Abstract
Mecamylamine (3-methylaminoisocamphane hydrochloride) is a nicotinic parasympathetic ganglionic blocker, originally utilized as a therapeutic agent to treat hypertension. Mecamylamine administration produces several deleterious side-effects at therapeutically relevant doses. As such, mecamylamine’s use as an antihypertensive agent was phased out, except in severe hypertension. Mecamylamine easily traverses the blood-brain barrier to reach the central nervous system (CNS), where it acts as a nicotinic acetylcholine receptor (nAChR) antagonist, inhibiting all known nAChR subtypes. Since nAChRs play a major role in numerous physiological and pathological processes, it is not surprising that mecamylamine has been evaluated for its potential therapeutic effects in a wide variety of CNS disorders, including addiction. Importantly, mecamylamine produces its therapeutic effects on the CNS at doses 3-fold lower than those used to treat hypertension, which diminishes the probability of peripheral side-effects. This review focuses on the pharmacological properties of mecamylamine, the differential effects of its stereoisomers, S(+)- and R(−)-mecamylamine, and the potential for effectiveness in treating CNS disorders, including nicotine and alcohol addiction, mood disorders, cognitive impairment and attention deficit hyperactivity disorder.
Keywords: mecamylamine, nicotinic acetylcholine receptors, smoking cessation, alcoholism, depression, anxiety
1.0 INTRODUCTION
The cholinergic neurotransmitter system plays a diverse role in numerous physiological processes of the central nervous system (CNS), including arousal, sleep, pain and cognitive function (Gotti and Clementi, 2004; Hogg et al., 2003), as well as in pathological conditions, including Alzheimer’s disease, Parkinson’s disease, depression and addiction (Newhouse et al., 1997; Picciotto and Zoli, 2008; Shytle et al., 2002e). Cholinergic signals are recognized and mediated by two pharmacologically distinct receptor classes: muscarinic and nicotinic acetylcholine receptors (nAChRs). Muscarinic receptors mediate numerous central and peripheral nervous system functions (Eglen, 2005), the manipulation of which can lead to severe side-effects. As a consequence, effort has focused on the discovery of both novel agonists and antagonists as therapeutic agents which target nAChRs (Arneric et al., 2007; Bencherif and Schmitt, 2002; Dwoskin and Bardo, 2009; Dwoskin and Crooks, 2001; Gotti et al., 2006; Levin and Rezvani, 2000).
One of the first widely-used therapeutic agents targeting nAChRs was the noncompetitive antagonist mecamylamine (Banerjee et al., 1990; Martin et al., 1989). Mecamylamine was introduced originally by Merck & Co., Inc. as an antihypertensive agent (Stone et al., 1956). Although similar to the ganglionic blocker and quaternary ammonium compound hexamethonium, mecamylamine, a secondary amine, is unique among antihypertensive agents. Mecamylamine is rapidly and completely absorbed from the gastrointestinal tract, and has both a fast onset (37 min) and a relatively long duration of action (22 hr; Ford et al., 1956). Unfortunately, mecamylamine lacks nAChR subtype selectivity, and thus exerts antagonist activity at parasympathetic ganglionic receptors, which at therapeutic doses results in undesirable side-effects, including constipation, urinary retention and dry mouth and skin (Shytle et al., 2002a). However, unlike other ganglionic blockers, mecamylamine easily traverses the blood-brain barrier, allowing inhibition of nAChRs in the CNS (Martin et al., 1989; Suchocki et al., 1991). More recent studies report that central effects of mecamylamine are obtained at 3-fold lower doses than those used to treat hypertension (2.5–10 mg/day versus 30–90 mg/day), resulting in fewer and more manageable peripheral side-effects (Shytle et al., 2002a, d, e). Since nAChRs play a major role in numerous physiological and pathological processes, it is not surprising that mecamylamine has been evaluated as a potential therapeutic agent for a wide variety of disorders that affect the CNS.
Targacept, Inc., which secured the intellectual property, regulatory documentation, contracts and inventory related to mecamylamine from Layton Biosciences, Inc., received approval from the United States Food and Drug Administration (FDA) to test the effectiveness of mecamylamine in treating Tourette’s syndrome, a neurological disorder characterized by involuntary movements (Sanberg et al., 2000). In 2002, Targacept, Inc. obtained use patents for the pure stereoisomers, S(+)- and R(−)-mecamylamine, for the treatment of a wide range of clinical conditions, including addiction, schizophrenia, hypertension and cancer (Shytle et al., 2002b, c). Additional use patents were secured for mecamylamine (racemic and stereoisomeric forms) to treat depression, bipolar disorder, obsessive compulsive disorder, attention deficit hyperactivity disorder (ADHD) and Alzheimer’s disease (Sanberg et al., 2005). While to date the only FDA-approved use of mecamylamine is for “the management of moderately severe hypertension and uncomplicated cases of malignant hypertension”, these patents have generated renewed excitement and investment in mecamylamine as a potential therapeutic for numerous CNS disorders.
In this review, we present an overview of the proposed therapeutic uses for mecamylamine, and both positive and negative results from preclinical and clinical studies. Also, results herein show an interaction between mecamylamine and nicotine, which likely will have an important impact on the development of mecamylamine in terms of doses employed and the conditions necessary to reveal efficacy. Future clinical studies should consider tobacco smoking status when selecting mecamylamine doses to be employed to obtain maximal efficacy for its proposed therapeutic uses.
2.0 NEUROPHARMACOLOGY
2.1 Mechanism of Action
Mecamylamine (3-ethylaminoisocamphane hydrochloride; Fig. 1, left) is a secondary amine that acts as a noncompetitive antagonist at all known nAChR subtypes (Connolly et al., 1992; Varanda et al., 1985). Interestingly, early studies reported that mecamylamine-induced inhibition of parasympathetic neurons from rat submandibular ganglia was the result of a competitive mechanism of action (Ascher et al., 1979; Gurney and Rang, 1984). However, mecamylamine inhibition of agonist-induced receptor activation could not be fully reversed, even after a 60-min washout, suggesting noncompetitive antagonism. In later studies utilizing cultures of parasympathetic neurons and medullary chromaffin cells, mecamylamine was shown to inhibit acetylcholine-evoked currents in a voltage-dependent manner, supporting a noncompetitive channel-blocking mechanism of action (Fieber and Adams, 1991; Nooney et al., 1992). Studies using cell expression systems, such as Xenopus oocytes expressing either rat or human nAChR subtypes, have demonstrated that mecamylamine inhibits both neuromuscular and neuronal nAChRs (Chavez-Noriega et al., 1997; Francis and Papke, 1996; Luetje and Patrick, 1991; Papke et al., 2008), providing additional evidence of its nonselective nAChR antagonist action.
Fig. 1.
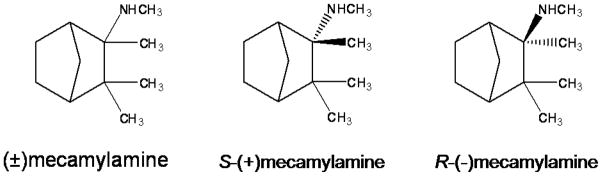
Chemical structures of racemic mecamylamine, S(+)-mecamylamine and R(−)-mecamylamine.
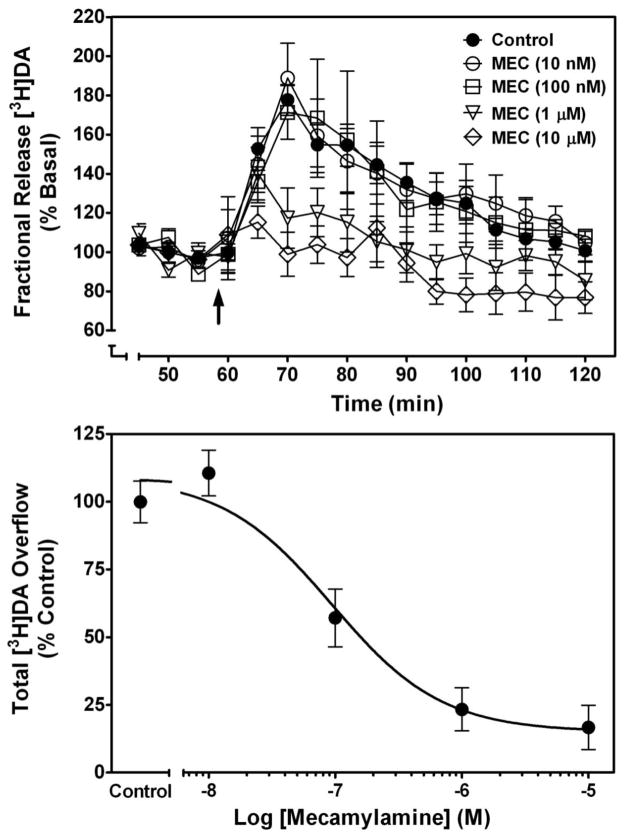
The mechanism of action of mecamylamine in the CNS has been evaluated in our laboratory by determining its ability to inhibit nicotine-evoked [3H]dopamine (DA) release from superfused rat striatal slices (Fig. 2). Using a previously published method (Grinevich et al., 2003), rat striatal slices were incubated with 0.1 μM [3H]DA (final concentration; dihydroxyphenylethylamine, 3, 4-[7-3H]-; specific activity 28.0 Ci/mmol; Perkin Elmer Life and Analytical Sciences, Inc; Boston, MA) for 30 min and then superfused (1 ml/min) for 60 min with Krebs’ buffer to establish a stable basal DA release rate. The superfusion buffer contained nomifensine (10 μM), a DA transporter inhibitor, and pargyline (10 μM), a monoamine oxidase inhibitor, to prevent reuptake and metabolism of DA, respectively, and to ensure that the [3H] collected in superfusate primarily represented parent neurotransmitter. Fractional release was calculated by dividing the amount of [3H] in each 5-min sample by the total tissue-[3H] at the time of sample collection. Basal [3H]DA outflow was calculated as the average fractional release in the two samples prior to addition of mecamylamine to the superfusion buffer. Total [3H]DA overflow was calculated by summing the increases in fractional release above basal release in response to nicotine in the absence and presence of mecamylamine.
Fig. 2. Mecamylamine inhibits nicotine-evoked [3H]DA overflow from superfused rat striatal slices in a concentration-dependent manner.
Time course (top) of mecamylamine-induced inhibition of nicotine-evoked [3H]DA overflow. Arrow indicates time point at which nicotine was added to the superfusion buffer. Data are expressed as fractional release as a percentage of basal (mean ± SEM); n = 6 rats. Mean basal [3H]outflow was 0.75 ± 0.02 fractional release as percentage of tissue [3H] content. Time course data were used to generate [3H]DA overflow data (bottom). Control represents [3H]DA overflow in response to 10 μM nicotine (total [3H]DA overflow as a percentage of tissue [3H]-content, mean ± S.E.M.). Control response to nicotine in the absence of antagonist was 3.06 ± 0.24 [3H]DA overflow. Concentration-response curves were generated using nonlinear regression. Data are expressed as percentage of control; n = 5/group.
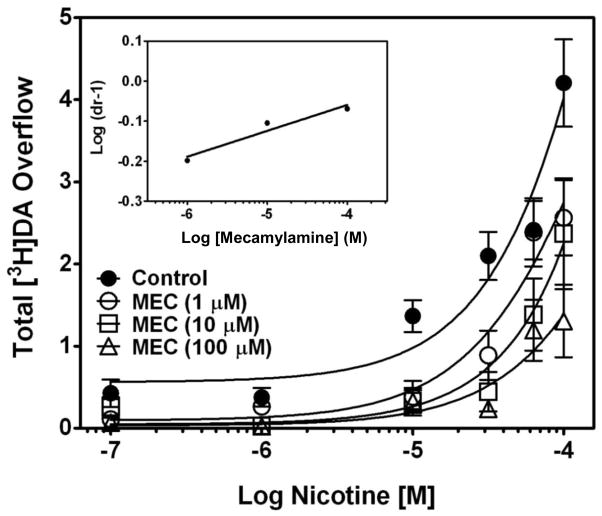
Using these methods, the ability of mecamylamine to inhibit nicotine-evoked [3H]DA release from striatal slices was determined across a range of mecamylamine concentrations (10 nM–10 μM). Nicotine (10 μM) was added to the buffer 45 min after mecamylamine, and superfusion continued for an additional 65 min. Peak fractional [3H]DA release was observed 12 min following the addition of nicotine to the buffer, and [3H]DA levels gradually returned to basal levels despite the constant presence of nicotine (Fig. 2, top). Mecamylamine inhibited nicotine-evoked [3H]DA release in a concentration-dependent manner, with maximum inhibition achieved at 10 μM. A two-way repeated measures analysis of variance (ANOVA) with time and concentration as within-subject factors revealed main effects of time (F15,330 = 24.0, p < 0.0001) and concentration (F4,22 = 1100, p < 0.0001), and a time x concentration interaction (F60,330 = 2.48, p < 0.0001). Nonlinear regression revealed a sigmoidal function for mecamylamine inhibition with an IC50 = 0.12 ± 0.04 μM and Imax = 85 ± 4% (Fig. 2, bottom). Analysis of total [3H]DA overflow using a one-way ANOVA with concentration as a within-subject factor revealed a concentration-dependent effect of mecamylamine (F4,12 = 24.1, p < 0.0001). Furthermore, Schild analysis was performed to evaluate the mechanism by which mecamylamine inhibited nicotine-evoked [3H]DA overflow. Concentration-response for nicotine (0.1–100 μM) was determined in the absence and presence of a single concentration of mecamylamine (1, 10, or 100 μM). For each mecamylamine concentration, the dose ratio (dr) was calculated as that producing an equivalent response to nicotine in the absence and presence of mecamylamine. The log(dr–1) was plotted as a function of log mecamylamine concentration to provide the Schild regression. Results indicated that mecamylamine produced a rightward and downward shift of the nicotine concentration-response curve, which was not surmounted by increasing nicotine concentrations (Fig. 3). Further, linear regression generated by the Schild analysis (Fig. 3, inset) revealed a slope (slope = 0.07 ± 0.02; r2 = 0.94) different from unity (t28 = 29.1; p < 0.001), consistent with noncompetitive inhibition (Arunlakshana and Schild, 1959; Kenakin and Boselli, 1989). These findings indicate that mecamylamine inhibits nicotine-evoked [3H]DA release from striatal slices via a noncompetitive mechanism of action.
Fig. 3. Schild analysis of mecamylamine inhibition of nicotine-evoked [3H]DA overflow from superfused rat striatal slices.
After collection of the third sample, slices were superfused with buffer in the absence or presence of mecamylamine (1, 10, 100 μM) for 45 min before the addition of nicotine (0.1–100 μM) to the buffer, and superfusion continued for an additional 45 min. For each nicotine concentration, control response is that for nicotine in the absence of mecamylamine. Control represents [3H]DA overflow in response to nicotine alone (total [3H]DA overflow as a percentage of tissue [3H]-content, mean ± S.E.M.); n = 5 rats/mecamylamine concentration; control, n = 12 rats (mecamylamine was between-groups factor, control was contemporaneous with each mecamylamine concentration). Concentration-response curves were generated by nonlinear regression. Inset shows the Schild regression in which the log of dr–1 was plotted as a function of log of mecamylamine concentration, and data were fit by linear regression.
Others have shown that mecamylamine binds to a site within the nAChR channel pore, leading to a shortened duration of the open-channel state (Martin et al., 1990; Nelson and Lindstrom, 1999; Peng et al., 1994; Shen and Horn, 1998). However, a simple channel blocking mechanism is not sufficient to explain mecamylamine-induced inhibition. As such, a “trapping” mechanism for mecamylamine has been described (Lingle, 1983; Ostroumov et al., 2008), whereby mecamylamine binds to a site located deep within the channel pore, interfering with the ability of cations to permeate the channel, but not preventing the channel from closing once the agonist is removed. As a result, mecamylamine is trapped within the channel. In order for mecamylamine to exit the channel, the agonist must bind to the agonist recognition site in an orthosteric manner to produce a conformational change, returning the receptor to the open channel state (Gurney and Rang, 1984; Lingle, 1983; Skorinkin et al., 2004). A caveat of these studies is that they were performed in either non-neuronal (chromaffin cells) or neuromuscular models, and the interpretation of the results extrapolated to suggest a trapping mechanism for mecamylamine at nAChRs in the CNS.
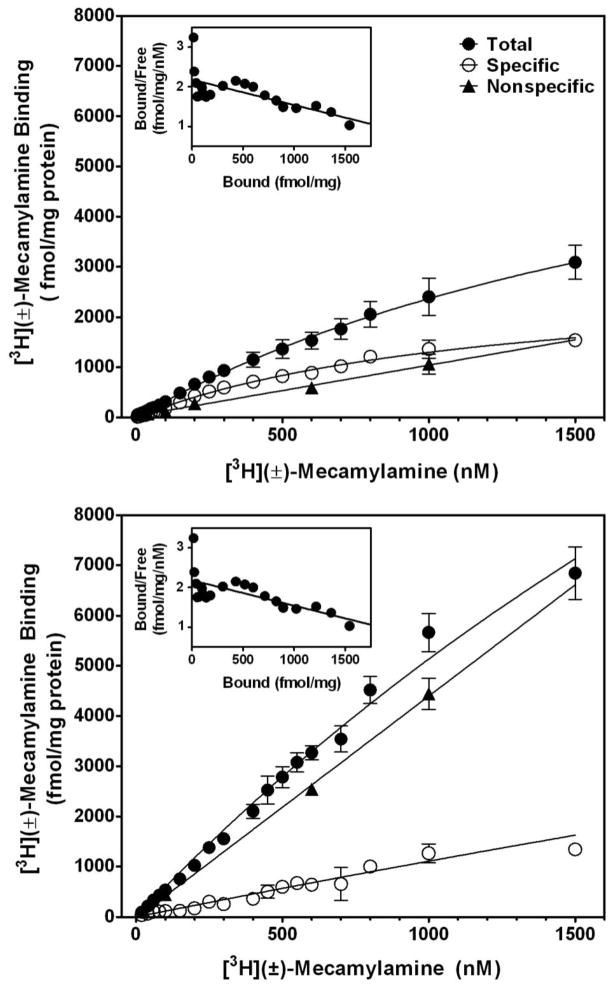
Recent in vitro work from our laboratory determining [3H]-mecamylamine binding to nAChRs expressed in rat brain membranes supports the hypothesis that mecamylamine is trapped within the pore of CNS nAChRs. Using a 20-point assay, saturation analysis of [3H]-mecamylamine binding was performed to determine the maximum number of [3H]-mecamylamine binding sites (Bmax), affinity (Kd), and whether the ligand binds to one or more sites (Fig. 4). Rat brain membranes were prepared from whole brain and suspended in 4.8 ml of incubation buffer, providing a protein concentration of 140–160 μg in 100 μl. For the 20-point saturation assay, two stock solutions of [3H]-mecamylamine (specific activities of 32.0 and 4.51 Ci/mmol; Perkin Elmer Life and Analytical Sciences, Inc; Boston, MA) were used to afford radioligand concentration ranges of 5–100 nM and 150–1500 nM, respectively. Samples were prepared by adding a single concentration of [3H]-mecamylamine in 50 μl of buffer, 50 μl of 2.5 mg/ml bovine serum albumin (BSA), 100 μl of membrane suspension into polypropylene tubes, and 50 μl of buffer, for a total volume of 250 μl. Nonspecific binding was determined using 500 μM cold mecamylamine. Samples were incubated at room temperature for 30 min, and incubation stopped by addition of 3 ml of ice-cold incubation buffer followed by immediate filtration through S&S glass fiber filters (grade #32; presoaked overnight in 0.5% polyethylenimine) using a cell harvester. Filters were rinsed 3 times with 3 ml of ice-cold buffer and transferred to scintillation vials, 4 ml scintillation cocktail was added, and radioactivity determined by liquid scintillation spectroscopy. Protein was measured using the Bradford dye-binding procedure (Bradford, 1976), with BSA as the standard. [3H]-Mecamylamine concentrations of up to 1500 nM were required to observe saturation (Fig. 4, top panel). Nonlinear regression revealed a Kd value of 1.27 ± 0.18 μM and a Bmax value of 2.92 ± 0.93 pM/mg protein. Data did not fit a two-site model (F2,56 = 0.1394; p = 0.87). Scatchard transformation suggested the presence of two binding sites (Fig. 4, top panel inset); however, nonlinear regression prohibited delineation of two linear representations of the Scatchard plot.
Fig. 4. Saturation analysis of racemic [3H]mecamylamine binding to rat brain membranes in the absence (top) and presence of 1 mM S(−)nicotine (bottom).
Saturation analysis was determined using a concentration range of 20–1500 nM of racemic [3H]-mecamylamine in both the absence (top) and presence of nicotine (1 mM; bottom). Nonspecific binding was measured with 100 μM (±)-mecamylamine. Data are expressed as fmol/mg protein and represent the mean ± S.E.M. of three independent experiments. Curves were generated using nonlinear regression for a one-site model. The inset illustrates the Scatchard transformation of the specific binding data.
Effects of nicotine on [3H]-mecamylamine binding were determined. Binding assays were conducted using 100 nM [3H]-mecamylamine in the presence of a range of nicotine concentrations (0.01 nM–1.0 mM). Only the highest S(−)nicotine concentration (1 mM) significantly increased the amount of specific [3H]-mecamylamine binding from 117 ± 0.51 to 306 ± 39.7 fmol/mg protein (p < 0.001; not shown). Saturation analysis of [3H]-mecamylamine binding to rat brain membranes in the presence of 1 mM nicotine revealed ~2-fold increase in total [3H]-mecamylamine binding (Fig. 4, bottom panel). Insufficient saturation of total [3H]-mecamylamine binding precluded delineation of accurate values for Bmax and Kd. However, the increase in [3H]-mecamylamine binding in the presence of nicotine provides evidence supporting binding within the nAChR channel, as channel opening increases in response to agonist. Thus, these findings support the hypothesis that mecamylamine binds within nAChR channels in the open conformational state, a prerequisite for trapping to occur.
2.2 Mecamylamine Stereoisomers
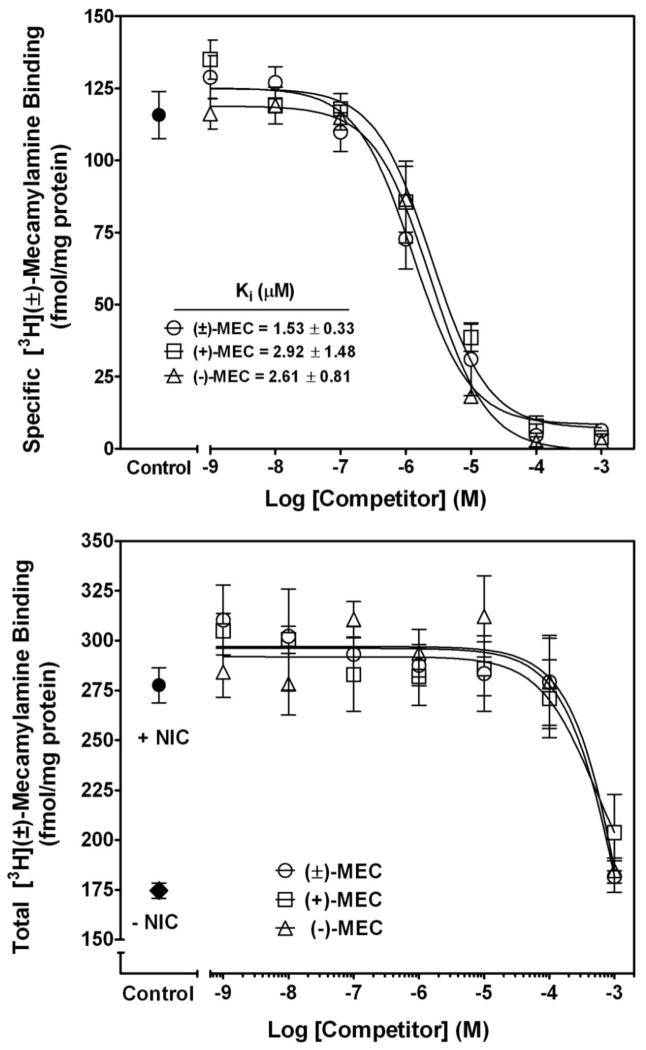
Over the past several decades, stereoselectivity has become an increasingly important consideration in the discovery and development of new therapeutic agents, largely due to improvements in stereoselective methods of synthesis and analyses (Jamali et al., 1989; Sastry, 1973; Smith, 1989; Waldeck, 2003), and to encouragement by the FDA with respect to new drug applications (De Camp, 1989). As a result, numerous racemic drugs, including mecamylamine, which previously received FDA approval, are being re-evaluated to determine potential benefits of the pure stereoisomers (Waldeck, 2003). Examination of the pharmacological properties of the mecamylamine stereoisomers (Fig. 1) began soon after the racemate was developed (Stone et al., 1962). Suchocki and colleagues (1991), using a mouse model, found no difference in potency between racemic mecamylamine, S(+)-mecamylamine or R(−)-mecamylamine with respect to inhibition of nicotine-induced decreases in spontaneous activity and antinociceptive effects. Similarly, no significant differences were observed between S(+)-mecamylamine and R(−)-mecamylamine inhibition of acetylcholine (ACh)-evoked currents in human α3β4, α4β2, α3β2 and α7nAChRs expressed in Xenopus oocytes, although S(+)-mecamylamine exhibited a significantly slower off-rate at α4β2 and α3β4 nAChRs (Papke et al., 2001). Ability of the racemate, S(+)-mecamylamine and R(−)-mecamylamine (1 nM–1 mM) to inhibit [3H]-mecamylamine binding to rat whole brain membranes revealed no differences in affinity (Ki = 1.53 ± 0.33 μM, 2.92 ± 1.48 μM and 2.61 ± 0.81 μM, respectively; Fig. 5, top panel). Ability of nicotine (1 mM) to alter S-(+)- and R-(−)mecamylamine inhibition of [3H]-mecamylamine binding revealed no differences between the stereoisomers and the racemate (Fig. 5, bottom panel). Consistent with our previous findings, stereoisomer concentrations required to observe inhibition of [3H]-mecamylamine binding were 3-orders of magnitude higher in the presence than in the absence of nicotine (compare Fig. 5 top and bottom panels). With respect to clinical relevance, these results suggest that the mecamylamine stereoisomers may have lower efficacy in smokers compared with non-smokers, and that higher doses of mecamylamine stereoisomers may be required to observe therapeutic efficacy. Unfortunately, as the dose is increased, the likelihood of nonspecific side-effects is elevated. Therefore, it may be important for clinical trials investigating benefit of mecamylamine stereoisomers to take into account smoking status, as this may impact the study outcome.
Fig. 5. High concentrations of mecamylamine stereoisomers are required to compete for racemic [3H]-mecamylamine binding to rat brain membranes in the presence of 1 mM S(−)nicotine.
Competitive binding assays were performed for racemic, S(+)- and R(−)-mecamylamine (1 nM - 1 mM) against 100 nM racemic [3H]-mecamylamine both in the absence (top) and presence of nicotine (1mM; bottom panel). Nonspecific binding was determined using 100 μM racemic mecamylamine. Data are expressed as fmol/mg protein and represent the mean ± SEM of three independent experiments. Curves were generated by nonlinear regression using a one-site model. Ki values are expressed as mean ± S.E.M. Ki values could not be calculated in the presence of nicotine.
In contrast to the previous research, stereoselective effects have been reported in other studies. S(+)-Mecamylamine was reported to inhibit low sensitivity α4β2 nAChRs with better efficacy than R(−)-mecamylamine, while acting as a positive allosteric modulator of high sensitivity α4β2 nAChRs (Fedorov et al., 2009). Low sensitivity α4β2 nAChRs have a stoichiometry of (α4)3(β2)2, lower affinity for nicotine (EC50 = 10 μM) and are highly permeable to calcium ions, whereas high sensitivity α4β2 nAChRs have a stoichiometry of (α4)2(β2)3, greater affinity for nicotine (EC50 = 1 μM), and have low calcium permeability (Tapia et al., 2007). Positive allosteric modulation of high sensitivity α4β2 nAChRs by S(+)-mecamylamine may be a contributing factor to the seemingly paradoxical procognitive effects of low-dose, racemic mecamylamine (see sections 3.8, 4.4), which is typically observed upon agonist activation of nAChRs. Interestingly, neuromuscular-type nAChRs appear to me more sensitive to R(−)-mecamylamine than to S(+)-mecamylamine (Papke et al., 2001), suggesting that S(+)-mecamylamine may be more effective at inhibiting neuronal nAChRs, and may be associated with a reduced side-effect profile.
3.0 PRECLINICAL BEHAVIORAL STUDIES
3.1 Nicotine Self-Administration
Nicotine, the major psychoactive compound in tobacco, is widely believed to play a primary role in maintaining smoking behavior (Rose, 2008). The use of mecamylamine in preclinical research has provided insights regarding the role of nAChRs in mediating the reinforcing properties of nicotine and the maintenance of smoking behavior (Katner et al., 2004; Lof et al., 2010; Newman et al., 2002; Palmatier et al., 2007; Phillips et al., 2007).
Numerous studies have shown that mecamylamine dose-dependently decreases intravenous nicotine self-administration (Le Foll and Goldberg, 2009, for review). Intravenous drug self-administration is the current gold-standard method to study the reinforcing properties of drugs. Self-administration involves implanting a catheter into the subject’s jugular vein for delivery of drug following response on the active lever in the operant chamber. Drugs that are positively reinforcing (e.g., nicotine) increase the probability of future responses on the active lever. Using a fixed ratio schedule, wherein a fixed number of responses are required to receive a nicotine infusion, pretreatment with mecamylamine decreases responding for nicotine in rats, mice, dogs and nonhuman primates (DeNoble and Mele, 2006; Fowler and Kenny, 2011; Glick et al., 1996; Martin-Garcia et al., 2009; Nakahara, 2004; Palmatier et al., 2007; Risner and Goldberg, 1983; Shoaib et al., 1997; Spealman and Goldberg, 1982; Stolerman et al., 1999). Mecamylamine also decreases the breakpoint obtained using progressive ratio schedules of nicotine reinforcement (Mansbach et al., 2000). The progressive ratio schedule requires the subject to increase the number of responses to some designated value to obtain each subsequent drug infusion (i.e., reinforcer). Furthermore, the progressive ratio schedule is used to determine the relative reinforcement efficacy of a particular reinforcer and the maximum effort the subject will expend to obtain that reinforcer.
Methods used to assess potential for relapse to nicotine-seeking are cue-, stress- and/or nicotine-induced reinstatement. In the reinstatement assay, subjects self-administer drug to stability, after which extinction is instituted, in which responding no longer delivers the drug reinforcer. Over time, the subject reduces the number of responses on the active lever as extinction is established and a low rate of responding is maintained. To assess relapse of drug-seeking behavior, a stressor, the drug itself, or an environmental cue previously associated with the self-administered drug is reintroduced, which leads to resumption of responding on the active lever, however, the drug continues to be unavailable. If responding is increased above the extinction level, this is interpreted as reinstatement of drug-seeking behavior. Mecamylamine decreases cue-induced reinstatement of responding for nicotine in this model (Liu et al., 2006; Liu et al., 2007). Thus, mecamylamine inhibition of nAChRs effectively decreases both nicotine reinforcement and nicotine-seeking behavior.
3.2 Nicotine Drug Discrimination
Another behavioral paradigm frequently employed in the preclinical literature to assess the effects of nicotine is the drug discrimination assay (Morrison and Stephenson, 1969). In these studies, food- or water-deprived subjects are trained to press the lever to obtain food or water. Once the operant response has been established, drug discrimination training ensues. Nicotine is injected prior to the session and responses on one (active) of the two levers in the operant chamber results in reinforcement, whereas responses on the other lever (inactive) has no programmed consequence. On alternating days, saline is injected prior to the session, and responses on the other lever result in reinforcement. Once the subject meets the training criteria, drug discrimination is evaluated. A test drug is injected prior to the session, and discrimination of the test drug as nicotine-like is indicated by the subject responding on the nicotine-associated rather than the saline-associated lever. To determine if the test drug inhibits the discriminative stimulus effects of nicotine, the test drug is administered prior to nicotine. If responding on the lever associated with nicotine is reduced, the results are interpreted as inhibition of the discriminative stimulus effects of nicotine. Pretreatment with racemic mecamylamine consistently blocked the discriminative effects of nicotine, which was not overcome by increasing the nicotine dose (Stolerman et al., 1983). Numerous studies have replicated these results (Brioni et al., 1994; James et al., 1994; Jutkiewicz et al, 2011; Olausson et al., 2004; Zaniewska et al., 2006). Thus, mecamylamine blockade of the discriminative stimulus effects of nicotine provides support for the involvement of nAChRs in this response to nicotine.
3.3 Nicotine-Mediated Classical Conditioning
Racemic mecamylamine also has been shown to block classical conditioning produced by nicotine. Classical conditioning involves the pairing of a conditioned stimulus with an unconditioned stimulus, which generates a specific response. Following a number of pairings, the conditioned stimulus generates the same response as the unconditioned stimulus. Thus, the response becomes a conditioned response and occurs in the absence of the unconditioned stimulus. A common behavioral task employing the classical conditioning approach is the conditioned place preference assay, which assesses the secondary, conditioned reward induced by a drug (Rossi and Reid, 1976). This assay is thought to assess DA-mediated incentive learning and to measure the rewarding effects of drugs (Bardo and Bevins, 2000; Di Chiara, 2000).
In the context of the current review, nicotine-induced conditioned place preference provides a measure of the secondary conditioned reward produced by nicotine. Subjects are typically initially allowed free access to the entire two- or three-chamber apparatus. Subsequently, nicotine is administered to the subject, and then the subject’s access is restricted to one of the environmentally-distinct chambers within the apparatus. On alternate sessions, saline is administered and the subject restricted to the other distinct chamber within the apparatus. On the test session, the subject has free access to the entire apparatus, and a greater amount of time spent in the chamber that was paired with nicotine indicates conditioned place preference has been established. Nicotine has been shown to produce conditioned place preference in rats, mice and non-human primates (see Le Foll and Goldberg, 2009 for review). Pretreatment with mecamylamine blocks the nicotine place preference (Fudala et al., 1985; Grabus et al., 2006; Iwamoto, 1990; Sershen et al., 2009), indicating that activation of nAChRs mediates the rewarding properties of nicotine.
Furthermore, mecamylamine has been shown to attenuate the reinstatement of nicotine conditioned place preference (Biala et al., 2010), which suggests that mecamylamine may have efficacy in attenuating nicotine relapse. In this assay, nicotine conditioned place preference is extinguished by allowing animals free access to the entire apparatus for several trials in the absence of drug pairing. Following extinction, the animals are primed with a small dose of nicotine, which reinstates the preference for the chamber formerly paired with nicotine. Importantly, in contrast with muscarinic acetylcholine receptors antagonists (e.g., atropine and scopolamine), mecamylamine does not produce conditioned place avoidance, i.e., mecamylamine alone does not decrease the amount of time spent in the mecamylamine-paired environment (Zarrindast et al., 2003). Thus, mecamylamine blocks the rewarding effects of nicotine, and this is not due to intrinsic aversive properties of mecamylamine.
Another behavioral task commonly used to measure the secondary conditioned effects of nicotine is conditioned taste avoidance (Garcia, 1985). In the conditioned taste avoidance assay, a substance with a benign or pleasant taste (e.g., saccharin; conditioned stimulus) is paired with a substance that causes gastric malaise (e.g., radiation; unconditioned stimulus). With pairings of the conditioned and unconditioned stimuli, the conditioned stimulus elicits the conditioned response. Nicotine, when paired with a saccharin solution, produced conditioned taste avoidance, and pretreatment with mecamylamine blocks the conditioned taste avoidance (Kumar et al., 1983). These initial results have been confirmed by others (Bevins et al., 2006; Iwamoto and Williamson, 1984; Rauhut et al., 2008), implicating nAChRs in mediating nicotine-induced conditioned avoidance. However, an alternative interpretation is that the decrease in consumption of saccharin-solution following nicotine administration is not conditioned taste avoidance, but an example of anticipatory contrast. The suppression of a conditioned stimulus (e.g., sweet taste of saccharin) is suggested to occur because the relative value of the reward from the conditioned stimulus is irrelevant compared with the reward from the highly preferred psychoactive drug (e.g., nicotine) that directly activates dopaminergic pathways in the brain (Grigson, 1997). Nicotine reward is known to be dopamine-dependent (Di Chiara et al., 2004). Thus, the decreased intake of saccharin-solution may be due to the rewarding effects of nicotine, rendering the reward obtained from saccharin irrelevant. Therefore, mecamylamine blockade of the rewarding properties of nicotine may be preventing formation of conditioned taste aversion.
Interestingly, mecamylamine also decreases conditioned place preference induced by morphine. Microinjection of mecamylamine (1 – 7.5 μg) into the ventral tegmental area and basolateral amygdala of rats dose-dependently decreases morphine-induced conditioned place preference (Rezayof et al., 2007; Zarrindast et al., 2005). nAChRs in the CA1 region of the dorsal hippocampus have been shown to play a role in morphine-induced conditioned place preference (Rezayof et al., 2006). Microinjections of nicotine (0.5, 0.75 and 1 μg) into the CA1 region potentiated the effect of an ineffective systemic morphine dose (0.5 mg/kg), and elicited conditioned place preference. Bilateral injection of mecamylamine (2, 4 and 8 μg) into the CA1 region decreased both morphine-induced conditioned place preference and the nicotine potentiation of the morphine response. These results indicate that morphine reward is at least partially mediated by nAChRs.
Together, this research shows that mecamylamine inhibits the reinforcing and rewarding effects of nicotine. Since mecamylamine acts as an antagonist at all nAChR subtypes, the specific nAChR subtypes involved have not been elucidated by this research. Studies are currently aimed at identifying specific nAChRs involved in the behavioral effects of nicotine using knockout mice, subtype-selective neurotoxic peptides and small molecules (Brunzell, 2012; Cahir et al. 2011; Exley et al., 2011; Neugebauer et al., 2006; Picciotto et al., 2001; Wooters et al., 2011). Discussion of this work is beyond the scope of the current review; however, results from these investigations may further define new pharmacological targets and augment the discovery of novel therapeutics as smoking cessation agents.
3.4 Alcohol Abuse
Alcohol and nicotine are the most commonly co-abused drugs (Ait-Daoud et al., 2005; Littleton et al., 2007; Meyerhoff et al., 2006). A growing body of literature suggests that nicotine and alcohol co-abuse is due to modulation of nAChR expression and function (Collins, 1990; Davis and de Fiebre, 2006; Dohrman and Reiter, 2003; Funk et al., 2006; Larsson and Engel, 2004). Mecamylamine decreases behaviors resulting from either acute or chronic alcohol administration. Hyperactivity produced by alcohol is decreased by mecamylamine at doses (1–6 mg/kg) that do not alter locomotor activity (Blomqvist et al., 1992; Larsson et al., 2002). Furthermore, mecamylamine decreased ethanol-induced psychomotor sensitization, without altering locomotor activity or blood alcohol levels (Bhutada et al., 2010). Mecamylamine prevented formation of ethanol conditioned place preference following restraint and forced swim stress (Bhutada et al., 2012), indicating that mecamylamine may be efficacious in reducing alcohol relapse following introduction to a stressful environment. Mecamylamine also decreased the locomotor response to alcohol in FAST mice (Kamens and Phillips, 2008), a selectively bred line with increased locomotor sensitivity to acute alcohol (Phillips, 1993). Interestingly, mecamylamine had no effect on hyperactivity induced by acute exposure to cocaine or methamphetamine in FAST mice (Kamens and Phillips, 2008), suggesting that nAChRs specifically mediate the response to alcohol in these animals.
Systemic administration of mecamylamine decreases voluntary alcohol intake in rats and mice using both a simple two bottle choice test and operant self-administration (Farook et al., 2009; Ford et al., 2009; Kuzmin et al., 2009; Le et al., 2000). Also, mecamylamine decreases alcohol intake in high alcohol-preferring C57Bl/6J mice using the “drinking in the dark” method, in which access to 20% ethanol for 2 or 4 hr during the dark cycle produces relevant blood alcohol concentrations (Hendrickson et al., 2009; Rhodes et al., 2005). Microinjections of mecamylamine into the ventral tegmental area and nucleus accumbens, two regions that play a critical role in addiction (Self, 2004; Vetulani, 2001), decreased alcohol self-administration in rats (Ericson et al., 1998; Löf et al, 2007; Nadal et al., 1998). Moreover, a recent microdialysis study demonstrated that microinjection of mecamylamine (100 μM) into anterior, but not posterior, ventral tegmental area is sufficient to decrease alcohol-evoked DA release in vivo (Ericson et al., 2008). Collectively, these studies suggest that nAChRs in ventral tegmental area and nucleus accumbens play an important role in the reinforcing effects of alcohol. Moreover, these results provide evidence for the use of mecamylamine as a therapeutic for alcohol addiction. However, the studies are limited because the effects of mecamylamine were not determined in a model in which animals are experienced with both nicotine and alcohol, as in the human condition. In may be that nAChR antagonists, such as mecamylamine, exhibit greater therapeutic benefit in the presence of nicotine, although higher doses of mecamylamine may be required. Future studies investigating effects of acute and chronic administration of alcohol plus nicotine are needed.
3.5 Methamphetamine Abuse
Nicotine and methamphetamine abuse appear to be linked (Goldsamt et al., 2005). Preclinical studies have begun to investigate interactions between these abused drugs. With respect to the locomotor stimulant effects of methamphetamine, mecamylamine did not alter methamphetamine-hyperactivity in FAST mice (Kamens and Phillips, 2008), suggesting that nAChRs play a limited role in this effect of methamphetamine. However, nicotine and methamphetamine substitute for one another in the drug discrimination assay (Gatch et al., 2008). Mecamylamine decreases the discriminative stimulus effects of methamphetamine in rats (Desai and Bergman, 2010). Furthermore, mecamylamine blocks the ability of nicotine to substitute for methamphetamine (Gatch et al., 2008). Thus, although locomotor stimulant effects of methamphetamine do not appear to involve nAChRs, nAChR activation underlies the interoceptive cues elicited by methamphetamine.
Mecamylamine also decreases methamphetamine self-administration in rats following local injection into the medial habenula or interpeduncular nucleus (Glick et al., 2008). This effect of mecamylamine was suggested to be the result of inhibition of α3β4* nAChRs, since this nAChR subtype is found in high density in these brain regions (Klink et al., 2001; Quick et al., 1999). When administered systemically, nicotine attenuated cue-induced and methamphetamine-induced reinstatement of methamphetamine-seeking in rats, although mecamylamine did not induce reinstatement (Hiranita et al, 2006). One interpretation of these findings is that nicotine decreases reinstatement of methamphetamine-seeking via activation of nAChRs, rather than by receptor desensitization. Collectively, these data demonstrate that nAChRs may be viable targets for the development of pharmacotherapeutic agents to treat methamphetamine abuse.
3.6 Cognition
Hippocampus and frontal cortex mediate mammalian cognitive processes and receive rich cholinergic input from the medial septal nucleus and nucleus basalis of Meynert. Literature has accumulated focusing on the influence of nicotine and nAChR antagonists on learning and memory. Many of these investigations employed the radial-arm maze to assess spatial and non-spatial learning and memory in rodents (Olton and Samuelson, 1976). The radial-arm maze consists of a central hub, from which a number of arms radiate. A food reward, which is not detectable from the central hub, is placed at the termini of a subset of arms. In the absence and presence of a test drug, the rodent is allowed access to the arms for a fixed duration of time with the goal of locating those arms which contain food. During successive trials, the animal returns to those arms which were previously baited. In a slightly modified version of the assay, the alternative arms are baited, and the animal must relearn the location of the food. Entry of arms lacking the food reward is considered an error, i.e., a reduction in choice accuracy. A preponderance of data demonstrate that nicotine enhances choice accuracy, while nAChR antagonists, including mecamylamine, impair choice accuracy, resulting in a greater number of errors (Levin et al., 1987, 1989, 1997; Levin and Rose, 1990; McGurk et al., 1991). Of importance, mecamylamine-induced inhibition of cognitive processes is dose-dependent. While high doses of mecamylamine negatively altered memory, low doses (< 1 mg/kg) enhanced memory (Levin et al., 1993; Levin and Caldwell, 2006). Corroborating evidence regarding the dose-related effects of mecamylamine on memory has been obtained in aged non-human primates, such that low doses of mecamylamine improved memory in the delayed matching to sample task (Terry et al., 1999).
Differential effects of mecamylamine on memory across a range of doses may be due to a greater sensitivity of specific nAChR subtypes to mecamylamine. Although mecamylamine is considered a nonselective nAChR antagonist, its inhibitory efficacy at α2β4 and α4β4 nAChRs is greater than at α2β2, α4β2 and α7 nAChRs (Chavez-Noriega et al., 1997). nAChR subtype heterogeneity occurs throughout the brain and local application of nAChR antagonists results in brain region-dependent alterations in memory demonstrated using the radial arm maze task (Levin et al., 2006). Another explanation for differential effects of mecamylamine doses on memory is that low doses of mecamylamine may produce effects similar to those following desensitization of nAChRs (Collins et al., 1988; Damaj et al., 1996; James et al., 1994). Thus, specific mechanisms underlying the differential dose-related effects of mecamylamine on memory and cognition have not been fully elucidated.
Similar to the improvements in cognition found following administration of low doses of mecamylamine, cognitive processes have been shown to be enhanced following low doses of methyllycaconitine, an α7-selective nAChR subtype antagonist (Hahn et al., 2011). Rats were trained to perform a 5-choice serial reaction time task, in which a light stimulus was randomly presented in one of several holes, and a nose poke in the illuminated hole either while illuminated or within five seconds of the light being extinguished resulted in delivery of a food pellet reward. An incorrect response was recorded when the rat nose-poked into any other hole, and a failure to issue a response was recorded as an omission error. Following stable responding, rats were systemically administered nicotine or vehicle in the presence of increasing doses of methyllycaconitine. As expected, nicotine reduced the number of omission errors, and this effect was reversed by methyllycaconitine in a dose-dependent manner. In the absence of nicotine, however, low doses of methyllycaconitine (0.4 and 1.3 mg/kg) improved response accuracy. In contrast, dihydro-β-erythroidine, a competitive antagonist at α4β2, α4β4, α3β2 and α2β2 nAChRs, neither increased the number of correct responses nor reduced the number of omission errors. Together, these results suggest that low doses of nAChR antagonists augment cognition and that the α7 nAChR subtype may serve a primary role in modulating facets of attentional awareness mediating response accuracy.
Another potential mechanism underlying the procognitive effects of low doses of mecamylamine may be activation of downstream signaling events resulting from nAChR antagonism. Alzheimer’s disease is characterized by impairments in cognition associated with a loss of basal forebrain cholinergic neurons (Etienne et al., 1986; Lehericy et al., 1993; Whitehouse et al., 1981), and a marked reduction in levels of nerve growth factor and its target receptor, TrkA (Boissiere et al., 1997; Mufson et al., 1997). Basal forebrain cholinergic neurons are highly dependent upon continuous exposure to nerve growth factor for proper maintenance and survival, and nicotine increases nerve growth factor levels in cortex, hippocampus and striatum (Bellurdo et al., 1998; French et al., 1999). Exposure of PC12 cells to nicotine (0.01–10 μM for 24 hr) resulted in increased TrkA expression and this effect was reversed by simultaneous exposure to mecamylamine (5 μM for 24 hr; Jonnala et al., 2002). Importantly, exposure to low concentrations of mecamylamine (10 and 100 nM) alone resulted in a marked elevation in TrkA receptor expression compared to control. To evaluate the effect of mecamylamine on TrkA receptor expression in vivo, rats were administered mecamylamine (24 mg/kg/day) via an indwelling catheter, and a modest elevation (20%) in TrkA receptor expression was found (Jonnala et al., 2002). Collectively, these studies provide some evidence supporting the idea that stimulation of downstream targets may underlie the positive effects of low doses of mecamylamine on cognition.
Thus, low doses of mecamylamine may provide cognitive benefits. However, when used therapeutically, the dose of mecamylamine should be considered carefully, since, as with other therapeutics, the beneficial effects of high doses must be balanced by potential negative side-effects, including negative effects on cognition. Additionally, consideration of the patient’s tobacco smoking status is important, as some of the negative effects on cognition induced by mecamylamine may be surmounted by nicotine (Levin et al., 1997b). Thus, the efficacy of mecamylamine as a therapeutic may be compromised by nicotine due to concurrent tobacco smoking.
3.7 Chronic Mecamylamine
A preponderance of studies evaluating effects of mecamylamine have investigated effects of acute administration, whereas few studies have evaluated effects of chronic mecamylamine administration. Continuous mecamylamine treatment has been reported to result in brain region- and subtype-specific upregulation of nicotinic receptors (Abdulla et al., 1996; Collins et al., 1994; Pauly et al., 1996; Peng et al., 1994). With respect to behavioral analyses, one study reported that similar to acute mecamylamine administration, chronic treatment of rats with mecamylamine (3 mg/kg/day) eliminated the improvement in working memory resulting from acute nicotine administration (Levin et al., 1993). Importantly, when mecamylamine alone was administered chronically, working memory was increased during the first week of treatment, however, tolerance developed to this effect in subsequent weeks of treatment. Further work revealed that chronic mecamylamine briefly increased choice accuracy, a facet of cognitive function, but with continued administration choice accuracy was hindered (Levin et al., 1997a). Transient improvements in working memory and choice accuracy may be prolonged with continuous administration of low-doses (< 1 mg/kg) of mecamylamine; however studies evaluating low doses have not been reported in the literature.
3.8 Antidepressant and Anxiolytic
While it is not possible to develop an animal model that measures “depression” per se (Cryan and Mombereau, 2004), several preclinical behavioral paradigms are used typically to identify antidepressant-like effects of drugs. Perhaps the most widely used assay is the forced swim test, in which a rodent is placed in a water-filled cylinder from which it cannot escape, and total distance travelled and length of time required for immobilization (i.e., cessation of escape-oriented movements) are measured (Porsolt et al., 1977). Administration of an antidepressant drug prior to the forced swim test increases both distance travelled and length of time until immobilization is reached (Cryan et al., 2005). Another behavioral assay used to measure antidepressant-like effects is the tail suspension test, wherein the animal (predominantly mice) is suspended in mid-air by the tail, and the length of immobilization time is measured. Similar to the results in the forced swim test, antidepressants decrease the length of time during which the animal is immobile (Caldarone et al., 2004; Holmes et al., 2002).
Importantly, preclinical research has demonstrated that many currently available antidepressants, including bupropion, reboxetine, fluoxetine, sertraline, desipramine, paroxetine, nefazodone and venlafaxine act as noncompetitive antagonists at nAChRs (Fryer and Lukas, 1999; Miller et al., 2002a, b). Thus, it is not surprising that mecamylamine (1–10 mg/kg) administered acutely increased distance travelled in the forced swim test and decreased the length of time immobilized in the tail suspension test (Andreasen et al., 2009; Caldarone et al., 2004). Mecamylamine decreased distance travelled in the open-field assay across the same dose range that increased distance travelled in the forced swim test, suggesting that these effects of mecamylamine are not due to nonspecific stimulant effects (Andreasen et al., 2009). Studies employing β2 or α7 nAChR subunit knockout mice found that while mecamylamine increased the time to immobilization for wild-type mice in the forced swim test, the β2 and α7 knockout mice were insensitive to this effect of mecamylamine. These results suggest that nAChRs containing β2 or α7 subunits may mediate the antidepressant-like effects of mecamylamine (Caldarone et al., 2004; Rabenstein et al., 2006). Further, mecamylamine potentiates the antidepressant-like effects of the selective serotonin reuptake inhibitor (SSRI), citalopram, and the tricyclic antidepressant, imipramine, in the tail suspension test (Popik et al., 2003). An interpretation of these results is that mecamylamine may potentiate the behavioral effects of these antidepressants by blocking serotonin reuptake (Ma et al., 2006). This mecamylamine-induced potentiation of the effects of citalopram was not replicated in a subsequent study; further, potentiation of the antidepressant-like effects of reboxetine, a selective norepinephrine reuptake inhibitor, were not observed in either the tail suspension test or forced swim test (Andreasen and Redrobe, 2009a). Thus, mecamylamine may have potential as an adjunct therapy for depression, however, discrepant results from the preclinical literature cast some doubt on this therapeutic application for mecamylamine.
Importantly, several preclinical studies have shown that repeated administration of nicotine produces antidepressant-like effects similar to those observed following acute mecamylamine administration (Djuric et al., 1999; Ferguson et al., 2000). This apparent contradiction, that a nAChR agonist and nAChR antagonist produce the same pharmacological effect, may be explained by agonist-induced receptor desensitization leading to functional antagonism of the receptor (Buccafusco et al., 2009; Gentry and Lukas, 2002; Giniatullin et al., 2005; Picciotto et al., 2008; Quick and Lester, 2002; Reitstetter et al., 1999). Classical work by Katz and Thesleff (1957) led to the concept of nAChR desensitization. Upon binding of nicotine to its recognition sites on the receptor, a rapid conformational change in the protein occurs; the ion channel opens allowing cation flux across the membrane, and the receptor shifts to a high-affinity desensitized state. Thus, upon initial receptor binding, nicotine acts as an agonist eliciting a cellular response (channel opening). Also, nicotine acts as a functional antagonist, inducing receptor desensitization and rendering the receptor unresponsive to subsequent exposure to agonist. Thus, nAChR antagonists such as mecamylamine mimic the desensitization produced by nicotine by prohibiting channel function, without providing an antecedent agonist-like response.
Observations that nAChR agonists and nAChR antagonists produce the same pharmacological effect are further complicated by studies showing that concentrations of agonist required to activate the receptors are significantly greater those which desensitize the receptor (Frazier et al., 2003; Grady et al., 1994). Thus, agonists, depending on the concentrations, can produce outcomes either similar or in opposition to the effect of antagonists in the nAChR system. With respect to the current topic, both agonist (e.g., low concentrations of nicotine or repeated administration) and antagonists (e.g., mecamylamine) produce antidepressant effects.
The role of nAChR desensitization in the antidepressant-like effects of nicotine is supported also by findings that acute mecamylamine reverses these antidepressant-like effects of nicotine in the Flinders Sensitive Line (FSL) of rats (Tizabi et al., 2000), another animal model of depression (Overstreet, 1993; Overstreet et al., 1995). However, results in the forced swim test appear to be dependent, at least in part, on the subject’s genetic background. Acute mecamylamine, but not nicotine, increased swim distance in inbred NMRI mice, whereas nicotine, but not mecamylamine, increased swim distance in outbred C57BL/6J mice, and both drugs increased swim distance in inbred BALB/c mice (Andreasen and Redrobe, 2009b). These results support previous observations that baseline activity exhibited by these strains covaries with drug responsiveness in this assay (Petit-Demouliere et al., 2005). Nonetheless, these findings indicate that the efficacy of mecamylamine as an antidepressant may in part depend upon prior history of nicotine use, as well as the genetic background of the individual.
The interaction of mecamylamine with targets downstream from AChRs also may contribute to its antidepressant effects. Depression has been correlated with the expression of brain-derived neurotrophic factor (BDNF), a protein supporting the survival of neurons and facilitating the differentiation and growth of new neurons. Specifically, clinical depression has been reported to be associated with low serum BDNF levels (Karege et al., 2002; Molendijk et al., 2011). Also, suicide victims prone to bouts of major depression have been shown to have reduced BDNF levels in both cerebral cortex and hippocampus (Dwivedi et al., 2003). Preclinical studies show that mice which were genetically modified to express low levels of BDNF display an increase in anxiety- and depression-like behaviors (Martinowich et al., 2007). Furthermore, several antidepressants, including fluoxetine and citalopram, elevate BDNF levels (Molteni et al., 2006; Russo-Neustadt et al., 2004), in addition to acting as nAChR antagonists (Fryer and Lukas, 1999).
Based on the above reports, one could hypothesize that the antidepressant-like properties of mecamylamine may be the result of elevations in BDNF expression. Several lines of evidence support this hypothesis. Using cell culture, differentiated SH-SY5Y cells treated with mecamylamine (0.01–10 μM) exhibited significantly increased BDNF transcript levels (Park et al., 2011). Results also showed that systemic administration to rats of a single low dose (0.3 mg/kg) of mecamylamine elevated cerebral cortical BDNF expression 200% relative to control (Park et al., 2011). Further, to assess the behavioral response to mecamylamine and the relationship to BDNF levels, this group of investigators employed the elevated plus maze. The elevated plus maze consists of two completely enclosed arms and two open exposed arms; the duration of time spent in the open and closed arms is quantified. Animals with low levels of anxiety spend more time in the open arms than in the closed arms. Results show that mecamylamine produced an anxiolytic effect, reducing the dexamethasone-induced increase in the amount of time spent in the closed arm of the elevated plus maze. Moreover, the observed anxiolytic effect of mecamylamine correlated with an increase in cortical BDNF levels obtained immediately following the behavioral test (Park et al., 2011). Thus, beneficial pharmacological effects of mecamylamine may be, in part, the result of effects on targets downstream from nAChRs, such as changes in BDNF expression.
3.9 Mecamylamine Stereoisomers and Behavior
Consistent with the majority of research performed using racemic mecamylamine, several preclinical studies have reported that each of the stereoisomeric forms of mecamylamine have antidepressant-like effects. For example, S-(+)mecamylamine increased the total distance travelled by both mice and rats in the forced swim test (Lippiello et al., 2008). S-(+)Mecamylamine also decreased anxiety-like behavior in rats, as evidenced by an increase in social interaction and an increase in percentage of time spent in the light side of the light/dark box, two assays revealing anxiety-like behavior in rodents (Lippiello et al., 2008). Conversely, R-(−)mecamylamine was anxiolytic in the forced swim test when evaluated using several strains of mice; however, this effect was not observed in all strains (Lippiello et al., 2008). Further, R-(−)mecamylamine was less potent (≥30-fold) than S-(+)mecamylamine in the forced swim and social interaction tests, and exhibited no activity in the light/dark box (Lippiello et al., 2008). Similarly, the S-(+)mecamylamine was more effective at blocking nicotine-induced seizures than R-(−)mecamylamine (Newman et al., 2001). Moreover, while S-(+)mecamylamine and R-(−)mecamylamine appear to have a similar potency in blocking nicotine-induced antinociception, only S-(+)mecamylamine decreased spontaneous locomotor activity (Suchocki et al., 1991). Taken together with results from cell expression systems that indicate S-(+)mecamylamine is more selective for neuronal nAChRs than R-(−)mecamylamine (Papke et al., 2001), S-(+)mecamylamine may better target nAChRs associated with anxiolytic/antidepressant-like effects and have the advantage of fewer peripheral side effects.
4.0 CLINICAL STUDIES
4.1 Smoking Cessation
Given the preclinical evidence demonstrating that mecamylamine decreases the reinforcing and rewarding effects of nicotine, it is not surprising that clinical studies have investigated the potential for mecamylamine to act as a smoking cessation agent. Early studies evaluated the ability of mecamylamine to treat nicotine dependence (Tennant et al., 1984; Tennant and Tarver, 1984). A group (n = 14) of nicotine-dependent subjects was given 5–10 mg mecamylamine daily for 21 days and the dose was escalated until patients either experienced a decrease in nicotine craving or until toxic effects were observed (Tennant et al., 1984). Results showed that 13 of 14 subjects (93%) had a reduction in nicotine craving and 7 subjects (50%) ceased smoking completely. In another study, subjects were administered 20 mg mecamylamine daily for 6 weeks or 50 mg/day for 3 weeks (Tennant and Tarver, 1984). Results showed that the higher mecamylamine dose reduced nicotine intake as measured by self-report and urine analysis, but that side-effects, including constipation, urinary retention, abdominal cramps and weakness, were pronounced. The lower dose (20 mg/day) of mecamylamine was less effective, but was better tolerated. Thus, while mecamylamine may be a viable treatment for some cases of recalcitrant nicotine dependence, a high prevalence of side-effects diminished its therapeutic value. In contrast, several studies reported that mecamylamine transiently increases tobacco consumption and nicotine intake (Pomerleau et al., 1987; Rose et al., 1998), consistent with the preclinical literature (Glick et al., 1996). The mecamylamine-induced increase in tobacco consumption was thought to be the result of an “extinction burst” and an attempt to overcome the consequent reduction in nicotine reward (Rose et al., 1998).
The side-effect profile of mecamylamine, together with the observed increased tobacco consumption, led to a lack of interest in this drug as a smoking cessation agent and treatment for nicotine dependence. However, interest in mecamylamine renewed in testing the hypothesis that a combination agonist-antagonist treatment may be effective as a smoking cessation therapy (Rose and Levin, 1991; Rose et al., 1994). The idea was that an antagonist would block the reinforcing effects of nicotine, while an agonist would minimize withdrawal symptoms. Nicotine replacement therapy (NRT; 21 mg/day) was given transdermally either alone or in combination with mecamylamine (5 mg oral tablet, twice daily for 2 weeks) to nicotine-dependent patients. Mecamylamine relieved craving and alterations in affect following the onset of smoking cessation. The low dose of mecamylamine resulted in minimal side-effects, including constipation, dry mouth and orthostatic hypotension. In another study by this group of investigators (Rose et al., 1998), the effectiveness of mecamylamine (10 mg/day) plus nicotine (21 mg/day) for 4 weeks prior to the initiation of smoking cessation resulted in a higher percentage of abstinent subjects compared with those that did not receive mecamylamine (47.5 vs. 27.5%). Also, the combined treatment of mecamylamine plus nicotine reduced smoking satisfaction and craving when compared with administration of either drug alone. Furthermore, continuous abstinence at 6 months was higher (40%) in individuals that received combined nicotine and mecamylamine treatment when compared with those that received nicotine only (20%), mecamylamine only (15%) or placebo (15%).
While these studies support the use of mecamylamine as an adjunct therapy with NRT for smoking cessation, the studies were limited by small numbers of subjects (n = 20–48). In a larger study, subjects (n = 233) received nicotine via transdermal patch (21 mg/24 hr) and/or mecamylamine (10 mg/day, oral), administered separately or concurrently, and the reward produced by cigarette smoking was rated using a 7-point scale that ranged from “not at all” to “extremely” (Rose and Behm, 2004). Results showed that nicotine alone, mecamylamine alone and the combined nicotine-mecamylamine treatment reduce smoking reward to the same degree. In agreement, another large (n = 180) study reported that the mecamylamine and nicotine combination therapy was no better than NRT alone in improving the chance of successfully quitting smoking (Glover et al., 2007). One explanation for these conflicting results may be that NRT produces nAChR desensitization, which leads to functional antagonism of the receptors, providing results analogous to those found following mecamylamine combination therapy (Gentry and Lukas, 2002; Quick and Lester, 2002; Picciotto et al., 2008). Thus, mecamylamine does not appear to be an effective adjunct therapy to NRT.
4.2 Alcohol Addiction
Similar to findings in the preclinical literature, reports from clinical studies indicate that mecamylamine has potential as a medication for the treatment of alcohol addiction. Mecamylamine decreased the rewarding effect of alcohol, alcohol-induced mood alterations and the self-reported desire to consume alcoholic beverages (Blomqvist et al., 2002). Self-report measures included the Drug Effect Questionnaire (Holdstock and de Wit, 1998), the Alcohol Sensation Scale (Maisto et al., 1980), and the Biphasic Alcohol Effects Scale (Martin et al., 1993). Mecamylamine also modified the pharmacokinetic profile of alcohol, as evidenced by decreased blood alcohol concentrations (Blomqvist et al., 2002). Furthermore, mecamylamine decreased the stimulant-like effects of alcohol, i.e., mood effects associated with the rising portion of the blood-alcohol concentration curve (Chi and de Wit, 2003; Young et al., 2005). While results from these clinical studies are encouraging, a small number of subjects were employed in each study (20–27 subjects/study), and neither alcohol-dependent subjects nor nicotine-dependent alcohol users were included. As such, the interpretation of these studies is limited. Given that both alcohol and nicotine alter nAChR expression and function (see above), alcohol-dependent individuals that regularly smoke tobacco are likely to have undergone neuroadaptations in response to repeated exposure to both of these abused drugs. Thus, future studies evaluating potential therapeutics, especially nAChR antagonists, for alcohol-dependence should consider pharmacological history as a critical factor in study design and outcome. Nevertheless, these results provide clinical evidence for a role for nAChRs in mediating the reinforcing properties of alcohol, and suggest that nAChR inhibition may provide an effective mechanism for the treatment of alcohol addiction.
4.3 Depression
In the early 1970’s, results from clinical studies (Janowsky et al., 1972a, b) led to the “cholinergic hypothesis of depression”, which states that increased cholinergic tone is an underlying mechanism of depression. Classical tricyclic antidepressants, as well as newer, more selective monoamine transporter inhibitors, have been shown to act as nAChR antagonists. Inhibition of hypercholinergic activity has been suggested as a potential approach to the treatment for depression (Lippiello et al., 2008; Shytle et al., 2002d). Several clinical trials have evaluated mecamylamine as a treatment for depression. Following 8 weeks of treatment, mecamylamine (2.5 mg/kg/day) decreased reports of “sudden mood changes” in patients with comorbid depression and Tourette’s syndrome (Shytle et al., 2002d). Mecamylamine (5 mg/day, orally, for 7 days; 10 mg/day, week 2–8, as tolerated) reduced depressive symptoms, as measured using the 17- and 21-item Hamilton Depression Rating Scale and the Beck Depression Inventory (BDI) II scale (George et al., 2008). While limited by small sample sizes (n = 38 and 21 at the end of treatment, respectively), results from these initial studies investigating the therapeutic benefit of mecamylamine in depression suggest that inhibition of nAChRs may be a novel approach to treatment.
Individuals diagnosed with major depressive disorder that do not respond to multiple antidepressant medications are classified as treatment resistant (Little, 2009). One therapeutic approach for treatment-resistant individuals is augmentation pharmacotherapy, which refers to administration of both non-standard antidepressants in combination with classical antidepressants (e.g., SSRIs), with the aim of enhancing effects of each medication (Carvalho et al., 2009). Mecamylamine has been evaluated as an augmentation therapeutic for depression (Bacher et al., 2009). Results from a recent Phase 2B clinical trial employing 579 subjects showed that the combination of S-(+)mecamylamine (TC-5214) and SSRI citalopram hydrobromide was more effective than citalopram alone, improving the primary outcome measure of depression (HAM-D rating scale) and secondary outcome measures including irritability, disability, cognition, severity of illness and global improvement (Targacept press release, July 15, 2009). Based on these promising results, the efficacy of TC-5214 as an adjunct therapy was evaluated in a series of five Phase III clinical trials employing patients with major depressive disorder who were not adequately responsive to either SSRIs or selective norepinephrine reuptake inhibitors (SNRIs). However, TC-5214, administered in either a fixed or flexible dosing schedule, in combination with a SSRI or SNRI, failed to show an advantage in comparison with placebo (AstraZeneca Clinical Study Report Synopses, D4130C00004, D4130C00005, D4130C00002, D4130C00003, D4130C00007, 2012).
While this discrepancy in outcome between the Phase II and III clinical trials was reason for disappointment, results of a contemporaneous study (AstraZeneca Clinical Study Report Synopsis, D4131C00001, 2012), which utilized TC-5214 as a monotherapy, provide some insight as to possible reasons for the failure of the S-(+)mecamylamine adjunct therapy to achieve relief of depressive symptoms. Subjects in the studies which employed TC-5214 as adjunct therapy exhibited a high placebo response rate that was not different from responses obtained in the experimental groups. In contrast, placebo response rate was lower in the TC-5214 monotherapy study. Parameter values for patients administered TC-5214 as a monotherapy suggested that a dose-related antidepressant effect may have begun to emerge as reflected by a least squares mean change in the Montgomery-Asberg depression rating scale from a placebo value of −7.6 to −11.2 for 4 mg twice daily dosage of TC-5214. Unfortunately, the TC-5214 monotherapy study may have been halted prematurely based on results from the contemporaneous evaluation of TC-5214 as an adjunct therapy. Since subjects in the placebo group of the adjunct therapy trials were receiving SSRI or SNRI treatment, it may be that manipulation of the serotonergic or noradrenergic circuitry interferes with the antidepressant action of TC-5214. Thus, a future study evaluating the efficacy of TC-5214 as a monotherapy in a group of antidepressant-naïve, depressed individuals may be of value in further determining the therapeutic value of S-(+)mecamylamine. Furthermore, TC-5214 may provide greater relief of depressive symptoms if tobacco smoking history were taken into account, since nicotine exposure decreases the efficacy of mecamylamine.
Extensive analysis of the results from the clinical trials for depression and work from additional preclinical studies led to the hypothesis that TC-5214 may have efficacy in treatment of overactive bladder (Targacept press release, Sep 05, 2012). Overactive bladder is characterized by increased frequency and urgency of urination that can progress to incontinence, and as such can diminish quality of life. Cholinergic receptors of various subtypes are expressed by urinary bladder epithelium and parasympathetic neurons which innervate the bladder, and stimulation of these receptors leads to bladder contraction and the urge to urinate (Beckel et al., 2005; Beckel and Birder, 2012; Biasi et al., 2000). The promising safety and tolerability profile generated by prior clinical trials, in combination with the pharmacokinetic data of TC-5214 which indicate a high degree of renal excretion, suggest that a low dose of S-(+)mecamylamine may be sufficient to treat overactive bladder in patients unable to tolerate currently available medication. Phase IIb clinical trials are expected to begin for this indication in 2013.
4.4 Cognitive and Attentional Spectrum Disorder
Preclinical results demonstrate that nicotine enhances several facets of cognitive function, including learning and memory (Levin, 2002). Due to these beneficial effects of nicotine, clinical research has focused primarily on the development of subtype-specific nAChR agonists as pharmacotherapeutics for diseases that are associated with cognitive impairments. To elucidate the mechanism underlying the procognitive effects of nicotine, mecamylamine has been employed as a nAChR antagonist to demonstrate nAChR involvement in healthy adults and cognitively impaired patients having various diseases (Dumas et al., 2006; Dumas et al., 2010; Green et al., 2005; Sacco et al., 2005; Sacco et al., 2006). Consistent with the idea that nAChR receptor activation improves cognition, an early study employing young, non-smoking males showed that mecamylamine dose-dependently increased errors in a repeated acquisition task, increased the number of false-alarms during the delay period in a recognition memory task, and increased response latency in both tasks (Newhouse et al., 1992). While a 20 mg dose of mecamylamine elevated the number of errors in a repeated acquisition task in healthy young and elderly subjects, only the elderly subjects showed impairments in learning following the 10 mg dose (Newhouse et al., 1994). Thus, the elderly appear to be more sensitive to the detrimental effects of mecamylamine in this regard. In another study employing young adults, mecamylamine increased latency to respond, but did not alter performance in an attention task, and only impaired delayed recall, but not immediate recall in a memory task (Pickworth et al., 1997). Interestingly, these effects on attention and memory were independent of smoking status. Thus, mecamylamine occupied an apparently sufficient number of receptors to produce detrimental effects, independent of the presence of nicotine.
Clinical trials evaluating mecamylamine for the treatment for attention disorders, including attention-deficit/hyperactivity disorder (ADHD), have been conducted. In the adult population, ADHD has been reported to be associated with tobacco smoking (Pomerleau et al., 1995). Adolescents who self-report symptoms consistent with a diagnosis of ADHD were found to be ~3-times more likely to both initiate tobacco smoking and be current smokers (Tercyak et al., 2002). These observations led to the hypothesis that individuals with ADHD may be self-medicating by smoking tobacco to alleviate their ADHD symptoms (Levin et al., 1996; Potter et al., 2006; Wilens et al., 1999). In support of this hypothesis, nicotine delivered via transdermal patch was found to improve attention similar to methylphenidate, a medication commonly prescribed for ADHD (Levin and Rezvani, 2002). If nicotine is producing beneficial effects in ADHD and the underlying mechanism is nAChR desensitization, mecamylamine may also be effective as a treatment or as an adjunct in attention-related disorders. However, studies evaluating effects of mecamylamine and employing a small number of healthy young subjects (n = 12–13) found no improvement in either memory or attention (Ellis et al., 2006; Potter and Newhouse, 2005). In concordance with preclinical work showing procognitive effects of low doses of mecamylamine (Levin et al., 1993; Levin and Caldwell, 2006; Terry et al., 1999), a clinical study evaluated the pharmacotherapeutic effects of mecamylamine as a treatment for attentional impairments in 15 non-smokers with ADHD (Potter et al., 2009). Low doses (< 1 mg/kg) of mecamylamine were administered and behavioral inhibition, recognition memory and delay aversion were assessed. Mecamylamine (0.5 mg) improved recognition memory, with a concurrent reduction in tolerance for delay, indicating that inhibition of nAChRs by low-dose mecamylamine results in procognitive effects. Mecamylamine at low doses may be acting as a mixed agonist/antagonist or nAChR inhibition may be mimicking nicotine-induced receptor desensitization. More recently, a high dose (20 mg) of mecamylamine was found to block the nicotine-induced increase in behavioral inhibition in the stop signal task in highly impulsive subjects diagnosed with ADHD (n = 11; Potter et al., 2012). These results suggest that the efficacy of nicotine as a therapeutic for ADHD may be related to nAChR activation, rather than nAChR desensitization.
Autism is an attention-related disorder on the opposite end of the “attentional spectrum” from ADHD. Autistic individuals are considered to be hyper-focused in contrast to those with ADHD, who are considered hypo-focused. Although based primarily on single case studies and anecdotal reports, mecamylamine has been hypothesized to be an efficacious therapy for autism (Dani and Bertrand, 2007; Lippiello, 2006). In a recent investigation, 10 children diagnosed with autism spectrum disorder were administered ascending fixed doses (0.5 mg/day-5 mg/day) of mecamylamine for a total of 18 weeks. Progress according to the Ohio Autism Clinical Impressions Scale was evaluated by a blinded independent clinician (Arnold et al., 2012). No differences were found between subjects receiving placebo versus those receiving mecamylamine. When evaluating the treatment group alone, 40% of the subjects demonstrated improvement that was sustained for the duration of the treatment. Of those four children, three received a maximum mecamylamine dose of only 0.13–0.15 mg/kg/day. These results illustrate that the beneficial effects of mecamylamine for this application may be experienced only with low doses (< 1 mg/kg).
4.5 Dosage-Related Effects
Review of the literature reveals that the majority of preclinical investigations in which positive effects of mecamylamine (e.g., reduction in drug self-administration, elimination of interoceptive cues associated with drug) were observed, animals were administered moderate to high doses (> 1 mg/kg) of mecamylamine. Furthermore, the majority of clinical studies investigating therapeutic efficacy of mecamylamine also employed moderate to high doses (5–50 mg/day). In contrast, positive therapeutic benefits on cognition were found only following administration of relatively low doses (< 1 mg/kg) of mecamylamine in studies employing animals and humans. Thus, it appears that procognitive effects of mecamylamine manifest at low doses (< 1 mg/kg), whereas moderate to high doses (> 1 mg/kg) are required for therapeutic benefits with respect to addiction-related indications. Difficulties arise relating the doses of mecamylamine (> 1 mg/kg) which decrease addiction related behaviors with concentration of mecamylamine (IC50 = 0.12 ± 0.04 μM; results from current review) that inhibit nicotine-evoked dopamine release from brain slices in vitro, since concentrations of mecamylamine in brain following peripheral administration in vivo have not been reported. However, the dose-related effects of mecamylamine on cognitive and drug abuse-related behaviors may be due to the involvement of distinct brain regions and differing nAChR populations, which exhibit different desensitization recovery rates (Giniatullin et al., 2005). Furthermore, nAChR subtypes display differential sensitivity to mecamylamine (Chavez-Noriega et al., 1997). Although low mecamylamine doses (< 1 mg/kg) are relatively selective for nAChRs (Clarke et al., 1994), a limitation of studies using high mecamylamine doses (> 1 mg/kg) is that such doses are not selective for nAChRs, and NMDA receptor interactions may be contributing to the effects of mecamylamine (Fu et al., 2008; O’Dell and Christensen, 1998; Papke et al., 2001).
5.0 CONCLUSIONS
The preclinical and clinical literature discussed in the current review suggest that mecamylamine may have therapeutic efficacy in a variety of CNS-related disorders. However, since much of the experimental data regarding the beneficial effects of mecamylamine have been obtained either from preclinical studies or clinical trials employing a small number of subjects, a comprehensive evaluation of the potential therapeutic benefit of mecamylamine has not been realized. The coincidence of smoking behavior in patients with depression, ADHD and alcoholism (Covey et al., 1998; Glassman, 1993; Meyerhoff et al., 2006; Pomerleau et al., 1995) leads to the suggestion that these individuals may be self-medicating with nicotine (Levin et al., 1996; Markou et al., 1998; Potter et al., 2006). However, an unresolved question is whether the beneficial effects of nicotine are due to nAChR activation or desensitization. As illustrated by the in vitro studies presented herein, nicotine exposure can modify mecamylamine binding to nAChRs. Considering these preclinical findings, future studies evaluating the effects of mecamylamine should consider tobacco smoking status, as well as nicotine replacement therapy (e.g. nicotine patch, nicotine gum, etc.), as an important factor when assessing mecamylamine efficacy. Thus, smoking history and status should be either an exclusionary factor or a covariate when determining the therapeutic benefit of mecamylamine and other nAChR antagonists being evaluated for clinical efficacy.
Although the future of mecamylamine as a therapeutic for the diseases and conditions discussed herein is questionable, the discovery of nAChR antagonists which are nAChR subtype selective holds promise. Both the preclinical and clinical literature indicate that the therapeutic efficacy of mecamylamine may be limited by anticholinergic side effects (e.g., constipation, hypotension), which result from inhibition of peripheral nAChRs. Furthermore, high doses of mecamylamine may produce cognitive deficits. Since mecamylamine interacts nonselectively with all known nAChR subtypes, the development of nAChR antagonists which exhibit selectivity for a specific nAChR subtype may augment its therapeutic benefits, while eliminating deleterious side-effects associated with mecamylamine. Questions regarding the therapeutic efficacy of subtype-selective nAChR antagonists will be addressed as novel compounds emerge from discovery and are developed to treat diseases in which the pathology is influenced by nicotinic receptors.
Highlights.
Mecamylamine inhibits all nicotinic acetylcholine receptor subtypes
Mecamylamine used in studies to assess effects on variety of addictions and disorders
Nicotine alters binding characteristics of mecamylamine
Researchers should determine smoking history of patients in future trials
Acknowledgments
The original research findings reported herein were supported by NIH grants U19 DA17548 and T32 DA16176, and a research contract from Layton Biosciences, Inc.
ABBREVIATIONS
- ACh
acetylcholine
- ADHD
attention deficit hyperactivity disorder
- ANOVA
analysis of variance
- BSA
bovine serum albumin
- CNS
central nervous system
- DA
dopamine
- dr
dose ratio
- FDA
United States Food and Drug Administration
- nAChR
nicotinic acetylcholine receptor
- SNRI
selective norepinephrine reuptake inhibitor
- SSRI
selective serotonin reuptake inhibitor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdulla FA, Bradbury E, Calaminici MR, Lippiello PM, Wonnacott S, Gray JA, Sinden JD. Relationship between up-regulation of nicotine binding sites in rat brain and delayed cognitive enhancement observed after chronic or acute nicotinic receptor stimulation. Psychopharmacology. 1996;124:323–331. doi: 10.1007/BF02247437. [DOI] [PubMed] [Google Scholar]
- Ait-Daoud N, Wiesbeck GA, Bienkowski P, Li MD, Pfutzer RH, Singer MV, Lesch OM, Johnson BA. Comorbid alcohol and nicotine dependence: From the biomolecular basis to clinical consequences. Alcohol Clin Exp Res. 2005;29:1541–1549. doi: 10.1097/01.alc.0000174692.20933.49. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Olsen GM, Wiborg O, Redrobe JP. Antidepressant-like effects of nicotinic acetylcholine receptor antagonists, but not agonists, in the mouse forced swim and mouse tail suspension tests. J Psychopharmacol. 2009;23:797–804. doi: 10.1177/0269881108091587. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Redrobe JP. Nicotine, but not mecamylamine, enhances antidepressant-like effects of citalopram and reboxetine in the mouse forced swim and tail suspension tests. Behav Brain Res. 2009a;197:150–6. doi: 10.1016/j.bbr.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Andreasen JT, Redrobe JP. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: Role of strain, test and sex. Behav Pharmacol. 2009b;20:286–295. doi: 10.1097/FBP.0b013e32832c713e. [DOI] [PubMed] [Google Scholar]
- Arneric SP, Holladay M, Williams M. Neuronal nicotinic receptors: A perspective on two decades of drug discovery research. Biochem Pharmacol. 2007;74:1092–1101. doi: 10.1016/j.bcp.2007.06.033. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Aman MG, Hollway J, Hurt E, Bates B, Li X, Farmer C, Anand R, Thompson S, Ramadan Y, Williams C. Placebo-controlled pilot trial of mecamylamine for treatment of autism spectrum disorder. J Child Adol Psychop. 2012;22(3):198–205. doi: 10.1089/cap.2011.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunlakshana O, Schild HO. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascher P, Large WA, Rang HP. Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol. 1979;295:139–170. doi: 10.1113/jphysiol.1979.sp012958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AstraZeneca Clinical Study Report Synopsis. 2012 Jun; D4130C00002, http://www.astrazenecaclinicaltrials.com/search/?itemId=9795370.
- AstraZeneca Clinical Study Report Synopsis. 2012 Jun; D4130C00003. http://www.astrazenecaclinicaltrials.com/search/?itemId=10941292.
- AstraZeneca Clinical Study Report Synopsis. 2012 Jun; D4130C00004. http://www.astrazenecaclinicaltrials.com/search/?itemId=9795478.
- AstraZeneca Clinical Study Report Synopsis. 2012 Jun; D4130C00005. http://www.astrazenecaclinicaltrials.com/search/?itemId=11399161.
- AstraZeneca Clinical Study Report Synopsis. 2012 Jul; D4131C00001. http://www.astrazenecaclinicaltrials.com/search/?itemId=12213831.
- Bacher I, Wu B, Shytle DR, George TP. Mecamylamine - a nicotinic acetylcholine receptor antagonist with potential for the treatment of neuropsychiatric disorders. Expert opinion on pharmacotherapy. 2009;10:2709–21. doi: 10.1517/14656560903329102. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Punzi JS, Kreilick K, Abood LG. [3H]Mecamylamine binding to rat brain membranes. Studies with mecamylamine and nicotine analogues. Biochem Pharmacol. 1990;40:2105–2110. doi: 10.1016/0006-2952(90)90241-c. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: What does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Beckel JM, Birder LA. Differential expression and function of nicotinic acetylcholine receptors in the urinary bladder epithelium of the rat. J Physiol. 2012;590:1465–1480. doi: 10.1113/jphysiol.2011.226860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckel JM, Kanai A, Lee SJ, de Groat WC, Birder LA. Expression of functional nicotinic acetylcholine receptors in rat urinary bladder epithelial cells. Am J Physiol Renal Physiol. 2005;290:F103–F110. doi: 10.1152/ajprenal.00098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellurdo N, Blum M, Mudo G, Andbjer B, Fuxe K. Acute intermittent nicotine treatment produces regional increases of basic fibroblast growth factor messenger RNA and protein in the tel- and diencephalons of the rat. Neuroscience. 1998;83:723–740. doi: 10.1016/s0306-4522(97)00323-0. [DOI] [PubMed] [Google Scholar]
- Bencherif M, Schmitt JD. Targeting neuronal nicotinic receptors: A path to new therapies. Curr Drug Targets CNS Neurol Disord. 2002;1:349–357. doi: 10.2174/1568007023339094. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM. Characterization of nicotine’s ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology. 2006;184:47–481. doi: 10.1007/s00213-005-0079-3. [DOI] [PubMed] [Google Scholar]
- Bhutada PS, Mundhada YR, Bansod KU, Dixit PV, Umathe SN, Mundhada DR. Inhibitory influence of mecamylamine on the development and the expression of ethanol-induced locomotor sensitization in mice. Pharmacol Biochem Behav. 2010;96:266–273. doi: 10.1016/j.pbb.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Bhutada P, Mundhada Y, Ghodki Y, Dixit P, Umathe S, Jain K. Acquisition, expression, and reinstatement of ethanol-induced conditioned place preference in mice: Effects of exposure to stress and modulation by mecamylamine. Psychopharmacology. 2012;26:315–323. doi: 10.1177/0269881111431749. [DOI] [PubMed] [Google Scholar]
- Biala G, Staniak N, Budzynska B. Effects of varenicline and mecamylamine on the acquisition, expression, and reinstatement of nicotine-conditioned place preference by drug priming in rats. Naunyn-Schmiedeberg’s Arch Pharmacol. 2010;381:361–370. doi: 10.1007/s00210-010-0498-5. [DOI] [PubMed] [Google Scholar]
- Biasi MD, Nigro F, Xu W. Nicotinic acetylcholine receptors in the autonomic control of bladder function. Eur J Pharmacol. 2000;393:137–140. doi: 10.1016/s0014-2999(00)00008-x. [DOI] [PubMed] [Google Scholar]
- Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Mecamylamine modifies the pharmacokinetics and reinforcing effects of alcohol. Alcohol Clin Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- Blomqvist O, Soderpalm B, Engel JA. Ethanol-induced locomotor activity: Involvement of central nicotinic acetylcholine receptors? Brain Res Bull. 1992;29:173–178. doi: 10.1016/0361-9230(92)90023-q. [DOI] [PubMed] [Google Scholar]
- Boissiere F, Hunot S, Faucheux B, Hersh LB, Agid Y, Hirsch EC. Trk neurotrophin receptors in cholinergic neurons of patients with Alzheimer’s disease. Dement Geriatr Cogn Disord. 1997;8:1–8. doi: 10.1159/000106594. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Kim DJ, O’Neill AB, Williams JE, Decker MW. Clozapine attenuates the discriminative stimulus properties of (−)-nicotine. Brain Res. 1994;643:1–9. doi: 10.1016/0006-8993(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Brunzell DH. Preclinical evidence that activation of mesolimbic alpha 6 subunit containing nicotinic acetylcholine receptors supports nicotine addiction phenotype. Nicotine Tob Res. 2012;14(11):1258–1269. doi: 10.1093/ntr/nts089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Beach JW, Terry AV. Desensitization of nicotinic acetylcholine receptors as a strategy for drug development. J Pharmacol Exper Ther. 2009;328:364–370. doi: 10.1124/jpet.108.145292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ. Beneficial effects of nicotine administered prior to a delayed matching-to-sample task in young and aged monkeys. Neurobiol Aging. 1991;12:233–238. doi: 10.1016/0197-4580(91)90102-p. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Jackson WJ, Terry AV, Marsh KC, Decker MW, Arneric SP. Improvement in performance of a delayed matching-to-sample task by monkeys following ABT-418: a novel cholinergic channel activator for memory enhancement. Psychopharmacology. 1995;120:256–266. doi: 10.1007/BF02311172. [DOI] [PubMed] [Google Scholar]
- Cahir E, Pillidge K, Drago J, Lawrence AJ. The necessity of α4* nicotinic receptors in nicotine-driven behaviors: Dissociation between reinforcing and motor effects of nicotine. Neuropsychopharmacology. 2011;36:1505–1517. doi: 10.1038/npp.2011.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarone BJ, Harrist A, Cleary MA, Beech RD, King SL, Picciotto MR. High-affinity nicotinic acetylcholine receptors are required for antidepressant effects of amitriptyline on behavior and hippocampal cell proliferation. Biol Psychiatry. 2004;56:657–664. doi: 10.1016/j.biopsych.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Carvalho AF, Machado JR, Cavalcante JL. Augmentation strategies for treatment-resistant depression. Current Opinion in Psychiatry. 2009;22:7–12. doi: 10.1097/YCO.0b013e32831be9ef. [DOI] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–56. [PubMed] [Google Scholar]
- Chi H, de Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- Clarke PBS, Chaudieu I, el-Bizri H, Boksa P, Quik M, Esplin BA, Capek R. The pharmacology of the nicotinic antagonist, chlorisondamine, investigated in rat brain and autonomic ganglion. Br J Pharmacol. 111:397–405. doi: 10.1111/j.1476-5381.1994.tb14748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AC. Interactions of ethanol and nicotine at the receptor level. Recent Dev Alcohol. 1990;8:221–231. [PubMed] [Google Scholar]
- Collins AC, Miner LL, Marks MJ. Genetic influences on acute responses to nicotine and nicotine tolerance in the mouse. Pharmacol Biochem Behav. 1988;30:269–278. doi: 10.1016/0091-3057(88)90455-8. [DOI] [PubMed] [Google Scholar]
- Collins AC, Yuehong Luo, Selvaag S, Marks MJ. Sensitivity to nicotine and brain nicotinic receptors are altered by chronic nicotine and mecamylamine infusion. J Pharmacol Exp Ther. 1994;271:125–133. [PubMed] [Google Scholar]
- Connolly J, Boulter J, Heinemann SF. Alpha 4–2 beta 2 and other nicotinic acetylcholine receptor subtypes as targets of psychoactive and addictive drugs. Br J Pharmacol. 1992;105:657–666. doi: 10.1111/j.1476-5381.1992.tb09035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covey LS, Glassman AH, Stetner F. Cigarette smoking and major depression. J Addict Dis. 1998;17:35–46. doi: 10.1300/J069v17n01_04. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C. In search of a depressed mouse: Utility of models for studying depression-related behavior in genetically modified mice. Mol Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Rev. 2005;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Welch SP, Martin BR. Characterization and modulation of acute tolerance to nicotine in mice. J Pharmacol Exp Ther. 1996;277:454–461. [PubMed] [Google Scholar]
- Dani JA, Bertrand D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annual review of pharmacology and toxicology. 2007;47:699–729. doi: 10.1146/annurev.pharmtox.47.120505.105214. [DOI] [PubMed] [Google Scholar]
- Davis TJ, de Fiebre CM. Alcohol’s actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- de Camp WH. The FDA perspective on the development of stereoisomers. Chirality. 1989;1:2–6. doi: 10.1002/chir.530010103. [DOI] [PubMed] [Google Scholar]
- DeNoble VJ, Mele PC. Intravenous nicotine self-administration in rats: Effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology. 2006;184:266–272. doi: 10.1007/s00213-005-0054-z. [DOI] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Drug discrimination in methamphetamine-trained rats: effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther. 2010;335:807–816. doi: 10.1124/jpet.110.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V, Fenu S, De Luca MA, Spina L, Cadoni C, Acquas E, Carboni E, Valentini V, Lecca D. Dopamine and drug addiction: The nucleus accumbens shell connection. Neuropharmacology. 2004;47 (Suppl 1):227–241. doi: 10.1016/j.neuropharm.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Djuric VJ, Dunn E, Overstreet DH, Dragomir A, Steiner M. Antidepressant effect of ingested nicotine in female rats of Flinders resistant and sensitive lines. Physiol Behav. 1999;67:533–537. doi: 10.1016/s0031-9384(99)00091-8. [DOI] [PubMed] [Google Scholar]
- Dohrman DP, Reiter CK. Ethanol modulates nicotine-induced upregulation of nAChRs. Brain Res. 2003;975:90–98. doi: 10.1016/s0006-8993(03)02593-9. [DOI] [PubMed] [Google Scholar]
- Dumas J, Hancur-Bucci C, Naylor M, Sites C, Newhouse P. Estrogen treatment effects on anticholinergic-induced cognitive dysfunction in normal postmenopausal women. Neuropsychopharmacology. 2006;31:2065–2078. doi: 10.1038/sj.npp.1301042. [DOI] [PubMed] [Google Scholar]
- Dumas JA, McDonald BC, Saykin AJ, McAllister TW, Hynes ML, West JD, Newhouse PA. Cholinergic modulation of hippocampal activity during episodic memory encoding in postmenopausal women: A pilot study. Menopause. 2010;17:852–859. doi: 10.1097/gme.0b013e3181e04db9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN, Pandey Tamminga, Ghanshyam N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry. 60:804–815. doi: 10.1001/archpsyc.60.8.804. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Bardo MT. Targeting nicotinic receptor antagonists as novel pharmacotherapies for tobacco dependence and relapse. Neuropsychopharmacology. 2009;34:244–246. doi: 10.1038/npp.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA. Competitive neuronal nicotinic receptor antagonists: A new direction for drug discovery. J Pharmacol Exp Ther. 2001;298:395–402. [PubMed] [Google Scholar]
- Eglen RM. Muscarinic receptor subtype pharmacology and physiology. Prog Med Chem. 2005;43:105–136. doi: 10.1016/S0079-6468(05)43004-0. [DOI] [PubMed] [Google Scholar]
- Ellis JR, Ellis KA, Bartholomeusz CF, Harrison BJ, Wesnes KA, Erskine FF, Vitetta L, Nathan PJ. Muscarinic and nicotinic receptors synergistically modulate working memory and attention in humans. Int J Neuropsychopharmacol. 2006;9:175–189. doi: 10.1017/S1461145705005407. [DOI] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Ericson M, Löf E, Stomberg R, Chau P, Soderpalm B. Nicotinic acetylcholine receptors in the anterior, but not posterior, ventral tegmental area mediate ethanol-induced elevation of accumbal dopamine levels. J Pharmacol Exp Ther. 2008;326:76–82. doi: 10.1124/jpet.108.137489. [DOI] [PubMed] [Google Scholar]
- Etienne P, Robitaille Y, Wood P, Gauthier S, Nair NPV, Quirion R. Nucleus basalis neuronal loss, neuritic plaques and choline acetyltransferase activity in advanced Alzheimer’s disease. Neuroscience. 1986;19:1279–1291. doi: 10.1016/0306-4522(86)90142-9. [DOI] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux JP, Maskos U, Cragg SJ, Faure P. Distinct contributions of nicotinic acetylcholine receptor subunit alpha4 and subunit alpha6 to the reinforcing effects of nicotine. Proc Natl Acad Sci USA. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farook JM, Lewis B, Gaddis JG, Littleton JM, Barron S. Effects of mecamylamine on alcohol consumption and preference in male C57BL/6J mice. Pharmacology. 2009;83:379–384. doi: 10.1159/000219488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov NB, Benson LC, Graef J, Lippiello PM, Bencherif M. Differential pharmacologies of mecamylamine enantiomers: Positive allosteric modulation and noncompetitive inhibition. J Pharmacol Exp Ther. 2009;328:525–532. doi: 10.1124/jpet.108.146910. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Brodkin JD, Lloyd GK, Menzaghi F. Antidepressant-like effects of the subtype-selective nicotinic acetylcholine receptor agonist, SIB-1508Y, in the learned helplessness rat model of depression. Psychopharmacology. 2000;152:295–303. doi: 10.1007/s002130000531. [DOI] [PubMed] [Google Scholar]
- Fieber LA, Adams DJ. Acetylcholine-evoked currents in cultured neurons dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Nickel JD, Mark GP, Strong MN, Yoneyama N, et al. The influence of mecamylamine on ethanol and sucrose self-administration. Neuropharmacology. 2009;57:250–8. doi: 10.1016/j.neuropharm.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford RV, Madison JC, Moyer JH. Pharmacology of mecamylamine. Am J Med Sci. 1956;232:129–143. doi: 10.1097/00000441-195608000-00002. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ. Intravenous nicotine self-administration and cue-induced reinstatement in mice: Effects of nicotine dose, rate of drug infusion and prior instrumental training. Neuropharmacology. 2011;61:687–698. doi: 10.1016/j.neuropharm.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis MM, Papke RL. Muscle-type nicotinic acetylcholine receptor delta subunit determines sensitivity to noncompetitive inhibitors, while gamma subunit regulates divalent permeability. Neuropharmacology. 1996;35:1547–56. doi: 10.1016/s0028-3908(96)00103-7. [DOI] [PubMed] [Google Scholar]
- Frazier CJ, Strowbridge BW, Papke RL. Nicotinic receptors on local circuit neurons in dentate gyrus: a potential role in regulation of granule cell excitability. J Neurophysiol. 2003;89:3018–3028. doi: 10.1152/jn.01036.2002. [DOI] [PubMed] [Google Scholar]
- French SJ, Humby T, Horner HC, Sofoniew VM, Rattray M. Hippocampal neurotrophic and trk receptor mRNA levels are altered by local administration of nicotine, carbachol and pilocarpine. Mol Brain Res. 1999;67:124–136. doi: 10.1016/s0169-328x(99)00048-0. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Antidepressants noncompetitively inhibit nicotinic acetylcholine receptor function. J Neurochem. 1999;72:1117–1124. doi: 10.1046/j.1471-4159.1999.0721117.x. [DOI] [PubMed] [Google Scholar]
- Fu H, Dou J, Li W, Luo J, Li KC, Lam CS, Lee NT, Li M, Han Y. Mecamylamine prevents neuronal apoptosis induced by glutamate and low potassium via differential anticholinergic-independent mechanisms. Neuropharmacology. 2008;54:755–765. doi: 10.1016/j.neuropharm.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Fudala PJ, Teoh KW, Iwamoto ET. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol, Biochem Behav. 1985;22:237–41. doi: 10.1016/0091-3057(85)90384-3. [DOI] [PubMed] [Google Scholar]
- Funk D, Marinelli PW, Le AD. Biological processes underlying co-use of alcohol and nicotine: neuronal mechanisms, cross-tolerance, and genetic factors. Alcohol Res Health. 2006;29:186–192. [PMC free article] [PubMed] [Google Scholar]
- Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA. A general theory of aversion learning. Ann N Y Acad Sci. 1985;443:8–21. doi: 10.1111/j.1749-6632.1985.tb27060.x. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Flores E, Forster MJ. Nicotine and methamphetamine share discriminative stimulus effects. Drug Alcohol Depen. 2008;93:63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry CL, Lukas RJ. Regulation of nicotinic acetylcholine receptor numbers and function by chronic nicotine exposure. Curr Drug Targets CNS Neurol Disord. 2002;1:359–385. doi: 10.2174/1568007023339184. [DOI] [PubMed] [Google Scholar]
- George TP, Sacco KA, Vessicchio JC, Weinberger AH, Shytle RD. Nicotinic antagonist augmentation of selective serotonin reuptake inhibitor-refractory major depressive disorder: a preliminary study. J Clin Psychopharmacol. 2008;28:340–344. doi: 10.1097/JCP.0b013e318172b49e. [DOI] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: Shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Glassman AH. Cigarette smoking: Implications for psychiatric illness. Am J Psych. 1993;150:546–553. doi: 10.1176/ajp.150.4.546. [DOI] [PubMed] [Google Scholar]
- Glick SD, Visker KE, Maisonneuve IM. An oral self-administration model of nicotine preference in rats: Effects of mecamylamine. Psychopharmacology. 1996;128:426–431. doi: 10.1007/s002130050153. [DOI] [PubMed] [Google Scholar]
- Glick SD, Sell EM, Maisonneuve IM. Brain regions mediating α3β4 nicotinic antagonist effects of 18-MC on methamphetamine and sucrose self-administration. Eur J Pharmacol. 2008;599:91–95. doi: 10.1016/j.ejphar.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover ED, Laflin MT, Schuh KJ, Schuh LM, Nides M, Christen AG, Glover PN, Strnad JV. A randomized, controlled trial to assess the efficacy and safety of a transdermal delivery system of nicotine/mecamylamine in cigarette smokers. Addiction. 2007;102:795–802. doi: 10.1111/j.1360-0443.2007.01763.x. [DOI] [PubMed] [Google Scholar]
- Goldsamt LA, O’Brien J, Clatts MC, McGuire LS. The relationship between club drug use and other drug use: a survey of New York City middle school students. Subst Use Misuse. 2005;40:1539–1555. doi: 10.1081/JA-200066886. [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: From structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Riganti L, Vailati S, Clementi F. Brain neuronal nicotinic receptors as new targets for drug discovery. Curr Pharm Des. 2006;12:407–428. doi: 10.2174/138161206775474486. [DOI] [PubMed] [Google Scholar]
- Grabus SD, Martin BR, Brown SE, Damaj MI. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology. 2006;184:456–63. doi: 10.1007/s00213-006-0305-7. [DOI] [PubMed] [Google Scholar]
- Grady SR, Marks MJ, Wonnacott S, Collins AC. Characterization of nicotinic receptor-mediated [3H]dopamine release from synaptosomes prepared from mouse striatum. J Neurochem. 1992;59:848–856. doi: 10.1111/j.1471-4159.1992.tb08322.x. [DOI] [PubMed] [Google Scholar]
- Green A, Ellis KA, Ellis J, Bartholomeusz CF, Ilic S, Croft RJ, Luan Phan K, Nathan PJ. Muscarinic and nicotinic receptor modulation of object and spatial n-back working memory in humans. Pharmacol Biochem Be. 2005;81:575–584. doi: 10.1016/j.pbb.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Grigson PS. Conditioned taste aversions and drugs of abuse: A reinterpretation. Behav Neurosci. 1997;111:129–136. [PubMed] [Google Scholar]
- Grinevich VP, Crooks PA, Sumithran SP, Haubner AJ, Ayers JT, Dwoskin LP. N-n-Alkylpyridinium analogs, a novel class of nicotinic receptor antagonists: Selective inhibition of nicotine-evoked [3H] dopamine overflow from superfused rat striatal slices. J Pharmacol Exp Ther. 2003;306:1011–20. doi: 10.1124/jpet.103.051789. [DOI] [PubMed] [Google Scholar]
- Gurney AM, Rang HP. The channel-blocking action of methonium compounds on rat submandibular ganglion cells. Br J Pharmacol. 1984;82:623–642. doi: 10.1111/j.1476-5381.1984.tb10801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Selective nicotinic receptor antagonists: effects on attention and nicotine-induced attentional enhancement. Psychopharmacology. 2011;217:75–82. doi: 10.1007/s00213-011-2258-8. [DOI] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology. 2009;204:563–72. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci. 2006;103:8523–8527. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogg RC, Raggenbass M, Bertrand D. Nicotinic acetylcholine receptors: From structure to brain function. Rev Physiol Biochem Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- Holdstock L, de Wit H. Individual differences in the biphasic effects of ethanol. Alcohol Clin Exp Res. 1998;22:1903–1911. [PubMed] [Google Scholar]
- Holmes A, Yang RJ, Murphy DL, Crawley JN. Evaluation of antidepressant-related behavioral responses in mice lacking the serotonin transporter. Neuropsychopharmacology. 2002;27:914–923. doi: 10.1016/S0893-133X(02)00374-3. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET. Nicotine conditions place preferences after intracerebral administration in rats. Psychopharmacology. 1990;100:251–7. doi: 10.1007/BF02244415. [DOI] [PubMed] [Google Scholar]
- Iwamoto ET, Williamson EC. Nicotine-induced taste aversion: Characterization and preexposure effects in rats. Pharmacol Biochem Behav. 1984;21:527–532. doi: 10.1016/s0091-3057(84)80034-9. [DOI] [PubMed] [Google Scholar]
- Jamali F, Mehvar R, Pasutto FM. Enantioselective aspects of drug action and disposition: Therapeutic pitfalls. J Pharm Sci. 1989;78:695–715. doi: 10.1002/jps.2600780902. [DOI] [PubMed] [Google Scholar]
- James JR, Villanueva HF, Johnson JH, Arezo S, Rosecrans JA. Evidence that nicotine can acutely desensitize central nicotinic acetylcholinergic receptors. Psychopharmacology. 1994;114:456–462. doi: 10.1007/BF02249336. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Hubbard B, Sekerke HJ. Cholinergic reversal of manic symptoms. Lancet. 1972a;1:1236–1237. doi: 10.1016/s0140-6736(72)90956-7. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, el-Yousef MK, Davis JM, Sekerke HJ. A cholinergic-adrenergic hypothesis of mania and depression. Lancet. 1972b;2:632–635. doi: 10.1016/s0140-6736(72)93021-8. [DOI] [PubMed] [Google Scholar]
- Jonnala RR, Terry AV, Buccafusco JJ. Nicotine increases the expression of high affinity nerve growth factor receptors in both in vitro and in vivo. Life Sci. 2002;70:1543–1554. doi: 10.1016/s0024-3205(01)01529-6. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Brooks EA, Kynaston AD, Rice KC, Woods JH. Patterns of nicotinic receptor antagonism: nicotine discrimination studies. J Pharmacol Exp Ther. 2011;339:194–202. doi: 10.1124/jpet.111.182170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, Phillips TJ. A role for neuronal nicotinic acetylcholine receptors in ethanol-induced stimulation, but not cocaine- or methamphetamine-induced stimulation. Psychopharmacology. 2008;196:377–87. doi: 10.1007/s00213-007-0969-7. [DOI] [PubMed] [Google Scholar]
- Karege F, Perret G, Bondolfi G, Schwald M, Bertschy G, Aubry JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res. 2002;109(2):143–148. doi: 10.1016/s0165-1781(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Katner SN, Davis SA, Kirsten AJ, Taffe MA. Effects of nicotine and mecamylamine on cognition in rhesus monkeys. Psychopharmacology. 2004;175:225–240. doi: 10.1007/s00213-004-1804-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Thesleff S. A study of the desensitization produced by acetylcholine at the motor-end plate. J Physiol. 1957;138(1):63–80. doi: 10.1113/jphysiol.1957.sp005838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T, Boselli C. Pharmacologic discrimination between receptor heterogeneity and allosteric interaction: Resultant analysis of gallamine and pirenzepine antagonism of muscarinic responses in rat trachea. J Pharmacol Exp Ther. 1989;250:944–952. [PubMed] [Google Scholar]
- Klink R, de Kerchove d EA, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Pratt JA, Stolerman IP. Characteristics of conditioned taste aversion produced by nicotine in rats. Br J Pharmacol. 1983;79:245–253. doi: 10.1111/j.1476-5381.1983.tb10518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Jerlhag E, Liljequist S, Engel J. Effects of subunit selective nACh receptors on operant ethanol self-administration and relapse-like ethanol-drinking behavior. Psychopharmacology. 2009;203:99–108. doi: 10.1007/s00213-008-1375-5. [DOI] [PubMed] [Google Scholar]
- Larsson A, Engel JA. Neurochemical and behavioral studies on ethanol and nicotine interactions. Neurosci Biobehav Rev. 2004;27:713–720. doi: 10.1016/j.neubiorev.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Larsson A, Svensson L, Soderpalm B, Engel JA. Role of different nicotinic acetylcholine receptors in mediating behavioral and neurochemical effects of ethanol in mice. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Goldberg SR. Effects of nicotine in experimental animals and humans: An update on addictive properties. Handbook of Exp Pharmacol. 2009;192:335–67. doi: 10.1007/978-3-540-69248-5_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehericy S, Hirsch EC, Cervera-Pierot P, Hersh LB, Bakchine S, Piette F, Duyckaerts C, Hauw JJ, Javoy-Agid F, Agid Y. Heterogeneity and selectivity of the degeneration of cholinergic neurons in the basal forebrain of patients with Alzheimer’s disease. J Comp Neurol. 1993;330:15–31. doi: 10.1002/cne.903300103. [DOI] [PubMed] [Google Scholar]
- Levin ED. Nicotinic receptor subtypes and cognitive function. J Neurobiol. 2002;53:633–640. doi: 10.1002/neu.10151. [DOI] [PubMed] [Google Scholar]
- Levin ED, Briggs SJ, Christopher NC, Rose JE. Chronic nicotinic stimulation and blockade effects on working memory. Behav Pharmacol. 1993;4:179–182. [PubMed] [Google Scholar]
- Levin ED, Caldwell DP. Low-dose mecamylamine improves learning of rats in the radial-arm maze repeated acquisition procedure. Neurobiol Learn Mem. 2006;86:117–122. doi: 10.1016/j.nlm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Levin ED, Castonguay M, Ellison GD. Effects of the nicotinic receptor blocker, mecamylamine, on radial arm maze performance in rats. Behav Neural Biol. 1987;48:206–212. doi: 10.1016/s0163-1047(87)90752-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Christopher NC, Briggs SJ. Chronic nicotinic agonist and antagonist effects on T-maze alternation. Physiol Behav. 1997a;61:863–866. doi: 10.1016/s0031-9384(96)00609-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Kaplan S, Boardman A. Acute nicotinic interactions with nicotinic and muscarinic antagonists: Working and reference memory effects in the 16-arm radial maze. Behav Pharmacol. 1997b;8:236–42. [PubMed] [Google Scholar]
- Levin ED, McClernon F, Rezvani A. Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, McGurk SR, South D, Butcher LL. Effects of combined nicotinic and muscarinic blockade on choice accuracy in the radial-arm maze. Behav Neural Biol. 1989;51:270–277. doi: 10.1016/s0163-1047(89)90917-5. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Development of nicotinic drug therapy for cognitive disorders. Eur J Pharmacol. 2000;393:141–146. doi: 10.1016/s0014-2999(99)00885-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic treatment for cognitive dysfunction. Curr Drug Targets CNS Neurol Disord. 2002;1:423–431. doi: 10.2174/1568007023339102. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rose JE. Anticholinergic sensitivity following chronic nicotine administration as measure by radial-arm maze performance in rats. Behav Pharmacol. 1990;1:511–520. [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose JE, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmacology. 1996;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Lingle C. Different types of blockade of crustacean acetylcholine-induced currents. J Physiol. 1983;339:419–437. doi: 10.1113/jphysiol.1983.sp014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippiello PM. Nicotinic cholinergic antagonists: A novel approach for the treatment of autism. Med Hypotheses. 2006;66:985–990. doi: 10.1016/j.mehy.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Lippiello PM, Beaver JS, Gatto GJ, James JW, Jordan KG, Traina VM, et al. TC-5214 (S-(+)-mecamylamine): A neuronal nicotinic receptor modulator with antidepressant activity. CNS Neurosci Ther. 2008;14:266–77. doi: 10.1111/j.1755-5949.2008.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little A. Treatment-resistant depression. Am Fam Physician. 2009;80:167–72. [PubMed] [Google Scholar]
- Littleton J, Barron S, Prendergast M, Nixon SJ. Smoking kills (alcoholics)! shouldn’t we do something about it? Alcohol. 2007;42:167–173. doi: 10.1093/alcalc/agm019. [DOI] [PubMed] [Google Scholar]
- Lof E, Olausson P, Stomberg R, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors are required for the conditioned reinforcing properties of sucrose-associated cues. Psychopharmacology. 2010;212:321–328. doi: 10.1007/s00213-010-1957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN. Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology. 2006;184:417–425. doi: 10.1007/s00213-005-0134-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology. 2007;32:710–718. doi: 10.1038/sj.npp.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löf E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, Soderpalm B. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology. 2007;195:333–343. doi: 10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- Luetje CW, Patrick J. Both alpha- and beta-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–45. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Pearson E, Isgor C, Tao R. Evidence of reuptake inhibition responsible for mecamylamine-evoked increases in extracellular serotonin. Brain Res. 2006;1073–1074:321–324. doi: 10.1016/j.brainres.2005.12.080. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Connors GJ, Tucker JA, McCollam JB, Adesso VJ. Validation of the Sensation Scale, a measure of subjective physiological responses to alcohol. Behav Res Ther. 1980;18:37–43. doi: 10.1016/0005-7967(80)90067-4. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Chambers LK, Rovetti CC. Effects of the competitive nicotinic antagonist erysodine on behavior occasioned or maintained by nicotine: Comparison with mecamylamine. Psychopharmacology. 2000;148:234–242. doi: 10.1007/s002130050047. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: A self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- Martin BR, Onaivi ES, Martin TJ. What is the nature of mecamylamine’s antagonism of the central effects of nicotine? Biochem Pharmacol. 1989;38:3391–3397. doi: 10.1016/0006-2952(89)90106-8. [DOI] [PubMed] [Google Scholar]
- Martin CS, Earleywine M, Musty RE, Perrine MW, Swift RM. Development and validation of the Biphasic Alcohol Effects Scale. Alcohol Clin Exp Res. 1993;17:140–146. doi: 10.1111/j.1530-0277.1993.tb00739.x. [DOI] [PubMed] [Google Scholar]
- Martin TJ, Suchocki J, May EL, Martin BR. Pharmacological evaluation of the antagonism of nicotine’s central effects by mecamylamine and pempidine. J Pharmacol Exp Ther. 1990;254:45–51. [PubMed] [Google Scholar]
- Martin-Garcia E, Barbano MF, Galeote L, Maldonado R. New operant model of nicotine-seeking behaviour in mice. Int J Neuropsychopharmacol. 2009;12:343–56. doi: 10.1017/S1461145708009279. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci. 2007;10(9):1089–1093. doi: 10.1038/nn1971. [DOI] [PubMed] [Google Scholar]
- McGurk SR, Levin ED, Butcher LL. Impairment of radial-arm maze performance in rats following lesions involving the cholinergic medial pathway: Reversal by arecoline and differential effects of muscarinic and nicotinic antagonists. Neuroscience. 1991;44:137–147. doi: 10.1016/0306-4522(91)90256-n. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ. Smoking comorbidity in alcoholism: Neurobiological and neurocognitive consequences. Alcohol Clin Exp Res. 2006;30:253–264. doi: 10.1111/j.1530-0277.2006.00034.x. [DOI] [PubMed] [Google Scholar]
- Miller DK, Sumithran SP, Dwoskin LP. Bupropion inhibits nicotine-evoked [3H]overflow from rat striatal slices preloaded with [3H]dopamine and from rat hippocampal slices preloaded with [3H]norepinephrine. J Pharmacol Exp Ther. 2002a;302:1113–22. doi: 10.1124/jpet.102.033852. [DOI] [PubMed] [Google Scholar]
- Miller DK, Wong EH, Chesnut MD, Dwoskin LP. Reboxetine: Functional inhibition of monoamine transporters and nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2002b;302:687–95. doi: 10.1124/jpet.302.2.687. [DOI] [PubMed] [Google Scholar]
- Molendijk ML, Bus BA, Spinhoven P, Penninx BW, Kenis G, Prickaerts J, Voshaar RO, Elzinga BM. Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features and pharmacological treatment. Mol Psychiatry. 2011;16(11):1088–1095. doi: 10.1038/mp.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CF, Stephenson JA. Nicotine injections as the conditioned stimulus in discrimination learning. Psychopharmacologia. 1969;15:351–360. doi: 10.1007/BF00403710. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Lavine N, Jaffar S, Kordower JH, Quirion R, Saragovi HU. Reduction in p140-TrkA receptor protein within the nucleus basalis and cortex in Alzheimer’s disease. Exp Neurol. 1997;146:91–103. doi: 10.1006/exnr.1997.6504. [DOI] [PubMed] [Google Scholar]
- Nadal R, Chappell AM, Samson HH. Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol Clin Exp Res. 1998;22:1190–1198. [PubMed] [Google Scholar]
- Nakahara D. Influence of nicotine on brain reward systems: Study of intracranial self-stimulation. Ann N Y Acad Sci. 2004;1025:489–490. doi: 10.1196/annals.1316.060. [DOI] [PubMed] [Google Scholar]
- Nelson ME, Lindstrom J. Single channel properties of human alpha3 AChRs: Impact of beta2, beta4 and alpha5 subunits. J Physiol. 1999;516:657–678. doi: 10.1111/j.1469-7793.1999.0657u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer NM, Zhang Z, Crook PA, Dwoskin LP, Bardo MT. Effect of a novel nicotinic receptor antagonist N,N-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology. 2006;184:426–434. doi: 10.1007/s00213-005-0163-8. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Corwin J, Lenox R. Acute nicotinic blockade produces cognitive impairment in normal humans. Psychopharmacology. 1992;108:480–484. doi: 10.1007/BF02247425. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Corwin J, Lenox R. Age-related effects of the nicotine antagonist mecamylamine on cognition and behavior. Neuropsychopharmacology. 1994;10:93–107. doi: 10.1038/npp.1994.11. [DOI] [PubMed] [Google Scholar]
- Newhouse PA, Potter A, Levin ED. Nicotinic system involvement in Alzheimer’s and Parkinson’s diseases. Implications for therapeutics. Drugs Aging. 1997;11:206–228. doi: 10.2165/00002512-199711030-00005. [DOI] [PubMed] [Google Scholar]
- Newman MB, Manresa JJ, Sanberg PR, Shytle RD. Nicotine induced seizures blocked by mecamylamine and its stereoisomers. Life Sci. 2001;69:2583–2591. doi: 10.1016/s0024-3205(01)01338-8. [DOI] [PubMed] [Google Scholar]
- Newman MB, Manresa JJ, Sanberg PR, Shytle RD. Anxiolytic effects of mecamylamine in two animal models of anxiety. Exp Clin Psychopharmacol. 2002;10:18–25. doi: 10.1037//1064-1297.10.1.18. [DOI] [PubMed] [Google Scholar]
- Nooney JM, Peters JA, Lambert JJ. A patch clamp study of the nicotinic acetylcholine receptor of bovine adrenomedullary chromaffin cells in culture. J Physiol. 1992;455:503–527. doi: 10.1113/jphysiol.1992.sp019314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology. 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- O’Dell TJ, Christensen BN. Mecamylamine is a selective non-competitive antagonist of n-methyl-d-aspartate and aspartate-induced currents in horizontal cells dissociated from catfish retina. Neurosci Lett. 1988;94:93–98. doi: 10.1016/0304-3940(88)90276-5. [DOI] [PubMed] [Google Scholar]
- O’Leary K, Parameswaran N, McIntosh JM, Quik M. Cotinine selectively activates a subpopulation of α3/α6β2 nicotinic receptors in monkey striatum. J PharmacolExp Ther. 2008;325:646–654. doi: 10.1124/jpet.108.136838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton DS, Samuelson RJ. Remembrance of places passed: Spatial memory in rats. J Exp Psychol Anim B. 1976;2:97–116. [Google Scholar]
- Ostroumov K, Shaikhutdinova A, Skorinkin A. Modeling study of mecamylamine block of muscle type acetylcholine receptors. Eur Biophys J. 2008;37:393–402. doi: 10.1007/s00249-007-0224-5. [DOI] [PubMed] [Google Scholar]
- Overstreet DH. The Flinders sensitive line rats: A genetic animal model of depression. Neurosci Biobehav Rev. 1993;17:51–68. doi: 10.1016/s0149-7634(05)80230-1. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Pucilowski O, Rezvani AH, Janowsky DS. Administration of antidepressants, diazepam and psychomotor stimulants further confirms the utility of Flinders Sensitive Line rats as an animal model of depression. Psychopharmacology. 1995;121:27–37. doi: 10.1007/BF02245589. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF. The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology. 2007;32:1098–1108. doi: 10.1038/sj.npp.1301228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Dwoskin LP, Crooks PA, Zheng G, Zhang Z, McIntosh JM, Stokes C. Extending the analysis of nicotinic receptor antagonists with the study of alpha6 nicotinic receptor subunit chimeras. Neuropharmacology. 2008;54:1189–200. doi: 10.1016/j.neuropharm.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papke RL, Sanberg PR, Shytle RD. Analysis of mecamylamine stereoisomers on human nicotinic receptor subtypes. J Pharmacol Exp Ther. 2001;297:646–656. [PubMed] [Google Scholar]
- Park DI, Kim HG, Jung WR, Shin MK, Kim KL. Mecamylamine attenuates dexamethasone-induced anxiety-like behavior in association with brain derived neurotrophic factor upregulation in rat brains. Neuropharmacology. 2011;61:276–282. doi: 10.1016/j.neuropharm.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Pauly JR, Marks MJ, Robinson SF, van de Kamp JL, Collins AC. Chronic nicotine and mecamylamine treatment increase brain nicotinic receptor binding without changing α4 or β2 mRNA levels. J Pharmacol Exp Ther. 1996;278:361–369. [PubMed] [Google Scholar]
- Peng X, Gerzanich V, Anand R, Whiting PJ, Lindstrom J. Nicotine-induced increase in neuronal nicotinic receptors results from a decrease in the rate of receptor turnover. Mol Pharmacol. 1994;46:523–530. [PubMed] [Google Scholar]
- Petit-Demouliere B, Chenu F, Bourin M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology. 2005;177:245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Ehrlichman RS, Siegel SJ. Mecamylamine blocks nicotine-induced enhancement of the P20 auditory event-related potential and evoked gamma. Neuroscience. 2007;144:1314–1323. doi: 10.1016/j.neuroscience.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips TJ. Use of genetically distinct mouse populations to explore ethanol reinforcement. Alcohol. 1993;2:451–5. [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: The role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Pickworth WB, Fant RV, Butschky MF, Henningfield JE. Effects of mecamylamine on spontaneous EEG and performance in smokers and non-smokers. Pharmacol Biochem Behav. 1997;56:181–187. doi: 10.1016/s0091-3057(96)00183-9. [DOI] [PubMed] [Google Scholar]
- Pivavarchyk R, Smith AM, Zhang Z, Zhou D, Wang X, Toyooka N, Tsuneki H, Sasaoka T, McIntosh JM, Crooks PA, Dwoskin LP. Indolizidine (−)-235B’ and related structural analogs: discovery of nicotinic receptor antagonists that inhibit nicotine-evoked [3H]dopamine release. Eur J Pharmacol. 2011;658:132–139. doi: 10.1016/j.ejphar.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerleau CS, Pomerleau OF, Majchrzak MJ. Mecamylamine pretreatment increases subsequent nicotine self-administration as indicated by changes in plasma nicotine level. Psychopharmacology. 1987;91:391–393. doi: 10.1007/BF00518198. [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Downey KK, Stelson FW, Pomerleau CS. Cigarette smoking in adult patients diagnosed with attention deficit hyperactivity disorder. J Subst Abuse Treat. 1995;7:373–378. doi: 10.1016/0899-3289(95)90030-6. [DOI] [PubMed] [Google Scholar]
- Popik P, Kozela E, Krawczyk M. Nicotine and nicotinic receptor antagonists potentiate the antidepressant-like effects of imipramine and citalopram. Br J Pharmacol. 2003;139:1196–1202. doi: 10.1038/sj.bjp.0705359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Potter AS, Bucci DJ, Newhouse PA. Manipulation of nicotinic acetylcholine receptors differentially affects behavioral inhibition in human subject with and without disordered baseline impulsivity. Psychopharmacology. 2012;220:331–340. doi: 10.1007/s00213-011-2476-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter A, Newhouse PA. Cognitive effects of acute nicotine and ultra low-dose mecamylamine in attention-deficit/hyperactivity disorder (ADHD) Biol Psychiatry. 2005;57:69S. [Google Scholar]
- Potter AS, Newhouse PA, Bucci DJ. Central nicotinic cholinergic systems: A role in the cognitive dysfunction in attention-deficit/hyperactivity disorder? Behav Brain Res. 2006;175:201–211. doi: 10.1016/j.bbr.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Potter AS, Ryan KK, Newhouse PA. Effects of acute ultra-low dose mecamylamine on cognition in adult attention-deficit/hyperactivity disorder (ADHD) Hum Psychopharmacol Clin Exp. 2009;24:309–317. doi: 10.1002/hup.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick MW, Ceballos RM, Kasten M, McIntosh JM, Lester RAJ. 34 subunit-containing nicotinic receptors dominate function in rat medial habenula neurons. Neuropharmacology. 1999;38:769–783. doi: 10.1016/s0028-3908(99)00024-6. [DOI] [PubMed] [Google Scholar]
- Quick MW, Lester RA. Desensitization of neuronal nicotinic receptors. J Neurobiol. 2002;53:457–478. doi: 10.1002/neu.10109. [DOI] [PubMed] [Google Scholar]
- Rabenstein RL, Caldarone BJ, Picciotto MR. The nicotinic antagonist mecamylamine has antidepressant-like effects in wild-type but not beta2- or alpha7-nicotinic acetylcholine receptor subunit knockout mice. Psychopharmacology. 2006;189:395–401. doi: 10.1007/s00213-006-0568-z. [DOI] [PubMed] [Google Scholar]
- Rapier C, Lunt GG, Wonnacutt S. Nicotinic modulation of [3H]dopamine release from striatal synaptosomes: pharmacological characterization. J Neurochem. 1990;54:937–945. doi: 10.1111/j.1471-4159.1990.tb02341.x. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Zentner IJ, Mardekian SK, Tanenbaum JB. Wistar Kyoto and Wistar rats differ in the affective and locomotor effects of nicotine. Physiol Behav. 2008;93:177–188. doi: 10.1016/j.physbeh.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Reitstetter R, Lukas RJ, Gruener R. Dependence of nicotinic acetylcholine receptor recovery from desensitization on the duration of agonist exposure. J Pharmacol Exp Ther. 1999;289:656–660. [PubMed] [Google Scholar]
- Rezayof A, Nazari-Serenjeh F, Zarrindast MR, Sepehri H, Delphi L. Morphine-induced place preference: involvement of cholinergic receptors of the ventral tegmental area. Eur J Pharmacol. 2007;562:92–102. doi: 10.1016/j.ejphar.2007.01.081. [DOI] [PubMed] [Google Scholar]
- Rezayof A, Zatali H, Haeri-Rohani A, Zarrindast MR. Dorsal hippocampal muscarinic and nicotinic receptors are involved in mediating morphine reward. Behav Brain Res. 2006;166:281–90. doi: 10.1016/j.bbr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Risner ME, Goldberg SR. A comparison of nicotine and cocaine self-administration in the dog: Fixed-ratio and progressive-ratio schedules of intravenous drug infusion. J Pharmacol Exp Ther. 1983;224:319–326. [PubMed] [Google Scholar]
- Rose JE. Disrupting nicotine reinforcement: From cigarette to brain. Ann N Y Acad Sci. 2008;1141:233–56. doi: 10.1196/annals.1441.019. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Extinguishing the rewarding value of smoke cues: Pharmacological and behavioral treatments. Nicotine Tob Res. 2004;6:523–532. doi: 10.1080/14622200410001696501. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC. Nicotine-mecamylamine treatment for smoking cessation: The role of pre-cessation therapy. Exp Clin Psychopharmacol. 1998;6:331–343. doi: 10.1037//1064-1297.6.3.331. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Levin ED, Stein RM, Ripka GV. Mecamylamine combined with nicotine skin patch facilitates smoking cessation beyond nicotine patch treatment alone. Clin Pharmacol Ther. 1994;56:86–99. doi: 10.1038/clpt.1994.105. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED. Concurrent agonist-antagonist administration for the analysis and treatment of drug dependence. Pharmacol Biochem Behav. 1991;41:219–226. doi: 10.1016/0091-3057(92)90086-u. [DOI] [PubMed] [Google Scholar]
- Rossi NA, Reid LD. Affective states associated with morphine injections. Physiol Psychol. 1976;4:269–74. [Google Scholar]
- Rowell PP, Duggan DS. Long-lasting inactivation of nicotinic receptor function in vitro by treatment with high concentrations of nicotine. Neuropharmacology. 1998;37:103–111. doi: 10.1016/s0028-3908(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Dudas MM, Seyal AA, Allen TM, Vessicchio JC, Wexler BE, George TP. Neuropsychological deficits in nonsmokers with schizophrenia: Effects of a nicotinic antagonist. Shizophr Res. 2006;85:213–221. doi: 10.1016/j.schres.2006.03.025. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Termine A, Seyal A, Dudas MM, Vessicchio JC, Krishnan-Sarin S, Jatlow PI, Wexler BE, George TP. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:649–659. doi: 10.1001/archpsyc.62.6.649. [DOI] [PubMed] [Google Scholar]
- Sanberg PR, Shytle RD, Silver AA. Office USPT, editor. Nicotine antagonists for nicotine-responsive neuropsychiatric disorders. United States: University of South Florida; 2000. p. 18. [Google Scholar]
- Sanberg PR, Shytle RD, Silver AA. Office USPT, editor. Method of treating cognitive deficits in learning and memory. United States: Targacept, Inc; 2005. p. 22. [Google Scholar]
- Sastry BV. Stereoisomerism and drug action in the nervous system. Ann Rev Pharmacol. 1973;13:253–267. doi: 10.1146/annurev.pa.13.040173.001345. [DOI] [PubMed] [Google Scholar]
- Self DW. Regulation of drug-taking and -seeking behaviors by neuroadaptations in the mesolimbic dopamine system. Neuropharmacology. 2004;47 (Suppl 1):242–255. doi: 10.1016/j.neuropharm.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Sershen H, Hashim A, Lajtha A. Differences between nicotine and cocaine-induced conditioned place preferences. Brain Res Bull. 2009 doi: 10.1016/j.brainresbull.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Shen WX, Horn JP. Mecamylamine selectively blocks nicotinic receptors on vasomotor sympathetic C neurons. Brain Res. 1998;788:118–124. doi: 10.1016/s0006-8993(97)01520-5. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: Strain and nicotine pre-exposure effects on acquisition. Psychopharmacology. 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Penny E, Silver AA, Goldman J, Sanberg PR. Mecamylamine (Inversine): an old antihypertensive with new research directions. J Hum Hypertens. 2002a;16:453–7. doi: 10.1038/sj.jhh.1001416. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Sanberg PR, Newman MB, Silver AA. Office USPT, editor. Exo-R-mecamylamine formulation and use in treatment. United States: Targacept, Inc; 2002b. p. 24. [Google Scholar]
- Shytle RD, Sanberg PR, Newman MB, Silver AA. Office USPT, editor. Exo-S-mecamylamine formulation and use in treatment. United States: Targacept, Inc; 2002c. p. 24. [Google Scholar]
- Shytle RD, Silver AA, Lukas RJ, Newman MB, Sheehan DV, Sanberg PR. Nicotinic acetylcholine receptors as targets for antidepressants. Mol Psychiatry. 2002d;7:525–35. doi: 10.1038/sj.mp.4001035. [DOI] [PubMed] [Google Scholar]
- Shytle RD, Silver AA, Sheehan KH, Sheehan DV, Sanberg PR. Neuronal nicotinic receptor inhibition for treating mood disorders: Preliminary controlled evidence with mecamylamine. Depress Anxiety. 2002e;16:89–92. doi: 10.1002/da.10035. [DOI] [PubMed] [Google Scholar]
- Singh A, Das DK, Kelley ME. Mecamylamine (Targacept) IDrugs. 2006;9:205–217. [PubMed] [Google Scholar]
- Skorinkin AI, Ostroumov KB, Shaikhutdinova AR, Giniatullin RA. Trapping blockage of muscle nicotinic cholinoreceptors by mecamylamine. Dokl Biol Sci. 2004;399:464–466. doi: 10.1007/s10630-005-0013-1. [DOI] [PubMed] [Google Scholar]
- Smith DF. The stereoselectivity of drug action. Pharmacol Toxicol. 1989;65:321–331. doi: 10.1111/j.1600-0773.1989.tb01182.x. [DOI] [PubMed] [Google Scholar]
- Spealman RD, Goldberg SR. Maintenance of schedule-controlled behavior by intravenous injections of nicotine in squirrel monkeys. J Pharmacol Exp Ther. 1982;223:402–408. [PubMed] [Google Scholar]
- Stolerman IP, Naylor C, Elmer GI, Goldberg SR. Discrimination and self-administration of nicotine by inbred strains of mice. Psychopharmacology. 1999;141:297–306. doi: 10.1007/s002130050837. [DOI] [PubMed] [Google Scholar]
- Stolerman IP, Pratt JA, Garcha HS, Giardini V, Kumar R. Nicotine cue in rats analyzed with drugs acting on cholinergic and 5-hydroxytryptamine mechanisms. Neuropharmacology. 1983;22:1029–1037. doi: 10.1016/0028-3908(83)90021-7. [DOI] [PubMed] [Google Scholar]
- Stone CA, Torchiana ML, Meckelnberg KL, Stavorski J, Sletzinger M, Stein GA, Ruyle WV, Reinhold DF, Gaines WA, Arnold H, Pfister K. Chemistry and structure-activity relationships of mecamylamine and derivatives. J Med Pharm Chem. 1962;91:665–690. doi: 10.1021/jm01239a001. [DOI] [PubMed] [Google Scholar]
- Stone CA, Torchiana ML, Navarro A, Beyer KH. Ganglionic blocking properties of 3-methylaminoisocamphane hydrochloride (mecamylamine): A secondary amine. J Pharmacol Exp Ther. 1956;117:169–183. [PubMed] [Google Scholar]
- Suchocki JA, May EL, Martin TJ, George C, Martin BR. Synthesis of 2-exo- and 2-endo-mecamylamine analogues. Structure-activity relationships for nicotinic antagonism in the central nervous system. J Med Chem. 1991;34:1003–1010. doi: 10.1021/jm00107a019. [DOI] [PubMed] [Google Scholar]
- Tapia L, Kuryatov A, Lindstrom J. Ca2+ permeability of the (alpha4)3(beta2)2 stoichiometry greatly exceeds that of (alpha4)2(beta2)3 human acetylcholine receptors. Mol Pharmacol. 2007;71:769–776. doi: 10.1124/mol.106.030445. [DOI] [PubMed] [Google Scholar]
- Targacept Inc. 2009 Jul 15; Press Release. http://www.targacept.com/wt/page/pr_1247655940.
- Targacept Inc. 2012 Sep 05; Press Release. http://www.targacept.com/wt/page/pr_1346813016.
- Tennant FS, Jr, Tarver AL. Withdrawal from nicotine dependence using mecamylamine: Comparison of three-week and six-week dosage schedules. NIDA Research Monograph. 1984;55:291–297. [PubMed] [Google Scholar]
- Tennant FS, Jr, Tarver AL, Rawson RA. Clinical evaluation of mecamylamine for withdrawal from nicotine dependence. NIDA Research Monograph. 1984;49:239–246. [PubMed] [Google Scholar]
- Tercyak KP, Lerman C, Audrain J. Association of attention-deficit/hyperactivity disorder symptoms with levels of cigarette smoking in a community sample of adolescents. J Am Acad Child Adolesc Psychiatry. 2002;41:799–805. doi: 10.1097/00004583-200207000-00011. [DOI] [PubMed] [Google Scholar]
- Terry AV, Buccafusco JJ, Prendergast MA. Dose-specific improvements in memory-related task performance by rats and aged monkeys administered the nicotinic-cholinergic antagonist mecamylamine. Drug Develop Res. 1999;47:127–136. [Google Scholar]
- Terry AV, Jackson WJ, Buccafusco JJ. Effects of concomitant cholinergic and adrenergic stimulation on learning and memory performance by young and aged monkeys. Cereb Cortex. 1993;3:304–312. doi: 10.1093/cercor/3.4.304. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Rezvani AH, Russell LT, Tyler KY, Overstreet DH. Depressive characteristics of FSL rats: Involvement of central nicotinic receptors. Pharmacol Biochem Behav. 2000;66:73–77. doi: 10.1016/s0091-3057(00)00236-7. [DOI] [PubMed] [Google Scholar]
- Varanda WA, Aracava Y, Sherby SM, VanMeter WG, Eldefrawi ME, Albuquerque EX. The acetylcholine receptor of the neuromuscular junction recognizes mecamylamine as a noncompetitive antagonist. Mol Pharmacol. 1985;28:128–137. [PubMed] [Google Scholar]
- Vetulani J. Drug addiction. Part II. Neurobiology of addiction. Pol J Pharmacol. 2001;53:303–317. [PubMed] [Google Scholar]
- Waldeck B. Three-dimensional pharmacology, a subject ranging from ignorance to overstatements. Pharmacol Toxicol. 2003;93:203–210. doi: 10.1046/j.1600-0773.2003.pto930502.x. [DOI] [PubMed] [Google Scholar]
- Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer disease; evidence for a selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ, Bostic J, Prince J, Monuteaux MC, Soriano J, Fine C, Abrams A, Rater M, Polisner D. A pilot controlled clinical trial of ABT-418, a cholinergic agonist, in the treatment of adults with attention deficit hyperactivity disorder. Am J Psychiatry. 1999;156:1931–1937. doi: 10.1176/ajp.156.12.1931. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Smith AM, Pivavarychyk M, Siripurapu KB, McIntosh JM, Zhang Z, Crooks PA, Bardo MT, Dwoskin LP. bPiDI: a novel selective α6β2* nicotinic receptor antagonist and preclinical candidate treatment for nicotine abuse. Br J Pharmacol. 2011;163:346–357. doi: 10.1111/j.1476-5381.2011.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young EM, Mahler S, Chi H, de Wit H. Mecamylamine and ethanol preference in healthy volunteers. Alcohol Clin Exp Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- Zaniewska M, McCreary AC, Przegalinski E, Filip M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur J Pharmacol. 2006;540:96–106. doi: 10.1016/j.ejphar.2006.04.034. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Faraji N, Rostami P, Sahraei H, Ghoshouni H. Cross-tolerance between morphine- and nicotine-induced conditioned place preference in mice. Pharmacol Biochem Behav. 2003;74:363–9. doi: 10.1016/s0091-3057(02)01002-x. [DOI] [PubMed] [Google Scholar]
- Zarrindast MR, Fattahi Z, Rostami P, Rezayof A. Role of the cholinergic system in the rat basolateral amygdala on morphine-induced conditioned place preference. Pharmacol Biochem Behav. 2005;82:1–10. doi: 10.1016/j.pbb.2005.02.018. [DOI] [PubMed] [Google Scholar]