Abstract
Purpose
To compare the chromatic contrast sensitivity function (CSF) for the blue-yellow opponent channel (BYOC) between female badminton players and non-athlete controls.
Methods
We recruited 40 young females (18-25 years old) who played badminton for at least 5 consecutive years as the test group, and 30 age-matched female controls who had no history of regular physical activity. The Pattern Generator™ system was used to test the CSF for the BYOC which was performed at three spatial frequencies (SFs) of 2 cycles per degree (cpd), 5 cpd, and 25 cpd.
Results
Comparison of BYOC thresholds showed significantly better results in the test group for all three SFs (P<0.001). Band pass shift (better CSF in the middle SF) was seen in the test group. The control group had low pass (better CSF in the low SF). Ocular motility (heterophoria, fusional convergence and divergence at far and near distances, and near point of convergence) was better in the test group, but the inter-group difference was not significant.
Conclusions
The BYOC threshold results for badminton players indicated a better visual performance which may be a result of enhanced performance of the parallel processing of the parvocellular and magnocellular systems. This may be inherent and/or acquired in badminton players. In addition, badminton players appear to have developed sensory-motor programmed activities. Testing the CSF for BYOC may be useful for athlete selection in different levels and/or used as a criterion for screening players in the field of badminton.
Keywords: Sports, Vision, Contrast Sensitivity, Badminton, Athletes
INTRODUCTION
Attention to the importance of vision in sports is not a new phenomenon [1]. Ancient athletes in ball sports attempted to focus their eyes on the ball [1]. In competitions such as shooting, chariot racing, and ball games, sharp visual acuity was considered a key to success. In the second century (131-201 A.D.), Galen believed that a close relationship existed between playing ball games and visual and physical abilities [1]. Despite this initial perception about the impact of vision on success in sports, scientific research in this area had been forgotten for many years. From the mid-20th century, the notion that sports is not a single dimension phenomenon was rekindled. Scientists have demonstrated that exercising sports may promote sensory-motor systems, and depending on its type and nature, a given sport may specifically improve certain sensory-motor mechanisms more than others [2–5].
Badminton involves dynamic sensory-motor interactions [6, 7], and various sensory-motor systems directly influence an athlete's performance and achievements [6–9]. In this regard, the visual system is one of the most important sensory-motor coordinators which is closely related to the proprioceptive and vestibular systems [10]. For badminton athletes, it is essential to fix their eyes on a fast-moving small shuttlecock, and determine its spatial position despite a lack of guidelines and spatial cues. At the same time, the player must be aware of the position of the net, the court, the opponent (and the teammate when playing doubles). The player continuously and simultaneously sees and interprets the visual information in different positions in the badminton court. In this regard, spatiotemporal properties of the environment, spatiotemporal performance of the visual system and contrast sensitivity may have important roles [11, 12].
Contrast sensitivity function (CSF) is one of the most important measures of visual system performance [13] which is determined according to a person's ability to discriminate luminance14, (luminance or achromatic contrast sensitivity) or color differences [15] (chromatic contrast sensitivity), resulting from the interaction of black–white, blue–yellow, and red–green opponent channels. The blue-yellow opponent channel (BYOC) is one of the most important mechanisms because under photopic conditions, yellow has the maximum luminous efficiency function or spectral sensitivity [16, 17], while blue shows the maximum dispersion in any optical system [16]. Therefore, testing the CSF for BYOC may be very important for evaluating the optical system and visual performance in different neural and optical processing situations, where a lower threshold shows better analysis and processing of visual information [13–16].
Considering contrast sensitivity an essential element of sports vision, scientists have tried to enhance the visual performance of athletes by improving CSF because they believe athlete performance and good CSF are strictly related to each other [18, 19]. Should the ocular pursuit of a fast moving object in badminton or shooting be related to good CSF [19–21], successful badminton players should have a better developed visual system and CSF. Testing the CSF for BYOC may be a good method for comparing visual system performance, which we used in this study to compare results between elite badminton players and non-athletes.
METHODS AND SUBJECTS
In this study, we enrolled 70 people between the ages of 18 and 25 years; 40 lady badminton players for the test group, and 30 women who did not perform any sports activities as non-athletes for the control group. The test group included sportswomen who had played for at least five consecutive years, five hours per week, and were members of the national badminton team and/or a high-ranking sports club.
Both groups had visual and ocular examinations. Inclusion criteria were good visual acuity (better than 20/25), low refractive error (hypermetropia < +0.75 D; myopia and oblique or against the rule astigmatism < -0.25 D; with the rule astigmatism < -0.50 D). None of the participants used optical correction. Exclusion criteria were any history of significant systemic or ocular diseases.
For all participants, we tested distant visual acuity (6 meters; TOPCON ACP-8 chart projector) and near visual acuity (40 cm; reduced Snellen chart). Refraction was tested using static retinoscopy (Heine Beta 200 retinoscope) and auto-refraction (TOPCON Auto Kerato-refractometer 8800) tests. Subjects also had the cover test and fusional measurements at far (6 meters) and near (40 cm); compensated heterophoria (latent ocular deviation) according to Sheard's and Percival's criteria (measurement of sensory-motor ability of the visual system to overcome motor disturbances with fusional vergences); slit lamp biomicroscopy (examination of the anterior segment of the eye) and direct ophthalmoscopy (examination of the posterior segment of the eye).
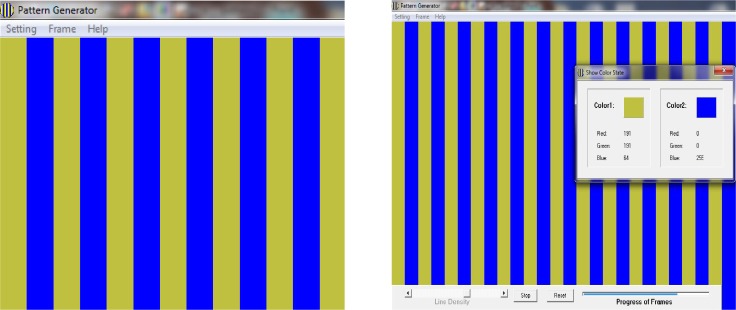
After confirmation of normal results for badminton players (test group) and non-athletes (control group), all participants were coded and referred to a blinded examiner who measured thresholds for the detection of increments using the Pattern Generator™ (Figure 1). The participants were seated three meters away from a 15” flat monitor. Grating patterns (parallel lines) with different spatial frequencies (width) and contrast were presented randomly to the subjects who informed the examiner whether they could detect gratings or not. The procedures were repeated three times for each threshold measurement, and the average of three measurements was recorded.
Fig. 1.
Pattern Generator™ test screen (left). Other spatial frequency in Pattern Generator™ screen that shows color coordination for each color and contrast level (right).
After confirmation of normal results for badminton players (test group) and non-athletes (control group), all participants were coded and referred to a blinded examiner who measured thresholds for the detection of increments using the Pattern Generator™ (Figure 1). The participants were seated three meters away from a 15” flat monitor. Grating patterns (parallel lines) with different spatial frequencies (width) and contrast were presented randomly to the subjects who informed the examiner whether they could detect gratings or not. The procedures were repeated three times for each threshold measurement, and the average of three measurements was recorded.
Contrast sensitivity (contrast sensitivity = 1/contrast threshold) was measured in three spatial frequencies (SFs) of low [2 cycle per degree (cpd)], intermediate (5 cpd), and high (25 cpd)]. These SFs were chosen according to the normal contrast sensitivity curve measured with grating stimuli. We recorded thresholds at each SF for both study groups. Statistical analysis was performed with the Stat graphics™ software. Results are described as mean, minimum, maximum, and standard deviations and we used the independent t-test to compare SFs in the two groups.
RESULTS
No statistically significant differences were noted between badminton players and non-athletes with respect to visual acuity, refractive error, or heterophoria (far and near). Measurements of fusional vergences at far and near for convergence and divergence showed better results for athletes. However, fusional vergence compensation according to Sheard's and Percival's criteria was necessary for all participants.
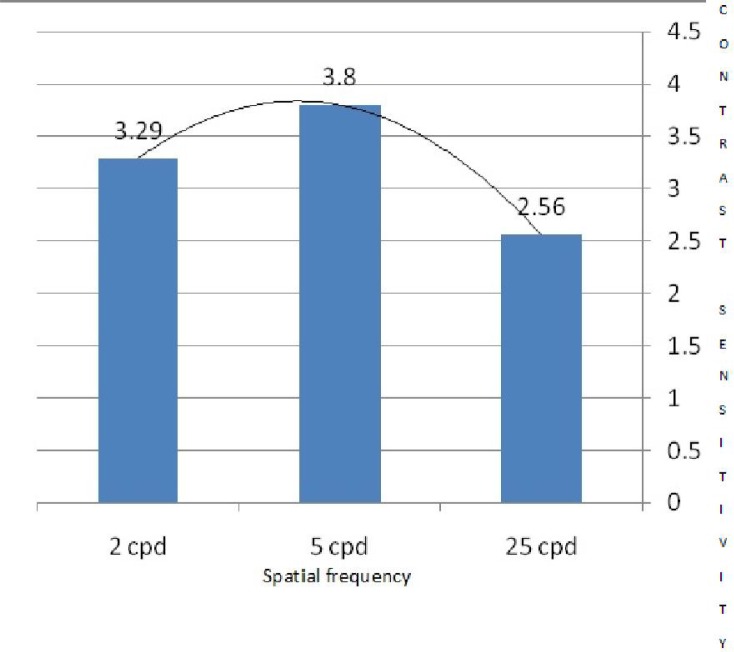
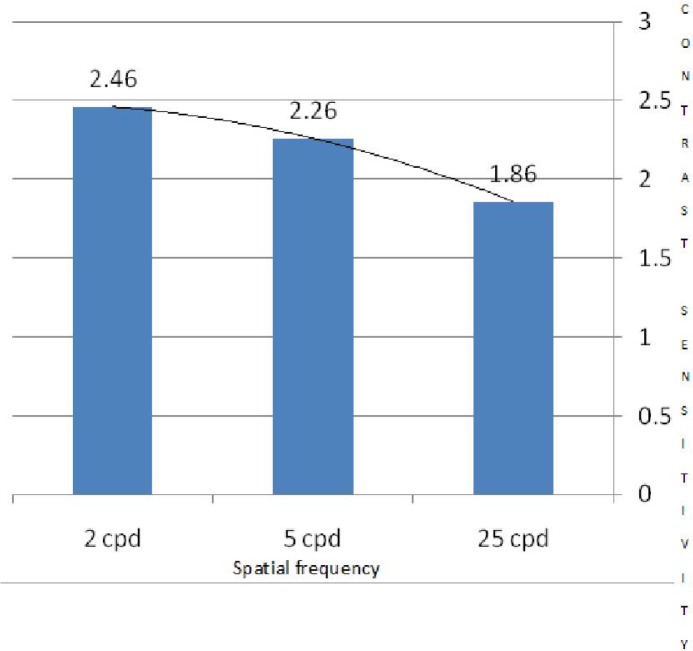
The mean contrast threshold in the test group at 2cpd, 5cpd, and 25cpd SFs was 30.4, 26.3, and 39.0, respectively; better results at 5cpd is indicative of band pass (better contrast sensitivity for middle SFs). In the control group, a better contrast threshold (40.7) was observed at 2 cpd or low pass (better contrast sensitivity for low SF). According to the independent t-test, the test group had significantly better mean contrast threshold at all SFs compared to the controlgroup (P<0.001) (Table 1). Results of CSF testing for BYOC in the two groups are demonstrated in Figures 2 and 3.
Table 1.
Descriptive results regarding contrast thresholds for the blue-yellow opponent channel in both groups
| Spatial frequency | Contrast threshold percent for athletes and (control) groups | t-test for inter-group difference | |||
|---|---|---|---|---|---|
| Mean | Maximum | Minimum | Standard deviation | ||
| 2 cpd* | 30.4 (40.7) | 50.9 (52.1) | 9 (14.1) | 10.2 (8.7) | p < 0.001 |
| 5 cpd | 26.3 (44.3) | 67.8 (67.8) | 7.1 (7.8) | 13.4 (16.2) | p < 0.001 |
| 25 cpd | 39 (53.8) | 74.1 (98.8) | 9 (32.1) | 17.3 (18.2) | p < 0.001 |
cpd: cycle per degree
Fig. 2.
Contrast sensitivity for blue-yellow opponent channel in badminton athletes (test group)
Fig. 3.
Contrast sensitivity for blue-yellow opponent channel in non-athletes (control group)
DISCUSSION
The findings of the present study indicated low pass CSF in the non-athlete control group (Fig. 3). In this regard, other studies report the same findings in normal subjects [22]. In normal non-athletes, BYOC contrast sensitivity is mediated by the koniocellular pathway that tends to exhibit a low pass curve [23] while band pass (better contrast sensitivity for middle SFs) is seen when testing with monochromatic gratings. The magnocellular pathway, mediates luminance and achromatic stimuli [24, 25].
In this study, the BYOC contrast sensitivity curves differed between the two groups (Fig. 2,3). Badminton players showed better contrast sensitivity for middle SFs, whereas non-athletes had better contrast sensitivity for low SFs (low pass). This indicates that the CSF for BYOC in athletes was similar to a monochromatic contrast sensitivity curve (black and white or different density of gray scales) in normal subjects[22]. This shift (from low to middle frequencies) may indicate dynamic interactions of specific visual pathways in badminton players [22]. However, the band pass (better contrast sensitivity for middle SFs) response and lower threshold in CSF for YBOC among badminton players may imply better visual capabilities and performance.
Our results of YBOC testing may indicate an interactive function of different visual neural subsystems, including the parvo-, magno- and koniocellular systems [26]. In fact, we live in a colorful world, thus color vision and contrast sensitivity are important issues. According to the visual information parallel processing theory [27], these subsystems may selectively show maximum response to specific stimuli. The parvocellular system may be very important in color discrimination, spatial resolution, depth perception, stereopsis and object detection [26], whereas the magnocellular system has shown a significant role in motion perception, luminance and contrast perception [28]. However, the koniocellular system may be activated in simultaneous presentation of blue and yellow colors [23]. Our results imply that visual information may be processed differently according to previous perceptual, sensory and motor experiences. This concept is confirmed by other studies [27, 29]. On the other hand, in the badminton athlete visual system, some specific interactions between these three subsystems may occur that is completely different from non-athletes. Obviously, these changes may improve athletic performance.
The visuomotor localization system may be differently modulated with luminance and color stimuli [30]. With any given visual environment and experience, specific improvement and capabilities may develop in the visual system [31]. The plasticity theory may explain visual development under specific visuomotor and perceptual situations [31]. Athletes provide a possible model to explore the plasticity of the visual cortex as athletic training in confrontational ball games is quite often accompanied by training of the visual system. Badminton is a dynamic sport in which the player focuses on different objects at the same time. The player's visual system needs to collect information from the shuttlecock, playing court, net position, match referee, and opposing player as quickly as possible. These environmental visual pieces of information, which are instantly transmitted through the parvo- and magnocellular systems to the visual cortex, determine the sensory-motor behavior of players [32]. Better performance of the parvo- and magnocellular systems may thus improve the badminton player's performance. As expected, our badminton players showed significantly better CSF for YBOC which is an index of parvo- and magnocellular performance.
Perceptual organization and programming in visual tasks may improve eye movement controls in visual system performance [33]. Some researchers believe that racket sport athletes may have better visual performance [34] which is necessary for achievements in their sport. However, visual skills are a multidisciplinary issue. Sensory and motor skill improvements should symmetrically develop in racket sport athletes [35]. Therefore, badminton athletes should be better in sensory (CSF for YBOC) and motor performance [31].
CONCLUSION
Better performance in sensory systems, such as the parallel parvo and magnocellular processing systems, may be inherent and/or acquired in badminton players. Undoubtedly, badminton and other racket sports may help develop neural programmed activities and sensory-motor reactions. This may be very important in clinical practice (improving visual performance with sports training) and useful for trainers in racket sports to improve sport performance with specific visual trainings. Further researches may be needed for specific modeling of visual performance and skills in athletes. Hence, modeling specific visual performance patterns can be suggested for every sport. Color CSF may be very important in this regard. Selective receptive field evaluation and interactive visual subsystem responses may be the most important advantages of color contrast sensitivity tests. Similarly, testing the CSF for YBOC may be used for athlete selection in different levels as a protocol and/or used as a criterion for screening superior talents in the field of badminton.
Our findings imply visual performance and contrast may be different in athletes and non-athlete groups. However, we suggest further studies in regard to visual skills, saccadic eye movement, facility of accommodation and sensory performance of visual system that may be evaluated by contrast sensitivity threshold. Other studies may be suggested in different lighting conditions so that photopic, mesopic and scotopic sensory visual responses may be compared. Specific lighting condition was the most important limitation of our study.
ACKNOWLEDGMENTS
The authors would like to thank Iranian Badminton Federation and all the badminton players for effective and valuable cooperation.
Conflict of interests: None
REFERENCES
- 1.Gregg J.R. Vision and sports. Boston: Butterworth's; 1987. pp. 1–7. [Google Scholar]
- 2.Chow JW, Carlton LG, Chae WS, et al. Movement characteristics of the tennis volley. Med Sci Sports Exerc. 1999;31:855–63. doi: 10.1097/00005768-199906000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Pita R, Aretouli E, Loukopoulou E, et al. Can ‘football-team color-code’ compensate for anomia? The case study of FN, a patient with color anomia. Neurocase. 2005;11:227–33. doi: 10.1080/13554790590944870. [DOI] [PubMed] [Google Scholar]
- 4.Zhang J, Watanabe K. Differences in saccadic latency and express saccades between skilled and novice ball players in tracking predictable and unpredictable targets at two visual angles. Percept Mot Skills. 2005;100:1127–36. doi: 10.2466/pms.100.3c.1127-1136. [DOI] [PubMed] [Google Scholar]
- 5.Martell SG, Vickers JN. Gaze characteristics of elite and near-elite athletes in ice hockey defensive tactics. Hum Mov Sci. 2004;22:689–712. doi: 10.1016/j.humov.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Edwards BJ, Lindsay K, Waterhouse J. Effect of time of day on the accuracy and consistency of the badminton serve. Ergonomics. 2005;48:1488–98. doi: 10.1080/00140130500100975. [DOI] [PubMed] [Google Scholar]
- 7.Toriola AL, Toriola OM, Dhaliwal HS, Igbokwe NU. Relationship between physical education students’ achievements in a French badminton service test and expert ratings of technique quality. Percept Mot Skills. 2004;98:406–8. doi: 10.2466/pms.98.2.406-408. [DOI] [PubMed] [Google Scholar]
- 8.Bebetsos E, Antoniou P. Psychological skills of Greek badminton athletes. Percept Mot Skills. 2003;97:1289–96. doi: 10.2466/pms.2003.97.3f.1289. [DOI] [PubMed] [Google Scholar]
- 9.Cabello Manrique D, Gonzalez-Badillo JJ. Analysis of the characteristics of competitive badminton. Br J Sports Med. 2003;37:62–6. doi: 10.1136/bjsm.37.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Munuera J, Morel P, Duhamel JR, Deneve S. Optimal sensorimotor control in eye movement sequences. J Neurosci. 2009;29:3026–35. doi: 10.1523/JNEUROSCI.1169-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anderson RS, Zlatkova MB, Beirne RO. The contrast sensitivity functions for detection and resolution of blue-on-yellow gratings in foveal and peripheral vision. Ophthalmic Physiol Opt. 2002;22:420–6. doi: 10.1046/j.1475-1313.2002.00068.x. [DOI] [PubMed] [Google Scholar]
- 12.McKeefry DJ, Murray IJ, Kulikowski JJ. Red-green and blue-yellow mechanisms are matched in sensitivity for temporal and spatial modulation. Vision Res. 2001;41(2):245–55. doi: 10.1016/s0042-6989(00)00247-9. [DOI] [PubMed] [Google Scholar]
- 13.Block SS, Beckerman SA, Berman PE. Vision profile of the athletes of the 1995 Special Olympics World Summer Games. J Am Optom Assoc. 1997;68:699–708. [PubMed] [Google Scholar]
- 14.Mullen KT, Sankeralli MJ, Hess RF. Color and luminance vision in human amblyopia: shifts in isoluminance, contrast sensitivity losses, and positional deficits. Vision Res. 1996;36:645–53. doi: 10.1016/0042-6989(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 15.Williams D, Sekiguchi N, Brainard D. Color, contrast sensitivity, and the cone mosaic. Proc Natl Acad Sci USA. 1993;90:9770–7. doi: 10.1073/pnas.90.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rabin J. Quantification of color vision with cone contrast sensitivity. Vis Neurosci. 2004;21:483–5. doi: 10.1017/s0952523804213128. [DOI] [PubMed] [Google Scholar]
- 17.Sharpe LT, Stockman A, Jagla W, Jagle H. A luminous efficiency function, V*(lambda), for daylight adaptation. J Vis. 2005;5:948–68. doi: 10.1167/5.11.3. [DOI] [PubMed] [Google Scholar]
- 18.Erickson GB, Horn FC, Barney T, et al. Visual performance with sport-tinted contact lenses in natural sunlight. Optom Vis Sci. 2009;86:509–16. doi: 10.1097/OPX.0b013e31819f9aa2. [DOI] [PubMed] [Google Scholar]
- 19.Laby DM, Kirschen DG, Pantall P. Eye Contact Lens. 2011. Mar 3, The Visual Function of Olympic-Level Athletes-An Initial Report. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman AB, Lust KL, Bullimore MA. Eye Contact Lens. 2011. Mar 3, Visual Acuity and Contrast Sensitivity Testing for Sports Vision. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Porisch E. Football players’ contrast sensitivity comparison when wearing amber sport-tinted or clear contact lenses. Optometry. 2007;78:232–5. doi: 10.1016/j.optm.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Mullen KT. The contrast sensitivity of human color vision to red-green and blue-yellow chromatic gratings. J Physiol. 1985;359:381–400. doi: 10.1113/jphysiol.1985.sp015591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sartucci F, Orlandi G, Lucetti C, et al. Changes in pattern electroretinograms to equiluminant red-green and blue-yellow gratings in patients with early Parkinson's disease. J Clin Neurophysiol. 2003;20:375–81. doi: 10.1097/00004691-200309000-00010. [DOI] [PubMed] [Google Scholar]
- 24.Rovamo JM, Kankaanpaa MI, Kukkonen H. Modelling spatial contrast sensitivity functions for chromatic and luminance-modulated gratings. Vision Res. 1999;39:2387–98. doi: 10.1016/s0042-6989(98)00273-9. [DOI] [PubMed] [Google Scholar]
- 25.Creutzfeldt O, Lee BB, Valberg A. Color and brightness signals of parvocellular lateral geniculate neurons. Exp Brain Res. 1986;63:21–34. doi: 10.1007/BF00235643. [DOI] [PubMed] [Google Scholar]
- 26.Hubel DH, Livingstone MS. Color and contrast sensitivity in the lateral geniculate body and primary visual cortex of the macaque monkey. J Neurosci. 1990;10:2223–37. doi: 10.1523/JNEUROSCI.10-07-02223.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis A, Anderson SJ. Color and luminance interactions in the visual perception of motion. Proc Biol Sci. 2002;269:1011–6. doi: 10.1098/rspb.2002.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shevell SK, Monnier P. Color shifts from S-cone patterned backgrounds: contrast sensitivity and spatial frequency selectivity. Vision Res. 2005;45:1147–54. doi: 10.1016/j.visres.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 29.Suero MI, Naranjo FL, Pardo PJ, Perez AL. Experimental study of the individual differences in chromatic perception through blue-yellow metameric matches of a white-light continuum. Ophthalmic Physiol Opt. 2010;30:646–52. doi: 10.1111/j.1475-1313.2010.00756.x. [DOI] [PubMed] [Google Scholar]
- 30.Ashida H, Yamagishi N, Anderson SJ. The relative contributions of color and luminance signals towards the visuomotor localization of targets in human peripheral vision. Exp Brain Res. 2007;183:425–34. doi: 10.1007/s00221-007-1059-0. [DOI] [PubMed] [Google Scholar]
- 31.Jin H, Xu G, Zhang JX, et al. Athletic training in badminton players modulates the early C1 component of visual evoked potentials: a preliminary investigation. Int J Psychophysiol. 2010;78:308–14. doi: 10.1016/j.ijpsycho.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Narasimhan S, Tripathy SP, Barrett BT. Loss of positional information when tracking multiple moving dots: the role of visual memory. Vision Res. 2009;49:10–27. doi: 10.1016/j.visres.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 33.Vergilino-Perez D, Findlay JM. Between-object and within-object saccade programming in a visual search task. Vision Res. 2006;46:2204–16. doi: 10.1016/j.visres.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 34.Babu RJ, Lillakas L, Irving EL. Dynamics of saccadic adaptation: differences between athletes and nonathletes. Optom Vis Sci. 2005;82:1060–5. doi: 10.1097/01.opx.0000192346.84549.6a. [DOI] [PubMed] [Google Scholar]
- 35.Di Russo F, Pitzalis S, Spinelli D. Fixation stability and saccadic latency in elite shooters. Vision Res. 2003;43:1837–45. doi: 10.1016/s0042-6989(03)00299-2. [DOI] [PubMed] [Google Scholar]