Abstract
Purpose
The aim of this study was to assess the effects of a six-month pedometer-based workplace intervention on changes in resting blood pressure (BP) and cardiorespiratory fitness (CRF).
Methods
A subsample of ASUKI Step participants (n= 355) were randomly selected to have changes in their BP and CRF monitored during the intervention. Pedometers were used to monitor steps taken with a goal of walking more than 10,000 steps/day. Systolic and diastolic BP were taken using an Omron automated BP cuff. Estimated VO2 max was obtained using the Åstrand-Rhyming cycle ergometer test. A multi-level growth modeling approach, and a mixed model ANOVA were used to predict changes in systolic and diastolic BP, and estimated VO2 max over time by steps, age, gender, and university site.
Results
Steps/day averaged 12,256 (SD = 3,180) during month 1 and steadily decreased to month 6. There were significant linear and quadratic trends in systolic and diastolic BP over time. Age was positively related to initial starting values for systolic and diastolic BP, and approached significance for systolic BP changes over time. Steps/day approached significance for linear changes in systolic BP. There was a significant difference between ASU and KI participants’ estimated VO2 max. There was a significant change over time in the estimated VO2 max. The number of steps taken was significantly related to changes in estimated VO2 max over time.
Conclusions
The results of the present study indicate that healthy individuals who took part in a pedometer intervention improved several cardiovascular disease risk factors.
Keywords: Workplace, Physical Activity, Pedometer Intervention, Cardiorespiratory Fitness, Blood Pressure, VO2 Max
INTRODUCTION
The functional consequences of physical training can be reflected by cardiorespiratory fitness (CRF) as an objective measure [1]. Recent studies showed that the link between poor CRF and cardiovascular mortality is mediated by the development of several cardiovascular disease risk factors such as adiposity, dyslipidemia, hypertension, and glucose intolerance [1–3]. Regular physical activity has been advised as an important lifestyle modification to prevent hypertension [4]. People of all age groups can improve their health and quality of life through regular lifelong moderate- and vigorous-intensity physical activity (PA) designed to increase CRF [5, 6].
Walking as a moderate-intensity form of aerobic PA is considered an important cornerstone in many PA promotion programs. As walking is popular and accessible to all with a low risk of injury, there is no need for expensive equipment, facilities or clothing to walk [7]. Walking can be done at a variety of intensities and speeds, individually or in a group. Brisk walking is associated with higher levels of CRF. In an intervention study designed to measure the amount of brisk walking needed to increase CRF, Anton et al. found that a minimum of 75 min of brisk walking per week was associated with significant improvements in CRF. While no differences were observed between walking durations of 75 and 150 minutes/week in improvements of CRF, those who walked longer durations showed larger improvements in CRF [8]. The assessment of PA in general, and walking in particular, using objective methods such as a pedometer has helped to clarify and more accurately determine the relationship between CRF and PA [9]. The relationships are strongest between PA and CRF when objective measures of PA are used and CRF is measured using maximal testing methods such as the multistage fitness test [9, 10].
Public health researchers suggest that walking at least 10,000 steps a day has beneficial health effects. Walking 10,000 steps per day expends about 2,000 kcal energy per week, and thus this amount of daily PA is suitable to improve health and prevent chronic diseases such as obesity and diabetes mellitus [11]. Using pedometers to track walking, Awain et al. showed that walking at least 10,000 steps per day resulted in reductions in elevated BP and an improved exercise capacity in hypertensive adults. Similar reductions in resting BP were not observed in normotensive subjects [12]. Other studies support walking at least 10,000 steps a day to promote health and prevent cardiovascular disease [7, 11, 12]. A systematic review including 27 randomized controlled trials provided evidence of the beneficial effects of walking interventions on lowering blood pressure (BP), and this systematic review explored that walking programs of moderate-high intensity may be more useful than walking at low-intensity on lowering BP [13]. Effects of walking interventions on BP measures may be moderated by the age of study participants. It is well documented that in adults, CRF declines and BP increases with age and they are influenced by lifestyle behaviors [14, 15].
Despite the documented role of walking as a method of reducing cardiovascular risk and the effect of walking on the sedentary population at risk for cardiovascular disease [16], routine prescription and/or promotion of PA programs are lacking empirical support [17]. The conflicting evidence in the effectiveness of walking interventions may be attributable to low compliance, underpowered studies, short follow-up, and small sample sizes [17].
The purpose of the current paper was to examine the effects of a six-month pedometer-based PA on resting BP and estimated VO2 max as determined by an ergometer submaximal exercise test. We hypothesized that a six-month pedometer-based PA would be positively associated with decrease in resting BP and improvement in CRF.
METHODS AND SUBJECTS
Overview of the study
The ASUKI Step study was an innovative six month pedometer-determined workplace intervention held on the campuses of Arizona State University (ASU) and the Karolinska Institutet (KI) with an overall goal to increase PA by walking 10,000 steps per day [18]. Participants were recruited by several methods, including newsletters, posters, mass email and electronic advertisements, fliers, and kick off seminars. Surveys were completed to assess demographic data and to identify correlates or predictors of walking behaviors. The intervention lasted six months with surveys completed at one week prior to initiation of the intervention, and after three, and six months using a web-based survey on the study website. A sub-sample of participants consisting of one participant randomly selected from each walking team, was created to examine changes in body composition, CRF, resting BP, and accelerometer-determined intensity and duration of movement over seven days. The ASUKI Step methods [18] and anthropometric changes in the Swedish material have been described by authors in more detail elsewhere[19].
Setting and sample
This study had a quasi-experimental design (pre-mid-posttest, no control group) with 2,118 university faculty, staff, and graduate student participants from the ASU (n = 712), and KI (n = 1406), aged 20 to 65 years. A sub-sample, included 355 ASUKI Step participants from the two universities (ASU = 141 and KI = 214), was randomly selected for fitness testing. Participants were notified by e-mail and invited to a testing laboratory on the ASU or KI campuses where they were working.
The ASUKI Step study and the sub-sample fitness testing protocols were approved by the Research Ethics Committees of the ASU and KI. All participants gave written informed consent before inclusion in the study, and were informed that they could leave the study any time.
ASUKI-Step Intervention and Fitness Sub-study
Upon enrolling in the study, participants received a pedometer and a study instruction booklet for the six-month pedometer program. Participants were instructed on appropriate use of the pedometer and asked to register the daily number of steps on a website developed for this study at the end of each day with a goal of walking more than 10,000 steps per day. Participants enrolled in teams of three to four persons per team with one person from each team followed more closely through physical measurements at the beginning (month one), midpoint (month three), and end of the study period (month six). The intervention period started in mid-March and ended in mid-September 2009. During this period, the seasons changed from winter to spring to summer.
ASUKI Step Fitness Sub-study Data Collection Instruments
The following measures were collected throughout the study period.
Physical Activity
PA categories were assessed with the self-administered, short version of the International Physical Activity Questionnaire (IPAQ) [20].
Pedometer-Determined Steps per day
The Yamax SW-200 pedometer was used to monitor steps taken. This pedometer is a valid and reliable tool for counting steps in healthy adults [11, 21]. The Yamax SW-200 pedometer is valid within ± 3% of actual steps taken during a self-paced walk on an individual and within 1% of actual steps for a group mean [22, 23]. Participants were asked to wear the pedometer every day on their waist band over the right hip and to register their daily number of steps on the ASUKI Step website throughout the study period. For other activities, such as bicycling or fitness classes, the participants were instructed to add 2700 “steps” per half hour of activity. Based on the step categories suggested by Tudor-Locke et al [24], participants were divided into four PA categories: sedentary (< 5,000 steps per day), low active/inactive (5,000-7,499 steps per day), somewhat active (7,500-9,999 steps per day), active (10,000-12,499 steps per day), and highly active (> 12,500 steps per day).
Anthropometric measures
Anthropometric measures were assessed at one, three, and six months and included height, weight, and Body Mass Index (BMI). All measurements were done in duplicate or triplicate if the first two measurements differed. Height (cm) was measured with bare feet using a Seca (MedexSupply, Road Rod, Monsey, NY) portable stadiometer. Body weight (kg) was determined by bioelectrical impedance analysis with the Tanita TBF-300A scale (TANITA Corporation: Arlington Heights, IL) [25]. BMI was computed as weight in kilograms divided by height in meters squared. Body mass was used to compute the estimated VO2 max in ml/kg/min.
Resting Blood Pressure
Systolic and diastolic BP were taken in the left arm using an Omron automated BP cuff (HEM-711 DLX and M6 Comfort) [26–28]. BP was taken after participants were seated for five minutes with an appropriately sized cuff. Two measures were made with a minimum of one minute between measurement trials. A third measure was taken if BP values were greater than 4 mmHg in difference. The main advantages of the OMRON automated device are accuracy comparable to manual mercury sphygmomanometery, with reduced potential for observer biases and less demand on research assistants in terms of training and effort in data collection [29] The OMRON device was also accepted according to the European Society of Hypertension International Validation protocol [30]. No other fitness assessment was performed until participants had their resting BP measured and deemed to be within the normal range. To define categories of BP and recommended follow-up the seventh report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure was used [31].
Assessment of CRF
Estimated maximal oxygen uptake (VO2 max) was obtained using the Åstrand-Rhyming cycle ergometer test at one and six months to examine CRF [32–34]. The test was conducted on a Monark bike ergometer, model 839E (Monark Exercise, Vansbro, Sweden), which was calibrated before each test session. The participants were asked to abstain from smoking and eating 2 hours before testing. Prior to testing participants were fitted with a heart rate monitor (Polar Electro Inc, Lake Success, NY) that linked the heart rate response to the ergometer using a telemetry system.
The participants also were given instructions for the cycle test, as well as how to report their perceived exertion using Borg's Rating of Perceived Exertion (RPE) scale [35]. The RPE uses a 6 – 20 point scale that describes exertion from very, very light to very, very hard. The cycle ergometer resistance was selected so participants would reach a steady-state heart rate of 120-150 beats per minutes (equivalent to 50-85% of their heart rate reserve computed as 220-age).
The pedal rate was set at 50-60 revolutions per minute using a metronome set at 100 bpm and work rate at 50-75 Watts for untrained participants or up to 100 Watts for moderately trained participants. The test was initiated at the established work rate and continued for 6 minutes to increase the heart rate to a target range of 125 beats per minute to 85% of age-predicted heart rate max. If the heart rate was lower or higher than the target range, the workload was adjusted to bring the heart rate into the desired range and an additional 6 minutes of cycling was performed.
The test was terminated when the difference in the heart rate between the 5th and 6th minutes of exercise was 5 beats or less. If the difference in the heart rate values was greater than 5 beats, the test was continued until the heart rate between successive minutes was less than 5 beats or a maximum of 12 minutes of cycling was completed. VO2 max (l.min−1) was estimated using the Åstrand-Rhyming nomogram from the steady state heart rate and the work rate [36]. VO2 max adjusted for body mass (ml.kg−1. min−1) was computed as (VO2 max in l min−1 x 1000)/kg body mass. Maximal oxygen consumption (VO2 max) is a generally accepted accurate index of CRF [37], also referred to as functional aerobic capacity. VO2 max is defined as the maximum capacity of person's body ability to transport and use oxygen to perform strenuous physical exercise [34]. VO2 max can be expressed as an absolute rate (l/min), or as a rate relative to one's body mass (ml/kg/min). The relative rate is often used for comparison of performance of endurance sports athletes.
Statistical analyses
Descriptive statistics including frequency, means, and standard deviations (SD) were used to describe participant characteristics. A multi-level growth modeling approach was used to show changes over time and predict changes over time by steps, age, gender, and site for resting systolic and diastolic BP. To better understand significant continuous predictors, conditional regression lines and contrasts for the intercepts and slopes were constructed using Cohen & Cohen's (1983) suggested values of one standard deviation above and one standard deviation below the mean for a continuous predictor[38]. To predict changes over time for estimated VO2 max a mixed model ANOVA was used. Analyses were performed using all available data, including participants who subsequently dropped out. Statistical intent-to-treat analyses using all available data were performed using SAS software version 9.2. The level of significance was set at P< 0.05 for all tests.
RESULTS
Descriptive statistics (means and standard deviations) at the beginning of the study are reported in Table 1. Most of the participants were female. The mean number of steps in month one exceeded the goal of 10,000 steps per day with KI participants recording more steps per day than ASU participants. A total of 251 ASU and KI faculty, staff, and PhD students out of the 355 randomly selected subgroup (ASU = 141, and KI = 214) completed the 6 month measurements with a total retention rate of 71.5%.
Table 1.
Descriptive statistics (mean and standard deviations) in the test group participants at month 1
| Variable | ASU (n = 141) | KI (n = 214) | All (N = 355) |
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| Age | 41.05 (11.39)* | 44.25 (12.30) | 42.98 (12.04) |
| Gender (% female) | 78.3% | 81.8% | 80.3% |
| Body Mass Index (kg.m−2 ) | 27.22 (7.14)* | 24.12 (3.62) | 25.35 (5.50) |
| Systolic Blood Pressure (mmHg) | 113.72 (13.41)* | 121.05 (16.99) | 118.14 (16.05) |
| Diastolic Blood Pressure (mmHg) | 77.56 (9.27)* | 79.79 (10.53) | 78.91 (10.10) |
| VO 2 max(ml . kg −1. min −1 ) | 34.16 (11.80)* | 37.82 (10.24) | 36.57 (10.56) |
| Mean steps/day | 10967 (3021)* | 13105 (2998) | 11256 (3180) |
Demonstrated a significant difference with P<0.05 between ASU and KI participants; SD: Standard Devition
Changes in Blood Pressure
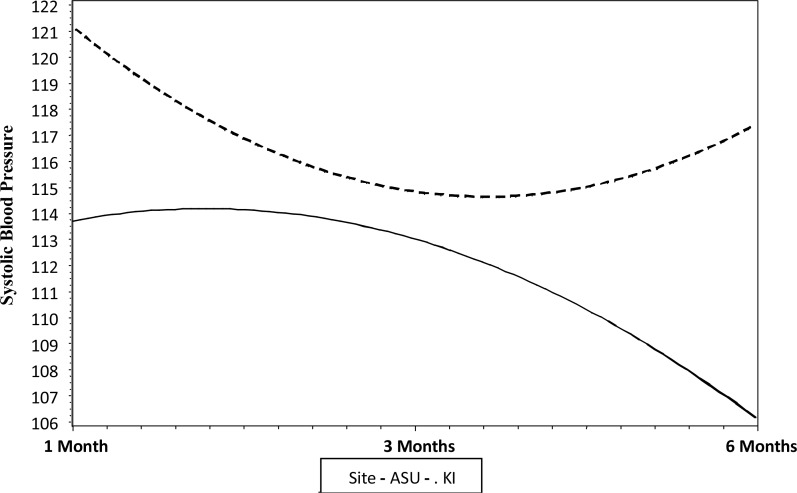
There was a significant change over time in systolic BP for the unconditional model, a model without predictors. On average, the starting systolic BP for participants was 118.14 (± 16.05) mmHg. Over time there was a significant linear decrease in systolic BP, with an average decrease of 5.57 mmHg every three months (t=−5.69, P=0.001). There also was a significant quadratic effect where the decrease in systolic BP slowed by 1.3 mmHg every three months (t=2.76, P=0.006). The random effects indicate that there were significant individual differences in the starting systolic BP (z=11.00, P=0.0001) and changes over time (z = −2.47, P=0.01). Variation in intercepts and slopes was related to the research site. For the KI site, the initial starting value of the systolic BP was significantly higher than ASU values (KI vs. ASU, P= 0.0001). The change over time for KI was U-shaped, concave upward, at first decreasing then increasing at the mid-study. ASU, in contrast, had a lower starting value of the systolic BP and the change over time was inverted U-shaped, concave downward, with values at first increasing and then decreasing at the mid-study (Fig. 1).
Fig. 1.
Changes over time in systolic BP for ASU and KI participants
Gender was related to individual differences in the initial starting value of systolic BP but was not related to changes over time. The gender difference in initial values did not differ by the research site. Age was significantly related to both the initial starting value (t =7.40, P=0.0001) and borderline significant for systolic BP changes over time (t=−1.82, P=0.07). Older age (one standard deviation above the mean age) was associated with a higher initial value for systolic BP. Also, older age saw a steeper linear slope with time (−8.08), whereas younger age (one standard deviation below the mean age) had a linear slope of −2.97. There was no significant quadratic slope for participants one standard deviation below the average age; whereas the average aged for participants one standard deviation above the mean age had a significant positive quadratic slope. These differences by age did not vary by research site. The number of steps was significantly related to initial starting values of systolic BP (t=1.99, P=0.05), and borderline significantly related to linear change over time (t=−1.86, P=0.06). The more steps one took the greater the linear decline in systolic BP. The relationship between steps and systolic BP did not vary by research site.
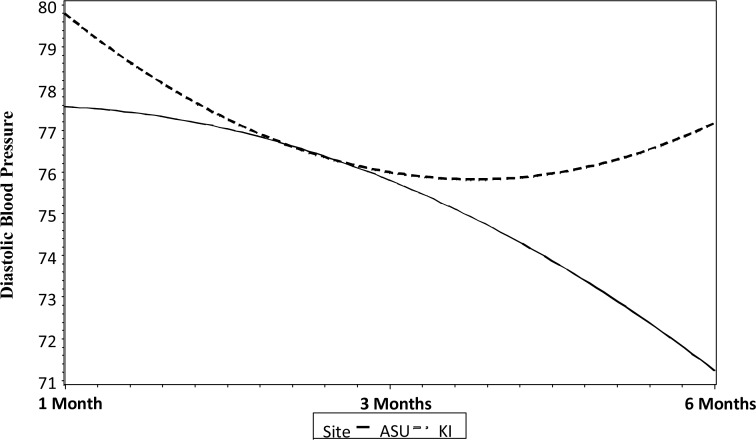
The results for the unconditional model for diastolic BP were similar to the results for systolic BP. There was a significant change over time in diastolic BP. On average the starting diastolic BP for participants was 78.91 (±10.10) mmHg. Over time there was a significant linear decrease in diastolic BP, with an average decrease of 4.03 mmHg every three months (t= −5.53, P=0.001). There also was a significant quadratic effect where the decrease in diastolic BP slowed by 1.04 mmHg every three months (t = 2.92, P=0.006). The random effects indicate that there were significant individual differences in the starting diastolic BP (z= 9.93, P=0.0001). Variation by research site was similar to the results for systolic BP. For KI the initial starting diastolic BP value was significantly higher than ASU (P=0.04). The change over time for KI was U-shaped, at first decreasing then increasing at the mid-study. ASU, in contrast, had a lower staring diastolic BP value and the change over time was inverted U-shaped, with values at first increasing and then decreasing at the mid study (Fig. 2).
Fig. 2.
Changes over time in diastolic BP for ASU and KI participants
Unlike systolic BP, gender was not related to one's initial starting diastolic BP value or to change in diastolic BP over time. Age was related to one's initial diastolic BP and diastolic BP increased with age. Age was not related to change over time in diastolic BP. Steps taken were not significantly related to initial diastolic BP value, but were related to both the linear and quadratic changes in diastolic BP over time, with more steps related to a greater linear decline in diastolic BP which slowed over time. Changes in resting systolic and diastolic BP measures in relation to time, steps taken, age, gender and site status using the conditional mixed model are reported in Table 2.
Table 2.
Changes in resting systolic and diastolic BP measures in relation to time, steps taken, age, gender and site status using the Conditional Mixed Model (N = 355)
| Effect | systolic blood pressure | diastolic blood pressure | ||||
|---|---|---|---|---|---|---|
| Coefficient | SE | P value | Coefficient | SE | P value | |
| Intercept | 118.9800 | 2.7946 | <0.0001 | 77.8314 | 1.9164 | <0.0001 |
| SITE | 5.3613 | 1.6198 | 0.0011 | 1.2894 | 1.1034 | 0.2 |
| female | −10.3880 | 1.9602 | <0.0001 | −2.1535 | 1.3340 | 0.1 |
| Centered Age | 0.4830 | 0.0653 | <0.0001 | 0.1981 | 0.0445 | <0.0001 |
| Steps | 0.0004 | 0.0002 | 0.0479 | 0.0002 | 0.0001 | 0.2 |
| time | 7.5726 | 3.7560 | 0.0446 | 5.5124 | 2.7458 | 0.04 |
| time 2 | −4.1966 | 1.6462 | 0.0114 | −4.1334 | 1.2256 | 0.0009 |
| SITE * time | −9.8710 | 2.0166 | <0.0001 | −5.0279 | 1.5050 | 0.001 |
| female * time | −1.2806 | 2.4423 | 0.6005 | −1.8105 | 1.8263 | 0.3 |
| CAge * time | −0.1485 | 0.0815 | 0.0697 | 0.0505 | 0.0609 | 0.4 |
| Steps * time | −0.0005 | 0.0003 | 0.0643 | −0.0004 | 0.0002 | 0.03 |
| SITE * time 2 | 5.0048 | 0.9712 | <0.0001 | 3.4937 | 0.7319 | <.0001 |
| female * time 2 | 0.4700 | 1.1833 | 0.6916 | 0.9958 | 0.8926 | 0.3 |
| CAge * time 2 | 0.0555 | 0.0391 | 0.1572 | −0.0311 | −0.0311 | 0.3 |
| Steps * time 2 | 0.0002 | 0.0001 | 0.1452 | 0.0002 | 0.0001 | 0.0001 |
SE: standard error
To better understand the differences between the effects of intervention on systolic and diastolic BP in normotensive and all participants, we reran the analyses by omitting the hypertensive subjects. Omitting the hypertensive subjects did not substantially affect our results. In addition, we have rerun the analyses with only the 251 participants (ASU = 86 and KI = 165) who completed the intervention. Omitting the dropped out subjects did not change the slope of the curves.
Changes in CRF
Three hundred and forty four ASUKI participants attended the first month cardiorespiratory fitness assessment with mean of estimated VO2 max = 35.68 ml.kg−1.min−1 (±10.80), and 251 participants attended the 6 months cardiorespiratory fitness assessment with the mean of estimated VO2 max = 35.38 ml.kg−1.min−1 (±9.98). The fixed effects tests showed that there was a significant difference between ASU and KI participants’ estimated VO2 max (F1,347=10.51, P= 0.001), ASU had a lower estimated VO2 max than KI. There was a significant change over time in the estimated VO2 max (F1,244 = 7.61, p .006). The time by site interaction was also significant, indicating that the change over time varied by site (F1,244=4.25, P=0.04), where VO2 max decreased for ASU but stayed essentially the same for KI participants. The number of steps taken was significantly related to changes in estimated VO2 max over time (F1,240=18.44, P=0.001), as steps increased estimated VO2 max increased. Gender was not related to changes in the estimated VO2 max over time. Age was a significant predictor of changes in VO2 max over time (F1,240= 5.20, P=0.02), where estimated VO2 max increased for older participants, and the increase was site specific (KI). Means and standard deviations of estimated VO2 max at one and six months are shown in Table 3.
Table 3.
Means and standard deviations (SD) of estimated VO2 max (ml.kg−1.min−1) at Months 1 and 6 for ASUKI Step study's participants
| SITE | n | Month 1 (mean ± SD) | n | Month 6 (mean ± SD) |
|---|---|---|---|---|
| ASU | 139 | 33.49 (11.03)* | 86 | 31.77 (8.64) |
| KI | 205 | 37.15 (10.40) | 165 | 37.27 (10.14) |
| ASUKI (All) | 344 | 35.68 (10.80)* | 251 | 35.38 (9.98) |
Demonstrated a significant differences with P< 0.05 between months 1 and 6 of intervention
SD: Standard Deviation
DISCUSSION
The major purpose of the study was to investigate the effects of pedometer-determined step counts on resting BP and estimated VO2 max as determined by an ergometer submaximal exercise test, during the six-month intervention period. The main results were observations of significant decreases in resting systolic and diastolic BP, while no significant improvements in estimated VO2 max in the ASUKI test group participants were observed. As would be expected, individuals with higher CRF levels at the start of the study on average reported more steps per day than participants with lower initial CRF levels. The study was unique since PA was recorded using pedometers during the 6 months intervention, and CRF was examined using an Åstrand-Rhyming cycle ergometer test.
Like many pedometer-based community intervention studies, the intervention period started with great enthusiasm. The participants walked a mean of 12,256 (SD = 3,180) reported steps per day and steadily decreased their walking duration to 8,586 (SD = 5,999) steps per day after the six month intervention. It is important to note that in this study, during month one of intervention, when participants’ motivation was likely at its highest, the majority of the test group participants (n =283, 79.7%) were classified in active or highly active in the pedometer step categories [24] During the final month of intervention, 61.5% participants averaged 10,000 or more steps per day. The goal of 10,000 steps per day was reached by the test group during a larger number of days than the other ASUKI participants. This indicates the importance of a thorough follow-up and continuous support in PA interventions.
The results show significant improvements in both systolic and diastolic BP. Over time, there were significant linear and quadratic decreases in both systolic and diastolic BP. This is similar to earlier pedometer interventions where decreases in systolic and diastolic BP have been reported, particularly when a specific target is set (e.g. 10,000 steps/day) [39–42]. The number of steps was borderline significantly related to linear change in systolic BP and significantly in related to both the linear and quadratic changes in diastolic BP over time.
BP is an indicator that can be improved rather quickly with exercise by adding daily moderate PA. It takes about 10 weeks for regular PA to have an impact on systolic and diastolic BP which indicates that changes in BP can be shown fast [43]. The differences between changes in the BP over time in ASU and KI participants indicated in Figures 1 and 2 may be related to the environmental temperature (heat in Arizona and cold in Stockholm). BP increases with cold and decreases with heat. In Stockholm, it was cold at the start of the study, warmer in the middle, and getting colder again at the end. In ASU it was cooler at the start, really hot at the middle, and still hot at the end of the study. Resting BP has been found to be higher in the winter season [44–48]. It is reported that hot weather can be associated not only with an attenuation of daytime and clinic BP but also with an increase in values of night time BP [49]. Previous studies declare that an increase in BP can indicate an existing hypothesis behind cold-related cardiovascular disease [50]. Generally, BP is worse in the winter and better in the summer. Seasonal changes in BP appear quickly if people move from a warmer weather to a colder weather, and more slowly when they move from a colder weather to a warmer weather [51]. Increased resting BP significantly elevates cardiovascular disease risk [52]. Estimates indicate that on a population level even small decrease in resting diastolic BP of 2 mmHg can lead to reduced chronic heart disease (CHD) risk by 6% [53]. Our results indicate that the change in weather cannot be the whole explanation for our BP reductions, since the effect was related to the number of steps.
In the present study there was no improvement in CRF. We saw no change in CRF over time for the KI participants in total or by gender. There was, however, a reduction in estimated VO2 max for ASU participants during the intervention period. One explanation for the significant decline in ASU participants’ estimated VO2 max can be related to a greater decrease in their reported daily average number of steps from 10,955 (± 3,035) steps per day at month one to 6,486 (± 5,509) steps per day in month 6 compared to KI participants who kept the goal of walking 10,000 steps per day and reported from 13,113 (± 2,993) steps per day in month one to 9,990 (± 5,957) steps per day in month 6. Estimated VO2 max increased for older KI participants during the intervention period. Interestingly, the older participants who had significantly greater increase in estimated VO2 max had a higher compliance to daily walking. Furthermore, the decline in CRF was greater for men than for women, similar to a previous study that reported reduction in CRF in men and women after 45 years at an accelerated rate and a reduction for males that was greater than that for females [15]. The increasing evidences indicate that regular PA can slow the age-related deterioration of several biological functions such as CRF [54].
The number of steps taken was significantly related to changes in estimated VO2 max over time, as steps increased estimated VO2 max increased. A meta-analysis of randomized, controlled trials that yielded over 40 studies using walking as an intervention, showed significant decreases in diastolic BP, and significant increases in CRF [17]. The findings from the meta-analysis showed that walking is a sufficient stimulus to improve CRF, and to reduce resting diastolic BP in sedentary but healthy individuals. The results of this meta-analysis study provide evidence of improvement of several known risk factors for cardiovascular disease in healthy but sedentary individuals who participated in regular brisk walking programs. It can reinforce the centrality of walking program in health promotion and importance of the efficacy of this type of PA to improve health among the sedentary majority [17].
Strengths and Limitations of the study
The major strengths of this study include continuous monitoring of PA using of pedometers and its long-term approach as a workplace pedometer intervention. The CRF and resting BP outcomes were measured by trained staff in a randomly selected sample, using objective and validated methods. However, this study has limitations which may have affected the internal validity of the study measures. Very few males (n = 67) volunteered for the study which limited the ability to view gender differences in the results. The dominance of female individuals in the walking intervention is similar to what has been seen in other studies and may indicate the intuitive sense that this form of PA is for females [17]. Despite the advantages of submaximal fitness tests with adults, laboratory measurements that examine actual individual's aerobic capacity are considered the gold standard for predicting CRF [1]. Furthermore, there are several limitations for Åstrand-Rhyming nomogram to predict VO2 max [55, 56]. The differences in individual maximal heart rate may be the most important. Previous studies have indicated that the nomogram may underestimate VO2 max. It is assumed that the nomogram is sub-optimal to compare between individuals but may have indications on a group level [55]. Direct methods to measure estimated VO2 max are superior in validity in comparison with other methods, but they may be less than optimal to use in a large samples of adults [55].
CONCLUSION
In conclusion, the results of this study indicate that generally healthy individuals who took part in a workplace intervention using pedometers improved several cardiovascular disease risk factors, and the goal of walking 10,000 steps per day could be effective in reducing resting systolic and diastolic BP in all participants, and increasing CRF, especially in the older age group who had the best compliance of walking 10,000 steps per day. Further studies with controls and follow-ups are suggested to substantiate the finding of this investigation and the effects of walking with the goal of 10,000 steps per day on CRF.
ACKNOWLEDGMENTS
The authors thank the data collectors, administrative support staff, and participants involved in the ASUKI Step study. ASUKI Step was funded by resources from Karolinska institutet human resources and health promotion department, and New Lifestyles Pedometers Inc.
Conflict of interests: None
REFERENCES
- 1.Cao ZB, Miyatake N, Higuchi M, et al. Predicting VO2max with an objectively measured physical activity in Japanese women. Med Sci Sports Exerc. 2010;42:179–86. doi: 10.1249/MSS.0b013e3181af238d. [DOI] [PubMed] [Google Scholar]
- 2.Aires L, Pratt M, Lobelo F, et al. Associations of cardiorespiratory fitness in children and adolescents with physical activity, active commuting to school, and screen time. J Phys Act Health. 2011;8:S198–205. [PubMed] [Google Scholar]
- 3.Chaudhary S, Kang MK, Sandhu JS. The effects of aerobic versus resistance training on cardiovascular fitness in obese sedentary females. Asian J Sports Med. 2010;1:177–84. doi: 10.5812/asjsm.34835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 5.Baert I, Feys H, Daly D, et al. Are patients 1 year post-stroke active enough to improve their physical health? Disabil Rehabil. 2012;34:574–80. doi: 10.3109/09638288.2011.613513. [DOI] [PubMed] [Google Scholar]
- 6.Saremi A, Shavandi N, Parastesh M, Daneshmand H. Twelve-week aerobic training decreases chemerin level and improves cardiometabolic risk factors in overweight and obese men. Asian J Sports Med. 2010;1:151–8. doi: 10.5812/asjsm.34860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel PZ, Brackbill RM, Heath GW. The epidemiology of walking for exercise: implications for promoting activity among sedentary groups. Am J Public Health. 1995;85:706–10. doi: 10.2105/ajph.85.5.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anton SD, Duncan GE, Limacher MC, et al. How much walking is needed to improve cardiorespiratory fitness? An examination of the 2008 Physical Activity Guidelines for Americans. Res Q Exerc Sport. 2011;82:365–70. doi: 10.1080/02701367.2011.10599766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Masurier GC, Corbin CB. Steps counts among middle school students vary with aerobic fitness level. Res Q Exerc Sport. 2006;77:14–22. doi: 10.1080/02701367.2006.10599327. [DOI] [PubMed] [Google Scholar]
- 10.Lubans DR, Morgan PJ, Callister R, Collins CE. The relationship between pedometer step counts and estimated VO2Max as determined by a submaximal fitness test in adolescents. Pediatr Exerc Sci. 2008;20:273–84. doi: 10.1123/pes.20.3.273. [DOI] [PubMed] [Google Scholar]
- 11.Crouter SE, Schneider PL, Karabulut M, Bassett DR., Jr Validity of 10 electronic pedometers for measuring steps, distance, and energy cost. Med Sci Sports Exerc. 2003;35:1455–60. doi: 10.1249/01.MSS.0000078932.61440.A2. [DOI] [PubMed] [Google Scholar]
- 12.Iwane M, Arita M, Tomimoto S, et al. Walking 10,000 steps/day or more reduces blood pressure and sympathetic nerve activity in mild essential hypertension. Hypertens Res. 2000;23:573–80. doi: 10.1291/hypres.23.573. [DOI] [PubMed] [Google Scholar]
- 13.Lee LL, Watson MC, Mulvaney CA, Tsai CC, Lo SF. The effect of walking intervention on blood pressure control: a systematic review. Int J Nurs Stud. 2010;47:1545–61. doi: 10.1016/j.ijnurstu.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Veerman DP, Imholz BP, Wieling W, et al. Effects of aging on blood pressure variability in resting conditions. Hypertension. 1994;24(1):120–30. doi: 10.1161/01.hyp.24.1.120. [DOI] [PubMed] [Google Scholar]
- 15.Jackson AS, Sui X, Hebert JR, et al. Role of lifestyle and aging on the longitudinal change in cardiorespiratory fitness. Arch Intern Med. 2009;169:1781–7. doi: 10.1001/archinternmed.2009.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aldred HE, Hardman AE, Taylor S. Influence of 12 weeks of training by brisk walking on postprandial lipemia and insulinemia in sedentary middle-aged women. Metabolism. 1995;44:390–7. doi: 10.1016/0026-0495(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 17.Murphy MH, Nevill AM, Murtagh EM, Holder RL. The effect of walking on fitness, fatness and resting blood pressure: a meta-analysis of randomised, controlled trials. Prev Med. 2007;44:377–85. doi: 10.1016/j.ypmed.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth BE, Der Ananian C, Soroush A, et al. “ASUKI Step” pedometer intervention in university staff: rationale and design. BMC Public Health. 2012;12:657. doi: 10.1186/1471-2458-12-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soroush A, Walker J, Poortvliet E, et al. The effects of a 6-month pedometer-determined physical activity intervention on body composition characteristics in Swedish adults: The ASUKI Step study. Int J Body Compos Res. 2012;10:47–54. [Google Scholar]
- 20.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–95. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 21.Schneider PL, Crouter SE, Bassett DR. Pedometer measures of free-living physical activity: comparison of 13 models. Med Sci Sports Exerc. 2004;36:331–5. doi: 10.1249/01.MSS.0000113486.60548.E9. [DOI] [PubMed] [Google Scholar]
- 22.Swartz AM, Bassett D, Jr, Moore J, Thompson D. Effects of body mass index on the accuracy of an electronic pedometer. Int J Sports Med. 2003;24:588–92. doi: 10.1055/s-2003-43272. [DOI] [PubMed] [Google Scholar]
- 23.Schneider P, Crouter S, Lukajic O, Bassett D., Jr Accuracy and reliability of ten pedometers for measuring steps over a 400- walk. Med Sci Sport Exerc. 2003;35:1799–84. doi: 10.1249/01.MSS.0000089342.96098.C4. [DOI] [PubMed] [Google Scholar]
- 24.Tudor-Locke C, Bassett DR., Jr How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 25.Ritchie JD, Miller CK, Smiciklas-Wright H. Tanita foot-to-foot bioelectrical impedance analysis system validated in older adults. J Am Diet Assoc. 2005;105:1617–9. doi: 10.1016/j.jada.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 26.Artigao LM, Llavador JJ, Puras A, et al. Evaluation and validation of Omron Hem 705 CP and Hem 706/711 monitors for self-measurement of blood pressure. Aten Primaria. 2000;25:96–102. doi: 10.1016/S0212-6567(00)78470-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Topouchian JA, El Assaad MA, Orobinskaia LV, et al. Validation of two automatic devices for self-measurement of blood pressure according to the International Protocol of the European Society of Hypertension: the Omron M6 (HEM-7001-E) and the Omron R7 (HEM 637-IT) Blood Press Monit. 2006;11:165–71. doi: 10.1097/01.mbp.0000209078.17246.34. [DOI] [PubMed] [Google Scholar]
- 28.Vera-Cala LM, Orostegui M, Valencia-Angel LI, et al. Accuracy of the Omron HEM-705 CP for blood pressure measurement in large epidemiologic studies. Arq Bras Cardiol. 2011;96:393–8. doi: 10.1590/s0066-782x2011005000038. [DOI] [PubMed] [Google Scholar]
- 29.Graciani A, Banegas JR, Lopez-Garcia E, et al. Assessment of a blood pressure measurement training programme for lay observers. Blood Press Monit. 2002;7:251–7. doi: 10.1097/00126097-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Grim CE, Grim CM. Omron HEM-711 DLX home Blood pressure monitor passes the European Society of Hypertension International Validation Protocol. Blood Press Monit. 2008;13:225–6. doi: 10.1097/MBP.0b013e3282feebd5. [DOI] [PubMed] [Google Scholar]
- 31.Cuddy ML. Treatment of hypertension: guidelines from JNC 7 (the seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure 1) J Pract Nurs. 2005;55:17–21. [PubMed] [Google Scholar]
- 32.Astrand PO, Ryhming I. A nomogram for calculation of aerobic capacity (physical fitness) from pulse rate during sub-maximal work. J Appl Physiol. 1954;7:218–21. doi: 10.1152/jappl.1954.7.2.218. [DOI] [PubMed] [Google Scholar]
- 33.Keller A, Hellesnes J, Brox JI. Reliability of the isokinetic trunk extensor test, Biering-Sorensen test, and Astrand bicycle test: assessment of intraclass correlation coefficient and critical difference in patients with chronic low back pain and healthy individuals. Spine (Phila Pa 1976 ). 2001;26:771–7. doi: 10.1097/00007632-200104010-00017. [DOI] [PubMed] [Google Scholar]
- 34.Patton JF, Vogel JA, Mello RP. Evaluation of a maximal predictive cycle ergometer test of aerobic power. Eur J Appl Physiol Occup Physiol. 1982;49:131–40. doi: 10.1007/BF00428971. [DOI] [PubMed] [Google Scholar]
- 35.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–81. [PubMed] [Google Scholar]
- 36.Kahn EB, Ramsey LT, Brownson RC, et al. The effectiveness of interventions to increase physical activity. A systematic review. Am J Prev Med. 2002;22(4 Suppl):73–107. doi: 10.1016/s0749-3797(02)00434-8. [DOI] [PubMed] [Google Scholar]
- 37.Latin RW, Elias BA. Predictions of maximum oxygen uptake from treadmill walking and running. J Sports Med Phys Fitness. 1993;33:34–9. [PubMed] [Google Scholar]
- 38.Cohen J, Cohen P, West S, Aiken L. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Oxford England: Lawrence Erlbaum; 1983. [Google Scholar]
- 39.Dishman RK, DeJoy DM, Wilson MG, Vandenberg RJ. Move to Improve: a randomized workplace trial to increase physical activity. Am J Prev Med. 2009;36:133–41. doi: 10.1016/j.amepre.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 40.Faghri PD, Omokaro C, Parker C, et al. E-technology and pedometer walking program to increase physical activity at work. J Prim Prev. 2008;29:73–91. doi: 10.1007/s10935-007-0121-9. [DOI] [PubMed] [Google Scholar]
- 41.Gemson DH, Commisso R, Fuente J, et al. Promoting weight loss and blood pressure control at work: impact of an education and intervention program. J Occup Environ Med. 2008;50:272–81. doi: 10.1097/JOM.0b013e318162f628. [DOI] [PubMed] [Google Scholar]
- 42.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 43.Grassi G, Seravalle G, Calhoun DA, Mancia G. Physical training and baroreceptor control of sympathetic nerve activity in humans. Hypertension. 1994;23:294–301. doi: 10.1161/01.hyp.23.3.294. [DOI] [PubMed] [Google Scholar]
- 44.Argani H, Javanshir MR. Seasonal variations of blood pressure in hemodialysis and renal transplant recipients. Transplant Proc. 2004;36:148–9. doi: 10.1016/j.transproceed.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 45.Sinha P, Kumar TD, Singh NP, Saha R. Seasonal variation of blood pressure in normotensive females aged 18 to 40 years in an urban slum of Delhi, India. Asia Pac J Public Health. 2010;22:134–45. doi: 10.1177/1010539509351190. [DOI] [PubMed] [Google Scholar]
- 46.Lewington S, Li L, Sherliker P, et al. Seasonal variation in blood pressure and its relationship with outdoor temperature in 10 diverse regions of China: the China Kadoorie Biobank. J Hypertens. 2012;30:1383–91. doi: 10.1097/HJH.0b013e32835465b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takenaka T, Kojima E, Sueyoshi K, et al. Seasonal variations of daily changes in blood pressure among hypertensive patients with end-stage renal diseases. Clin Exp Hypertens. 2010;32:227–33. doi: 10.3109/10641963.2010.491887. [DOI] [PubMed] [Google Scholar]
- 48.Madsen C, Nafstad P. Associations between environmental exposure and blood pressure among participants in the Oslo Health Study (HUBRO) Eur J Epidemiol. 2006;21:485–91. doi: 10.1007/s10654-006-9025-x. [DOI] [PubMed] [Google Scholar]
- 49.Modesti PA, Morabito M, Bertolozzi I, et al. Weather-related changes in 24-hour blood pressure profile: effects of age and implications for hypertension management. Hypertension. 2006;47:155–61. doi: 10.1161/01.HYP.0000199192.17126.d4. [DOI] [PubMed] [Google Scholar]
- 50.Halonen JI, Zanobetti A, Sparrow D, et al. Relationship between outdoor temperature and blood pressure. Occup Environ Med. 2011;68:296–301. doi: 10.1136/oem.2010.056507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hayashi T, Ohshige K, Sawai A, et al. Seasonal influence on blood pressure in elderly normotensive subjects. Hypertens Res. 2008;31:569–74. doi: 10.1291/hypres.31.569. [DOI] [PubMed] [Google Scholar]
- 52.He J, Whelton PK. Elevated systolic blood pressure and risk of cardiovascular and renal disease: overview of evidence from observational epidemiologic studies and randomized controlled trials. Am Heart J. 1999;138:211–9. doi: 10.1016/s0002-8703(99)70312-1. [DOI] [PubMed] [Google Scholar]
- 53.Cook NR, Cohen J, Hebert PR, et al. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–9. [PubMed] [Google Scholar]
- 54.De Vito G, Hernandez R, Gonzalez V, et al. Low intensity physical training in older subjects. J Sports Med Phys Fitness. 1997;37:72–7. [PubMed] [Google Scholar]
- 55.Ekblom OB, Bak EA, Ekblom BT. Cross-sectional trends in cardiovascular fitness in Swedish 16-year-olds between 1987 and 2007. Acta Paediatr. 2011;100:565–9. doi: 10.1111/j.1651-2227.2010.02135.x. [DOI] [PubMed] [Google Scholar]
- 56.Sarelius IH, Quinn JP. Estimation of maximal oxygen uptake in New Zealanders of three age groups. NZ Med J. 1975;81:549–52. [PubMed] [Google Scholar]