Abstract
The sympathetic nervous system is an important regulator of coronary blood flow. The cold pressor test (CPT) is a powerful sympathoexcitatory stressor. We tested the hypotheses that: (1) CPT-induced sympathetic activation elicits coronary vasodilatation in young adults that is impaired with advancing age and (2) combined α- and β-adrenergic blockade diminishes/abolishes these age-related differences. Vascular responses of the left anterior descending artery to the CPT were determined by transthoracic Doppler echocardiography before (pre-blockade) and during (post-blockade) systemic co-administration of α- and β-adrenergic antagonists in young (n= 9; 26 ± 1 years old, mean ± SEM) and older healthy men (n= 9; 66 ± 2 years old). Coronary vascular resistance (CVR; mean arterial pressure/coronary blood velocity) was used as an index of vascular tone. CPT decreased CVR (i.e. coronary vasodilatation occurred) in young (Δ–33 ± 6%), but not older men (Δ–3 ± 4%; P < 0.05 vs. young) pre-blockade. Adrenergic blockade abolished CPT-induced coronary vasodilatation in young men (Δ–33 ± 6%vs. Δ 0 ± 6%, pre-blockade vs. post-blockade, respectively; P < 0.05) such that responses post-blockade mirrored those of older men (Δ–3 ± 4%vs. Δ 8 ± 9%; both P > 0.05 compared to young pre-blockade). Impaired CPT-induced coronary vasodilatation could not be explained by a reduced stimulus for vasodilatation as group and condition effects persisted when CVR responses were expressed relative to myocardial oxygen demand (rate–pressure product). These data indicate that the normal coronary vascular response to sympathetic activation in young men is pronounced vasodilatation and this effect is lost with age as the result of an adrenergic mechanism. These findings may help explain how acute sympathoexcitation may precipitate angina and coronary ischaemic events, particularly in older adults.
Key points
The sympathetic nervous system is an important regulator of coronary blood flow.
How ageing may effect sympathetic nervous system regulation of coronary blood flow during physiological stress is unknown.
We measured coronary vascular responses to sympathetic activation before and during systemic α- and β-adrenergic receptor blockade in young and older healthy men.
Our results indicate that the normal coronary vascular response to sympathetic activation in young men is pronounced vasodilatation and that this effect is lost with advancing age, as the result of an apparent adrenergic mechanism.
These findings may help explain how acute sympathetic activation may trigger angina and coronary ischaemic events, particularly in older adults.
Introduction
The sympathetic nervous system is an important modulator of coronary blood flow (Berne, 1964; Feigl, 1967; Barbato, 2009). In humans the cold pressor test (CPT; i.e. placing the hand in ice water) elicits a pronounced physiological response (Hines & Brown, 1936), which includes sympathetic nervous system activation (Robertson et al. 1979; Victor et al. 1987; Monahan et al. 2004) that increases arterial blood pressure (BP) and the rate–pressure product (RPP; i.e. increased myocardial oxygen demand; Antony et al. 1994; Dubois-Rande et al. 1995). Coronary vasodilatation (i.e. increased myocardial oxygen supply) allows increases in myocardial oxygen demand to be met during the CPT in young to middle-aged healthy adults (Chandraratna et al. 1999). In contrast, impaired coronary vasodilatation or even paradoxical coronary vasoconstriction occurs during the CPT in numerous disease states (Mudge et al. 1976; Nabel et al. 1988; Zeiher et al. 1989; Antony et al. 1994; Tousoulis et al. 1997; Nitenberg et al. 2004b). Measuring coronary vasomotor responses to sympathetic activation during the CPT is important as these responses have prognostic significance (Schachinger et al. 2000; Nitenberg et al. 2004a,b) and may provide insight into how acute sympathetic activation may precipitate angina and/or coronary ischaemic events in humans (Mudge et al. 1976; Heusch, 1990; Remme, 1998).
Previously we have shown that skin surface cooling elicits greater increases in BP and myocardial oxygen demand (i.e. RPP) in older than in young adults (Hess et al. 2009; Wilson et al. 2010; Gao et al. 2012). However, myocardial oxygen supply did not increase during skin surface cooling in older adults, as it did in the young (Gao et al. 2012). These findings suggest that ageing may be associated with an altered coronary vasomotor response to acute sympathetic activation. As vasomotor responses to cold stress represent a highly integrative response (Nabel et al. 1988; Zeiher et al. 1989; Tousoulis et al. 1997; Schindler et al. 2003), altered coronary haemodynamic responses in older adults may directly or indirectly reflect detrimental effects of ageing on adrenergic function. Presently, the effect ageing exerts on the coronary vasomotor response to the CPT and the contribution of adrenergic mechanisms to this response is unknown.
Accordingly, the purpose of the present study was to determine coronary vasomotor responses to acute sympathetic activation elicited by the CPT in young and older healthy adults before and during combined systemic α- and β-adrenergic receptor blockade. We hypothesized that: (1) CPT-induced sympathetic activation elicits coronary vasodilatation in healthy young adults, the magnitude of which is reduced with advancing age and (2) combined systemic α- and β-adrenergic receptor blockade would reduce/abolish these age-related differences. Support for these hypotheses could implicate altered adrenergic function (directly) and/or endothelial dysfunction (indirectly) as key mechanism(s) contributing to impaired coronary vasodilatation during sympathetic activation with advancing age in humans.
Methods
Ethical approval
The experiments conformed to guidelines set forth in the latest revision of the Declaration of Helsinki. The Institutional Review Board at the Pennsylvania State University College of Medicine reviewed and approved the studies. Signed informed consent was obtained from all subjects prior to testing.
Subjects
Nine young and 9 older healthy males volunteered for this study. Inclusion criteria included: healthy (as assessed by review of medical history and physical examination), 20–35 (young) or 55–79 years of age (older), arterial blood pressure (BP) at rest <140/90 mmHg, non-smoker, non-obese (body mass index <30 kg m−2), not endurance trained, and not taking any medication.
Protocol
Subjects refrained from alcohol (24 h), caffeine (12 h), and food ingestion (4 h) prior to testing. Upon arrival in the laboratory subjects were positioned supine and instrumented for the study by placing BP cuffs on the left arm and left hand, inserting an intravenous catheter, and applying electrocardiogram electrodes. Initial measurements (pre-blockade) were obtained at least 30 min after instrumentation with the subject in the left lateral decubitus position. After obtaining sufficient resting data the subject's right hand, up to the level of the wrist, was placed in ice water for 2 min (cold pressor test; CPT). Pre-blockade measurements were repeated after administration of adrenergic antagonists (post-blockade; see next paragraph) (Sielatycki et al. 2011).
β−Adrenergic blockade was accomplished by intravenous infusion of propranolol (priming dose; 0.25 mg kg−1 over 15 min). α−Adrenergic blockade was accomplished by intravenous infusion of phentolamine (priming dose; 0.1428 mg kg−1 over 5 min) after the priming dose of propranolol. Maintenance doses of each drug (propranolol 0.004 mg kg−1 min−1; phentolamine 0.01428 mg kg−1 min−1) began immediately after completion of the priming doses and continued until data collection was complete. Propranolol (Bell et al. 2001; Monroe et al. 2001) and phentolamine doses (Halliwill et al. 2000; Sugawara et al. 2007) were chosen based on previously published studies.
Measurements
BP and heart rate
BP was measured over the brachial artery using a semi-automated device (Dinamap; GE Medical system, Milwaukee, WI, USA) in triplicate before (∼5 min preceding) and on a beat-by-beat basis (Finometer Finapres Medical Systems, Amsterdam, the Netherlands) prior to (3 min preceding) and during (2 min) the CPT trials. Heart rate was determined via a 3-lead electrocardiogram throughout.
Echocardiography and coronary haemodynamics
Transthoracic Doppler echocardiography was performed using a digital ultrasound system (iE33, Philips Ultrasound, Bothell, WA, USA) and a broadband sector ultrasound transducer (S8-3 for coronary flow velocity, 8.0–3.0 MHz; Momen et al. 2009, 2010a; Gao et al. 2012). Coronary blood velocity (CBV) was measured in the distal portion of the left anterior descending artery using colour Doppler flow mapping set at ±19 cm s−1 to provide optimal imaging (Momen et al. 2009, 2010a; Gao et al. 2012). After locating the distal portion of the left anterior descending artery in the region of the apex of the left ventricle, care was taken to position the transducer in a manner that allowed a long axis view of the artery. With a sample volume (2.0 mm) positioned over the colour signal in the left anterior descending artery, CBV was measured at end-expiration. The Doppler signal profiles of coronary blood flow during the diastolic portion of each cardiac cycle was analysed using ProSolv 3.0 software.
Data collection and analysis
Physiological data (BP and heart rate) were recorded at 400 Hz (MacLab 8e, ADInstruments). Echocardiography data were digitally recorded.
RPP was calculated as systolic BP × heart rate. Peak CBV was measured during diastole. Coronary vascular resistance (CVR) was calculated as mean BP/peak CBV. To quantify peak vascular responses to the CPT, the peak CBV measured was used (pre-blockade and post-blockade). A stimulus–response ratio was calculated ((CVR/RPP) × 1000) to allow comparisons of vascular responses pre-blockade and post-blockade relative to stimulus intensity.
Statistical analysis
Differences in subject characteristics were determined by unpaired t test. Responses both within (pre-blockade vs. post-blockade and rest vs. CPT) and between groups (young vs. older) were assessed using repeated measures ANOVA. Specific contrasts were made using Newman–Keuls post hoc tests when significant interactions were identified (group × drug × time in young and older men or drug × time in young or older men). Statistical significance was accepted when P < 0.05. All data are presented as mean ± SEM.
Results
Subject characteristics
Young (26 ± 1 years old) and older (66 ± 2 years old) men were of similar stature (181.3 ± 2.8 vs. 178.5 ± 1.0 cm for young and older, respectively), body mass (82.0 ± 4.8 vs. 84.1 ± 2.5 kg), and body mass index (24.7 ± 0.9 vs. 26.4 ± 0.8 kg m−2; all P > 0.05). BP at rest increased with advancing age and was found to be in the ‘normal’ range in young men and ‘prehypertensive’ range in older men (Chobanian et al. 2003) before infusion of adrenergic antagonists (Table 1).
Table 1.
Heart rate, blood pressure, and rate–pressure product at rest before (Pre-blockade) and during β-adrenergic blockade and α- and β-adrenergic blockade
| Pre-blockade | β-Blockade | α- andβ-blockade | |
|---|---|---|---|
| Young men | |||
| Heart rate (beats min−1) | 59 ± 3 | 49 ± 2* | 53 ± 2*† |
| ΔHeart rate, (beats min−1) | — | −10 ± 2* | −6 ± 2*† |
| Systolic BP (mmHg) | 118 ± 5 | 116 ± 4 | 111 ± 4*† |
| ΔSystolic BP (mmHg) | — | −2 ± 2 | −7 ± 2*† |
| Diastolic BP (mmHg) | 62 ± 1 | 61 ± 2 | 58 ± 2*† |
| ΔDiastolic BP (mmHg) | — | −1 ± 1 | −4 ± 2*† |
| Mean BP (mmHg) | 85 ± 2 | 84 ± 1 | 81 ± 2*† |
| ΔMean BP (mmHg) | — | −1 ± 1 | −4 ± 1*† |
| RPP (mmHg (beats min−1)) | 7003 ± 543 | 5743 ± 344* | 5958 ± 309* |
| ΔRPP, (mmHg (beats min−1)) | — | −1260 ± 287* | −1045 ± 309* |
| Older men | |||
| Heart rate (beats min−1) | 62 ± 4 | 54 ± 4* | 59 ± 5† |
| ΔHeart rate, (beats min−1) | — | −8 ± 2* | −3 ± 2† |
| Systolic BP (mmHg) | 128 ± 3‡ | 126 ± 3 | 114 ± 3*† |
| ΔSystolic BP (mmHg) | — | −2 ± 1 | −14 ± 2*†§ |
| Diastolic BP (mmHg) | 76 ± 2‡ | 75 ± 3 | 68 ± 2*† |
| ΔDiastolic BP (mmHg) | — | −1 ± 2 | −8 ± 1*†§ |
| Mean BP (mmHg) | 95 ± 2‡ | 93 ± 2 | 86 ± 2*† |
| ΔMean BP (mmHg) | — | −2 ± 1 | −9 ± 1*†§ |
| RPP (mmHg (beats min−1)) | 7933 ± 572‡ | 6835 ± 555* | 6700 ± 550* |
| ΔRPP (mmHg (beats min−1)) | — | −1098 ± 206* | −1232 ± 162* |
Data were obtained: (1) Pre-blockade (i.e. before infusion of any medications), (2) after infusion of β-adrenergic receptor antagonist priming dose (β-blockade), and (3) after infusion of β- and α-adrenergic receptor antagonist priming doses. BP, blood pressure; RPP, rate–pressure product; Δ, change from Pre-blockade. *P < 0.05 vs. Pre-blockade (same age group). †P < 0.05 vs. β-blockade (same age group). ‡P < 0.05 vs. young (Pre-blockade). §P < 0.05 vs. change in young (same time point).
Effect of adrenergic blockade on systemic haemodynamics at rest
β-Adrenergic blockade decreased (P < 0.05) heart rate in young and older men, but did not alter BP in either group. In contrast, during combined α- and β-adrenergic blockade, BP was lowered (P < 0.05) in young and older men, although the magnitude of this decrease was greater in older men (P < 0.05). RPP decreased similarly during β-adrenergic blockade and during combined α- and β-adrenergic blockade in young and older men (Table 1).
Systemic haemodynamic response to the CPT before and during adrenergic blockade
CPT increased heart rate, BP and RPP in young and older adults (pre-blockade). The CPT-induced pressor effect (i.e. increase in BP during the CPT) was greater in older than in young men (pre-blockade). The tachycardia associated with the CPT tended to be greater in young than in older men (pre-blockade; group × time interaction, P= 0.10). RPP increased similarly in young and older men (pre-blockade) during the CPT. Combined α- and β-adrenergic blockade significantly blunted systemic haemodynamic responses (heart rate, BP and RPP) to the CPT in both young and older men (Fig. 1). When haemodynamic responses to the CPT were compared post-blockade (young vs. older) no age-related differences were observed.
Figure 1. Responses to the cold pressor test (CPT) before (Pre-blockade) and during (Post-blockade) systemic α- and β-adrenergic blockade in young and older men.
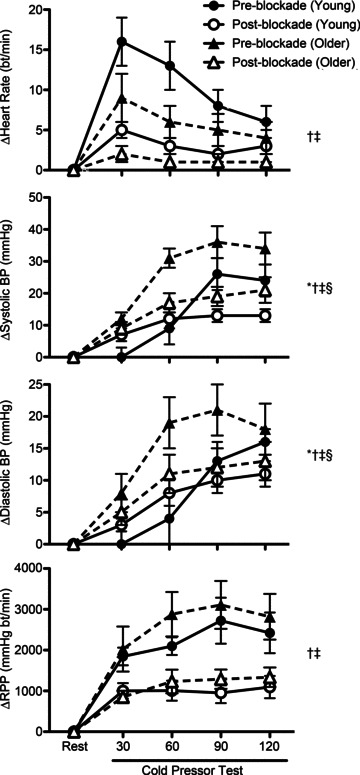
Data represent changes (Δ) from levels at rest over 30 s intervals during the CPT. CPT increased (P < 0.05) heart rate, blood pressure (BP) and rate–pressure product (RPP; systolic BP × heart rate) in young and older men. CPT-induced increases in systolic and diastolic BP were greater in older than in young men (Pre-blockade). α- and β-adrenergic blockade blunted CPT-induced increases in heart rate, BP and RPP in both groups. Responses were similar in young and older men Post-blockade. *P < 0.05 group (young vs. older) main effect (Pre-blockade). †P < 0.05 drug (Pre-blockade vs. Post-blockade) × time interaction (within young). ‡P < 0.05 drug (Pre-blockade vs. Post-blockade) × time interaction (within older). §P < 0.05 group × drug × time interaction.
Coronary haemodynamic response to the CPT before and during adrenergic blockade
CPT increased CBV in young and older adults (pre-blockade and post-blockade). Increases in CBV during the CPT (ΔCBV and ΔCBV (%)) were reduced with age (young pre-blockade vs. older pre-blockade; P < 0.05 group × time interaction) and by adrenergic blockade (young pre-blockade vs. young post-blockade and older pre-blockade vs. older post-blockade; P < 0.05 drug × time interaction in young and older, respectively). Adrenergic blockade abolished age-associated differences in the CBV response to the CPT observed pre-blockade (Fig. 2).
Figure 2. Coronary blood velocity (CBV) responses to CPT in young (filled bars) and older men (open bars) before (Pre) and during (Post) systemic α- and β-adrenergic blockade.
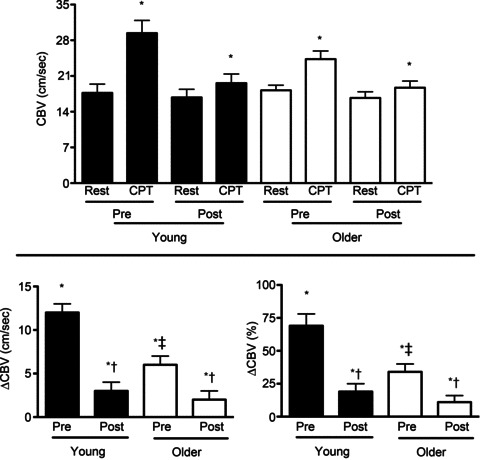
CBV was measured in the distal left anterior descending artery using transthoracic Doppler echocardiography. Data were obtained before (Rest) and at peak response during the stressor (CPT). CBV at rest was similar in young and older men at all time points. CPT increased (P < 0.05) CBV in young and older men Pre and Post. Increases in CBV during CPT were greater in young than in older men (Pre). Adrenergic blockade abolished these age-associated differences in the CBV response to the CPT. Pre, pre-blockade; Post, post-blockade. *P < 0.05 compared to rest (same condition; Pre or Post). †P < 0.05 compared to Pre (same age group; young or older). ‡P < 0.05 compared to response in young (same condition; Pre or Post).
CPT decreased CVR (P < 0.05) in young men (pre-blockade), indicative of coronary vasodilatation (Fig. 3). In contrast CVR was unchanged by the CPT in older men (pre-blockade). Adrenergic blockade abolished coronary vasodilatation to the CPT in young men (young post-blockade). In older men adrenergic blockade produced either no change (ΔCVR; P= 0.19) or a slight increase in the coronary vasomotor response to the CPT (ΔCVR (%); P= 0.045). Adrenergic blockade (post-blockade) abolished age-associated differences in the CVR response to the CPT observed pre-blockade (Fig. 3). These ageing and adrenergic blockade effects persisted even when vascular responses were expressed relative to the stimulus for the vasomotor response (i.e. CVR/RPP, ΔCVR/ΔRPP and ΔCVR (%)/ΔRPP (%); Fig. 4).
Figure 3. Coronary vascular resistance (CVR) responses to CPT in young (filled bars) and older men (open bars) before (Pre) and during (Post) combined systemic α- and β-adrenergic blockade.
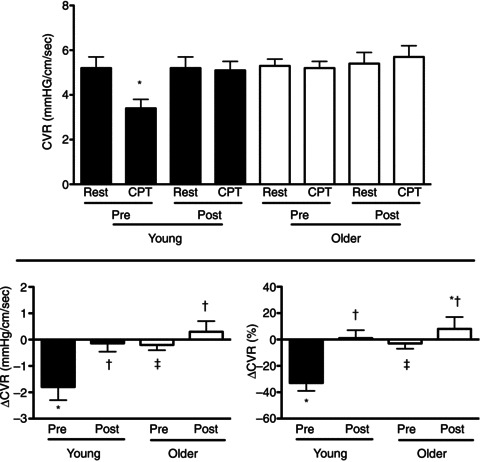
CVR was calculated as mean arterial pressure/coronary blood velocity. Coronary blood velocity was measured in the distal left anterior descending artery using transthoracic Doppler echocardiography. Data were obtained before (Rest) and at peak responses during the stressor (CPT). CVR at rest was similar in young and older men at all time points. CPT decreased (P < 0.05) CVR, indicating coronary vasodilatation occurred in the young Pre, but not Post. In contrast CVR was unchanged by the CPT in older men Pre and Post. CPT-induced decreases in CVR were greater in young compared to older men (Pre). Absolute (ΔCVR) and relative changes (ΔCVR (%)) in CVR during the CPT in older men were increased Post (increase greater than zero) indicating a coronary vasoconstrictor response. Pre, pre-blockade; Post, post-blockade. *P < 0.05 compared to rest (same condition; Pre or Post). †P < 0.05 compared to Pre (same age group; young or older). ‡P < 0.05 compared to response in young (same condition; Pre or Post).
Figure 4. Increases in CVR relative to increases in rate–pressure product (RPP) during CPT in young (filled bars) and older men (open bars) before (Pre) and during (Post) combined systemic α- and β-adrenergic blockade.
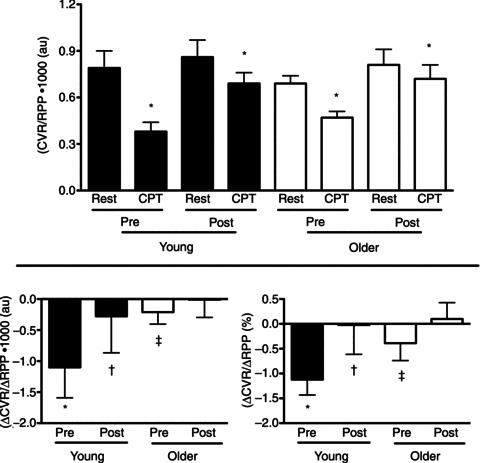
CVR was calculated as mean arterial pressure/coronary blood velocity. RPP was calculated as the product of systolic blood pressure and heart rate. Coronary blood velocity was measured in the distal left anterior descending artery using transthoracic Doppler echocardiography. CVR/RPP decreased (P < 0.05) during CPT in young and older men. Consistent with the pronounced coronary vasodilatation that occurred during the CPT in young adults (Pre) (Figs 2 and 3) the magnitude of this effect was largest in this group (Pre). As these data express responses (CVR) relative to changes in stimulus (RPP) they indicate that impaired coronary vasodilatation during the CPT in young (Post) and older men (Pre and Post) is not simply explained by a reduced stimulus for vasodilatation. Pre, pre-blockade; Post, post-blockade. *P < 0.05 compared to rest (same condition; Pre or Post). †P < 0.05 compared to Pre (same age group; young or older). ‡P < 0.05 compared to response in young (same condition; Pre or Post).
Discussion
This study reports a number of novel findings. First, the pronounced coronary vasodilator response to sympathetic activation during the CPT observed in young men is abolished by advancing age. Second, impaired coronary vasodilatation during the CPT in older men cannot be explained by a blunted increase in myocardial oxygen demand, as RPP increases at least as much in older men as it does in young men. Moreover, this effect persisted when the vascular response was expressed relative to the stimulus (e.g. CVR/RPP, ΔCVR/ΔRPP and ΔCVR (%)/ΔRPP (%)). Lastly, in young men systemic α- and β-adrenergic blockade mimics the effect ageing exerts on the coronary vasomotor responses to the CPT (i.e. it abolishes coronary vasodilatation during the CPT), suggesting a critical role for an adrenergic mechanism in these age-associated differences.
Prior studies examining coronary vascular responses to physiological stressors such as the CPT have largely relied on invasive methods to assess coronary blood flow. Thus, most data have been collected in clinical populations in whom diagnostic cardiac catheterization was otherwise indicated. Thus, some of the measured responses may have been made in ‘healthy arterial segments’ (i.e. arterial segments without significant stenosis; Mudge et al. 1976; Nabel et al. 1988; Zeiher et al. 1989; Tousoulis et al. 1997), but not in ‘healthy adults’per se. Advances in transthoracic Doppler echocardiography now makes it possible to reliably measure CBV non-invasively in humans (Hozumi et al. 1998a,b; Momen et al. 2007, 2009, 2010b; Gao et al. 2012). Thus, the ability to study coronary haemodynamics in nearly any population, such as young and older healthy adults, and to address fundamental physiological questions, such as the effects of healthy ageing and the role of adrenergic mechanisms on coronary vascular control, is now possible.
Coronary vascular responses to the CPT represent a complex integrative physiological response. At least three mechanisms may favour coronary vasodilatation during the CPT. First, as metabolism is a primary determinant of coronary flow (Feigl, 1983), increases in epicardial coronary shear stress during the CPT, as a result of downstream metabolic vasodilatation, should elicit endothelium-dependent flow-induced dilatation (Dubois-Rande et al. 1995; Nitenberg et al. 1995; Tousoulis et al. 1997). Consistent with this suggestion administration of the arginine analogue NG-monomethyl-l-arginine (l-NMMA) blunts coronary vasodilatation during the CPT (Tousoulis et al. 1997). Moreover, full expression of vasodilatation during the CPT requires an intact endothelium (Zeiher et al. 1989, 1991). Second, adrenaline (epinephrine) released during the CPT (Robertson et al. 1979) should activate β2-adrenergic receptors located on coronary vascular smooth muscle cells favouring vasodilatation (Nabel et al. 1988). Consistent with this suggestion intracoronary administration of propranolol blunts coronary vasodilatation during the CPT in healthy coronary arterial segments (Nabel et al. 1988). Lastly, noradrenaline (norepinephrine) release during the CPT (Robertson et al. 1979) could stimulate nitric oxide synthesis via stimulation of α2-adrenergic receptors located on coronary artery endothelial cells (Berkenboom et al. 1991).
Additionally, at least three mechanisms may limit coronary vasodilatation or contribute to coronary vasoconstriction during the CPT. First, in the presence of endothelial dysfunction reduced nitric oxide bioavailability (Tousoulis et al. 1997) and enhanced generation of endothelium-derived contracting factors (Vanhoutte & Tang, 2008) could limit coronary vasodilatation or produce coronary vasoconstriction during the CPT. Second, noradrenaline release, as the result of CPT-induced sympathoexcitation (Robertson et al. 1979), should stimulate α1- and α2-adrenergic receptors located on vascular smooth muscle cells limiting vasodilatation favouring vasoconstriction (Feigl, 1967; Chilian, 1991; Jones et al. 1993; Barbato, 2009). The magnitude of this α-adrenergically mediated effect during the CPT could be amplified in the setting of endothelial dysfunction as the results of a reduced ability of nitric oxide to oppose noradrenaline-induced vasoconstriction (Tesfamariam & Cohen, 1988; Berkenboom et al. 1991; Vita et al. 1992; Jones et al. 1993). Lastly, myogenic mechanisms should be engaged during the CPT, as the result of increased perfusion pressure, favouring vasoconstriction (Kuo et al. 1990). Ultimately, the integrative coronary vascular response to the CPT will most likely be the net result of the engagement of all of these potential vasodilator/vasoconstrictor mechanisms.
It is likely that at least several mechanisms described in the previous two paragraphs contributed to the impaired coronary vasodilatation we observed during the CPT in older men. First, since cardiac β-adrenergic sensitivity declines with age (Bertel et al. 1980) and β-mediated vasodilatation contributes critically to the vascular response during the CPT (Nabel et al. 1988) it is likely that age-related changes in cardiac β-adrenergic sensitivity contributed to the impaired coronary vasodilatation we observed in older men during the CPT. Second, downstream metabolic (Feigl, 1983) and β-mediated vasodilatation (Hamdad et al. 1996) during the CPT should increase epicardial shear stress and mediate a nitric oxide-dependent flow-induced vasodilatation (Drexler et al. 1989). Thus, factors that reduce nitric oxide bioavailability (i.e. produce endothelial dysfunction), such as ageing (Seals et al. 2011), should decrease CPT-induced coronary vasodilatation. Consistent with this suggestion acute administration of the potent antioxidant ascorbic acid, which improves endothelial dysfunction and increases nitric oxide bioavailability, produces a more favourable coronary vasomotor response to the CPT in hypercholesterolaemic and hypertensive humans (Jeserich et al. 1999).
It is possible that other mechanisms contributed to impaired coronary vasodilatation during the CPT in older men. CPT-induced sympathetic activation (Robertson et al. 1979) would favour coronary vasoconstriction via stimulation of α1- and α2-adrenergic receptors on coronary vascular smooth muscle cells (Mudge et al. 1976; Jones et al. 1993). This effect could be enhanced with age via unopposed vasoconstriction secondary to endothelial dysfunction (Vita et al. 1992; Jones et al. 1993). Additionally, as α2-adrenergic receptors on endothelial cells modulate nitric oxide release (Berkenboom et al. 1991), sympathetic activation associated with the CPT could contribute to coronary vasodilatation (Cocks & Angus, 1983) and decreased sensitivity of these receptors with age could impair this response. Presently, we are unaware of any data demonstrating what effect ageing exerts on α1- and α2-adrenergic receptor sensitivity on coronary vascular smooth muscle cells and endothelial cells in humans. Thus, we can only speculate that these mechanisms may have played a role in our observed responses.
It is unclear if the magnitude of sympathetic activation during the CPT is altered by age. Increases in directly recorded muscle sympathetic nerve activity during the CPT may be similar (absolute increase) or blunted (relative increase) in older as compared to young adults (Ng et al. 1994). The former response is believed to be more indicative of the degree of sympathetic activation during stress as the latter response is largely influenced by the pronounced increase in muscle sympathetic nerve activity at rest with age (Ng et al. 1994). In contrast increases in plasma noradrenaline concentrations during the CPT have been reported to be similar (Ng et al. 1994) or increased (Palmer et al. 1978) in older as compared to young adults. Importantly, none of these data address the effect ageing exerts on the cardiac sympathetic responses to the CPT. During other forms of physiological stress (e.g. mental stress and exercise) greater increases in cardiac noradrenaline spillover (Esler et al. 1995b) and blunted increases in adrenal adrenaline spillover (Esler et al. 1995a) have been reported in older as compared to young adults. Thus, we are unsure how possible age-related differences in the sympathetic nervous system response to the CPT might have affected our findings.
Several lines of evidence strongly suggest that the age-related impairment in coronary vasodilatation during the CPT cannot be explained by a reduced stimulus for vasodilatation. First, CPT increases RPP (i.e. myocardial oxygen demand) similarly in young and older men before (pre-blockade) and during adrenergic blockade (post-blockade), although RPP responses are reduced in both groups post-blockade. Second, impaired coronary vasodilatation during the CPT persists when changes in CVR (response) are expressed relative to changes in RPP (stimulus). Lastly, greater pressor response to the CPT in older as compared to young men (pre-blockade) suggest greater increases in afterload occurred in older men than young men during the CPT. Collectively, these findings indicate that the stimulus for coronary vasodilatation was at least as great in older as compared to young men during the CPT providing strong support for our hypothesis that ageing is associated with impaired coronary vasodilatation during sympathetic activation.
This study has several limitations. First, we studied only healthy men. Thus, our findings may not be representative of responses in other populations. Second, we did not determine individual effects of β- and α-adrenergic blockade on coronary responses to the CPT. The latter effect would be difficult to address as administration of an α-adrenergic blocker alone leaves β-adrenergic receptor effects intact and unopposed making interpretation of findings difficult. Third, adrenergic blockade was probably incomplete as evidenced by a residual increase in BP during the CPT in the post-blockade condition. However, had we completely blocked the pressor response to the CPT we would have also abolished the stimulus (increase in RPP) for the response we were measuring (changes in CBV or CVR). The only way to overcome this limitation would be to administer adrenergic antagonists directly into the coronary arteries, which was not possible in the present study. Lastly, CBV rather than coronary blood flow in the left anterior descending artery was measured. This was done as the ability to accurately and reliably measure coronary artery diameters in the range of 0.8–2.0 mm is limited by the resolution of the imaging modality employed. However, this factor is unlikely to have influenced our results as measures of coronary blood flow and CBV are strongly correlated, even at high flow rates (Marcus et al. 1981; Wilson et al. 1985).
In conclusion, our findings indicate that the: (1) normal coronary vascular response to sympathetic activation during the CPT is vasodilatation in young men and that this vasomotor response is abolished by advancing age in healthy men, (2) impaired coronary vasodilatation during the CPT in older adults is not the result of a reduced stimulus, as myocardial oxygen demand (e.g. RPP) increases at least as much in older as compared to young men and persists when changes in vasomotor tone are expressed relative to myocardial oxygen demand (e.g. CVR/RPP), and (3) impaired coronary vasodilatation during acute sympathetic activation with age is abolished by adrenergic blockade (systemic α- and β-adrenergic). These data strongly suggest that an adrenergic mechanism contributes to the impaired coronary vasodilator responses to the CPT with advancing age in humans. These findings may help us better understand how acute sympathetic activation may precipitate angina and coronary ischaemic events, particularly in older adults.
Translational perspective
The sympathetic nervous system is an important regulator of coronary blood flow. The effect ageing exerts on sympathetic modulation of coronary blood flow during physiological stress in humans is unknown. We tested the hypotheses that sympathetic activation, induced by having subjects perform a cold pressor test (CPT; submerge their hand in ice water), elicits coronary vasodilatation in young adults that is impaired with advancing age in healthy adults and that combined α- and β-adrenergic blockade diminishes/abolishes these age-related differences. Our results indicate that the CPT produces pronounced coronary vasodilatation in young, but not older men before adrenergic blockade. Adrenergic blockade abolished CPT-induced coronary vasodilatation in young men, such that responses during blockade mirrored those observed in older men. Importantly, impaired coronary vasodilatation during the CPT could not be explained by a reduced stimulus, as group and condition effects persisted when vasomotor responses were expressed relative to myocardial oxygen demand. These data indicate that the normal coronary vascular response to sympathetic activation in young men is pronounced vasodilatation and this effect is lost with age as the result of an adrenergic mechanism. These findings may help explain how/why acute sympathetic activation may precipitate angina and coronary ischaemic events, particularly in older adults. Future studies will be needed to address the consequences of these changes and the potential for various factors (e.g. diet, exercise, etc.) to favourably modify these age-related effects with the aim being to improve cardiovascular health and reduce cardiovascular risk.
Acknowledgments
This research was supported by grants from the National Institutes of Health (HL092309, AG024420, HL096570, HL07022, and TR000127) and the American Heart Association (12GRNT10220000).
Glossary
- BP
blood pressure
- CBV
coronary blood velocity
- CPT
cold pressor test
- CVR
coronary vascular resistance
- RPP
rate–pressure product
Author contributions
K.D.M. contributed to the conception and design of the study, analysis and interpretation of data, and drafted the article. R.P.F contributed to the analysis and interpretation of data and revised the article critically for important intellectual content. L.I.S. contributed to the conception and design of the study, interpretation of data, and revised the article critically for important intellectual content. Z.G. contributed to analysis and interpretation of data and revised the article critically for important intellectual content. All authors approved the final version of the article. Experiments were performed in the Clinical Research Center at the Penn State College of Medicine, Hershey, PA, USA.
References
- Antony I, Aptecar E, Lerebours G, Nitenberg A. Coronary artery constriction caused by the cold pressor test in human hypertension. Hypertension. 1994;24:212–219. doi: 10.1161/01.hyp.24.2.212. [DOI] [PubMed] [Google Scholar]
- Barbato E. Role of adrenergic receptors in human coronary vasomotion. Heart. 2009;95:603–608. doi: 10.1136/hrt.2008.150888. [DOI] [PubMed] [Google Scholar]
- Bell C, Seals DR, Monroe MB, Day DS, Shapiro LF, Johnson DG, Jones PP. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab. 2001;86:4440–4444. doi: 10.1210/jcem.86.9.7855. [DOI] [PubMed] [Google Scholar]
- Berkenboom G, Unger P, Fang ZY, Fontaine J. Endothelium-derived relaxing factor and protection against contraction to norepinephrine in isolated canine and human coronary arteries. J Cardiovasc Pharmacol. 1991;17(Suppl. 3):S127–S132. [Google Scholar]
- Berne RM. Regulation of coronary blood flow. Physiol Rev. 1964;44:1–29. doi: 10.1152/physrev.1964.44.1.1. [DOI] [PubMed] [Google Scholar]
- Bertel O, Buhler FR, Kiowski W, Lutold BE. Decreased beta-adrenoreceptor responsiveness as related to age, blood pressure, and plasma catecholamines in patients with essential hypertension. Hypertension. 1980;2:130–138. doi: 10.1161/01.hyp.2.2.130. [DOI] [PubMed] [Google Scholar]
- Chandraratna PA, Nimalasuriya AR, Vlachonassios KD, Mathews SJ, Kedes W, Marwah OS, Saad M. Usefulness of the response of flow velocity in the left anterior descending coronary artery to the cold pressor test for evaluating endothelium-dependent vascular relaxation in the coronary microvasculature by transesophageal echocardiography in subjects with angiographically normal coronary arteries. Am J Cardiol. 1999;84:1362–1365. doi: 10.1016/s0002-9149(99)00576-7. A1368. [DOI] [PubMed] [Google Scholar]
- Chilian WM. Functional distribution of alpha 1- and alpha 2-adrenergic receptors in the coronary microcirculation. Circulation. 1991;84:2108–2122. doi: 10.1161/01.cir.84.5.2108. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. Seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Cocks TM, Angus JA. Endothelium-dependent relaxation of coronary arteries by noradrenaline and serotonin. Nature. 1983;305:627–630. doi: 10.1038/305627a0. [DOI] [PubMed] [Google Scholar]
- Drexler H, Zeiher AM, Wollschlager H, Meinertz T, Just H, Bonzel T. Flow-dependent coronary artery dilatation in humans. Circulation. 1989;80:466–474. doi: 10.1161/01.cir.80.3.466. [DOI] [PubMed] [Google Scholar]
- Dubois-Rande JL, Dupouy P, Aptecar E, Bhatia A, Teiger E, Hittinger L, Berdeaux A, Castaigne A, Geschwind H. Comparison of the effects of exercise and cold pressor test on the vasomotor response of normal and atherosclerotic coronary arteries and their relation to the flow-mediated mechanism. Am J Cardiol. 1995;76:467–473. doi: 10.1016/s0002-9149(99)80132-5. [DOI] [PubMed] [Google Scholar]
- Esler M, Kaye D, Thompson J, Jennings G, Cox H, Turner A, Lambert G, Seals D. Effects of aging on epinephrine secretion and regional release of epinephrine from the human heart. J Clin Endocrinol Metab. 1995a;80:435–442. doi: 10.1210/jcem.80.2.7852502. [DOI] [PubMed] [Google Scholar]
- Esler MD, Thompson JM, Kaye DM, Turner AG, Jennings GL, Cox HS, Lambert GW, Seals DR. Effects of aging on the responsiveness of the human cardiac sympathetic nerves to stressors. Circulation. 1995b;91:351–358. doi: 10.1161/01.cir.91.2.351. [DOI] [PubMed] [Google Scholar]
- Feigl EO. Sympathetic control of coronary circulation. Circ Res. 1967;20:262–271. doi: 10.1161/01.res.20.2.262. [DOI] [PubMed] [Google Scholar]
- Feigl EO. Coronary physiology. Physiol Rev. 1983;63:1–205. doi: 10.1152/physrev.1983.63.1.1. [DOI] [PubMed] [Google Scholar]
- Gao Z, Wilson TE, Drew RC, Ettinger J, Monahan KD. Altered coronary vascular control during cold stress in healthy older adults. Am J Physiol Heart Circ Physiol. 2012;302:H312–H318. doi: 10.1152/ajpheart.00297.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwill JR, Minson CT, Joyner MJ. Effect of systemic nitric oxide synthase inhibition on postexercise hypotension in humans. J Appl Physiol. 2000;89:1830–1836. doi: 10.1152/jappl.2000.89.5.1830. [DOI] [PubMed] [Google Scholar]
- Hamdad N, Ming Z, Parent R, Lavallee M. Beta 2-adrenergic dilation of conductance coronary arteries involves flow-dependent NO formation in conscious dogs. Am J Physiol Heart Circ Physiol. 1996;271:H1926–H1937. doi: 10.1152/ajpheart.1996.271.5.H1926. [DOI] [PubMed] [Google Scholar]
- Hess KL, Wilson TE, Sauder CL, Gao Z, Ray CA, Monahan KD. Aging affects the cardiovascular responses to cold stress in humans. J Appl Physiol. 2009;107:1076–1082. doi: 10.1152/japplphysiol.00605.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch G. Alpha-adrenergic mechanisms in myocardial ischemia. Circulation. 1990;81:1–13. doi: 10.1161/01.cir.81.1.1. [DOI] [PubMed] [Google Scholar]
- Hines EA, Brown G. The cold pressor test for measuring the reactivity of the blood pressure. Data concerning 571 normal hypertensive subjects. Am Heart J. 1936;11:1–9. [Google Scholar]
- Hozumi T, Yoshida K, Akasaka T, Asami Y, Ogata Y, Takagi T, Kaji S, Kawamoto T, Ueda Y, Morioka S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: comparison with invasive technique. J Am Coll Cardiol. 1998a;32:1251–1259. doi: 10.1016/s0735-1097(98)00389-1. [DOI] [PubMed] [Google Scholar]
- Hozumi T, Yoshida K, Ogata Y, Akasaka T, Asami Y, Takagi T, Morioka S. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation. 1998b;97:1557–1562. doi: 10.1161/01.cir.97.16.1557. [DOI] [PubMed] [Google Scholar]
- Jeserich M, Schindler T, Olschewski M, Unmussig M, Just H, Solzbach U. Vitamin C improves endothelial function of epicardial coronary arteries in patients with hypercholesterolaemia or essential hypertension–assessed by cold pressor testing. Eur Heart J. 1999;20:1676–1680. doi: 10.1053/euhj.1999.1689. [DOI] [PubMed] [Google Scholar]
- Jones CJ, DeFily DV, Patterson JL, Chilian WM. Endothelium-dependent relaxation competes with alpha 1- and alpha 2-adrenergic constriction in the canine epicardial coronary microcirculation. Circulation. 1993;87:1264–1274. doi: 10.1161/01.cir.87.4.1264. [DOI] [PubMed] [Google Scholar]
- Kuo L, Chilian WM, Davis MJ. Coronary arteriolar myogenic response is independent of endothelium. Circ Res. 1990;66:860–866. doi: 10.1161/01.res.66.3.860. [DOI] [PubMed] [Google Scholar]
- Marcus M, Wright C, Doty D, Eastham C, Laughlin D, Krumm P, Fastenow C, Brody M. Measurements of coronary velocity and reactive hyperemia in the coronary circulation of humans. Circ Res. 1981;49:877–891. doi: 10.1161/01.res.49.4.877. [DOI] [PubMed] [Google Scholar]
- Momen A, Gahremanpour A, Mansoor A, Kunselman A, Blaha C, Pae W, Leuenberger UA, Sinoway LI. Vasoconstriction seen in coronary bypass grafts during handgrip in humans. J Appl Physiol. 2007;102:735–739. doi: 10.1152/japplphysiol.00618.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen A, Gao Z, Cohen A, Khan T, Leuenberger UA, Sinoway LI. Coronary vasoconstrictor responses are attenuated in young women as compared with age-matched men. J Physiol. 2010a;588:4007–4016. doi: 10.1113/jphysiol.2010.192492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen A, Kozak M, Leuenberger UA, Ettinger S, Blaha C, Mascarenhas V, Lendel V, Herr MD, Sinoway LI. Transthoracic Doppler echocardiography to noninvasively assess coronary vasoconstrictor and dilator responses in humans. Am J Physiol Heart Circ Physiol. 2010b;298:H524–H529. doi: 10.1152/ajpheart.00486.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momen A, Mascarenhas V, Gahremanpour A, Gao Z, Moradkhan R, Kunselman A, Boehmer JP, Sinoway LI, Leuenberger UA. Coronary blood flow responses to physiological stress in humans. Am J Physiol Heart Circ Physiol. 2009;296:H854–H861. doi: 10.1152/ajpheart.01075.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KD, Wilson TE, Ray CA. Omega-3 fatty acid supplementation augments sympathetic nerve activity responses to physiological stressors in humans. Hypertension. 2004;44:732–738. doi: 10.1161/01.HYP.0000145292.38579.f4. [DOI] [PubMed] [Google Scholar]
- Monroe MB, Seals DR, Shapiro LF, Bell C, Johnson D, Parker Jones P. Direct evidence for tonic sympathetic support of resting metabolic rate in healthy adult humans. Am J Physiol Endocrinol Metab. 2001;280:E740–E744. doi: 10.1152/ajpendo.2001.280.5.E740. [DOI] [PubMed] [Google Scholar]
- Mudge GH, Jr, Grossman W, Mills RM, Jr, Lesch M, Braunwald E. Reflex increase in coronary vascular resistance in patients with ischemic heart disease. N Engl J Med. 1976;295:1333–1337. doi: 10.1056/NEJM197612092952401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel EG, Ganz P, Gordon JB, Alexander RW, Selwyn AP. Dilation of normal and constriction of atherosclerotic coronary arteries caused by the cold pressor test. Circulation. 1988;77:43–52. doi: 10.1161/01.cir.77.1.43. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Sympathetic neural reactivity to stress does not increase with age in healthy humans. Am J Physiol Heart Circ Physiol. 1994;267:H344–H353. doi: 10.1152/ajpheart.1994.267.1.H344. [DOI] [PubMed] [Google Scholar]
- Nitenberg A, Antony I, Aptecar E, Arnoult F, Lerebours G. Impairment of flow-dependent coronary dilation in hypertensive patients. Demonstration by cold pressor test induced flow velocity increase. Am J Hypertens. 1995;8:13S–18S. doi: 10.1016/0895-7061(95)00028-n. [DOI] [PubMed] [Google Scholar]
- Nitenberg A, Chemla D, Antony I. Epicardial coronary artery constriction to cold pressor test is predictive of cardiovascular events in hypertensive patients with angiographically normal coronary arteries and without other major coronary risk factor. Atherosclerosis. 2004a;173:115–123. doi: 10.1016/j.atherosclerosis.2003.12.030. [DOI] [PubMed] [Google Scholar]
- Nitenberg A, Valensi P, Sachs R, Cosson E, Attali JR, Antony I. Prognostic value of epicardial coronary artery constriction to the cold pressor test in type 2 diabetic patients with angiographically normal coronary arteries and no other major coronary risk factors. Diabetes Care. 2004b;27:208–215. doi: 10.2337/diacare.27.1.208. [DOI] [PubMed] [Google Scholar]
- Palmer GJ, Ziegler MG, Lake CR. Response of norepinephrine and blood pressure to stress increases with age. J Gerontol. 1978;33:482–487. doi: 10.1093/geronj/33.4.482. [DOI] [PubMed] [Google Scholar]
- Remme WJ. The sympathetic nervous system and ischaemic heart disease. Eur Heart J. 1998;19(Suppl. F):F62–71. [PubMed] [Google Scholar]
- Robertson D, Johnson GA, Robertson RM, Nies AS, Shand DG, Oates JA. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation. 1979;59:637–643. doi: 10.1161/01.cir.59.4.637. [DOI] [PubMed] [Google Scholar]
- Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- Schindler TH, Nitzsche EU, Munzel T, Olschewski M, Brink I, Jeserich M, Mix M, Buser PT, Pfisterer M, Solzbach U, Just H. Coronary vasoregulation in patients with various risk factors in response to cold pressor testing: contrasting myocardial blood flow responses to short- and long-term vitamin C administration. J Am Coll Cardiol. 2003;42:814–822. doi: 10.1016/s0735-1097(03)00851-9. [DOI] [PubMed] [Google Scholar]
- Seals DR, Jablonski KL, Donato AJ. Aging and vascular endothelial function in humans. Clin Sci (Lond) 2011;120:357–375. doi: 10.1042/CS20100476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sielatycki JA, Shamimi-Noori S, Pfeiffer MP, Monahan KD. Adrenergic mechanisms do not contribute to age-related decreases in calf venous compliance. J Appl Physiol. 2011;110:29–34. doi: 10.1152/japplphysiol.00930.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara J, Komine H, Hayashi K, Yoshizawa M, Otsuki T, Shimojo N, Miyauchi T, Yokoi T, Maeda S, Tanaka H. Systemic α-adrenergic and nitric oxide inhibition on basal limb blood flow: effects of endurance training in middle-aged and older adults. Am J Physiol Heart Circ Physiol. 2007;293:H1466–H1472. doi: 10.1152/ajpheart.00273.2007. [DOI] [PubMed] [Google Scholar]
- Tesfamariam B, Cohen RA. Inhibition of adrenergic vasoconstriction by endothelial cell shear stress. Circ Res. 1988;63:720–725. doi: 10.1161/01.res.63.4.720. [DOI] [PubMed] [Google Scholar]
- Tousoulis D, Davies G, Tentolouris C, Crake T, Toutouzas P. Inhibition of nitric oxide synthesis during the cold pressor test in patients with coronary artery disease. Am J Cardiol. 1997;79:1676–1679. doi: 10.1016/s0002-9149(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Tang EH. Endothelium-dependent contractions: when a good guy turns bad! J Physiol. 2008;586:5295–5304. doi: 10.1113/jphysiol.2008.161430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor RG, Leimbach WN, Jr, Seals DR, Wallin BG, Mark AL. Effects of the cold pressor test on muscle sympathetic nerve activity in humans. Hypertension. 1987;9:429–436. doi: 10.1161/01.hyp.9.5.429. [DOI] [PubMed] [Google Scholar]
- Vita JA, Treasure CB, Yeung AC, Vekshtein VI, Fantasia GM, Fish RD, Ganz P, Selwyn AP. Patients with evidence of coronary endothelial dysfunction as assessed by acetylcholine infusion demonstrate marked increase in sensitivity to constrictor effects of catecholamines. Circulation. 1992;85:1390–1397. doi: 10.1161/01.cir.85.4.1390. [DOI] [PubMed] [Google Scholar]
- Wilson RF, Laughlin DE, Ackell PH, Chilian WM, Holida MD, Hartley CJ, Armstrong ML, Marcus ML, White CW. Transluminal, subselective measurement of coronary artery blood flow velocity and vasodilator reserve in man. Circulation. 1985;72:82–92. doi: 10.1161/01.cir.72.1.82. [DOI] [PubMed] [Google Scholar]
- Wilson TE, Gao Z, Hess KL, Monahan KD. Effect of aging on cardiac function during cold stress in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1627–R1633. doi: 10.1152/ajpregu.00099.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiher AM, Drexler H, Wollschlaeger H, Saurbier B, Just H. Coronary vasomotion in response to sympathetic stimulation in humans: importance of the functional integrity of the endothelium. J Am Coll Cardiol. 1989;14:1181–1190. doi: 10.1016/0735-1097(89)90414-2. [DOI] [PubMed] [Google Scholar]
- Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]


