Abstract
We investigated the role of myoendothelial projections (MPs) in endothelial cell (EC) feedback response to smooth muscle cell (SMC) stimulation using mathematical modelling. A previously developed compartmental EC–SMC model is modified to include MPs as subcellular compartments in the EC. The model is further extended into a 2D continuum model using a finite element method (FEM) approach and electron microscopy images to account for MP geometry. The EC and SMC are coupled via non-selective myoendothelial gap junctions (MEGJs) which are located on MPs and allow exchange of Ca2+, K+, Na+ and Cl− ions and inositol 1,4,5-triphosphate (IP3). Models take into consideration recent evidence for co-localization of intermediate-conductance calcium-activated potassium channels (IKCa) and IP3 receptors (IP3Rs) in the MPs. SMC stimulation causes an IP3-mediated Ca2+ transient in the MPs with limited global spread in the bulk EC. A hyperpolarizing feedback generated by the localized IKCa channels is transmitted to the SMC via MEGJs. MEGJ resistance (Rgj) and the density of IKCa and IP3R in the projection influence the extent of EC response to SMC stimulation. The predicted Ca2+ transients depend also on the volume and geometry of the MP. We conclude that in the myoendothelial feedback response to SMC stimulation, MPs are required to amplify the SMC initiated signal. Simulations suggest that the signal is mediated by IP3 rather than Ca2+ diffusion and that a localized rather than a global EC Ca2+ mobilization is more likely following SMC stimulation.
Key points
Endothelial microprojections (MPs) are cellular extensions of endothelial cells (ECs) that may be involved in regulation of smooth muscle cell (SMC) constriction in blood vessels.
We developed computational models to investigate the role of MPs in generating EC feedback during SMC stimulation. The models account for the geometry of MPs and heterogeneous distribution of membrane channels and receptors in an EC.
Simulations show that SMC stimulation causes calcium release in and around EC MPs that activates hyperpolarizing currents in ECs and moderates SMC constriction.
The results help us better understand the mechanisms that regulate blood flow and pressure.
Introduction
Myoendothelial communication plays an important role in vascular tone regulation. Complex bidirectional pathways exist between the endothelial cell (EC) and smooth muscle cell (SMC) layers which regulate SMC constriction and vessel diameter. Agonist stimulation of SMCs may lead to a calcium (Ca2+) response in ECs sufficient to initiate Ca2+-dependent vasodilatory signals such as the endothelium-derived hyperpolarizing factor (EDHF). Such EC response can moderate SMC constriction and is referred to as myoendothelial feedback. The presence of endothelial feedback after SMC stimulation was first suggested by Dora et al. (1997) and remains a topic of active investigation (Dora et al. 2008; Ledoux et al. 2008a; Sandow et al. 2009b; Tran et al. 2012). Different aspects of this feedback response remain unresolved including the identity of the main mediator in SMC-to-EC communication (Ca2+ or inositol 1,4,5-triphosphate (IP3)) and the characteristics of the EC response, as well as whether attenuation of this mechanism contributes to vessel pathology (Sandow et al. 2009b; Kerr et al. 2012).
Myoendothelial projections (MPs) are cellular extensions from ECs and/or SMC that extend over the internal elastic lamina and come in close contact with the other cell type (Heberlein et al. 2009; Sandow et al. 2009b). In spite of their early discovery in 1957 (Moore & Ruska, 1957), the functional importance of MPs has only recently begun to be appreciated (Kerr et al. 2012). Electron microscopy studies confirm the presence of MPs in many different vessels, including in rat mesenteric arteries (Ledoux et al. 2008a; Sandow et al. 2009b; Tran et al. 2012). MPs vary both in number and size among different vascular beds, age, species, sex and diseased states of animals (Sandow et al. 2009a). An increase in incidences of MPs with decrease in vessel diameter is often reported (Heberlein et al. 2009; Sandow et al. 2009a), which correlates with an increase in EDHF action (Garland et al. 1995; Shimokawa et al. 1996; Hill et al. 2000, 2002) but not with the activity of the endothelium-derived relaxing factor (EDRF) whose efficacy often diminishes with decrease in vessel size (Luksha et al. 2009).
Recent immunohistochemical labelling studies show evidence for localization of IP3 receptors (IP3Rs), intermediate conductance Ca2+-activated potassium channels (IKCa) and connexins in MPs (Crane et al. 2003; Sandow et al. 2006; Dora et al. 2008; Isakson, 2008; Ledoux et al. 2008a; Sandow et al. 2009b). Myoendothelial gap junctions (MEGJs) are often located on top of these projections and are responsible for electrochemical communication between ECs and SMCs (Heberlein et al. 2009; Sandow et al. 2009a,b). It has been proposed that a small amount of IP3 and/or Ca2+ entering from the stimulated SMC can accumulate in the restricted space of MPs and activate localized IP3Rs, leading to rapid release of Ca2+ from the endoplasmic reticulum (ER) stores and amplification of Ca2+ response. In support of this theory, spontaneous and agonist initiated local Ca2+ events like ‘pulsars’ (Ledoux J, 2008b) and ‘wavelets’ (Tran et al. 2012) have been shown to occur in and around MP sites in ECs following both EC as well as SMC stimulation. The occurrence of these Ca2+ events in the vicinity of localized IKCa channels is consistent with a role for the EDHF in closing the myoendothelial feedback loop. Endothelial nitric oxide synthase (eNOS) may also colocalize at MPs, but evidence indicates a limited role for NO in the feedback response (Nausch et al. 2012).
Mathematical modelling can assist in further analysis of the experimental data, but few theoretical studies have investigated EC–SMC communication. In a recent study, Brasen et al. (2012) describe a 2D-axisymmetric model incorporating the anatomical structure of MPs to show the spatiotemporal modulation of Ca2+ and IP3 in EC, SMC and MPs during EC and SMC stimulation. In this model, they highlight the competence of MPs in rectification of Ca2+ signals by the virtue of their structure and location. However, the role of the membrane potential (Vm), the effect of plasma membrane channels, the localization of channels and receptors, and the myoendothelial feedback in the heterocellular communication have yet to be modelled. In our previously developed EC–SMC model (Kapela et al. 2009), we integrated detailed single cell EC (Silva et al. 2007) and SMC (Kapela et al. 2008) models with electrical, chemical and NO coupling pathways. Agonist stimulation of ECs increased Ca2+ in ECs, activated EDHF and EDRF pathways, hyperpolarized SMC, and reduced SMC Ca2+. Ca2+ fluxes from stimulated SMC or EC did not elevate global Ca2+ in the other cell. EC feedback response to SMC stimulation was observed only when we significantly increased gap junction permeability to IP3 and EC IP3R density relatively to previously suggested values.
In this study, we develop computational models to investigate the role of MPs in myoendothelial communication. We extend our previously developed EC–SMC model to incorporate a subcompartment within the EC that simulates the presence of MPs (Fig. 1A). We also present a continuum model capable of incorporating accurate MP geometry from electron microscopy images (Fig. 2A) and account for spatial localization of IKCa and IP3Rs as suggested in experimental studies (Crane et al. 2003; Sandow et al. 2006; Isakson, 2008; Ledoux et al. 2008a). The current models provide theoretical insights into the role of MPs.
Figure 1.
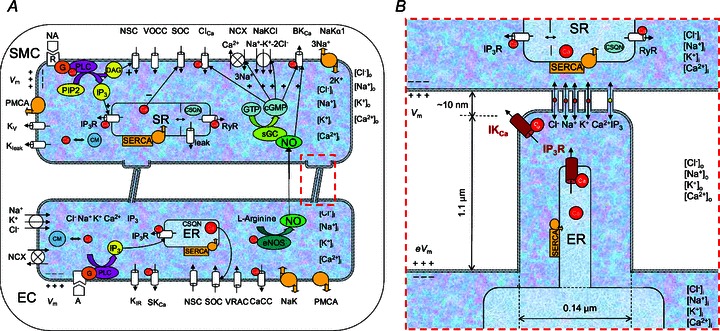
A, schematic diagram showing all the channels and pumps incorporated in the EC–MP–SMC compartmental model. B, characteristics of the EC microprojections (MP). Localized (high density) IKCa channels and IP3Rs are present in the MP. All other channels and pumps are distributed proportionally to the volumes of the EC bulk and MP (the other channels in MP are not shown for clarity).
Figure 2.
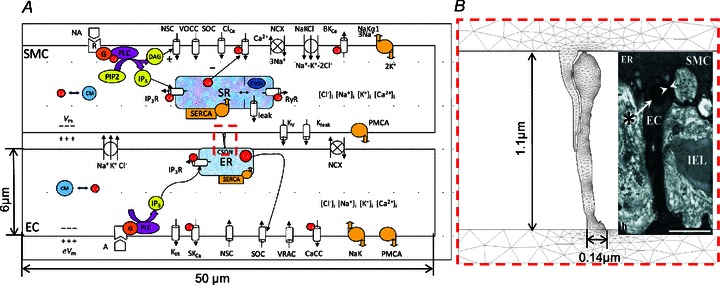
A, 2D FEM model geometry with SMC and EC as rectangular segments and with a MP whose shape is imported from electron microscopy image by (Sandow et al. 2009b) (inset in B). Ion channel currents are uniformly distributed along the top and bottom boundaries of each cell. B, EC projection with mesh. IKCa channels and IP3Rs are localized in the MP. All other channels and pumps are also present in MP membrane, but proportionally to MP volume. The ER is homogeneously spread across the entire EC including the MPs.
Methods
Compartmental model
The current model is based on our previously developed two-cell EC–SMC model (Kapela et al. 2009). The earlier models were optimized for a specific tissue (i.e. model descriptions and parameters were based on experimental data obtained primarily from rat mesenteric resistance arteries). We modified the EC by partitioning it into two compartments representing the bulk EC and the MP as shown in Fig. 1A. Parametric studies were performed to determine the influence of certain parameters on model responses.
Geometric parameters
Figure 1B shows the dimensions used to calculate effective volume, area and diffusion parameters for the EC MP compartment. The length and width of MPs are taken from electron microscopy images of rat mesenteric artery (Sandow et al. 2009b). 2.7 MEGJs per endothelial cell were assumed (Dora et al. 2008) and that every projection contains one gap junction. The total EC volume (1 pl; Schuster et al. 2003) is divided into bulk EC volume and a much smaller MP volume. MP volume is calculated by assuming the MP to be cylindrically shaped with dimensions as shown in Fig. 1B. Membrane area, whole cell capacitance and the ER/SR are divided between the two compartments proportionally to their volume ratios. This allows for appropriate division of intracellular fluxes between the two compartments.
Ionic channel distributions
The model schematic is shown in Fig. 1A. The whole cell conductances of the channels and pumps are maintained the same as in the previous EC and SMC models (Silva et al. 2007; Kapela et al. 2008). The following channels are assumed uniformly distributed across the membrane of EC and their maximum conductances are scaled by the bulk and MP volumes: Inwardly rectifying potassium channel (Kir), non-selective cation channel (NSC), small conductance calcium activated potassium channel (SKCa), store-operated channel (SOC), calcium activated chloride channel (CaCl), sodium-potassium pump (NaKα1), Na+–Ca2+exchanger (NCX), plasma membrane Ca2+-ATPase (PMCA), sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA). IKCa (Crane et al. 2003; Sandow et al. 2006, 2009b; Dora et al. 2008; Ledoux et al. 2008a) and IP3R (Isakson, 2008; Ledoux et al. 2008a; Sandow et al. 2009b) are localized to MPs as shown in Fig. 1B. The maximum conductance of IKCa channel (GIKCa) has been measured in porcine arteries (Bychkov et al. 2002), and was rescaled in our lumped EC model (Silva et al. 2007). Immunohistochemistry data suggest a high presence of IKCa channels and IP3Rs in the vicinity of MPs (Sandow et al. 2006; Dora et al. 2008; Isakson, 2008; Ledoux et al. 2008a; Sandow et al. 2009b). IKCa labelling is very punctuated and colocalized with MPs in some vascular beds, e.g. rat mesenteric arteries (Dora et al. 2008; Ledoux et al. 2008a; Sandow et al. 2009b), but more dispersed in others (McNeish et al. 2006; Tran et al. 2012) and therefore their distribution between MPs and the rest of the cell is likely to vary. Thus, under control conditions we assume that the majority of IKCa channels are concentrated in MPs (Table 1) and we also performed simulations for different levels of IKCa localization. Similarly, a significant percentage (i.e. 10% under control conditions) of the total IP3Rs is assumed to be localized inside the MPs and the remaining distributed in the bulk of EC. This corresponds to a much higher IP3R density in MP than in the bulk of EC. (The distribution of IP3Rs is controlled by the maximum current parameter (IIP3R,max, Table 1).) The degree of localization (10%) was assumed based on the fact that IP3Rs are not as highly colocalized with MPs as IKCa (Ledoux et al. 2008a; Sandow et al. 2009b). In the SMC model, single cytosolic compartment integrates currents through the following channels: voltage-dependent L-type calcium channel (VOCC), delayed rectifier potassium channel (Kv), NSC, ATP activated potassium channel (KATP), large conductance calcium activated potassium channel (BKCa), SOC, CaCl, NCX, and PMCA and NaKα1 pumps. Norepinephrine (NA) stimulation generates IP3 through Ca2+-dependent phospholipase-C (PLC). MEGJs connect the MP compartment to the SMC (Sandow & Hill, 2000; Dora et al. 2003). Permeability of gap junctions is calculated based on experimental data for the total MEGJ resistance (Rgj; Yamamoto et al. 2001). MEGJs are assumed to be non-selective and permeable to Na+, K+, Ca2+, Cl− ions and IP3. The EDRF–NO pathway is blocked for all simulations.
Table 1.
List of parameters describing dimensions of MPs and microdomains along with values of diffusion constants of ions and IP3 in the cytosol
| Parameter | Value | Description |
|---|---|---|
| GIKCa | Bulk EC: 0.0 nS | Maximum IKCa channel conductance |
| MP: 1.72 nS (Kapela et al. 2009) | ||
| IIP3R,max | Bulk EC: 1.053 ×103 pA mm−1 | Maximum current through IP3R per concentration difference |
| MP: 0.117 × 103 pA mm−1 | ||
| Kd_act | Low: 130 nm (Jacobsen et al. 2007) | [Ca2+] for half-maximal activation of IP3R |
| High: 350 nm (assumed) | ||
| f | Control: 1.75 | Diffusional restriction between MP and bulk EC |
| No MP : 10−3 | ||
| DCa | 300 μm2 s−1 (Allbritton et al. 1992) | Diffusivity of free calcium in cytosol |
| DK | 744 μm2 s−1 | Diffusivity of potassium in cytosol |
| DNa | 505 μm2 s−1 | Diffusivity of sodium in cytosol |
| DCl | 900 μm2 s−1 | Diffusivity of chloride in cytosol |
| DIP3 | 283 μm2 s−1 (Allbritton et al. 1992) | Diffusivity of IP3 in cytosol |
| Amp | 0.0154 μm2 (Sandow & Hill, 2000) | Area of a single MP |
| Lmp | 3.5 μm (Sandow & Hill, 2000) | Diffusion length from MP to bulk EC |
| Nmp | 2.7 (Dora et al. 2008) | Number of MEGJ/EC |
| F | 96487.0 C mol−1 | Faraday's constant |
| R | 8341 mJ (mol K)-1 | Universal gas constant |
| T | 293 K | Temperature |
| zK, zNa, zCa, zCl | 1, 1, 2, –1 | Ion valency of K+, Na+, Ca2+, Cl− |
Coupling between MPs and bulk EC: diffusion currents
We used (electro) diffusion equations to describe fluxes of the four ions and IP3. The equations account for fluxes between two compartments and their dependence on Vm as well as concentration difference:
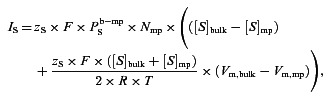 |
(1) |
| (2) |
where S represents Na+, K+, Ca2+ and Cl−. Suffixes ‘mp’ and ‘bulk’ represent the MP and bulk compartments in EC. 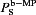 is the permeability of species S between the MP and the bulk of the cell. A first approximation for its value can be acquired from the species diffusivity (Ds), MP cross-sectional area (Amp) and the MP length (Lmp), i.e.
is the permeability of species S between the MP and the bulk of the cell. A first approximation for its value can be acquired from the species diffusivity (Ds), MP cross-sectional area (Amp) and the MP length (Lmp), i.e.
A correction factor (f) was also utilized to account for the effects of buffering/degradation/extrusion and/or the presence of subcellular organelles that can further limit the exchange of species between the two compartments.
The ER in MP is coupled with the bulk ER using diffusion equation for Ca2+ transport:
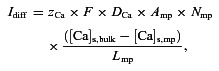 |
(3) |
where [Ca]s is the concentration of Ca2+ in the ER stores. All parameter values and their description are listed in Table 1.
Calcium-induced calcium release (CICR)
The default IP3R kinetics in the EC bulk and MP is the same as in the previous EC-SMC model (Silva et al. 2007; Kapela et al. 2009), comprising of IP3-dependent activation and Ca2+-dependent inactivation terms:
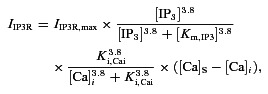 |
(4) |
where [Ca]S and [Ca]i represent the ER and cytosolic calcium concentrations respectively and [IP3] represents intracellular IP3 concentration. Km,IP3 and Ki,Cai are the half-activation and -inhibition constants for IP3 activation and Ca2+-dependent inhibition of the IP3R current.
To test the potential of intercellular Ca2+ flux to induce CICR through IP3Rs, the IP3R current in the EC was modified to include a calcium-dependent activation component (PCa) as shown in eqns (5) and (6).
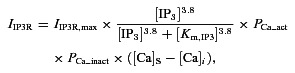 |
(5) |
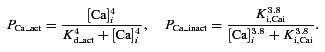 |
(6) |
Two values of Kd_act were examined as listed in Table 1. The lower value of Kd_act was taken from a model of SMCs (Jacobsen et al. 2007), and the second value is an average from various cell types (Finch et al. 1991; Atri et al. 1993).
Numerical methods
All other model equations are described in detail in previous studies (Silva et al. 2007; Kapela et al. 2008, 2009). The compartmental EC and SMC models are implemented using 20 and 26 ordinary differential equations, respectively. Table 1 lists any changed or newly introduced parameter values. The rest of the parameter values remain unchanged from the previous EC–SMC model. The equations are coded in Fortran 90 and solved numerically using Gear's backward differentiation formula method for stiff systems (IMSL Numerical Library routine). The maximum time step was 1 ms and the tolerance for convergence was 0.0005.
Finite element method (FEM) model
The compartmental EC–MP model described above does not allow us to predict intracellular concentration gradients, the spatiotemporal nature of Ca2+ mobilization and the localization of the Ca2+ signal. Furthermore, preliminary simulations revealed that the feedback response depends significantly on the permeability between the MP and the bulk EC cytosol (adjusted through the factor ‘f’’). Calculation of fluxes between two unequal compartments (MP and bulk EC) needs to take into account a variable diffusion area, boundary fluxes, buffering of Ca2+ and degradation of IP3 which are difficult to accurately capture through lumped diffusion parameters used in the compartmental model. Thus, we developed a FEM model to account for the spatial gradients of Ca2+ inside the EC MP and for the diffusion of species from the MP to the bulk EC with higher accuracy. A 2D model also allows us to incorporate accurate MP geometry by importing MP images from experimental studies and to account for spatial localizations of IKCa and IP3Rs within the MPs.
The model is developed using the Chemical Engineering module of COMSOL, similarly to (Kapela & Tsoukias, 2011). While a 3D cylindrical axisymmetric model of EC and SMC was implemented in (Kapela & Tsoukias, 2011), here we assumed a simplified geometry and EC and SMC are represented as rectangular structures with dimensions as shown in Fig. 2A. The model implements only half of the EC and SMC. The results in the remaining half are assumed to be symmetrical. The shapes of the projections are imported from experimental electron microscopy images (Heberlein et al. 2009; Sandow et al. 2009b; Tran et al. 2012; Fig. 5). Volume of MP is calculated assuming the MP to be cylindrical with diameter and length as shown in Fig. 2B. Two rectangular domains connect the SMC and EC MPs to represent MEGJs. Differences in height (depth) of the cell's bulk, MPs, and MEGJs have been accounted for by appropriate scaling of fluxes at the boundaries between MEGJ and MP and at boundary between MP and bulk cytosol.
Figure 5.
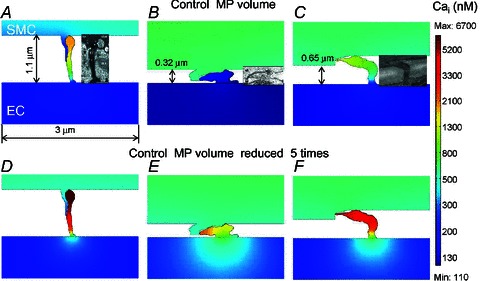
Predicted Ca2+ concentration inside the MP after NA stimulation of SMC for different MP geometries: (A–C) control MP volume and shape, (D–F) MP volume reduced five-fold. Insets in (A–C) show the original electron microscopy images of the projections reproduced with permission from John Wiley and Sons, and The American Physiological Society (Heberlein et al. 2009; Sandow et al. 2009b; Tran et al. 2012).
Nernst-Planck equations describe the electrodiffusion of the ions in the EC, MP, SMC and MEGJs:
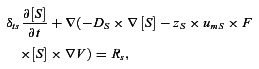 |
(7) |
where [S] is the intracellular concentration of the four ionic species (Ca2+, Na+, K+, Cl−), Ds is the diffusivity of the respective ions, 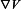 is the electrical gradient, zS is the charge, F is the Faraday constant, and umS is the mobility of the respective ion. Rs is the source/sink term which includes expressions for Ca2+ release/uptake from the ER/SR, and Ca2+ buffering in EC bulk and MP. In SMC, fast Ca2+ buffering is represented by the Ca2+-dependent function δts. Diffusion of IP3 and buffered Ca2+ in the cytosol, and Ca2+ in ER/SR is implemented using diffusion equations inside the SMC, EC, MP and MEGJs domains:
is the electrical gradient, zS is the charge, F is the Faraday constant, and umS is the mobility of the respective ion. Rs is the source/sink term which includes expressions for Ca2+ release/uptake from the ER/SR, and Ca2+ buffering in EC bulk and MP. In SMC, fast Ca2+ buffering is represented by the Ca2+-dependent function δts. Diffusion of IP3 and buffered Ca2+ in the cytosol, and Ca2+ in ER/SR is implemented using diffusion equations inside the SMC, EC, MP and MEGJs domains:
| (8) |
where [S] is the concentration of either species (IP3, Ca2+S, Ca2+buffered). In EC, IP3 production is introduced by the Rs term, while in SMC agonist receptors and IP3 formation are described by an embedded weak boundary equation.
All model equations describing the membrane and store channels are the same as in the compartmental model. The membrane currents are defined as boundary fluxes across the top and bottom boundaries of EC, SMC and all boundaries of the MP. The left and right boundaries of the cell are assumed insulated. To distribute the channels uniformly, the currents are divided between the MP and bulk cytosol according to the volumes of MP and bulk cytosol. Similarly to the compartmental model, the whole maximum conductance of IKCa channels (GIKCa) and 10% of IP3Rmax current were placed in EC MPs. 900 MΩ is used as control Rgj unless stated otherwise.
The equations are solved using the SPOOLES direct solver with absolute and relative tolerances of 10−3 and 10−2 respectively. The solution time was 30 s with a time stepping of 0.1 s. The different geometries were divided into ∼1000 elements. Unless stated otherwise, the NO pathway is blocked for all simulations.
Results
Global vs. local Ca2+ and IP3 changes
Figure 3 shows Ca2+ and IP3 changes in bulk EC and MP compartments during SMC (Fig. 3A) and EC (Fig. 3B) agonist stimulations. Following SMC stimulation with NA (Fig. 3A), the IP3 diffusing from SMC into the EC MP causes a high IP3 transient in the MP, which does not spread into the bulk of EC. A large Ca2+ transient is generated in the MP by activation of IP3Rs localized in the MP. Like IP3, Ca2+ in the MP does not spread much into the bulk EC compartment. IKCa channels localized in the MPs are activated by the Ca2+ transients and generate hyperpolarizing current that spreads through gap junctions to moderate SMC depolarization (Fig. 3C). The high Ca2+ and IP3 transients in the MP after NA stimulation of SMC are attributed to the small volume of the MP, the localization of IP3Rs and the restricted diffusion between the MP and bulk EC. Thus, the magnitude of the Ca2+ and IP3 transients depend on the assumed value of effective permeability 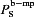 . Simulations with a wide range of permeability values were performed by varying parameter f (data not shown). Correction factor f < 0.1 makes the bulk and MP well mixed and effectively lacking a functional MP compartment, while f≫ 1.75 (control) creates excessive electrical resistance and the two compartments are no longer near isopotential.
. Simulations with a wide range of permeability values were performed by varying parameter f (data not shown). Correction factor f < 0.1 makes the bulk and MP well mixed and effectively lacking a functional MP compartment, while f≫ 1.75 (control) creates excessive electrical resistance and the two compartments are no longer near isopotential.
Figure 3.
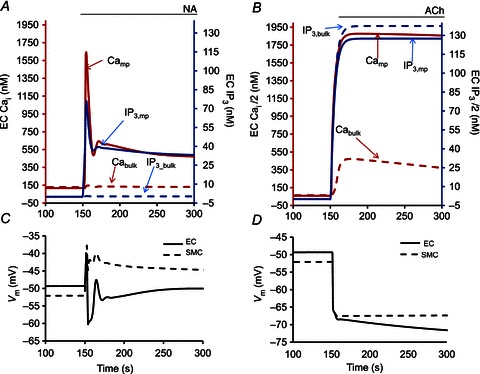
Compartmental model results showing Ca2+ and IP3 concentrations in the EC MP (continuous lines) and EC bulk (dashed lines) during 1 μm NA stimulation of SMC (A) and ACh stimulation of EC (B). Corresponding Vm in EC (continuous lines) and SMC (dashed lines) are shown in C and D. MEGJ resistance is maintained at 900 MΩ with restricted diffusion between the projection and bulk EC. 10% of IP3Rs are concentrated in the MP compartment.
Acetylcholine (ACh) stimulation of bulk EC compartment increases the global Ca2+ concentration through the production of IP3 (Fig. 3B) and opening of IP3Rs in ER. Diffusion of IP3 from the bulk to MP generates a Ca2+ increase ∼7 times higher than in bulk EC because of the localization of IP3Rs in the small volume of MPs and restricted diffusion between the two compartments. The Ca2+ increase in the bulk and MP compartments opens SKCa and IKCa channels and hyperpolarizes the EC. This change in EC Vm is transmitted through MEGJs as EDHF and hyperpolarizes SMC (Fig. 3D).
In agreement with the compartmental model, the 2D FEM model predicts that NA stimulation of SMC generates high IP3 (Fig. 4C) and Ca2+ (Fig. 4A) transients inside the EC MP with minimal spread into the bulk EC. Simulations with ACh stimulation (Fig. 4B and D) are also consistent with the compartmental model (Fig. 3B). In Fig. 5, we examined the effect of MP geometry on the spread of Ca2+ from the MP into the bulk EC. The three MP geometries examined are from the electron microscopy studies of rat mesenteric arteries (Sandow et al. 2009b), mouse cremaster arterioles (Heberlein et al. 2009), and hamster vessels (Tran et al. 2012). Under control MP volume, the long MP with comparatively small diffusion area yielded a large Ca2+ transient which did not spread much into the bulk of EC (Fig. 5A). The transients decreased with smaller heights and larger surface areas of the MPs (Fig. 5B and C). The short MP with large surface area did not have a significant Ca2+ transient in the MP (Fig. 5B). If the volume of each MP was reduced 5 times, effectively making it flatter, the predicted Ca2+ transients in the MPs increased significantly in all three cases (Fig. 5D–F), but the MP with higher surface area (Fig. 5E) allowed for a comparatively bigger spread of Ca2+ into the bulk of EC.
Figure 4.
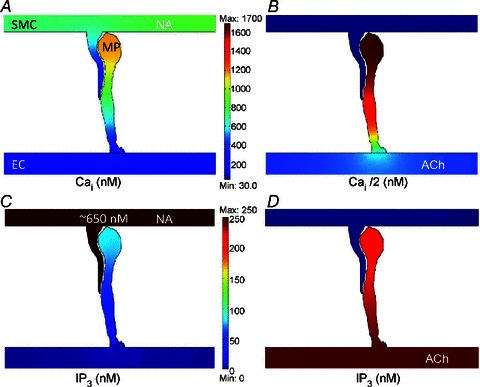
2D FEM model results for NA stimulation of SMC (A, C) and ACh stimulation of EC (B, D). Predicted Ca2+ concentration inside the MP after NA stimulation of SMC (A) and bulk EC stimulation with ACh (B). The corresponding IP3 transients in the MP for SMC and EC stimulation are shown in C and D, respectively. Ca2+ and IP3 concentration profiles are shown 7.5 s after 1 μm NA stimulation of SMC and 2 s after ACh stimulation of EC, respectively.
Effect of MPs
Figure 6 shows the effect of EC MPs on feedback in SMC Vm. In the presence of MPs, an approximately 2–3 mV feedback can be achieved, compared to the model with a single well-mixed EC compartment (Fig. 6A, continuous vs. dashed lines). The feedback is lost in the model without MPs because NA cannot generate a Ca2+ transient in the single compartment model (Fig. 6B, dashed vs. continuous lines). In fact, the global EC Ca2+ decreases in the model without MPs (Fig. 6B, dashed line) because of SMC depolarization transmitted through MEGJs to EC. Large increases in the gap junction permeability (∼102-fold; Rgj= 9 MΩ), and maximum IP3R current (∼103-fold) in the single EC compartment model could compensate partially for the absence of MPs (Fig. 6A and B, dotted lines). From the results, it is evident that the presence of MPs facilitates the production of a hyperpolarizing feedback from EC to SMC following SMC stimulation.
Figure 6.
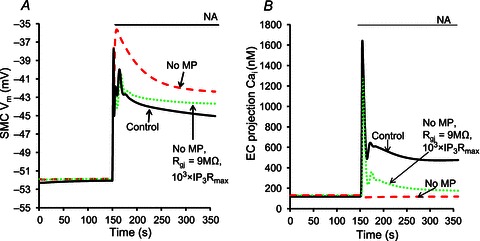
SMC Vm (A) and EC MP Ca2+ (B) under three scenarios: no MP (dashed lines), control (continuous lines), and no MP with MEGJ resistance reduced from control 900 MΩ to 9 MΩ and the maximum IP3R current increased 103-fold (dotted lines).
IP3 vs. Ca2+ signalling
Figure 7 examines the relative contributions of IP3 and Ca2+ diffusion from stimulated SMC to EC to the generation of MP Ca2+ transients and the myoendothelial feedback. Average Ca2+ from MP in the 2D FEM model after SMC stimulation is plotted for different MEGJ resistances before (Fig. 7A) and after (Fig. 7B) blockade of intercellular IP3 diffusion. Under control Rgj (900 MΩ as estimated by Yamamoto et al. 2001), IP3 diffusion appears to be the major signalling molecule (Fig. 7A, continuous line), as the Ca2+ transient achieved by intercellular Ca2+ diffusion during IP3 blockade (Fig. 7B, continuous line) is insignificant. In contrast, blocking Ca2+ diffusion did not cause a significant change in the MP Ca2+ transient (Fig. 7B, inset, dashed line vs. continuous line). Along with the Ca2+ transient, the feedback in SMC Vm is also abolished by blockade of IP3 diffusion from SMC to EC (Fig. 7C, continuous vs. dashed lines). At low Rgj values (250 MΩ > Rgj > 50 MΩ, based on 70 MΩ from Sandow & Hill, 2000), The Ca2+ transient arising purely from diffusion of Ca2+ (Fig. 7B) becomes significant and might cause a minor feedback. In general, the predicted Ca2+ transient in MP increases with decrease in Rgj and is mediated mainly by IP3 rather than Ca2+ diffusion.
Figure 7.
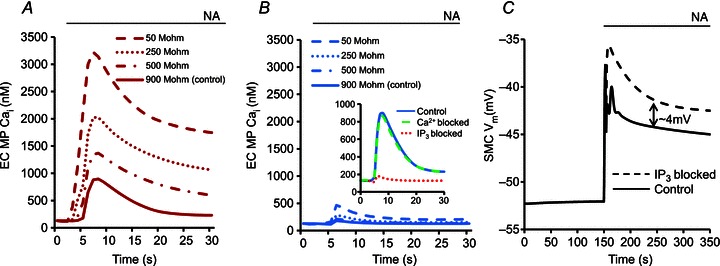
Ca2+ transients in the MP after NA stimulation of SMC in the 2D FEM model under control conditions (A) and with IP3 diffusion across MEGJs blocked (B), for different MEGJ resistances. The variation in Rgj is based on resistances estimated in experimental studies (Sandow & Hill, 2000; Yamamoto et al. 2001). C, SMC Vm under control conditions (continuous line) and with IP3 diffusion from SMC to EC blocked in the compartmental model after NA stimulation of SMC. The arrow line (⇕) shows the magnitude of the feedback mediated by the IP3 diffusion.
Effect of IKCa distribution
Experimental studies suggest that the quantity of IKCa channels in MPs is likely to vary between vascular beds and vessel types (Crane et al. 2003; Sandow et al. 2006; Dora et al. 2008; Ledoux et al. 2008a; Tran et al. 2012). Figure 8 shows the effect of varying IKCa distribution between MP and bulk EC on myoendothelial feedback in the compartmental model. The myoendothelial feedback after SMC stimulation increases with increased localization of IKCa channels in the MP (Fig. 8A), although the amount of IKCa channels in the MP does not affect the Ca2+ transient in the MP (Fig. 8B). The maximum feedback is achieved under control conditions with 100% IKCa channels present in the MP (Fig. 8A, continuous line). With uniform distribution of IKCa channels (Fig. 8A, dashed line), the model loses its ability to generate feedback and resembles the output of the model with no MP (Fig. 6A, dashed line).
Figure 8.
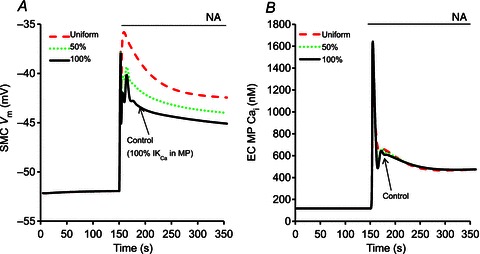
Compartmental model results showing SMC Vm (A) and Ca2+ concentration in the EC MP (B) after stimulation with NA under different IKCa distribution in EC. Uniform IKCa (dashed lines), 50% of IKCa channels in MP (dotted lines), and all IKCa channels in MP (continuous lines).
Effect of Rgj
Values for the myoendothelial Rgj reported in the literature vary significantly. A lower value of 70 MΩ has been reported based on morphological observations. A higher value of 900 MΩ presents perhaps a better estimate as it was derived from electrical measurements (Sandow & Hill, 2000; Yamamoto et al. 2001; de Wit & Griffith, 2010). The effect of varying Rgj on the feedback is shown in more detail in Fig. 9. In all simulations, both IP3 and Ca2+ coupling are present and the permeability of MEGJs to IP3 and Ca2+ is inversely proportional to the assumed MEGJ resistance. Consistent with Fig. 7A, reduction of Rgj enhances the feedback in SMC Vm (Fig. 9A) and SMC Ca2+ (Fig. 9B). For low values of MEGJ resistances (<250 MΩ), IP3 diffusion from SMC induces large Ca2+ responses in MP (Fig. 7A) and hyperpolarizing current from IKCa channels can overcome NA-induced SMC depolarization (Fig. 9A). This non-physiological response suggests that the combination of high IP3R density in MP and low Rgj are not physiological. The magnitude of the feedback in SMC Vm and SMC Ca2+ vs. Rgj is shown in Fig. 9C and D. The feedbacks are calculated as the difference between SMC Vm (or Ca2+) in the model with given Rgj and SMC Vm (or Ca2+) in the model with no MP after 200 s of NA stimulation of SMC. The simulations without MP (and Rgj= 900 MΩ) are taken as the reference because no feedback response is generated in this scenario.
Figure 9.
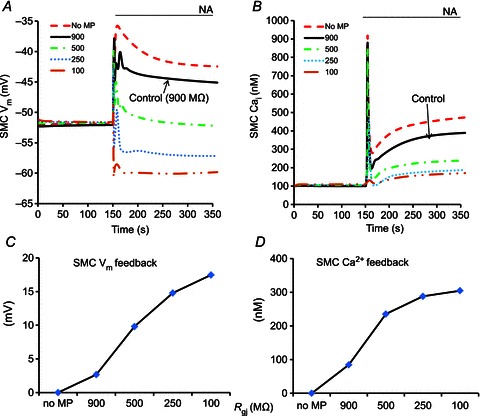
Compartmental model predictions of SMC Vm (A) and SMC Ca2+ transients (B) after SMC stimulation with NA for different Rgj values as shown in panel keys. The magnitude of the feedback in SMC Vm (C) and Ca2+ (D) is calculated at the end of simulation as a difference between the model with given Rgj and the ‘no MP’ model(dashed lines).
Effect of IP3Rs
Immunohistochemical labelling techniques report a significant localization of IP3Rs in the MPs, but their density and activation characteristics remain unclear and it is likely that they vary across different vascular beds (Isakson, 2008; Ledoux et al. 2008a; Ledoux J, 2008b; Sandow et al. 2009b). Figure 10 shows the predicted magnitude of the myoendothelial feedback as a function of IP3R distribution. In these simulations, the IP3R current was calculated according to eqn (4), which includes only IP3-dependent (but not Ca2+-dependent) activation. Greater feedback is achieved with higher IP3R density in the MP and around 6 mV of hyperpolarizing feedback can be achieved with 30% IP3R localization in MP (Fig. 10C, long dashed line). Under control conditions (10% IP3Rs in MP), the maximum feedback achieved is around 3 mV (Fig. 9A, continuous vs. dashed lines) under the assumed parameter values.
Figure 10.
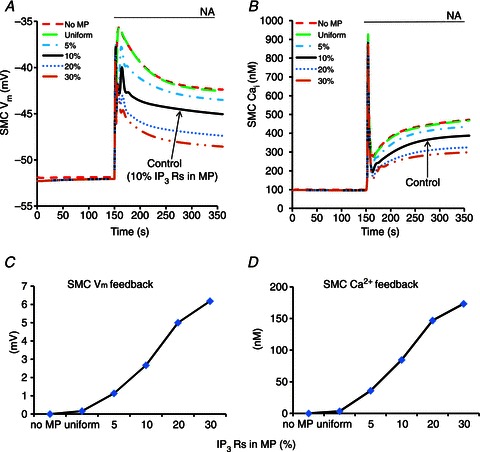
Compartmental model predictions of SMC Vm (A) and SMC Ca2+ transients (B) for different IP3R densities inside the MP as shown in panel keys. The magnitude of the feedback in SMC Vm (C) and SMC Ca2+ (D) is calculated at the end of simulation as a difference between the model with given IP3R distribution and the ‘no MP’ model(dashed lines).
Figure 11 examines whether the incorporation of Ca2+-dependent activation of IP3Rs and presence of basal IP3 can increase the role of intercellular Ca2+ diffusion in the feedback response. In these simulations, the IP3R current was calculated according to eqn (5), which includes both IP3- and Ca2+-dependent activations. SMC Vm at rest and following NA stimulation (1 μm) and is plotted as a function of basal IP3 concentration in EC (i.e. EC prestimulation). Simulations are repeated after blocking Ca2+ diffusion (dashed line), blocking IP3 diffusion (dotted line) and blocking both Ca2+ and IP3 diffusion (dashed-dotted line) between bulk EC and MP. The difference between the dashed-dotted and continuous lines is the amount of feedback. Simulations are performed for two different sensitivities of IP3R activation to Ca2+ (i.e. Kd_act).
Figure 11.
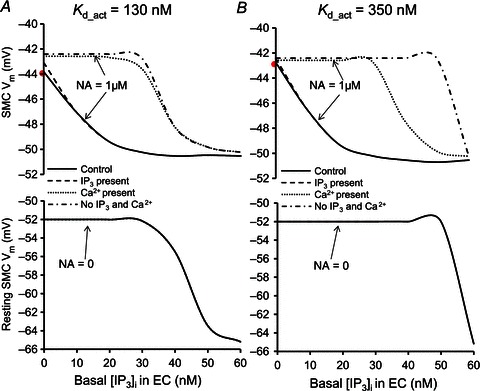
Compartmental model predictions of SMC Vm after 200 s of NA stimulation and at rest (NA = 0) for different basal IP3 concentrations in the EC, with control MEGJ coupling (continuous lines), IP3 diffusion alone (Ca2+ diffusion blocked) (dashed lines), Ca2+ diffusion alone (IP3 diffusion blocked) (dotted lines), and no IP3 or Ca2+ diffusion (dashed-dotted lines). A, simulations with low EC IP3R Kd_act= 130 nm. B, simulations with high EC IP3R Kd_act= 350 nm.
A first observation is that for both Kd_act values (Fig. 11A and B) SMC hyperpolarization when IP3 exchange is present is the same as in control (dashed and continuous lines). Thus, IP3 diffusion alone can produce maximum feedback response regardless of the contribution or not from Ca2+. With a low Ca2+-dependent IP3R activation parameter (Kd_act= 130 nm), Ca2+ diffusion alone does not generate significant feedback at any basal IP3 in EC (Fig. 11A, dotted vs. dashed-dotted line). IP3 diffusion generates feedback of a few millivolts at low basal IP3, and larger feedback at intermediate concentrations of basal IP3 (∼25 nm; Fig. 11A, dashed vs. dashed-dotted line). Ca2+ diffusion has a small synergistic effect with IP3 diffusion at low basal IP3, but it has no effect at intermediate and high basal IP3 (Fig. 11A, dashed vs. continuous line). High basal IP3 (>35 nm) activates EC independently from SMC and limits the feedback response.
With a high Ca2+-dependent activation parameter (Kd_act= 350 nm), Ca2+ diffusion alone could generate significant feedback in the presence of appropriate basal IP3 in EC (Fig. 11B, dotted vs. dashed-dotted line). EC prestimulation with basal IP3 in the range of 30–45 nm partially activates IP3Rs and elevates resting Ca2+ near Kd_act. At this point IP3Rs in MP are sensitized to small Ca2+ fluxes from SMC, and significant myoendothelial feedback can be mediated without IP3 coupling. IP3 diffusion generates weak feedback at low basal IP3, and large feedback at intermediate concentrations of basal IP3 (<45 nm; Fig. 11B, dashed vs. dashed-dotted line). In the presence of IP3 coupling, Ca2+ diffusion has little or no effect on the feedback (Fig. 11A, dashed vs. continuous line). In general, basal IP3 can sensitize EC to IP3 and Ca2+ fluxes from SMC. However, IP3 diffusion can initiate a significant feedback response under many scenarios of parameter values and conditions, while Ca2+ diffusion is required only when IP3 diffusion is blocked and its potential for feedback is conditional on an appropriate value for the sensitivity of the IP3R to Ca2+ and the existence of basal IP3 levels within a narrow concentration window.
Discussion
The primary aim of this study was to understand the role of MPs in myoendothelial signalling. Using the proposed models, we examined the effect of MPs on the intracellular Ca2+ gradients in EC during SMC stimulation and the resulting NO-independent vasodilatory feedback response from EC to SMC. Simulations show the effect of MP geometry, MEGJ coupling, Rgj, IKCa distribution, IP3R density and activation parameters, and basal IP3.
EC-to-SMC communication
Endothelial control of vascular tone is attributed to an increase in EC Ca2+ followed by the activation of Ca2+-dependent vasodilatory pathways such as EDRF, prostacyclin (PGI2) and EDHF (Dora et al. 1997; Sandow, 2004; Feletou & Vanhoutte, 2006) among others. Both the compartmental and 2D FEM models could simulate SMC relaxation induced by ACh stimulation of EC and mediated by EDHF, consistent with experimental data and the previous EC–SMC model (Dora et al. 1997; Oishi et al. 2001; Schuster et al. 2001; Lamboley et al. 2005; Kapela et al. 2009). ACh stimulation increased IP3 and Ca2+ in EC bulk and MP (Fig. 4A and B), leading to activation of SKCa and IKCa channels. The hyperpolarizing current from SKCa and IKCa channels was transmitted by the MEGJs to SMC and reduced SMC Ca2+. Presence of MP did not compromise EDHF, and EC MP was effectively isopotential with EC bulk.
SMC-to-EC communication
An increasing amount of evidence shows the existence of local EC Ca2+ events such as ‘pulsars’ and ‘wavelets’ in and around EC MPs following SMC stimulation without significant global spread (Kansui et al. 2008; Ledoux et al. 2008b; Tran et al. 2012). Similarly, following SMC stimulation in the models, the IP3 and Ca2+ increase in EC was confined to the MPs and its spread into the bulk EC was rather limited (Fig. 3A, 4A and C). The amplitude of Ca2+ transients in the MPs depended upon the size and shape of the projection (Fig. 5) and the area available for diffusion of ions into the bulk EC. Theoretical estimations suggest that passive diffusion of Ca2+ and/or IP3 into EC is insufficient for a global Ca2+ mobilization (Nagaraja et al. 2012). The small size of MP as compared to the bulk cytosol and restricted diffusivity from MP into bulk EC allows for significant accumulation of IP3 and/or Ca2+ following SMC stimulation. RyRs and/or IP3Rs like those found in MPs can further amplify the weak fluxes of Ca2+ and IP3 to induce local Ca2+ transients and EC hyperpolarization (Isakson, 2008; Ledoux et al. 2008a).
In earlier studies, a global Ca2+ increase in EC after SMC stimulation has also been reported (Dora et al. 1997; Yashiro & Duling, 2000; Schuster et al. 2001; Tuttle & Falcone, 2001; Jackson et al. 2008). The reason for this discrepancy may be attributed to differences in the experimental techniques employed. Our results suggest that if a global response does happen, a mechanism to amplify the small local events is necessary. The presence of a currently unidentified regeneration mechanism cannot be excluded. In fact, in a recent theoretical study Brasen et al. (2012) showed that a Ca2+ transient in the MP, induced by IP3 elevation in SMC, could subsequently spread into the entire EC as a Ca2+ wave through CICR in IP3Rs. CICR was possible because of sensitization of EC bulk with basal IP3 levels (0.1 μm) and not because of IP3 spread from the SMC and MP to EC bulk. This study did not account for membrane electrophysiology. In our model, sub-threshold levels of IP3 enhanced significantly the feedback without generating global EC Ca2+, while further elevation of basal IP3 hyperpolarized resting Vm (Fig. 11). These results show that fine tuning of the system's excitability may allow facilitation of Ca2+ spread, but at least in our model, NA-induced Ca2+ transients are more likely to have limited spread, in agreement with recent experimental studies (Kansui et al. 2008; Ledoux et al. 2008b; Tran et al. 2012).
MPs amplify feedback
In the model, the local Ca2+ transients and IKCa channels in the EC MPs generated a hyperpolarizing feedback in SMC of around 3 mV (Fig. 6A, continuous line). In simulations with no diffusional resistance between the MPs and bulk EC and thus effectively no MPs, the feedback was lost (Fig. 6A, dashed line), in spite of the IP3R and IKCa localization still present near the MEGJs. In the absence of a restricted space like the MPs, the IP3 and Ca2+ entering the MPs from the stimulated SMC rapidly diffused into the entire endothelial cell and failed to produce a local or global Ca2+ increase (Fig. 6B, dashed line), because of dilution in large cytosolic volume, Ca2+ sequestration and buffering, and IP3 degradation by one to five phosphatases present in the cytosol. Increasing the gap junction permeability 102-fold and maximum IP3R current 103-fold failed to fully compensate the lack of MPs (Fig. 6A, continuous vs. dotted lines). Thus, it appears that in conjunction with the localization of IKCa and IP3Rs, the presence of a restricted space like a MP within the EC is essential for Ca2+ mobilization in the EC and facilitation of feedback following SMC stimulation. It is important to note here that the differences in Ca2+ and IP3 concentration between the MPs and bulk EC, as well as the spread of MP Ca2+ into the bulk EC, will depend on the relative size, surface area and volume of the MP with respect to the bulk of EC (Fig. 5).
Ca2+ accumulation may be further enhanced by the close proximity of ER and mitochondria to the plasma membrane. The presence of organelles at the base of the MP, for example, will restrict diffusion to the bulk of EC, enabling significant Ca2+ transients in the vicinity of MP which could activate components that are not necessarily placed within the MP. Thus, in general, small spaces restricted by MP and/or subcellular structures seem to be imperative for amplification of SMC initiated signals causing local Ca2+ events and the experimentally observed feedback of a few millivolts (Tran et al. 2012). The inhibition of SKCa/IKCa channels enhanced NA-induced depolarization by ∼3.5 mV and was associated with about 10% smaller diameter of NA-constricted vessels (Tran et al. 2012). Since the resistance to flow is inversely proportional to diameter to the fourth power (i.e. Poiseuille equation), this change corresponds to a very significant (more than 50%) increase in resistance.
MEGJs and Rgj
MEGJs are the primary NO-independent pathway for transfer of agonist induced EC hyperpolarization to the SMC (Sokoya et al. 2006; Isakson et al. 2007; Kansui et al. 2008). Their presence has been documented in various vascular beds (Figueroa et al. 2004), including rat mesenteric arteries (Hill et al. 2000; Sandow & Hill, 2000), using direct as well as indirect methods like electron microscopy and dye coupling between two cells (Little et al. 1995; Beny, 1997). MEGJs also transmit Vm changes from SMC to EC, although differences in input resistances of endothelial and smooth muscle layers can cause asymmetric ME responses (Emerson & Segal, 2000; Diep et al. 2005). Furthermore, the highest occurrences of MEGJs have been observed in small arteries and arterioles coincidental with a high number of MPs and high EDHF activity (Hwa et al. 1994; Sandow et al. 2009a). Myoendothelial signalling is highly diminished or even abolished in the presence of gap junction blockers like 18-glycyrrhetinic acid (18-GA) and carbenoxolone (Hill et al. 2000; Goto et al. 2002; Hill et al. 2002; Dora et al. 2003; Sandow et al. 2004; Mather et al. 2005; Sokoya et al. 2006; Dora et al. 2008), but not after eNOS inhibition (Nausch et al. 2012). Some vessels may lack MEGJs and functional ME coupling (Sandow et al. 2002; Siegl et al. 2005). Gap junctions are believed to be non-selective in nature, with similar permeabilities for small ions (Christ et al. 1996; Brink et al. 2000). Experimentally estimated values of Rgj are spread over a large range, 70–900 MΩ, depending on the particular tissue and experimental conditions and the type of cells being examined (Sandow & Hill, 2000; Yamamoto et al. 2001; de Wit & Griffith, 2010). In the simulations, a decrease in Rgj is associated with increased permeability of MEGJs to Ca2+ and IP3 and and increase in Ca2+ transients in the EC MP (Fig. 7A). This in turn led to higher feedback in terms of SMC Ca2+ and SMC Vm (Fig. 9). Under control density of IP3Rs and IKCa channels in MP and with Ca2+ and IP3 diffusion through MEGJs, resistances below 500 MΩ caused Ca2+ transients in the MP to be high enough to counteract the SMC depolarization to NA stimulation (Fig. 9A). This suggests lower IP3R density in MPs and/or higher Rgj.
Localization of IP3Rs
IP3R localization will be an important determinant of amplitude of Ca2+ transients in the MP (Lamboley et al. 2005; Isakson, 2008; Sandow et al. 2009b). While a significant number of IP3R clusters seem to be present along the projections (Ledoux et al. 2008a; Sandow et al. 2009b), IP3Rs in the rest of the EC are primarily responsible for experimentally observed global Ca2+ increase in EC after ACh stimulation, which is most likely initiated from the luminal side of the EC, opposite to the location of MPs. Results from the compartmental model showed an increase in feedback in terms of SMC Vm and SMC Ca2+ with increase in IP3R localization in the MP, predicting a hyperpolarizing feedback of up to 6 mV with 30% IP3R localization (Fig. 10C). ER is a dynamic and flexible structure spread throughout the EC and undergoing constant change. ER provides a single continuous space for the movement of Ca2+ ions and can direct Ca2+ movement in the cell by concentrating IP3Rs in a particular cellular region (Diambra & Marchant, 2009; Taylor et al. 2009). Perhaps, for this reason, ER localizes at the base and inside of some projections so as to increase the density of IP3R in the projection to form a local regulating module to enhance the feedback of EC to SMC. With better quantification of the relative densities of these receptors in the MP as compared to the whole EC, we can obtain a better estimate of the feedback capacity of MPs.
Localization of IKCa channels
The co-localization of IKCa channels close to IP3Rs is crucial for facilitation of feedback by MPs. Experimental studies in some tissues show a spatial separation of the two types of KCa channels expressed in the ECs (Crane et al. 2003; Sandow et al. 2006; Dora et al. 2008). In rat mesenteric arteries, IKCa channels are predominantly expressed in and around the MPs (Dora et al. 2008; Ledoux et al. 2008a; Sandow et al. 2009b), while SKCa channels are mostly confined to the bulk of EC near EC–EC tight junctions (Crane et al. 2003; Sandow et al. 2006). MPs contain most of the IKCa channels that along with SKCa channels are responsible for EC hyperpolarization during EC stimulation (Doughty et al. 1999; Crane et al. 2003; Tran et al. 2012). This segregation of IKCa and SKCa channels might be a way to separate the respective roles of the very closely related and similarly activated KCa channels. Because of their localization in the MP, IKCa channels might be engaged in a local feedback mechanism through MP Ca2+. Model predictions showed that, during SMC stimulation, the concentration of Ca2+ in the MPs increased as high as the global Ca2+ concentration in EC during ACh (a potent vasodilator) stimulation of EC (Fig. 3A vs. B) Therefore, this unique orientation of IKCa channels near the local Ca2+ transients might dismiss the need of a global response. This is also reflected in the fact that for the same Ca2+ transient in the MP (Fig. 8B), the feedback generated with the control model (100% IKCa channels localized to MP) was lost with uniform distribution of these channels (Fig. 8A, continuous vs. dashed lines). The feedback progressively decreased with a decrease in the number of IKCa channels localized in the MP. For these simulations, Rgj and IP3R density were maintained at their respective control values.
IP3 diffusion
Experimental results suggest that Ca2+ mobilization in EC after SMC stimulation can be attributed mostly to diffusion of IP3 rather than Ca2+ (Lamboley et al. 2005; Isakson et al. 2007; Tran et al. 2012). Vascular co-culture studies by Isakson et al. showed that IP3R blockade in ECs inhibits Ca2+ flashes in ECs induced by agonist stimulation of BAPTA-loaded SMCs (Isakson et al. 2007; Isakson, 2008). A similar blocking of IP3Rs in SMC following EC stimulation, however, did not alter SMC response due to lack of IP3R localization on the SMC side of the myoendothelial junction and an abundance of IP3 metabolizer 5-phosphatase (Isakson, 2008). Experiments on rat mesenteric arteries by Lamboley et al. (2005) showed that blocking of any component of the IP3 signalling pathway (PLC inhibition in SMC, IP3R blocking in EC) led to a severe reduction in Ca2+ transients in ECs following SMC stimulation. In a recent study in hamster vessels, Tran et al. (2012) showed that blockade of EC IP3Rs inhibits feedback in SMC Vm (by ∼4 mV) during SMC stimulation with an IP3 releasing vasoconstrictor (PE), as seen in our simulations (Fig. 7C, dashed line). A similar blockade of EC IP3Rs did not alter SMC depolarization and constriction due to a non-IP3 releasing voltage-dependent potassium channel (Kv) blocker, 4-aminopyridine (4-AP) (Tran et al. 2012). In agreement with the experimental observations, the 2D FEM model results under control (Fig. 7A) and blocked IP3 diffusion (Fig. 7B) conditions for different Rgj values predicted a significant reduction (∼4 times) in Ca2+ transients by blockade of IP3 diffusion from SMC to EC, which suggests that the majority of Ca2+ transient can be attributed to the diffusion of IP3 and not Ca2+.
Ca2+ diffusion
Earlier studies suggested that Ca2+ mobilization in EC after SMC stimulation is mediated by diffusion of Ca2+ ions (Dora et al. 1997; Schuster et al. 2001), but this hypothesis has not been supported experimentally. In our control model, blocking of Ca2+ diffusion from SMC to EC did not alter the MP Ca2+ transient (Fig. 7B, inset). Ca2+ diffusion alone appears to produce some Ca2+ increase in the MP only at low values of Rgj (<50 MΩ; Fig. 7B, dashed line). In the model with Ca2+- and IP3-activated IP3Rs (eqn (5)), Ca2+ diffusion can activate IP3Rs in synergy with IP3 diffusion (Fig. 11A) or in the presence of basal IP3 levels in the EC (Fig. 11B). The basal concentration of IP3 needs to be adequate enough to sensitize IP3R in ECs to weak Ca2+ flux, but at the same time it should not increase the resting EC MP Ca2+ levels and induce CICR prior to SMC stimulation (Nagaraja et al. 2012). Hence, there appears to be a narrow window of basal IP3 levels for which Ca2+ might be able to contribute to the feedback response. The adequacy of basal IP3 will be influenced by the degree of IP3R localization and the half-maximum activation concentration (Kd_act) of both IP3 and Ca2+ for the IP3R. So far, there is no direct evidence for simultaneous existence of all these conditions in the EC. In our model, for a limited set of parameters (Kd_act= 350 nm; basal IP3 between 30–50 nm), Ca2+ diffusion could produce a hyperpolarizing feedback of up to 6 mV for control values of Rgj, IP3R and IKCa localization (Fig. 11B, dotted line). However, for this high value of Kd_act, the IP3R activation and feedback (∼0.5 mV) are much smaller in the absence of basal IP3 compared to the control model.
Limitations
A number of parameter values have not been accurately quantified. In this study, we try to remain consistent with our previous EC–SMC model with respect to parameter values and whole cell currents (i.e. most parameters were derived in this earlier study from rat mesenteric artery data). The important deviations were the maximum IP3R current and permeability of MEGJs to IP3. These parameters had been increased 103- and 102-fold in the earlier EC–SMC model to compensate for the lack of MPs and to allow for myoendothelial feedback. The models’ behaviour is sensitive to a number of tissue specific parameters such as channel conductances, myoendothelial IP3 permeability, dimension and number of MPs, localization and density of IP3Rs, SKCa/IKCa channels, and MEGJs inside the MPs. This limitation was partially addressed through parametric studies that examine the effect of parameter uncertainty on the models’ predictions.
The compartmental model captures well the electrical aspects of the myoendothelial coupling, but cannot accurately simulate subcellular concentration gradients and exchange of species. The 2D model is better suited to simulate Ca2+ transients in the vicinity of MPs and to account for the MP geometry and the spatial distribution of channels and receptors. The 2D model is limited, however, by the absence of quantification for the spatial distribution of these important cellular components. The integrated CICR mechanism is not sufficient to facilitate significant Ca2+ spread in the models. Other regenerative mechanisms might also exist that could enhance Ca2+ and IP3 diffusion. For example, IP3 generation by Ca2+ (via Ca2+-dependent PLC) in ECs might lead to global Ca2+ mobilization in the EC. At this point, however, such mechanisms have not been established experimentally.
By assuming only a single EC and SMC pair, the model simulates an average behaviour spatiotemporally. That is, every EC in the vessel is hyperpolarized to the same level for the entire duration. In reality, only a small percentage of ECs will exhibit NA-induced MP Ca2+ wavelets at any given time (Tran et al. 2012). Depending on the number of active sites, the duration and frequency of the events, the IKCa current required per MP must be stronger to compensate for current loss to other ECs. The model does not account for the transient/stochastic behaviour of Ca2+ mobilization seen in the experimental studies. Figures 8–10 show, however, that a single MP is capable of generating much stronger hyperpolarizing currents.
Conclusions
The models developed in this study present a first attempt to capture the role of MPs in the myoendothelial feedback. The models predict that even in the absence of NO signalling pathway, EC is able to generate the few-millivolt (i.e. 2–3 mV) feedback observed experimentally, thanks to the presence of signalling microdomains composed of MPs with small cytosolic volume, restricted permeability to bulk EC, and localized IP3Rs and IKCa channels. In agreement with recent experimental evidence (Tran et al. 2012) the model predicts that IP3, rather than Ca2+, diffusion from SMC to EC is the mechanism responsible for the Ca2+ transients in the EC MPs after SMC agonist stimulation. These Ca2+ transients are localized primarily within the MPs, with limited spread into EC bulk. A global spread would thus require the presence of a regeneration mechanism in the bulk of EC. The amount of feedback depends on various parameters, but simulations suggest that MPs are required for a significant feedback. Thus, our results highlight the importance of MPs in myoendothelial communication by amplifying local Ca2+ increase and facilitating the generation of feedback response to SMC constriction.
Acknowledgments
This work was supported by the National Institutes of Health grant SC1HL095101 (N.M.T.), and an operating grant from the Canadian Institute of Health Research (D.G.W.). S.N. was supported by a Dissertation Year Fellowship from the University Graduate School of Florida International University.
Glossary
- ACh
acetylcholine
- CICR
calcium-induced calcium release
- EC
endothelial cell
- EDHF
endothelium-derived hyperpolarizing factor
- EDRF
endothelium-derived relaxing factor
- ER
endoplasmic reticulum
- FEM
finite element method
- IKCa
intermediate-conductance Ca2+-activated K+ channels
- IP3
inositol 1,4,5-triphosphate
- IP3R
IP3 receptor
- MEGJ
myoendothelial gap junctions
- MP
myoendothelial projection
- NA
noradrenaline
- SMC
smooth muscle cell
Author contributions
S.N., A.K., C.H.T., D.G.W. and N.M.T are responsible for conception and design of the research. S.N., A.K. and N.M.T implemented the models, performed the simulations, and drafted the manuscript. C.H.T. and D.G.W. edited and revised the manuscript. S.N., A.K., C.H.T., D.G.W. and N.M.T approved the final version of the manuscript.
References
- Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Atri A, Amundson J, Clapham D, Sneyd J. A single-pool model for intracellular calcium oscillations and waves in the Xenopus laevis oocyte. Biophys J. 1993;65:1727–1739. doi: 10.1016/S0006-3495(93)81191-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beny J. Electrical coupling between smooth muscle cells and endothelial cells in pig coronary arteries. Pflugers Arch. 1997;433:364–367. doi: 10.1007/s004240050289. [DOI] [PubMed] [Google Scholar]
- Brasen JC, Jacobsen JC, Holstein-Rathlou NH. The nanostructure of myoendothelial junctions contributes to signal rectification between endothelial and vascular smooth muscle cells. PLoS One. 2012;7:e33632. doi: 10.1371/journal.pone.0033632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brink PR, Ricotta J, Christ GJ. Biophysical characteristics of gap junctions in vascular wall cells: implications for vascular biology and disease. Braz J Med Biol Res. 2000;33:415–422. doi: 10.1590/s0100-879x2000000400007. [DOI] [PubMed] [Google Scholar]
- Bychkov R, Burnham MP, Richards GR, Edwards G, Weston AH, Feletou M, Vanhoutte PM. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br J Pharmacol. 2002;137:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ GJ, Spray DC, el-Sabban M, Moore LK, Brink PR. Gap junctions in vascular tissues. Evaluating the role of intercellular communication in the modulation of vasomotor tone. Circ Res. 1996;79:631–646. doi: 10.1161/01.res.79.4.631. [DOI] [PubMed] [Google Scholar]
- Crane GJ, Gallagher N, Dora KA, Garland CJ. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- Diambra L, Marchant JS. Localization and socialization: experimental insights into the functional architecture of IP3 receptors. Chaos. 2009;19:037103. doi: 10.1063/1.3147425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep HK, Vigmond EJ, Segal SS, Welsh DG. Defining electrical communication in skeletal muscle resistance arteries: a computational approach. J Physiol. 2005;568:267–281. doi: 10.1113/jphysiol.2005.090233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Doyle MP, Duling BR. Elevation of intracellular calcium in smooth muscle causes endothelial cell generation of NO in arterioles. Proc Natl Acad Sci U S A. 1997;94:6529–6534. doi: 10.1073/pnas.94.12.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Gallagher NT, McNeish A, Garland CJ. Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries. Circ Res. 2008;102:1247–1255. doi: 10.1161/CIRCRESAHA.108.172379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dora KA, Sandow SL, Gallagher NT, Takano H, Rummery NM, Hill CE, Garland CJ. Myoendothelial gap junctions may provide the pathway for EDHF in mouse mesenteric artery. J Vasc Res. 2003;40:480–490. doi: 10.1159/000074549. [DOI] [PubMed] [Google Scholar]
- Doughty JM, Plane F, Langton PD. Charybdotoxin and apamin block EDHF in rat mesenteric artery if selectively applied to the endothelium. Am J Physiol Heart Circ Physiol. 1999;276:H1107–H1112. doi: 10.1152/ajpheart.1999.276.3.H1107. [DOI] [PubMed] [Google Scholar]
- Emerson GG, Segal SS. Electrical coupling between endothelial cells and smooth muscle cells in hamster feed arteries: role in vasomotor control. Circ Res. 2000;87:474–479. doi: 10.1161/01.res.87.6.474. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now. Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Figueroa XF, Isakson BE, Duling BR. Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 2004;19:277–284. doi: 10.1152/physiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- Finch EA, Turner TJ, Goldin SM. Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science. 1991;252:443–446. doi: 10.1126/science.2017683. [DOI] [PubMed] [Google Scholar]
- Garland CJ, Plane F, Kemp BK, Cocks TM. Endothelium-dependent hyperpolarization: a role in the control of vascular tone. Trends Pharmacol Sci. 1995;16:23–30. doi: 10.1016/s0165-6147(00)88969-5. [DOI] [PubMed] [Google Scholar]
- Goto K, Fujii K, Kansui Y, Abe I, Iida M. Critical role of gap junctions in endothelium-dependent hyperpolarization in rat mesenteric arteries. Clin Exp Pharmacol Physiol. 2002;29:595–602. doi: 10.1046/j.1440-1681.2002.03689.x. [DOI] [PubMed] [Google Scholar]
- Heberlein KR, Straub AC, Isakson BE. The myoendothelial junction: breaking through the matrix. Microcirculation. 2009;16:307–322. doi: 10.1080/10739680902744404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Hickey H, Sandow SL. Role of gap junctions in acetylcholine-induced vasodilation of proximal and distal arteries of the rat mesentery. J Auton Nerv Syst. 2000;81:122–127. doi: 10.1016/s0165-1838(00)00113-2. [DOI] [PubMed] [Google Scholar]
- Hill CE, Rummery N, Hickey H, Sandow SL. Heterogeneity in the distribution of vascular gap junctions and connexins: implications for function. Clin Exp Pharmacol Physiol. 2002;29:620–625. doi: 10.1046/j.1440-1681.2002.03699.x. [DOI] [PubMed] [Google Scholar]
- Hwa JJ, Ghibaudi L, Williams P, Chatterjee M. Comparison of acetylcholine-dependent relaxation in large and small arteries of rat mesenteric vascular bed. Am J Physiol Heart Circ Physiol. 1994;266:H952–H958. doi: 10.1152/ajpheart.1994.266.3.H952. [DOI] [PubMed] [Google Scholar]
- Isakson BE. Localized expression of an Ins(1,4,5)P3 receptor at the myoendothelial junction selectively regulates heterocellular Ca2+ communication. J Cell Sci. 2008;121:3664–3673. doi: 10.1242/jcs.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res. 2007;100:246–254. doi: 10.1161/01.RES.0000257744.23795.93. [DOI] [PubMed] [Google Scholar]
- Jackson WF, Boerman EM, Lange EJ, Lundback SS, Cohen KD. Smooth muscle alpha1D-adrenoceptors mediate phenylephrine-induced vasoconstriction and increases in endothelial cell Ca2+ in hamster cremaster arterioles. Br J Pharmacol. 2008;155:514–524. doi: 10.1038/bjp.2008.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen JC, Aalkjaer C, Nilsson H, Matchkov VV, Freiberg J, Holstein-Rathlou NH. Activation of a cGMP-sensitive calcium-dependent chloride channel may cause transition from calcium waves to whole cell oscillations in smooth muscle cells. Am J Physiol Heart Circ Physiol. 2007;293:H215–H228. doi: 10.1152/ajpheart.00726.2006. [DOI] [PubMed] [Google Scholar]
- Kansui Y, Garland CJ, Dora KA. Enhanced spontaneous Ca2+ events in endothelial cells reflect signalling through myoendothelial gap junctions in pressurized mesenteric arteries. Cell Calcium. 2008;44:135–146. doi: 10.1016/j.ceca.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of Ca2+ dynamics in rat mesenteric smooth muscle cell: agonist and NO stimulation. J Theor Biol. 2008;253:238–260. doi: 10.1016/j.jtbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Kapela A, Bezerianos A, Tsoukias NM. A mathematical model of vasoreactivity in rat mesenteric arterioles: I. Myoendothelial communication. Microcirculation. 2009;16:694–713. doi: 10.3109/10739680903177539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapela A, Tsoukias NM. Multiscale FEM modeling of vascular tone: from membrane currents to vessel mechanics. IEEE Trans Biomed Eng. 2011;58:3456–3459. doi: 10.1109/TBME.2011.2162513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr PM, Tam R, Ondrusova K, Mittal R, Narang D, Tran CH, Welsh DG, Plane F. Endothelial feedback and the myoendothelial projection. Microcirculation. 2012;19:416–422. doi: 10.1111/j.1549-8719.2012.00187.x. [DOI] [PubMed] [Google Scholar]
- Lamboley M, Pittet P, Koenigsberger M, Sauser R, Beny JL, Meister JJ. Evidence for signaling via gap junctions from smooth muscle to endothelial cells in rat mesenteric arteries: possible implication of a second messenger. Cell Calcium. 2005;37:311–320. doi: 10.1016/j.ceca.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Ledoux J, Boney AD, Hannah RM, Tallini YN, Kotlikoff MI, Nelson MT. Ca2+ pulsars: spatially restricted, IP3R-mediated Ca2+ release important for endothelial function. J Vasc Res. 2008b;45(Suppl. 1):102. [Google Scholar]
- Ledoux J, Taylor MS, Bonev AD, Hannah RM, Solodushko V, Shui B, Tallini Y, Kotlikoff MI, Nelson MT. Functional architecture of inositol 1,4,5-trisphosphate signaling in restricted spaces of myoendothelial projections. Proc Natl Acad Sci U S A. 2008a;105:9627–9632. doi: 10.1073/pnas.0801963105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TL, Xia J, Duling BR. Dye tracers define differential endothelial and smooth muscle coupling patterns within the arteriolar wall. Circ Res. 1995;76:498–504. doi: 10.1161/01.res.76.3.498. [DOI] [PubMed] [Google Scholar]
- Luksha L, Agewall S, Kublickiene K. Endothelium-derived hyperpolarizing factor in vascular physiology and cardiovascular disease. Atherosclerosis. 2009;202:330–344. doi: 10.1016/j.atherosclerosis.2008.06.008. [DOI] [PubMed] [Google Scholar]
- McNeish AJ, Sandow SL, Neylon CB, Chen MX, Dora KA, Garland CJ. Evidence for involvement of both IKCa and SKCa channels in hyperpolarizing responses of the rat middle cerebral artery. Stroke. 2006;37:1277–1282. doi: 10.1161/01.STR.0000217307.71231.43. [DOI] [PubMed] [Google Scholar]
- Mather S, Dora KA, Sandow SL, Winter P, Garland CJ. Rapid endothelial cell-selective loading of connexin 40 antibody blocks endothelium-derived hyperpolarizing factor dilation in rat small mesenteric arteries. Circ Res. 2005;97:399–407. doi: 10.1161/01.RES.0000178008.46759.d0. [DOI] [PubMed] [Google Scholar]
- Moore DH, Ruska H. The fine structure of capillaries and small arteries. J Biophys Biochem Cytol. 1957;3:457–462. doi: 10.1083/jcb.3.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraja S, Kapela A, Tsoukias NM. Intercellular communication in the vascular wall: a modeling perspective. Microcirculation. 2012;19:391–402. doi: 10.1111/j.1549-8719.2012.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausch LW, Bonev AD, Heppner TJ, Tallini Y, Kotlikoff MI, Nelson MT. Sympathetic nerve stimulation induces local endothelial Ca2+ signals to oppose vasoconstriction of mouse mesenteric arteries. Am J Physiol Heart Circ Physiol. 2012;302:H594–H602. doi: 10.1152/ajpheart.00773.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi H, Budel S, Schuster A, Stergiopulos N, Meister JJ, Beny JL. Cytosolic-free calcium in smooth-muscle and endothelial cells in an intact arterial wall from rat mesenteric artery in vitro. Cell Calcium. 2001;30:261–267. doi: 10.1054/ceca.2001.0233. [DOI] [PubMed] [Google Scholar]
- Sandow SL. Factors, fiction and endothelium-derived hyperpolarizing factor. Clin Exp Pharmacol Physiol. 2004;31:563–570. doi: 10.1111/j.1440-1681.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Goto K, Rummery NM, Hill CE. Developmental changes in myoendothelial gap junction mediated vasodilator activity in the rat saphenous artery. J Physiol. 2004;556:875–886. doi: 10.1113/jphysiol.2003.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Gzik DJ, Lee RM. Arterial internal elastic lamina holes: relationship to function. J Anat. 2009a;214:258–266. doi: 10.1111/j.1469-7580.2008.01020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Haddock RE, Hill CE, Chadha PS, Kerr PM, Welsh DG, Plane F. What's where and why at a vascular myoendothelial microdomain signalling complex. Clin Exp Pharmacol Physiol. 2009b;36:67–76. doi: 10.1111/j.1440-1681.2008.05076.x. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function. J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- Schuster A, Beny JL, Meister JJ. Modelling the electrophysiological endothelial cell response to bradykinin. Eur Biophys J. 2003;32:370–380. doi: 10.1007/s00249-003-0279-x. [DOI] [PubMed] [Google Scholar]
- Schuster A, Oishi H, Beny JL, Stergiopulos N, Meister JJ. Simultaneous arterial calcium dynamics and diameter measurements: application to myoendothelial communication. Am J Physiol Heart Circ Physiol. 2001;280:H1088–H1096. doi: 10.1152/ajpheart.2001.280.3.H1088. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, Takayanagi T, Nagao T, Egashira K, Fujishima M, Takeshita A. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Siegl D, Koeppen M, Wolfle SE, Pohl U, de Wit C. Myoendothelial coupling is not prominent in arterioles within the mouse cremaster microcirculation in vivo. Circ Res. 2005;97:781–788. doi: 10.1161/01.RES.0000186193.22438.6c. [DOI] [PubMed] [Google Scholar]
- Silva HS, Kapela A, Tsoukias NM. A mathematical model of plasma membrane electrophysiology and calcium dynamics in vascular endothelial cells. Am J Physiol Cell Physiol. 2007;293:C277–C293. doi: 10.1152/ajpcell.00542.2006. [DOI] [PubMed] [Google Scholar]
- Sokoya EM, Burns AR, Setiawan CT, Coleman HA, Parkington HC, Tare M. Evidence for the involvement of myoendothelial gap junctions in EDHF-mediated relaxation in the rat middle cerebral artery. Am J Physiol Heart Circ Physiol. 2006;291:H385–H393. doi: 10.1152/ajpheart.01047.2005. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Taufiq Ur R, Pantazaka E. Targeting and clustering of IP3 receptors: key determinants of spatially organized Ca2+ signals. Chaos. 2009;19:037102. doi: 10.1063/1.3127593. [DOI] [PubMed] [Google Scholar]
- Tran CH, Taylor MS, Plane F, Nagaraja S, Tsoukias NM, Solodushko V, Vigmond EJ, Furstenhaupt T, Brighan M, Welsh DG. Endothelial Ca2+ wavelets and the induction of myoendothelial feedback. Am J Physiol Cell Physiol. 2012;302:C1226–C1242. doi: 10.1152/ajpcell.00418.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle JL, Falcone JC. Nitric oxide release during alpha1-adrenoceptor-mediated constriction of arterioles. Am J Physiol Heart Circ Physiol. 2001;281:H873–H881. doi: 10.1152/ajpheart.2001.281.2.H873. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Klemm MF, Edwards FR, Suzuki H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol. 2001;535:181–195. doi: 10.1111/j.1469-7793.2001.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashiro Y, Duling BR. Integrated Ca2+ signaling between smooth muscle and endothelium of resistance vessels. Circ Res. 2000;87:1048–1054. doi: 10.1161/01.res.87.11.1048. [DOI] [PubMed] [Google Scholar]


