Abstract
BACKGROUND
Lipids and other biologically active substances accumulate in platelet concentrates (PCs) during storage. Some of these substances have been suggested to modulate immune responses and to play a pathogenic role in the development of transfusion-related acute lung injury. This study compared the content and impact of some biological response modifiers in PCs treated with pathogen reduction (PR) technology and nontreated PCs.
STUDY DESIGN AND METHODS
Apheresis PCs (n = 12) were split in two: one split was subjected to PR treatment (INTERCEPT, Cerus Corp.) and the other split was left untreated. Basic characterization and content of vascular endothelial growth factor (VEGF) and sCD154 were measured. Lipopolysaccharide (LPS)-induced secretion of interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α) was measured after incubation of heparinized whole blood with platelet (PLT) supernatants. The supernatants’ neutrophil (PMN)-priming capacity, and thereby activation of the NADPH oxidase, was measured as the rate of superoxide anion production after formyl-Met-Leu-Phe activation. Lipids were extracted from the supernatants on Day 6 and tested for PMN-priming activity.
RESULTS
Supernatants from PR-treated PCs demonstrated significantly higher mean PLT volume (MPV)3−, and significantly and O2, lower pH, CO2, and HCO less LPS-induced TNF-α secretion compared to untreated PCs. No differences in swirling, PLT count, potassium levels, glucose consumption, lactate production, IL-10, VEGF, sCD154, or PMN-priming activity were found between the groups over time.
CONCLUSION
INTERCEPT PR treatment caused no substantial differences in PCs, except for minor changes in MPV and metabolic variables. Further studies are needed to explain the differences in the LPS-induced TNF-α secretion.
The platelet storage lesion (PSL) is a term often used for the sum of the deleterious changes in structure and function that occur to platelets (PLTs) in platelet concentrates (PCs) during storage under blood bank conditions. One of the features of the PSL is gradual accumulation in the PCs of various biological response modifiers.1–3 It has been suggested that some of these may induce immunosuppression in the recipients, a condition often referred to as transfusion-related immunomodulation (TRIM).4
Both lysophosphatidylcholines5 and soluble CD154 (sCD40 ligand) accumulate during storage of PCs and have been shown to be involved in transfusion-related acute lung injury (TRALI)6,7 and other adverse transfusion reactions.8,9 TRALI is a rare but potentially fatal complication of transfusion and has for years been the most common cause of transfusion-related fatalities in the United States.10 Most frequently implicated in TRALI are products with high plasma content, such as fresh-frozen plasma (FFP) or PCs.
In addition to substances implicated in TRALI other important biological response modifiers may accumulate in PCs during storage. One of these substances is vascular endothelial growth factor (VEGF), which has numerous biological effects:11 VEGF has been suggested to be implicated in atherosclerosis,12 acts as an angiogenic factor upon vascular injury,12 stimulates angiogenesis associated with tumor growth, and may be assumed to decrease effectiveness of cancer therapy by binding monoclonal antibodies directed against growth factors of tumor origin.11
Despite numerous studies on the immunomodulative effects of transfusion the total impact of TRIM is not fully elucidated.13 Some early studies report positive effects, where transfusion has been linked to improved clinical outcome in renal transplantation,14,15 while others suggest significant immunosuppression in recipients, possibly leading to increased rate of recurrence of cancer16 and postoperative bacterial infections.17,18
Storage of PCs at 22 ± 2°C with constant agitation is associated with a significant risk for transfusion-associated septicemia. A strategy to decrease this risk is to implement one of two pathogen reduction (PR) systems approved for PCs. Both the INTERCEPT Blood System (Cerus Corp., Concord, CA) and the Mirasol Pathogen Reduction Technology (Mirasol PRT, TerumoBCT, Lake-wood, CO) target DNA and RNA in pathogens and white blood cells (WBCs), thereby inhibiting transcription and translation.
A large number of reports and clinical studies conclude that PR treatment of PCs is well suited for use. However, there are clear indications of enhanced storage lesion in the PR-treated PCs19,20 although these changes are considered to be of minor clinical importance.21
The aim of this work was to investigate whether the INTERCEPT PR process has an impact on the amount of accumulated lysophosphatidylcholines, sCD154, VEGF, or other immunomodulative substances in apheresis PCs, compared to untreated PCs. Furthermore, we have examined to what extent accumulated biological response modifiers in PCs affect the cytokine release of heparinized whole blood after addition of LPS. Finally, we assessed the capacity of the PC supernatants to prime neutrophils (PMNs), which is the most important cell type in the development of TRALI.
MATERIALS AND METHODS
Study design and donors
The study was a paired single-blind comparison; each plateletpheresis product was split into 2 units: one subunit was subjected to photochemical PR technology, whereas the other was left untreated.
All donors (n = 12) were eligible for PLT apheresis according to the Guidelines for Norwegian Transfusion Service and they gave written consent for participation in the project. The donors were not previously transfused males with a body weight of more than 75 kg and a PLT count of more than 250 × 109/L. The reason for choosing male donors was first to facilitate collection of the target value, which was set to 500 × 109 to 520 × 109 PLTs, and second to avoid alloantibodies, such as HLA and HNA antibodies, which could possibly interfere with some of our assays.
Heparinized blood for the cytokine release experiment was obtained from one established male blood donor, who met the general blood donor requirements and gave written consent for the use of blood for research purposes. For the priming experiments, heparinized whole blood was obtained from healthy donors after obtaining informed consent under a protocol approved by the Colorado Multiple Institution Review Board.
PLT collection, pathogen inactivation, coding, and storage
The plateletpheresis products were harvested and leukoreduced on a cell separator (Fenwal Amicus, Baxter Healthcare Corp., Deerfield, IL) at the Blood Bank, Haukeland University Hospital, Bergen, and suspended in approximately 35% plasma and 65% PLT additive solution (PAS-III Intersol, Baxter Healthcare Corp.). The apheresis collection day was defined as Day 0. The PLT target value was set to 500 × 109 to 520 × 109 in a volume of 410 mL. All harvestings were completed within 75 minutes.
Each PLT bag was assigned a project number. Immediately after donation a 5-mL sample was aseptically collected from the PLT bag and transferred to a culture bottle for monitoring of bacterial contamination (BacT/ALERT, bioMérieux, Durham, NC). The PLT storage bags were well and gently mixed and divided into two split products of equal size; all split products were given a code that was not revealed for the first author of this article before all collected data were analyzed. One split product was photochemically PR treated (INTERCEPT), whereas the other was left untreated. The PCs were stored at 22 ± 2°C with horizontal agitation overnight. Two or three PCs were collected per day on 5 different days. On Day 1, the day after collection, the PCs were shipped to the Blood Bank in Oslo by car and plane. During the shipment, which lasted less than 5 hours, the PCs were subjected to the agitation caused by the transportation only. The used PAS, Intersol, contains phosphate; this has been shown to be of great importance when agitation is interrupted.22 PCs were stored at 22 ± 2°C with horizontal agitation upon arrival.
Sampling
On Days 1, 4, and 6 samples were aseptically collected for basic characterization and metabolic analyses. Additionally, a 6-mL sample was collected from each split product on Days 1, 4, and 6 and centrifuged twice at 5000 × g for 7 minutes at 20°C. The cell- and debris-free supernatant was split in aliquots and stored at −86°C until further analysis. One set of aliquots was shipped on dry ice to Denver, Colorado, for priming analysis. Upon thawing in Denver the PLT supernatants were centrifuged at 12,500 × g to remove possible remaining PLT contamination and acellular debris.
Basic characterization and metabolic measures
Swirling was scored visually on a scale from 0 to 3.23 PLT count and mean PLT volume (MPV) were measured on a cell counter (COBAS Argos, Roche ABX Hematologie, Montpellier, France). pH, blood gas analyses, potassium, glucose, and lactate were measured on a blood gas analyzer (ABL700 series, Radiometer, Copenhagen, Denmark).
Measuring sCD154 and VEGF content
The contents of sCD154 and VEGF were measured by enzyme-linked immunosorbent assay (ELISA; human sCD40L, BMS239INST, Bender Medsystems GmbH, Vienna, Austria; and human VEGF, DVE00, R&D Systems, Inc., Minneapolis, MN), according to the manufacturers’ instructions and read on a microplate reader (BioTek XL 800, Bio Tek Instruments, Inc., Winooski, VT).
Testing the effect of additional biological response modifiers
The possible effect of biological response modifiers on cytokine secretion was analyzed as previously described.24 Briefly, fresh heparinized human whole blood was incubated with 10% cell-free supernatant from the PCs and 10 ng/mL (final concentration) lipopolysaccharide (LPS; L6529, Sigma, St Louis, MO) for tumor necrosis factor-α (TNF-α) testing and 100 ng/mL LPS (final concentration) for interleukin-10 (IL-10) testing. Samples were incubated for 6 hours (TNF-α) and 24 hours (IL-10), at 37°C in 5% CO2 humidified atmosphere with occasional gentle shaking. The supernatant/whole blood ratio (1/9) reflected a typical PLT transfusion volume relative to the total blood volume of an adult. Adequate controls (without LPS or without supernatant) were included. After incubation, the samples were centrifuged for 10 minutes at 1000 × g at 20°C and the supernatants were stored at −86°C until further analysis. The levels of IL-10 and TNF-α were analyzed by ELISA (human IL-10 instant ELISA, BMS215INST, Bender Medsystems GmbH; and TNF-α [human] enzyme immunoassay kit 589201, Cayman Chemical Co., Ann Arbor, MI), according to the manufacturers’ instructions and read on a BioTek XL 800 reader.
To examine the possible cytotoxicity of the LPS concentrations used in these experiments, cell viability was tested before and after incubation with LPS. General viability was determined using an integrated fluorescence microscope (NucleoCounter NC-100, ChemoMetec A/S, Allerød, Denmark): mixtures of whole blood, PLT supernatant, and LPS were treated and stabilized according to the manufacturer’s instructions and added to propidium iodide in the NucleoCassette. Cellular DNA was stained in the WBCs and viability was calculated automatically, subtracting the number of nonviable cells from the total cell count. By this analysis LPS caused less than 1% cell death. The specific viability was also tested by flow cytometry: 100 μL of whole blood was stained with 10 μL of fluorescein isothiocyanate–conjugated anti-CD45 (345808, Becton Dickinson, Franklin Lakes, NJ) and 10 μL phycoerythrin-conjugated anti-CD14 (345785, Becton Dickinson). After 15 minutes of incubation at 20°C, the red blood cells (RBCs) were lysed and the sample was fixed using a reagent system (IMMUNOPREP, 7546946, Beckman Coulter, Inc., Fullerton, CA). Forty microliters of 7-aminoactinomycin D (559925, Becton Dickinson, Pharmingen) were added, and the samples were incubated for 10 minutes at 20°C before analysis was performed on a flow cytometer (Epics XL.MCL, Beckman Coulter). Adequate isotype controls were run in parallel and control cells (Stem-Trol, IM3632, Beckman Coulter) were included as control of the function of 7-aminoactinomycin D. Flow cytometry testing of cell viability following LPS treatment revealed greater than 90% viable monocytes, greater than 99% viable lymphocytes, and greater than 99% viable granulocytes. Thus, we consider that the cell viability was acceptable after exposure to the used LPS concentrations.
Assays for neutrophil priming
Unless otherwise indicated all reagents were purchased from Sigma Chemical Corp. All reagents were endotoxin free, and all solutions were made from sterile water (United States Pharmacopeia [USP] from Baxter Health-care Corp.).5,25 All buffers were made from the following stock USP solutions: 10% CaCl2, 23.4% NaCl, 50% MgSO4 (American Reagent Laboratories, Inc., Shirley, NY); sodium phosphates (278 mg/mL monobasic and 142 mg/mL dibasic); and 50% dextrose (Abbott Laboratories, North Chicago, IL).5,25 Furthermore, all solutions were sterile-filtered with disposable sterilization filter units (Nalgene MF75 series, Fisher Scientific Corp., Pittsburgh, PA). Ficoll-Paque was purchased from Amersham Biosciences (Piscataway, NJ). Plastic microplates, manufactured by Nunc were purchased from Life Sciences Products, Inc. (Frederick, CO).
PMNs were isolated from heparinized whole blood employing dextran sedimentation, Ficoll-Hypaque gradient centrifugation, and hypotonic lysis of contaminating RBCs, as previously described.5,25 PMNs were warmed to 37°C and incubated with buffer (Krebs-Ringer phosphate buffer with dextrose, pH 7.4) and the supernatants (10% vol/vol) for 5 minutes. Samples were washed at 1800 × g for 3 minutes.5 FFP was used as a negative control and FFP plus 2 μmol/L PLT-activating factor as a positive control. The PMNs were centrifuged at 2100 × g for 3 minutes and resuspended in fresh, warm Krebs-Ringer phosphate buffer with dextrose, pH 7.4. The maximal rate of super-oxide anion production in response to 1 μmol/L formyl-Met-Leu-Phe (fMLP) was measured in real time, as the reduction of cytochrome c read at 550 nm in a microplate reader (Molecular Dynamics, Sunnyvale, CA).5 Samples were tested against 3 PMN donors and all analyses were run in duplicates.
Priming was operationally defined as augmentation of the fMLP-activated respiratory burst, which is the rate of super oxide anion production after activation by fMLP.5 In addition, the plasma lipids were extracted from the Day 6 supernatant samples, by the method of Bligh and Dyer26 using a 1:1:1 chloroform:methanol:water with 2.0% acetic acid extraction. The lipids in the chloroform-soluble phase were removed, dried, solubilized in 1.25% essentially fatty acid–free human albumin, and tested for priming activity to ascertain any effects from the PR system on the lipids that are generated during storage of PCs.5
Statistical analysis
The statistical analyses were performed with computer software (Microsoft Office Excel 2007, Microsoft Corp., Redmond, WA; SPSS 15.0 for Windows, SPSS, Inc., Chicago, IL; and Statview, 5.0.1.0, SAS Institute, Inc., Cary, NC). Repeated measures analyses of variance (ANOVA) were used to compare groups over time for data that met the assumptions for the ANOVA model. When checking normality assumptions for the model, we found that further improvement could be achieved by using logarithmic transformations for PLT count, IL-10 concentration, neutrophil-priming activity (nmol O2−/min/PLTs × 109), and sCD154. For MPV, pH, potassium, and swirling we used the following nonparametric approach: area under the curve (AUC) was calculated for each concentrate and groups were compared by performing Wilcoxon’s signed rank test on the AUC, to show main effect. To assess if there was a significant development over time, Friedman’s test was performed on all observations in the two groups together for all time points. As alternative to the interaction term in ANOVA, we calculated the difference at all three time points between each pair of observations (INTERCEPT PCs and untreated PCs) and used Friedman’s test on the difference between groups. Paired t test was used to compare the lipid-priming results from Day 6. Results were presented as mean ± 95% confidence interval (CI) for normally distributed values and median ± 95% CI for values not conforming to the normal distribution. Median values from the priming activity measurements, VEGF, and sCD154 as well as LPS-induced release of IL-10 and TNF-α, which were run in duplicates or triplicates, were used for statistical analysis. Significance was defined as p values of less than 0.05.
RESULTS
Descriptive and metabolic measures
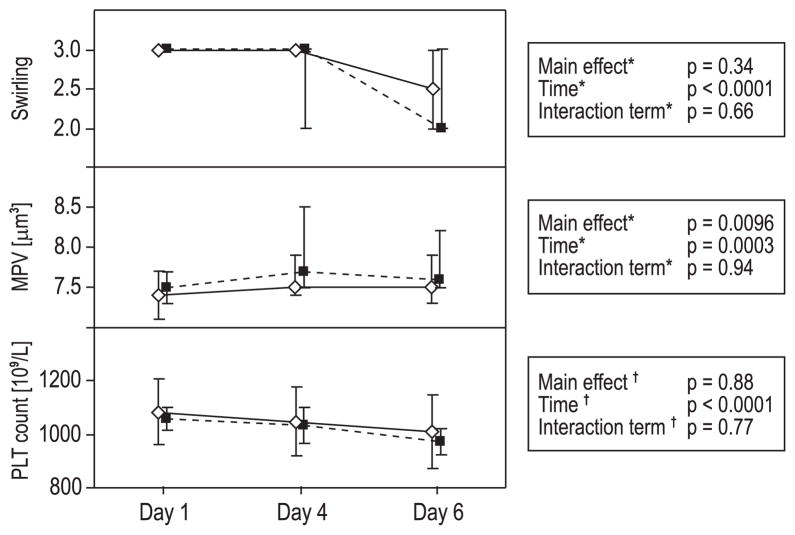
Swirling decreased significantly during storage (time, p < 0.0001); the differences between the groups were not significant over time (Fig. 1). Median MPV was higher in the PR-treated PCs (main effect, p = 0.0096) and increased significantly over time (time, p = 0.0003). PLT concentration decreased significantly during storage (time, p < 0.0001); none of these differences were significant over time between the groups (Fig. 1).
Fig. 1.
Swirling, MPV, and PLT concentration. Mean ± 95% CI (PLT conc.) and median ± 95% CI (swirling and MPV) for Days 1, 4, and 6. (◇) Untreated PLTs; (■) INTERCEPT PR-treated PLTs. *Nonparametric methods; see text for further details. †Repeated-measures ANOVA. Swirling decreased during storage, but no significant differences were seen between the groups over time. MPV was significantly higher in the PR-treated PCs. MPV increased and PLT concentration decreased significantly over time, but no significant differences were seen between the groups over time.
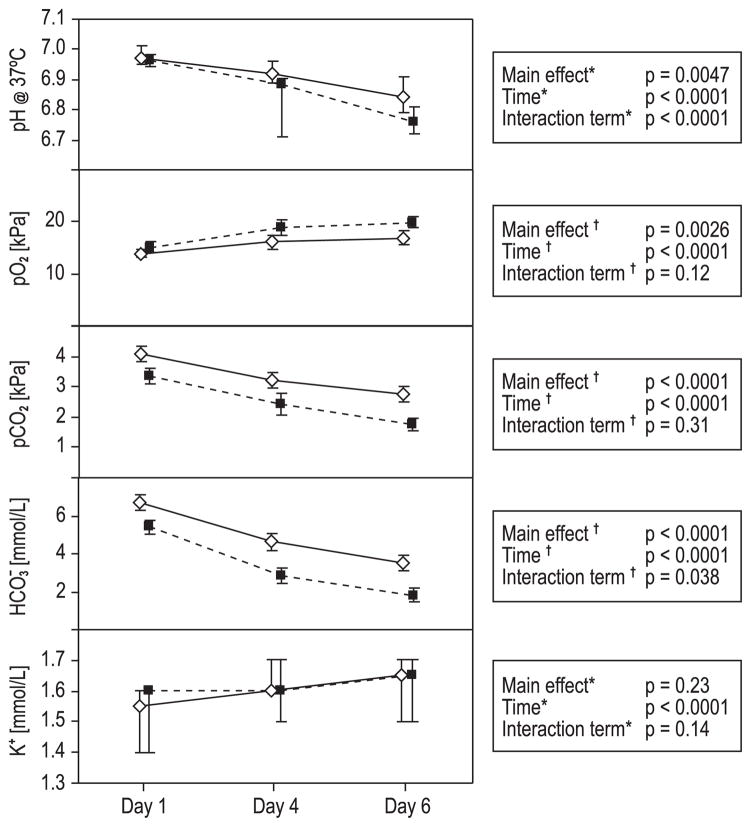
pH37°C was significantly lower in the PR-treated PCs (main effect, p = 0.0047) and decreased significantly during storage (time, p < 0.0001). The untreated PCs demonstrated significantly higher pH compared to the PR-treated PCs over time (interaction term, p < 0.0001). All values were acceptable according to the European guidelines27 (Fig. 2).
Fig. 2.
Blood gas analysis and potassium content. Mean ± 95% CI (pO2, pCO2, HCO3−) and median ± 95% CI (pH and potassium) for Days 1, 4, and 6. (◇) Untreated PLTs; (■) INTERCEPT PR-treated PLTs. *Nonparametric methods; see text for further details. †Repeated-measures ANOVA. pH decreased significantly during storage, and pH was significantly lower in the PR-treated PLTs. The untreated PCs demonstrated significantly higher pH compared to the PR-treated PCs over time. pO2 increased significantly and pCO2 decreased significantly during storage. Although untreated PCs had significantly higher carbon dioxide levels and significantly lower oxygen levels than the PR-treated PCs, these differences were not significant between groups over time. Bicarbonate levels were significantly higher in the untreated PCs, but decreased in both groups over time; the change over time also differed significantly between groups. The content of free potassium increased significantly during storage; the change was not significant between the groups over time.
The levels of oxygen increased significantly (time, p < 0.0001) and levels of carbon dioxide decreased significantly (time, p < 0.0001) during storage. The untreated PCs revealed higher carbon dioxide levels and lower oxygen levels than the PR-treated PCs (main effects, p < 0.0001 and p = 0.0026, respectively), but these differences were not significant between the groups over time (Fig. 2). The levels of bicarbonate were significantly higher in the untreated PCs (main effect, p < 0.0001) and decreased significantly during storage (time, p < 0.0001); the difference between the groups was significant over time as well (interaction term, p = 0.038; Fig. 2). The content of free potassium increased significantly during storage (time, p < 0.0001), but was not significantly different between the groups over time (Fig. 2). Glucose consumption and lactate production were calculated for the periods between Days 1 and 4 and between Days 4 and 6; the levels were not significantly different between the groups over time (Fig. 3).
Fig. 3.
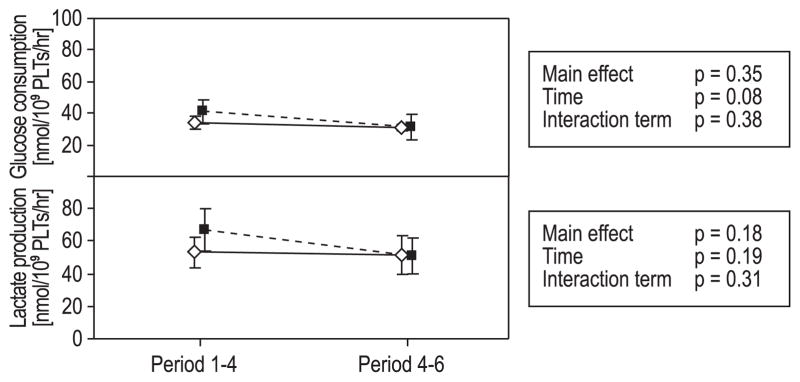
Glucose consumption and lactate production.
Mean ± 95% CI for Period 1 to 4 (from Day 1 to Day 4) and Period 4 to 6 (from Day 4 to Day 6). (◇) Untreated PLTs; (■) INTERCEPT PR-treated PLTs. Statistical analyses performed with repeated-measures ANOVA. The consumption of glucose decreased significantly over time. Neither changes in glucose consumption nor lactate production were significant between the groups over time.
Biological response modifiers
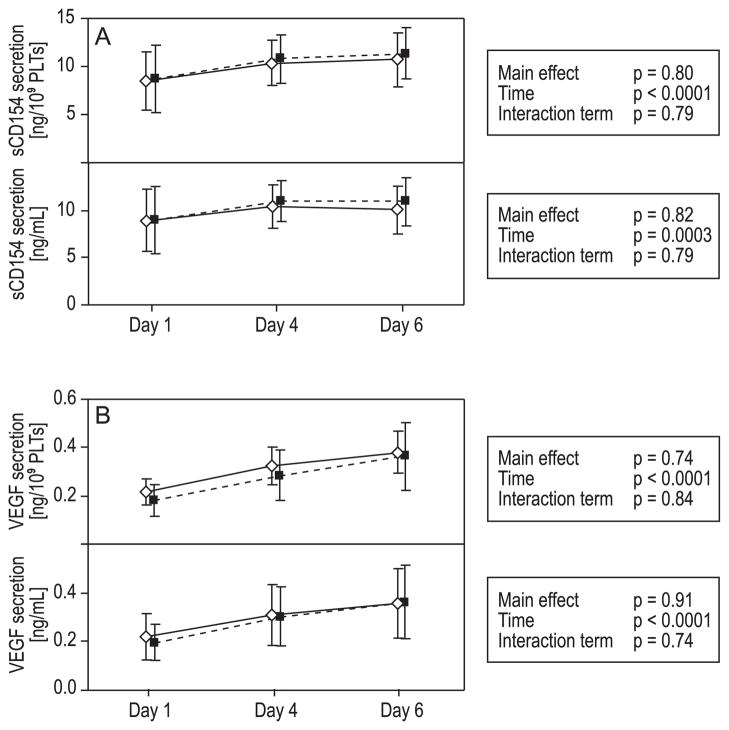
We have chosen to present the results of sCD154 and VEGF in ng/109 PLTs as well as in ng/mL plasma volume. The contents of both sCD154 and VEGF increased significantly during storage (time, p < 0.0001/p = 0.0003 and time, p < 0.0001/<0.0001, respectively; Fig. 4). The increases were not significant between the groups over time.
Fig. 4.
Secretion of sCD154 and VEGF. Mean ± 95% CI for Days 1, 4, and 6. (◇) Untreated PLTs; (■) INTERCEPT PR-treated PLTs. Statistical analyses performed with repeated-measures ANOVA. Both content of sCD154 (A) and VEGF (B) increased significantly over time, but the differences were not significant between the groups over time.
LPS-induced release of TNF-α and IL-10
Cells in the heparinized whole blood, which was mixed with supernatants from PLTs that underwent PR treatment, secreted significantly lower levels of TNF-α after LPS stimulation compared to cells in whole blood mixed with supernatants from untreated PLTs (main effect, p = 0.0095 and interaction term p = 0.032; Fig. 5). Although the LPS-induced release of IL-10 decreased when using 6-day-old supernatants from untreated PCs, the overall difference between groups and changes between groups over time were not significant (Fig. 5).
Fig. 5.
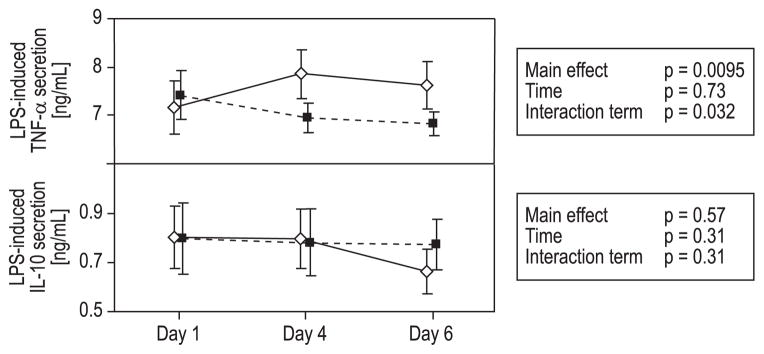
LPS-induced secretion of TNF-α and IL-10.
Mean ± 95% CI for Days 1, 4, and 6. (◇) Untreated PLTs; (■) INTERCEPT PR-treated PLTs. Statistical analyses performed with repeated-measures ANOVA. The INTERCEPT PR-treated PCs induced a significantly lower secretion of TNF-α than the untreated PCs. The difference between the groups was also significant over time. No significant differences were seen in the IL-10 secretion.
Plasma priming activity in stored PCs
Supernatants from all stored PCs demonstrated a significant increase of PMN-priming activity during storage both when measured as pmol O2−/min/PLTs × 109 (time, p = 0.0006) and pmol O2−/min/plasma volume (time, p = 0.02). No significant differences were seen between the groups over time (Fig. 6). Regarding the priming activity of the plasma lipids measured on Day 6, no significant differences were found between the groups (Table 1), indicating that the priming activity was not affected by the INTERCEPT PR system.
Fig. 6.
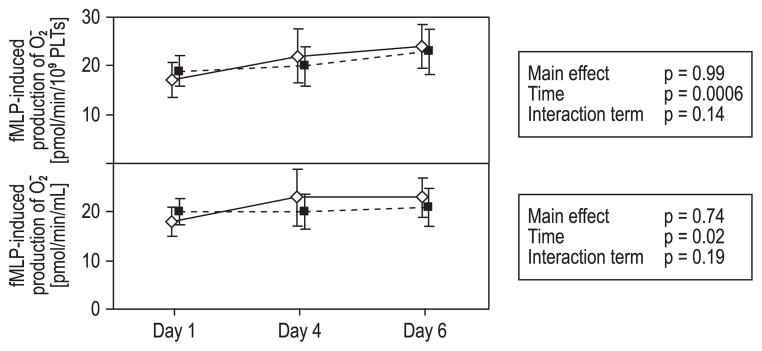
Neutrophil priming by supernatants from apheresis PCs. Mean ± 95% CI for Days 1, 4, and 6. (◇) Untreated PLTs; (■) INTERCEPT PR-treated PLTs. Statistical analyses performed with repeated-measures ANOVA. Sample priming increased significantly over time, but no significant differences were seen between the groups or between the groups over time.
TABLE 1.
Priming activity of plasma lipids on Day 6*
| Superoxide production | Untreated PLTs (n = 12) | INTERCEPT PR–treated PLTs (n = 12) | p value |
|---|---|---|---|
| pmol O2−/min/PLTs × 109 (95% CI) | 23 (19–27) | 24 (20–27) | 0.78 |
| pmol O2−/min/mL (95% CI) | 22 (20–24) | 23 (20–26) | 0.44 |
Results for lipid-priming activity are given as mean ± 95% CI.
DISCUSSION
Photochemical PR technology has been introduced to reduce the risk of transfusion-associated infections. Although promising, these technologies adversely affect PLT quality.21,28 In this study we investigated biological response modifiers in PR-treated and untreated PCs and compared the results in a blinded manner. To mimic the situation after transfusion we incubated whole blood with PLT supernatants and assessed the total effect of the biological response modifiers in the PCs by measuring the LPS-induced production of both a proinflammatory (TNF-α) and an anti-inflammatory (IL-10) cytokine.
The supernatants from both groups of PCs influenced the LPS-induced secretion of cytokines. This indicates that some immunomodulatory components accumulate in the PCs. No differences were seen between the groups over time concerning the release of IL-10, but the supernatants from PR-treated PLTs caused a significantly lower LPS-induced secretion of TNF-α compared to the untreated PLTs. These data could be interpreted in two different ways. One possible cause of the difference could be the presence of increased levels of biological response modifiers in the supernatant of the PR-treated PCs, which could lead to a lower secretion of TNF-α. An alternative explanation could be that the PSL in the untreated PCs have progressed further and thereby released substances, which could have increased the secretion of TNF-α. However, the fact that the results from our metabolic analyses, which revealed minor adverse metabolic changes in the PR-treated PCs, and that several other studies have concluded that PR treatment accelerates the PSL,19–21,28 makes the latter interpretation more unlikely. However, additional studies are needed to settle which interpretation is correct.
The PLT α-granules contain growth factors, and during the PSL, the content of PLT granules is released into the PC supernatant.3,29 The clinical effect of transfusing amounts of growth factors is not completely understood. VEGF normally induces vasculogenesis and angiogenesis,30 but when overexpressed, VEGF can contribute to disease, such as atherosclerosis and tumor growth.11,12 Although there was no difference in VEGF levels between groups, we found a gradual increase in VEGF levels during storage, a finding that is in concordance with an earlier study on apheresis PLTs.11
CD154 is a ligand for CD40 on antigen-presenting cells and is expressed on activated T cells, PLTs, mast cells, macrophages, basophils, NK cells, and B lymphocytes.
During collection and storage of PCs CD154 is proteolytically cleaved from the surface of PLTs,31 an event that results in accumulation of sCD154 to very high levels in the supernatant during storage, shown to reach maximum levels on Day 3 after collection.9 sCD154 can both activate macrophages causing production and release of several proinflammatory cytokines and prime the PMN oxidase, an event that is crucial in the pathogenesis of TRALI.6 We did not find a significant difference between PR-treated and untreated PLTs with regard to sCD154 content, but like others, we found a gradual increase during storage.3,6
In this study PMN-priming activity increased in all PCs during storage. The activity was dominantly of lipid nature, and lipids have the capability to cause TRALI in susceptible hosts.32 However, no significant differences in the measurements of the lipid priming activity were found between the PR-treated PLTs and the untreated PLTs. These results indicate that the priming activity was not affected by treatment with the INTERCEPT PR system. Two other studies that tested the PMN-priming activity in PCs reached similar conclusions. In the first study PCs collected with Trima and Amicus equipment were treated with either the INTERCEPT Blood System or the Mirasol PR System;33 the other study34 tested split PC units treated with riboflavin and ultraviolet light as in the Mirasol PR system. Altogether, these studies indicate that either of the two PR technologies cause PMN priming to the same degree as in untreated PCs and thus it is unlikely that the PR treatment will cause enhanced risk for development of TRALI.
Metabolic measures in our study disclosed that the untreated PCs had higher pH, pCO2, and HCO3− concentration and lower pO2 than PR-treated PCs; these changes are small and may not be of major clinical importance. However, these findings correspond with previous results suggesting that PR treatment accelerates the PSL.21
Many studies confirm that the concentrations of immunomodulative substances, for example, VEGF and sCD154 are very high in apheresis PCs, independent of PR treatment, and the immunomodulative consequences for the recipients may warrant further investigations on how to improve clinical outcome. Removal of supernatants containing these mediators by washing the blood products before transfusion has been tried. Two trials conducted on 43 adult acute leukemia patients35 and 162 children undergoing cardiac surgery36 did not reveal significant differences, but a tendency toward better clinical outcome for the patients, who received washed RBC and PLT components. Larger studies with sufficient power will be necessary to show whether transfusion of washed blood components will prove significance.
In conclusion, according to this study the INTERCEPT Blood System for PR of PCs seems to affect the metabolism of the PLTs, which is in accordance with previous studies. In addition, supernatants from PCs PR treated with the INTERCEPT Blood System caused a lower LPS-induced secretion of TNF-α, compared to untreated PCs. However, further studies are needed to confirm and explain the difference in the secretion of this proinflammatory cytokine. Furthermore, as the content of sCD154 and the PMN-priming activity was unaffected by use of the INTERCEPT system, our study does not indicate that this type of PR treatment is associated with increased risk of TRALI.
Acknowledgments
The authors thank the nurses and medical technologists at the Department of Immunology and Transfusion Medicine, Oslo University Hospital, Ullevaal, and at the Blood Bank, Haukeland University Hospital in Bergen for help and cooperation with collection and management of apheresis products.
ABBREVIATIONS
- fMLP
formyl-Met-Leu-Phe
- LPS
lipopolysaccharide
- MPV
mean PLT volume
- PC(s)
platelet concentrate(s)
- PR
pathogen reduction
- PSL
platelet storage lesion
- TRIM
transfusion-related immunomodulation
- VEGF
vascular endothelial growth factor
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest relevant to this manuscript in TRANSFUSION.
References
- 1.Silliman CC, Thurman GW, Ambruso DR. Stored blood components contain agents that prime the neutrophil NADPH oxidase through the platelet-activating-factor receptor. Vox Sang. 1992;63:133–6. doi: 10.1111/j.1423-0410.1992.tb02500.x. [DOI] [PubMed] [Google Scholar]
- 2.Heddle NM. Pathophysiology of febrile nonhemolytic transfusion reactions. Curr Opin Hematol. 1999;6:420–6. doi: 10.1097/00062752-199911000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Cognasse F, Boussoulade F, Chavarin P, Acquart S, Fabrigli P, Lamy B, Garraud O. Release of potential immunomodulatory factors during platelet storage. Transfusion. 2006;46:1184–9. doi: 10.1111/j.1537-2995.2006.00869.x. [DOI] [PubMed] [Google Scholar]
- 4.Blajchman MA. Transfusion immunomodulation or TRIM: what does it mean clinically? Hematology. 2005;10 (Suppl 1):208–14. doi: 10.1080/10245330512331390447. [DOI] [PubMed] [Google Scholar]
- 5.Silliman CC, Dickey WO, Paterson AJ, Thurman GW, Clay KL, Johnson CA, Ambruso DR. Analysis of the priming activity of lipids generated during routine storage of platelet concentrates. Transfusion. 1996;36:133–9. doi: 10.1046/j.1537-2995.1996.36296181925.x. [DOI] [PubMed] [Google Scholar]
- 6.Khan SY, Kelher MR, Heal JM, Blumberg N, Boshkov LK, Phipps R, Gettings KF, McLaughlin NJ, Silliman CC. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–62. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139–59. doi: 10.1016/j.blre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg N, Gettings KF, Turner C, Heal JM, Phipps RP. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–21. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaufman J, Spinelli SL, Schultz E, Blumberg N, Phipps RP. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–96. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 10.FDA. Fatalities reported to FDA following blood collection and transfusion: annual summary for fiscal year 2010. 2011 [cited 2012 Jan 10]. Available from: URL: http://www.fda.gov/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/ucm254802.htm.
- 11.Kanter J, Khan SY, Kelher M, Gore L, Silliman CC. Oncogenic and angiogenic growth factors accumulate during routine storage of apheresis platelet concentrates. Clin Cancer Res. 2008;14:3942–7. doi: 10.1158/1078-0432.CCR-07-4824. [DOI] [PubMed] [Google Scholar]
- 12.Arisato T, Hashiguchi T, Sarker KP, Arimura K, Asano M, Matsuo K, Osame M, Maruyama I. Highly accumulated platelet vascular endothelial growth factor in coagulant thrombotic region. J Thromb Haemost. 2003;1:2589–93. doi: 10.1046/j.1538-7836.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- 13.Blajchman MA. Immunomodulation and blood transfusion. Am J Ther. 2002;9:389–95. doi: 10.1097/00045391-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Opelz G, Terasaki PI. Dominant effect of transfusions on kidney graft survival. Transplantation. 1980;29:153–8. doi: 10.1097/00007890-198002000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Blajchman MA, Bordin JO. Mechanisms of transfusion-associated immunosuppression. Curr Opin Hematol. 1994;1:457–61. [PubMed] [Google Scholar]
- 16.Burrows L, Tartter P, Aufses A. Increased recurrence rates in perioperatively transfused colorectal malignancy patients. Cancer Detect Prev. 1987;10:361–9. [PubMed] [Google Scholar]
- 17.Vamvakas EC, Blajchman MA. Deleterious clinical effects of transfusion-associated immunomodulation: fact or fiction? Blood. 2001;97:1180–95. doi: 10.1182/blood.v97.5.1180. [DOI] [PubMed] [Google Scholar]
- 18.Blumberg N. Deleterious clinical effects of transfusion immunomodulation: proven beyond a reasonable doubt. Transfusion. 2005;2 (Suppl):33S–9S. doi: 10.1111/j.1537-2995.2005.00529.x. [DOI] [PubMed] [Google Scholar]
- 19.van Rhenen D, Gulliksson H, Cazenave JP, Pamphilon D, Ljungman P, Kluter H, Vermeij H, Kappers-Klunne M, de Greef G, Laforet M, Lioure B, Davis K, Marblie S, Mayaudon V, Flament J, Conlan M, Lin L, Metzel P, Buchholz D, Corash L euroSPRITE trial. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood. 2003;101:2426–33. doi: 10.1182/blood-2002-03-0932. [DOI] [PubMed] [Google Scholar]
- 20.Snyder E, McCullough J, Slichter SJ, Strauss RG, Lopez-Plaza I, Lin JS, Corash L, Conlan MG. Clinical safety of platelets photochemically treated with amotosalen HCl and ultraviolet A light for pathogen inactivation: the SPRINT trial. Transfusion. 2005;45:1864–75. doi: 10.1111/j.1537-2995.2005.00639.x. [DOI] [PubMed] [Google Scholar]
- 21.Hervig T, Seghatchian J, Apelseth TO. Current debate on pathogen inactivation of platelet concentrates—to use or not to use? Transfus Apher Sci. 2010;43:411–4. doi: 10.1016/j.transci.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer PF, Liefting LA, Pietersz RN. The effect of interruption of agitation on in vitro measures of platelet concentrates in additive solution. Transfusion. 2007;47:955–9. doi: 10.1111/j.1537-2995.2007.01226.x. [DOI] [PubMed] [Google Scholar]
- 23.Vetlesen A, Mirlashari MR, Torsheim IA, Kjeldsen-Kragh J. Platelet activation and residual activation potential during storage of hyperconcentrated platelet products in two different platelet additive solutions. Transfusion. 2005;45:1349–55. doi: 10.1111/j.1537-2995.2005.00218.x. [DOI] [PubMed] [Google Scholar]
- 24.Widing L, Bechensteen AG, Mirlashari MR, Vetlesen A, Kjeldsen-Kragh J. Evaluation of nonleukoreduced red blood cell transfusion units collected at delivery from the placenta. Transfusion. 2007;47:1481–7. doi: 10.1111/j.1537-2995.2007.01287.x. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin NJ, Banerjee A, Kelher MR, Gamboni-Robertson F, Hamiel C, Sheppard FR, Moore EE, Silliman CC. Platelet-activating factor-induced clathrin-mediated endocytosis requires beta-arrestin-1 recruitment and activation of the p38 MAPK signalosome at the plasma membrane for actin bundle formation. J Immunol. 2006;176:7039–50. doi: 10.4049/jimmunol.176.11.7039. [DOI] [PubMed] [Google Scholar]
- 26.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 27.European Committee (Partial Agreement) on Blood Transfusion (CD-P-TS) 2010 European Directorate for the Quality of Medicines & HealthCare (EDQM) 16. Council of Europe; 2011. Guide to the preparation, use and quality assurance of blood components. [Google Scholar]
- 28.Kerkhoffs JL, van Putten WL, Novotny VM, Te Boekhorst PA, Schipperus MR, Zwaginga JJ, van Pampus LC, de Greef GE, Luten M, Huijgens PC, Brand A, van Rhenen DJ Dutch–Belgian HOVON cooperative group. Clinical effectiveness of leucoreduced, pooled donor platelet concentrates, stored in plasma or additive solution with and without pathogen reduction. Br J Haematol. 2010;150:209–17. doi: 10.1111/j.1365-2141.2010.08227.x. [DOI] [PubMed] [Google Scholar]
- 29.Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev. 2009;23:177–89. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferrara N, Gerber HP. The role of vascular endothelial growth factor in angiogenesis. Acta Haematol. 2001;106:148–56. doi: 10.1159/000046610. [DOI] [PubMed] [Google Scholar]
- 31.Phipps RP, Kaufman J, Blumberg N. Platelet derived CD154 (CD40 ligand) and febrile responses to transfusion. Lancet. 2001;357:2023–4. doi: 10.1016/s0140-6736(00)05108-4. [DOI] [PubMed] [Google Scholar]
- 32.Silliman CC, Bjornsen AJ, Wyman TH, Kelher M, Allard J, Bieber S, Voelkel NF. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43:633–40. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 33.Ambruso DR, Thurman G, Tran K, Marschner S, Gathof B, Janetzko K, Goodrich RP. Generation of neutrophil priming activity by cell-containing blood components treated with pathogen reduction technology and stored in platelet additive solutions. Transfusion. 2011;51:1220–7. doi: 10.1111/j.1537-2995.2010.02983.x. [DOI] [PubMed] [Google Scholar]
- 34.Silliman CC, Khan SY, Ball JB, Kelher MR, Marschner S. Mirasol Pathogen Reduction Technology treatment does not affect acute lung injury in a two-event in vivo model caused by stored blood components. Vox Sang. 2010;98:525–30. doi: 10.1111/j.1423-0410.2009.01289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blumberg N, Heal JM, Rowe JM. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia (ISRCTN76536440) BMC Blood Disord. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cholette JM, Henrichs KF, Alfieris GM, Powers KS, Phipps R, Spinelli SL, Swartz M, Gensini F, Daugherty LE, Nazarian E, Rubenstein JS, Sweeney D, Eaton M, Lerner NB, Blumberg N. Washing red blood cells and platelets transfused in cardiac surgery reduces postoperative inflammation and number of transfusions: results of a prospective, randomized, controlled clinical trial. Pediatr Crit Care Med. 2011 Sep 15; doi: 10.1097/PCC.0b013e31822f173c. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]