Abstract
EZH2 is a Polycomb group protein that exerts oncogenic functions in breast cancer, where its overexpression is associated with metastatic disease. While it reportedly acts a transcriptional repressor through trimethylation of histone H3 at lysine 27, EZH2 may exhibit context-dependent activating functions. Despite associations with worse outcome and metastasis in breast cancer, a functional role of EZH2 in breast cancer metastasis in vivo has not been demonstrated. Furthermore, whether EZH2 regulates cancer cell phenotype and motility are unknown. In this study, we discovered that knockdown of EZH2 induces a phenotypic reprograming from mesenchymal to epithelial, reduces motility, and blocks invasion in breast cancer cell lines. In vivo, EZH2 downregulation in MDA-MB-231 cells decreases spontaneous metastasis to the lungs. We uncover an unexpected role of EZH2 in inducing the p38 mitogen-activated protein kinase signaling pathway, an important regulator of breast cancer invasion and metastasis. In breast cancer cells EZH2 binds to phosphorylated-p38 (p-p38) in association with other core members of the Polycomb Repressive Complex 2 (PRC2), EED and SUZ12, and EZH2 overexpression leads to increased levels of p-p38 and of activated, downstream pathway proteins. The effect on p-p38 was confirmed in vivo where it correlated with decreased spontaneous metastasis. In clinical specimens of matched primary and invasive breast carcinomas, we found that EZH2 expression was upregulated in 100% of the metastases, and that EZH2 and p-p38 were coexpressed in 63% of cases, consistent with the functional results. Together our findings reveal a new mechanism by which EZH2 functions in breast cancer, and provide direct evidence that EZH2 inhibition reduces breast cancer metastasis in vivo.
Keywords: breast cancer, EZH2, p38, cell motility, metastasis
Introduction
Breast cancer is the 2nd most common cause of cancer-related deaths for women in the United States [1]. Despite advances in breast cancer detection and treatment strategies, metastatic breast cancer is essentially incurable and the 5 year survival rate for women diagnosed with distant metastatic disease is only 23% [2]. The degree of breast cancer cell differentiation directly impacts its metastatic ability; the more undifferentiated the primary invasive carcinoma, the greater likelihood to develop metastasis [3]. Thus, it is not surprising that dysregulation of cell type identity and differentiation programs directly impact breast cancer metastasis.
Polycomb group proteins are major regulators of cellular memory that function in multimeric complexes to regulate the expression of specific genes, mainly through transcriptional repression. Enhancer of Zeste Homolog 2 (EZH2) is the catalytic core member of the Polycomb Repressive Complex 2 (PRC2), which catalyzes the trimethylation of histone H3 lysine27 (H3K27me3) [4–6]. Although primarily functioning in gene repression, EZH2 has been shown to exhibit gene activating functions, at times through mechanisms independent of its histone methyltransferase activity [7–10]. EZH2 is highly expressed in a wide range of human cancers and has been shown to mediate the expression of target genes involved in tumorigenesis, including cell cycle regulation and proliferation, stem cell maintenance, cell differentiation, and neoplastic cell transformation [11–13]. EZH2 protein is overexpressed in 55% of invasive breast carcinomas, and is significantly associated with poorly differentiated, estrogen receptor negative (ER−) tumors. We have demonstrated that EZH2 is an independent prognostic biomarker in breast cancer as women with tumors expressing high EZH2 have worse disease free and overall survival than women with tumors expressing low EZH2 [14–18]. Despite these associations, direct demonstration that EZH2 downregulation decreases breast cancer metastasis is lacking.
The p38 mitogen-activated protein kinase (MAPK) signaling pathway plays a complex and key role in cancer progression by translating extracellular signals into cellular responses through phosphorylation of specific serine and threonine residues of downstream effector proteins, especially transcription factors and protein kinases. Four p38 isoforms have been identified, whose implications in tumorigenesis may depend on cell context and tumor type [19,20]. Once activated, p38 has been associated with regulation of the epithelial-to-mesenchymal transition (EMT), invasion and motility of cancer cells, all cellular processes that are crucial to metastasis [20,19]. Recently, elevated p38γ expression was shown to be associated with a lower overall survival of patients with breast cancer [21].
In this study, we demonstrate a previously undescribed function of EZH2: its role in cancer cell motility and cell phenotype. EZH2 knockdown in breast cancer cells induces a mesenchymal-to-epithelial transition (MET), decreases cancer cell motility and the speed of movement. We provide first evidence that EZH2 knockdown in breast cancer cells reduces lung metastasis in vivo. Mechanistically, EZH2 binds to phosphorylated p38 (p-p38) and upregulates p38 downstream signaling, while EZH2 inhibition in breast cancer cells decreases p-p38 binding, expression, and downstream signaling. The relevance of our in vivo and in vitro studies to human breast cancer is highlighted by the finding that human breast cancer distant metastases express high levels of EZH2 and p-p38. Taken together, this study identifies a novel function of EZH2 in controlling p-p38 activity, breast cancer cell motility, invasion and metastasis.
Materials and Methods
Cell culture
Breast cancer cell lines MDA-MB-231 and MCF7 and mammary epithelial cell line MCF10A were obtained from the American Type Culture Collection. All cell lines were grown under recommended conditions. The SUM149 breast cancer cell line was obtained from the S. Ethier laboratory (Karmanos Cancer Institute) and cultured as previously reported [22].
EZH2 knockdown using stable short-hairpin interfering RNA in lentivirus was completed as previously reported [17]. Cells were transduced and selected for antibiotic resistance with puromycin (Sigma-Aldrich, #P9620). EZH2 knockdown was also achieved using 3-Deazaneplanocin A (Cayman Chemical, #13828) at 1μM for 5 days treating every other day. As previously reported, transient EZH2 overexpression was achieved through infection with an EZH2-encoding, myc-tagged pCMV for 48 hours, kind gift of A. Chinnaiyan [23,24]. The p-p38 inhibitors, SB203580 (Cell Signaling, #5633) or SB202190 (abcam, #120638) were used at 10 or 20μM for 48 hours.
Western blotting and immunoprecipitations
Cells were lysed in RIPA lysis buffer with protease and phosphatase inhibitors (Thermo Scientific, #89900, #78410 & #78420) and Western blot analyses were carried out using 50μg of whole cell extract. Samples were separated by SDS-PAGE gels and transferred onto PVDF membranes; membranes were blocked and incubated with primary antibodies in 3% BSA (Sigma-Aldrich, #A3059) in TBS-T (Bio-Rad, #161-0372, with 0.05% Tween20) at 4°C overnight. Protein signals were visualized via chemiluminescence as described by manufacturer (Thermo Scientific, #32106). β-Actin-HRP (Santa Cruz, #47778) was used to confirm equal loading. Cell Signaling antibodies: rabbit monoclonals EZH2 (#5246), E-cadherin (#3195), SUZ12 (#3737), p38β (#2339), p38δ (#2308), MAPKAPK-2 (#3042), Snail1 (#3879); rabbit polyclonals p38 (#9212), p38α (#9218), p38γ (#2307), phospho-HSP27 (Ser82, #2401), phospho-MAPKAPK-2 ( #3007); mouse monoclonals Snail1 (#3895) and HSP27 (#2402). Abcam antibodies: rabbit monoclonal Cytokeratin-18 (#32118), rabbit polyclonal EED (#4469) and mouse monoclonal trimethyl-HistoneH3 ( #6002). Additionally: rabbit polyclonal ACTIVE®-p38 MAPK (pTGpY, Promega, #V1211) and rabbit monoclonal Vimentin (Epitomics, #2707-1).
Immunoprecipitations (IPs) were conducted following protocol instructions (Sigma-Aldrich, #IP50). Protein was extracted from 70% confluent cells, and protein extracts were precleared with Protein G agarose for 3 hours and incubated with antibody (normal mouse IgG [Santa Cruz, #2025], p38 [Novus Biologicals, #NBP1-97545], EED [abcam, #4469], EZH2, phospho-p38, or SUZ12 [Cell Signaling, #5246, #9216, #3737, respectively]) overnight at 4°C. Next day, protein–antibody complexes were captured with Protein G agarose beads for 2 hours, washed in stringent conditions and eluted. Inputs and IPs were separated as by described Western blot protocol. Immunoprecipitated EED was detected using Clean-Blot™ IP HRP (Thermo Scientific, #21230) to avoid interference from denatured IgG.
Invasion and motility assays
In vitro invasion was done using Matrigel Invasion Chambers (BD Biosciences, #354480) according to the manufacturer’s instructions, in triplicate. Invasive cells on lower sides of chambers were crystal violet stained, air-dried and photographed. They were quantified using ImageJ to count colored pixels, or for colorimetric assays, inserts were treated with 10% acetic acid to remove dye and absorbance was measured at 560 nm.
Random motion cell motility assays were completed as previously described [21]. Briefly, cells were plated on collagen-coated chambered coverslips at low density attaching overnight. Next day, cells were imaged every 10 minutes at 37°C for 24 hours using the DeltaVision RT Live Cell Imaging System (Applied Precision, GE Healthcare) equipped with a UPlanAo 20X/0.7 NA lens at the University of Michigan Microscopy and Image analysis Laboratory. DIC images were acquired using SoftWoRx 3.5.1 software, and cell movements were quantified using MTrackJ /ImageJ software.
Spontaneous metastasis assay / Human breast tissue and immunohistochemistry
Ten-week-old severe combined immunodeficiency mice (Jackson Laboratories) were used for examining tumorigenicity as previously reported [17]. Additional methods on the spontaneous metastasis assay, including information on tumor staining and staining quantifications, can be found in the Supplementary Methods.
A high-density tissue microarray containing 16 human primary invasive breast carcinomas with matched metastases was employed as previously reported [25,26]. Immunohistochemistry on formalin-fixed, paraffin-embedded tissue blocks was performed using anti-EZH2 (Cell Signaling, #5246, 1:150) and anti-phospho-p38 MAPK (Cell Signaling, #9216, 1:3000). EZH2 and p-p38 expression was evaluated as low or high based on intensity of staining and percentage of staining cells, following published literature [27]. The complete clinical and pathological information on these tumors is shown in Supplementary Table 1.
Results
EZH2 knockdown induces a mesenchymal-to-epithelial transition and decreases the ability of breast cancer cells to move
EZH2 overexpressing breast carcinomas have aggressive clinical behavior, high frequency of estrogen receptor negative status (ER−) and are associated with a high propensity to metastasize [14]. However, direct evidence that EZH2 regulates cancer cell phenotype and motility are lacking. To test the hypothesis that EZH2 knockdown may reduce the ability of breast cancer cells to move and invade into the surrounding tissues, we employed breast cancer cell lines MDA-MB-231 and SUM149, both of which are ER−, invasive, tumorigenic in vivo and express high levels of EZH2 protein in comparison to nontumorigenic breast cell lines [17]. We utilized two independent and complementary methods to downregulate EZH2 protein levels in breast cancer cells: stable expression of a short hairpin RNA interference (shRNA) in a lentiviral vector and pharmacologic inhibition using 3-Deazaneplanocin A (DZNeP), a histone methyltransferase inhibitor which disrupts PRC2 (Fig. 1a and Supplementary Fig. 1a).
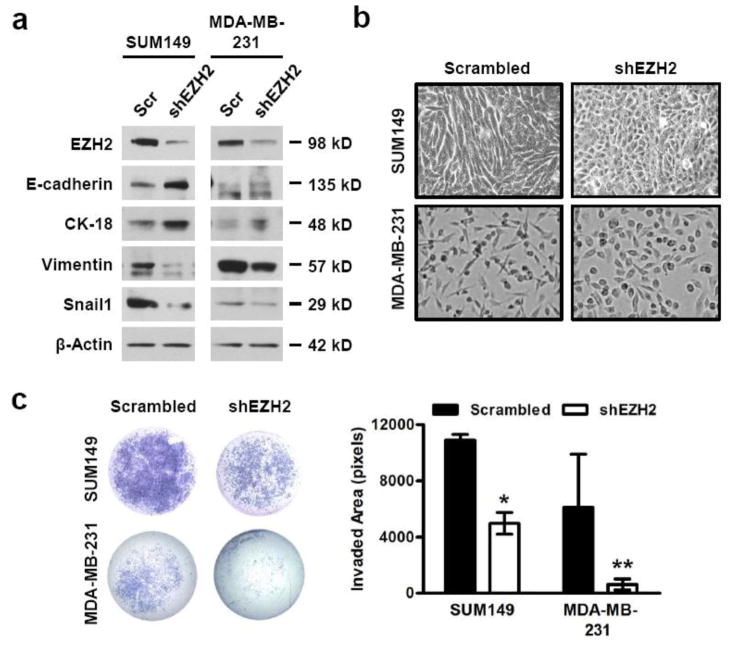
Figure 1. EZH2 knockdown induces a MET and decreases invasion in breast cancer cells.
(a) Immunoblots of SUM149 and MDA-MB-231 breast cancer cells show that downregulation of EZH2 protein with EZH2-targeted shRNA [shEZH2] leads to a protein expression profile indicative of epithelial differentiation compared to scrambled control shRNA [Scr]. E-cadherin and Cytokeratin-18 [CK-18] represent epithelial marker proteins, and Vimentin and Snail1 represent mesenchymal marker proteins. (b) Representative phase contrast images show that EZH2 knockdown (KD) in SUM149 and MDA-MB-231 cells leads to a morphological change from mesenchymal-like to epithelial when compared to controls [200X magnification]. (c) EZH2 KD reduces invasion of SUM149 and MDA-MB-231 cells compared to controls using a reconstituted Boyden basement membrane invasion chamber assay. Left, representative images of entire invaded and stained chambers are shown; right, mean invaded area ± SD was calculated by quantifying stained image pixels using ImageJ. [Student’s t-test, *p<0.0002, **p=0.03]
EZH2 knockdown through either shRNA or DZNeP treatment was sufficient to induce a morphologic and a molecular mesenchymal-to-epithelial transition (MET) of SUM149 and MDA-MB-231 cells when compared to scrambled shRNA or untreated controls, respectively (Fig. 1a–1b and Supplementary Fig. 1a–1b). The observed morphologic change was associated with a protein expression profile characteristic of MET: increased expression of the epithelial markers Cytokeratin-18 and E-cadherin and decreased expression of the mesenchymal markers Vimentin and Snail1 (Fig. 1a and Supplementary Fig. 1a). Knockdown of EZH2 with shRNA or DZNeP significantly reduced invasion in MDA-MB-231 and SUM149 cells when compared to corresponding controls (Fig. 1c and Supplementary Fig. 1c).
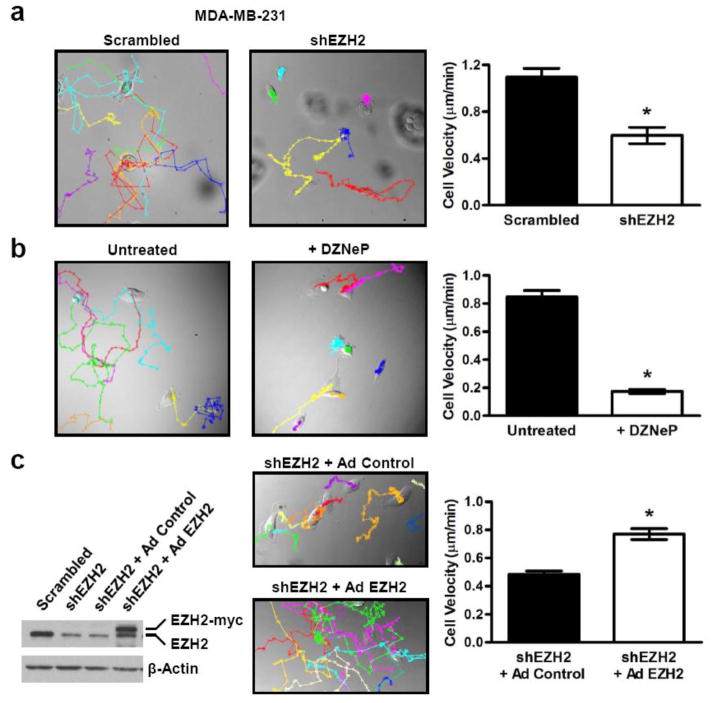
We next investigated the role of EZH2 on cell motility, a critical step in metastasis. Random cell motion was quantified using live cell imaging with time-lapse microscopy as previously described [21]. EZH2 downregulation by shRNA or DZNeP in MDA-MB-231 cells significantly decreased the average cell velocity when compared to controls (Fig. 2a–2b). Furthermore, rescue of EZH2 expression using a myc-tagged EZH2 adenovirus partially reversed the decreased motility induced by EZH2 knockdown in MDA-MB-231 cells (Fig. 2c). Collectively, these experiments show that EZH2 downregulation promotes a MET and reduces the motility and invasiveness of breast cancer cells.
Figure 2. EZH2 knockdown decreases breast cancer cell motility.
(a & b) Left, representative images displaying MTrackJ individual MDA-MB-231 cell tracks, colored dots and connecting lines, from 24 hour time-lapse videos of (a) scrambled shRNA control and shEZH2 or (b) untreated and DZNeP treated cells [200X magnification]. Each dot represents a 10 minute time span and closely spaced dots indicate less movement over the elapsed time versus widely spaced dots. Right, bar graphs show that EZH2 KD cells are significantly slower than controls as demonstrated by the average cell velocity ± SEM [Student’s t-test, *p<1×10−5, n ≥25 cells per condition]. (c) Transient rescue of EZH2 expression in MDA-MB-231 EZH2 KD cells using a myc-tagged EZH2-encoding adenovirus reverses the decreased motility of EZH2 KD cells. Representative images displaying cell tracks of shEZH2 cells infected with either control or EZH2 adenovirus [200X magnification]. The bar graph shows that shEZH2 cells with EZH2 adenoviral rescue are significantly faster than control adenoviral infected cells as demonstrated by the average cell velocity ± SEM [Student’s t-test, *p<9×10−10, n ≥90 cells per condition].
EZH2 regulates the levels of phosphorylated p38 protein and signaling pathway
p38 has emerged as an important regulator of cell migration and metastasis in breast cancer models [20,21,27]. Whether EZH2 influences the levels and function of p38 in human breast cancer is unknown. We found that EZH2 downregulation with shRNA or DZNeP reduced p-p38 protein and the phosphorylation of downstream targets MAPKAPK-2 (MK2) and Heat Shock Protein 27 (HSP27) in breast cancer cell lines when compared to controls (Fig. 3a and Supplementary Fig. 2a). No significant effect on the levels of total p38 was observed by EZH2 knockdown. Conversely, adenoviral overexpression of myc-tagged EZH2 in nontumorigenic MCF10A breast cells and in MCF7 breast cancer cells consistently led to upregulation of p-p38 protein levels when compared to controls (Supplementary Fig. 2b).
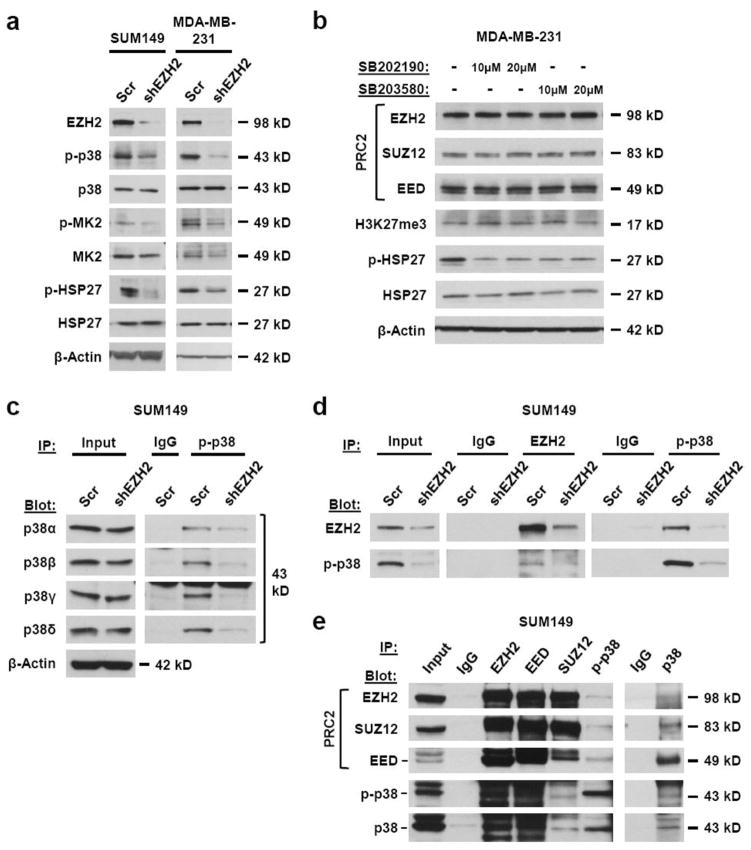
Figure 3. EZH2 regulates the activation of the p38 MAPK signaling pathway and binds to phosphorylated p38 (p-p38).
(a) Immunoblots of SUM149 and MDA-MB-231 breast cancer cells show downregulation of EZH2 protein with EZH2-targeted shRNA [shEZH2] have decreased levels of p-p38 and its activity as demonstrated by the phosphorylation of downstream signaling targets, MK2 and HSP27 when compared to scrambled shRNA control cells [Scr]. (b) Immunoblots show that inhibition of p-p38 activity in MDA-MB-231 cells with SB202190 or SB203580 at two different concentrations for 48 hours does not affect the levels of EZH2, SUZ12, EED or H3K27me3. (c) Activated, phosphorylated levels of all four p38 isoforms, but not total isoform protein levels, are decreased in SUM149 shEZH2 cells when compared to scrambled shRNA control cells. Total p-p38 was immunoprecipitated from whole cell extracts followed by Western blot analysis for the four individual isoforms. (d) Co-immunoprecipitations from whole cell extracts of SUM149 shEZH2 and scrambled shRNA control cells show that endogenous EZH2 immunoprecipitates with endogenous p-p38. Extracts were immunoprecipitated with EZH2, p-p38 or control IgG and bound proteins were revealed by Western blot via antibodies against EZH2 and p-p38. (e) Co-immunoprecipitations from whole cell extracts of SUM149 cells show that EZH2 binds p38/p-p38 in association with PRC2 members SUZ12 and EED. Extracts were immunoprecipitated with EZH2, p38, p-p38, EED, SUZ12 or control IgG and bound proteins were revealed by Western blot via antibodies against EZH2, p38, p-p38, EED and SUZ12.
To further define the mechanistic link between EZH2 and p38, we tested whether p-p38 regulates the levels of EZH2 and other core components of PRC2. Treatment with SB203580 or SB202190, which inhibit the ability of activated p-p38 to phosphorylate downstream targets, such as HSP27, had no effect on EZH2, SUZ12, EED, or H3K27me3 protein levels in MDA-MB-231 cells. These data indicate that EZH2 levels in breast cancer cells are not affected by the p38 signaling pathway (Fig. 3b).
We next investigated whether EZH2 regulates a specific p38 isoform by testing the effect of EZH2 knockdown on the expression of phosphorylated p38α, p38β, p38γ and p38δ. As phospho-specific isoform antibodies are not available, total p-p38 was immunoprecipitated from whole cell extracts of MDA-MB-231 and SUM149 cells expressing scrambled or EZH2-targeted shRNA followed by Western blot analysis for the four isoforms. EZH2 knockdown decreased the phosphorylated levels of all isoforms when compared to controls, while total p38 isoform protein levels remained unaffected (Fig. 3c and Supplementary Fig. 3a). Further supporting a non-transcriptional role for EZH2 in the regulation of p-p38, quantitative real-time RT-PCR showed that neither knockdown nor overexpression of EZH2 affected the mRNA levels of the p38 isoforms when compared to controls (Supplementary Fig. 3b). Collectively, these results show that EZH2 regulates the phosphorylated levels of all p38 isoforms in breast cancer cells and suggest either an indirect transcriptional or a post-transcriptional regulatory mechanism.
EZH2 protein binds with phosphorylated p38 protein
To further understand the mechanism by which EZH2 regulates p-p38, we tested the hypothesis that EZH2 may bind to p-p38 in breast cancer cells. Immunoprecipitation and Western blot analyses revealed that endogenous EZH2 protein interacts with p-p38 in SUM149 and MDA-MB-231 breast cancer cells; and that the binding is reduced by EZH2 knockdown, thereby supporting the specificity of the interaction (Fig. 3d–3e). Furthermore, reciprocal immunoprecipitation experiments demonstrated that EZH2 protein binds to p38/p-p38 protein in association with EED and SUZ12 (Fig. 3e). As PRC2 functions in protein methylation, we hypothesized that PRC2 may methylate p38. Even though all p-p38 isoforms were affected by EZH2 protein expression, we chose to analyze p38α because it is the most abundant and ubiquitously expressed [28]. An in vitro methylation assay shows that addition of PRC2 leads to p38α protein methylation (Supplementary Fig. 3c). Collectively, these data demonstrate that EZH2 binds to p-p38 in association with PRC2, and show that PRC2 can methylate p38α in vitro, which paves the way for future mechanistic investigations.
EZH2 knockdown is sufficient to reduce distant metastasis
EZH2 knockdown was shown to decrease primary breast cancer tumor growth, but whether EZH2 downregulation impacts distant metastasis in breast cancer is unknown [17]. Compared to the scrambled shRNA control, EZH2 knockdown reduced the ability of MDA-MB-231 cells to form spontaneous lung metastasis when injected into the mammary fat pads of NOD/SCID mice (Fig. 4a–4b). Histological analysis of lung tissues collected when primary tumors reached 2 cm3, revealed that 8 of 10 (80%) MDA-MB-231/control mice developed metastases compared with 6 of 10 (60%) of MDA-MB-231/shEZH2 mice. Although no significant difference was observed in the number of mice developing lung metastases between conditions, the metastatic burden as determined by the number of lung metastases per mouse was significantly reduced in shEZH2 mice in comparison to control mice (Fig. 4b). In addition, the metastases formed by shEZH2 cells were smaller than controls; the average sizes of the largest lung metastasis per mouse were 304 μm and 737 μm from shEZH2 and control mice, respectively.
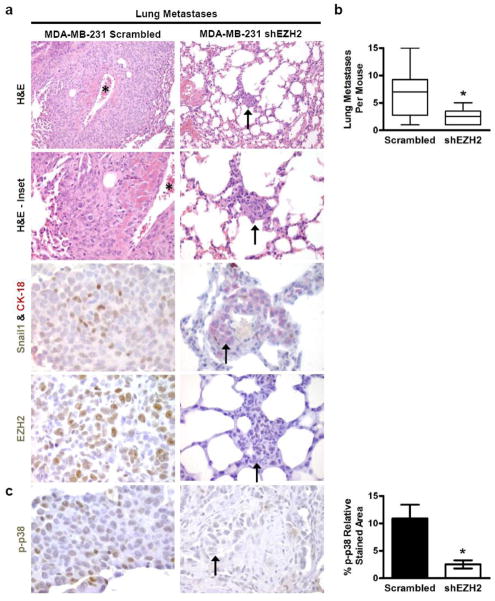
Figure 4. EZH2 knockdown in MDA-MB-231 cells is sufficient to reduce distant metastasis.
(a) Representative photomicrographs of mouse lung metastases of MDA-MB-231 scrambled shRNA control or EZH2-targeted shRNA [shEZH2] cells. EZH2 KD changed the tumor morphology from poorly circumscribed and highly invasive areas towards small, circumscribed foci. The asterisk shows a vessel encased by metastatic carcinoma. The arrows indicate metastases formed by MDA-MB-231 shEZH2 cells. Double immunostain with anti-CK-18 and anti-Snail1 antibodies show that shEZH2 metastases exhibit upregulation of CK-18 and decreased expression of Snail1 in the nuclei of cancer cells compared to controls [H&E: 200X magnification; H&E-Inset: 400X magnification; CK-18&Snail1, EZH2: 600X magnification]. (b) EZH2 KD significantly reduced the number of lung metastases per mouse. The shows that MDA-MB-231 shEZH2 cells formed significantly fewer lung metastases compared to controls. Whiskers indicate the minimum and maximum number of lung metastases per mouse for each condition [Student’s t-test, *p<0.05]. (c) Left, photomicrographs of lung metastases of MDA-MB-231 control and shEZH2 cells exhibit decreased p-p38 protein [600X magnification]. Right, bar graph shows p-p38 protein expression ± SEM in shEZH2 and control lung metastases quantified using FRIDA software [Student’s t-test, *p=0.01].
Consistent with our functional findings, pathological analyses revealed a change in the invasive pattern of breast cancer cells at the metastatic site. The metastases formed by control cells exhibited irregular and infiltrative borders, and encased pre-existing normal structures such as bronchioles and blood vessels (Fig. 4a). In contrast, metastases formed by shEZH2 cells were smaller and circumscribed, with round borders and minimal parenchymal infiltration (Fig. 4a). Consistent with the in vitro data and the histopathological findings, metastases formed by shEZH2 cells exhibited increased Cytokeratin-18 and decreased Snail1 proteins compared to metastases formed by the controls, as demonstrated by double immunohistochemical analyses (Fig. 4a); this effect was also observed in the primary xenografts (Supplementary Fig. 4a). EZH2 knockdown metastases had significantly reduced cell proliferation compared to controls (Supplementary Fig. 4b). Supporting our in vitro observations and mechanistic studies, shEZH2 lung metastases had decreased p-p38 levels when compared to controls (Fig. 4c).
Human breast cancer metastasis exhibit high EZH2 and p-p38 protein expression
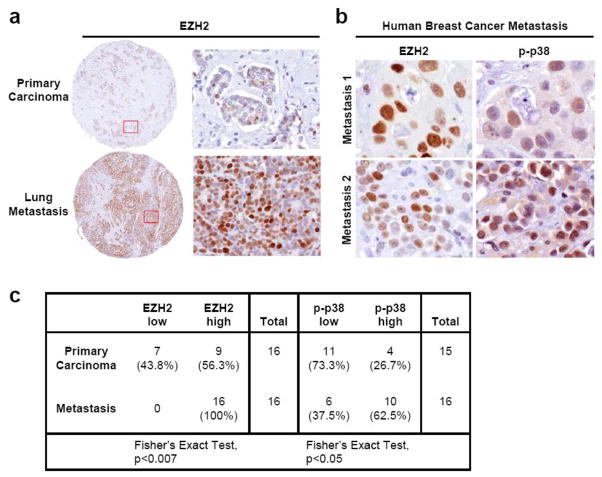
The relevance of these novel findings to human breast cancer was validated by examining the expression of EZH2 and p-p38 proteins in a unique cohort of primary invasive carcinomas and their matched metastases from 16 patients, arrayed in tissue microarrays [25,26]. When present, both proteins predominantly localized to the nuclei of breast cancer cells (Fig. 5a–5b). EZH2 and p-p38 were scored as exhibiting low or high expression according to a previously validated schema [14,27]. We found that EZH2 was significantly upregulated in all metastases when compared to primary carcinomas, and that EZH2 and p-p38 were co-expressed in 63% of the metastases (Fig. 5c). The complete clinical and pathological information on these tumors is shown in Supplementary Table 1.
Figure 5. EZH2 and p-p38 are significantly upregulated in human breast cancer metastases when compared to matched primary tumors from the same patient.
(a) Representative images of matched primary human breast carcinomas and metastases (n=16 patients) immunostained for EZH2 [100X magnification, Inset: 400X magnification]. EZH2 is upregulated in the metastasis compared to the primary tumor. (b) Representative images of two metastases showing concordant high EZH2 and p-p38 expression [600X magnification] (c) The table shows the distribution of EZH2 and p-p38 protein expression in the 16 primary breast carcinomas and matched metastases; 62.5% of metastases exhibited high expression of both EZH2 and p-p38.
Discussion
The data presented here reveal the previously undescribed findings that downregulation of EZH2 leads to MET and decreases motility in breast cancer cells. We show for the first time that EZH2 knockdown is sufficient to reduce distant metastasis in vivo. We uncovered a novel mechanism of EZH2 function by which EZH2 protein binds to p-p38 and leads to upregulated expression of p-p38 protein and its signaling pathway in breast cancer cells.
It has become increasingly evident that cancer cell plasticity influences the biologic behavior of breast cancer by allowing the conversion between epithelial and mesenchymal states [29]. EMT describes the reversible and dynamic process in which epithelial cells, characterized as organized and polarized cells closely attached by intercellular adhesion complexes, undergo a change into mesenchymal-like cells, characterized by a lack of polarization and intercellular junctions. The process of epithelial cancer cells acquiring attributes of mesenchymal-like cells and being driven towards a motile state and metastasis is referred to as oncogenic EMT [29]. One of the hallmarks of EMT is the reduction of normal expression of the cell-cell junction protein E-cadherin [30]. We and other investigators have reported that EZH2 overexpression induces invasion in nontumorigenic breast and prostate cells, and decreases the expression of E-cadherin [23]. However, whether EZH2 can influence EMT and motility of breast cancer cells has not been previously considered. In this study, we show that EZH2 downregulation in breast cancer cell lines is sufficient to reprogram the phenotype of the cells from a spindle to an epithelial morphology with upregulation of E-cadherin and Cytokeratin-18, and downregulation of the mesenchymal protein Vimentin and the EMT transcription factor Snail. The molecular and morphologic features indicative of EMT are tightly associated with the ability of cancer cells to migrate and invade, enabling metastasis. We found that EZH2 downregulation decreased invasion and motility in breast cancer cell lines. Time-lapse microscopy demonstrated that EZH2 downregulation decreased average cell velocity compared to controls.
p38 has been established as a regulator of transitions between epithelial and mesenchymal states as well as cancer cell migration [21,20,27]. Activated p-p38 regulates transcription factors responsible for E-cadherin repression including Snail, Slug and Twist inducing a mesenchymal-like phenotype [31–34]. During TGF-β-induced EMT, p38 activation increases breast cancer lung metastasis [35]. p38α activity is required for the invasive capability of breast, pancreatic, hepatocellular, and head and neck squamous carcinoma cell lines, in part through regulation of matrix metalloproteinases implicated in extracellular remodeling and degradation [27,36–44]. Also, p38δ has been proposed to regulate the invasion of squamous cell carcinoma, while p38γ has been associated with Ras-induced invasion [37,45]. Recently, down-regulation of p38γ markedly decreased the cell motility of breast cancer cells in vitro [21]. Despite the important role of p38 in motility, invasion and metastasis of human cancer, the mechanisms regulating its activation are still being defined. Through a combination of knockdown and overexpression strategies, EZH2 emerges as a novel regulator of p-p38 protein levels and the levels of its downstream signaling proteins in nontumorigenic breast cells and breast cancer cell lines.
The mechanisms implicated in the oncogenic role of EZH2 need further investigation. EZH2 has been considered largely a transcriptional repressor of tumor suppressor genes as part of PRC2, but recent evidence supports contextual, activating functions of EZH2 [7–10,46]. Here, we demonstrate that EZH2 regulates p-p38 via a non-transcriptional mechanism. We found that EZH2 had no effect on the mRNA levels of p38 isoforms using quantitative RT-PCR. Unexpectedly, in breast cancer cells, endogenous EZH2, EED and SUZ12 proteins bind to p-p38 protein, and that downregulation of EZH2 abrogates the binding of EZH2 and p-p38. Our in vitro methylation assay results suggest that PRC2 may methylate p-p38, and paves the way for future studies. Our data lead to the novel hypothesis that EZH2 in association with other PRC2 members may influence p-p38 activity, which is under investigation in our laboratory.
Our group has previously reported that high EZH2 protein expression is associated with the development of metastasis in breast cancer and worse clinical outcome [14]. Data presented here show for the first time that EZH2 knockdown reduces the number of distant breast cancer metastases in vivo. EZH2 knockdown in highly aggressive MDA-MB-231 cells decreased the metastatic burden and reduced the invasiveness of breast cancer cells at the metastatic site, as well as the expression of p-p38. In paired human samples of primary and metastatic carcinomas, EZH2 was significantly overexpressed at the metastatic site. Furthermore, co-expression of EZH2 and p-p38 were detected in 63% of the metastatic carcinomas.
In conclusion, our results demonstrate a previously unknown mechanism of EZH2 function in breast cancer metastasis. We have shown that EZH2 inhibition in breast cancer cell lines leads to a phenotypic change from mesenchymal to epithelial, with reduced motility, invasion and metastasis. We uncover a previously unknown molecular mechanism by which EZH2 binds to p-p38 and regulates the activation of the p38 signaling pathway. From a clinical perspective, the role of EZH2 in p38 signaling is of particular interest as activation of this pathway can be detectable and targetable in tumors to halt breast cancer metastasis.
Supplementary Material
Acknowledgments
We thank Xin Li for help in mouse colony maintenance and executing the spontaneous metastasis assay. We thank Wei Huang and Paul Moore for helpful experimental suggestions and support. We thank Yali Dou and Bo Zhou for assistance with the in vitro methylation assay. This work was supported by the Department of Defense Breast Cancer Research Program through a Predoctoral Traineeship award (BC093828; to H.M.M) and National Institutes of Health grants R01 CA107469, R01 CA125577 and U01 CA154224 (to C.G.K.), and the University of Michigan’s Cancer Center Support Grant (5 P30 CA46592).
Abbreviation
- EMT
Epithelial-to-Mesenchymal Transition
- EZH2
Enhancer of Zeste Homolog 2
- MET
Mesenchymal-to-Epithelial Transition
- p-p38 MAPK
phosphorylated p38 mitogen-activated protein kinase
- PRC2
Polycomb Repressive Complex 2
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53 (1):5–26. doi: 10.3322/canjclin.53.1.5. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Breast Cancer Facts & Figures 2011–2012. 2012. [Google Scholar]
- 3.Hayes DF, Isaacs C, Stearns V. Prognostic factors in breast cancer: current and new predictors of metastasis. J Mammary Gland Biol Neoplasia. 2001;6 (4):375–392. doi: 10.1023/a:1014778713034. [DOI] [PubMed] [Google Scholar]
- 4.Laible G, Wolf A, Dorn R, Reuter G, Nislow C, Lebersorger A, Popkin D, Pillus L, Jenuwein T. Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres. EMBO J. 1997;16(11):3219–3232. doi: 10.1093/emboj/16.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ringrose L, Paro R. Epigenetic regulation of cellular memory by the Polycomb and Trithorax group proteins. Annu Rev Genet. 2004;38:413–443. doi: 10.1146/annurev.genet.38.072902.091907. [DOI] [PubMed] [Google Scholar]
- 6.Satijn DP, Otte AP. Polycomb group protein complexes: do different complexes regulate distinct target genes? Biochim Biophys Acta. 1999;1447(1):1–16. doi: 10.1016/s0167-4781(99)00130-x. S0167-4781(99)00130-X [pii] [DOI] [PubMed] [Google Scholar]
- 7.Tonini T, Bagella L, D’Andrilli G, Claudio PP, Giordano A. Ezh2 reduces the ability of HDAC1-dependent pRb2/p130 transcriptional repression of cyclin A. Oncogene. 2004;23(28):4930–4937. doi: 10.1038/sj.onc.1207608. 1207608 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Shi B, Liang J, Yang X, Wang Y, Zhao Y, Wu H, Sun L, Zhang Y, Chen Y, Li R, Hong M, Shang Y. Integration of estrogen and Wnt signaling circuits by the polycomb group protein EZH2 in breast cancer cells. Mol Cell Biol. 2007;27(14):5105–5119. doi: 10.1128/MCB.00162-07. MCB.00162-07 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su IH, Dobenecker MW, Dickinson E, Oser M, Basavaraj A, Marqueron R, Viale A, Reinberg D, Wulfing C, Tarakhovsky A. Polycomb group protein ezh2 controls actin polymerization and cell signaling. Cell. 2005;121(3):425–436. doi: 10.1016/j.cell.2005.02.029. S0092-8674(05)00196-0 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Asangani IA, Ateeq B, Cao Q, Dodson L, Pandhi M, Kunju LP, Mehra R, Lonigro RJ, Siddiqui J, Palanisamy N, Wu YM, Cao X, Kim JH, Zhao M, Qin ZS, Iyer MK, Maher CA, Kumar-Sinha C, Varambally S, Chinnaiyan AM. Characterization of the EZH2-MMSET Histone Methyltransferase Regulatory Axis in Cancer. Mol Cell. 2012 doi: 10.1016/j.molcel.2012.10.008. S1097-2765(12)00861-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang CJ, Hung MC. The role of EZH2 in tumour progression. Br J Cancer. 2012;106(2):243–247. doi: 10.1038/bjc.2011.551. bjc2011551 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauvageau M, Sauvageau G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. Cell Stem Cell. 2010;7(3):299–313. doi: 10.1016/j.stem.2010.08.002. S1934-5909(10)00394-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chase A, Cross NC. Aberrations of EZH2 in cancer. Clin Cancer Res. 2011;17(9):2613–2618. doi: 10.1158/1078-0432.CCR-10-2156. 1078-0432.CCR-10-2156 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, Ghosh D, Sewalt RG, Otte AP, Hayes DF, Sabel MS, Livant D, Weiss SJ, Rubin MA, Chinnaiyan AM. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100(20):11606–11611. doi: 10.1073/pnas.1933744100. 1933744100 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP, Akslen LA. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24(2):268–273. doi: 10.1200/JCO.2005.01.5180. JCO.2005.01.5180 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Collett K, Eide GE, Arnes J, Stefansson IM, Eide J, Braaten A, Aas T, Otte AP, Akslen LA. Expression of enhancer of zeste homologue 2 is significantly associated with increased tumor cell proliferation and is a marker of aggressive breast cancer. Clin Cancer Res. 2006;12(4):1168–1174. doi: 10.1158/1078-0432.CCR-05-1533. 12/4/1168 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez ME, Li X, Toy K, DuPrie M, Ventura AC, Banerjee M, Ljungman M, Merajver SD, Kleer CG. Downregulation of EZH2 decreases growth of estrogen receptor-negative invasive breast carcinoma and requires BRCA1. Oncogene. 2009;28(6):843–853. doi: 10.1038/onc.2008.433. onc2008433 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding L, Erdmann C, Chinnaiyan AM, Merajver SD, Kleer CG. Identification of EZH2 as a molecular marker for a precancerous state in morphologically normal breast tissues. Cancer Res. 2006;66(8):4095–4099. doi: 10.1158/0008-5472.CAN-05-4300. 66/8/4095 [pii] [DOI] [PubMed] [Google Scholar]
- 19.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat Rev Cancer. 2009;9(8):537–549. doi: 10.1038/nrc2694. nrc2694 [pii] [DOI] [PubMed] [Google Scholar]
- 20.del Barco Barrantes I, Nebreda AR. Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans. 2012;40(1):79–84. doi: 10.1042/BST20110676. BST20110676 [pii] [DOI] [PubMed] [Google Scholar]
- 21.Rosenthal DT, Iyer H, Escudero S, Bao L, Wu Z, Ventura AC, Kleer CG, Arruda EM, Garikipati K, Merajver SD. p38gamma promotes breast cancer cell motility and metastasis through regulation of RhoC GTPase, cytoskeletal architecture, and a novel leading edge behavior. Cancer Res. 2011;71(20):6338–6349. doi: 10.1158/0008-5472.CAN-11-1291. 0008-5472.CAN-11-1291 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleer CG, Zhang Y, Pan Q, Merajver SD. WISP3 (CCN6) is a secreted tumor-suppressor protein that modulates IGF signaling in inflammatory breast cancer. Neoplasia. 2004;6(2):179–185. doi: 10.1593/neo.03316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cao Q, Yu J, Dhanasekaran SM, Kim JH, Mani RS, Tomlins SA, Mehra R, Laxman B, Cao X, Kleer CG, Varambally S, Chinnaiyan AM. Repression of E-cadherin by the polycomb group protein EZH2 in cancer. Oncogene. 2008;27(58):7274–7284. doi: 10.1038/onc.2008.333. onc2008333 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419(6907):624–629. doi: 10.1038/nature01075. nature01075 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Cimino-Mathews A, Hicks JL, Illei PB, Halushka MK, Fetting JH, De Marzo AM, Park BH, Argani P. Androgen receptor expression is usually maintained in initial surgically resected breast cancer metastases but is often lost in end-stage metastases found at autopsy. Hum Pathol. 2012;43(7):1003–1011. doi: 10.1016/j.humpath.2011.08.007. S0046-8177(11)00343-1 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singhi AD, Cimino-Mathews A, Jenkins RB, Lan F, Fink SR, Nassar H, Vang R, Fetting JH, Hicks J, Sukumar S, De Marzo AM, Argani P. MYC gene amplification is often acquired in lethal distant breast cancer metastases of unamplified primary tumors. Mod Pathol. 2012;25(3):378–387. doi: 10.1038/modpathol.2011.171. modpathol2011171 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal A, Huang W, Li X, Toy KA, Nikolovska-Coleska Z, Kleer CG. CCN6 modulates BMP signaling via the Smad-independent TAK1/p38 pathway, acting to suppress metastasis of breast cancer. Cancer Res. 2012;72(18):4818–4828. doi: 10.1158/0008-5472.CAN-12-0154. 0008-5472.CAN-12-0154 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cuadrado A, Nebreda AR. Mechanisms and functions of p38 MAPK signalling. Biochem J. 2010;429(3):403–417. doi: 10.1042/BJ20100323. BJ20100323 [pii] [DOI] [PubMed] [Google Scholar]
- 29.Drasin DJ, Robin TP, Ford HL. Breast cancer epithelial-to-mesenchymal transition: examining the functional consequences of plasticity. Breast Cancer Res. 2011;13(6):226. doi: 10.1186/bcr3037. bcr3037 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tiwari N, Gheldof A, Tatari M, Christofori G. EMT as the ultimate survival mechanism of cancer cells. Semin Cancer Biol. 2012;22(3):194–207. doi: 10.1016/j.semcancer.2012.02.013. S1044-579X(12)00049-1 [pii] [DOI] [PubMed] [Google Scholar]
- 31.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. doi: 10.1016/j.cell.2009.11.007. S0092-8674(09)01419-6 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Hong J, Zhou J, Fu J, He T, Qin J, Wang L, Liao L, Xu J. Phosphorylation of serine 68 of Twist1 by MAPKs stabilizes Twist1 protein and promotes breast cancer cell invasiveness. Cancer Res. 2011;71(11):3980–3990. doi: 10.1158/0008-5472.CAN-10-2914. 0008-5472.CAN-10-2914 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hipp S, Berg D, Ergin B, Schuster T, Hapfelmeier A, Walch A, Avril S, Schmalfeldt B, Hofler H, Becker KF. Interaction of Snail and p38 mitogen-activated protein kinase results in shorter overall survival of ovarian cancer patients. Virchows Arch. 2010;457(6):705–713. doi: 10.1007/s00428-010-0986-5. [DOI] [PubMed] [Google Scholar]
- 34.Zohn IE, Li Y, Skolnik EY, Anderson KV, Han J, Niswander L. p38 and a p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125(5):957–969. doi: 10.1016/j.cell.2006.03.048. S0092-8674(06)00563-0 [pii] [DOI] [PubMed] [Google Scholar]
- 35.Parvani JG, Taylor MA, Schiemann WP. Noncanonical TGF-beta signaling during mammary tumorigenesis. J Mammary Gland Biol Neoplasia. 2011;16(2):127–146. doi: 10.1007/s10911-011-9207-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsieh YH, Wu TT, Huang CY, Hsieh YS, Hwang JM, Liu JY. p38 mitogen-activated protein kinase pathway is involved in protein kinase Calpha-regulated invasion in human hepatocellular carcinoma cells. Cancer Res. 2007;67(9):4320–4327. doi: 10.1158/0008-5472.CAN-06-2486. 67/9/4320 [pii] [DOI] [PubMed] [Google Scholar]
- 37.Junttila MR, Ala-Aho R, Jokilehto T, Peltonen J, Kallajoki M, Grenman R, Jaakkola P, Westermarck J, Kahari VM. p38alpha and p38delta mitogen-activated protein kinase isoforms regulate invasion and growth of head and neck squamous carcinoma cells. Oncogene. 2007;26(36):5267–5279. doi: 10.1038/sj.onc.1210332. 1210332 [pii] [DOI] [PubMed] [Google Scholar]
- 38.Demuth T, Reavie LB, Rennert JL, Nakada M, Nakada S, Hoelzinger DB, Beaudry CE, Henrichs AN, Anderson EM, Berens ME. MAP-ing glioma invasion: mitogen-activated protein kinase kinase 3 and p38 drive glioma invasion and progression and predict patient survival. Mol Cancer Ther. 2007;6(4):1212–1222. doi: 10.1158/1535-7163.MCT-06-0711. 1535-7163.MCT-06-0711 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Hsieh MJ, Chen KS, Chiou HL, Hsieh YS. Carbonic anhydrase XII promotes invasion and migration ability of MDA-MB-231 breast cancer cells through the p38 MAPK signaling pathway. Eur J Cell Biol. 2010;89(8):598–606. doi: 10.1016/j.ejcb.2010.03.004. S0171-9335(10)00070-1 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Johansson N, Ala-aho R, Uitto V, Grenman R, Fusenig NE, Lopez-Otin C, Kahari VM. Expression of collagenase-3 (MMP-13) and collagenase-1 (MMP-1) by transformed keratinocytes is dependent on the activity of p38 mitogen-activated protein kinase. J Cell Sci. 2000;113(Pt 2):227–235. doi: 10.1242/jcs.113.2.227. [DOI] [PubMed] [Google Scholar]
- 41.Kumar P, Yadav A, Patel SN, Islam M, Pan Q, Merajver SD, Teknos TN. Tetrathiomolybdate inhibits head and neck cancer metastasis by decreasing tumor cell motility, invasiveness and by promoting tumor cell anoikis. Mol Cancer. 2010;9:206. doi: 10.1186/1476-4598-9-206. 1476-4598-9-206 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006;25(21):2987–2998. doi: 10.1038/sj.onc.1209337. 1209337 [pii] [DOI] [PubMed] [Google Scholar]
- 43.Park SY, Jeong KJ, Panupinthu N, Yu S, Lee J, Han JW, Kim JM, Lee JS, Kang J, Park CG, Mills GB, Lee HY. Lysophosphatidic acid augments human hepatocellular carcinoma cell invasion through LPA1 receptor and MMP-9 expression. Oncogene. 2011;30(11):1351–1359. doi: 10.1038/onc.2010.517. onc2010517 [pii] [DOI] [PubMed] [Google Scholar]
- 44.Dreissigacker U, Mueller MS, Unger M, Siegert P, Genze F, Gierschik P, Giehl K. Oncogenic K-Ras down-regulates Rac1 and RhoA activity and enhances migration and invasion of pancreatic carcinoma cells through activation of p38. Cell Signal. 2006;18(8):1156–1168. doi: 10.1016/j.cellsig.2005.09.004. S0898-6568(05)00239-1 [pii] [DOI] [PubMed] [Google Scholar]
- 45.Loesch M, Chen G. The p38 MAPK stress pathway as a tumor suppressor or more? Front Biosci. 2008;13:3581–3593. doi: 10.2741/2951. 2951 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shin YJ, Kim JH. The role of EZH2 in the regulation of the activity of matrix metalloproteinases in prostate cancer cells. PLoS One. 2012;7(1):e30393. doi: 10.1371/journal.pone.0030393. PONE-D-11-21823 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.