Abstract
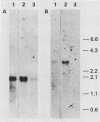
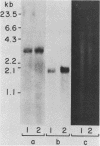
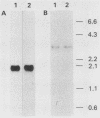
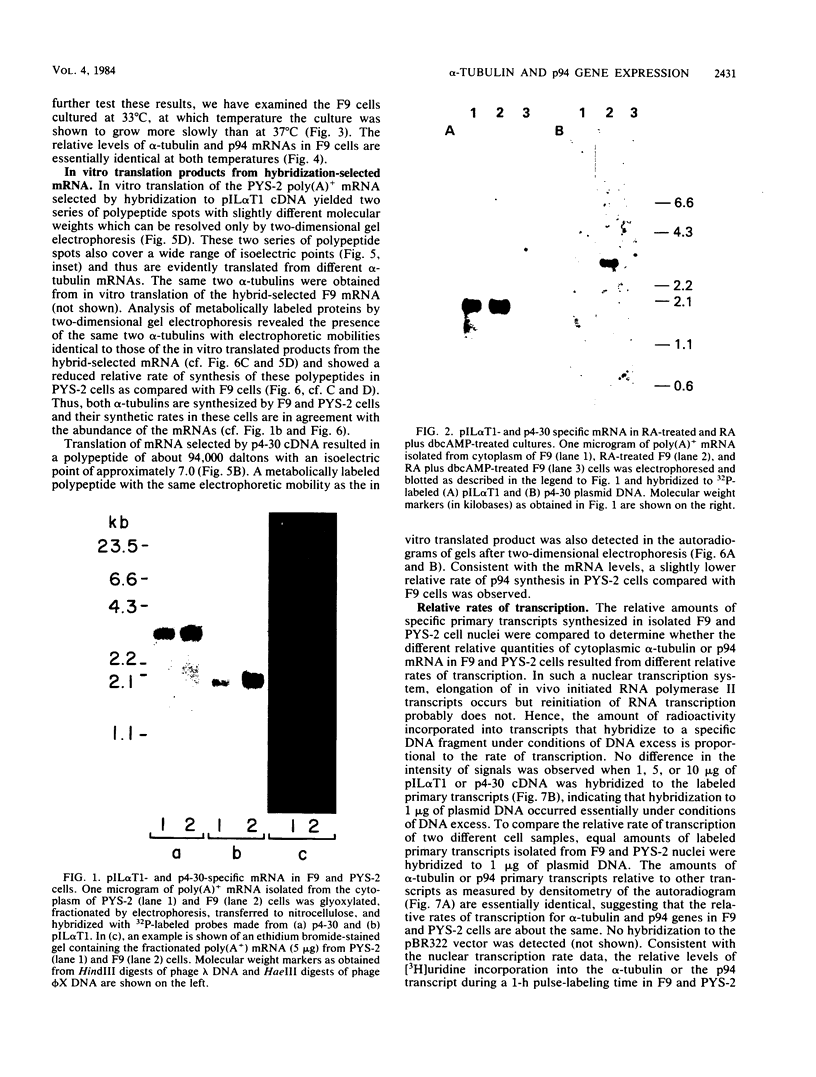
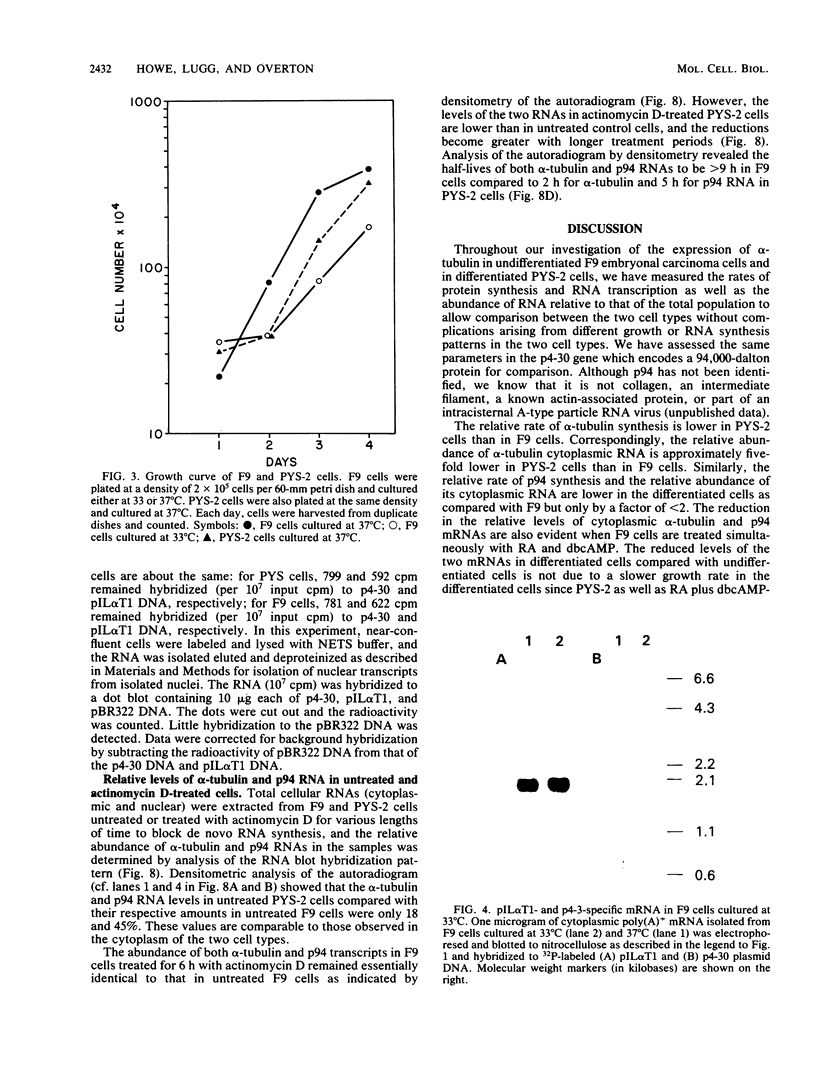
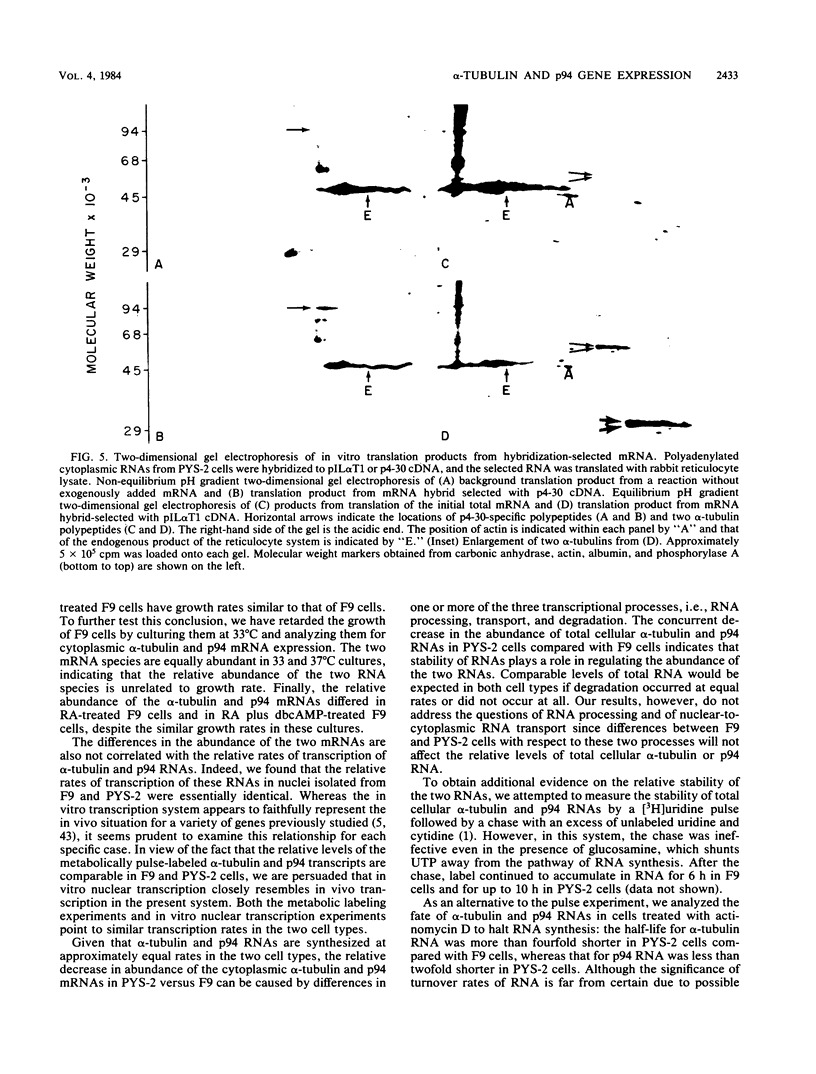
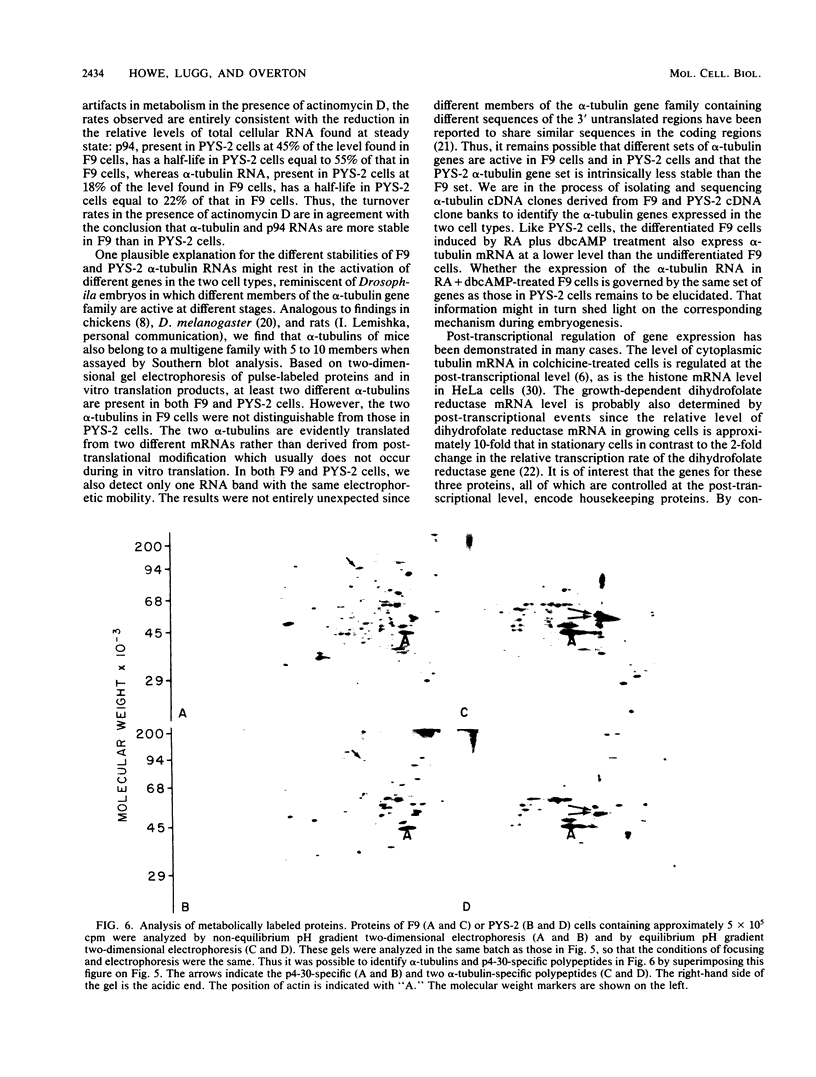
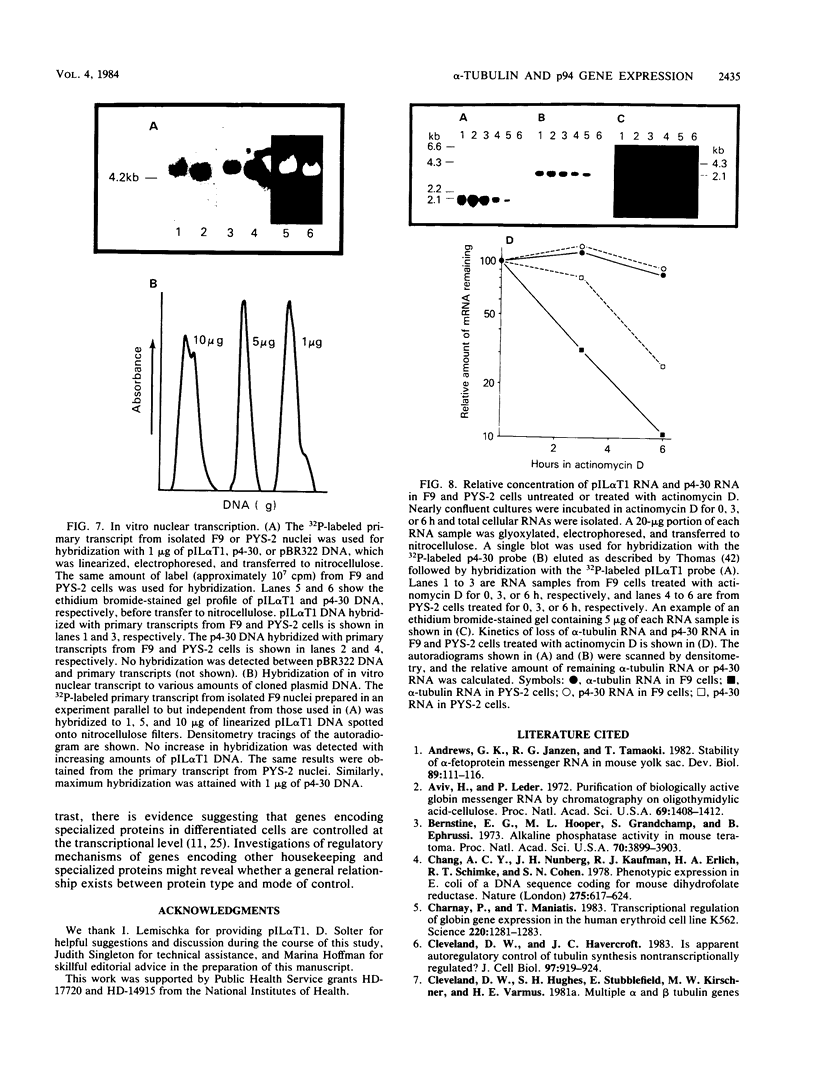
Changes in the expression of the genes encoding alpha-tubulin and a 94,000-dalton protein (p94) specified by a cDNA clone, p4-30, were examined in a differentiated teratocarcinoma-derived parietal endoderm cell line, PYS-2, and an undifferentiated teratocarcinoma stem cell line, F9. Relative to other proteins or mRNA species, the synthesis rate of the alpha-tubulins and of p94, as well as the levels of their corresponding cytoplasmic mRNAs, were lower in PYS-2 than in F9 cells. The decrease was greater for the relative abundance of cytoplasmic alpha-tubulin mRNA than for p94 mRNA. Similarly, induction of differentiation of F9 cells by simultaneous exposure to retinoic acid (RA) and dibutyryl cyclic AMP resulted in reduced relative levels of the cytoplasmic mRNAs for these proteins. The reduction in abundance of the two RNA species was not due to a decrease in growth rate since the differentiated cells, PYS-2, RA-treated F9, and RA plus dibutyryl cyclic AMP-treated F9 cells, grew at a rate similar to that of undifferentiated F9 cells. However, induction of differentiation of F9 cells by treatment with RA alone did not cause down-regulation of the two RNA species. The relative levels of total cellular RNA encoding alpha-tubulin and p94 in PYS-2 cells were also lower than those in F9 cells to an extent comparable to the decrease in the cytoplasmic RNAs. Since the apparent relative rates of RNA transcription were similar in both cell types, we conclude that the reduction in relative levels of the alpha-tubulin and p94 RNAs in the cell depends largely on the relative stability of the two RNAs and not on the relative rates of transcription. The faster disappearance of the two RNA species relative to other cellular RNAs from actinomycin D-treated PYS-2 compared with F9 cells is consistent with this interpretation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews G. K., Janzen R. G., Tamaoki T. Stability of alpha-fetoprotein messenger RNA in mouse yolk sac. Dev Biol. 1982 Jan;89(1):111–116. doi: 10.1016/0012-1606(82)90299-8. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berstine E. G., Hooper M. L., Grandchamp S., Ephrussi B. Alkaline phosphatase activity in mouse teratoma. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3899–3903. doi: 10.1073/pnas.70.12.3899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Nunberg J. H., Kaufman R. J., Erlich H. A., Schimke R. T., Cohen S. N. Phenotypic expression in E. coli of a DNA sequence coding for mouse dihydrofolate reductase. Nature. 1978 Oct 19;275(5681):617–624. doi: 10.1038/275617a0. [DOI] [PubMed] [Google Scholar]
- Charnay P., Maniatis T. Transcriptional regulation of globin gene expression in the human erythroid cell line K562. Science. 1983 Jun 17;220(4603):1281–1283. doi: 10.1126/science.6574602. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Havercroft J. C. Is apparent autoregulatory control of tubulin synthesis nontranscriptionally regulated? J Cell Biol. 1983 Sep;97(3):919–924. doi: 10.1083/jcb.97.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. Unpolymerized tubulin modulates the level of tubulin mRNAs. Cell. 1981 Aug;25(2):537–546. doi: 10.1016/0092-8674(81)90072-6. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Wilde C. D., Chow L. T., Wefald F. C. Structural variation among human beta-tubulin genes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4877–4881. doi: 10.1073/pnas.78.8.4877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Getz M. J., Birnie G. D., Young B. D., MacPhail E., Paul J. A kinetic estimation of base sequence complexity of nuclear poly(A)-containing RNA in mouse Friend cells. Cell. 1975 Feb;4(2):121–129. doi: 10.1016/0092-8674(75)90118-x. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan B. L. High molecular weight extracellular proteins synthesized by endoderm cells derived from mouse teratocarcinoma cells and normal extraembryonic membranes. Dev Biol. 1980 May;76(2):275–285. doi: 10.1016/0012-1606(80)90379-6. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Gmür R., Solter D. Cytoplasmic and nuclear protein synthesis during in vitro differentiation of murine ICM and embryonal carcinoma cells. Dev Biol. 1980 Feb;74(2):351–363. doi: 10.1016/0012-1606(80)90437-6. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Solter D. Changes in cell surface proteins during differentiation of mouse embryonal carcinoma cells. Dev Biol. 1981 May;84(1):239–243. doi: 10.1016/0012-1606(81)90390-0. [DOI] [PubMed] [Google Scholar]
- Howe C. C., Solter D. Cytoplasmic and nuclear protein synthesis in preimplantation mouse embryos. J Embryol Exp Morphol. 1979 Aug;52:209–225. [PubMed] [Google Scholar]
- Howe C. C., Solter D. Identification of noncollagenous basement membrane glycopolypeptides synthesized by mouse parietal entoderm and an entodermal cell line. Dev Biol. 1980 Jun 15;77(2):480–487. doi: 10.1016/0012-1606(80)90489-3. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. Developmental regulation of Drosophila alpha-tubulin genes. Cell. 1982 May;29(1):91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. alpha-Tubulin genes of Drosophila. Cell. 1981 Apr;24(1):97–106. doi: 10.1016/0092-8674(81)90505-5. [DOI] [PubMed] [Google Scholar]
- Kaufman R. J., Sharp P. A. Growth-dependent expression of dihydrofolate reductase mRNA from modular cDNA genes. Mol Cell Biol. 1983 Sep;3(9):1598–1608. doi: 10.1128/mcb.3.9.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehman J. M., Speers W. C., Swartzendruber D. E., Pierce G. B. Neoplastic differentiation: characteristics of cell lines derived from a murine teratocarcinoma. J Cell Physiol. 1974 Aug;84(1):13–27. doi: 10.1002/jcp.1040840103. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., van deSande H. Chain length determination of small double- and single-stranded DNA molecules by polyacrylamide gel electrophoresis. Biochemistry. 1975 Aug 26;14(17):3787–3794. doi: 10.1021/bi00688a010. [DOI] [PubMed] [Google Scholar]
- Melli M., Spinelli G., Arnold E. Synthesis of histone messenger RNA of HeLa cells during the cell cycle. Cell. 1977 Sep;12(1):167–174. doi: 10.1016/0092-8674(77)90194-5. [DOI] [PubMed] [Google Scholar]
- Mischke D., Pardue M. L. Organization and expression of alpha-tubulin genes in Drosophila melanogaster. One member of the alpha-tubulin multigene family is transcribed in both oogenesis and later embryonic development. J Mol Biol. 1982 Apr 15;156(3):449–466. doi: 10.1016/0022-2836(82)90260-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Peterson J. L., McConkey E. H. Non-histone chromosomal proteins from HeLa cells. A survey by high resolution, two-dimensional electrophoresis. J Biol Chem. 1976 Jan 25;251(2):548–554. [PubMed] [Google Scholar]
- Raff E. C., Fuller M. T., Kaufman T. C., Kemphues K. J., Rudolph J. E., Raff R. A. Regulation of tubulin gene expression during embryogenesis in Drosophila melanogaster. Cell. 1982 Jan;28(1):33–40. doi: 10.1016/0092-8674(82)90372-5. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeburg P. H., Shine J., Martial J. A., Baxter J. D., Goodman H. M. Nucleotide sequence and amplification in bacteria of structural gene for rat growth hormone. Nature. 1977 Dec 8;270(5637):486–494. doi: 10.1038/270486a0. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan M., Burke M. The free heavy chain of vertebrate skeletal myosin subfragment 1 shows full enzymatic activity. J Biol Chem. 1982 Jan 25;257(2):1102–1105. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strickland S., Mahdavi V. The induction of differentiation in teratocarcinoma stem cells by retinoic acid. Cell. 1978 Oct;15(2):393–403. doi: 10.1016/0092-8674(78)90008-9. [DOI] [PubMed] [Google Scholar]
- Strickland S., Smith K. K., Marotti K. R. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980 Sep;21(2):347–355. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- Sánchez F., Natzle J. E., Cleveland D. W., Kirschner M. W., McCarthy B. J. A dispersed multigene family encoding tubulin in Drosophila melanogaster. Cell. 1980 Dec;22(3):845–854. doi: 10.1016/0092-8674(80)90561-9. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M., Belayew A. Transcriptional control of the murine albumin/alpha-fetoprotein locus during development. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5254–5257. doi: 10.1073/pnas.79.17.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida N., Kominami R., Hatanaka M., Uesugi S. Identification of unintegrated forms of Kirsten murine sarcoma viral DNA and restriction endonuclease cleavage map of linear DNA. J Virol. 1981 May;38(2):797–803. doi: 10.1128/jvi.38.2.797-803.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Wilde C. D., Chow L. T., Wefald F. C., Cowan N. J. Structure of two human alpha-tubulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(1):96–100. doi: 10.1073/pnas.79.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]