Abstract
Purpose
The aim of this study was to investigate whether supplementation of carbohydrate together with peptide glutamine would prevent anaerobic power decrease during repeated competitions.
Methods
Twenty eight physical education male students voluntarily participated in the study. Subjects were randomly divided on a maximal power (Max power) output value basis into four groups: 1) G group (oral ingestion of glutamine at the dose of 0.25 g/kg body mass in 250 ml of water), 2) M group (a single carbohydrate at a concentration of 50g of maltodextrin in 250 ml of water), 3) GM group (carbohydrate at a concentration of 50g of maltodextrin + glutamine at the dose of 0.25 g/kg body mass in 250 ml of water) and, 4) P group (just 250 ml of water and 30 gram sweetener). Each subject performed three times Running-based Anaerobic Sprint Test (RAST) with intervals of 1 hour. Max power, Minimal power (Min power) and fatigue were calculated for each participant.
Results
There was a significant decrease in Max and Min power in P group in time series (P<0.05). Furthermore, regarding the Max and Min power, there was significant difference between P and GM group in third bout indicating stronger influence of combination of maltodextrin and glutamine in comparison with pure consumption of glutamine and maltodextrin (P< 0.05).
Conclusions
It seems acute supplementation of glutamine and maltodextrin combination, 2 hours before exercise is more efficient in prevention of anaerobic power decrease than consumption of a pure carbohydrate or glutamine in repeated bouts of RAST protocol. Thus, supplementation with both carbohydrate and peptide glutamine improved the physical performance of athletes during repeated competitions. Obviously, it is necessary to do further studies.
Keywords: Carbohydrate, Peptide, Maximal Power, Minimal Power, Fatigue Index
INTRODUCTION
Glutamine is an important constituent of proteins and is a precursor for the synthesis of amino acids, nucleotides, nucleic acids, amino sugars, and several other biologically important molecules [1]. It is the most abundant amino acid in plasma as well as skeletal muscle and accounts for more than 60% of the total intramuscular free amino acid pool [2, 3].Glutamine is largely synthesized in skeletal muscles and precursors to gluconeogenesis in the liver. Physical exercise is known to affect glutamine synthesis and to modulate glutamine uptake [4]. Strenuous physical exercise as well as exhaustive training programs lead to glutamine depletion due to lowered synthesis and enhanced uptake by liver and immune cells [4].
This might be the reason why Glutamine is a popular dietary supplement consumed for purported ergogenic benefits of increased strength, quicker recovery, decreased frequency of respiratory infections, and prevention of overtraining [5]. However, a lack of evidence has been demonstrated for definitive positive ergogenic benefits as a result of glutamine supplementation [5].
On the other hand, Carbohydrate ingested before and during exercise provides an alternate source of muscle fuel that can support moderate and moderately high-intensity physical activity [6]. Maltodextrin is a carbohydrate polymer utilized preferentially as diet recourse in exercise. The gastric emptying for glucose polymer is faster than glucose solutions, avoiding a sudden drop in blood glucose and hyperinsulinaemia induced hypoglycaemia during exercise [7]. Athletes in long-duration types of sports (e.g. runners, triathletes and cyclists) benefit from the use of carbohydrate and glutamine together. Glutamine is an intermediate metabolite in the Krebs cycle and thus acts in gluconeogenesis by saving phosphocreatine (CP) deposits and glycogen in muscle fibers, particularly type I (aerobic) fibers, thereby increasing the tolerance to exercise [8–10]. However, there are few studies about the effect of glutamine on the performance of athletes. Moreover, the related mechanisms are still unclear. Also, there is a lack of research about the use of glutamine in some sports in which athletes need to do power-like competitions more than twice within 3-5 hours. In some sports such as martial arts, wrestling, swimming and others of that kind, athletes compete more than twice in short intervals within 3-5 hours. These types of athletes need to keep anaerobic power in optimum range for any competition. Maintenance of power during consecutive competition is a critical requisite to success. We think there is a paucity of practical investigations about the effect of combination of glutamine and carbohydrate supplementation on such sports, in which athletes have to compete consequently within a few hours. Furthermore, we believe in this study, there are some novelties such as utilization of supplementation 2 hours before exercise, conducting three repeated bouts of anaerobic power-like trial and use of standard RAST protocol. Therefore, the aim of the present study is to investigate whether supplementation of peptide glutamine and carbohydrate would prevent anaerobic power decrease during repeated competitions.
METHODS AND SUBJECTS
Participants
This was a double-blind, placebo-controlled study. Twenty-eight male physical education students voluntarily participated in the study. All were well-trained physical education students who were participating in a training regimen (three or more days a week for at least an hour). Descriptive characteristics of the subjects are shown in Table 1. The inclusion criteria were age range between 20-30 years, absence of cigarette and water pipe use; and VO2max more than 40 ml/kg/min.
Table 1.
Subjects’ physical characteristics for all groups
| Characteristics | Group P (n = 7) | Group M (n = 7) | Group G (n = 7) | Group GM (n = 7) |
|---|---|---|---|---|
| Age (yr) | 22.14 (4.10) | 23.86 (3.89) | 23.29 (3.53) | 22.14 (2.85) |
| Mass (kg) | 72.71 (8.01) | 73.00 (10.42) | 82.14 (16.24) | 74.00 (8.08) |
| Height (cm) | 177. 71 (4.78) | 177.57 (6.18) | 179.14 (6.99) | 177.29 (5.02) |
| Body mass index (kg/m 2 ) | 22.79 (2.49) | 23.37 (3.52) | 25.36 (3.31) | 23.35 (2.14) |
| %Fat | 10.90 (3.48) | 13.88 (4.71) | 12.66 (1.69) | 13.88 (3.77) |
| VO2 max (ml.kg -1 .min -1 ) | 47.56 (4.14) | 48.46 (4.55) | 47.66 (4.82) | 46.81 (4.23) |
Data are declared as mean ± SD. One-way ANOVA demonstrated no significant differences among groups (P<0.05) G group (Glutamine), M group (Maltodextrin), GM group (Maltodextrin + Glutamine), P group (water and sweetener)
Procedure
Prior to participating, the subjects read and signed an informed consent form and completed a physical activity and health readiness questionnaire that was previously approved by the researchers and previous investigators’ experiences [11]. Furthermore, before undergoing the tests, the subjects were given explanations about the assessment procedures, the study objectives, and the possible benefits and risks. Measurement of body composition was obtained prior to test using the three-site skin-fold caliper method [12]. Each subject was weighed prior to the test. All the participants completed a Running-based Anaerobic Sprint Test (RAST) [13]. Then, maximum power (Max power) output was calculated in pre-test for each subject. Before testing, each subject performed a sub-maximal sprint to familiarize themselves with the test procedure. Immediately after baseline testing, subjects were randomly divided, on a Max power output value basis, to four groups: 1) G group (oral ingestion of glutamine at the dose of 0.25 g/kg body mass in 250 ml of water +30 gram sweetener), 2) M group (a single carbohydrate at a concentration of 50g of maltodextrin in 250 ml of water + 30 gram sweetener), 3) GM group (carbohydrate at a concentration of 50g of maltodextrin + glutamine at the dose of 0.25 g/kg body mass in 250 ml of water + 30 gram sweetener) and, 4) P group (250 ml of water + 30 gram sweetener) in a double-blind fashion. These supplements were administered two hours before test. Recent research has indicated that high doses of oral glutamine supplementation (0.3 g/kg) do not cause any harmful side effects [14]. Two hours after supplementation, each subject performed RAST three times with 1-hour intervals. The subject warmed up for a period of five minutes, which was followed by a three-minute recovery.
Running-based Anaerobic Sprint Test (RAST)
It has been shown that this test can replace the Wingate test as an estimate of anaerobic power and capacity because reliability of RAST is high (r = 0.88) [13]. The test consists of six times 35m discontinuous sprints. Each sprint represents a maximal effort with 10 seconds allowed between each sprint for the turnaround. After completion of each RAST, subjects were allowed a 5 minute cool-down period at low to moderate aerobic power immediately followed by a 45 minute inactive recovery period. Maximal and minimal power (Min power) output was expressed in watts (W) and defined as the highest and lowest power output in six times 35m discontinuous sprints, respectively. Fatigue index indicates the decrease in power from the highest to the lowest anaerobic power output. Fatigue index was defined as the difference between the Max and Min power output divided by total time for 6 sprints. Power output and fatigue index were calculated by the following equations: 1) Power: Weight (kg) x Distance (m2)/Time (s3) and 2) Fatigue index (W/S): (Max power – Min power)/Total time for 6 sprints (s). Max and Min power output and fatigue index was calculated for each RAST to determine statistically significant differences among groups.
Statistical Analysis
Results are expressed as means ± SD, and P < 0.05 was considered statistically significant. An independent two-way analysis of variance with repeated measures was used to compare results among treatments and over time. Where significant F ratios were found, a Tukey Honest Significant Difference test was used to determine location of variance. When there were only single comparisons, a student's t-test with Bonferroni correction for correlated data was used to determine whether any differences between treatments existed.
RESULTS
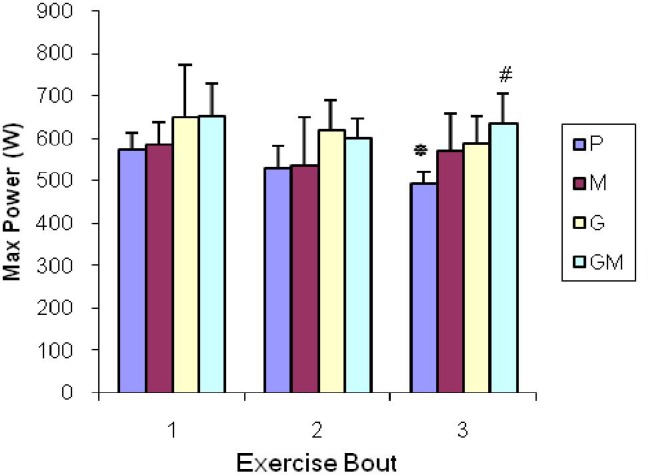
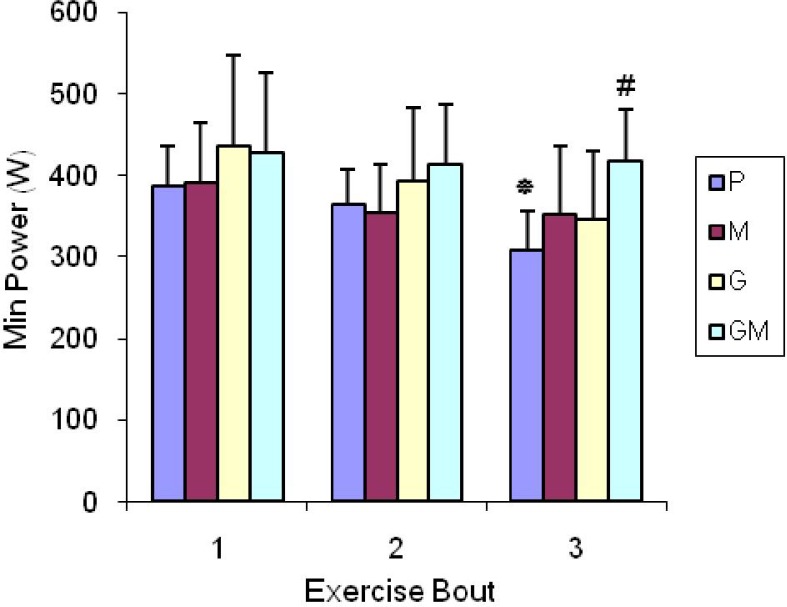
The results showed that Max and Min Power outputs decreased significantly in P group in time series (P< 0.05) (Figs. 1 and 2). However, no significant decreases were observed in them in time series in other groups (P< 0.05). Furthermore, related to Max and Min power, there were significant differences between P and GM group in the third bout indicating stronger influence of combination of maltodextrin and glutamine in comparison with consumption of glutamine and maltodextrin alone (P< 0.05) (Figs. 1 and 2). There were no significant differences in time series and among groups in relation to fatigue index (P>0.05).
Fig. 1.
Max Power changes in time series and among groups. Values represent means ± SD (n = 7)
*Values indicate Max Power decline at third time compared with first and second time in P group (P<0.05). # Value indicates decline of Max Power in P group compared with GM groups (P< 0.05).
Fig. 2.
Min Power changes in time series and among groups. Values represent means ± SD (n = 7)
* Values indicate Min power decline at third time compared with first and second time in P group (P<0.05). # Value indicates decline of Min Power in P group compared with GM group (P< 0.05).
DISCUSSION
This hypothesis suggests that acute supplementation of a glutamine and maltodextrin combination, 2 hours before exercise is possibly more efficient to prevent anaerobic power decrease than consumption of carbohydrate or glutamine alone in repeated bouts of RAST protocol. According to Fig. 1, there is a significant decrease in Max power in P group in time series (P<0.05). However, no decline was seen in time series in other groups indicating a possible effect of supplementations in sustaining Max power. In addition, there is significant difference between the P and GM group in the third bout, indicating stronger influence of the combination of maltodextrin and glutamine in comparison with pure consumption of glutamine or maltodextrin only (P< 0.05). According to Figure 2, the results of Min power and Max power are the same. Regarding fatigue index, there are no significant differences in time series and among groups (P> 0.05). There are some explanations about the effect of all supplements compared with placebo and stronger effect of the combination of maltodextrin and glutamine:
Preview studies showed that the beneficial effect of pre-exercise CHO feeding could be attributed to the increase in liver glycogen and alternatively an increase muscle glycogen for potential oxidation during exercise [15]. In addition, the observed improvements in performance with CHO ingestion have been attributed to maintenance of plasma glucose and glycogen availability [16]. In this context, CHO supplementation has been shown to increase the amount of work that can be performed [17–19]. However, several studies have not observed positive effects of pre-exercise CHO feedings [20, 21]. Differences in the training status of the subjects, amount of CHO ingested and the failure of the pre-exercise CHO feeding to alter glycogen metabolism may have been the potential causes for the discrepancy between these studies.
Furthermore, the type of CHO supplementation is very important. It has been suggested that because of their lower osmolalities, glucose polymer solutions (i.e. maltodextrins) would be preferable to isocaloric glucose solutions as a source of ingested CHO before and during exercise [22]. Ruffo et al, Observed that maltodextrin supplementation before exercise increased muscle and hepatic glycogen storage [7]. It seems the present study can be explained similar to other studies; in which prevention of Max and Min power decrease in M group is due to increased muscle and hepatic glycogen storage or maintenance of plasma glucose and glycogen availability.
On the other hand, glutamine supplementation managed to prevent decrease of Max and Min power in comparison with P group (Figures 1 and, 2). It has been shown that ingestion of pure glutamine has been able to promote muscle glycogen re-synthesis during recovery from exhaustive exercise [23]. It has also been reported that glutamine stimulates the activity of hepatic glycogen synthesis [24]. Glutamine is readily taken up into skeletal muscle via the high-capacity, sodium-dependent system, [25] resulting in an increased intramuscular glutamine concentration [26]. Variner et al found that intravenous administration of glutamine promotes muscle glycogen re-synthesis during recovery after exhaustive exercise, possibly because glutamine acts as a glycogenic substrate and/or through an activator of glycogen synthase[27]. Strenuous physical exercise as well as exhaustive training programs lead to glutamine depletion due to lowered synthesis and enhanced uptake by liver and immune cells [4]. Moreover, one interesting study carried out among tri-athletes found that glutamine supplementation was efficiently absorbed and was not used for enterocyte proliferation[28], thus making it easier for the athletes receiving the supplementation to maintain their glycemic levels and ensuring greater exercise tolerance [29].
Finally, GM supplementation has been shown to be more effective than separate G or M supplementation (Figures 1 and 2). The stronger effect of GM supplementation is possibly due to greater storage of carbohydrate in sites other than skeletal muscle, the most likely candidate being the liver [23, 30]. Studies developed among cyclists and runners have also found greater tolerance to effort when these athletes were supplemented with peptide glutamine and carbohydrate. This is because glutamine is an intermediate metabolite of the Krebs cycle, and is thus thought to act in gluconeogenesis, thereby increasing the efficiency of this metabolic process [8, 9]. Hence, a higher efficiency of gluconeogenesis for energy production by muscle glycogen is of fundamental importance for higher performance in sports. Additionally, in soccer players, a tendency towards a lower subjective perception of sub-maximal effort was observed among those who received glutamine and maltodextrin supplementation compared with those who received pure maltodextrin supplementation [29].
It is important to critically evaluate the results and the whole study. The present study has certain limitations that need to be taken into account when considering the study itself and its contributions to the field. Peptide glutamine has often been used for immunological support in immunosuppressed patients. Only a few studies have used peptide glutamine as an ergogenic aid for improving athletic performance. In our study, we used this supplement in acute manner with excellent results, though in a small sample. However, we do not know whether chronic use of this supplement would lead to the same results in our study population. Thus, we suggest that more research with different designs should be carried out in order to compare whether other models of peptide glutamine use might be more efficient in increasing the levels of anaerobic power among athletes. This field of research remains open and some of the limitations of this current study may be seen as fruitful avenues for future research on this topic.
CONCLUSION
It seems acute supplementation of glutamine and maltodextrin combination, 2 hours before exercises is more efficient in prevention of anaerobic power decrease than consumption of carbohydrate or glutamine alone in repeated bouts of RAST protocol. Thus, supplementation with both carbohydrate and peptide glutamine improved the physical performance of athletes during repeated competitions.
ACKNOWLEDGMENTS
The authors would like to thank the Ardabil Branch, Islamic Azad University officials for their kind cooperation. This study received specific grant from Ardabil Branch, Islamic Azad University.
Conflict of interests: None
REFERENCES
- 1.Fasina YO, Bowers JB, Hess JB, McKee SR. Effect of dietary glutamine supplementation on Salmonella colonization in the ceca of young broiler chicks. Poult Sci. 2010;89:1042–1048. doi: 10.3382/ps.2009-00415. [DOI] [PubMed] [Google Scholar]
- 2.Antonio J, Street C. Glutamine: A potentially useful supplement for athletes. Can J Appl Physiol. 1999;24:1–14. doi: 10.1139/h99-001. [DOI] [PubMed] [Google Scholar]
- 3.Kreider R. Dietary supplements and the promotion of muscle growth with resistance exercise. Sports Med. 1999;27:97–110. doi: 10.2165/00007256-199927020-00003. [DOI] [PubMed] [Google Scholar]
- 4.Agostini F, Biolo G. Effect of physical activity on glutamine metabolism. Curr Opin Clin Nutr Metab Care. 2010;13:58–64. doi: 10.1097/MCO.0b013e328332f946. [DOI] [PubMed] [Google Scholar]
- 5.Phillips GC. Glutamine: the nonessential amino acid for performance enhancement. Curr Sports Med Rep. 2007;6:265–8. doi: 10.1007/s11932-007-0043-6. [DOI] [PubMed] [Google Scholar]
- 6.Coyle EF. New dimensions in carbohydrates. Washington, DC: A symposium sponsored by the American Society for Clinical Nutrition, Inc., and the Sugar Association; 1993. Dec, Substrate utilization in active persons. [Google Scholar]
- 7.Ruffo AM, Osiecki R, Fernandes LC, et al. Moderate to High Dose of Maltodextrin Before Exercise Improves Glycogen Availability in Soleus and Liver After Prolonged Swimming in Rats. J Exerc Physiol online. 2009;12:30–8. [Google Scholar]
- 8.Owen MD, Kregel KC, Wall PT, Gisolf CV. Effects of carbohydrate ingestion on thermoregulation, gastric emptying, and plasma volume during exercise in the heat. Med Sci Sports Exerc. 1986;18:568–75. [PubMed] [Google Scholar]
- 9.Ryan AJ, Bleider TL, Carter JE, Gisolf CV. Gastric Effects of carbohydrate ingestion on thermoregulation, gastric emptying, and plasma volume during exercise in the heat. Med Sci Sports Exerc. 1989;21:52–8. [Google Scholar]
- 10.Tsintzas K, Williams C, Constantin-Teodosiu D, et al. Phosphocreatine degradation in type I and II muscle fibres during submaximal exercise in man: effect of carbohydrate ingestion. J Physiol. 2001;537:305–11. doi: 10.1111/j.1469-7793.2001.0305k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wasserman K, Hansen J, Sue DY, et al. Principals of exercise testing and interpretation. 3rd ed. Philadelphia: Lippincott, Williams & wilkins; 1999. [Google Scholar]
- 12.Diboll DC, Moffit JK. A comparison of bioelectrical impedance and NEAR-Infrared Interact ance to skinfold measure in determining minimum wrestling weight in collegiate wrestlers. Int Electron J. 2003;6:26–36. [Google Scholar]
- 13.Zacharogiannis E, Paradisis G, Tziortzis S. An Evaluation of Tests of Anaerobic Power and Capacity. Med Sci Sports Exerc. 2004;36:116. [Google Scholar]
- 14.Piattolly T, Welsch MA. L-glutamine supplementation: Effects on recovery from exercise. Med Sci Sports Exerc. 2004;36:853. [Abstract] [Google Scholar]
- 15.Alberici JC, Farrell PA, Kris-Etherton PM, Shively CA. Effects of preexercise candy bar ingestion on glycemic response, substrate utilisation, and performance. IntJ Sports Nutr. 1993;3:323–33. doi: 10.1123/ijsn.3.3.323. [DOI] [PubMed] [Google Scholar]
- 16.Coggan AR, Coyle EF. Reversal of fatigue during prolonged exercise by carbohydrate infusion or ingestion. J Appl Physiol. 1987;63:2388–95. doi: 10.1152/jappl.1987.63.6.2388. [DOI] [PubMed] [Google Scholar]
- 17.Ivy JL, Costill DL, Fink WJ, Lower RW. Influence of caffeine and carbohydrate feedings on endurance performance. Med Sci Sports. 1979;11:6–11. [PubMed] [Google Scholar]
- 18.Neufer PD, Costill DL, Flynn MG, et al. Improvements in exercise performance:Effects of carbohydrate feedings and diet. J Appl Physiol. 1987;62:983–8. doi: 10.1152/jappl.1987.62.3.983. [DOI] [PubMed] [Google Scholar]
- 19.Wright DA, Sherman WM, Dernbach AR. Carbohydrate feedings before, during, or in combination improve cycling endurance performance. J Appl Physiol. 1991;71:1082–8. doi: 10.1152/jappl.1991.71.3.1082. [DOI] [PubMed] [Google Scholar]
- 20.Hargreaves M, Costill DL, Fink WJ, et al. Effect of pre-exercise carbohydrate feedings on endurance cycling performance. Med Sci Sports Exert. 1987;19:33–6. [PubMed] [Google Scholar]
- 21.Coyle EF, Coggan AR, Hemmert MK, et al. Substrate usage during prolonged exercise following a pre-exercise meal. J Appl Physiol. 1985;59:429–33. doi: 10.1152/jappl.1985.59.2.429. [DOI] [PubMed] [Google Scholar]
- 22.Leese GP, Bowtell J, Mudambo S, et al. Post-exercise gastric emptying of carbohydrate solutions determined using the “C acetate breath test. Eur J Appl Physiol. 1995;71:306–10. doi: 10.1007/BF00240409. [DOI] [PubMed] [Google Scholar]
- 23.Bowtell JL, Gelly K, Jackman ML, et al. Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. J Appl Physiol. 1999;86:1770–7. doi: 10.1152/jappl.1999.86.6.1770. [DOI] [PubMed] [Google Scholar]
- 24.Meijer AJ, Baquet A, Gustafson L, et al. Mechanism of activation of liver glycogen synthase by swelling. J Biol Chem. 1992;267:5823–8. [PubMed] [Google Scholar]
- 25.Ahmed A, Maxwell DL, Taylor PM, Rennie MJ. Glutamine transport in human skeletal muscle. Am J Physiol. 1993;264:993–1000. doi: 10.1152/ajpendo.1993.264.6.E993. [DOI] [PubMed] [Google Scholar]
- 26.Low SY, Rennie MJ, Taylor PM. Modulation of glycogen synthesis in rat skeletal muscle by changes in cell volume. J Physiol. 1996;495:299–303. doi: 10.1113/jphysiol.1996.sp021594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Varnier M, Leese GP, Thompson J, Rennie MJ. Stimulatory effect of glutamine on glycogen accumulation in human skeletal muscle. Am J Physiol. 1995;269:E309–15. doi: 10.1152/ajpendo.1995.269.2.E309. [DOI] [PubMed] [Google Scholar]
- 28.Peres FP. Efeitos da suplementação da glutaminapeptídeo e carboidratosna performance de triatletas de alto nível; Piracicaba, SP: Dissertação de mestrado; 2004. p. 71. [Google Scholar]
- 29.Favano A, Santos-Silva PR, Nakano EY, et al. Peptide glutamine supplementation for tolerance of intermittent exercise in soccer players. Clinics. 2008;63:27–32. doi: 10.1590/s1807-59322008000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zawadzki KM, Yaspelkis BB, Ivy JL. Carbohydrate protein complex increases the rate of muscle glycogen storage after exercise. J Appl Physiol. 1992;72:1854–9. doi: 10.1152/jappl.1992.72.5.1854. [DOI] [PubMed] [Google Scholar]