Abstract
Ligating the femoral artery for 72 h in decerebrated rats exaggerates the exercise pressor reflex. The sensory arm of this reflex is comprised of group III and IV afferents, which can be either sensitized or stimulated by PGE2. In vitro studies showed that endoperoxide (EP) 3 and 4 receptors were responsible for the PGE2-induced sensitization of rat dorsal root ganglion cells. This in vitro finding prompted us to test the hypothesis that blockade of EP3 and/or EP4 receptors attenuated the exaggerated exercise pressor reflex in rats with ligated femoral arteries. We measured the cardiovascular responses to static hindlimb contraction or tendon stretch before and after femoral arterial injection of L798106 (an EP3 antagonist) or L161982 (an EP4 antagonist). The pressor and cardioaccelerator responses to either contraction or tendon stretch were not attenuated by L798106 in either the ligated or freely perfused rats. Likewise in five rats whose hindlimb muscles were freely perfused, the pressor and cardioaccelerator responses to either contraction or tendon stretch were not attenuated by L161982. In the six ligated rats, however, the pressor response to contraction was attenuated by L161982, averaging 37 ± 3 mmHg before, 18 ± 2 mmHg afterward (P < 0.05). Western blotting analysis revealed that ligation of the femoral artery for 72 h increased the EP4 receptor protein in the L4 and L5 dorsal root ganglia over their freely perfused counterparts by 24% (P < 0.05). We conclude that EP4 receptors, but not EP3 receptors, play an important role in the exaggerated exercise pressor reflex found in rats with ligated femoral arteries.
Key points
In decerebrated rats, the exercise pressor reflex arising from a hindlimb whose femoral artery was occluded for 72 h was significantly higher than that arising from a hindlimb whose femoral artery was freely perfused.
Blockade of endoperoxide 4 receptors, but not blockade of endoperoxide 3 receptors, prevented the exaggerated exercise pressor reflex in rats with ligated femoral arteries.
Blockade of endoperoxide 3 or 4 receptors in rats with freely perfused femoral arteries had no effect on the exercise pressor reflex.
Western immunoblots showed that ligation of the femoral artery for 72 h increased the endoperoxide 4 receptor protein in the L4 and L5 dorsal root ganglia over their freely perfused counterparts by 24% (P < 0.05).
Introduction
The exercise pressor reflex is evoked by contraction of hindlimb skeletal muscle and is manifested by increases in arterial pressure and heart rate (HR). These reflex increases, in turn, have been shown to increase arterial blood flow to the exercising muscles, an effect that maintains performance and delays fatigue (O’Leary & Sheriff, 1995; O’Leary et al. 1999; Amann et al. 2009, 2011). Group III and IV muscle afferents comprise the afferent limb of the exercise pressor reflex arc (Coote & Pérez-González, 1970; McCloskey & Mitchell, 1972). Group III afferents have thinly myelinated axons and are sensitive to mechanical stimuli, whereas group IV afferents have unmyelinated axons and are sensitive to metabolic stimuli (Kaufman et al. 1983). Group III and IV afferents, taken together, are termed thin fibre afferents.
The responses of thin fibre afferents to contraction are influenced by the metabolic milieu surrounding their endings in skeletal muscle. One group of metabolites that appear to play an important role in determining the responsiveness of thin fibre muscle afferents to contraction are cyclooxygenase products of arachidonic acid (Rotto et al. 1990b,a; Hayes et al. 2006). PGE2, which in human skeletal muscle accounts for the largest proportion of cyclooxygenase products of arachidonic acid (Berlin et al. 1979), may be especially important in determining these responses to contraction. Contraction of hindlimb skeletal muscle is well known to increase the concentration of PGE2 (Symons et al. 1991; McCord et al. 2008) as well as to increase the concentration of its precursor, arachidonic acid (Rotto et al. 1989). In addition, cyclooxygenase blockade with either indomethacin or sodium meclofenamate has been shown to attenuate the exercise pressor reflex (Stebbins et al. 1988b; Middlekauff & Chiu, 2004), findings that are consistent with but do not prove the hypothesis that PGE2 plays a role in evoking the exercise pressor reflex.
Ligation of the femoral artery of a rat for 72 h has been shown to simulate the arterial blood flow pattern in hindlimb muscle seen in peripheral artery disease. Specifically, blood flow to the limb whose femoral artery is ligated is adequate to meet metabolic demand at rest, but is inadequate to meet demand during exercise (Yang et al. 2000; Prior et al. 2004). In this preparation, the exercise pressor reflex in decerebrated rats is substantially larger than that in decerebrated rats with patent femoral arteries (Tsuchimochi et al. 2010, 2011). This finding as well as those described above suggested to us that the exaggerated exercise pressor reflex caused by ligating the femoral artery for 72 h was due to a PGE2-induced sensitization of group III and IV muscle afferents.
In a variety of tissues, PGE2 binds to endoperoxide (EP) receptors, of which there are several subtypes. Dorsal root ganglion cells in rats have been shown to possess the EP1, EP2, EP3C and EP4 receptor (Southall & Vasko, 2001; Kopp et al. 2004). In vitro, only the EP3C and the EP4 receptor were found to be responsible for the PGE2-induced sensitization of rat dorsal root ganglion cells (Southall & Vasko, 2001). These findings prompted us to test the hypothesis that blockade of EP3 and/or EP4 receptors attenuated the exaggerated exercise pressor reflex in rats with ligated femoral arteries.
Methods
All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the Pennsylvania State University, Hershey Medical Center. Adult male rats (Sprague–Dawley, n= 68, weighing between 320 g and 500 g) were used in this study. The rats were housed in a temperature-controlled room (24 ± 1°C) with a 12:12 h light–dark cycle. Rats were fed a standard diet and tap water ad libitum. Seventy-two hours before an experiment, some of the rats underwent surgery to ligate the left femoral artery according to the procedure described previously (Tsuchimochi et al. 2010). Briefly, rats were anaesthetized initially with a mixture of 4% isoflurane in 100% oxygen. A cone was then placed over the nose while the left femoral artery was isolated and then tightly ligated with 4-0 silk suture just distal to the inguinal ligament. The rats were allowed to recover 72 h before the experiments were started. Femoral artery ligation has no effect on normal cage activity. Ligation procedure has been shown to reduce blood flow reserve capacity to about 10–20% of normal but maintains sufficient blood flow to meet resting requirements (Yang et al. 2000; Prior et al. 2004).
Surgical preparation
On the day of the experiment, rats were anaesthetized initially with a mixture of 4% isoflurane and 100% oxygen. The trachea was cannulated, and the lungs were ventilated mechanically (Harvard Apparatus, Hollistan, MA) with 2% isoflurane in 100% oxygen. The right jugular vein and common carotid artery were cannulated (PE-50) for the delivery of drugs and fluids and the measurement of arterial blood pressure, respectively. The carotid arterial catheter was connected to a pressure transducer (model P23 XL, Statham, Franklin Lakes, NJ, USA). HR was calculated beat to beat from the arterial pressure pulse (Gould Biotach, Cleveland, OH, USA). Arterial blood gases and pH were measured by an automated blood gas analyser (model ABL-700, Radiometer, Cleveland, OH, USA). P!co2 and arterial pH were maintained within 33–40 mmHg and 7.35–7.42, respectively, by either adjusting ventilation or by intravenous administration of sodium bicarbonate (8.5%). A rectal temperature probe was inserted and the core body temperature of the animal was maintained at 37–38°C by a water-perfused heating pad and a lamp.
In the freely perfused rats, we cannulated (PE-10) the right femoral artery in a retrograde direction and advanced the tip to the bifurcation of the abdominal aorta. This allowed us to inject drugs into the arterial supply of the left hindlimb. On the other hand, in the ligated rats, we cannulated the ipsilateral left femoral artery peripheral to the previously ligated site and advanced the tip of the cannula so that it was located just before the popliteal artery. These procedures allowed the injection of substance into the circulation of the left hindlimb via the femoral artery. After either cannulation, we placed a snare around the abdominal aorta and the inferior vena cava just above the aortic bifurcation. When tightened, the snare helped to keep the injectate within the circulation of the left hindlimb.
Our method of injecting EP receptor antagonists into the right femoral artery of freely perfused rats resulted in distribution of the antagonist over the entire left hindlimb. In contrast, our method of injecting these antagonists into the left femoral artery of ligated rats resulted in the distribution of the antagonist only to that portion of the left hindlimb that was perfused below the inguinal ligament. This difference raised the possibility that injection of the antagonist resulted in it reaching a higher concentration in hindlimb muscles of the ligated rats than in the hindlimb muscles of freely perfused rats. To control for this possibility, we placed a 5 mm piece of PE50 tubing soaked in heparin (100 units ml−1) into the left femoral artery just distal to the inguinal ligament in five freely perfused rats. The tubing allowed some blood to flow through it, although clotting was evident when we inspected it at the end of the experiment. We then placed a second cannula (PE10) adjacent to the PE50 tubing so that we could inject L161962 (1 μg) in the same manner as we did in the ligated rats. For purposes of description, we termed injections of L161962 into the right femoral artery as ‘retrograde’ and injections of the antagonist into the left femoral artery as ‘anterograde.’
The rat was placed in a Kopf stereotaxic frame. Dexamethasone (0.2 mg) was injected intravenously just before the decerebration procedure to minimize brainstem oedema. The left common carotid artery was tied off and a precollicular decerebration was performed. The plane of section was less than 1 mm anterior to the superior colliculi. All neural tissue rostral to the section was removed, bleeding was controlled and the cranial cavity was packed with cotton. Rats were decerebrated instead of anaesthetized because the preponderance of evidence indicates that anaesthesia prevents the exercise pressor reflex in this species (Smith et al. 2001).
A laminectomy exposing the lower lumbar and sacral portions of the spinal cord (L1–L5) was performed. The rat was then secured in a customized spinal frame by clamps placed on rostral lumbar vertebrae and the pelvis. Using the skin on the back, we formed a pool that was filled with warm (37°C) mineral oil. The dura was cut and reflected allowing visual identification of the spinal roots. The left L4 and L5 ventral roots were identified and cut close to their exits from the spinal cord. The calcaneal bone of a left hindlimb was severed, and the triceps surae muscles were isolated. Once the surgeries were completed, the anaesthesia was withdrawn; the lungs were ventilated with room air. After decerebration, we waited a minimum of 60 min before beginning any experimental protocol.
Experimental protocols
Efficacy of antagonists
To test the efficacy of the two EP antagonists, we evaluated the peak pressor responses to the femoral arterial injection of PGE2 (10–20 μg; 200 μl) before and after intra-arterial injection of either L798106 (1 μg; 200 μl), an EP3 receptor antagonist, or L161982 (1 μg; 200 μl), an EP4 receptor antagonist. We did not perform a laminectomy on these rats; likewise the calcaneal tendon was not severed.
Reflex protocol
The peripheral cut ends of the L4 and L5 ventral roots were placed on shielded stimulating electrodes. The left calcaneal tendon was attached to a force transducer (model FT 10, Grass, Quincy, MA, USA), which in turn was attached to a rack-and -pinion. The tendon was stretched so that baseline tension was set between 80 and 100 g. Static contraction was evoked by electrically stimulating (40 Hz, 0.1 ms, ∼2 times motor threshold) the L4 and L5 ventral roots (Smith et al. 2001; Tsuchimochi et al. 2010). The muscle mechanoreceptor reflex was evoked by stretching the triceps surae muscles by manually turning the rack-and-pinion that was attached to the calcaneal tendon (Stebbins et al. 1988a). Baseline tension was set between 80 and 100 g. Both muscle contraction and tendon stretch lasted for 60 s. The order of presentation of the two stimuli was varied randomly.
We first determined the pressor and cardioaccelerator responses to static contraction and tendon stretch before and after blockade of EP3 receptors with L798106 (1 μg; 200 μl), a selective EP3 receptor antagonist (Juteau et al. 2001). In these rats, we tightened the snare placed around the abdominal aorta and inferior vena cava just before the femoral arterial injection of the antagonist. The snare was maintained for 5 min, after which it was released and the hindlimb was reperfused for 25 min.
We next determined the pressor and cardioaccelerator responses to static contraction and tendon stretch before and after blockade of EP4 receptors with L161982 (1 μg; 200 μl), a selective EP4 receptor antagonist (Machwate et al. 2001; Kopp et al. 2004). In these rats, which were not given L798106, we tightened the snare placed around the abdominal aorta and the inferior vena cava just before femoral arterial injection of the EP4 antagonist. The snare, which partially trapped the antagonist in the circulation of the hindlimb, was maintained for 5 min, after which it was released and the hindlimb was reperfused for 25 min.
In four ligated rats, we injected L161982 (1 μg; 200 μl) into the jugular vein to test whether our findings with femoral arterial injection of L161982 could be explained by its circulation to the spinal cord and brainstem. In four other ligated rats, we injected the vehicle for L161982 to test whether our findings with static contraction were repeatable. Static contraction and tendon stretch were evoked at least 25 min after the injection.
Immediately before the end of each experiment in which we injected L798106 or L161982 into the hindlimb circulation, we injected blue dye retrogradely into the femoral arterial catheter. In each case, the triceps surae muscles were stained blue, verifying that L798106 or L161982 had access to this muscle group. Both antagonists were purchased from (Sigma-Aldrich, St. Louis, MO, USA) and were injected in all experiments as a 5% DMSO–95% saline solution. At the conclusion of the experiment, the rat was humanely killed with an overdose of pentobarbital followed by an injection of saturated KCl solution.
Western blot analysis
A total of 13 rats were ligated as described above under isoflurane anaesthesia (4%) balanced with oxygen. They were killed 72 h later under isoflurane anaesthesia (4%), after which the L4 and L5 dorsal root ganglia (DRG) were collected from the freely perfused and ligated sides of each rat. After removal, the DRG were kept cold in Hank's buffer salt solution. A total of 26 samples were collected (13 freely perfused and 13 ligated; each sample contained L4 and L5 DRG) to compare protein expression for EP3 (n= 7) and EP4 (n= 6) between freely perfused and ligated hindlimbs. Total protein from DRG tissue was prepared with the Nucleospin RNA/Protein Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions. Protein concentration was determined using Qubit 2.0 Fluorometer (Life Technologies, Grand Island, NY, USA). Protein samples (25 μg) were separated on NuPAGE 10% Bis-Tris pre-cast gels (Life Technologies) by gel electrophoresis employing 90 V for 110 min at 4°C for EP3 or 200 V for 55 min at room temperature for EP4, and then transferred on to PVDF membranes. The membranes were blocked for 60 min at room temperature in Tris-buffered saline-Tween 20 supplemented with 7% milk for EP3 or 5% horse serum for EP4. Thereafter, the membranes were incubated with either anti-EP3 (1:200; Millipore Inc., Billerica, MA, USA) rabbit polyclonal or anti-EP4 (1:250; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) mouse monoclonal antibodies for 60 min at room temperature. Next, the membranes were incubated with horseradish peroxidase conjugated antirabbit (for EP3) or antimouse (for EP4) IgG antibody (1:5000; GE Healthcare, Piscataway, NJ, USA) for 60 min. Membrane bound proteins were visualized using enhanced chemiluminescent reagent, SuperSignal West Femto (Thermo Scientific, Rockford, IL, USA), and acquired with a ChemiDoc-It imaging system (UVP, LLC, Upland, CA, USA) equipped with a 16-bit CCD camera. The membranes were then stripped for 5 min at room temperature with Restore Western Blot Stripping Buffer (Thermo Scientific) and retested with anti-actin mouse monoclonal antibody (1:2500; Abcam, Cambridge, MA, USA) to normalize for protein loading. The protein bands were quantified and processed using VisionWorksLS software (UVP).
Data analysis
Arterial blood pressure, HR, and tension developed by the triceps surae muscles were recorded with a Spike 2 data acquisition system (CED, Cambridge, UK) and stored on a computer hard drive (Dell). Mean arterial pressure (MAP) is expressed in mmHg and HR in beats per minute (b.p.m.). Baseline values for MAP and HR were determined immediately before contraction, which was not initiated until these values were stable. Contraction-evoked pressor and cardioaccelerator responses were analysed as peaks. The tension-time index (TTI) was calculated by integrating the area between the tension trace and the baseline level (Spike 2) and is expressed in kg·s.
All values are expressed as means ±s.e.m. Statistical analyses of arterial pressure, HR and tension development were performed with a two-way ANOVA with one repeated measure. When applicable, a Holm–Sidak's multiple comparison post hoc test was performed. Statistical analyses of Western blots were performed with a paired t test. The criterion for statistical significance was set at P < 0.05.
Results
Our first task was to establish a dose of EP3 and EP4 antagonist, which when injected into the femoral artery, prevented the pressor responses to femoral arterial injection of PGE2 (10–20 μg). We found that there was a marked difference between the ligated and freely perfused rats. Specifically, the first injection of PGE2 in the ligated rats (n= 11) evoked a pressor response, whereas the first injection in the freely perfused rats (n= 10) evoked a depressor response (Fig. 1A and B). In the ligated rats, a dose of 1 μg of either L798106 (EP3 antagonist) or L161982 (EP4 antagonist) significantly decreased the pressor response to PGE2 (Fig. 1C and D). Likewise, in the freely perfused rats, either L798106 or L161982 significantly decreased the pressor response to PGE2, but this pressor response arose only after repeated injections of this prostanoid (Fig. 1C and D).
Figure 1. Effects of EP3 and EP4 antagonists on the pressor responses to femoral arterial injection of PGE2 (10–20 μg).
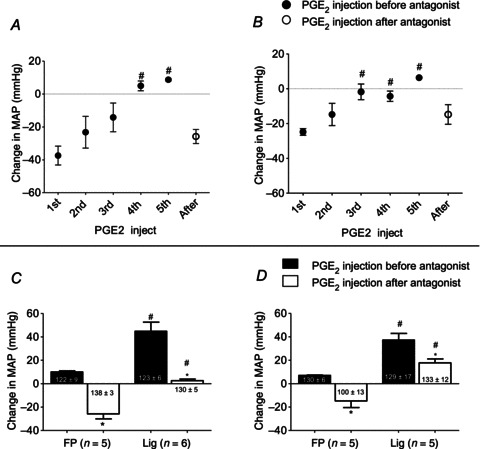
A, changes in MAP for five sequential injections of PGE2, after which the EP3 antagonist, L798106 (1 μg), was injected into the femoral artery of five rats with FP femoral arteries. B, changes in MAP for five sequential injections of PGE2, after which the EP4 antagonist, L161982 (1 μg), was injected into the femoral artery of five rats with FP femoral arteries. A and B, number sign (#) signifies that the pressor response was significantly greater (P < 0.05) than the depressor response to the first injection. C, summary data for effect of the EP3 antagonist on the pressor responses to PGE2 injection in five rats with patent femoral arteries and in five rats with Lig arteries before (filled bars) and after (open bars) injection of the EP3 antagonist. Note that the pressor responses to PGE2 in the FP rats represent that of the fifth sequential injection of the prostanoid shown in (A), whereas the pressor responses in the ‘Lig’ rats represents that of the first injection of PGE2. D, summary data for effect of the EP4 antagonist on the pressor responses to PGE2 injection in five rats with patent femoral arteries and six rats with Lig arteries before (filled bars) and after (open bars) injection of the EP4 antagonist. Note that the pressor responses to PGE2 in the FP rats represents that of the fifth sequential injection of the prostanoid shown in (A), whereas the pressor responses in the Lig rats represents that of the first injection of PGE2. In (C) and (D) and in subsequent figures, numbers within or underneath the vertical bars represent baseline means ±s.e. C and D, number sign (#) signifies a significant difference (P < 0.05) between the pressor response to PGE2 injection in the Lig rats and that to injection in their FP counterparts. Asterisk (*) signifies a significant difference (P < 0.05) within either the ‘Lig’ or ‘freely perfused’ rats between changes in MAP before and after the EP antagonist. EP, endoperoxide; FP, freely perfused; Lig, ligated; MAP, mean arterial pressure.
Effect of endoperoxide 3 antagonist on the exercise pressor and muscle mechanoreceptor reflexes
We examined the effects of L798106 on the exercise pressor reflex, induced by static contraction, and the muscle mechanoreflex, induced by tendon stretch. In the five rats whose femoral arteries were patent as well as in the five rats whose femoral arteries were ligated, static contraction and tendon stretch increased MAP and HR above baseline levels both before and after L798106. The magnitudes of the pressor and cardioaccelerator responses to either contraction or tendon stretch were not attenuated by L798106 in either the ‘ligated or patent’ group (Fig. 2A, B, D and E). The TTIs were not different before and after L798106 for either contraction or stretch (Fig. 2C and F). For tendon stretch, however, the TTIs for the rats whose femoral arteries were ligated were significantly greater than those for the rats whose femoral arteries were freely perfused (P < 0.05). L798106 did not attenuate the pressor or cardioaccelerator responses to this large stimulus (i.e. tendon stretch) in rats with ligated femoral arteries. The greater pressor and cardioaccelerator responses to stretch in rats with ligated femoral arteries than in rats with patent femoral arteries was probably due to the greater stimulus in the former than in the latter; nevertheless, the possibility exists that these augmented responses were in some small part caused by ligation of the femoral artery. Regardless of the cause, L798106 had no effect on their magnitude.
Figure 2. Summary data showing that femoral arterial injection of the EP3 antagonist, L798106 (1 μg), had no effect on the pressor responses to either static contraction or tendon stretch in either five rats with FP or in five rats with Lig femoral arteries.
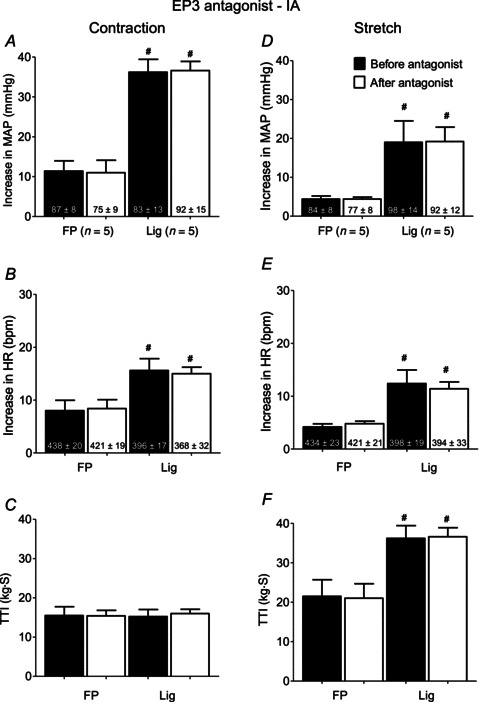
Filled bars represent mean values before injection of the EP3 antagonist. Open bars represent mean values after injection of the antagonist. Number sign (#) represents a significant difference (P < 0.02) between the Lig mean value and its FP counterpart. EP, endoperoxide; FP, freely perfused; HR, heart rate; Lig, ligated; MAP, mean arterial pressure; TTI, tension-time index.
Effect of endoperoxide 4 antagonist on the exercise pressor and muscle mechanoreceptor reflexes
In five rats whose femoral arteries were patent, static contraction and tendon stretch increased MAP and HR above baseline levels both before and after L161982 (Fig. 3A, B, D, and E). The magnitudes of the pressor and cardioaccelerator responses to contraction and stretch were not attenuated by retrograde injections of L161982 into the right femoral artery (Fig. 3A, B, D and E; Fig. 4). The TTIs were not different before and after L161982 for either contraction or stretch (Fig. 3C and F).
Figure 3. Summary data showing that femoral arterial injection of the EP4 antagonist, L161982 (1 μg), attenuated the pressor response to static contraction in six rats with Lig femoral arteries, but had no effect on the pressor response to contraction in five rats whose femoral arteries were FP.
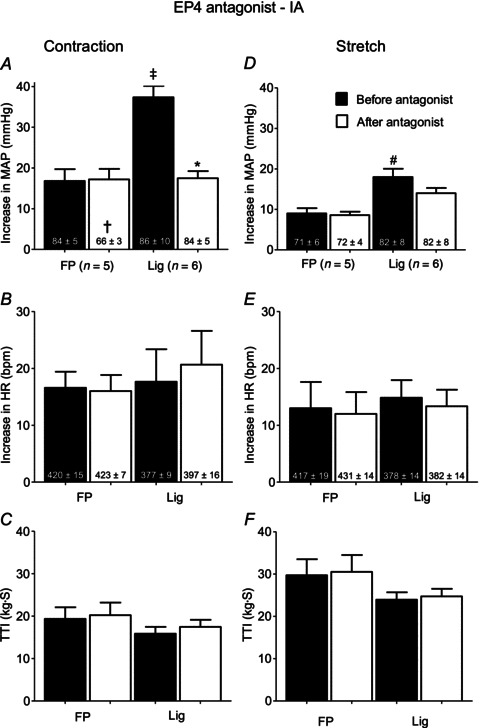
Filled bars represent mean values before injection of the EP4 antagonist. Open bars represent mean values after injection of the antagonist. Cross (†) represents a significant difference (P < 0.05) between the FP baseline value before and after the antagonist. Asterisk (*) represents a significant difference (P < 0.05) between the pressor response to contraction before the EP4 antagonist and the pressor response to contraction afterwards. Double cross (‡) signifies that the EP4 antagonist significantly decreased the pressor response to contraction in the Lig rats but had no effect on the pressor response to contraction in the FP rats (P < 0.001). Number sign (#) represents a significant difference between FP & Lig before injection of EP4 antagonist. EP, endoperoxide; FP, freely perfused; HR, heart rate; Lig, ligated; MAP, mean arterial pressure; TTI, tension-time index.
Figure 4. Example of the attenuating effect by the endoperoxide 4 antagonist, L161982 (1 μg), on the pressor response to static contraction in a rat whose femoral artery was ligated (A and B) and the lack of this effect by the endoperoxide 4 antagonist in another rat whose femoral artery was patent (C and D).
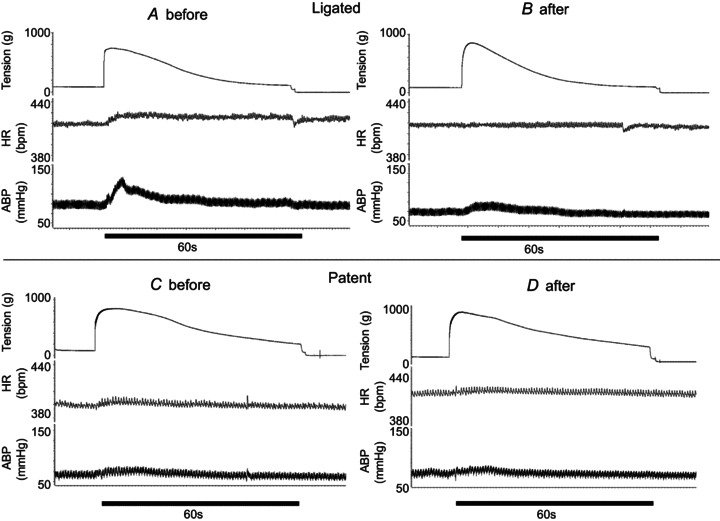
ABP, arterial blood pressure; HR, heart rate.
In another set of five rats whose femoral arteries were patent, we injected L161982 (1 μg) in an anterograde manner into the left femoral artery. We performed these injections as a control for the possibility that retrograde injection of the antagonist into the right femoral artery altered the distribution of the antagonist when compared to that of left femoral arterial injection. In these five rats, anterograde injection of L161982 had no effect on the exercise pressor reflex, although the TTIs were significantly less (P < 0.05) than those evoked by contraction in rats receiving retrograde injection of the antagonist (Fig. 5).
Figure 5. Summary data showing that in rats with ‘patent femoral arteries’ that neither retrograde nor anterograde injection of the EP4 antagonist (1 μg) attenuated the exercise pressor reflex.
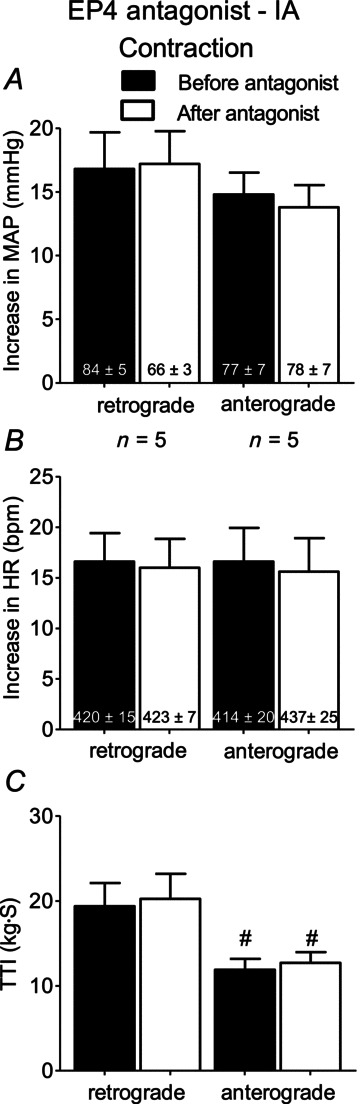
Number signs (#) represent significant differences (P < 0.05) in TTIs between retrograde and anterograde conditions both before and after injection of L161982, EP4 antagonist. For purposes of comparison, the data shown in Fig. 3 A–C (i.e. retrograde injection) have been repeated in this figure. Cross (†) represents a significant difference (P < 0.05) between the freely perfused baseline value before and after the antagonist. EP, endoperoxide; HR, heart rate; MAP, mean arterial pressure; TTI, tension-time index.
In the five rats whose femoral artery was ligated, static contraction increased MAP above baseline levels both before and after L161982 (P < 0.05; Fig. 3A). However, the magnitude of the pressor response to contraction was attenuated by L161982 (Figs 3A and 4). The magnitude of the cardioaccelerator response to contraction was not attenuated by L161982 (Fig. 3B). The TTIs for static contraction were not different before and after L161982 (Fig. 3C). We found that the pressor response to static contraction was greater in rats whose femoral artery had been ligated 72 h before the start of the experiment (n= 6) than that in rats whose hindlimbs were freely perfused (P < 0.05; Fig. 3A). There was no difference in the cardioaccelerator response to contraction between the two groups (Fig. 3B).
We found that the pressor response to tendon stretch was greater in rats whose femoral artery was ligated 72 h before the start of the experiment (n= 6) than that in rats whose hindlimbs were freely perfused (P < 0.05; Fig. 3D). There was no difference in the cardioaccelerator response to stretch between the two groups (Fig. 3E). In the rats whose femoral artery was ligated, stretch increased MAP above baseline levels both before and after L161982 (P < 0.05; Fig. 3D). The magnitudes of responses of the pressor and cardioaccelerator responses to stretch were not attenuated by L161982 (Fig. 3D and E). The TTIs for stretch were not different before and after L161982 (Fig. 3F).
Femoral arterial injection of the vehicle for L161982 in four rats whose femoral artery was ligated 72 h before the start of the experiment had no significant effect on either the pressor or cardioaccelerator components of the exercise pressor reflex. Specifically, contraction before injection of the vehicle significantly increased MAP by 33 ± 4 mmHg over its baseline of 97 ± 9 mmHg (P < 0.05); contraction after injection of the vehicle increased MAP by 31 ± 3 mmHg over its baseline of 90 ± 8 mmHg (P < 0.05). Likewise, contraction before the vehicle significantly increased HR by 21 ± 1 b.p.m. over its baseline of 454 ± 25 b.p.m. (P < 0.05); contraction after injection of the vehicle increased HR by 19 ± 1 b.p.m. over its baseline of 444 ± 39 b.p.m. (P > 0.05). The TTIs averaged 14.5 ± 2 kg s before the vehicle was injected and 14.7 ± 2 kg s−1 afterwards (P > 0.05).
Intravenous L161982
We found that intravenous injection L161982 had no effect on the pressor response to static contraction in rats whose hindlimbs had been ligated 72 h before the start of the experiment (n= 4; Fig. 6A). Likewise, we found that intravenous injection of L161982 had no effect on the pressor responses to tendon stretch in rats whose hindlimbs had been ligated 72 h before the start of the experiment (n= 4; Fig. 6D).
Figure 6. Summary data showing that intravenous injection of the EP4 antagonist, L161982 (1 μg), had no effect on the pressor response to static contraction in the rats with ligated femoral arteries (n= 4).
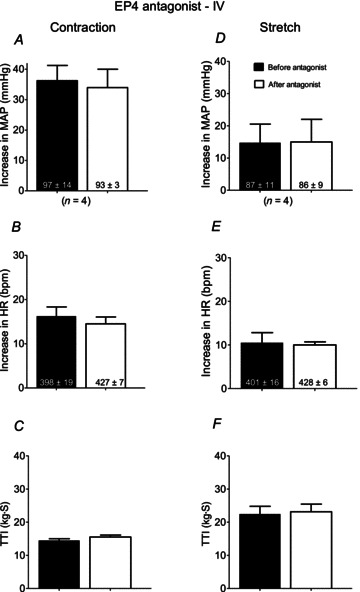
Filled bars represent mean values before injection of the EP4 antagonist. Open bars represent mean values after injection of the antagonist. EP, endoperoxide; HR, heart rate; MAP, mean arterial pressure; TTI, tension-time index.
Western blot
The ratio of the binding of the EP3 receptor antibody to the binding of the actin antibody in protein extracted from the L4 and L5 DRG innervating the hindlimbs whose femoral arteries were ligated averaged 1.43 ± 0.24, whereas the ratio of the binding of the EP3 receptor antibody to the binding of the actin antibody in protein extracted from the DRG innervating the hindlimbs whose femoral arteries were patent averaged 1.39 ± 0.28 (n= 7; P= 0.459; Fig. 7). In contrast, the ratio of the binding of the EP4 receptor antibody to the binding of the actin antibody in protein extracted from the L4 and L5 DRG innervating the hindlimbs whose femoral arteries were ligated averaged 9.23 ± 1.84, whereas the ratio of the binding of the EP4 receptor antibody to the binding of the actin antibody in protein extracted from the DRG innervating the hindlimbs whose femoral arteries were patent averaged 7.28 ± 1.11 (n= 6; P= 0.037; Fig. 7). Simply put, ligation significantly increased the EP4 receptor in the DRG by 27%, whereas ligation non-significantly increased the EP3 receptor in these ganglia by only 3%.
Figure 7. Detection of protein expression of the EP4 and EP3 receptors by Western blot analysis in L4–L5 DRG tissue.
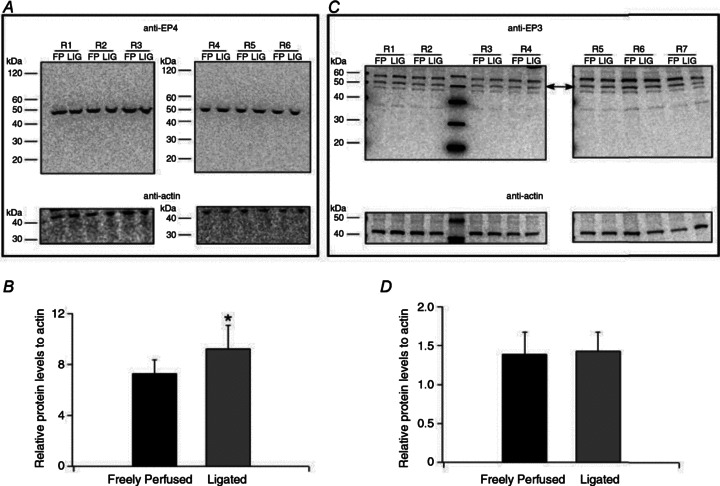
A and C, Western blot experiments illustrating results with anti-EP4 (A) and anti-EP3 (C) in DRG tissue isolated from six (A) and seven (C) rats with FP and LIG femoral arteries for 72 h. Lines indicate approximate molecular weights (kDa). The arrows in (C) indicate the approximate molecular weight (53 kDa) of EP3 receptors. B and D, densitometric analysis of Western blots for EP3, EP4 and actin. Each EP4 and EP3 value was normalized to actin and the ratio of the receptor protein levels to actin are plotted. The values represent the mean (±s.e.). *P < 0.05 compared to FP DRG tissue. DRG, dorsal root ganglia; EP, endoperoxide; FP, freely perfused; LIG, ligated.
Discussion
Using antisense oligonucleotides, Southall and Vasko reported that knockdown of EP3C and EP4 receptors in cultured rat DRG cells prevented two effects indicative of neuronal excitation. The two effects were PGE2-induced production of cyclic AMP and PGE2-induced release of the neuromodulators, substance P and calcitonin gene-related peptide (Southall & Vasko, 2001). This in vitro study led us to examine the role played by these receptors in evoking the exercise pressor reflex, which was evoked by static contraction of the hindlimb muscles in rats with patent femoral arteries as well as in rats with ligated femoral arteries. We found that in vivo blockade of EP4 receptors, but not blockade of EP3 receptors, attenuated the exaggerated exercise pressor reflex in rats with ligated femoral arteries. In contrast, blockade of either EP receptor did not attenuate the reflex in rats with patent femoral arteries.
We needed to establish that the dose of EP receptor antagonist used in our experiments was effective in attenuating thin fibre afferent input. Our criterion was that the antagonist used was effective in blocking the pressor response to a femoral arterial injection of PGE2. We chose this criterion because it can be easily interpreted as the reflex effect evoked by stimulating thin fibre somatic afferents in the hindlimb. We chose not to use a depressor response to PGE2 injection because this effect is caused by vasodilation, induced by relaxation of smooth muscle, and is not indicative of afferent excitation. In rats with patent femoral arteries, our first injection of PGE2 decreased arterial pressure, whereas in rats with ligated femoral arteries, our first injection increased arterial pressure. The former finding caused us to inject PGE2 several times before a pressor response was obtained in rats with patent arteries. Even then, the pressor response was small when compared with that evoked by PGE2 injection in rats with ligated arteries. Nevertheless, the doses of EP receptor antagonists used in our experiments were found to prevent pressor responses to injection of PGE2, thereby satisfying our criterion for adequate blockade.
In our experiments, blockade of either EP3 or EP4 receptors prevented the pressor response to exogenous injection of PGE2, but only blockade of EP4 receptors attenuated the pressor response to contraction. The lack of effect of EP3 receptor blockade on the exercise pressor reflex might be surprising because contraction of skeletal muscle is well known to produce PGE2. We can offer two possible explanations for this apparent discrepancy, although both must be considered as speculation. The first is that the sensitivity of EP4 receptors on thin fibre muscle afferents to PGE2 is greater than that of EP3 receptors. We think that this is a possibility because the amounts of PGE2 injected into the femoral artery in our experiments were large (i.e. 10–20 μg) and probably exceeded the contraction-induced concentration (i.e. 200 pg ml−1) of PGE2 in the muscle interstitial space, the location of the endings of group III and IV afferents (McCord et al. 2008). The second explanation is that PGE2 stimulated cutaneous, joint as well as muscle afferents to produce a pressor response. The possibility exists that EP receptors on thin fibre cutaneous and joint afferents have a different sensitivity to PGE2 than do thin fibre muscle afferents. Both possibilities may have functioned in our experiments. We would like to emphasize, however, that the purpose of injecting PGE2 into the femoral artery was not to create a physiological or pathophysiological concentration of the prostanoid or to stimulate selectively a type of somatic afferent. Instead, our purpose was to establish that the dose of EP antagonist injected was functioning effectively to prevent a pressor response to PGE2.
In addition to its ability to stimulate or more frequently sensitize thin fibre somatic afferents there is substantial evidence that PGE2 has important effects on synaptic transmission in the dorsal horn of the spinal cord. For example, nociceptive stimulation of the hindpaw of anaesthetized rats increased the concentration of PGE2, but not of PGF2α or 6-keto PGF1α, in the lumbar spinal perfusate (Coderre et al. 1990). In addition, preventing prostaglandin production in the spinal cord with cyclooxygenase inhibitors decreased pain-related behaviour evoked by noxious stimulation of the periphery (Malmberg & Yaksh, 1992). These findings raised the possibility that in our experiments L161982, the EP4 receptor antagonist, circulated to the spinal cord and/or brainstem to exert its inhibitory effects on the exercise pressor reflex in rats with ligated femoral arteries. To assess this possibility we injected the antagonist intravenously and found that it had no effect on the exercise pressor reflex. Consequently, we conclude that attenuation of the reflex in rats with ligated femoral arteries occurred at the afferent ending in the hindlimb and did not occur in the spinal cord or brainstem.
We found that the EP4 receptor played an important role in causing the exaggerated exercise pressor reflex in rats whose femoral arteries were ligated for 72 h before the start of the experiment, but appeared to play no role in evoking the reflex in rats whose femoral arteries were freely perfused (Tsuchimochi et al. 2010). In fact, blockade of this receptor in the ligated rats seemed to restore the exercise pressor reflex to the level found in rats with freely perfused femoral arteries. Two possibilities may explain the differential role played by the EP4 receptor in evoking the exercise pressor reflex between freely perfused and ligated rats. The first possibility is that ischaemia increased the production of PGE2 by the contracting muscles (Symons et al. 1991) to the threshold concentration needed to stimulate EP4 receptors on the endings of thin fibre muscle afferents. This possibility seems somewhat remote because in previous experiments we found that ligation of the femoral artery for only 3 min instead of 3 days did not result in an exaggerated exercise pressor reflex (Tsuchimochi et al. 2010). The second possibility is that the number of EP4 receptors on the endings of these afferents may have increased over the 72 h period when the femoral artery was ligated. Although both possibilities may have acted together, our finding that the EP4 receptor protein increased in the DRG is supportive of this second possibility. The activation of EP4 receptors, which are coupled to Gαs proteins (Southall & Vasko, 2001), leads to stimulation of adenylate cyclase and subsequent elevation of intracellular cAMP. Thus, the 25% increase in EP4 receptor protein expression in the ligated group may have led to a greater amplification of the signalling cascade pathways associated with this receptor.
In rats classified as having ‘patent femoral arteries,’ we found that neither retrograde nor anterograde injection of the EP4 receptor antagonist had any effect on the exercise pressor reflex. We also found that the magnitude of the exercise pressor reflex did not differ between rats receiving either type of injection. Nevertheless, the TTIs in rats receiving anterograde injections of the antagonist were significantly lower than those in rats receiving retrograde injections. We speculate that the smaller TTI in rats receiving anterograde injections of the antagonist was probably caused by a clotting-induced decrease in blood flow to the hindlimb muscles in the PE tubing placed in the femoral artery. If our speculation is correct, it implies that the contracting hindlimb muscles of rats receiving anterograde injections had a higher degree of ischaemia when compared to the contracting hindlimb muscles of rats receiving retrograde injections. Apparently, this relative degree of ischaemia had little effect on the magnitude of the exercise pressor reflex. We previously found that occluding the circulation to the contracting hindlimb 3 min before as well as during its initiation did not increase the exercise pressor reflex in decerebrate rats (Tsuchimochi et al. 2010).
Large and possibly unphysiological of doses of PGE2 or arachidonic acid, its precursor, have been shown to stimulate about half of the group III and IV muscle afferents tested (Mense, 1981; Rotto & Kaufman, 1988; Rotto et al. 1990a). This stimulatory effect accounts, at least in part, for the pressor responses evoked by injection of these substances in our experiments. The literature suggests that PGE2 or arachidonic acid, in concentrations in either the physiological or pathophysiological range, is effective in sensitizing thin fibre afferents innervating muscles (Rotto et al. 1990b,a; Hayes et al. 2006). Frequently, this afferent sensitization is found when muscles or nerves are injured or inflamed (Schafers et al. 2004; Ma et al. 2010; St-Jacques & Ma, 2011). The level of injury or inflammation existing in the rats used in our experiments was probably small if it existed at all. Specifically, ligation of one femoral artery for 72 h has been shown to allow adequate arterial perfusion of the hindlimb muscles in cage restrained rats (Yang et al. 2000; Prior et al. 2004). Consequently, any muscle damage might be expected to be minimal.
Regardless of whether the femoral artery was ligated or was freely perfused, we found that blockade of either EP3 or EP4 receptors did not attenuate the muscle mechanoreceptor reflex, which was evoked by tendon stretch (Stebbins et al. 1988a). This finding is somewhat surprising because cyclooxygenase products of arachidonic acid seem to play a role in determining the sensitivity of group III mechanoreceptors to tendon stretch in cats with freely perfused muscles (Rotto et al. 1990b). Although there have been no equivalent reflex studies performed on the muscle mechanoreceptor reflex in cats, our findings in rats raise the possibility that a species difference may exist between the two.
In both cats and healthy humans, cyclooxygenase blockade has been shown to attenuate the exercise pressor reflex (Stebbins et al. 1988b; Middlekauff & Chiu, 2004; Cui et al. 2007). Our findings suggest that blockade of the EP4 receptor is not responsible for this attenuation in the healthy state. Blockade of the thromboxane A2 receptor has been shown to attenuate the exercise pressor reflex in rats with freely perfused arteries and therefore may play an important role in sensitizing group III and IV muscle afferents to contraction in health (Leal et al. 2011). In the pathophysiological state, such as claudication caused by an occluded arterial blood supply to the legs, the evidence from rats whose femoral arteries have been ligated for 72 h suggests that both the EP4 and thromboxane A2 receptor play roles in causing the exaggerated exercise pressor reflex found in both rats (Tsuchimochi et al. 2010) and humans (Baccelli et al. 1999).
Glossary
- HR
heart rate
- MAP
mean arterial pressure
- TTI
tension–time index
Additional information
Competing interests
No conflicts of interests are declared by the authors.
Author contributions
K.Y. contributed to the conception, design, analysis and interpretation of the study, and reviewed the manuscript for its intellectual content. J.S.K. contributed to the analysis and interpretation of the data and reviewed the manuscript for its intellectual content. A.J.S. contributed to the analysis and interpretation of the data and reviewed the manuscript for its intellectual content. V.R.V. contributed to the analysis and interpretation of the data and reviewed the article for its intellectual content. M.P.K. contributed to the conception, analysis and interpretation of the study and drafted the article. All authors approved the final version of the manuscript.
Funding
This work was supported by NIH grants HL-096570 and AR-059397.
References
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–283. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol. 2011;589:3855–3866. doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology. 1999;50:361–374. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- Berlin T, Cronestrand R, Nowak J, Sonnenfeld T, Wennmalm A. Conversion of arachidonic acid to prostaglandins in homogenates of human skeletal muscle and kidney. Acta Physiol Scand. 1979;106:441–445. doi: 10.1111/j.1748-1716.1979.tb06424.x. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Gonzales R, Goldyne ME, West J, Levine JD. Noxious stimulus-induced increase in spinal prostaglandin E2 is noradrenergic terminal-dependent. Neurosci Lett. 1990;115:253–258. doi: 10.1016/0304-3940(90)90464-k. [DOI] [PubMed] [Google Scholar]
- Coote JH, Pérez-González JF. The response of some sympathetic neurones to volleys in various afferent nerves. J Physiol. 1970;208:261–278. doi: 10.1113/jphysiol.1970.sp009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, McQuillan P, Momen A, Blaha C, Moradkhan R, Mascarenhas V, Hogeman C, Krishnan A, Sinoway LI. The role of the cyclooxygenase products in evoking sympathetic activation in exercise. Am J Physiol Heart Circ Physiol. 2007;293:H1861–H1868. doi: 10.1152/ajpheart.00258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Cyclooxygenase blockade attenuates responses of group III and IV muscle afferents to dynamic exercise in cats. Am J Physiol Heart Circ Physiol. 2006;290:H2239–H2246. doi: 10.1152/ajpheart.01274.2005. [DOI] [PubMed] [Google Scholar]
- Juteau H, Gareau Y, Labelle M, Sturino CF, Sawyer N, Tremblay N, Lamontagne S, Carriere MC, Denis D, Metters KM. Structure-activity relationship of cinnamic acylsulfonamide analogues on the human EP3 prostanoid receptor. Bioorg Med Chem. 2001;9:1977–1984. doi: 10.1016/s0968-0896(01)00110-9. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kopp UC, Cicha MZ, Nakamura K, Nusing RM, Smith LA, Hokfelt T. Activation of EP4 receptors contributes to prostaglandin E2-mediated stimulation of renal sensory nerves. Am J Physiol Renal Physiol. 2004;287:F1269–F1282. doi: 10.1152/ajprenal.00230.2004. [DOI] [PubMed] [Google Scholar]
- Leal AK, McCord JL, Tsuchimochi H, Kaufman MP. Blockade of the TP receptor attenuates the exercise pressor reflex in decerebrated rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2011;301:H2140–H2146. doi: 10.1152/ajpheart.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Chabot JG, Vercauteren F, Quirion R. Injured nerve-derived COX2/PGE2 contributes to the maintenance of neuropathic pain in aged rats. Neurobiol Aging. 2010;31:1227–1237. doi: 10.1016/j.neurobiolaging.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Machwate M, Harada S, Leu CT, Seedor G, Labelle M, Gallant M, Hutchins S, Lachance N, Sawyer N, Slipetz D, Metters KM, Rodan SB, Young R, Rodan GA. Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2) Mol Pharmacol. 2001;60:36–41. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord JL, Hayes SG, Kaufman MP. PPADS does not block contraction-induced prostaglandin E2 synthesis in cat skeletal muscle. Am J Physiol Heart Circ Physiol. 2008;295:H2043–H2045. doi: 10.1152/ajpheart.00904.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. Sensitization of group IV muscle receptors to bradykinin by 5-hydroxytryptamine and prostaglandin E-2. Brain Res. 1981;225:95–105. doi: 10.1016/0006-8993(81)90320-6. [DOI] [PubMed] [Google Scholar]
- Middlekauff HR, Chiu J. Cyclooxygenase products sensitize muscle mechanoreceptors in healthy humans. Am J Physiol Heart Circ Physiol. 2004;287:H1944–H1949. doi: 10.1152/ajpheart.00329.2004. [DOI] [PubMed] [Google Scholar]
- O’Leary DS, Augustyniak RA, Ansorge EJ, Collins HL. Muscle metaboreflex improves O2 delivery to ischemic active skeletal muscle. Am J Physiol Heart Circ Physiol. 1999;276:H1399–H1403. doi: 10.1152/ajpheart.1999.276.4.H1399. [DOI] [PubMed] [Google Scholar]
- O’Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs. Am J Physiol Heart Circ Physiol. 1995;268:H980–H986. doi: 10.1152/ajpheart.1995.268.3.H980. [DOI] [PubMed] [Google Scholar]
- Prior BM, Lloyd PG, Ren J, Li H, Yang HT, Laughlin MH, Terjung RL. Time course of changes in collateral blood flow and isolated vessel size and gene expression after femoral artery occlusion in rats. Am J Physiol Heart Circ Physiol. 2004;287:H2434–H2447. doi: 10.1152/ajpheart.00398.2004. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effects of metabolic products of muscular contraction on the discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–2313. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Massey KD, Burton KP, Kaufman MP. Static contraction increases arachidonic acid levels in gastrocnemius muscles of cats. J Appl Physiol. 1989;66:2721–2724. doi: 10.1152/jappl.1989.66.6.2721. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Hill JM, Schultz HD, Kaufman MP. Cyclooxygenase blockade attenuates the responses of group IV muscle afferents to static contraction. Am J Physiol Heart Circ Physiol. 1990a;259:H745–H750. doi: 10.1152/ajpheart.1990.259.3.H745. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Schultz HD, Longhurst JC, Kaufman MP. Sensitization of group III muscle afferents to static contraction by products of arachidonic acid metabolism. J Appl Physiol. 1990b;68:861–867. doi: 10.1152/jappl.1990.68.3.861. [DOI] [PubMed] [Google Scholar]
- Schafers M, Marziniak M, Sorkin LS, Yaksh TL, Sommer C. Cyclooxygenase inhibition in nerve-injury- and TNF-induced hyperalgesia in the rat. Exp Neurol. 2004;185:160–168. doi: 10.1016/j.expneurol.2003.09.015. [DOI] [PubMed] [Google Scholar]
- Smith SA, Mitchell GS, Garry MG. Electrically induced static exercise elicits a pressor response in the decerebrate rat. J Physiol. 2001;537:961–970. doi: 10.1111/j.1469-7793.2001.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall MD, Vasko MR. Prostaglandin receptor subtypes, EP3C and EP4, mediate the prostaglandin E2-induced cAMP production and sensitization of sensory neurons. J Biol Chem. 2001;276:16083–16091. doi: 10.1074/jbc.M011408200. [DOI] [PubMed] [Google Scholar]
- St-Jacques B, Ma W. Role of prostaglandin E2 in the synthesis of the pro-inflammatory cytokine interleukin-6 in primary sensory neurons: an in vivo and in vitro study. J Neurochem. 2011;118:841–854. doi: 10.1111/j.1471-4159.2011.07230.x. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Brown B, Levin D, Longhurst JC. Reflex effect of skeletal muscle mechanoreceptor stimulation on the cardiovascular system. J Appl Physiol. 1988a;65:1539–1547. doi: 10.1152/jappl.1988.65.4.1539. [DOI] [PubMed] [Google Scholar]
- Stebbins CL, Maruoka Y, Longhurst JC. Prostaglandins contribute to cardiovascular reflexes evoked by static muscular contraction. Circ Res. 1988b;59:645–654. doi: 10.1161/01.res.59.6.645. [DOI] [PubMed] [Google Scholar]
- Symons JD, Theodossy SJ, Longhurst JC, Stebbins CL. Intramuscular accumulation of prostaglandins during static contraction of the cat triceps surae. J Appl Physiol. 1991;71:1837–1842. doi: 10.1152/jappl.1991.71.5.1837. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol. 2010;299:H106–H113. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Leal AK, Kaufman MP. Dorsal root tetrodotoxin-resistant sodium channels do not contribute to the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. Am J Physiol Heart Circ Physiol. 2011;300:H652–H663. doi: 10.1152/ajpheart.00859.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HT, Feng Y, Allen LA, Protter A, Terjung RL. Efficacy and specificity of bFGF increased collateral flow in experimental peripheral arterial insufficiency. Am J Physiol Heart Circ Physiol. 2000;278:H1966–H1973. doi: 10.1152/ajpheart.2000.278.6.H1966. [DOI] [PubMed] [Google Scholar]


