0 | (0.7 to 1.3) | 1.2 | (0.7 to 2.0) | 1.0 | (0.8 to 1.3) | ||||||
| Smoking (ever) | 0.8 | (0.5 to 1.4) | 0.4 | (0.2 to 1.2) | 0.7 | (0.4 to 1.2) | ||||||
| Pack-years (continuous, 35 pack-yrs) | 1.2 | (0.9 to 1.6) | 1.2 | (0.7 to 2.2) | 1.2 | (0.9 to 1.6) | ||||||
| Alcohol drinking (two drinks or more/day) | 1.5 | (0.8 to 2.6) | 2.0 | (0.7 to 5.3) | 1.5 | (0.9 to 2.6) | ||||||
| Diagnosis of hypertension | 5.6 | (3.1 to 10.3) | 3.4 | (1.2 to 9.7) | 4.9 | (2.8 to 8.5) | ||||||
| Systolic blood pressure (continuous, 20 mmHg) | 0.8 | (0.6 to 1.0) | 0.6 | (0.4 to 1.1) | 0.8 | (0.6 to 1.0) | ||||||
| Diastolic blood pressure (continuous, 20 mmHg) | 0.8 | (0.6 to 1.0) | 1.0 | (0.6 to 1.6) | 0.8 | (0.6 to 1.0) | ||||||
| Diabetes | 1.2 | (0.7 to 2.0) | 1.7 | (0.7 to 4.2) | 1.3 | (0.8 to 2.1) | ||||||
| Statin use | 5.1 | (3.3 to 7.9) | 2.8 | (1.3 to 6.1) | 4.8 | (3.2 to 7.3) | ||||||
| Serum total cholesterol (continuous, 150 mg/dl) | 1.0 | (0.8 to 1.2) | 1.0 | (0.6 to 1.5) | 1.0 | (0.8 to 1.2) | ||||||
| Serum HDL (continuous, 15 mg/dl) | 0.7 | (0.5 to 0.9) | 0.7 | (0.4 to 1.1) | 0.7 | (0.5 to 0.9) | ||||||
| Total homocysteine (continuous, 4 nmol/ml) | 1.1 | (0.9 to 1.3) | 1.2 | (0.8 to 1.7) | 1.1 | (0.9 to 1.3) | ||||||
| Glomerular filtration rate (continuous, 25 ml/min/1.73m2) | 0.8 | (0.5 to 1.2) | 0.7 | (0.4 to 1.3) | 0.8 | (0.5 to 1.1) | ||||||
| Percent neutrophils (continuous, 10%) | 1.1 | (0.9 to 1.4) | 2.2 | (1.4 to 3.5) | 1.2 | (1.0 to 1.5) | ||||||
| Percent lymphocytes (continuous, 10%) | 0.6 | (0.3 to 1.2) | 1.3 | (0.3 to 5.3) | 0.6 | (0.3 to 1.2) | ||||||
|
| ||||||||||||
| LINE-1 methylation (continous, -2.5 %mC)c | 1.31 | (1.07 to 1.61) | 1.36 | (1.04 to 1.78) | 1.37 | (0.96 to 1.96) | 1.90 | (1.16 to 3.10) | 1.31 | (1.07 to 1.58) | 1.40 | (1.08 to 1.80) |
The multivariable models included all the listed variables as independent variables. For continuous independent variables other than LINE-1, the table reports ORs and 95 CIs for an increase equal to the number of units indicated. The number of units was selected as a round number that approximates the interquartile range of each of the variables. The exact interquartile ranges were the following: age, 9 years; BMI, 4.8 kg/m2; pack-years, 35; HDL, 15 mg/dl; total cholesterol 138 mg/dl; total homocysteine, 3.5 nmol/ml; glomerular filtration rate, 22.9 ml/min/1.73m2; percent neutrophils, 11%; percent lymphocytes, 10%.
Reference category.
Results from a second set of models fitting LINE-1 methylation as a continuous independent variable. ORs and 95% CI estimates per each 2.5 %5mC decrease in LINE-1 methylation (LINE-1 interquartile range was 2.55 %5mC).
Longitudinal Risk of non-fatal Ischemic Heart Disease and Stroke
Thirty-six incident cases of non-fatal ischemic heart disease and eight cases of stroke were identified in the follow-up of the 356 subjects free of baseline ischemic heart disease/stroke who were included in the incidence analysis. Because longitudinal data included a relatively small number of events, our analyses were based on methylation levels categorized according to the median value among participants free of ischemic heart disease and stroke at baseline.
Participants with low baseline LINE-1 methylation exhibited a higher risk of ischemic heart disease and stroke during follow-up (Table 3). In unadjusted analyses, participants with low LINE-1 methylation had a increased risk of developing ischemic heart disease (HR = 2.7 [95% CI = 1.3 to 5.6]) compared with subjects with high LINE-1 methylation. The corresponding risk for stroke was approximately 5-fold higher (4.9 [1.0 to 24.5]). Overall, participants with low LINE-1 methylation had a 3-fold higher risk of developing either ischemic heart disease or stroke (3.0 [1.6 to 5.8]). The corresponding adjusted risk estimates were 4.0 (1.8 to 8.9) for ischemic heart disease, 5.7 (0.8 to 39.8) for stroke, and 4.1 (1.9 to 8.7) for the composite of ischemic heart disease and stroke in multivariable models.
Table 3. Association of LINE-1 methylation and other risk factors with incidence of ischemic heart disease or stroke—Crude estimates and multivariable models based on the subset of individuals free of baseline disease.
| Ischemic Heart Disease | Stroke | Ischemic Heart Disease or Stroke | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| crude | multivariablea | crude | multivariablea | crude | multivariablea | |||||||
| HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | |
| LINE-1 methylation | ||||||||||||
| 86.3-77.5 (%5mC)b | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| 77.4-68.1 (%5mC) | 2.7 | (1.3 to 5.6) | 4.0 | (1.8 to 8.9) | 4.9 | (1.0 to 24.5) | 5.7 | (0.8 to 39.8) | 3.0 | (1.6 to 5.8) | 4.1 | (1.9 to 8.7) |
| Age (continuous, 10 yrs) | 1.3 | (0.6 to 2.7) | 1.0 | (0.2 to 6.1) | 1.2 | (0.6 to 2.3) | ||||||
| BMI (continuous, 5 kg/m2) | 1.0 | (0.6 to 1.7) | 0.3 | (0.1 to 1.9) | 0.9 | (0.5 to 1.5) | ||||||
| Smoking (ever) | 1.4 | (0.5 to 4.1) | 1.9 | (0.1 to 33.8) | 1.4 | (0.5 to 3.6) | ||||||
| Pack-years (continuous, 35 pack-yrs) | 1.3 | (0.6 to 2.6) | 2.2 | (0.4 to 11.4) | 1.4 | (0.7 to 2.8) | ||||||
| Alcohol drinking (two drinks or more/day) | 0.4 | (0.1 to 1.4) | 1.7 | (0.2 to 13.4) | 0.7 | (0.3 to 1.9) | ||||||
|
|
||||||||||||
| Diagnosis of hypertension | 1.1 | (0.4 to 2.5) | 1.3 | (0.2 to 9.9) | 1.2 | (0.5 to 2.5) | ||||||
| Systolic blood pressure (continuous, 20 mmHg) | 1.2 | (0.7 to 2.2) | 2.9 | (0.8 to 10.8) | 1.3 | (0.8 to 2.2) | ||||||
| Diastolic blood pressure (continuous, 20 mmHg) | 0.5 | (0.2 to 0.8) | 0.2 | (0.1 to 0.8) | 0.4 | (0.2 to 0.7) | ||||||
| Diabetes | 2.2 | (0.8 to 6.1) | 3.2 | (0.4 to 25.5) | 2.6 | (1.1 to 6.3) | ||||||
| Statin use | 0.5 | (0.2 to 1.6) | 2.4 | (0.3 to 16.8) | 0.6 | (0.2 to 1.6) | ||||||
| Serum total cholesterol (continuous, 150 mg/dl) | 1.1 | (0.7 to 1.6) | 0.5 | (0.1 to 2.7) | 1.0 | (0.7 to 1.5) | ||||||
| Serum HDL (continuous, 15 mg/dl) | 0.8 | (0.5 to 1.3) | 0.8 | (0.3 to 2.1) | 0.8 | (0.5 to 1.2) | ||||||
| Total homocysteine (continuous, 4 nmol/ml) | 1.1 | (0.6 to 1.8) | 1.0 | (0.3 to 4.0) | 1.1 | (0.7 to 1.7) | ||||||
| Glomerular filtration rate (continuous, 25 ml/min/1.73m2) | 1.0 | (0.5 to 2.0) | 1.8 | (0.3 to 10.4) | 1.0 | (0.5 to 1.8) | ||||||
| Percent neutrophils (continuous, 10%) | 1.7 | (1.0 to 2.8) | 1.6 | (0.6 to 4.7) | 1.6 | (1.0 to 2.6) | ||||||
| Percent lymphocytes (continuous, 10%) | 0.4 | (0.1 to 2.0) | 0.1 | (0.01 to 0.4) | 0.3 | (0.1 to 1.0) | ||||||
|
| ||||||||||||
| LINE-1 methylation (continous, -2.5 %mC)c | 1.48 | (1.06 to 2.07) | 1.91 | (1.21 to 3.04) | 1.63 | (0.88 to 3.04) | 1.80 | (0.72 to 4.46) | 1.50 | (1.10 to 2.04) | 1.80 | (1.20 to 2.69) |
The multivariable models included all the listed variables as independent variables. For continuous independent variables other than LINE-1, the table reports Hazard Ratios (HRs) and 95 CIs for an increase equal to the number of units indicated. The number of units was selected as a round number that approximates the interquartile range of each of the variables. The exact interquartile ranges were the following: age, 9 years; BMI, 4.8 kg/m2; pack-years, 35; HDL, 15 mg/dl; total cholesterol 138 mg/dl; total homocysteine, 3.5 nmol/ml; glomerular filtration rate, 22.9 ml/min/1.73m2; percent neutrophils, 11%; percent lymphocytes, 10%.
Reference category.
Results from a second set of models fitting LINE-1 methylation as a continuous independent variable. HRs and 95% CI estimates per each 2.5 %5mC decrease in LINE-1 methylation (LINE-1 interquartile range was 2.55 %5mC
For 24 of the 44 participants who developed ischemic heart disease or stroke during follow-up, a second blood DNA sample was obtained after diagnosis (time from baseline, 33-80 months; median, 62 months). Mean LINE-1 methylation was not different in the samples collected after IHD/stroke diagnosis (mean = 76.9 [95% CI = 76.4 to 77.4]) compared with baseline (77.2 [76.6 to 77.7]). Age-adjusted means were also very similar in the two set of samples (after diagnosis, mean = 76.8 [95% CI = 76.3 to 77.3]; at baseline, mean = 77.0 [76.5 to 77.5]).
Risk of Death from Ischemic Heart Disease and Stroke
Eighty-six of the 705 subjects included in the mortality analysis died during the follow-up, including 35 deaths from ischemic heart disease and 10 from stroke. Participants with lower LINE-1 methylation at baseline had higher ischemic heart disease and stroke mortality (Table 4), as well as higher mortality from any cause (Table 5). In unadjusted analyses, participants with LINE-1 methylation below the medians had higher mortality from ischemic heart disease (HR = 3.4 [95% CI = 1.5 to 7.9]). Stroke mortality was also increased with lower LINE-1 methylation (2.0 [0.5 to 7.9]), but the HR estimates were imprecise due to the small number of stroke deaths. Overall, participants with low LINE-1 methylation had a 3-fold higher mortality from either ischemic heart disease or stroke (3.0 [1.5 to 6.1]). We also found that low LINE-1 methylation was associated with a moderate increase in mortality from causes not related to ischemic heart disease or stroke (1.6 [0.8to 3.0]), and 2.1-fold higher risk of death from any cause (2.1 [1.3 to 3.4]) (Table 5). Adjusted estimates from multivariable models were HR = 3.3 (CI = 1.3 to 8.4) for ischemic heart disease mortality, 2.8 (0.6 to 14.3) for stroke mortality, 2.9 (CI = 1.3 to 6.2) for either ischemic heart disease or stroke mortality, 1.5 (0.8 to 3.0) for mortality from causes other than ischemic heart disease or stroke, and 2.0 (1.2 to 3.3) for any cause of death.
Table 4. Association of LINE-1 methylation and other risk factors with mortality from ischemic heart disease or stroke – Crude estimates and multivariable models.
| Ischemic Heart Disease | Stroke | Ischemic Heart Disease or Stroke | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| crude | multivariablea | crude | multivariablea | crude | multivariablea | |||||||
| HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | |
| LINE-1 methylation | ||||||||||||
| 86.3-77.5 (%5mC)b | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | ||||||
| 77.4-68.1 (%5mC) | 3.4 | (1.5 to 7.9) | 3.3 | (1.3 to 8.4) | 2.0 | (0.5 to 7.9) | 2.8 | (0.6 to 14.3) | 3.0 | (1.5 to 6.1) | 2.9 | (1.3 to 6.2) |
| Age (continuous, 10 yrs) | 2.6 | (1.3 to 5.2) | 47.0 | (5.5 to 403) | 4.1 | (2.1 to 7.8) | ||||||
| BMI (continuous, 5 kg/m2) | 1.2 | (0.7 to 1.9) | 0.4 | (0.1 to 1.5) | 1.0 | (0.6 to 1.5) | ||||||
| Smoking (ever) | 1.7 | (0.6 to 4.5) | 2.8 | (0.3 to 24.6) | 1.7 | (0.7 to 3.9) | ||||||
| Pack-years (continuous, 35 pack-yrs) | 1.1 | (0.8 to 1.7) | 0.9 | (0.2 to 3.0) | 1.1 | (0.8 to 1.6) | ||||||
|
|
||||||||||||
| Alcohol drinking (two drinks or more/day) | 0.6 | (0.2 to 2.3) | 5.8 | (0.6 to 54.1) | 1.1 | (0.4 to 3.0) | ||||||
| Diagnosis of hypertension | 2.7 | (0.8 to 9.5) | 1.0 | (0.1 to 11.4) | 2.4 | (0.8 to 7.1) | ||||||
| Systolic blood pressure (continuous, 20 mmHg) | 0.8 | (0.5 to 1.4) | 0.2 | (0.1 to 0.8) | 0.7 | (0.4 to 1.1) | ||||||
| Diastolic blood pressure (continuous, 20 mmHg) | 1.3 | (0.8 to 2.2) | 6.0 | (1.7 to 21.9) | 1.7 | (1.1 to 2.7) | ||||||
| Diabetes | 1.0 | (0.4 to 2.6) | 21.4 | (2.5 to 184) | 1.9 | (0.8 to 4.2) | ||||||
| Statin use | 0.9 | (0.4 to 2.1) | 2.0 | (0.4 to 11.3) | 1.1 | (0.5 to 2.2) | ||||||
| Serum total cholesterol (continuous, 150 mg/dl) | 1.0 | (0.7 to 1.4) | 0.6 | (0.2 to 1.8) | 0.9 | (0.7 to 1.3) | ||||||
| Serum HDL (continuous, 15 mg/dl) | 0.9 | (0.5 to 1.5) | 0.2 | (0.1 to 1.2) | 0.7 | (0.5 to 1.2) | ||||||
| Total homocysteine (continuous, 4 nmol/ml) | 1.0 | (0.7 to 1.6) | 1.0 | (0.3 to 2.9) | 1.0 | (0.7 to 1.5) | ||||||
| Glomerular filtration rate (continuous, 25 ml/min/1.73m2) | 0.6 | (0.3 to 1.2) | 1.1 | (0.2 to 5.6) | 0.7 | (0.4 to 1.3) | ||||||
| Percent neutrophils (continuous, 10%) | 1.1 | (0.7 to 1.6) | 1.7 | (0.7 to 3.8) | 1.2 | (0.8 to 1.7) | ||||||
| Percent lymphocytes (continuous, 10%) | 1.1 | (0.8 to 1.6) | 0.6 | (0.2 to 1.4) | 0.9 | (0.7 to 1.3) | ||||||
|
| ||||||||||||
| LINE-1 methylation (continous, -2.5 %mC)c | 1.60 | (1.07 to 2.38) | 1.73 | (1.06 to 2.83) | 1.28 | (0.59 to 2.82) | 1.00 | (0.32 to 3.08) | 1.53 | (1.07 to 2.18) | 1.50 | (0.96 to 2.34) |
The multivariable models included all the listed variables as independent variables. For continuous independent variables other than LINE-1, the table reports Hazard Ratios (HRs) and 95 CIs for an increase equal to the number of units indicated. The number of units was selected as a round number that approximates the interquartile range of each of the variables. The exact interquartile ranges were the following: age, 9 years; BMI, 4.8 kg/m2; pack-years, 35; HDL, 15 mg/dl; total cholesterol 138 mg/dl; total homocysteine, 3.5 nmol/ml; glomerular filtration rate, 22.9 ml/min/1.73m2; percent neutrophils, 11%, percent lymphocytes, 10%.
Reference category.
Results from a second set of models fitting LINE-1 methylation as a continuous independent variable. HRs and 95% CI estimates per each 2.5 %5mC decrease in LINE-1 methylation (LINE-1 interquartile range was 2.55 %5mC).
Table 5. Association of LINE-1 methylation and other risk factors with non-cardiovascular and total mortality—Crude estimates and multivariable models.
| Non cardiovascular mortality | Total Mortality | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| crude | multivariablea | crude | multivariablea | |||||
| HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | HR | (95%CI) | |
| LINE-1 methylation | ||||||||
| 86.3-77.5 (%5mC)b | 1.0 | 1.0 | 1.0 | 1.0 | ||||
| 77.4-68.1 (%5mC) | 1.6 | (0.8 to 2.9) | 1.5 | (0.8 to 3.0) | 2.1 | (1.3 to 3.4) | 2.0 | (1.2 to 3.3) |
| Age (continuous, 10 yrs) | 2.5 | (1.4 to 4.6) | 3.2 | (2.1 to 5.0) | ||||
| BMI (continuous, 5 kg/m2) | 1.3 | (0.8 to 1.9) | 1.1 | (0.8 to 1.5) | ||||
| Smoking (ever) | 1.5 | (0.7 to 3.5) | 1.6 | (0.9 to 2.8) | ||||
| Pack-years (continuous, 35 pack-yrs) | 1.3 | (0.9 to 1.7) | 1.2 | (1.0 to 1.5) | ||||
| Alcohol drinking (two drinks or more/day) | 0.9 | (0.3 to 2.4) | 1.0 | (0.5 to 1.9) | ||||
|
|
||||||||
| Diagnosis of hypertension | 0.5 | (0.2 to 1.0) | 0.9 | (0.5 to 1.7) | ||||
| Systolic blood pressure (continuous, 20 mmHg) | 1.0 | (0.6 to 1.7) | 0.8 | (0.6 to 1.1) | ||||
| Diastolic blood pressure (continuous, 20 mmHg) | 1.7 | (1.0 to 2.8) | 1.7 | (1.2 to 2.3) | ||||
| Diabetes | 1.7 | (0.7 to 3.8) | 1.8 | (1.0 to 3.1) | ||||
| Statin use | 0.9 | (0.4 to 2.0) | 1.0 | (0.6 to 1.7) | ||||
| Serum total cholesterol (continuous, 150 mg/dl) | 0.8 | (0.5 to 1.2) | 0.9 | (0.7 to 1.1) | ||||
| Serum HDL (continuous, 15 mg/dl) | 1.0 | (0.6 to 1.5) | 0.9 | (0.6 to 1.2) | ||||
| Total homocysteine (continuous, 4 nmol/ml) | 1.1 | (0.8 to 1.4) | 1.1 | (0.9 to 1.3) | ||||
| Glomerular filtration rate (continuous, 25 ml/min/1.73m2) | 0.5 | (0.3 to 0.9) | 0.6 | (0.4 to 0.9) | ||||
| Percent neutrophils (continuous, 10%) | 1.2 | (0.8 to 1.7) | 1.2 | (0.9 to 1.5) | ||||
| Percent lymphocytes (continuous, 10%) | 0.8 | (0.5 to 1.3) | 0.9 | (0.7 to 1.2) | ||||
|
| ||||||||
| LINE-1 methylation (continous, -2.5 %mC)c | 1.44 | (0.99 to 2.11) | 1.56 | (0.99 to 2.46) | 1.49 | (1.14 to 1.93) | 1.51 | (1.10 to 2.08) |
The multivariable models included all the listed variables as independent variables. For continuous independent variables other than LINE-1, the table reports Hazard Ratios (HRs) and 95 CIs for an increase equal to the number of units indicated. The number of units was selected as a round number that approximates the interquartile range of each of the variables. The exact interquartile ranges were the following: age, 9 years; BMI, 4.8 kg/m2; pack-years, 35; HDL, 15 mg/dl; total cholesterol 138 mg/dl; total homocysteine, 3.5 nmol/ml; glomerular filtration rate, 22.9 ml/min/1.73m2; percent neutrophils, 11%, percent lymphocytes, 10%.
Reference category.
Results from a second set of models fitting LINE-1 methylation as a continuous independent variable. HRs and 95% CI estimates per each 2.5 %5mC decrease in LINE-1 methylation (LINE-1 interquartile range was 2.55 %5mC).
Additional models testing for the interaction between LINE-1 methylation and baseline ischemic heart disease or stroke showed that the association between hypomethylation and mortality was not different in subjects with or without baseline disease (data not shown).
Sensitivity Analyses and Other Results
We adjusted for age in our primary set of analyses by fitting age as a continuous variable in multivariable models. Using alternative modeling of age (e.g., categorical variable based on quintiles, or linear and quadratic age terms) caused only slight changes in risk estimates. Because total homocysteine might be considered as a variable in the causal pathway linking LINE-1 methylation to cardiovascular disease, its inclusion as a dependent variable in multivariable models may not be appropriate. As a sensitivity analysis, we re-ran all the multivariable models after removing total homocysteine from the independent variables. In these models, the estimates for the association of LINE-1 methylation with prevalence, incidence and mortality of ischemic heart disease and stroke were remarkably similar to those shown in the paper (data not shown). Also, adding to the models the time between sample collection and DNA methylation analysis produced only minimal changes in the risk estimates. Models fitting LINE-1 methylation using a smoothing function showed only minor deviations from linearity (eFigures 1-4, http:links.lww.com). The study cohort investigated in the present study is made up mostly (96%) of white men. Excluding non-whites from the analyses or adjusting for ethnicity in multivariable analyses did not affect the results. In addition to LINE-1 methylation, we have measures of Alu methylation, a second type of repetitive element, for this cohort. A set of analysis on Alu methylation, similar to those reported in this paper for LINE-1 methylation, did not show any association with prevalence, incidence or mortality from ischemic heart disease or stroke (data not shown).
Discussion
Our study shows that individual differences in repetitive element DNA methylation predict the risk of developing ischemic heart disease and stroke in elderly men. The associations of LINE-1 hypomethylation with ischemic heart disease and stroke, both in cross-sectional and longitudinal analyses, suggest that DNA hypomethylation anticipates disease diagnosis. Hypomethylation may help identify individuals at risk well before the onset of clinical disease. This conclusion is supported by our observation of no changes in average methylation in a subset of subjects for whom blood DNA samples were collected before and after the diagnosis of heart disease or stroke. Epigenetic marks can be inherited and are largely established in utero or during early life. Thus, DNA hypomethylation may be part of the processes that determine transgenerational risks, as well as of the effects of in utero and early life conditions on adult disease.1,28
Hypomethylated DNA has been shown to be prone to mutations or aberrant gene expression patterns in vascular tissue, leading to the transition from normal phenotype to vascular fibrocellular lesions by increasing proliferation of vascular smooth cells and lipid deposition.29 Although the correlation of DNA methylation in blood and in vascular tissue is undetermined, blood DNA methylation content and repetitive element methylation both undergo progressive decline as people age,15,30 which may be related with the risk of common age-related disease.
In our data, lower LINE-1 methylation predicted the risk of cardiovascular disease independent of established risk factors. Epigenetic modifications—in contrast with genetic changes—are potentially reversible. Our results, if confirmed, would indicate the potential for lifestyle or pharmacological interventions to reverse deleterious epigenetic features.1 Although DNA methylation analysis of LINE-1 sequences has been frequently used to estimate global genomic methylation content,2,3 the observed association with heart disease and stroke may result directly from altered LINE-1 organization and function. Hypomethylation of repetitive DNA sequences is expected to lead to the transcriptional activation of those repetitive sequences that still contain active promoters.4-6,31 Repetitive elements have been demonstrated to be activated during conditions of cellular stress,5,32 and LINE-1 expression has been recently identified as a mediator of ischemic heart damage.33 However, it remains to be determined whether LINE-1 hypomethylation is associated with specific functions that mediate increased cardiovascular risk, and what the nature of these functions might be. Because we measured LINE-1 methylation in peripheral blood leukocytes, the potential functions of LINE-1 repetitive elements associated with hypomethylation in this cell type will be likely related to mechanisms different from those operating in the heart. In our data, LINE-1 methylation showed very few associations with the baseline characteristics we evaluated, including several established risk factors for cardiovascular disease. In particular, we did not find any association with plasma homocysteine, which has been inversely related with global or LINE-1 methylation in experimental models and human studies.9,11,13 Specific characteristics of this population, including older age, might have contributed to discrepancies with other studies.
Hypomethylation of repetitive elements has been found in association with hypermethylation of specific genes, particularly in studies conducted on cancer tissues.1 Further research is warranted to evaluate whether blood LINE-1 hypomethylation is associated with increased methylation of genes that are hypermethylated in atherosclerotic tissues.34,35
In previous work on 1,097 blood DNA samples from this same cohort, including multiple measures of LINE-1 methylation from a subset of the study subjects, we found that LINE-1 methylation showed a moderate cross-sectional association with age.15 At the examination taken as baseline, a 10-year increase in age was associated with −0.23 % 5mC lower LINE-1 methylation. All multivariable models we fitted included age as an independent variable. Therefore, the associations between LINE-1 methylation and cardiovascular risks appear independent from age.
We also recently found that participants in the Normative Aging Study exposed to higher levels of particulate air pollution from vehicular traffic in the week before the blood drawing exhibited lower LINE-1 methylation.18 This finding was replicated in a study of foundry workers exposed to airborne metal-rich particulate.36 Whereas air-pollution exposure is an established risk factor for atherosclerotic cardiovascular disease, the short time scale in our air pollution analysis points to rapid and transient changes that are unlikely to be responsible for cardiovascular events occurring several years later, such as those investigated in the current study.
Our study has the advantage of being based on a population of aging men unselected on the basis of disease status. However, our results can be generalized only to older white men. Future studies should address the role of repetitive-element hypomethylation among women, as well as in various age and ethnic groups. Results on hypomethylation-related risk of stroke were based on a limited number of cases, and should be replicated in larger samples. Because we measured methylation in blood DNA—a source of DNA that is easily obtainable and does not require processing before DNA extraction—our results might have reflected shifts in the proportions of white-blood-cell subsets caused by alterations related to impending disease onset. This scenario, however, predicts that the strength of the association of lower DNA methylation with higher incidence and mortality of ischemic heart disease and stroke should be maximal immediately after DNA methylation measurement and decline afterwards.37 In contrast, we did not see such a decline, and adjustment in multivariable analysis for percent neutrophils and lymphocytes (the major white blood cell types) did not cause major changes in the risk estimates. A limitation of the present study is that the ischemic heart disease and stroke diagnoses did not differentiate underlying disease. The diagnoses of ischemic heart disease and stroke might have included events occurring in subjects with little or no atherosclerosis. Because previous animal and human studies have specifically related DNA hypomethylation to atherosclerosis,7,22 this might have lead to a dilution of the hypomethylation-related risk estimates.
In summary, we showed that epigenetic changes measured in blood DNA predict the risk of common age-related diseases, such as ischemic heart disease and stroke. Our results indicate blood repetitive-element hypomethylation is a novel risk factor for cardiovascular risk and survival, and may contribute to better cardiovascular risk stratification as simple, standardized assays of DNA methylation become available. At the same time, our results give further substance to ongoing endeavors to develop interventions and treatments that act through epigenetic mechanisms.
Supplementary Material
Figure 1.
Dot plots of LINE-1 methylation at the baseline examination according to the presence of A, ischemic heart disease, B, stroke, or C, ischemic heart disease or stroke. Horizontal lines are placed at the median (dotted) and the lower and upper quartiles (solid) of the distributions.
Acknowledgments
Supported by the National Institute of Environmental Health Sciences (NIEHS) grants ES015172-01, ES00002; Environmental Protection Agency (EPA) grants EPA R83241601 and R827353; CARIPLO Foundation grant 2007-5469, and MIUR PRIN2007 grant 2S2HT8. The VA Normative Aging Study is supported by the Cooperative Studies Program/Epidemiology Research and Information Center of the U.S. Department of Veterans Affairs and is a component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC).
References
- 1.Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature. 2007;447(7143):433–40. doi: 10.1038/nature05919. [DOI] [PubMed] [Google Scholar]
- 2.Yang AS, Estecio MR, Doshi K, Kondo Y, Tajara EH, Issa JP. A simple method for estimating global DNA methylation using bisulfite PCR of repetitive DNA elements. Nucleic Acids Res. 2004;32(3):e38. doi: 10.1093/nar/gnh032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weisenberger DJ, Campan M, Long TI, Kim M, Woods C, Fiala E, Ehrlich M, Laird PW. Analysis of repetitive element DNA methylation by MethyLight. Nucleic Acids Res. 2005;33(21):6823–36. doi: 10.1093/nar/gki987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostertag EM, Kazazian HH., Jr Biology of mammalian L1 retrotransposons. Annu Rev Genet. 2001;35:501–38. doi: 10.1146/annurev.genet.35.102401.091032. [DOI] [PubMed] [Google Scholar]
- 5.Schulz WA. L1 retrotransposons in human cancers. J Biomed Biotechnol. 2006;2006(1):83672. doi: 10.1155/JBB/2006/83672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han JS, Szak ST, Boeke JD. Transcriptional disruption by the L1 retrotransposon and implications for mammalian transcriptomes. Nature. 2004;429(6989):268–74. doi: 10.1038/nature02536. [DOI] [PubMed] [Google Scholar]
- 7.Turunen MP, Aavik E, Yla-Herttuala S. Epigenetics and atherosclerosis. Biochim Biophys Acta. 2009;1790(9):886–91. doi: 10.1016/j.bbagen.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 8.Hiltunen MO, Yla-Herttuala S. DNA methylation, smooth muscle cells, and atherogenesis. Arterioscler Thromb Vasc Biol. 2003;23(10):1750–3. doi: 10.1161/01.ATV.0000092871.30563.41. [DOI] [PubMed] [Google Scholar]
- 9.Yideng J, Jianzhong Z, Ying H, Juan S, Jinge Z, Shenglan W, Xiaoqun H, Shuren W. Homocysteine-Mediated Expression of SAHH, DNMTs, MBD2, and DNA Hypomethylation Potential Pathogenic Mechanism in VSMCs. DNA Cell Biol. 2007;26t(8):603–11. doi: 10.1089/dna.2007.0584. [DOI] [PubMed] [Google Scholar]
- 10.Hiltunen MO, Turunen MP, Hakkinen TP, Rutanen J, Hedman M, Makinen K, Turunen AM, Aalto-Setala K, Yla-Herttuala S. DNA hypomethylation and methyltransferase expression in atherosclerotic lesions. Vasc Med. 2002;7(1):5–11. doi: 10.1191/1358863x02vm418oa. [DOI] [PubMed] [Google Scholar]
- 11.Castro R, Rivera I, Struys EA, Jansen EE, Ravasco P, Camilo ME, Blom HJ, Jakobs C, Tavares de Almeida I. Increased homocysteine and S-adenosylhomocysteine concentrations and DNA hypomethylation in vascular disease. Clin Chem. 2003;49(8):1292–6. doi: 10.1373/49.8.1292. [DOI] [PubMed] [Google Scholar]
- 12.Lund G, Andersson L, Lauria M, Lindholm M, Fraga MF, Villar-Garea A, Ballestar E, Esteller M, Zaina S. DNA methylation polymorphisms precede any histological sign of atherosclerosis in mice lacking apolipoprotein E. J Biol Chem. 2004;279(28):29147–54. doi: 10.1074/jbc.M403618200. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, Chen MF, Pai A, John SW, Smith RS, Bottiglieri T, Bagley P, Selhub J, Rudnicki MA, James SJ, Rozen R. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10(5):433–43. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 14.Fuke C, Shimabukuro M, Petronis A, Sugimoto J, Oda T, Miura K, Miyazaki T, Ogura C, Okazaki Y, Jinno Y. Age related changes in 5-methylcytosine content in human peripheral leukocytes and placentas: an HPLC-based study. Ann Hum Genet. 2004;68(Pt 3):196–204. doi: 10.1046/j.1529-8817.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- 15.Bollati V, Schwartz J, Wright R, Litonjua A, Tarantini L, Suh H, Sparrow D, Vokonas P, Baccarelli A. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130(4):234–9. doi: 10.1016/j.mad.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ting Hsiung D, Marsit CJ, Houseman EA, Eddy K, Furniss CS, McClean MD, Kelsey KT. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16(1):108–14. doi: 10.1158/1055-9965.EPI-06-0636. [DOI] [PubMed] [Google Scholar]
- 17.Ingrosso D, Cimmino A, Perna AF, Masella L, De Santo NG, De Bonis ML, Vacca M, D'Esposito M, D'Urso M, Galletti P, Zappia V. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet. 2003;361(9370):1693–9. doi: 10.1016/S0140-6736(03)13372-7. [DOI] [PubMed] [Google Scholar]
- 18.Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Rapid DNA methylation changes after exposure to traffic particles. Am J Respir Crit Care Med. 2009;179(7):572–8. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104(32):13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniades C, Antonopoulos AS, Tousoulis D, Marinou K, Stefanadis C. Homocysteine and coronary atherosclerosis: from folate fortification to the recent clinical trials. Eur Heart J. 2009;30(1):6–15. doi: 10.1093/eurheartj/ehn515. [DOI] [PubMed] [Google Scholar]
- 21.Marti-Carvajal AJ, Sola I, Lathyris D, Salanti G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database Syst Rev. 2009(4):CD006612. doi: 10.1002/14651858.CD006612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;135(1):5–8. doi: 10.1093/jn/135.1.5. [DOI] [PubMed] [Google Scholar]
- 23.Bell B, Rose C, Damon A. The normative aging study: an interdisciplinary and longitudinal study of health and aging. Aging Hum Dev. 1972;3:4–17. [Google Scholar]
- 24.Kawachi I, Sparrow D, Spiro A, 3rd, Vokonas P, Weiss ST. A prospective study of anger and coronary heart disease. The Normative Aging Study. Circulation. 1996;94(9):2090–5. doi: 10.1161/01.cir.94.9.2090. [DOI] [PubMed] [Google Scholar]
- 25.Todaro JF, Shen BJ, Niaura R, Spiro A, 3rd, Ward KD. Effect of negative emotions on frequency of coronary heart disease (The Normative Aging Study) Am J Cardiol. 2003;92(8):901–6. doi: 10.1016/s0002-9149(03)00967-6. [DOI] [PubMed] [Google Scholar]
- 26.Kubzansky LD, Sparrow D, Vokonas P, Kawachi I. Is the glass half empty or half full? A prospective study of optimism and coronary heart disease in the normative aging study. Psychosom Med. 2001;63(6):910–6. doi: 10.1097/00006842-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, Cavallo D, Byun HM, Jiang J, Marinelli B, Pesatori AC, Bertazzi PA, Yang AS. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res. 2007;67(3):876–80. doi: 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- 28.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in Utero and Early-Life Conditions on Adult Healt and Disease. New Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Newman PE. Can reduced folic acid and vitamin B12 levels cause deficient DNA methylation producing mutations which initiate atherosclerosis? Med Hypotheses. 1999;53(5):421–4. doi: 10.1054/mehy.1998.0794. [DOI] [PubMed] [Google Scholar]
- 30.Bjornsson HT, Sigurdsson MI, Fallin MD, Irizarry RA, Aspelund T, Cui H, Yu W, Rongione MA, Elkstrom TJ, Harris TB, Launer LJ, Eiriksdottir G, Leppert MF, Sapienza C, Gudnason V, Feinberg AP. Intra-individual change over time in DNA methylation with familial clustering. Jama. 2008;299(24):2877–83. doi: 10.1001/jama.299.24.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jasinska A, Krzyzosiak WJ. Repetitive sequences that shape the human transcriptome. FEBS Lett. 2004;567(1):136–41. doi: 10.1016/j.febslet.2004.03.109. [DOI] [PubMed] [Google Scholar]
- 32.Li TH, Schmid CW. Differential stress induction of individual Alu loci: implications for transcription and retrotransposition. Gene. 2001;276(1-2):135–41. doi: 10.1016/s0378-1119(01)00637-0. [DOI] [PubMed] [Google Scholar]
- 33.Lucchinetti E, Feng J, Silva R, Tolstonog GV, Schaub MC, Schumann GG, Zaugg M. Inhibition of LINE-1 expression in the heart decreases ischemic damage by activation of Akt/PKB signaling. Physiol Genomics. 2006;25(2):314–24. doi: 10.1152/physiolgenomics.00251.2005. [DOI] [PubMed] [Google Scholar]
- 34.Zhu S, Goldschmidt-Clermont PJ, Dong C. Inactivation of monocarboxylate transporter MCT3 by DNA methylation in atherosclerosis. Circulation. 2005;112(9):1353–61. doi: 10.1161/CIRCULATIONAHA.104.519025. [DOI] [PubMed] [Google Scholar]
- 35.Post WS, Goldschmidt-Clermont PJ, Wilhide CC, Heldman AW, Sussman MS, Ouyang P, Milliken EE, Issa JP. Methylation of the estrogen receptor gene is associated with aging and atherosclerosis in the cardiovascular system. Cardiovasc Res. 1999;43(4):985–91. doi: 10.1016/s0008-6363(99)00153-4. [DOI] [PubMed] [Google Scholar]
- 36.Tarantini L, Bonzini M, Apostoli P, Pegoraro V, Bollati V, Marinelli B, Cantone L, Rizzo G, Hou LF, Schwartz J, Bertazzi PA, Baccarelli A. Effects of Particulate Matter on Genomic DNA Methylation Content and iNOS Promoter Methylation. Environ Health Perspect. 2009;117:217–222. doi: 10.1289/ehp.11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cawthon RM, Smith KR, O'Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–5. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.