Abstract
Background and Objectives
Plasma and platelet concentrates are disproportionately implicated in transfusion-related acute lung injury (TRALI). Platelet-derived pro-inflammatory mediators, including soluble CD40 ligand (sCD40L), accumulate during storage. We hypothesized that platelet contamination induces sCD40L generation that causes neutrophil [polymorphonuclear leucocyte (PMN)] priming and PMN-mediated cytotoxicity.
Materials and Methods
Plasma was untreated, centrifuged (12 500 g) or separated from leucoreduced whole blood (WBLR) prior to freezing. Platelet counts and sCD40L concentrations were measured 1–5 days post-thaw. The plasma was assayed for PMN priming activity and was used in a two-event in vitro model of PMN-mediated human pulmonary microvascular endothelial cell (HMVEC) cytotoxicity.
Results
Untreated plasma contained 42 ± 4.2 × 103/μl platelets, which generated sCD40L accumulation (1.6-eight-fold vs. controls). Priming activity and HMVEC cytotoxicity were directly proportional to sCD40L concentration. WBLR and centrifugation reduced platelet and sCD40L contamination, abrogating the pro-inflammatory potential.
Conclusion
Platelet contamination causes sCD40L accumulation in stored plasma that may contribute to TRALI. Platelet reduction is potentially the first TRALI mitigation effort in plasma manufacturing.
Keywords: plasma, sCD40L, transfusion-related acute lung injury
Introduction
Transfusion-related acute lung injury (TRALI) is the leading cause of transfusion-associated morbidity and mortality worldwide [1–4]. TRALI is defined as the development of acute lung injury (ALI): non-cardiogenic severe hypoxaemia (PaO2/FiO2 < 300 mmHg) and bilateral pulmonary infiltrates seen on radiograph, during or within 6 h of transfusion [3, 5]. Although the exact aetiology of TRALI has yet to be elucidated, there appear to be both antibody and antibody-negative mechanisms [6–8]. In all proposed mechanisms of TRALI, the neutrophil [polymorphonuclear leucocyte (PMN)] is the effector cell. Additionally, TRALI is thought to be the result of two distinct clinical events: the first is the patient's clinical condition, an underlying disease that leads to the recruitment and sequestration of PMNs to the pulmonary microvasculature, and the second event is the passive transfer of antibodies or biological response modifiers (BRMs) from the transfused component that activate the recipient's primed, adherent PMNs, resulting in PMN-mediated destruction of endothelial cells, capillary leak, and ALI [9, 10].
Platelets produce several BRMs that are released during activation. Vascular endothelial growth factor (VEGF) is a platelet-derived growth factor implicated in both angiogenesis and pulmonary capillary permeability [11–13]. Platelet factor 4 (PF4) is a cytokine also stored in the α-granules of platelets and released during activation. Its primary role is to upregulate thrombosis formation by binding heparin [14]. Soluble CD40 ligand (sCD40L) is a transmembrane protein released from the platelet in its soluble form during platelet activation and it upregulates both inflammation and thrombosis [15].
Current TRALI mitigation strategies focus on the deferral of high-risk plasma donors, including multiparous women who have an increased likelihood of having anti-leucocyte antibodies [16–19]. This `male-only' plasma strategy has effectively reduced antibody-mediated TRALI and overall number of TRALI-related deaths [2, 20]. Despite these efforts, there are still TRALI patients who received `low-risk' plasma units and this incidence is unchanged by donor-targeted mitigation strategies [21]. In order to further decrease the incidence of TRALI from both `high-' and `low-risk' plasma units, there need to be mitigation strategies that address the aetiology of non-antibody-mediated TRALI.
We hypothesize that (i) there is significant platelet contamination in plasma separated from whole blood (WB) by centrifugation, per industry standards, and that these platelets release BRMs that induce PMN cytotoxicity, (ii) whole-blood leucoreduction (WBLR) or cell depletion centrifugation will decrease platelet contamination of frozen plasma, leading to less VEGF, PF4, and sCD40L and less accumulation of these proteins in thawed plasma over 5-day storage period at 4°C, and (iii) reduction of platelets and platelet-derived BRMs (PDBRMs) in FDA-licensed plasma will reduce its pro-inflammatory potential, leading to fewer plasma transfusion-associated reactions, particularly TRALI.
Materials and methods
Control units and plasma centrifugation plasma
Ten units of WB were drawn from volunteer donors (five women and five men). These units underwent standard centrifugation at Bonfils Blood Center for the primary separation of red cells from plasma in the production of FDA-licensed products. Following separation, the plasma unit was divided into two samples – one that underwent additional centrifugation prior to freezing and the other that did not. The additional centrifugation was performed at 12 500 g for 6 min at 4°C to achieve cell depletion. Samples were subsequently frozen in accordance with industry guidelines for the preparation of frozen plasma [22].
Whole-blood leucoreduction
A total of 28 units of WB were drawn from volunteer donors (17 women and 11 men). Ten of these units were filtered with the PALL BP4 leucoreduction filter (430–40) (PALL Corp. Port Washington, NY). Following collection, the filter line was connected in sterile fashion to the WB unit and to a satellite bag per the manufacturer's specifications (detailed in the package insert). Nine units were filtered with the Leukoflex (LST) (MacoPharma, Mouvaux, France) per manufacturer's specifications (detailed in the package insert). Nine units were filtered using the Imuflex WB-RP bag system with integral WB leucocyte reduction filter (Terumo Medical Corp., Somerset, NJ) in accordance with the manufacturer's specifications (detailed in the package insert). These three filters employ different filtering mechanisms and are used during different points in the collection or manufacturing process, making it important to evaluate all three. The WB was then centrifuged as described previously to have primary separation of the plasma from the red-blood-cells (RBCs). The final processing was performed in a Compomat G4 (Fresenius Kabi, Bad Hamburg, Germany). All plasma units were frozen to −18°C after processing and within 8–24 h of collection and stored at −80°C. The three filters will be referred to as filters A, B and C, respectively.
Plasma storage and thaw
Samples were removed from the −80°C freezer and thawed in a 37°C shaking water bath for 15 min. They were then stored at 4°C per industry standards for the specified amount of time: 1, 3 or 5 days.
Quantification of platelet and PDBRM contamination
For each of the preparations, platelet levels were counted using an AcT5DiffAL (Beckman-Coulter, Brea, CA). In addition, commercially available ELISA was used for the quantification of PDBRMs in the units of frozen plasma: PF4 (ELISA; American Diagnostica, Stamford, CT), sCD40L and VEGF (ELISA; R&D Systems, Minneapolis, MN).
Flow cytometry
To confirm the presence of whole platelets, 100 μl of plasma containing approximately 1 × 106 platelets was incubated with phycoerythrin (PE)-labelled anti-CD41 antibodies or PE-labelled isotype antibodies for negative control (AbD Serotek, Oxford, UK) on ice for 30 min. Paraformaldehyde was added for 5 min, then the sample was diluted to 1% paraformaldehyde using Krebs–Ringer phosphate with 2% dextrose (KRPD). The final sample was diluted to 1:10 with KRPD and analysed in the BD FACSCanto™ II). Flow cytometry data were analysed using with the BD FACSDiva™ (BD Biosciences, San Jose, CA).
PMN isolation and priming and activation assays
Heparinized WB was drawn from healthy human donors, after informed consent, in accordance with a protocol approved by the Colorado Multiple Institutional Review Board at the University of Colorado Denver. PMNs were isolated by standard techniques, including dextran sedimentation, Ficoll-Hypaque gradient centrifugation and hypotonic lysis of contaminating red-blood-cells [23]. Priming assays were completed by incubating PMNs at 37°C for 5 min with 10% plasma. PMNs were then activated by 1-μm formyl-Met-Leu-Phe (fMLP), and the maximal rate of superoxide anion released was measured as the super-oxide dismutase-inhibitable reduction of cytochrome c, as previously published [23, 24]. Priming activity was calculated as the augmentation of the fMLP-activated respiratory burst in buffer-primed control cells.
Human pulmonary microvascular endothelial cell (HMVEC) damage assay
The HMVEC damage assay was performed in accordance with previously published studies [25, 26]. HMVECs were grown to ≥90% confluence in 12-well plates. The wells were incubated with either endotoxin from Salmonella enteroides (LPS) or with buffer for 6 h at 37°C, 5% CO2. PMNs (1 × 106) were added, at a 10:1 effector cell-to-target cell ratio, and allowed to settle for 30 min [25]. After settling, the PMNs were exposed to buffer or 10% plasma that had been stored for 1, 3 or 5 days. Following a 30-min incubation, the supernatants were forcefully decanted by quickly inverting the plates onto absorbent towels, and warm KRPD buffer was added. Viable HMVECs were counted over a 1-mm2 surface area by three separate observers to exclude bias. Controls consisted of HMVECs alone without PMNs, HMVECs with PMNs only, and HMVECs incubated with PMNs and LPS only.
Statistical methods
The results were analysed by one-way ANOVA with a Newman–Keuls post hoc test for multiple comparisons. A P-value < 0.05 was considered significant for raw data, and a P-value < 0.01 was considered significant for normalized data (i.e. priming and HMVEC analyses).
Results
Platelet contamination in FDA-licensed plasma
Untreated plasma units had significant platelet contamination of 42.4 ± 4.2 × 103/μl (n = 10) prior to freezing. This number was not significantly different to the concentration of platelets on the day following thaw, 44.1 ± 2.9 × 103/μl. Over the post-thaw refrigerated storage period, there was a significant decrease to 27 ± 4.0 × 103/μl over 3 days and to 23.2 ± 3.1 × 103/μl over 5 days (Fig. 1). Both centrifugation and WBLR resulted in a significant decrease in number of platelets. Centrifuged samples had 1.7 ± 0.8 × 103/μl, 2.2 ± 0.9 × 103/μl and 2.0 ± 0.7 × 103/μl platelet concentrations (n = 10) on 1, 3 and 5 days of storage, respectively. There was no statistical difference between platelet concentrations over the storage period. WBLR resulted in complete elimination of platelets with a concentration 0.0 × 103/μl in all samples from all three filters (n = 10, 9 and 9 for filters A, B and C, respectively) (Fig. 1).
Fig. 1.
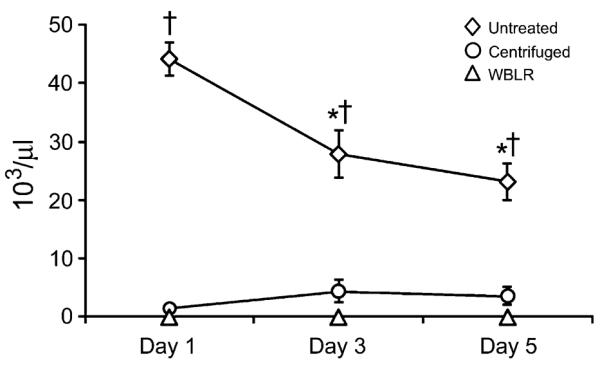
Change in platelet concentration over the 5-day storage period in untreated (n = 10), centrifuged (n = 10), and leucoreduced whole blood (WBLR) (n = 28) plasma samples. The y-axis represents platelet count in ×103/μl. *Decreased from day 1 of storage. †Increased over centrifugation and WBLR samples (P < 0.05).
Flow cytometry with PE-labelled anti-CD41 antibodies confirmed the presence of whole platelets in the untreated plasma samples. There was minimal PE signal in the centrifuged or WBLR samples, confirming the efficacy of these platelet reduction methods. Flow cytometry results of representative units are shown in Fig. 2.
Fig. 2.
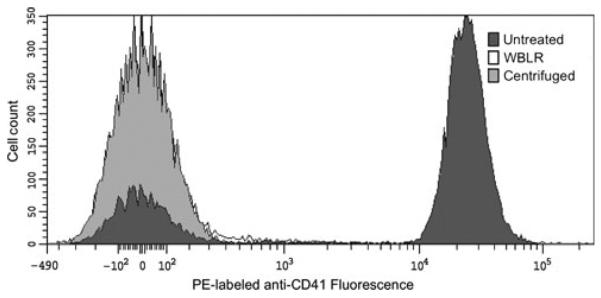
Flow cytometry to evaluate platelets in untreated and treated units. Plasma samples were incubated with phycoerythrin (PE)-labelled antibodies: anti-CD41 or isotype antibody. Each curve of the three curves represents the PE-labelled anti-CD41 signal corrected for the isotype control. Representative units in each treatment group are shown: untreated (dark grey-filled), leucoreduced whole blood (WBLR) (white-filled) and centrifugation (light grey-filled). Consistent with the platelet counts of approximately 0 (as measured by Coulter counter), there was no peak representing PE-labelled anti-CD41 antibodies in either of the treatment groups, which caused significant overlap in the curves. The untreated sample showed a dramatic increase in signal over the treated samples.
Platelet-derived BRM contamination in plasma
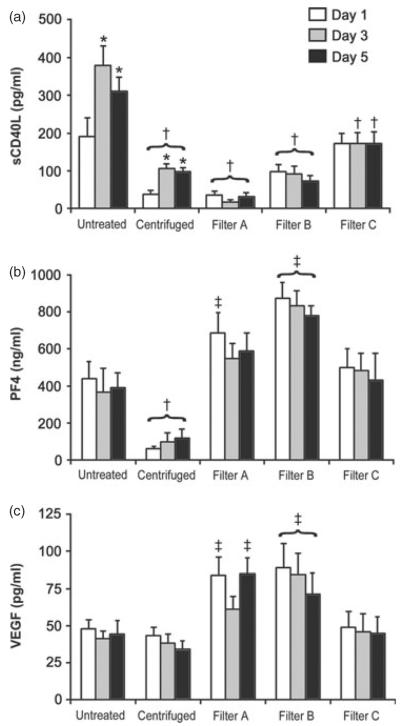
Untreated samples had a sCD40L concentration 1 day post-thaw of 190.2 ± 49.7 pg/ml, significantly more than samples that underwent centrifugation (37.8 ± 11.2 pg/ml) or leucoreduction by filters A and B (36.5 ± 10.4 and 97.0 ± 20.1 pg/ml, respectively). There was no difference between untreated plasma and filter C plasma on day 1 (190.2 ± 49.7 vs. 172 ± 27.9 pg/ml, respectively). However, after 3 and 5 days of post-thaw storage, sCD40L in the untreated plasma increased significantly and was higher than all of the centrifugation and WBLR samples. After 5 days of storage, the concentration of sCD40L in the untreated samples increased 1.6 ± 1.0-fold (range 1–10.5-fold) to 311.2 ± 36.5 pg/ml. Centrifuged samples had approximately a 2.6 ± 0.5-fold (range one to sixfold) increase to 98.6 ± 9.3 pg/ml over the same 5-day period, but comparing each day to the untreated plasma, the centrifuged samples had significantly lower levels of sCD40L. There was no significant change over the 5-day storage period in the samples undergoing WBLR. After 5 days of storage, untreated plasma had significantly more sCD40L than any of the treated samples (Fig. 3a).
Fig. 3.
(a–c) Concentrations of PDBRMs over the 5-day storage period of untreated, centrifuged and leucoreduced whole blood (WBLR) plasma samples expressed as the mean ± SEM. (a) soluble CD40 ligand (sCD40L), (b) Platelet factor 4 (PF4) and (c) vascular endothelial growth factor (VEGF). *Greater than day 1 of same preparation. †Lower than untreated plasma on equivalent storage day. ‡Greater than untreated plasma on equivalent storage day (P < 0.05).
Neither PF4 nor VEGF accumulated over the 5-day period of plasma storage in the untreated plasma or the centrifugation or WBLR groups (Fig. 3b,c, respectively). Samples leucoreduced by filters A or B had higher PF4 and VEGF concentrations than the untreated plasma samples. This increase was not seen in the samples from filter C or centrifugation. In fact, samples that underwent centrifugation were found to have significantly less PF4 than untreated samples. This effect was not seen with VEGF in those samples. There was no relationship between either VEGF or PF4 concentrations and PMN priming activity.
Plasma priming activity
PMN priming activity correlated with sCD40L concentration. PMN priming activity increased as sCD40L concentration increased, with 5-day-old untreated plasma having almost twofold more priming activity than buffer-primed control. The pattern of PMN priming activity matched that of sCD40L concentration in the plasma units. Units that underwent either centrifugation or leucoreduction showed no priming activity when compared to buffer alone and caused significantly less PMN priming when compared to untreated plasma on equivalent storage days. The exception to this finding was that 1-day-old plasma from filter C was equivalent to 1-day-old untreated plasma with respect to priming activity (Fig. 4). To account for variability in differences in baseline PMN activity, the primary data were normalized to the paired buffer-primed controls.
Fig. 4.
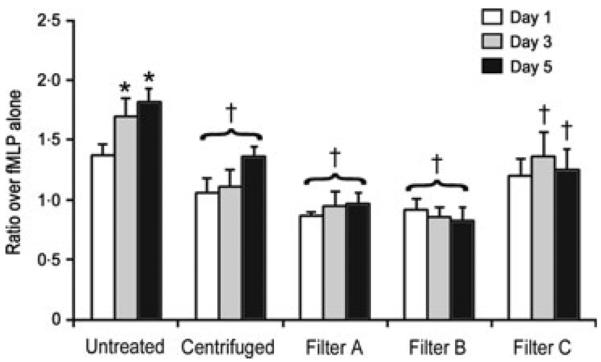
PMN priming activity represented as fold over negative control (PMNs incubated with buffer and LPS only) expressed as the mean ± SEM. *Greater than day 1 of same preparation. †Lower than untreated plasma on equivalent storage day (P < 0.01).
A two-event in vitro model of PMN cytotoxicity
The HMVEC damage assay was run in 12-well plates and controls included HMVECs alone; HMVECs with PMNs and buffer alone; and HMVECs with PMNs, LPS and buffer. The remaining nine wells had HMVECS, PMNs, LPS and 10% plasma. There were three units of plasma per plate, each with 1-, 3- and 5-day-old plasma. The same three plasma units were used on each plate and chosen because they had the highest concentration of sCD40L and highest PMN priming activity. To account for inter-plate HMVEC density variation, all wells on the plate were normalized to the well containing only HMVECs, which was corrected to 200 cells/mm2. The number of surviving HMVECs in the 5-day-old plasma was almost 50% less than the untreated HMVECs. Additionally, there was a 33% decrease in surviving HMVEC cells incubated with 5-day-old plasma than with LPS alone and 1-day-old plasma (Fig. 5).
Fig. 5.
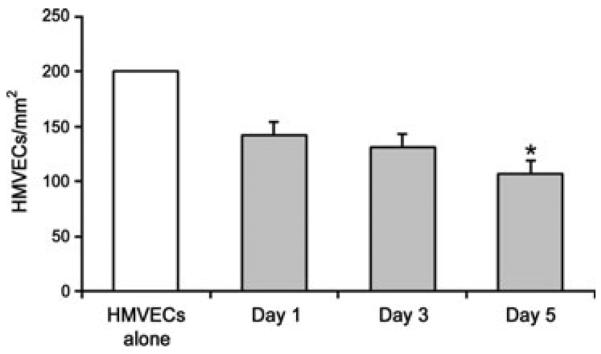
Human pulmonary microvascular endothelial cell (HMVEC) destruction by PMNs activated by plasma and LPS. The data are expressed as the mean ± SEM of cells remaining. Data are represented as normalized to 200 cells/m2 in the HMVECs-alone control well. *Lower than plasma stored for 1 and 3 days and LPS alone (P < 0.01).
Discussion
Of all transfused products, those with >30 ml of plasma (frozen plasma and platelet concentrates) are responsible for a disproportionate number of TRALI cases and are associated with higher mortality rates [2, 27–29]. Plasma accounts for approximately 13% of the total number of blood components transfused [30], but surveil-lance data from the United States, United Kingdom, New Zealand, Germany, and the Netherlands show that frozen plasma products have been implicated in approximately 45–60% of TRALI cases and fatalities [1, 2, 28, 29, 31, 32].
Antibody-mediated TRALI is the most common aetiology of TRALI; in 60–80% of TRALI cases, both the anti-leucocyte antibody and the cognate antigen could be identified [31, 33, 34]. Therefore, blood centres worldwide have implemented a TRALI mitigation strategy of deferring donors who are at high risk of having anti-leucocyte antibodies, such as multiparous women [2, 35, 36]. This mitigation has successfully decreased the number of TRALI cases, though it has not completely eliminated them [2, 20, 37]. Preliminary data presented by the TRALI Specialized Centers of Clinically Oriented Research (SCCOR) group at the AABB National meeting in October 2010 demonstrated that the implementation of a policy eliminating `high-risk' donors (based on likelihood of having anti-leucocyte antibodies, i.e. multiparous women) resulted in approximately 90% fewer cases of TRALI caused by `high-risk' plasma. However, the rate of TRALI caused by `low-risk' units had no change in incidence. The SCCOR data suggest that anti-leucocyte antibodies are not responsible for all TRALI cases whose incidence is unaffected by current TRALI mitigation steps. Prior to this policy, `high-risk' plasma units accounted for 62% of TRALI cases, and following its implementation, they only accounted for 8% of TRALI cases [21].
There continues to be variation in component preparation in the United States, Canada and Europe. Universal leucodepletion is becoming common at many blood centres around the world [38–40]. The point in the manufacturing process at which components are leucoreduced varies from country to country, and many of the surveys performed do not differentiate centres that leucoreduce WB vs. packed red-blood-cells. In Europe, the amount of plasma that is reported to be leucoreduced varies from 20% to 100% depending on country [40]. The data from this study can be used to support those blood centres which already practice whole-blood leucoreduction that results in platelet-depleted plasma and can potentially encourage other centres to consider switching to leucoreducing WB, rather than packed red-blood-cells.
The cases of antibody-negative TRALI are likely caused by BRMs and cytokines that cause the activation of primed PMNs, leading to the release of the microbicidal arsenal of reactive oxygen species and proteases from primed PMNs. As platelets play an important role in the inflammatory response, they can serve as a source of these BRMs found in plasma. In 1996, the AABB introduced thawed plasma [41], which is frozen plasma that has been thawed and stored up to 5 days at 1–6°C. This product is not currently FDA-licenced, though many blood banks and hospitals have adopted its use in an effort to decrease product waste and save money [42, 43].
The data presented in this study demonstrate that there is sufficient platelet contamination in FDA-licensed, whole-blood-derived plasma units to have measurable levels of platelet-derived pro-inflammatory mediators. As the flow cytometry results demonstrate, platelets did survive the freeze–thaw cycle in untreated samples. Both the decrease in platelet count and concomitant increase in sCD40L over the 5-day storage period are likely due to platelet activation and subsequent aggregation or apoptosis. Soluble CD40L accumulates during storage of cellular blood components: WB, platelet concentrates and packed RBCs [26, 44, 45], but to date, only the presented data have examined sCD40L accumulation during the storage of plasma.
Soluble CD40L is an important platelet-derived BRM, which may be responsible for many inflammatory transfusion reactions [26, 46]. Platelet activation causes the release of CD40L in its soluble form, which upregulates adhesion molecules on local endothelial cells and subsequently promotes recruitment and extravasation of PMNs [15, 47]. Soluble CD40L likely binds to PMNs via the CD40 surface receptor, resulting in priming of the fMLP-activated oxidative burst [26]. Our data showed that priming activity occurred from plasma with as little as 290 pg/ml of sCD40L (data not shown), which is much lower than the previously published threshold [26]. This is likely because those priming assays were performed with recombinant, monomeric sCD40L, which has significantly decreased bioactivity as compared to native (dimeric or trimeric) sCD40L, which was present in the plasma samples described in this study [26, 46]. Both the presented and previous data suggest that there is a threshold concentration at which sCD40L causes PMN priming and may explain why there was a significant decrease in priming activity in the both centrifuged and WBLR plasma samples when compared to the untreated plasma.
In a prospective cohort study of patients who had a platelet transfusion-associated febrile or allergic reaction, the mean concentration of sCD40L was 1.24-fold greater in units implicated in a reaction than those that were not (P < 0.015) [46]. This modest increase demonstrates that even small reductions in sCD40L concentration can potentially decrease the pro-inflammatory property of a transfused component. We found that the untreated plasma had 1.9- to five-fold greater sCD40L concentrations on day 1 (filter C excepted) and 1.8- to 28-fold greater levels by day 5 of storage than the centrifuged or WBLR samples.
A recent publication by Tuinman et al. [48] found that blockage of the sCD40L interaction with the vascular endothelium with ciglitazone did not reduce the inflammation in a model of antibody-mediated TRALI. Their model differs from ours in two important ways: (i) their model focuses on platelet–endothelium interactions, whereas our model is PMN-based and (ii) their use of MHC-I antibodies induced a TRALI process independent of the presence or absence of sCD40L. The question our work addresses is not whether sCD40L potentiates antibody-mediated TRALI, but rather whether it is a sufficient stimulus to be the second hit in the two-hit model of TRALI pathogenesis. This point is proven by the HMVEC assay where endothelial cell samples incubated with both LPS and plasma have increased PMN-mediated cell cytotoxicity when compared to LPS alone.
The increased levels of VEGF and PF4 in the WBLR samples are most likely secondary to platelet activation and degranulation during the filtration process. Only filters A and B showed increased VEGF and PF4, while filter C did not. This discrepancy is likely due to inherent filter differences as the system for filter C is in line between the donor and collection bag, whereas filters A and B utilize a satellite bag connection for filtration. This difference in filtration mechanism is also likely the cause of the increased sCD40L levels in filter C samples. Both the VEGF and sCD40L concentrations in our samples were consistent with the findings of Glenister and Sparrow [49], who measured platelet-derived cytokines in the supernatant of leucoreduced (LR) packed red-blood-cells. Like our findings, they determined that the type of LR filter played a role in the concentration and accumulation of platelet-derived cytokines.
There appeared to be a small, non-significant decrease in both VEGF and PF4 in samples from filters A and B over the 5-day storage period. The degree of PMN priming in the plasma samples did not correlate with either VEGF or PF4 levels, suggesting that sCD40L is a more effective pro-inflammatory mediator of innate immunity. The observed increases in its concentration and its ability to induce PMN priming predicted that thawed stored plasma that have significant platelet contamination may participate in PMN-mediated cytotoxicity, resulting in TRALI as the direct result of the increases in sCD40L [26, 50].
Previous data have shown in a stepwise fashion that in vitro PMN priming activity is predictive of in vitro destruction of HMVECs in a two-hit model, which uses the LPS-elicited cellular inflammatory response as the first hit and the BRM in question as the second [25, 26, 50–52]. The HMVEC damage assay performed in this study demonstrated a significant increase in PMN-mediated endothelial cytotoxicity when exposed to 5-day-old untreated plasma and LPS when compared to 1- and 3-day-old plasma and LPS alone. This assay demonstrates a clear link between PMN priming activity and degree of PMN-mediated endothelial cell destruction in an in vitro model, as previously published by our laboratory [25, 26]. Furthermore, a similar two-step in vivo model showed rats pretreated with LPS develop ALI in a manner and time-course similar to that of TRALI when exposed to either an anti-leucocyte antibody or pro-inflammatory BRMs [25, 50, 52, 53]. This relationship between the in vitro and in vivo models supports our conclusion that older, non-cell-depleted plasma which causes PMN priming and HMVEC damage contains BRMs that are likely the cause of antibody-negative TRALI.
Our data demonstrate that contaminating platelets contribute to plasma's pro-inflammatory properties. Platelets are well known for their prominent role in haemostasis and thrombosis and recently have been recognized for their role in mediating inflammation [54]. This role has been confirmed in vivo because platelets were required for the recruitment and activation of PMNs in the pulmonary microvasculature [55, 56]. The role platelets play in inflammation is both direct and indirect (i.e. through the release of BRMs). The presented data implicate sCD40L as the most likely causative platelet-derived BRM of the ones we tested, though there may be others that contribute to plasma's pro-inflammatory properties. Further work is needed to examine this effect in vivo as a further means of elucidating the role the accumulation of specific BRMs in plasma play in the pathogenesis of TRALI.
Male-only or male-predominant donor policies have succeeded in decreasing antibody-mediated TRALI incidence. The challenge for blood banks is to institute additional changes to the collection and manufacturing of frozen plasma that will be effective in decreasing the incidence of antibody-negative TRALI. The data presented in this study provide evidence that the pro-inflammatory potential of FDA-licensed plasma is likely mediated by contaminating platelets and platelet-derived BRMs. The addition of a platelet and platelet-derived BRM reduction step, such as prestorage WBLR or centrifugation, in the production of frozen plasma could reduce the number of non-antibody TRALI cases. Together, the deferral of high-risk donors, along with the addition of a TRALI mitigation step in the production process, have the potential to eliminate almost all cases of TRALI associated with the transfusion of frozen plasma.
Acknowledgements
Dr. Bercovitz is supported by the National Blood Foundation Research Scientist Training Grant. We would like to thank the Production and Quality Control Departments for their assistance in the acquisition and processing of the blood components. We would like to thank the Specialized Donation Department at Bonfils Blood Centre for their assistance in arranging volunteer donations. This work was also supported in part by a grant from National Institutes of Health, NIGMS (P50 GM49222).
Funding
National Blood Foundation Scientific Research Training Grant, Bonfils Blood Center, and a grant from NIGMS, NIH (P50 GM49222).
Footnotes
Conflict of interest
Leucoreduction filters provided by MacoPharma, Mouvaux, France; PALL Corp. Port Washington, NY; Terumo Medical Corp., Somerset, NJ.
Copyright of Vox Sanguinis is the property of Wiley-Blackwell and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
References
- 1.Keller-Stanislawski B, Lohmann A, Gunay S, et al. The German haemovigi-lance system – reports of serious adverse transfusion reactions between 1997 and 2007. Transfus Med. 2009;19:340–349. doi: 10.1111/j.1365-3148.2009.00947.x. [DOI] [PubMed] [Google Scholar]
- 2.Chapman CE, Stainsby D, Jones H, et al. Ten years of hemovigilance reports of transfusion-related acute lung injury in the United Kingdom and the impact of preferential use of male donor plasma. Transfusion. 2009;49:440–452. doi: 10.1111/j.1537-2995.2008.01948.x. [DOI] [PubMed] [Google Scholar]
- 3.Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med. 2005;33:721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 4. [accessed 1 June 2011];Fatalities Reported to the FDA Following Blood Collection and Transfusion. 2008. http://www.fda.gov/Biologics BloodVaccines/SafetyAvailability/Repor taProblem/TransfusionDonationFataliti es/ucm113649.htm (Last)
- 5.Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European Consensus conference on acute respiratory distress syndrome: definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994;9:72–81. doi: 10.1016/0883-9441(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 6.Silliman CC. Transfusion-related acute lung injury. Transfus Med Rev. 1999;13:177–186. doi: 10.1016/s0887-7963(99)80031-5. [DOI] [PubMed] [Google Scholar]
- 7.Urahama N, Tanosaki R, Masahiro K, et al. TRALI after the infusion of marrow cells in a patient with acute lymphoblastic leukemia. Transfusion. 2003;43:1553–1557. doi: 10.1046/j.1537-2995.2003.00542.x. [DOI] [PubMed] [Google Scholar]
- 8.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105:2266–2273. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 9.Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion. 1985;25:573–577. doi: 10.1046/j.1537-2995.1985.25686071434.x. [DOI] [PubMed] [Google Scholar]
- 10.Fung YL, Silliman CC. The role of neutrophils in the pathogenesis of transfusion-related acute lung injury. Transfus Med Rev. 2009;23:266–283. doi: 10.1016/j.tmrv.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Salgado R, Benoy I, Bogers J, et al. Platelets and vascular endothelial growth factor (VEGF): a morphological and functional study. Angiogenesis. 2001;4:37–43. doi: 10.1023/a:1016611230747. [DOI] [PubMed] [Google Scholar]
- 12.Bates DO. Vascular endothelial growth factors and vascular permeability. Cardiovasc Res. 2010;87:262–271. doi: 10.1093/cvr/cvq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ancelin M, Chollet-Martin S, Herve MA, et al. Vascular endothelial growth factor VEGF189 induces human neutrophil chemotaxis in extravascular tissue via an autocrine amplification mechanism. Lab Invest. 2004;84:502–512. doi: 10.1038/labinvest.3700053. [DOI] [PubMed] [Google Scholar]
- 14.Maurer A-M, Bin Z, Zhong Chao H. Roles of platelet factor 4 in hematopoiesis and angiogenesis. Growth Factors. 2006;24:242–252. doi: 10.1080/08977190600988225. [DOI] [PubMed] [Google Scholar]
- 15.Henn V, Steinbach S, Buchner K, et al. The inflammatory action of CD40 ligand (CD154) expressed on activated human platelets is temporally limited by coex-pressed CD40. Blood. 2001;98:1047–1054. doi: 10.1182/blood.v98.4.1047. [DOI] [PubMed] [Google Scholar]
- 16.Densmore TL, Goodnough LT, Ali S, et al. Prevalence of HLA sensitization in female apheresis donors. Transfusion. 1999;39:103–106. doi: 10.1046/j.1537-2995.1999.39199116901.x. [DOI] [PubMed] [Google Scholar]
- 17.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49:1825–1835. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Insunza A, Romon I, Gonzalez-Ponte ML, et al. Implementation of a strategy to prevent TRALI in a regional blood centre. Transfus Med. 2004;14:157–164. doi: 10.1111/j.0958-7578.2004.00492.x. [DOI] [PubMed] [Google Scholar]
- 19.Powers A, Stowell CP, Dzik WH, et al. Testing only donors with a prior history of pregnancy or transfusion is a logical and cost-effective transfusion-related acute lung injury prevention strategy. Transfusion. 2008;48:2549–2558. doi: 10.1111/j.1537-2995.2008.01902.x. [DOI] [PubMed] [Google Scholar]
- 20.Wiersum-Osselton JC, Middelburg RA, Beckers EAM, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion. 2011;51:1278–1283. doi: 10.1111/j.1537-2995.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 21.Looney MR. Personal communication. 2010.
- 22.American Association of Blood Banks . Technical Manual of the American Association of Blood Banks. 17th edn AABB; Washington, DC: 2011. pp. 203–204.pp. 279–280. [Google Scholar]
- 23.Silliman CC, Clay KL, Thurman GW, et al. Partial characterization of lipids that develop during the routine storage of blood and prime the neutrophil NADPH oxidase. J Lab Clin Med. 1994;124:684–694. [PMC free article] [PubMed] [Google Scholar]
- 24.Elzi DJ, Hiester AA, Silliman CC. Receptor-mediated calcium entry is required for maximal effects of platelet activating factor primed responses in human neutrophils. Biochem Biophys Res Commun. 1997;240:763–765. doi: 10.1006/bbrc.1997.7740. [DOI] [PubMed] [Google Scholar]
- 25.Wyman TH, Bjornsen AJ, Elzi DJ, et al. A two-insult in vitro model of PMN-mediated pulmonary endothelial damage: requirements for adherence and chemokine release. Am J Physiol Cell Physiol. 2002;283:C1592–C1603. doi: 10.1152/ajpcell.00540.2001. [DOI] [PubMed] [Google Scholar]
- 26.Khan SY, Kelher MR, Heal JM, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gajic O, Rana R, Winters JL, et al. Transfusion-related acute lung injury in the critically ill: prospective nested case-control study. Am J Respir Crit Care Med. 2007;176:886–891. doi: 10.1164/rccm.200702-271OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eder AF, Herron R, Strupp A, et al. Transfusion-related acute lung injury surveillance (2003–2005) and the potential impact of the selective use of plasma from male donors in the American Red Cross. Transfusion. 2007;47:599–607. doi: 10.1111/j.1537-2995.2007.01102.x. [DOI] [PubMed] [Google Scholar]
- 29.Holness L, Knippen MA, Simmons L, et al. Fatalities caused by TRALI. Transfus Med Rev. 2004;18:184–188. doi: 10.1016/j.tmrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Whitaker BI, King MR, Leiberg LL, et al. 2007 National Blood Collection and Utilization Survey Report. Department of Health and Human Services; 2008. [Google Scholar]
- 31.van Stein D, Beckers EA, Sintnicolaas K, et al. Transfusion-related acute lung injury reports in the Netherlands: an observational study. Transfusion. 2010;50:213–220. doi: 10.1111/j.1537-2995.2009.02345.x. [DOI] [PubMed] [Google Scholar]
- 32.Dunn P, Dinesh D. Transfusion-related acute lung injury (TRALI): a review of investigations by the National Tissue Typing Laboratory of cases reported in New Zealand since June 2004. N Z Med J. 2008;121:42–47. [PubMed] [Google Scholar]
- 33.Zupanska B, Uhrynowska M, Michur H, et al. Transfusion-related acute lung injury and leucocyte-reacting antibodies. Vox Sang. 2007;93:70–77. doi: 10.1111/j.1423-0410.2007.00920.x. [DOI] [PubMed] [Google Scholar]
- 34.Keller-Stanislawski B, Reil A, Gunay S, et al. Frequency and severity of transfusion-related acute lung injury - German haemovigilance data (2006–2007) Vox Sang. 2010;98:70–77. doi: 10.1111/j.1423-0410.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 35.Eder AF, Benjamin RJ. TRALI risk reduction: donor and component management strategies. J Clin Apher. 2009;24:122–129. doi: 10.1002/jca.20198. [DOI] [PubMed] [Google Scholar]
- 36.Eder AF, Herron RM, Jr, Strupp A, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006–2008) Transfusion. 2010;50:1732–1742. doi: 10.1111/j.1537-2995.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 37.Hume HA. TRALI: moving toward prevention. Transfusion. 2009;49:402–405. doi: 10.1111/j.1537-2995.2008.02090.x. [DOI] [PubMed] [Google Scholar]
- 38.The 2009 national blood collection and utilization survey report. US Department of Health and Human Services, Office of the Assistant Secretary for Health; Washington, DC: 2011. Report of the US Department of Health and Human Services. [Google Scholar]
- 39.Services CB. [accessed 19 August 2011];Circular of information. 2004 http://www.bloodservices.ca/Cen treApps/.../Circular-of-Information_SH_E.pdf (Last)
- 40.Chin Tad Muon M. Leukodepletion. In: Rouger PHC, editor. Blood Transfusions in Europe, The White Book 2005. Elsevier; Paris: 2005. pp. 159–165. [Google Scholar]
- 41.AABB . Standards for Blood Banks and Transfusion Services. 17th edn American Association of Blood Banks; Bethesda, MD: 1996. [Google Scholar]
- 42.Gerdes J, Bertolani B, Berg M. Thawed plasma an alternative to fresh frozen plasma. J Trauma Nurs. 2005;12:57–58. doi: 10.1097/00043860-200512020-00007. [DOI] [PubMed] [Google Scholar]
- 43.Wehrli G, Taylor NE, Haines AL, et al. Instituting a thawed plasma procedure: it just makes sense and saves cents. Transfusion. 2009;49:2625–2630. doi: 10.1111/j.1537-2995.2009.02342.x. [DOI] [PubMed] [Google Scholar]
- 44.Kanter J, Khan SY, Kelher M, et al. Oncogenic and angiogenic growth factors accumulate during routine storage of apheresis platelet concentrates. Clin Cancer Res. 2008;14:3942–3947. doi: 10.1158/1078-0432.CCR-07-4824. [DOI] [PubMed] [Google Scholar]
- 45.Kaufman J, Spinelli SL, Schultz E, et al. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–796. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 46.Blumberg N, Gettings KF, Turner C, et al. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–1821. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 47.Pamukcu B, Lip GY, Snezhitskiy V, et al. The CD40-CD40L system in cardiovascular disease. Ann Med. 2011;47:331–340. doi: 10.3109/07853890.2010.546362. [DOI] [PubMed] [Google Scholar]
- 48.Tuinman PR, Gerards MC, Jongsma G, et al. Lack of evidence of CD40 ligand involvement in transfusion-related acute lung injury. Clin Exp Immunol. 2011;165:278–284. doi: 10.1111/j.1365-2249.2011.04422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glenister KM, Sparrow RL. Level of platelet-derived cytokines in leukoreduced red blood cells is influenced by the processing method and type of leukoreduction filter. Transfusion. 2010;50:185–189. doi: 10.1111/j.1537-2995.2009.02353.x. [DOI] [PubMed] [Google Scholar]
- 50.Kelher MR, Masuno T, Moore EE, et al. Plasma from stored packed red blood cells and MHC class I antibodies causes acute lung injury in a 2-event in vivo rat model. Blood. 2009;113:2079–2087. doi: 10.1182/blood-2008-09-177857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silliman CC, Voelkel NF, Allard JD, et al. Plasma and lipids from stored packed red blood cells cause acute lung injury in an animal model. J Clin Invest. 1998;101:1458–1467. doi: 10.1172/JCI1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silliman CC, Curtis BR, Kopko PM, et al. Donor antibodies to HNA-3a implicated in TRALI reactions prime neutrophils and cause PMN-mediated damage to human pulmonary microvascular endothelial cells in a two-event in vitro model. Blood. 2007;109:1752–1755. doi: 10.1182/blood-2006-05-025106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Silliman CC, Bjornsen AJ, Wyman TH, et al. Plasma and lipids from stored platelets cause acute lung injury in an animal model. Transfusion. 2003;43:633–640. doi: 10.1046/j.1537-2995.2003.00385.x. [DOI] [PubMed] [Google Scholar]
- 54.von Hundelshausen P, Weber C. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res. 2007;100:27–40. doi: 10.1161/01.RES.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 55.Asaduzzaman M, Lavasani S, Rahman M, et al. Platelets support pulmonary recruitment of neutrophils in abdominal sepsis. Crit Care Med. 2009;37:1389–1396. doi: 10.1097/CCM.0b013e31819ceb71. [DOI] [PubMed] [Google Scholar]
- 56.Rahman M, Zhang S, Chew M, et al. Platelet-derived CD40L (CD154) mediates neutrophil upregulation of Mac-1 and recruitment in septic lung injury. Ann Surg. 2009;250:783–790. doi: 10.1097/SLA.0b013e3181bd95b7. [DOI] [PubMed] [Google Scholar]