 |
Dr. Brian P. Kavanagh graduated from University College Dublin (Ireland) in 1985. Following residency in Internal Medicine in Dublin and in Anaesthesia (residency and fellowship) in Toronto, he trained in Critical Care Medicine in Stanford. He returned to the Toronto General Hospital in 1994 and in 1999 moved to the Hospital for Sick Children where he is a clinician-scientist and holds the Dr. Geoffrey Barker Chair in Critical Care Medicine. He is a professor of anesthesia, medicine and physiology, and in 2006 was appointed chair of the Department of Anesthesia at the University of Toronto. His laboratory investigates molecular and physiological mechanisms of ventilator-induced lung injury and the mechanisms of action of CO2 on the lung, and is supported by two operating grants and previous career awards from the Canadian Institutes for Health Research and from the Ontario Government. Dr. Kavanagh regularly presents at international meetings. He chairs the executive committee for the Critical Care Canada-Forum and is past program chair for the critical care assembly at the American Thoracic Society. Dr. Kavanagh is the physician lead for the Organ Donation Program and chairs the Organ & Tissue Donation committee at the Hospital for Sick Children. He serves or has served on the editorial advisory boards of the American Journal of Respiratory and Critical Care Medicine, Intensive Care Medicine, and is an associate editor of Anesthesiology.
The term ‘permissive hypercapnia’ was coined after two case series by Hickling and colleagues in the early 1990s that suggested that limitation of airway pressure and tidal volume, with a tolerant approach to elevations in arterial CO2, was associated with lower hospital mortality than predicted by ‘acute physiological and chronic health evaluation (APACHE) II’ scores (Hickling et al. 1990, 1994). In fact directly analogous findings had been reported in the mid 1980s, whereby lowering tidal volumes in status asthmaticus (Darioli & Perret, 1984) and in persistent pulmonary hypertension of the newborn (Wung et al. 1985) was associated with hypercapnia and an apparent improvement in survival. Subsequently, two large scale randomized clinical trials (RCTs) proved that reducing tidal volumes in acute respiratory distress syndrome (ARDS) patients, resulting in greater or lesser degrees of hypercapnia, can improve patient survival (Amato et al. 1998; ARDS-Network, 2000). Furthermore, laboratory studies have documented clear direct beneficial effects of hypercapnia in some circumstances (Shibata et al. 1998; Laffey et al. 2000c), while buffering hypercapnic acidosis (HCA) attenuates its benefit (Laffey et al. 2000a), and hypocapnia can be harmful (Laffey et al. 2000b). HCA is not without risks (Doerr et al. 2005; O’Croinin et al. 2008); however, advances in our understanding of its mechanisms of action (Takeshita et al. 1999; O’Toole et al. 2009), together with strategies that can minimize harmful effects (Chonghaile et al. 2008), will enable us to shift the balance towards benefit in ARDS. Several clinical issues are directly important in ARDS, and so in this paper we consider hypercapnia under the following headings.
Hypercapnia improves blood flow and tissue oxygenation
HCA increases arterial and tissue oxygenation in pre-clinical studies (Swenson et al. 1994; Wang et al. 2008) and in healthy humans (Akca et al. 2002) via important mechanisms. First, it potentiates hypoxic pulmonary vasoconstriction (Swenson et al. 1994) and increases local alveolar ventilation (Domino et al. 1998) by inhibition of airway tone: the net effect is augmented ventilation/perfusion (V/Q) matching and enhanced arterial oxygenation. Second, hypercapnia-mediated increases in cardiac output augment systemic oxygen delivery by several mechanisms, including sympato-adrenalmediated release of catecholamines. Indeed, an increase in 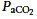 of approximately 10 mmHg increases the cardiac index by 14% in mechanically ventilated patients (Akca et al. 2002; Mekontso Dessap et al. 2009). Third, it shifts the oxyhaemoglobin dissociation curve rightwards, facilitating O2 release (the Bohr effect) and thereby increasing tissue O2 availability (Turek & Kreuzer, 1981). Finally, hypercapnia causes microvascular vasodilatation, promoting oxygen delivery and tissue perfusion (Komori et al. 2007).
of approximately 10 mmHg increases the cardiac index by 14% in mechanically ventilated patients (Akca et al. 2002; Mekontso Dessap et al. 2009). Third, it shifts the oxyhaemoglobin dissociation curve rightwards, facilitating O2 release (the Bohr effect) and thereby increasing tissue O2 availability (Turek & Kreuzer, 1981). Finally, hypercapnia causes microvascular vasodilatation, promoting oxygen delivery and tissue perfusion (Komori et al. 2007).
Hypercapnia reduces inflammation and oxidant-induced injury
HCA affords protection against inflammation-induced organ injury. This effect was first described for ischaemia–reperfusion injury of the heart and liver (Kitakaze et al. 1988; Currin et al. 1991), and subsequently in kidney, brain and lung (Laffey et al. 2000c); many oxidative and inflammatory cascades are blunted, as is the lethal intracellular calcium influx associated with abrupt cellular re-oxygenation. HCA reduces the severity of injury in pre-clinical ARDS models, including ventilator-induced lung injury (VILI) (Sinclair et al. 2002), bacterial pneumonia (Ni Chonghaile et al. 2008) and systemic sepsis (Costello et al. 2009). Hypercapnia also inhibits hypoxia-induced chronic pulmonary hypertension in adult and newborn rodents (Kantores et al. 2006; Masood et al. 2009), and provides protection against chronic neonatal lung injury (Masood et al. 2009).
HCA can potently suppress inflammation. Because proteins have pH optima in the near physiological range, it is not surprising that acidosis reduces radical oxygen and nitrogen species generation, diminishes proinflammatory cytokine and chemokine production, impairs neutrophil chemotaxis, and inhibits many proteases, nucleases, and phospholipases activated in injured cells (Somero, 1986; Nishio et al. 2001). While the effects of in vivo hypercapnia are likely to occur in part through the alteration of pH, recent evidence suggests that CO2 may also directly regulate gene expression. Hypercapnia suppresses the activity of nuclear factor-kappa B (NF-κB), a major transcription factor that regulates genes responsible for immunity and inflammation, including proinflammatory cytokines, via a pH-independent mechanism (O’Toole et al. 2009; Cummins et al. 2010). Such a mechanism may explain the protective effects of HCA in pre-clinical ARDS models (Contreras et al. 2012).
Striking a balance – avoiding harm with hypercapnia
Inflammation and repair pathways are not separate processes, and a balance must be struck between inhibiting inflammation and maintaining host defence and repair mechanisms. A key concern is whether HCA, while suppressing inflammation, might impair the host response to infection and/or slow repair following injury. Pre-clinical studies demonstrate that the effects of HCA on bacterial injury may vary, ranging from benefit to harm; the impact appears to depend on important factors including stage of infection (early vs. established), site of infection (pulmonary vs. extra-pulmonary) and concomitant antibiotic therapy (use vs. non-use).
For example, in early severe bacterial pneumonia, HCA (compared with normocapnic conditions) reduces the severity of lung injury and the associated vigorous host inflammatory response (Ni Chonghaile et al. 2008). However, HCA did not increase pulmonary bacterial load in these studies (O’Croinin et al. 2005; Ni Chonghaile et al. 2008). In contrast, in the context of prolonged bacterial pneumonia, environmental hypercapnia increased E. coli bacterial load (as well as the severity of lung injury), possibly via impairment of neutrophil phagocytosis (O’Croinin et al. 2008). Reassuringly, early institution of appropriate antibiotic therapy abolished these deleterious effects of hypercapnia, reducing lung injury and lung bacterial load to degrees observed with normocapnia (O’Croinin et al. 2008). Of note, in the setting of early (or late) systemic infection, hypercapnia reduced injury (Costello et al. 2009). At a cellular level, HCA impairs ex vivo and in vivo wound healing of the airway and alveolar epithelia (Doerr et al. 2005; O’Toole et al. 2009), and reduces alveolar fluid clearance (Briva et al. 2007). Greater understanding of the mechanisms of action of CO2 in sepsis and lung inflammation may enable better titration of benefits vs. risks in ARDS.
Hypercapnia – ventilator management
Hypercapnia is common in ARDS. Managing elevated 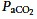 by increasing tidal volume is now known to be unacceptable; however, management by increasing the respiratory rate, although common (Checkley et al. 2008) is of uncertain impact. For example, increasing respiratory frequency from 12 to 30 breaths per minute adds over 25,000 additional opening and closing cycles per day to an already injured lung, and laboratory data suggest that this approach is associated with additional lung injury (Hotchkiss et al. 2000).
by increasing tidal volume is now known to be unacceptable; however, management by increasing the respiratory rate, although common (Checkley et al. 2008) is of uncertain impact. For example, increasing respiratory frequency from 12 to 30 breaths per minute adds over 25,000 additional opening and closing cycles per day to an already injured lung, and laboratory data suggest that this approach is associated with additional lung injury (Hotchkiss et al. 2000).
Permissive hypercapnia – clinical evidence of real benefit
Clinical trials of lung-protective ventilation are confounded by the inability to dissect the effects of permissive hypercapnia from effects of tidal volume (Amato et al. 1998). In the ARMA trial (ARDS-Network, 2000), the effects of hypercapnia or acidosis were partially ‘treated’ by increasing respiratory rate and administering sodium bicarbonate. However, a multivariate analysis of this study (ARDS-Network, 2000), after controlling for other variables predictive of mortality, found that the patients who had moderate HCA (pH 7.15–7.35, 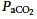 45–65 mmHg) on study day 1 had a significantly lower odds ratio of death at 28 days, but only in the 12 ml kg−1 tidal volume group, a result consistent with a protective effect of HCA in ventilator-induced lung injury (Kregenow et al. 2006). While not proof of cause and effect, these data support the idea that hypercapnia might contribute direct benefit in ARDS patients.
45–65 mmHg) on study day 1 had a significantly lower odds ratio of death at 28 days, but only in the 12 ml kg−1 tidal volume group, a result consistent with a protective effect of HCA in ventilator-induced lung injury (Kregenow et al. 2006). While not proof of cause and effect, these data support the idea that hypercapnia might contribute direct benefit in ARDS patients.
Conclusion
Hypercapnic acidosis is not without risks (Doerr et al. 2005; O’Croinin et al. 2008), and clinical decision-making regarding hypercapnia and ventilation in ARDS patients requires an understanding of its risk–benefit profile. Hypercapnia may be indirectly beneficial in ARDS by facilitating a reduction of the intensity of mechanical ventilation. Hypercapnia also has potent direct effects, and careful scientific evidence has accumulated, showing how HCA can independently diminish lung and systemic inflammation, which could mitigate development or progression of ARDS (Takeshita et al. 1999; Contreras et al. 2012). Limiting the severity and duration of hypercapnia, together with careful infection surveillance, may minimize the risk of harm. We conclude that permissive hypercapnia, used appropriately, may diminish lung injury, and provide incremental benefit beyond tidal volume reduction in ARDS.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word’. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;591/11/2763
Acknowledgments
J. G. Laffey is supported by a Merit award and G. F. Curley by a Clinician Scientist Transition award, from the Department of Anesthesia at the University of Toronto.
References
- Akca O, Doufas AG, Morioka N, Iscoe S, Fisher J, Sessler DI. Hypercapnia improves tissue oxygenation. Anesthesiology. 2002;97:801–806. doi: 10.1097/00000542-200210000-00009. [DOI] [PubMed] [Google Scholar]
- Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- ARDS-Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Briva A, Vadasz I, Lecuona E, Welch LC, Chen J, Dada LA, Trejo HE, Dumasius V, Azzam ZS, Myrianthefs PM, Batlle D, Gruenbaum Y, Sznajder JI. High CO2 levels impair alveolar epithelial function independently of pH. PLoS One. 2007;2:e1238. doi: 10.1371/journal.pone.0001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley W, Brower R, Korpak A, Thompson BT. Effects of a clinical trial on mechanical ventilation practices in patients with acute lung injury. Am J Respir Crit Care Med. 2008;177:1215–1222. doi: 10.1164/rccm.200709-1424OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonghaile MN, Higgins BD, Costello J, Laffey JG. Hypercapnic acidosis attenuates lung injury induced by established bacterial pneumonia. Anesthesiology. 2008;109:837–848. doi: 10.1097/ALN.0b013e3181895fb7. [DOI] [PubMed] [Google Scholar]
- Contreras M, Ansari B, Curley G, Higgins BD, Hassett P, O’Toole D, Laffey JG. Hypercapnic acidosis attenuates ventilation-induced lung injury by a nuclear factor-κB-dependent mechanism. Crit Care Med. 2012;40:2622–2630. doi: 10.1097/CCM.0b013e318258f8b4. [DOI] [PubMed] [Google Scholar]
- Costello J, Higgins B, Contreras M, Chonghaile MN, Hassett P, O’Toole D, Laffey JG. Hypercapnic acidosis attenuates shock and lung injury in early and prolonged systemic sepsis. Crit Care Med. 2009;37:2412–2420. doi: 10.1097/CCM.0b013e3181a385d3. [DOI] [PubMed] [Google Scholar]
- Cummins EP, Oliver KM, Lenihan CR, Fitzpatrick SF, Bruning U, Scholz CC, Slattery C, Leonard MO, McLoughlin P, Taylor CT. NF-κB links CO2 sensing to innate immunity and inflammation in mammalian cells. J Immunol. 2010;185:4439–4445. doi: 10.4049/jimmunol.1000701. [DOI] [PubMed] [Google Scholar]
- Currin RT, Gores GJ, Thurman RG, Lemasters JJ. Protection by acidotic pH against anoxic cell killing in perfused rat liver: evidence for a pH paradox. FASEB J. 1991;5:207–210. doi: 10.1096/fasebj.5.2.2004664. [DOI] [PubMed] [Google Scholar]
- Darioli R, Perret C. Mechanical controlled hypoventilation in status asthmaticus. Am Rev Respir Dis. 1984;129:385–387. doi: 10.1164/arrd.1984.129.3.385. [DOI] [PubMed] [Google Scholar]
- Doerr CH, Gajic O, Berrios JC, Caples S, Abdel M, Lymp JF, Hubmayr RD. Hypercapnic acidosis impairs plasma membrane wound resealing in ventilator-injured lungs. Am J Respir Crit Care Med. 2005;171:1371–1377. doi: 10.1164/rccm.200309-1223OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino KB, Emery MJ, Swenson ER, Hlastala MP. Ventilation heterogeneity is increased in hypocapnic dogs but not pigs. Respir Physiol. 1998;111:89–100. doi: 10.1016/s0034-5687(97)00103-5. [DOI] [PubMed] [Google Scholar]
- Hickling KG, Henderson SJ, Jackson R. Low mortality associated with low volume pressure limited ventilation with permissive hypercapnia in severe adult respiratory distress syndrome. Intensive Care Med. 1990;16:372–377. doi: 10.1007/BF01735174. [DOI] [PubMed] [Google Scholar]
- Hickling KG, Walsh J, Henderson S, Jackson R. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22:1568–1578. doi: 10.1097/00003246-199422100-00011. [DOI] [PubMed] [Google Scholar]
- Hotchkiss JR, Jr, Blanch L, Murias G, Adams AB, Olson DA, Wangensteen OD, Leo PH, Marini JJ. Effects of decreased respiratory frequency on ventilator-induced lung injury. Am J Respir Crit Care Med. 2000;161:463–468. doi: 10.1164/ajrccm.161.2.9811008. [DOI] [PubMed] [Google Scholar]
- Kantores C, McNamara PJ, Teixeira L, Engelberts D, Murthy P, Kavanagh BP, Jankov RP. Therapeutic hypercapnia prevents chronic hypoxia-induced pulmonary hypertension in the newborn rat. Am J Physiol Lung Cell Mol Physiol. 2006;291:L912–L922. doi: 10.1152/ajplung.00480.2005. [DOI] [PubMed] [Google Scholar]
- Kitakaze M, Weisfeldt ML, Marban E. Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts. J Clin Invest. 1988;82:920–927. doi: 10.1172/JCI113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori M, Takada K, Tomizawa Y, Nishiyama K, Kawamata M, Ozaki M. Permissive range of hypercapnia for improved peripheral microcirculation and cardiac output in rabbits. Crit Care Med. 2007;35:2171–2175. doi: 10.1097/01.ccm.0000281445.77223.31. [DOI] [PubMed] [Google Scholar]
- Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med. 2006;34:1–7. doi: 10.1097/01.ccm.0000194533.75481.03. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Engelberts D, Kavanagh BP. Buffering hypercapnic acidosis worsens acute lung injury. Am J Respir Crit Care Med. 2000a;161:141–146. doi: 10.1164/ajrccm.161.1.9905080. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Engelberts D, Kavanagh BP. Injurious effects of hypocapnic alkalosis in the isolated lung. Am J Respir Crit Care Med. 2000b;162:399–405. doi: 10.1164/ajrccm.162.2.9911026. [DOI] [PubMed] [Google Scholar]
- Laffey JG, Tanaka M, Engelberts D, Luo X, Yuan S, Tanswell AK, Post M, Lindsay T, Kavanagh BP. Therapeutic hypercapnia reduces pulmonary and systemic injury following in vivo lung reperfusion. Am J Respir Crit Care Med. 2000c;162:2287–2294. doi: 10.1164/ajrccm.162.6.2003066. [DOI] [PubMed] [Google Scholar]
- Masood A, Yi M, Lau M, Belcastro R, Shek S, Pan J, Kantores C, McNamara PJ, Kavanagh BP, Belik J, Jankov RP, Tanswell AK. Therapeutic effects of hypercapnia on chronic lung injury and vascular remodeling in neonatal rats. Am J Physiol Lung Cell Mol Physiol. 2009;297:L920–L930. doi: 10.1152/ajplung.00139.2009. [DOI] [PubMed] [Google Scholar]
- Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L, Vieillard-Baron A. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–1858. doi: 10.1007/s00134-009-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Chonghaile M, Higgins BD, Costello JF, Laffey JG. Hypercapnic acidosis attenuates severe acute bacterial pneumonia-induced lung injury by a neutrophil-independent mechanism. Crit Care Med. 2008;36:3135–3144. doi: 10.1097/CCM.0b013e31818f0d13. [DOI] [PubMed] [Google Scholar]
- Nishio K, Suzuki Y, Takeshita K, Aoki T, Kudo H, Sato N, Naoki K, Miyao N, Ishii M, Yamaguchi K. Effects of hypercapnia and hypocapnia on [Ca2+]i mobilization in human pulmonary artery endothelial cells. J Appl Physiol. 2001;90:2094–2100. doi: 10.1152/jappl.2001.90.6.2094. [DOI] [PubMed] [Google Scholar]
- O’Croinin DF, Hopkins NO, Moore MM, Boylan JF, McLoughlin P, Laffey JG. Hypercapnic acidosis does not modulate the severity of bacterial pneumonia-induced lung injury. Crit Care Med. 2005;33:2606–2612. doi: 10.1097/01.ccm.0000186761.41090.c6. [DOI] [PubMed] [Google Scholar]
- O’Croinin DF, Nichol AD, Hopkins N, Boylan J, O’Brien S, O’Connor C, Laffey JG, McLoughlin P. Sustained hypercapnic acidosis during pulmonary infection increases bacterial load and worsens lung injury. Crit Care Med. 2008;36:2128–2135. doi: 10.1097/CCM.0b013e31817d1b59. [DOI] [PubMed] [Google Scholar]
- O’Toole D, Hassett P, Contreras M, Higgins BD, McKeown ST, McAuley DF, O’Brien T, Laffey JG. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-κB dependent mechanism. Thorax. 2009;64:976–982. doi: 10.1136/thx.2008.110304. [DOI] [PubMed] [Google Scholar]
- Shibata K, Cregg N, Engelberts D, Takeuchi A, Fedorko L, Kavanagh BP. Hypercapnic acidosis may attenuate acute lung injury by inhibition of endogenous xanthine oxidase. Am J Respir Crit Care Med. 1998;158:1578–1584. doi: 10.1164/ajrccm.158.5.9804039. [DOI] [PubMed] [Google Scholar]
- Sinclair SE, Kregenow DA, Lamm WJ, Starr IR, Chi EY, Hlastala MP. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2002;166:403–408. doi: 10.1164/rccm.200112-117OC. [DOI] [PubMed] [Google Scholar]
- Somero GN. Protons, osmolytes, and fitness of internal milieu for protein function. Am J Physiol Regul Integr Comp Physiol. 1986;251:R197–R213. doi: 10.1152/ajpregu.1986.251.2.R197. [DOI] [PubMed] [Google Scholar]
- Swenson ER, Robertson HT, Hlastala MP. Effects of inspired carbon dioxide on ventilation-perfusion matching in normoxia, hypoxia, and hyperoxia. Am J Respir Crit Care Med. 1994;149:1563–1569. doi: 10.1164/ajrccm.149.6.8004314. [DOI] [PubMed] [Google Scholar]
- Takeshita K, Suzuki Y, Nishio K, Aoki T, Takeuchi O, Toda K, Sato N, Naoki K, Kudo H, Yamaguchi K. Hyperoxia and hypercapnic acidosis differentially alter nuclear factor-kappa B activation in human pulmonary artery endothelial cells. Adv Exp Med Biol. 1999;471:265–270. doi: 10.1007/978-1-4615-4717-4_32. [DOI] [PubMed] [Google Scholar]
- Turek Z, Kreuzer F. Effect of shifts of the O2 dissociation curve upon alveolar-arterial O2 gradients in computer models of the lung with ventilation-perfusion mismatching. Respir Physiol. 1981;45:133–139. doi: 10.1016/0034-5687(81)90055-4. [DOI] [PubMed] [Google Scholar]
- Wang Z, Su F, Bruhn A, Yang X, Vincent JL. Acute hypercapnia improves indices of tissue oxygenation more than dobutamine in septic shock. Am J Respir Crit Care Med. 2008;177:178–183. doi: 10.1164/rccm.200706-906OC. [DOI] [PubMed] [Google Scholar]
- Wung JT, James LS, Kilchevsky E, James E. Management of infants with severe respiratory failure and persistence of the fetal circulation, without hyperventilation. Pediatrics. 1985;76:488–494. [PubMed] [Google Scholar]


