Abstract
Infection with human immunodeficiency virus-1 (HIV-1) often leads to HIV-associated neurocognitive disorders (HAND) prior to the progression to acquired immunodeficiency syndrome (AIDS). At the cellular level, mitogen-activated protein kinases (MAPK) provide a family of signal transducers that regulate many processes in response to extracellular stimuli and environmental stress, such as viral infection. Recently, evidence has accumulated suggesting that p38 MAPK plays crucial roles in various pathological processes associated with HIV infection, ranging from macrophage activation to neurotoxicity and impairment of neurogenesis to lymphocyte apoptosis. Thus, p38 MAPK, which has generally been linked to stress-related signal transduction, may be an important mediator in the development of AIDS and HAND.
Keywords: HIV-1, Infection, AIDS, NeuroAIDS, HAND, Signaling, p38 MAPK, Macrophages/microglia, Neurotoxicity, Progenitors, Neurogenesis, Neurodegeneration
Introduction
Almost 30 years after emerging into an epidemic, infection with the human immunodeficiency virus-1 (HIV-1) and acquired immunodeficiency syndrome (AIDS) constitutes a persistent health problem worldwide. HIV-1 compromises not only the immune system of its human host, thus eventually leading to AIDS, but the virus also induces a variety of neurological problems and neurocognitive impairments that have recently been categorized as HIV-associated neurocognitive disorders (HAND; Antinori et al. 2007). According to standardized measures of dysfunction, HAND defines three categories of disorders: (1) asymptomatic neurocognitive impairment, (2) mild neurocognitive disorder, and (3) HIV-associated dementia (HAD). While this classification scheme improves the assessment of the overall clinical situation for HIV disease of the central nervous system (CNS), the underlying cellular and molecular mechanisms of HAND remain incompletely understood.
However, several lines of evidence strongly suggest that HIV-1 associated neurodegeneration and consequent HAND may occur via at least two major mechanisms (Kaul 2008). The first is neurotoxicity as a consequence of either direct exposure to HIV-1 and its fragments or indirect injury through neurotoxins released by infected or immune-stimulated, inflammatory microglia and macrophages (MΦ) in the brain (Giulian et al. 1990; Kaul et al. 2001; Gonzalez-Scarano and Martin-Garcia 2005; Lindl et al. 2010). The second strike of HIV at the brain constitutes the impairment of neurogenesis (Krathwohl and Kaiser 2004b; Tran et al. 2005; Poluektova et al. 2005; Okamoto et al. 2007). In addition, evidence has been accumulating that at the molecular level, both pathological mechanisms critically depend on HIV coreceptors, including CCR5, CCR3 and CXCR4, (Meucci et al. 1998; Kaul and Lipton 1999; Kaul et al. 2001, 2005; Gonzalez-Scarano and Martin-Garcia 2005) and downstream activation of mitogen-activated protein kinases (MAPKs), in particular the stress-related p38 MAPK (Kaul and Lipton 1999; Singh et al. 2005; Sui et al. 2006; Kaul et al. 2007; Okamoto et al. 2007; Kaul 2008; Eggert et al. 2010; Gelbard et al. 2010; Medders et al. 2010).
Therefore, this article will review recent progress in understanding the apparent role of MAPKs in the neuropathological mechanisms associated with HIV-1 infection and presumably underlying the development of HAND with an emphasis on p38 MAPK.
HIV-1 infection, associated neuropathology and development of HAND
CD4 and HIV-1/gp120 coreceptors, in particular chemokine receptors CXCR4 (CD184) and CCR5 (CD195), are likely the first sites of host–virus interaction. HIV-1/gp120 associates with CD4 receptors, which are only located on cells of immune lineage, to efficiently engage chemokine coreceptors and infect its primary target cells, macrophages/ microglia, and T-lymphocytes (Bleul et al. 1996; Alkhatib et al. 1996; Dragic et al. 1996; Kaul et al. 2001; Gonzalez-Scarano and Martin-Garcia 2005). In the brain, CCR3, besides CCR5, seems to facilitate HIV infection of microglia (He et al. 1997), and while it is generally believed that CCR5-preferring (R5) HIV are macrophage-tropic (M-tropic) and CXCR4-preferring virus strains (X4) infect T-lymphocytes (T-tropic), the reality seems less clear-cut. In fact, it has been found that syncytia-inducing viruses initially thought to be only X4-tropic can also be dualtropic and infect via CXCR4 or CCR5 (Simmons et al. 1996), and M- and T-tropic simian immunodeficiency virus (SIV) strains can use CCR5 (Edinger et al. 1997). In any case, most HIV-infected CD4+ T-lymphocytes seem to be efficient propagators of the virus, but also rapidly die from apoptosis, except for a certain number of memory cells that constitute a quiescent, latent reservoir (Pantaleo and Fauci 1995; Chun and Fauci 1999; Alexaki et al. 2008). In contrast, macrophages infected with HIV-1 are a long-lived reservoir and presumably carry the virus into the brain where they can pass it on to local macrophages and microglia (Koenig et al. 1986; Kaul et al. 2001; Gonzalez-Scarano and Martin-Garcia 2005).
Interestingly, CCR5 and CXCR4 are present not only on macrophages and microglia but also on neurons and astrocytes which lack CD4 expression (Lavi et al. 1997; Halks-Miller et al. 1997; Rottman et al. 1997; Kaul et al. 2007). In fact, interaction between HIV-1 gp120 and CXCR4/CCR5, independent of CD4, has been shown to cause activation of the G protein-coupled receptors and contribute to intracellular Ca2+ accumulation and signaling (Hesselgesser et al. 1997). However, infection of astrocytes by the virus, which usually remains non-productive, seems to require a mannose receptor (Liu et al. 2004). On the other hand, direct interaction of gp120 with neuronal chemokine receptors (Hesselgesser et al. 1997, 1998) may contribute to neuronal injury, while activation of macrophage HIV coreceptors and subsequent release of neurotoxins, such as excitotoxins, chemokines and/or pro-inflammatory cytokines, presumably provide the predominant trigger for neuronal injury and death, as previously suggested (Giulian et al. 1993; Kaul and Lipton 1999; Porcheray et al. 2006; O’Donnell et al. 2006; Cheung et al. 2008; Eggert et al. 2010; Medders et al. 2010).
Development of HAND in patients during life time has been correlated to a post mortem neuropathological diagnosis that is often referred to as HIV encephalitis (HIVE). The salient pathological features comprise infiltration predominantly by monocyte lineage cells, such as blood-derived macrophages, activated resident microglia, microglial nodules, multinucleated giant cells, widespread reactive astrocytosis, myelin pallor, and decreased synaptic and dendritic density, combined with selective neuronal loss (Petito et al. 1986; Masliah et al. 1997). Surprisingly, ante mortem measures of cognitive dysfunction and HAND do not correlate well with numbers of HIV-infected cells, multinucleated giant cells, or viral antigens in brain tissue (Glass et al. 1995; Achim et al. 1994; Wiley et al. 1994; Masliah et al. 1997). Instead, increased numbers of microglia (Glass et al. 1995), decreased synaptic and dendritic density, selective neuronal loss(Masliah et al. 1997; Achim et al. 1994; Wiley et al. 1994), elevated tumor necrosis factor (TNF)-α messenger (mRNA) in microglia and astrocytes (Wesselingh et al. 1997), and evidence of excitatory neurotoxins in cerebrospinal fluid (CSF) and serum (Heyes et al. 1991) represent pathological features best complementing clinical signs of HAND. An important role for monocyte-lineage cells is further supported by two studies suggesting that the amount of pro-viral HIV DNA in peripherally circulating monocytes and macrophages correlates well with HAND (Shiramizu et al. 2005, 2006).
HIV-1’s genome encodes nine gene products with structural, regulatory, or accessory functions (recently reviewed in (Ellis et al. 2007)). Most of these viral factors have been linked to distinct functions during infection and in the viral life cycle. Whereas various HIV proteins affect the course of viral infection and disease, the envelope gp120 seems to play a major role in the induction of apoptosis in infected and uninfected bystander lymphocytes (Perfettini et al. 2005a, b). On the other hand, six HIV proteins have been reported to directly or indirectly affect neurons and glia (Ellis et al. 2007). Besides intact HIV-1 itself, the two proteins most intensely investigated regarding their contribution to brain injury and HAND are the structural envelope protein gp120 and the regulatory protein “transactivator of transcription”, Tat (Brenneman et al. 1988; Giulian et al. 1990, 1993; Kruman et al. 1998; Liu et al. 2000; Kaul et al. 2001; Mattson et al. 2005). Several in vitro and in vivo studies have revealed that the envelope glycoprotein gp120 and Tat of various HIV-1 strains produce injury and apoptosis in both primary human and rodent neurons (Brenneman et al. 1988; Lannuzel et al. 1995; Meucci and Miller 1996; Singh et al. 2005; Shi et al. 1996; Giulian et al. 1993; Toggas et al. 1994; Meucci et al. 1998; Kaul and Lipton 1999; Kaul et al. 2007; Hesselgesser et al. 1998; Kruman et al. 1998; Liu et al. 2000). Recently, we have shown that both CCR5 and CXCR4 can mediate the neurotoxic effect of gp120 depending on the coreceptor usage of the virus strain from which the envelope protein was originally isolated (Kaul et al. 2007). Interestingly, in macrophages HIV-1 infection and exposure to just the envelope protein gp120 seem to trigger a similar neurotoxic phenotype (Giulian et al. 1990, 1993), and Tat alone also causes activation and release of cytokines as it can occur upon viral infection (Sui et al. 2006).
However, in contrast to the viral envelope protein, the regulatory Tat protein can apparently interact with several cellular binding partners on the surface as well as inside a cell because it can freely diffuse through membranes (Fawell et al. 1994). Therefore, Tat can in principle interact with all cells although the consequences may vary between the individual cell types and depending on what Tat-interacting cellular factors are present. Cell surface receptors that have been reported to provide binding sites for Tat include chemokine receptors, such as the CCR2, CCR3, and the HIV coreceptor CXCR4, the low-density lipoprotein receptor-related protein (LRP), group I metabotropic glutamate receptors and N-methyl-D-aspartate (NMDA) type ionotropic glutamate receptors (Albini et al. 1998; Ghezzi et al. 2000; Xiao et al. 2000; Eugenin et al. 2007; Neri et al. 2007; Li et al. 2008). Intracellular proteins that have been found to directly interact with Tat comprise the glucagon synthase kinase 3β and various transcription factors, such as nuclear receptor chicken ovalbumin upstream promoter transcription factor and AFF4 (Maggirwar et al. 1999; Rohr et al. 2000; He et al. 2010). All these possible interactions of Tat provide a wealth of potential pathways for the viral protein to promote HIV-1 infection and the associated injury of the nervous system.
In addition, transgenic mice expressing HIV/gp120 or Tat in their brains, both manifest several neuropathological features observed in AIDS brains, such as decreased synaptic and dendritic density, increased numbers of activated microglia, and pronounced astrocytosis (Toggas et al. 1994; Kim et al. 2003). HIV/gp120-transgenic mice also develop significant physiological and behavioral changes, such as reduced escape latency, swimming velocity, and spatial retention at 12 months of age (Mucke et al. 1995; Krucker et al. 1998; Asensio et al. 2001; D’hooge et al. 1999). The relevance of this model is further supported by the finding that the neuronal damage initiated by HIV-infected human macrophages intracerebrally administered into mice with severe combined immunodeficiency also closely resembles the neuropathology of human AIDS brains and HIV/gp120tg mice (Persidsky et al. 1996). HIV/Tat-transgenic mice also display failure to thrive, impaired movement, seizures and premature death (Kim et al. 2003). Intact HIV-1 as well as its components gp120 and Tat have all been linked to intracellular signal transduction prominently involving MAPKs among other factors.
Mitogen-activated protein kinases
MAPKs are a family of intracellular serine/threonine phospho-kinases that mediate the cellular response to stimuli and stresses originating in the extracellular environment. Stimuli known to activate these kinases include growth factors, pro-inflammatory cytokines, infections, neurotransmitters, hormones, or chemical and physical stresses, such as change of osmotic conditions and ultraviolet light (Pearson et al. 2001; Chang and Karin 2001; Kumar et al. 2003; Chopra et al. 2008). The currently known MAPKs have been assigned to six groups, ERK1/2, cJun N-terminal kinase (JNK)1/2/3, p38α/β/γ/δ, ERK5, NLK, and ERK3α/β, and constitute the bottom part of a set of three-step signaling cascades which generally consist of upstream MAPK-activating kinases (MAPKK or MEK/MKK) and MKK-activating kinases (MAPKKK or MEKK; Pearson et al. 2001; Chang and Karin 2001). MEKKs are also known as mixed lineage kinases (MLKs) as they can activate more than one signaling cascade (Gallo and Johnson 2002). MAPK cascades are expressed in all cell types and regulate cell cycle and proliferation, differentiation and development, transcription, mRNA stability, translation, migration, inflammation, and cell survival and apoptosis. The MAPKs accomplish all these effects by phosphorylating cytoplasmic and nuclear targets, such as downstream kinases, transcription factors, and a variety of other proteins with diverse biological functions (Pearson et al. 2001; Chang and Karin 2001; Kumar et al. 2003; Chopra et al. 2008). Generally, the activity of the various MAPKs is regulated by tyrosine-, serine- or threonine phosphatases, or dual-specificity phosphatases (MAPK phosphatases; Pearson et al. 2001).
The biological roles of MAPKs very much depend on cell type and context, but activation of ERK has mostly been linked to cell survival and proliferation while JNK and p38 MAPK have primarily been implicated in stress- and apoptosis-related signaling (Xia et al. 1995; Chang and Karin 2001). The stress-activated protein kinase or p38 MAPK is besides JNK the most studied of its kind in pathological or disease conditions, including neurodegeneration (Gallo and Johnson 2002; Kumar et al. 2003; Chopra et al. 2008). Since several lines of evidence suggest a pronounced involvement of p38 MAPK at different levels in HIV infection and the associated pathological processes, we focus here primarily on p38 MAPK.
There are two well-studied signaling pathways that regulate the activation of p38 MAPK. The canonical activation pathway of p38 MAPK includes the serine/ threonine kinases (MKKKs or MLKs), which phosphorylate and activate the dual specificity MAPK kinases (MKKs such as MKK3, MKK4, and MKK6), which in turn phosphorylate p38 MAPK isoforms (Gallo and Johnson 2002; Kumar et al. 2003; Chopra et al. 2008). Alternatively, recent studies have shown that a non-canonical activation pathway of p38 MAPK involves auto-phosphorylation aided by the association of the kinase with TGFβ-activated protein kinase 1-binding protein 1 (TAB1) (Ge et al. 2002; Kang et al. 2006). Both pathways lead to dual phosphorylation of the kinase at Thr 180 and Tyr 182 residues, which is required for activation of p38 MAPK (Ono and Han 2000). The canonical activation pathway can be triggered by many stimuli and is involved in multiple biological responses, including inflammation (Lee et al. 1999). However, the role of TAB1 triggered auto-phosphorylation still remains elusive (Kumar et al. 2003; Chopra et al. 2008).
The currently appreciated functions of activated p38 MAPK include phosphorylation of downstream kinases and transcription factors affecting cellular activation pathways and gene expression, stabilization of mRNA exerting control of translation, regulation of cell cycle, proliferation and development, and control of cell survival and apoptosis (Chang and Karin 2001; Gallo and Johnson 2002; Kumar et al. 2003; Chopra et al. 2008).
The activation of p38 MAPK results in diverse responses via phosphorylation of its substrates, mostly kinases and transcription factors. The kinase substrates of p38 MAPK include MAPK-activated protein kinase 2 (MAPKAPK2 or MK2), MK3, and MK5. Activation of p38 MAPK has been shown to increase cytokine production by direct phosphorylation of downstream factors affecting either transcriptional regulation or stabilization of mRNA, or both events, especially in monocytic cells (Dean et al. 1999; Ono and Han 2000). There are four isoforms of p38 MAPK (α, β, γ, and δ), of which the α-isoform (MAPK14) is basically expressed in every cell type. In contrast, the β- (MAPK11), γ- (MAPK12), and δ- (MAPK13) isoforms are primarily found in endothelial and T-cells, skeletal and cardiac muscle, and monocytes/macrophages, neutrophils and T-cells, respectively (Chopra et al. 2008).
The α-isoform (MAPK14) and β- isoform (MAPK11) have been identified as the main targets of potent anti-inflammatory imidazoles, such as the compound SB203580 (Lee et al. 1999). Past studies have identified p38α MAPK as the principal isoform responsible for the inflammatory response (Kang et al. 2008). In fact, several inhibitors of p38α MAPK have been developed for the treatment of inflammatory disorders, such as psoriasis, arthritis, or chronic obstructive pulmonary disease with mixed success in clinical trials—so far—due to possible side effects (Mayer and Callahan 2006). While therefore p38 MAPK appears not to be an easy to handle therapeutic target, the previous clinical trials have generated important information guiding the development of improved inhibitors. A search for better drugs that allow interfering with the activity of p38 MAPK seems most desirable since this kinase has also been implicated in HIV-1 infection and the associated pathological consequences in the periphery and the central nervous system.
Involvement of p38 MAPK in HIV infection and development of HAND
Three studies investigating the mechanism of HIV-1 infection have found that inhibition of p38 MAPK suppresses viral replication and cytopathic effects in T-lymphocytes and monocytes/macrophages possibly by preventing the activation of the HIV-1 long terminal repeat, impairing reverse transcription and compromising proviral integration (Kumar et al. 1996; Cohen et al. 1997; Muthumani et al. 2004). Accordingly, in an HIV-1 JR-CSF transgenic mouse model, in which monocytic cells produce infectious virus, bacterial lipopolysaccharide (LPS, endotoxin) in combination with granulocyte/macrophage colony-stimulating factor increases viral expression in a p38 MAPK-dependent fashion (Osiecki et al. 2005), and a different HIV-transgenic mouse model carrying a polymerase-deficient viral genome of the strain NL-4-3 confirms these findings (Kadoki et al. 2010). In an apparent paradox, LPS alone, which by itself is the classical inducer of an inflammatory response, can inhibit HIV-1 infection in macrophages also in a p38 MAPK-dependent fashion. In fact, SB203580, a pharmacological inhibitor of p38 MAPK, was found in the same study to restore permissiveness for infection in the presence of LPS (Zybarth et al. 1999). Thus, the exact effect of p38 MAPK on HIV-1 infection is context dependent.
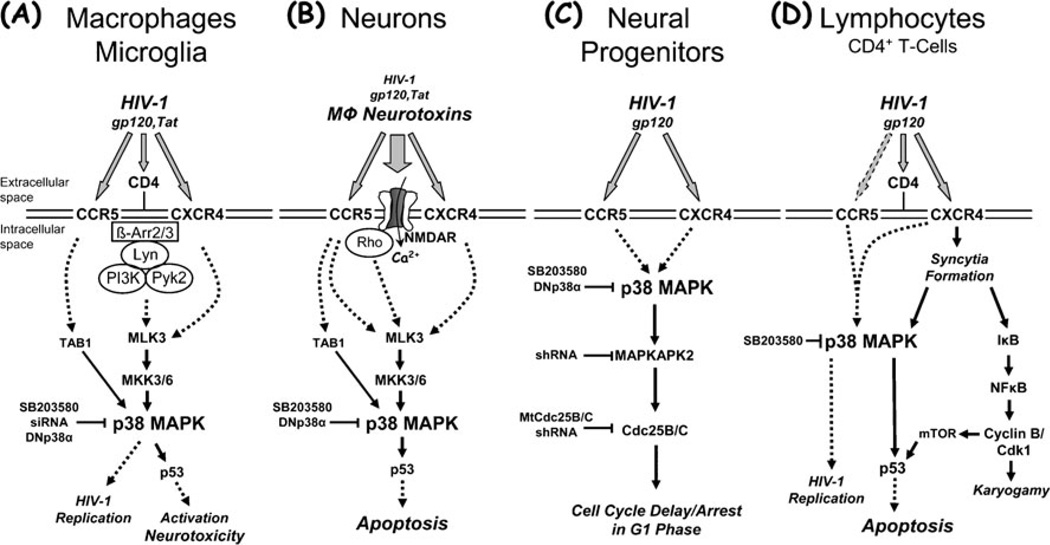
However, we and others have found an essential role for p38 MAPK in the induction of neuronal injury and apoptotic death by HIV-1 gp120, Tat and the intact virus (Kaul and Lipton 1999; Kaul et al. 2007; Choi et al. 2007; Medders et al. 2010; Singh et al. 2005; Sui et al. 2006; Eggert et al. 2010; Fig. 1). In addition, p38 MAPK plays an important role in the activation of the monocytic cell lineage that leads to neurotoxin production (Kaul and Lipton 1999; Medders et al. 2010). Similarly, others reported that HIV-1 infection as well as Tat and gp120 all activate p38 MAPK and upstream, MLK 3 in infected or stimulated, neurotoxic monocytes, and macrophages (Sui et al. 2006; Eggert et al. 2010). Moreover, we have observed that p38 MAPK signaling is essential upon exposure to HIV gp120 for both the neurotoxic phenotype of macrophages and microglia, and the subsequent induction of macrophage toxin-initiated neuronal apoptosis (Medders et al. 2010). The requirement of p38 MAPK activity in both macrophages and neurons for HIV gp120-triggered neurotoxicity to occur is therefore the same that has been reported for the transcription factor p53 (Garden et al. 2004).
Fig. 1.
Summary of the apparent roles for p38 MAPK in HIV-1 infection and associated pathological processes potentially underlying brain injury, including macrophage activation and neurotoxicity, impairment of neurogenesis, and lymphocyte apoptosis. Activation of p38 MAPK by HIV-1 or its components can possibly contribute to the development of HAND and AIDS via various pathways. HIV-1 infection and replication in macrophages is associated with activation and production of neurotoxins (a). Macrophage neurotoxins induced by intact HIV-1 or its components, such as the envelope protein gp120 or the regulatory factor Tat, cause neuronal injury and death, and a direct effect of viral factors may contribute as well (b). HIV-1 or its coat protein gp120 inhibit proliferation of neural progenitors and thus compromise neurogenesis (c), and HIV-1 infection and replication in lymphocytes is associated with syncytia formation and apoptosis (d). HIV-infected and immune-activated, brain-infiltrating macrophages (MΦ) and microglia release presumably neurotoxic factors (reviewed in (Kaul et al. 2001; Gonzalez-Scarano and Martin-Garcia 2005; Kraft-Terry et al. 2009; Lindl et al. 2010)). The list of currently suspected neurotoxins includes quinolinic acid and other excitatory amino acids, such as glutamate and l-cysteine, arachidonic acid, platelet activating factor, a small molecule named ‘NTox’, free radicals, TNF-α, among others. These factors from MΦ/microglia (and also possibly from stimulated astrocytes) contribute to neuronal injury, dendritic and synaptic damage and loss, and apoptosis as well as possibly to astrocytosis. MΦ/microglia and lymphocytes express CCR5 and CXCR4 chemokine receptors on their surface in addition to CD4, and viral gp120 interacts via these receptors. Entry of HIV-1 into MΦ/microglia and lymphocytes occurs via gp120 binding, and therefore it is not surprising that gp120 (and Tat) are capable of activating uninfected MΦ/microglia to release similar factors to those secreted in response to productive HIV infection. In the same way, it may not be surprising that gp120 triggers apoptosis in infected and uninfected lymphocytes. Interestingly, although both CCR5-preferring and CXCR4-preferring HIV-1 variants infect lymphocytes depending on their expression profile of chemokine receptors, syncytia formation apparently occurs exclusively via CXCR4 and HIV/gp120 that preferentially interact with this receptor. Interestingly, Tat can apparently freely cross through cellular membranes by itself and thus potentially affect all cell types. However, Tat also interacts with chemokine receptors, including CXCR4, and NMDA receptors. Neurons (and astrocytes) also possess CXCR4 and CCR5 on their surface, raising the possibility of direct interaction with gp120 in the absence of CD4. Neuronal injury involves overactivation of NMDARs with resultant excessive influx of Ca2+ (reviewed in (Kaul et al. 2001, 2005; Kraft-Terry et al. 2009; Lindl et al. 2010)). This in turn leads to overactivation of a variety of potentially harmful signaling systems, among p38 MAPK. Interference of HIV-1/gp120 with the function of neural progenitor cells induces delay or even arrest of proliferation and promotes quiescence, but apparently does not result in apoptosis (Krathwohl and Kaiser 2004a, b; Okamoto et al. 2007). Solid arrows show direct interaction or effect, while dotted arrows indicate indirect or potential direct interaction. β-Arr β-Arrestin, Cdk1 cyclin-dependent kinase 1, DNp38α mutant, dominant-negative interfering p38α MAPK isoform, IκB inhibitor of NFκBMtCdc25B/C mutant Cdc25B/C, mTOR mammalian target of rapamycin, NFκB nuclear factor κB, shRNA small hairpin RNA, siRNA small interfering RNA, TAB1 transforming growth factor-β-activated protein kinase (TAK1) binding protein 1
The exact molecular mechanism linking in macrophages p38 MAPK with HIV coreceptors that are engaged by viral gp120 remains currently uncertain. However, previous studies have shown that possible mechanisms of HIV-1 neuropathogenesis involve the activation and perturbation of numerous intracellular signaling pathways by HIV-1/ gp120 in association with the release of neurotoxic factors from activated macrophages and microglia (Giulian et al. 1993; Kaul and Lipton 1999; Zheng et al. 1999; Del Corno et al. 2001; Porcheray et al. 2006; O’Donnell et al. 2006). As such, Src family kinase Lyn, PI3K20 (Cheung et al. 2008), Akt (Kaul et al. 2007), the focal adhesion-related proline-rich tyrosine kinase Pyk2 (Del Corno et al. 2001; Cheung et al. 2008), phosphatidylcholine phospholipase C (Fantuzzi et al. 2008), proteins of the MAPK family (Kaul and Lipton 1999; Del Corno et al. 2001; Kaul et al. 2007; Perfettini et al. 2005a; Cheung et al. 2008; Sui et al. 2006; Eggert et al. 2010), and the transcription factor p53 (Garden et al. 2004; Perfettini et al. 2005a) have all been implicated as potential contributors to gp120-induced macrophage activation and neurotoxicity and provide a potential link between HIV coreceptors and p38 MAPK activation. Accordingly, HIV-1 gp120 has been shown to stimulate, besides neurotoxicity, production of proinflammatory cytokines from monocytic cells, which also could be prevented by p38 MAPK inhibition (Fantuzzi et al. 2008; Cheung et al. 2008; Lee et al. 2005; Fig. 1a).
As is the case with HIV coreceptors stimulated by viral gp120 in the presence and absence of CD4, the precise mechanism of p38 MAPK activation in macrophages by Tat remains to be elucidated. However, the aforementioned finding that Tat directly interacts with chemokine receptors CCR2, CCR3, and, in particular, the major HIV coreceptor CXCR4 provides a possible explanation that suggests a pathway similar to that triggered by gp120 (Albini et al. 1998; Xiao et al. 2000; Ghezzi et al. 2000). That interpretation also fits with reports that showed activation of MLK3, p38 MAPK, and JNK in monocytes and macrophages upon exposure to Tat and linked the signaling pathways to neurotoxicity (Sui et al. 2006; Eggert et al. 2010; Fig. 1a). Moreover, a receptor-mediated effect of Tat would also be in line with a report showing induction of CXCL10 in macrophages by HIV Tat and interferon (IFN)γ via a signaling mechanism that involved MEK1/2, p38MAPK, and JAK/signal transducer and activator of transcription (STAT; Dhillon et al. 2008). However, since Tat can move through cell membranes and directly interact with intracellular components, including kinases and transcription factors, it seems very much possible that this viral regulatory protein may activate p38 MAPK in macrophages and potentially other immune cells also in ways that have yet to be characterized (Brana et al. 1999; Maggirwar et al. 1999; Rohr et al. 2000).
HIV-1 infection leads in most cases to a massive demise of CD4+ T-cells by programmed cell death and eventually to AIDS (Pantaleo and Fauci 1995; Perfettini et al. 2005a, b). Since an intact lymphocyte compartment seems to be a pre-requisite for a healthy and fully functional central nervous system, the lasting diminution of CD4+ T-cells could potentially also contribute to the development of HAND (Kipnis et al. 2008). In any case, several studies have identified the viral envelope gp120 as one of the major triggers of apoptosis in the lymphocyte population, affecting both infected and uninfected “bystander” cells (Perfettini et al. 2005a, b; Trushin et al. 2007). Interestingly, those studies have provided evidence that gp120 exerts its deadly effect via a pathway that involves engagement of HIV coreceptors and downstream activation in parallel of NFkB and p38 MAPK pathways and a direct phosphorylation and pro-apoptotic activation of p53 by mammalian target of rapamycin and p38 MAPK (Perfettini et al. 2005a, b). Importantly, one of the studies has demonstrated activated, phosphorylated p38 MAPK and p53 in multinucleated giant cells in the cortex of patients with HIV-associated dementia, the most severe manifestation of HAND, but not healthy controls (Perfettini et al. 2005a). Altogether, these observations strongly support a role for p38 MAPK and p53 in the development of AIDS and HAND (Fig. 1).
There exists also a substantial amount of evidence associating elevated p38 MAPK activation with inflammation of the brain, as that seen in HIVE patients and Alzheimer’s disease (AD; Kaminska et al. 2009). Activation of p38 MAPK signaling also occurred in neurons that were cocultured with LPS-activated microglia and this signaling was vital to induction of neuronal death (Xie et al. 2004). Amyloid-β-induced activation of p38 MAPK resulted in upregulation of pro-inflammatory cytokines TNFα, inducible NO synthase (iNOS), and IL-1β, and subsequent neuronal death (Delgado et al. 2008). Our and other groups have shown that inhibition of p38 MAPK activity in vitro with pharmacological inhibitor SB203580 or dominant negative p38αAF (TGY substituted with AGF) prevented neuronal apoptosis induced by HIV-1 gp120 or glutamate (Kaul and Lipton 1999; Singh et al. 2005; Kaul et al. 2007; Choi et al. 2007; Chaparro-Huerta et al. 2008; Fig. 1b). A separate study showed that in neurons HIV-1 Tat and gp120 both activate MLK 3, which controls p38 MAPK and JNK pathways (Sui et al. 2006; Gallo and Johnson 2002). The same and one additional report also found that inhibition or MLK 3 protected neurons from toxicity of gp120, Tat and HIV-1 infected macrophages (Sui et al. 2006; Eggert et al. 2010). Of note, while independent pharmacological inhibition of p38 MAPK or JNK can both ameliorate the toxicity of Tat in cerebellar granular neurons, only blockade of p38 MAPK, but not JNK, can prevent Tat-induced death in striatal neurons (Singh et al. 2005; Sui et al. 2006).
Interestingly, primary neuronal cell cultures and macrophages, perhaps gradually more than monocytic THP-1 cells, all display a substantial level of active p38 MAPK at baseline (Singh et al. 2005; Kaul et al. 2001; Semenova et al. 2007; Choi et al. 2007; Kaul et al. 2007; Medders et al. 2010; Eggert et al. 2010). Given the diverse physiological functions in which p38 MAPK has been implicated, the observation of a clearly detectable baseline activity may not be surprising. On the other hand, little is known about what drives and maintains this baseline activity and what the exact function of this “tonic” kinase activity is. One possible explanation is as yet undefined housekeeping functions of p38 MAPK. In fact, the general genetic knockout of p38α, but not of the other isoforms, is embryonally lethal (Adams et al. 2000; Tamura et al. 2000; Chang and Karin 2001; Kumar et al. 2003). In contrast, conditional knockouts of p38α in epidermal, myeloid, and even monocytic cells are non-lethal but affect innate immune functions and inflammation (Kim et al. 2008; Kang et al. 2008). In neurons, p38α activity is regulated by intracellular Ca2+ concentration and the small GTPase Rho and linked to the activity of NMDA type of glutamate receptors and excitotoxicity (Semenova et al. 2007). Indeed, several studies have suggested that excitoxicity itself constitutes one important mechanism of HIV-1 induced neurotoxicity (Dreyer et al. 1990; O’Donnell et al. 2006; Kaul et al. 2001; Lindl et al. 2010). The link between glutamate receptors, influx of Ca2+, Rho, and activation of p38α certainly suggests that in neurons synaptic activity may be a major regulator of the kinase’s baseline activity. Our studies indicate that in the context of HIV/gp120-induced, macrophage-mediated neurotoxicity inhibition of p38 MAPK prevents neuronal death, but surprisingly, neurons surviving the viral envelope’s toxicity in the absence of kinase inhibitor display a higher level of active p38 MAPK compared to control cells (Medders et al. 2010). Similarly, macrophages also show a sustained increase of active p38 MAPK over up to 4 days of exposure to HIV gp120 (Medders et al. 2010), or 24 h after infection with intact virus (Eggert et al. 2010). Whereas a heightened level of active kinase in macrophages can easily be explained as a sign of continuing immune activation, the lasting elevation of p38 MAPK activity in neurons raises the question of how that condition is possible without inducing neuronal apoptosis. While we cannot yet provide an answer to this question, our findings suggest that in neurons an adaptive mechanism exists that allows for survival in the presence of an increased fraction of active of p38 MAPK (Medders et al. 2010). The fact that blockade of p38 MAPK activity allows for neurons to survive at control levels, suggests that a failure to reduce p38 MAPK activity to baseline or to trigger the above proposed adaptive process may leave neurons vulnerable to induction of apoptosis by HIV/gp120-initiated macrophage toxins. Of note, our studies also indicate that gp120 directly interacts with neurons and possibly astrocytes in the absence of microglia (and CD4), and that interaction also results in activation of neuronal p38 MAPK. However, that increase of kinase activity is short-lived and does not result in neuronal death in mixed neuronal-astrocytic cell cultures (Medders et al. 2010).
In the case of Tat, interaction with the NMDA receptor, and consequent modulation of intracellular Ca2+ homeostasis, provides a possible explanation for the activation of p38 MAPK in neurons (Li et al. 2008; Neri et al. 2007; Fig. 1b). Tat has also been reported to initiate in neurons a macromolecular complex comprising LRP, postsynaptic density protein-95, NMDA receptors, and neuronal nitric oxide synthase that locates to the plasma membrane (Eugenin et al. 2007). Interestingly, that protein complex seems to cause apoptosis not only in NMDA receptor-expressing neurons but also in neurons lacking that type of ionotropic glutamate receptor and astrocytes. Regarding Tat’s pathological effect on intracellular ion homeostasis, other reports showed the viral protein to activate neuronal ryanodine receptors, which in turn induced an unfolded protein response and mitochondrial hyper-polarization (Norman et al. 2008). Tat was also able to increase intracellular Ca2+, to trigger mitochondrial production of reactive oxygen species and peroxynitrite, and caspase activation and eventually neuronal cell death (Kruman et al. 1998). Independently, Tat has been found to induce neuronal expression of TNFα and cause neurotoxicity in a pathway involving NFκB (New et al. 1998). Another detrimental pathway directly triggered by Tat in neurons includes increased expression of components of the p53 pathway, p53, and p73, followed by retraction of neurites and expression of the pro-apoptotic Bcl2 family protein Bax (Mukerjee et al. 2008). Finally, Tat has also been found to affect neuronal synapses by dysregulating microRNAs. Altered expression of neuronal miR-128 inhibits expression of the presynaptic protein SNAP25 (Eletto et al. 2008). Although none of these studies on Tat’s direct effect(s) on neurons addressed the activation of p38 MAPK per se, all the presented data and pathways share a common phenomenon, namely cellular stress and deterioration, which makes the presumed involvement of the stress-associated kinase very feasible. In fact, the studies discussed in this paragraph all provide potential explanations for the activation of p38 MAPK and JNK following direct exposure to Tat that has been reported for striatal neurons (Singh et al. 2005).
Astrocytes are widely believed to be ill-suited for productive HIV-1 infection which may only occur under certain circumstances, such as stimulation of the glial cells with IFNγ prior to contact with virus (Blumberg et al. 1994; Carroll-Anzinger and al-Harthi 2006; Carroll-Anzinger et al. 2007). In any case, infection of astrocytes by the virus appears to be mediated by a mannose receptor (Liu et al. 2004) while a direct interaction of gp120 with astrocytic chemokine receptors may also indirectly contribute to neuronal injury independently of whether or not an infection occurs (Klein et al. 1999; Lazarini et al. 2000; Li et al. 2007). Although we observed a clearly detectable amount of p38 MAPK protein in astrocytes, exposure to HIV gp120 did not result in a significant increase in the amount of active kinase in this cell type (Medders et al. 2010). However, increased active p38 MAPK and JNK have been reported in brains of SIV-infected macaques with encephalitis (Barber et al. 2004). Interestingly, p38 MAPK has also been implicated in the reaction of astrocytes exposed to HIV-1 or Tat, such as the production and release of CCL2, CXCL8/IL-8 and CXCL10 (Kutsch et al. 2000; Williams et al. 2009a, b; Zheng et al. 2008).
Comparable to the direct interaction of HIV/gp120 with neurons and astrocytes, recent evidence strongly suggests that the virus and its envelope protein can directly interfere with biological functions of neural stem and progenitor cells (reviewed in (Kaul 2008)). Stem cells are thought to be undifferentiated, multipotent, and to possess unlimited capacity of self-renewal, whereas progenitor cells are considered to represent a population with limited ability to proliferate and committed to a restricted number of differentiation pathways (Morrison et al. 1997). Both of these cell types lack CD4, but express the two major HIV coreceptors. Of those, the CXCR4-CXCL12/SDF-1 receptor–ligand axis serves important roles in the physiological function of hematopoietic and neural stem cells (Asensio and Campbell 1999; Tran and Miller 2003; Lazarini et al. 2003; Belmadani et al. 2005). In addition, we have observed that activation of CCR5 can cross-desensitize CXCR4 in cerebrocortical neuronal cell cultures (Kaul et al. 2007). Thus, whatever the coreceptor usage of a given HIV envelope may be, both CCR5-preferring HIV/gp120 and CXCR4-preferring virus can potentially interfere with physiological processes depending on CXCR4-mediated signaling. Moreover, it has been reported that human neural progenitor cells are permissive to HIV-1 whereas the virus apparently fails to productively infect neurons (Lawrence et al. 2004; Mattson et al. 2005; Schwartz and Major 2006). In any case, HIV-1/gp120 and chemokines can affect human or rodent neural progenitor cells (Krathwohl and Kaiser 2004a; Krathwohl and Kaiser 2004b; Okamoto et al. 2007). Comparable to what has been observed by others in brain specimen from HAND/HAD patients, we also found a reduction of proliferating neural progenitors in the hippocampal dentate gyrus of transgenic mice that express HIV/ gp120 in the brain in comparison to non-transgenic controls (Krathwohl and Kaiser 2004b; Okamoto et al. 2007). In an immunofluorescence study, we found that progenitors express both HIV coreceptors, CXCR4 and CCR5, and treatment with HIV-1/gp120 in vitro reduced the proliferation of adult progenitors without producing apoptosis (Okamoto et al. 2007). Further analysis revealed that gp120 inhibits proliferation of neural progenitor cells through activation of a pathway that engages in the following order p38MAPK, MAPKAPK2 (MK2), a cell cycle checkpoint kinase, and Cdc25B/C which in turn causes a delay or even arrest of the cell cycle in the G1 phase (Fig. 1c). The compromised proliferation of neural progenitors imposed by gp120 diminishes the number of progenitor cells for subsequent differentiation into neurons, thus quantitatively impairing neurogenesis (Okamoto et al. 2007). Of note, neurogenesis in the hippocampal dentate gyrus is considered to be essential for learning and memory formation, neurocognitive functions which are impaired in HAND. The apparent ability of HIV-1 and its envelope to interfere with the normal function of neural progenitor cells raises the possibility that the development of HAND entails not only injury and death of existing neurons in response to soluble, macrophage-derived neurotoxins initiated by HIV-1 gp120, Tat or the intact virus, but also virus-induced disturbance of tissue homeostasis and renewal mechanisms in the CNS.
While the here discussed literature clearly supports the notion that activation of p38 MAPK may play a critical role in HIV-1 infection and development of AIDS and HAND, there still remains a lot to be learned about signaling pathways downstream of the MAPK, in particular in immune and neural cell types. The transcription factor p53 and the kinase MAPKAPK2 are the two downstream substrates of p38 MAPK that have been most directly implicated in HIV infection, AIDS and HAND: the transcription factor in macrophages, lymphocytes and neurons, the kinase in neural progenitors (Garden et al. 2004; Perfettini et al. 2005a; Okamoto et al. 2007). However, the induction of cytokines and chemokines by HIV-1, gp120 or Tat in immune cells and astrocytes that has been linked to p38 MAPK suggests that MAPKAPK2-dependent signaling also occurs in these cell types (Pearson et al. 2001; Koistinaho and Koistinaho 2002; Kumar et al. 2003; Mayer and Callahan 2006; Chopra et al. 2008). Downstream targets of p38α MAPK are found in all cell types, including immune and neural cells, and comprise besides kinases, transcription factors and a spectrum of other proteins (Chopra et al. 2008). In contrast, substrates of the other p38 MAPK isoforms are more restricted to the respective cell types in which the kinases are expressed, but those downstream targets are largely shared with p38α MAPK. For example, activating transcription factor-2 can in principle be phosphorylated in macrophages and CD4+ T cells by p38α and δ but proinflammatory signaling in general has mostly been linked to activation of the α isoform (Hale et al. 1999; Chopra et al. 2008). Interestingly, p38 MAPK also directly activates cytosolic phospholipase A2 and STAT-1 and thus plays a role in IFN signaling (Goh et al. 1999; Kovarik et al. 1999; Li et al. 2004). Regarding neuronal signal transduction other than that occurring in neural progenitor cells, two substrates of p38α MAPK have recently drawn scrutiny in the context of neuronal development and survival on one and neurodegeneration on the other hand: myocyte-enhancing factor −2 C, a transcription factor, and the microtubule-associated protein Tau (Mao et al. 1999; Goedert et al. 1997; Munoz and Ammit 2010). In fact, increased phosphorylation of Tau has not only been found in AD but also in neuropathological changes induced by HIV gp120 (Kang et al. 2010). However, it remains to be shown how much p38 MAPK contributes to pathological Tau phosphorylation in neurons in AD and HAND.
Potential therapeutic strategies for targeting p38 MAPK in HIV infection and development of HAND
The mixed success of p38 MAPK inhibitors in clinical trials for several diseases other than HIV infection and HAND was due to concerns about possible side effects (Mayer and Callahan 2006; Chopra et al. 2008). Yet, the potentially important role of p38 MAPK in HIV-1 infection and development of AIDS and HAND, in combination with the lack of a specific therapy for neuroAIDS and HAND warrant a continued search for improved inhibitors of the kinase and better therapeutic strategies. The multitude of physiological processes to which p38 MAPK contributes, the strong context dependence of the kinase’s biological function, and the presence of three isoforms with cell type-restricted expression patterns, all may pose challenges and opportunities at the same time. The universal expression of p38α and its prominent role in inflammation may require the development of new drug delivery approaches rather than new inhibitors. Novel delivery strategies should allow for targeting of specific cell types or tissues. That may currently seem most feasible for certain peripheral immune cells, where inhibition of p38 MAPK could help to limit the spread of the virus by complementing the effects of HAART, but it could eventually also become possible for the central nervous system in its entirety or even for specific neural cell types. Intranasal application, such as recently shown for erythropoietin in combination with insulin-like growth factor-1, may be one route to enhanced drug delivery to the central nervous system (Kang et al. 2010), but additional innovative approaches will be required to accomplish cell type-specific delivery of p38 MAPK inhibitors within the brain.
Most previously tested p38 MAPK inhibitors primarily interact with the α- and β-isoforms and predominantly act as ATP competitiors (Kumar et al. 2003; Mayer and Callahan 2006; Chopra et al. 2008). This situation leaves the other isoforms yet to be explored as targets, such as p38δ which is strongly expressed in two major HIV targets, macrophages and CD4+ T cells (Hale et al. 1999). Furthermore, compounds blocking other parts of p38 MAPK than the ATP binding site could also provide new and improved therapeutic properties. For that reason, dominant negative-interfering mutants (DN), such as p38αAF, could be helpful, in particular if the mutant proteins could be targeted to specific cell types and tissues (Medders et al. 2010). It remains to be seen for example whether the above discussed intranasal application can facilitate the delivery of DN p38 mutants into the brain.
Interestingly, protective inhibition of p38 MAPK can apparently also be achieved by compounds that were not initially developed as kinase inhibitors or may have more than one mechanism of action. An example of such a scenario is provided by minocycline, a tetracycline antibiotic that readily crosses the blood–brain barrier and has been clinically used since the 1970s (Cappel and Klastersky 1971). Minocycline has been found to protect neurons against excitotoxicity and to prevent excitotoxin-induced p38 MAPK activation, and production of nitric oxide (NO) and IL-1 as well as proliferation in microglia (Tikka et al. 2001). In cerebellar granular neurons, the tetracycline abrogates NO-induced death by inhibiting p38 MAPK (Lin et al. 2001), and in glia the antibiotic reduces expression of iNOS and caspase-1 (Du et al. 2001). In addition, one study found that minocycline protects neurons against glutamate-induced excitotoxicity by not only preventing activation of p38 MAPK but also stabilizing the level of active Akt (Pi et al. 2004). Potentially important for clinical application in HIV-1 infection and HAND, the tetracycline reduces active p38 MAPK, inflammatory markers and brain injury in a model of SIV encephalitis (SIVE), and inhibits in vitro SIV and HIV infection (Zink et al. 2005). A more recent report proposes as the underlying mechanism a suppression of SIVE involving reduction of NO production and inhibition of apoptosis signal-regulating kinase-1 with consequent reduction of active p38 MAPK and JNK (Follstaedt et al. 2008). While all these studies clearly associate the protective effect of minocycline with a reduction of p38 MAPK activity, it remains unclear if the tetracycline inhibits the kinase in a direct or indirect fashion. In fact, it has been suggested that the cytoprotective effect of minocycline occurs at the mitochondrial level by prevention of a pro-apoptotic cell stress pathway that comprises a permeability transition event at the organelle’s membrane followed by the release of cytochrome C and activation of caspase-3 (Zhu et al. 2002). Since microglia and astrocytes can be activated by distressed neurons, a reduction of active p38 MAPK in glial cells and neurons could indirectly result from the prevention by minocycline of injurious neuronal stress which in turn ameliorates glial activation. However, regarding inhibition of HIV or SIV infection by the tetracycline, it is unknown if a mitochondria-based mechanism is involved. Thus, despite the fact that questions remain about the exact protective mechanism, minocycline is being evaluated in a clinical trial for a therapeutic effect in HIV-1 infection of the central nervous system (ClinicalTrials.gov Identifier: NCT01064752).
In summary, signaling via p38 MAPK constitutes a common crucial theme in HIV-1 infection and development of AIDS and HAND. Hence, the continued quest for better p38 MAPK inhibitors and strategies for their targeted delivery remains an urgent matter.
Acknowledgments
MK is supported by grants from the National Institutes of Health, MH087332, DA026306, and DA029480.
Footnotes
Conflict of interest disclosure The authors state that they have no conflict of interest.
Contributor Information
Kathryn E. Medders, Infectious and Inflammatory Disease Center, Sanford-Burnham Medical Research Institute, 10901 North Torrey Pines Road, La Jolla, CA 92037, USA
Marcus Kaul, Infectious and Inflammatory Disease Center, Sanford-Burnham Medical Research Institute, 10901 North Torrey Pines Road, La Jolla, CA 92037, USA, mkaul@sanfordburnham.org; Department of Psychiatry, University of California, San Diego, 9500 Gilman Drive, La Jolla, CA 92093, USA.
References
- Achim CL, Wang R, Miners DK, Wiley CA. Brain viral burden in HIV infection. J Neuropathol Exp Neurol. 1994;53:284–294. doi: 10.1097/00005072-199405000-00010. [DOI] [PubMed] [Google Scholar]
- Adams RH, Porras A, Alonso G, Jones M, Vintersten K, Panelli S, Valladares A, Perez L, Klein R, Nebreda AR. Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol Cell. 2000;6:109–116. [PubMed] [Google Scholar]
- Albini A, Ferrini S, Benelli R, Sforzini S, Giunciuglio D, Aluigi MG, Proudfoot AE, Alouani S, Wells TN, Mariani G, Rabin RL, Farber JM, Noonan DM. HIV-1 Tat protein mimicry of chemokines. Proc Natl Acad Sci USA. 1998;95:13153–13158. doi: 10.1073/pnas.95.22.13153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res. 2008;6:388–400. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhatib G, Combadiere C, Broder CC, Feng Y, Kennedy PE, Murphy PM, Berger EA. CC CKR5: a RANTES, MIP-1alpha, MIP-1beta receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asensio VC, Campbell IL. Chemokines in the CNS: plurifunctional mediators in diverse states. Trends Neurosci. 1999;22:504–512. doi: 10.1016/s0166-2236(99)01453-8. [DOI] [PubMed] [Google Scholar]
- Asensio VC, Maier J, Milner R, Boztug K, Kincaid C, Moulard M, Phillipson C, Lindsley K, Krucker T, Fox HS, Campbell IL. Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro. J Virol. 2001;75:7067–7077. doi: 10.1128/JVI.75.15.7067-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber SA, Uhrlaub JL, DeWitt JB, Tarwater PM, Zink MC. Dysregulation of mitogen-activated protein kinase signaling pathways in simian immunodeficiency virus encephalitis. Am J Pathol. 2004;164:355–362. doi: 10.1016/S0002-9440(10)63125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmadani A, Tran PB, Ren D, Assimacopoulos S, Grove EA, Miller RJ. The chemokine stromal cell-derived factor-1 regulates the migration of sensory neuron progenitors. J Neurosci. 2005;25:3995–4003. doi: 10.1523/JNEUROSCI.4631-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer TA. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- Blumberg BM, Gelbard HA, Epstein LG. HIV-1 infection of the developing nervous system: central role of astrocytes in pathogenesis. Virus Res. 1994;32:253–267. doi: 10.1016/0168-1702(94)90044-2. [DOI] [PubMed] [Google Scholar]
- Brana C, Biggs TE, Barton CH, Sundstrom LE, Mann DA. A soluble factor produced by macrophages mediates the neurotoxic effects of HIV-1 Tat in vitro. AIDS. 1999;13:1443–1452. doi: 10.1097/00002030-199908200-00002. [DOI] [PubMed] [Google Scholar]
- Brenneman DE, Westbrook GL, Fitzgerald SP, Ennist DL, Elkins KL, Ruff MR, Pert CB. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature. 1988;335:639–642. doi: 10.1038/335639a0. [DOI] [PubMed] [Google Scholar]
- Cappel R, Klastersky J. Bacteriologic and clinical evaluation of minocycline, a new tetracycline. Curr Ther Res Clin Exp. 1971;13:227–233. [PubMed] [Google Scholar]
- Carroll-Anzinger D, al-Harthi L. Gamma interferon primes productive human immunodeficiency virus infection in astrocytes. J Virol. 2006;80:541–544. doi: 10.1128/JVI.80.1.541-544.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll-Anzinger D, Kumar A, Adarichev V, Kashanchi F, al-Harthi L. Human immunodeficiency virus-restricted replication in astrocytes and the ability of gamma interferon to modulate this restriction are regulated by a downstream effector of the Wnt signaling pathway. J Virol. 2007;81:5864–5871. doi: 10.1128/JVI.02234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- Chaparro-Huerta V, Flores-Soto ME, Gudiño-Cabrera G, Rivera-Cervantes MC, Bitzer-Quintero OK, Beas-Zárate C. Role of p38 MAPK and pro-inflammatory cytokines expression in glutamate-induced neuronal death of neonatal rats. Int J Dev Neurosci. 2008;26:487–495. doi: 10.1016/j.ijdevneu.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Cheung R, Ravyn V, Wang L, Ptasznik A, Collman RG. Signaling mechanism of HIV-1 gp120 and virion-induced IL-1beta release in primary human macrophages. J Immunol. 2008;180:6675–6684. doi: 10.4049/jimmunol.180.10.6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WT, Kaul M, Kumar S, Wang J, Kumar IM, Dong CZ, An J, Lipton SA, Huang Z. Neuronal apoptotic signaling pathways probed and intervened by synthetically and modularly modified (SMM) chemokines. J Biol Chem. 2007;282:7154–7163. doi: 10.1074/jbc.M611599200. [DOI] [PubMed] [Google Scholar]
- Chopra P, Kanoje V, Semwal A, Ray A. Therapeutic potential of inhaled p38 mitogen-activated protein kinase inhibitors for inflammatory pulmonary diseases. Expert Opin Investig Drugs. 2008;17:1411–1425. doi: 10.1517/13543784.17.10.1411. [DOI] [PubMed] [Google Scholar]
- Chun TW, Fauci AS. Latent reservoirs of HIV: obstacles to the eradication of virus. Proc Natl Acad Sci USA. 1999;96:10958–10961. doi: 10.1073/pnas.96.20.10958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PS, Schmidtmayerova H, Dennis J, Dubrovsky L, Sherry B, Wang H, Bukrinsky M, Tracey KJ. The critical role of p38 MAP kinase in T cell HIV-1 replication. Mol Med. 1997;3:339–346. [PMC free article] [PubMed] [Google Scholar]
- D’hooge R, Franck F, Mucke L, De Deyn PP. Age-related behavioural deficits in transgenic mice expressing the HIV-1 coat protein gp120. Eur J Neurosci. 1999;11:4398–4402. doi: 10.1046/j.1460-9568.1999.00857.x. [DOI] [PubMed] [Google Scholar]
- Dean JL, Brook M, Clark AR, Saklatvala J. p38 mitogen-activated protein kinase regulates cyclooxygenase-2 mRNA stability and transcription in lipopolysaccharide-treated human monocytes. J Biol Chem. 1999;274:264–269. doi: 10.1074/jbc.274.1.264. [DOI] [PubMed] [Google Scholar]
- Del Corno M, Liu QH, Schols D, De Clercq E, Gessani S, Freedman BD, Collman RG. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood. 2001;98:2909–2916. doi: 10.1182/blood.v98.10.2909. [DOI] [PubMed] [Google Scholar]
- Delgado M, Varela N, Gonzalez-Rey E. Vasoactive intestinal peptide protects against beta-amyloid-induced neurodegeneration by inhibiting microglia activation at multiple levels. Glia. 2008;56:1091–1103. doi: 10.1002/glia.20681. [DOI] [PubMed] [Google Scholar]
- Dhillon N, Zhu X, Peng F, Yao H, Williams R, Callen S, Ladner AO, Buch S, Qiu J. Molecular mechanism(s) involved in the synergistic induction of CXCL10 by human immunodeficiency virus type 1 Tat and interferon-gamma in macrophages. J Neurovirol. 2008;14:196–204. doi: 10.1080/13550280801993648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragic T, Litwin V, Allaway GP, Martin SR, Huang Y, Nagashima KA, Cayanan C, Maddon PJ, Koup RA, Moore JP, Paxton WA. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- Dreyer EB, Kaiser PK, Offermann JT, Lipton SA. HIV-1 coat protein neurotoxicity prevented by calcium channel antagonists. Science. 1990;248:364–367. doi: 10.1126/science.2326646. [DOI] [PubMed] [Google Scholar]
- Du Y, Ma Z, Lin S, Dodel RC, Gao F, Bales KR, Triarhou LC, Chernet E, Perry KW, Nelson DL, Luecke S, Phebus LA, Bymaster FP, Paul SM. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson’s disease. Proc Natl Acad Sci USA. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edinger AL, Amedee A, Miller K, Doranz BJ, Endres M, Sharron M, Samson M, Lu ZH, Clements JE, Murphey-Corb M, Peiper SC, Parmentier M, Broder CC, Doms RW. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci USA. 1997;94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert D, Dash PK, Gorantla S, Dou H, Schifitto G, Maggirwar SB, Dewhurst S, Poluektova L, Gelbard HA, Gendelman HE. Neuroprotective activities of CEP-1347 in models of neuroAIDS. J Immunol. 2010;184:746–756. doi: 10.4049/jimmunol.0902962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eletto D, Russo G, Passiatore G, Del VL, Giordano A, Khalili K, Gualco E, Peruzzi F. Inhibition of SNAP25 expression by HIV-1 Tat involves the activity of mir-128a. J Cell Physiol. 2008;216:764–770. doi: 10.1002/jcp.21452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci USA. 2007;104:3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi L, Spadaro F, Purificato C, Cecchetti S, Podo F, Belardelli F, Gessani S, Ramoni C. Phosphatidylcholine-specific phospholipase C activation is required for CCR5-dependent, NF-kB-driven CCL2 secretion elicited in response to HIV-1 gp120 in human primary macrophages. Blood. 2008;111:3355–3363. doi: 10.1182/blood-2007-08-104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S, Seery J, Daikh Y, Moore C, Chen LL, Pepinsky B, Barsoum J. Tat-mediated delivery of heterologous proteins into cells. Proc Natl Acad Sci USA. 1994;91:664–668. doi: 10.1073/pnas.91.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follstaedt SC, Barber SA, Zink MC. Mechanisms of minocycline-induced suppression of simian immunodeficiency virus encephalitis: inhibition of apoptosis signal-regulating kinase 1. J Neurovirol. 2008;14:376–388. doi: 10.1080/13550280802199898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol. 2002;3:663–672. doi: 10.1038/nrm906. [DOI] [PubMed] [Google Scholar]
- Garden GA, Guo W, Jayadev S, Tun C, Balcaitis S, Choi J, Montine TJ, Moller T, Morrison RS. HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J. 2004;18:1141–1143. doi: 10.1096/fj.04-1676fje. [DOI] [PubMed] [Google Scholar]
- Ge B, Gram H, Di PF, Huang B, New L, Ulevitch RJ, Luo Y, Han J. MAPKK-independent activation of p38alpha mediated by TAB1-dependent autophosphorylation of p38alpha. Science. 2002;295:1291–1294. doi: 10.1126/science.1067289. [DOI] [PubMed] [Google Scholar]
- Gelbard HA, Dewhurst S, Maggirwar SB, Kiebala M, Polesskaya O, Gendelman HE. Rebuilding synaptic architecture in HIV-1 associated neurocognitive disease: a therapeutic strategy based on modulation of mixed lineage kinase. Neurotherapeutics. 2010;7:392–398. doi: 10.1016/j.nurt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi S, Noonan DM, Aluigi MG, Vallanti G, Cota M, Benelli R, Morini M, Reeves JD, Vicenzi E, Poli G, Albini A. Inhibition of CXCR4-dependent HIV-1 infection by extracellular HIV-1 Tat. Biochem Biophys Res Commun. 2000;270:992–996. doi: 10.1006/bbrc.2000.2523. [DOI] [PubMed] [Google Scholar]
- Giulian D, Vaca K, Noonan CA. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- Giulian D, Wendt E, Vaca K, Noonan CA. The envelope glycoprotein of human immunodeficiency virus type 1 stimulates release of neurotoxins from monocytes. Proc Natl Acad Sci USA. 1993;90:2769–2773. doi: 10.1073/pnas.90.7.2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Goedert M, Hasegawa M, Jakes R, Lawler S, Cuenda A, Cohen P. Phosphorylation of microtubule-associated protein tau by stress-activated protein kinases. FEBS Lett. 1997;409:57–62. doi: 10.1016/s0014-5793(97)00483-3. [DOI] [PubMed] [Google Scholar]
- Goh KC, Haque SJ, Williams BR. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 1999;18:5601–5608. doi: 10.1093/emboj/18.20.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81. doi: 10.1038/nri1527. [DOI] [PubMed] [Google Scholar]
- Hale KK, Trollinger D, Rihanek M, Manthey CL. Differential expression and activation of p38 mitogen-activated protein kinase alpha, beta, gamma, and delta in inflammatory cell lineages. J Immunol. 1999;162:4246–4252. [PubMed] [Google Scholar]
- Halks-Miller M, Hesselgesser J, Miko IJ, Horuk R. Chemokine receptors in developing human brain. Methods Enzymol. 1997;288:27–38. doi: 10.1016/s0076-6879(97)88005-6. [DOI] [PubMed] [Google Scholar]
- He J, Chen Y, Farzan M, Choe H, Ohagen A, Gartner S, Busciglio J, Yang X, Hofmann W, Newman W, Mackay CR, Sodroski J, Gabuzda D. CCR3 and CCR5 are coreceptors for HIV-1 infection of microglia. Nature. 1997;385:645–649. doi: 10.1038/385645a0. [DOI] [PubMed] [Google Scholar]
- He N, Liu M, Hsu J, Xue Y, Chou S, Burlingame A, Krogan NJ, Alber T, Zhou Q. HIV-1 Tat and host AFF4 recruit two transcription elongation factors into a bifunctional complex for coordinated activation of HIV-1 transcription. Mol Cell. 2010;38:428–438. doi: 10.1016/j.molcel.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesselgesser J, Halks-Miller M, DelVecchio V, Peiper SC, Hoxie J, Kolson DL, Taub D, Horuk R. CD4-independent association between HIV-1 gp120 and CXCR4: functional chemokine receptors are expressed in human neurons. Curr Biol. 1997;7:112–121. doi: 10.1016/s0960-9822(06)00055-8. [DOI] [PubMed] [Google Scholar]
- Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–598. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Martin A, Price RW, Salazar AM, Sidtis JJ, Yergey JA, Mouradian MM, Sadler AE, Keilp J, Rubinow D, Markey SP. Quinolinic acid in cerebrospinal fluid and serum in HIV-1 infection: relationship to clinical and neurological status. Ann Neurol. 1991;29:202–209. doi: 10.1002/ana.410290215. [DOI] [PubMed] [Google Scholar]
- Kadoki M, Choi BI, Iwakura Y. The mechanism of LPS-induced HIV type I activation in transgenic mouse macrophages. Int Immunol. 2010;22:469–478. doi: 10.1093/intimm/dxq032. [DOI] [PubMed] [Google Scholar]
- Kaminska B, Gozdz A, Zawadzka M, Ellert-Miklaszewska A, Lipko M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat Rec Hoboken. 2009;292:1902–1913. doi: 10.1002/ar.21047. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Seit-Nebi A, Davis RJ, Han J. Multiple activation mechanisms of p38alpha mitogen-activated protein kinase. J Biol Chem. 2006;281:26225–26234. doi: 10.1074/jbc.M606800200. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Chen J, Otsuka M, Mols J, Ren S, Wang Y, Han J. Macrophage deletion of p38alpha partially impairs lipopolysaccharide-induced cellular activation. J Immunol. 2008;180:5075–5082. doi: 10.4049/jimmunol.180.7.5075. [DOI] [PubMed] [Google Scholar]
- Kang YJ, Digicaylioglu M, Russo R, Kaul M, Achim CL, Fletcher L, Masliah E, Lipton SA. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann Neurol. 2010;68:342–352. doi: 10.1002/ana.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M. HIV’s double strike at the brain: neuronal toxicity and compromised neurogenesis. Front Biosci. 2008;13:2484–2494. doi: 10.2741/2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Lipton SA. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc Natl Acad Sci USA. 1999;96:8212–8216. doi: 10.1073/pnas.96.14.8212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaul M, Zheng J, Okamoto S, Gendelman HE, Lipton SA. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–892. doi: 10.1038/sj.cdd.4401623. [DOI] [PubMed] [Google Scholar]
- Kaul M, Ma Q, Medders KE, Desai MK, Lipton SA. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007;14:296–305. doi: 10.1038/sj.cdd.4402006. [DOI] [PubMed] [Google Scholar]
- Kim BO, Liu Y, Ruan Y, Xu ZC, Schantz L, He JJ. Neuropathologies in transgenic mice expressing human immunodeficiency virus type 1 Tat protein under the regulation of the astrocyte-specific glial fibrillary acidic protein promoter and doxycycline. Am J Pathol. 2003;162:1693–1707. doi: 10.1016/S0002-9440(10)64304-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C, Sano Y, Todorova K, Carlson BA, Arpa L, Celada A, Lawrence T, Otsu K, Brissette JL, Arthur JS, Park JM. The kinase p38 alpha serves cell type-specific inflammatory functions in skin injury and coordinates pro- and anti-inflammatory gene expression. Nat Immunol. 2008;9:1019–1027. doi: 10.1038/ni.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Derecki NC, Yang C, Scrable H. Immunity and cognition: what do age-related dementia, HIV-dementia and ‘'chemo-brain’ have in common? Trends Immunol. 2008;29:455–463. doi: 10.1016/j.it.2008.07.007. [DOI] [PubMed] [Google Scholar]
- Klein RS, Williams KC, Alvarez-Hernandez X, Westmoreland S, Force T, Lackner AA, Luster AD. Chemokine receptor expression and signaling in macaque and human fetal neurons and astrocytes: implications for the neuropathogenesis of AIDS. J Immunol. 1999;163:1636–1646. [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40:175–183. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- Kovarik P, Stoiber D, Eyers PA, Menghini R, Neininger A, Gaestel M, Cohen P, Decker T. Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen- activated protein kinase whereas IFN-gamma uses a different signaling pathway. Proc Natl Acad Sci USA. 1999;96:13956–13961. doi: 10.1073/pnas.96.24.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64:133–145. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004a;22:109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV-1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004b;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Krucker T, Toggas SM, Mucke L, Siggins GR. Transgenic mice with cerebral expression of human immunodeficiency virus type-1 coat protein gp120 show divergent changes in short- and long-term potentiation in CA1 hippocampus. Neuroscience. 1998;83:691–700. doi: 10.1016/s0306-4522(97)00413-2. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Kumar S, Orsini MJ, Lee JC, McDonnell PC, Debouck C, Young PR. Activation of the HIV-1 long terminal repeat by cytokines and environmental stress requires an active CSBP/p38 MAP kinase. J Biol Chem. 1996;271:30864–30869. doi: 10.1074/jbc.271.48.30864. [DOI] [PubMed] [Google Scholar]
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726. doi: 10.1038/nrd1177. [DOI] [PubMed] [Google Scholar]
- Kutsch O, Oh J, Nath A, Benveniste EN. Induction of the chemokines interleukin-8 and IP-10 by human immunodeficiency virus type 1 tat in astrocytes. J Virol. 2000;74:9214–9221. doi: 10.1128/jvi.74.19.9214-9221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannuzel A, Lledo PM, Lamghitnia HO, Vincent JD, Tardieu M. HIV-1 envelope proteins gp120 and gp160 potentiate NMDA [Ca2+]i increase, alter [Ca2+]i homeostasis and induce neurotoxicity in human embryonic neurons. Eur J Neurosci. 1995;7:2285–2293. doi: 10.1111/j.1460-9568.1995.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Lavi E, Strizki JM, Ulrich AM, Zhang W, Fu L, Wang Q, O’Connor M, Hoxie JA, Gonzalez-Scarano F. CXCR-4 (fusin), a coreceptor for the type 1 human immunodeficiency virus (HIV-1), is expressed in the human brain in a variety of cell types, including microglia and neurons. Am J Pathol. 1997;151:1035–1042. [PMC free article] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Casanova P, Tham TN, De CE, renzana-Seisdedos F, Baleux F, Dubois-Dalcq M. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–125. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- Lazarini F, Tham TN, Casanova P, Arenzana-Seisdedos F, Dubois-Dalcq M. Role of the alpha-chemokine stromal cell-derived factor (SDF-1) in the developing and mature central nervous system. Glia. 2003;42:139–148. doi: 10.1002/glia.10139. [DOI] [PubMed] [Google Scholar]
- Lee JC, Kassis S, Kumar S, Badger A, Adams JL. p38 mitogen-activated protein kinase inhibitors—mechanisms and therapeutic potentials. Pharmacol Ther. 1999;82:389–397. doi: 10.1016/s0163-7258(99)00008-x. [DOI] [PubMed] [Google Scholar]
- Lee C, Tomkowicz B, Freedman BD, Collman RG. HIV-1 gp120-induced TNF-{alpha} production by primary human macrophages is mediated by phosphatidylinositol-3 (PI-3) kinase and mitogen-activated protein (MAP) kinase pathways. J Leukoc Biol. 2005;78:1016–1023. doi: 10.1189/jlb.0105056. [DOI] [PubMed] [Google Scholar]
- Li Y, Sassano A, Majchrzak B, Deb DK, Levy DE, Gaestel M, Nebreda AR, Fish EN, Platanias LC. Role of p38alpha map kinase in type I interferon signaling. J Biol Chem. 2004;279:970–979. doi: 10.1074/jbc.M309927200. [DOI] [PubMed] [Google Scholar]
- Li J, Bentsman G, Potash MJ, Volsky DJ. Human immunodeficiency virus type 1 efficiently binds to human fetal astrocytes and induces neuroinflammatory responses independent of infection. BMC Neurosci. 2007;8:31. doi: 10.1186/1471-2202-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, Shankar SK, Mahadevan A, Satishchandra P, Nath A. NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci. 2008;28:12190–12198. doi: 10.1523/JNEUROSCI.3019-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S, Zhang Y, Dodel R, Farlow MR, Paul SM, Du Y. Minocycline blocks nitric oxide-induced neurotoxicity by inhibition p38 MAP kinase in rat cerebellar granule neurons. Neurosci Lett. 2001;315:61–64. doi: 10.1016/s0304-3940(01)02324-2. [DOI] [PubMed] [Google Scholar]
- Lindl KA, Marks DR, Kolson DL, Jordan-Sciutto KL. HIV-associated neurocognitive disorder: pathogenesis and therapeutic opportunities. J Neuroimmune Pharmacol. 2010;5:294–309. doi: 10.1007/s11481-010-9205-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 Tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Liu Y, Liu H, Kim BO, Gattone VH, Li J, Nath A, Blum J, He JJ. CD4-independent infection of astrocytes by human immunodeficiency virus type 1: requirement for the human mannose receptor. J Virol. 2004;78:4120–4133. doi: 10.1128/JVI.78.8.4120-4133.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Mao Z, Bonni A, Xia F, Nadal-Vicens M, Greenberg ME. Neuronal activity-dependent cell survival mediated by transcription factor MEF2. Science. 1999;286:785–790. doi: 10.1126/science.286.5440.785. [DOI] [PubMed] [Google Scholar]
- Masliah E, Heaton RK, Marcotte TD, Ellis RJ, Wiley CA, Mallory M, Achim CL, McCutchan JA, Nelson JA, Atkinson JH, Grant I. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC group. The HIV Neurobehavioral Research Center. Ann Neurol. 1997;42:963–972. doi: 10.1002/ana.410420618. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Haughey NJ, Nath A. Cell death in HIV dementia. Cell Death Differ. 2005;12:893–904. doi: 10.1038/sj.cdd.4401577. [DOI] [PubMed] [Google Scholar]
- Mayer RJ, Callahan JF. p38 MAP kinase inhibitors: a future therapy for inflammatory diseases. Drug Discov Today Ther Strateg. 2006;3:49–54. [Google Scholar]
- Medders KE, Sejbuk NE, Maung R, Desai MK, Kaul M. Activation of p38 MAPK is required in monocytic and neuronal cells for HIV glycoprotein 120-induced neurotoxicity. J Immunol. 2010;185:4883–4895. doi: 10.4049/jimmunol.0902535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Miller RJ. Gp120-induced neurotoxicity in hippocampal pyramidal neuron cultures: protective action of TGF-beta1. J Neurosci. 1996;16:4080–4088. doi: 10.1523/JNEUROSCI.16-13-04080.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Bushell TJ, Gray PW, Miller RJ. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc Natl Acad Sci USA. 1998;95:14500–14505. doi: 10.1073/pnas.95.24.14500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- Mucke L, Abraham CR, Ruppe MD, Rockenstein EM, Toggas SM, Mallory M, Alford M, Masliah E. Protection against HIV-1 gp120-induced brain damage by neuronal expression of human amyloid precursor protein. J Exp Med. 1995;181:1551–1556. doi: 10.1084/jem.181.4.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukerjee R, Deshmane SL, Fan S, Del VL, White MK, Khalili K, Amini S, Sawaya BE. Involvement of the p53 and p73 transcription factors in neuroAIDS. Cell Cycle. 2008;7:2682–2690. doi: 10.4161/cc.7.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz L, Ammit AJ. Targeting p38 MAPK pathway for the treatment of Alzheimer’s disease. Neuropharmacology. 2010;58:561–568. doi: 10.1016/j.neuropharm.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Muthumani K, Wadsworth SA, Dayes NS, Hwang DS, Choo AY, Abeysinghe HR, Siekierka JJ, Weiner DB. Suppression of HIV-1 viral replication and cellular pathogenesis by a novel p38/ JNK kinase inhibitor. AIDS. 2004;18:739–748. doi: 10.1097/00002030-200403260-00004. [DOI] [PubMed] [Google Scholar]
- Neri E, Musante V, Pittaluga A. Effects of the HIV-1 viral protein TAT on central neurotransmission: role of group I metabotropic glutamate receptors. Int Rev Neurobiol. 2007;82:339–356. doi: 10.1016/S0074-7742(07)82018-6. [DOI] [PubMed] [Google Scholar]
- New DR, Maggirwar SB, Epstein LG, Dewhurst S, Gelbard HA. HIV-1 Tat induces neuronal death via tumor necrosis factor-alpha and activation of non-N-methyl-D-aspartate receptors by a NFkappaB-independent mechanism. J Biol Chem. 1998;273:17852–17858. doi: 10.1074/jbc.273.28.17852. [DOI] [PubMed] [Google Scholar]
- Norman JP, Perry SW, Reynolds HM, Kiebala M, De Mesy Bentley KL, Trejo M, Volsky DJ, Maggirwar SB, Dewhurst S, Masliah E, Gelbard HA. HIV-1 Tat activates neuronal ryanodine receptors with rapid induction of the unfolded protein response and mitochondrial hyperpolarization. PLoS ONE. 2008;3:e3731. doi: 10.1371/journal.pone.0003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell LA, Agrawal A, Jordan-Sciutto KL, Dichter MA, Lynch DR, Kolson DL. Human immunodeficiency virus (HIV)-induced neurotoxicity: roles for the NMDA receptor subtypes. J Neurosci. 2006;26:981–990. doi: 10.1523/JNEUROSCI.4617-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S, Kang YJ, Brechtel CW, Siviglia E, Russo R, Clemente A, Harrop A, McKercher S, Kaul M, Lipton SA. HIV/gp120 decreases adult neural progenitor cell proliferation via checkpoint kinase-mediated cell-cycle withdrawal and G1 arrest. Cell Stem Cell. 2007;1:230–236. doi: 10.1016/j.stem.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/s0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Osiecki K, Xie L, Zheng JH, Squires R, Pettoello-Mantovani M, Goldstein H. Identification of granulocyte-macrophage colony-stimulating factor and lipopolysaccharide-induced signal transduction pathways that synergize to stimulate HIV type 1 production by monocytes from HIV type 1 transgenic mice. AIDS Res Hum Retroviruses. 2005;21:125–139. doi: 10.1089/aid.2005.21.125. [DOI] [PubMed] [Google Scholar]
- Pantaleo G, Fauci AS. Apoptosis in HIV infection. Nat Med. 1995;1:118–120. doi: 10.1038/nm0295-118. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers GT, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Perfettini JL, Castedo M, Nardacci R, Ciccosanti F, Boya P, Roumier T, Larochette N, Piacentini M, Kroemer G. Essential role of p53 phosphorylation by p38 MAPK in apoptosis induction by the HIV-1 envelope. J Exp Med. 2005a;201:279–289. doi: 10.1084/jem.20041502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfettini JL, Castedo M, Roumier T, Andreau K, Nardacci R, Piacentini M, Kroemer G. Mechanisms of apoptosis induction by the HIV-1 envelope. Cell Death Differ. 2005b;12(Suppl 1):916–923. doi: 10.1038/sj.cdd.4401584. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Limoges J, McComb R, Bock P, Baldwin T, Tyor W, Patil A, Nottet HS, Epstein L, Gelbard H, Flanagan E, Reinhard J, Pirruccello SJ, Gendelman HE. Human immunodeficiency virus encephalitis in SCID mice. Am J Pathol. 1996;149:1027–1053. [PMC free article] [PubMed] [Google Scholar]
- Petito CK, Cho ES, Lemann W, Navia BA, Price RW. Neuropathology of acquired immunodeficiency syndrome (AIDS): an autopsy review. J Neuropathol Exp Neurol. 1986;45:635–646. doi: 10.1097/00005072-198611000-00003. [DOI] [PubMed] [Google Scholar]
- Pi R, Li W, Lee NT, Chan HH, Pu Y, Chan LN, Sucher NJ, Chang DC, Li M, Han Y. Minocycline prevents glutamate-induced apoptosis of cerebellar granule neurons by differential regulation of p38 and Akt pathways. J Neurochem. 2004;91:1219–1230. doi: 10.1111/j.1471-4159.2004.02796.x. [DOI] [PubMed] [Google Scholar]
- Poluektova L, Meyer V, Walters L, Paez X, Gendelman HE. Macrophage-induced inflammation affects hippocampal plasticity and neuronal development in a murine model of HIV-1 encephalitis. Glia. 2005;52:344–353. doi: 10.1002/glia.20253. [DOI] [PubMed] [Google Scholar]
- Porcheray F, Samah B, Leone C, Dereuddre-Bosquet N, Gras G. Macrophage activation and human immunodeficiency virus infection: HIV replication directs macrophages towards a pro-inflammatory phenotype while previous activation modulates macrophage susceptibility to infection and viral production. Virology. 2006;349:112–120. doi: 10.1016/j.virol.2006.02.031. [DOI] [PubMed] [Google Scholar]
- Rohr O, Schwartz C, Hery C, Aunis D, Tardieu M, Schaeffer E. The nuclear receptor chicken ovalbumin upstream promoter transcription factor interacts with HIV-1 Tat and stimulates viral replication in human microglial cells. J Biol Chem. 2000;275:2654–2660. doi: 10.1074/jbc.275.4.2654. [DOI] [PubMed] [Google Scholar]
- Rottman JB, Ganley KP, Williams K, Wu L, Mackay CR, Ringler DJ. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am J Pathol. 1997;151:1341–1351. [PMC free article] [PubMed] [Google Scholar]
- Schwartz L, Major EO. Neural progenitors and HIV-1-associated central nervous system disease in adults and children. Curr HIV Res. 2006;4:319–327. doi: 10.2174/157016206777709438. [DOI] [PubMed] [Google Scholar]
- Semenova MM, Maki-Hokkonen AM, Cao J, Komarovski V, Forsberg KM, Koistinaho M, Coffey ET, Courtney MJ. Rho mediates calcium-dependent activation of p38alpha and subsequent excitotoxic cell death. Nat Neurosci. 2007;10:436–443. doi: 10.1038/nn1869. [DOI] [PubMed] [Google Scholar]
- Shi B, De GU, He J, Wang S, Lorenzo A, Busciglio J, Gabuzda D. Apoptosis induced by HIV-1 infection of the central nervous system. J Clin Invest. 1996;98:1979–1990. doi: 10.1172/JCI119002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19:45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiramizu B, Ratto-Kim S, Sithinamsuwan P, Nidhinandana S, Thitivichianlert S, Watt G, Desouza M, Chuenchitra T, Sukwit S, Chitpatima S, Robertson K, Paul R, Shikuma C, Valcour V. HIV DNA and dementia in treatment-naive HIV-1-infected individuals in Bangkok, Thailand. Int J Med Sci. 2006;4:13–18. doi: 10.7150/ijms.4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Wilkinson D, Reeves JD, Dittmar MT, Beddows S, Weber J, Carnegie G, Desselberger U, Gray PW, Weiss RA, Clapham PR. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either Lestr or CCR5 as coreceptors for virus entry. J Virol. 1996;70:8355–8360. doi: 10.1128/jvi.70.12.8355-8360.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh IN, El-Hage N, Campbell ME, Lutz SE, Knapp PE, Nath A, Hauser KF. Differential involvement of p38 and JNK MAP kinases in HIV-1 Tat and gp120-induced apoptosis and neurite degeneration in striatal neurons. Neuroscience. 2005;135:781–790. doi: 10.1016/j.neuroscience.2005.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui Z, Fan S, Sniderhan L, Reisinger E, Litzburg A, Schifitto G, Gelbard HA, Dewhurst S, Maggirwar SB. Inhibition of mixed lineage kinase 3 prevents HIV-1 Tat-mediated neurotoxicity and monocyte activation. J Immunol. 2006;177:702–711. doi: 10.4049/jimmunol.177.1.702. [DOI] [PubMed] [Google Scholar]
- Tamura K, Sudo T, Senftleben U, Dadak AM, Johnson R, Karin M. Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell. 2000;102:221–231. doi: 10.1016/s0092-8674(00)00027-1. [DOI] [PubMed] [Google Scholar]
- Tikka T, Fiebich BL, Goldsteins G, Keinanen R, Koistinaho J. Minocycline, a tetracycline derivative, is neuroprotective against excitotoxicity by inhibiting activation and proliferation of microglia. J Neurosci. 2001;21:2580–2588. doi: 10.1523/JNEUROSCI.21-08-02580.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, Rall GF, Abraham CR, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Miller RJ. The HIV-1 coat protein gp120 regulates CXCR4-mediated signaling in neural progenitor cells. J Neuroimmunol. 2005;160:68–76. doi: 10.1016/j.jneuroim.2004.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushin SA, Algeciras-Schimnich A, Vlahakis SR, Bren GD, Warren S, Schnepple DJ, Badley AD. Glycoprotein 120 binding to CXCR4 causes p38-dependent primary T cell death that is facilitated by, but does not require cell-associated CD4. J Immunol. 2007;178:4846–4853. doi: 10.4049/jimmunol.178.8.4846. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Takahashi K, Glass JD, McArthur JC, Griffin JW, Griffin DE. Cellular localization of tumor necrosis factor mRNA in neurological tissue from HIV-infected patients by combined reverse transcriptase/polymerase chain reaction in situ hybridization and immunohistochemistry. J Neuroimmunol. 1997;74:1–8. doi: 10.1016/s0165-5728(96)00160-9. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Masliah E, Achim CL. Measurement of CNS HIV burden and its association with neurologic damage. Adv Neuro-immunol. 1994;4:319–325. doi: 10.1016/s0960-5428(06)80272-x. [DOI] [PubMed] [Google Scholar]
- Williams R, Dhillon NK, Hegde ST, Yao H, Peng F, Callen S, Chebloune Y, Davis RL, Buch SJ. Proinflammatory cytokines and HIV-1 synergistically enhance CXCL10 expression in human astrocytes. Glia. 2009a;57:734–743. doi: 10.1002/glia.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R, Yao H, Dhillon NK, Buch SJ. HIV-1 Tat cooperates with IFN-gamma and TNF-alpha to increase CXCL10 in human astrocytes. PLoS ONE. 2009b;4:e5709. doi: 10.1371/journal.pone.0005709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xiao H, Neuveut C, Tiffany HL, Benkirane M, Rich EA, Murphy PM, Jeang KT. Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc Natl Acad Sci USA. 2000;97:11466–11471. doi: 10.1073/pnas.97.21.11466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Smith CJ, Van Eldik LJ. Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia. 2004;45:170–179. doi: 10.1002/glia.10314. [DOI] [PubMed] [Google Scholar]
- Zheng J, Ghorpade A, Niemann D, Cotter RL, Thylin MR, Epstein L, Swartz JM, Shepard RB, Liu X, Nukuna A, Gendelman HE. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J Virol. 1999;73:8256–8267. doi: 10.1128/jvi.73.10.8256-8267.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng JC, Huang Y, Tang K, Cui M, Niemann D, Lopez A, Morgello S, Chen S. HIV-1-infected and/or immune-activated macrophages regulate astrocyte CXCL8 production through IL-1beta and TNF-alpha: involvement of mitogen-activated protein kinases and protein kinase R. J Neuro-immunol. 2008;200:100–110. doi: 10.1016/j.jneuroim.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S, Stavrovskaya IG, Drozda M, Kim BY, Ona V, Li M, Sarang S, Liu AS, Hartley DM, Wu DC, Gullans S, Ferrante RJ, Przedborski S, Kristal BS, Friedlander RM. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA. 2005;293:2003–2011. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]
- Zybarth G, Reiling N, Schmidtmayerova H, Sherry B, Bukrinsky M. Activation-induced resistance of human macrophages to HIV-1 infection in vitro. J Immunol. 1999;162:400–406. [PubMed] [Google Scholar]