Abstract
Objectives
To determine the incidence and clinical characteristics of cutaneous nontuberculous mycobacterial (NTM) infection during the past 30 years and whether the predominant species have changed.
Patients and Methods
Using Rochester Epidemiology Project data, we identified Olmsted County, Minnesota, residents with cutaneous NTM infections between January 1, 1980, and December 31, 2009, examining the incidence of infection, patient demographic and clinical features, the mycobacterium species, and therapy.
Results
Forty patients (median age, 47 years; 58% female [23 of 40]) had positive NTM cultures plus 1 or more clinical signs. The overall age- and sex-adjusted incidence of cutaneous NTM infection was 1.3 per 100,000 person-years (95% CI, 0.9–1.7 per 100,000 person-years). The incidence increased with age at diagnosis (P = .003) and was higher in 2000 to 2009 (2.0 per 100,000 person-years; 95% CI, 1.3–2.8 per 100,000 person-years) than in 1980 to 1999 (0.7 per 100,000 person-years; 95% CI, 0.3–1.1 per 100,000 person-years) (P = .002). The distal extremities were the most common sites of infection (27 of 39 patients [69%]). No patient had human immunodeficiency virus infection, but 23% (9 of 39) were immunosuppressed. Of the identifiable causes, traumatic injuries were the most frequent (22 of 29 patients [76%]). The most common species were Mycobacterium marinum (17 of 38 patients [45%]) and Mycobacterium chelonae/Mycobacterium abscessus (12 of 38 patients [32%]). In the past decade (2000–2009), 15 of 24 species (63%) were rapidly growing mycobacteria compared with only 4 of 14 species (29%) earlier (1980–1999) (P = .04).
Conclusion
The incidence of cutaneous NTM infection increased nearly 3-fold during the study period. Rapidly growing mycobacteria were predominant during the past decade.
The nontuberculous mycobacteria (NTM) compose a diverse group of environmental organisms that can produce clinical disease in any body tissue, including skin and soft tissue.1 These slender, nonmotile, acid-fast bacilli are traditionally divided into 2 groups on the basis of their speed of growth on media: rapidly growing mycobacteria (RGM) or slowly growing mycobacteria (SGM).2–4 Cutaneous infections caused by NTM, especially those that are multifocal or caused by RGM, are encountered in immunosuppressed patients. In immunocompetent patients, NTM infections may develop after traumatic injury, surgery, or cosmetic procedures.5,6 The primary lesions may evolve after weeks to months, resulting in localized abscesses, ulcers, nodules, or granulomas.6
There has been an increase in the number of reports in the medical literature of cutaneous infection caused by NTM, 5–8 yet a population-based assessment of incidence with clinical correlation and analyses is lacking, to our knowledge. We sought to determine whether there has been a statistically significant increase in the incidence of skin and soft tissue infection (SSTI) attributable to NTM and whether the predominant species have changed in residents of Olmsted County, Minnesota, over 30 years.
PATIENTS AND METHODS
After approval by the institutional review boards of Olmsted Medical Center and Mayo Clinic, we collected inpatient and outpatient medical records of residents of Olmsted County from the Rochester Epidemiology Project (REP). The REP is an informatics tool that indexes medical diagnoses made at health care facilities (clinics, hospitals, and nursing homes) or during autopsies for all residents of Olmsted County.
Using the REP database, we retrieved all the medical records for Olmsted County residents who had a diagnosis of SSTI caused by NTM between January 1, 1980, and December 31, 2009. Medical records were reviewed only for patients who had consented to the use of their records for research. Nonresidents of Olmsted County were excluded.
We defined an incident case as the confirmed first lifetime diagnosis of cutaneous NTM infection. From the medical records of these patients, we abstracted data that included patient demographic characteristics; mycobacterium species; immune status or use of immunosuppressive medications; characteristics, site, and clinical features of infection; antimicrobial drug therapy; and surgical procedures. Definite infection was confirmed by a positive culture concomitant with a compatible clinical sign of infection, such as ulcers, abscesses, or granulomas. The exclusion criteria included growth in culture of isolated single NTM colonies without a compatible clinical sign.
The microbiology laboratory (mycobacteriology laboratory in the Division of Clinical Microbiology, Mayo Clinic, Rochester, MN) identified organism species by standard criteria, such as morphologic structure, growth rate, mycolic acid analysis, and biochemical test results (eg, arylsulfatase and nitrate reduction). Beginning on November 9, 1999, the laboratory started using 16S ribosomal RNA (rRNA) gene sequencing to identify NTM. Given that Mycobacterium chelonae and Mycobacterium abscessus are identical under 16S rRNA gene sequencing, they are usually reported as M chelonae/M abscessus, which is the terminology we use herein.9
Incidence rates per 100,000 person-years were calculated overall and by decade using a numerator composed of the incident cases of cutaneous NTM infection and a denominator composed of the age- and sex-specific estimates of the Olmsted County population. Census data from 1980, 1990, and 2000 were used to estimate the population at risk, with linear interpolation for intercensal years. The US white population in 2000 was used for age-and sex-adjusted incidence rates. The relationships of the incidence of cutaneous NTM infection with age at diagnosis, sex, and year of diagnosis were assessed by fitting generalized linear models using the SAS GENMOD procedure (SAS Institute, Inc). Incident cases were grouped into 3 age intervals (0–39, 40–59, and ≥60 years) and 2 distinct year-of-diagnosis intervals (1980–1999 and 2000–2009). The models fit the natural logarithm of crude incidence rates as a linear function of age at diagnosis, sex, and year of diagnosis, with a Poisson distribution used to model the error structure. The significance of the linear trends for the features of interest and the interaction terms among these features were assessed using likelihood ratio statistics. Comparisons among features were evaluated using the 2-sample t test, the χ2 test, and the Fisher exact test. All the analyses were performed using a software program (SAS, version 9.2; SAS Institute, Inc), with P<.05 considered statistically significant.
RESULTS
A search of the REP database yielded 360 medical records of patients identified as having any form of NTM infection. Records were excluded if infections (1) were not cutaneous or (2) did not meet the inclusion criteria of positive growth in culture concomitant with 1 or more correlated clinical signs. Forty cases of definite SSTI caused by NTM were reviewed and analyzed.
Infections were recorded in 23 female patients (58%) and 17 male patients (43%). The median age of the 40 patients was 47 years (range, 6–92 years). The overall sex-and age-adjusted incidence of cutaneous NTM infection between 1980 and 2009 was 1.3 per 100,000 person-years (95% CI, 0.9–1.7 per 100,000 person-years) (Table 1). The age-adjusted incidence was not significantly different in females vs males (1.4 vs 1.0 per 100,000 person-years; P = .50). However, the incidence of cutaneous NTM infection increased significantly with age at diagnosis (P = .003; Table 1). There were no significant associations among age at diagnosis, sex, and year of diagnosis (Table 2, Figure). Specifically, the increased incidence by age and by year of diagnosis was similar for females and males.
TABLE 1.
Incidence of Cutaneous Nontuberculous Mycobacterial Infection per 100,000 Person-Years by Year of Diagnosis, Stratified by Age (1980–2009)
| Age (y) | 1980–2009
|
1980–1999
|
2000–2009
|
|||
|---|---|---|---|---|---|---|
| No. | Rate | No. | Rate | No. | Rate | |
| 0–39 | 13 | 0.6 | 7 | 0.5 | 6 | 0.8 |
|
| ||||||
| 40–59 | 16 | 1.9 | 5 | 1.1 | 11 | 2.9 |
|
| ||||||
| ≥60 | 11 | 2.2 | 2 | 0.7 | 9 | 4.3 |
|
| ||||||
| Total | 40 | 1.3a | 14 | 0.7a | 26 | 2.0a |
Age and sex adjusted to the 2000 US white population.
TABLE 2.
Characteristics of Patients With Cutaneous Nontuberculous Mycobacterial Infection (1980–2009)a
| Characteristic | Valueb |
|---|---|
| Age at diagnosis (y), mean (median; range) | 48.3 (47; 6–92) |
|
| |
| Age at diagnosis (y) (n = 40) | |
| 0–39 | 13 (33) |
| 40–59 | 16 (40) |
| ≥60 | 11 (28) |
|
| |
| Sex (n = 40) | |
| Female | 23 (58) |
| Male | 17 (43) |
|
| |
| Year of diagnosis (n = 40) | |
| 1980–1989 | 5 (13) |
| 1990–1999 | 9 (23) |
| 2000–2009 | 26 (65) |
|
| |
| Race/ethnicity (n = 32) | |
| White | 31 (97) |
| American Indian/Alaskan native | 1 (3) |
|
| |
| Site (n = 39)c | |
| Distal extremity | 27 (69) |
| Forearm | 9 (23) |
| Trunk | 5 (13) |
| Arm | 1 (3) |
| Leg | 1 (3) |
|
| |
| No. of sites (n = 39) | |
| Single | 31 (79) |
| Multiple | 8 (21) |
|
| |
| Immunosuppressed (n = 39) | |
| No | 30 (77) |
| Yes | 9 (23) |
|
| |
| Setting (n = 29) | |
| Traumatic injury | 22 (76) |
| Cosmetic procedure | 4 (14) |
| Surgery | 3 (10) |
|
| |
| Mycobacterium species (n = 38) | |
| Mycobacterium marinum | 17 (45) |
| Mycobacterium chelonae/Mycobacterium abscessus | 12 (32) |
| Mycobacterium fortuitum/Mycobacterium peregrinum | 6 (16) |
| Mycobacterium avium-intracellulare | 1 (3) |
| Mycobacterium smegmatis | 1 (3) |
| Mycobacterium szulgai | 1 (3) |
|
| |
| Species (n = 38) | |
| Slow growing | 19 (50) |
| Rapid growing | 19 (50) |
|
| |
| Clinical presentation (n = 40)d | |
| Subcutaneous abscess | 16 (40) |
| Cellulitis | 11 (28) |
| Wound infection | 10 (25) |
| Dermatitis | 3 (8) |
| Nodules | 2 (5) |
| Inflammation (erythema, swelling) | 2 (5) |
| Erythema | 2 (5) |
| Papule | 1 (3) |
|
| |
| Pathologic examination (n = 40) | |
| No | 22 (55) |
| Yes | 18 (45) |
|
| |
| Granulomatous inflammation identified on pathologic examination (n = 18) | |
| No | 6 (33) |
| Yes | 12 (67) |
|
| |
| Treatment (n = 39) | |
| Antimicrobial drug plus surgery | 22 (56) |
| Antimicrobial drug | 17 (44) |
|
| |
| Surgical treatment (n = 22) | |
| Patients with single lesions | 17 (77) |
| Patients with multiple lesions | 5 (23) |
|
| |
| No. of antibiotic drug courses (n = 83) | |
| Monotherapy | 32 (39) |
| Combination therapy | 51 (61) |
Values are presented as No. (percentage) unless indicated otherwise.
Percentages may not total to 100% due to rounding.
Patients with multiple sites are listed in more than 1 category.
Some patients had multiple skin findings.
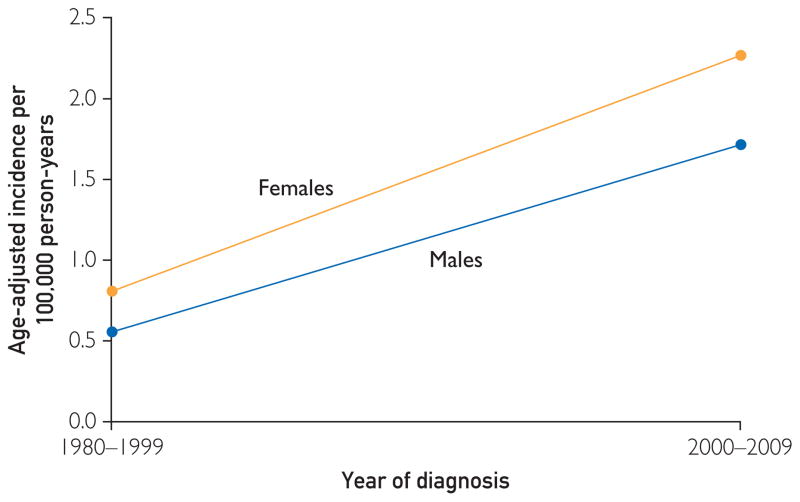
FIGURE.
Incidence of cutaneous nontuberculous mycobacterial infection per 100,000 person-years by year of diagnosis and sex (1980–2009).
The Figure shows the incidence of cutaneous NTM infection for 1980 to 1999 and 2000 to 2009. The incidence was significantly higher in 2000 to 2009 than in 1980 to 1999 (P = .002), increasing over time from 0.7 (95% CI, 0.3–1.1) to 2.0 (95% CI, 1.3–2.8) per 100,000 person-years.
The demographic and clinical features of the 40 patients are summarized in Table 2. Of 32 patients (80%) whose race/ethnicity was documented, 31 (97%) were identified as white. The location of the NTM infection was documented in 39 patients (98%). The most common infection site was a distal extremity (27 patients; 69%), with 25 patients (93%) having hand involvement and 2 (7%) foot involvement. Of 39 patients (98%) for whom immune status was known, 9 (23%) were immunosuppressed. No patient had human immunodeficiency virus infection. Three of the immunosuppressed patients (33%) had multiple sites of infection compared with 5 of the 30 nonimmunosuppressed patients (17%) (P = .35, Fisher exact test).
The cause of cutaneous NTM was documented in 29 patients (73%), with traumatic injury being the most common (22 patients; 76%), followed by a cosmetic procedure (4 patients; 14%) and surgery (3 patients; 10%). Detailed descriptions of the infections were not uniformly available because of the retrospective nature of the study and the use of medical records to determine clinical presentation. Documented skin findings at presentation included subcutaneous abscesses (16 patients; 40%), cellulitis (11 patients; 28%), wound infections (10 patients; 25%), dermatitis (3 patients; 8%), nodules (2 patients; 5%), inflammation (2 patients; 5%), erythema (2 patients; 5%), and papules (1 patient; 3%).
Species causing NTM infection were identified in 38 patients (95%). The most common NTM pathogens were Mycobacterium marinum (17 patients; 45%), M chelonae/M abscessus (12 patients; 32%), and Mycobacterium fortuitum/Mycobacterium peregrinum (6 patients; 16%). During the last decade of the study (2000–2009), 15 of 24 species (63%) were RGM (M chelonae/M abscessus, M fortuitum/M peregrinum, and Mycobacterium smegmatis) compared with only 4 of 14 species (29%) diagnosed in 1980 to 1999 (P = .04, χ2 test) (Table 3). Features of the identified SGM (M marinum, Mycobacterium avium-intracelulare, and Mycobacterium szulgai) and RGM (M chelonae/M abscessus, M fortuitum/M peregrinum, and M smegmatis) were compared. Differences in mean age and sex at diagnosis between the 2 categories were not statistically significant. There was, however, a statistically significant difference in the setting of the NTM infection, with RGM associated more with cosmetic and surgical procedures (P = .02, χ2 test). Seven of 15 RGM infections (47%) followed cosmetic or surgical procedures, whereas SGM infections were limited to patients with traumatic injuries. The number of infected sites also differed significantly by type, with 11 of 18 SGM infections (61%) and 18 of 19 RGM infections (95%) being single site (P = .02, χ2 test).
TABLE 3.
Comparison of Mycobacterium Species by Year of Diagnosis for 38 Patients With Cutaneous Nontuberculous Mycobacterial Infection
| Mycobacterium species | Year of diagnosis, No. (%)
|
P value | |
|---|---|---|---|
| 1980–1999 (n = 14) | 2000–2009 (n = 24) | ||
| By name | .03 | ||
| Mycobacterium marinum | 9 (64) | 8 (33) | |
| Mycobacterium chelonae/Mycobacterium abscessus | 1 (7) | 11 (46) | |
| Mycobacterium fortuitum/Mycobacterium peregrinum | 2 (14) | 4 (17) | |
| Mycobacterium avium-intracellulare | 0 | 1 (4) | |
| Mycobacterium smegmatis | 1 (7) | 0 | |
| Mycobacterium szulgai | 1 (7) | 0 | |
|
| |||
| By rate of growth | .04 | ||
| Slow | 10 (71) | 9 (38) | |
| Rapid | 4 (29) | 15 (63) | |
None of the cultures grew more than 1 species of mycobacteria. Of 36 patient samples sent for acid-fast bacillus testing, 18 results (50%) were positive. Skin samples from 18 patients (45%) were evaluated by pathologic examination, with 12 (67%) demonstrating granulomatous inflammation.
Treatment information was available for 39 patients (98%). Seventeen patients (44%) were treated with antimicrobial drug therapy alone, whereas 22 (56%) were treated with combined antimicrobial drug therapy and surgery. No one was treated with surgery alone. Of the 39 patients receiving antimicrobial drug therapy, 18 (46%) were treated with antimicrobial monotherapy. A total of 83 courses of antimicrobial drug treatment were delivered to the 39 patients (a course equaling ≥7 days of therapy). Thirty-three courses consisted of monotherapy and 50 of combination therapy (defined as ≥2 agents). The antimicrobial drugs prescribed included macrolides (32 courses), fluoroquinolones (22 courses), tetracyclines (17 courses), trimethoprim-sulfamethoxazole (10 courses), rifampin (7 courses), ethambutol (6 courses), aminoglycosides (3 courses), and cephalosporins (1 course). The antimicrobials most frequently used as single agents included macrolides (12 courses), tetracyclines (10 courses), and trimethoprim-sulfamethoxazole (7 courses).
Follow-up information was available for 36 patients (90%). The median duration of patient follow-up was 102 days (range, 11–358 days). Successful treatment was documented in all 36 patients. Five patients died within 2 years of their NTM diagnosis of causes unrelated to cutaneous NTM infection.
DISCUSSION
The results of this study show that in Olmsted County, the incidence of cutaneous NTM infection in 2000 to 2009 (2.0 per 100,000 person-years) was nearly 3-fold higher than that in 1980 to 1999 (0.7 per 100,000 person-years). This finding provides population-based data to substantiate the presumed increased incidence of cutaneous NTM infection. This report based case definition on culture and clinical data rather than on laboratory results alone.10
During the entire study period, M marinum was the most frequently encountered species (45%), a finding that concurs with previous reports of this species as the most common cause of mycobacterial SSTIs.11,12 However, there was an increased incidence of the RGM species M chelonae/M abscessus, M fortuitum/M peregrinum, and M smegmatis in the latter third of the review period, with these species composing 29% of infections between 1980 and 1999 vs 63% of infections between 2000 and 2009. A recent study examining NTM diagnoses made between 2005 and 2006 also found a predominance of RGM infections.13 This change in species incidence may foreshadow alterations in the clinical presentation of NTM infections because RGM are more likely to be single-site infections, whereas SGM are more likely to present with multiple-site infections. In the present study, only 5% of RGM infections involved multiple sites, whereas 39% of SGM infections were multisite (P = .02).
The incidence of NTM infection in the present population is likely underreported given that the inclusion criteria required a positive culture, thereby excluding patients in whom NTM diagnosis was not considered and, thus, no cultures were obtained. Additional limitations include the retrospective nature of the study, with its reliance on medical records that were not complete for every patient. Also of note is Olmsted County’s demographic composition, which is 89% white,14 so these results may not be generalizable to other populations.
Previous reports have suggested that cutaneous NTM infection occurs more commonly in women,13 but we found no statistically significant difference in incidence by sex. The mean age (48.3 years) and the median age (47 years) of the present patients were comparable with those found in other NTM studies.10,11,13 The incidence of NTM infection did increase with age in this population (P = .003). Given that the average age of the US population is increasing, a potential cause for the increased incidence of NTM infection may be the aging of the American populace.15
Some researchers have related the increase in NTM infections to a growing population of immunosuppressed patients.6–8,11,16 However, only 23% of the present patients were immunosuppressed, and none had a known human immunodeficiency virus infection, although an increased prevalence of this infection is often used to explain the increased incidence of NTM infection. Patients with identified immunosuppression included 4 persons with solid organ transplants, 1 with IgA deficiency, 1 with systemic lupus erythematosus, and 2 with long-term systemic corticosteroid use for undocumented underlying disease. The difference in the number of infection sites (single vs multiple) did not differ significantly between immunosuppressed and immunocompetent patients, despite previous reports to the contrary.17,18
Cutaneous NTM infections have usually been reported in the medical literature as occurring after surgical procedures.5,19–24 However, recent speculation attributes increased rates of such infections to an increase in cosmetic procedures, such as tattooing,25–29 pedicures,30,31 mesotherapy,12,32 liposuction,33–35 and body piercing,36 and to growing reliance on complementary and alternative medicine techniques, such as acupuncture.37,38 The present findings show that a slightly higher proportion of cosmetic procedures (14%) than surgical procedures (10%) was linked to NTM infections. The RGM species were involved in all infections that followed either cosmetic or surgical procedures. Indeed, procedural outbreaks of cutaneous NTM infection are most often caused by RGM.39 Thus, an increase in the number of such procedures could lead to an increase in the incidence of NTM infection. The fact remains, however, that most (76%) of the patients had infection secondary to isolated traumatic injuries, which supports the idea that increased clinical awareness may be at least partly responsible for the increasing incidence.
Whereas other investigators have questioned whether advances in laboratory techniques have led to an apparent increase in incidence,8,40 we found that the mycobacteria that are predominant in SSTIs are species that were easily identifiable using biochemical and high-performance liquid chromatography and that newer methods do not significantly enhance the ability to identify these species. The nucleic acid sequencing of 16S rRNA for identification of Mycobacterium species allows faster identification of species,41,42 but the overall identification to the species level is nearly the same as before the use of sequencing for the species predominant in SSTIs. In the laboratory, before implementing sequencing, only 5.3% of all mycobacteria isolates could not be identified to the species level by traditional phenotypic methods. After sequencing was introduced, that value decreased to 1.8%.9 Therefore, sequencing allowed species-level identification of only 3.5% more mycobacteria than did traditional methods. Most important for the purposes of this study, all the samples that could not be identified to the species level would still have been identified as mycobacteria and would have been included in this study.
Previously, cutaneous NTM infections have been found most commonly on the lower extremities or the trunk.17,18,42 However, the present findings that upper distal extremities are the most frequently (64%) affected site are similar to those of another study13 and may reflect the high rate of traumatic injury identified in this study.
Fewer than half of the patients (45%) with infections that we identified underwent a biopsy for pathologic examination. Of these specimens, 67% were found to have granulomatous inflammation, a proportion that falls within the range of rates reported in similar retrospective reviews.11,17 The tendency for granuloma formation may be affected by the immunologic status of the patient, with immunosuppressed patients being less likely to have granulomas.43 Only 3 immunosuppressed patients had biopsies performed, so we cannot comment on the rate of granuloma formation in immunocompetent vs immunosuppressed patients. Acid-fast bacilli were identified in half of the patients (50%) tested, which is higher than the reported percentage from the same studies. Because underdiagnosis of NTM infection may be ascribed partly to a lack of clinical suspicion,12,18 physicians responsible for diagnosing lesions should be aware of the need to send specimens with suspected NTM infection for pathologic examination.
These findings demonstrate the lack of consensus on the treatment of NTM infections that has been noted in the medical literature.2,11,25 Although all patients with documentation of outcome responded to therapy, questions remain regarding the appropriate duration of antibiotic drug therapy, monotherapy vs combination therapy, route of antibiotic drug therapy, use of surgical treatment, and use of surgery combined with antibiotic agents. Further study is warranted on the most effective treatment for NTM SSTIs, especially given the demonstrated increase in incidence of this form of infection.
CONCLUSION
The incidence of cutaneous NTM infection has increased nearly 3-fold in Olmsted County during the past 30 years, from 0.7 per 100,000 person-years between 1980 and 1999 to 2.0 per 100,000 person-years between 2000 and 2009. There was no significant difference between males and females in the rate of NTM infection, although the incidence did increase with age. The RGM infections made up the greatest proportion of cases in the latter third of the study’s time frame, caused all cases of infection after cosmetic or surgical procedures, and were more often associated with single-site than multisite infections. The NTM infections predominated on the distal extremities; otherwise, the clinical presentation of NTM was highly variable and included subcutaneous abscesses, nodules, dermatitis, cellulitis, and folliculitis. All health care professionals should be aware of the increasing incidence of cutaneous NTM infection to facilitate prompt diagnosis.
Supplementary Material
Acknowledgments
Grant Support: This study was made possible by the Rochester Epidemiology Project (Principal Investigators: Walter A. Rocca, MD, MPH, and Barbara P. Yawn, MD, MSc). Research reported in this publication was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations and Acronyms
- NTM
nontuberculous mycobacteria
- REP
Rochester Epidemiology Project
- RGM
rapidly growing mycobacteria
- rRNA
ribosomal RNA
- SGM
slowly growing mycobacteria
- SSTI
skin and soft tissue infection
References
- 1.Brown-Elliott BA, Wallace RJ., Jr . Infections Caused by Nontuberculous Mycobacteria. 6. Philadelphia, PA: Elsevier; 2005. [Google Scholar]
- 2.Bhambri S, Bhambri A, Del Rosso JQ. Atypical mycobacterial cutaneous infections. Dermatol Clin. 2009;27(1):63–73. doi: 10.1016/j.det.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 3.Runyon EH. Anonymous mycobacteria in pulmonary disease. Med Clin North Am. 1959;43(1):273–290. doi: 10.1016/s0025-7125(16)34193-1. [DOI] [PubMed] [Google Scholar]
- 4.Runyon EH. Identification of mycobacterial pathogens utilizing colony characteristics. Am J Clin Pathol. 1970;54(4):578–586. doi: 10.1093/ajcp/54.4.578. [DOI] [PubMed] [Google Scholar]
- 5.Fisher EJ, Gloster HM., Jr Infection with mycobacterium abscessus after Mohs micrographic surgery in an immunocompetent patient. Dermatol Surg. 2005;31(7):790–794. doi: 10.1097/00042728-200507000-00014. [DOI] [PubMed] [Google Scholar]
- 6.Sniezek PJ, Graham BS, Busch HB, et al. Rapidly growing mycobacterial infections after pedicures. Arch Dermatol. 2003;139(5):629–634. doi: 10.1001/archderm.139.5.629. [DOI] [PubMed] [Google Scholar]
- 7.Jogi R, Tyring SK. Therapy of nontuberculous mycobacterial infections. Dermatol Ther. 2004;17(6):491–498. doi: 10.1111/j.1396-0296.2004.04051.x. [DOI] [PubMed] [Google Scholar]
- 8.Kullavanijaya P. Atypical mycobacterial cutaneous infection. Clin Dermatol. 1999;17(2):153–158. doi: 10.1016/s0738-081x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- 9.Hall L, Doerr KA, Wohlfiel SL, Roberts GD. Evaluation of the Micro-Seq system for identification of mycobacteria by 16S ribosomal DNA sequencing and its integration into a routine clinical mycobacteriology laboratory. J Clin Microbiol. 2003;41(4):1447–1453. doi: 10.1128/JCM.41.4.1447-1453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Brien DP, Currie BJ, Krause VL. Nontuberculous mycobacterial disease in northern Australia: a case series and review of the literature. Clin Infect Dis. 2000;31(4):958–967. doi: 10.1086/318136. [DOI] [PubMed] [Google Scholar]
- 11.Dodiuk-Gad R, Dyachenko P, Ziv M, et al. Nontuberculous mycobacterial infections of the skin: a retrospective study of 25 cases. J Am Acad Dermatol. 2007;57(3):413–420. doi: 10.1016/j.jaad.2007.01.042. [DOI] [PubMed] [Google Scholar]
- 12.van Dissel JT, Kuijper EJ. Rapidly growing mycobacteria: emerging pathogens in cosmetic procedures of the skin. Clin Infect Dis. 2009;49(9):1365–1368. doi: 10.1086/606051. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49(12):e124–e129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 14.US Census Bureau. State and County QuickFacts: Minnesota (Olmsted County) US Census Bureau; [Accessed November 19, 2012]. website. http://quickfacts.census.gov/qfd/states/27/27109.html. [Google Scholar]
- 15.US Census Bureau. Table 1, Annual estimates of the resident population by sex and five-year age groups for the United States: April 1, 2000 to July 1, 2009 (NC-EST2009-01) US Census Bureau; [Accessed November 19, 2012]. website. http://www.census.gov/popest/data/historical/2000s/vintage_2009/ [Google Scholar]
- 16.Weitzul S, Eichhorn PJ, Pandya AG. Nontuberculous mycobacterial infections of the skin. Dermatol Clin. 2000;18(2):359–377. xi–xii. doi: 10.1016/s0733-8635(05)70182-0. [DOI] [PubMed] [Google Scholar]
- 17.Lee WJ, Kang SM, Sung H, et al. Non-tuberculous mycobacterial infections of the skin: a retrospective study of 29 cases. J Dermatol. 2010;37(11):965–972. doi: 10.1111/j.1346-8138.2010.00960.x. [DOI] [PubMed] [Google Scholar]
- 18.Uslan DZ, Kowalski TJ, Wengenack NL, Virk A, Wilson JW. Skin and soft tissue infections due to rapidly growing mycobacteria: comparison of clinical features, treatment, and susceptibility. Arch Dermatol. 2006;142(10):1287–1292. doi: 10.1001/archderm.142.10.1287. [DOI] [PubMed] [Google Scholar]
- 19.Angeli K, Lacour JP, Mantoux F, et al. Mycobacterium fortuitum skin infection occurring after a facelift [in French] Ann Dermatol Venereol. 2004;131(2):198–200. doi: 10.1016/s0151-9638(04)93571-5. [DOI] [PubMed] [Google Scholar]
- 20.Behroozan DS, Christian MM, Moy RL. Mycobacterium fortuitum infection following neck liposuction: a case report. Dermatol Surg. 2000;26(6):588–590. doi: 10.1046/j.1524-4725.2000.99165.x. [DOI] [PubMed] [Google Scholar]
- 21.Buckley R, Cobb MW, Ghurani S, Brock NF, Harford RR. Mycobacterium fortuitum infection occurring after a punch biopsy procedure. Pediatr Dermatol. 1997;14(4):290–292. doi: 10.1111/j.1525-1470.1997.tb00960.x. [DOI] [PubMed] [Google Scholar]
- 22.Coney PM, Thrush S. Cutaneous Mycobacterium fortuitum complicating breast reconstruction. J Plast Reconstr Aesthet Surg. 2007;60(10):1162–1163. doi: 10.1016/j.bjps.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 23.Ena P, Sechi LA, Molicotti P, Ortu S, Zanetti S. Cutaneous Mycobacterium chelonae I infection extending in the lower extremities in a renal transplanted patient. J Eur Acad Dermatol Venereol. 2005;19(4):504–505. doi: 10.1111/j.1468-3083.2005.01131.x. [DOI] [PubMed] [Google Scholar]
- 24.Freudenberger RS, Simafranca SM. Cutaneous infection with rapidly-growing mycobacterial infection following heart transplant: a case report and review of the literature. Transplant Proc. 2006;38(5):1526–1529. doi: 10.1016/j.transproceed.2006.02.126. [DOI] [PubMed] [Google Scholar]
- 25.Drage LA, Ecker PM, Orenstein R, Phillips PK, Edson RS. An outbreak of Mycobacterium chelonae infections in tattoos. J Am Acad Dermatol. 2010;62(3):501–506. doi: 10.1016/j.jaad.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 26.Jacob CI. Tattoo-associated dermatoses: a case report and review of the literature. Dermatol Surg. 2002;28(10):962–965. doi: 10.1046/j.1524-4725.2002.02066.x. [DOI] [PubMed] [Google Scholar]
- 27.Kazandjieva J, Tsankov N. Tattoos: dermatological complications. Clin Dermatol. 2007;25(4):375–382. doi: 10.1016/j.clindermatol.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Wolf R, Wolf D. A tattooed butterfly as a vector of atypical Mycobacteria. J Am Acad Dermatol. 2003;48(5, suppl):S73–S74. doi: 10.1067/mjd.2003.166. [DOI] [PubMed] [Google Scholar]
- 29.Wong HW, Tay YK, Sim CS. Papular eruption on a tattoo: a case of primary inoculation tuberculosis. Australas J Dermatol. 2005;46(2):84–87. doi: 10.1111/j.1440-0960.2005.00147.x. [DOI] [PubMed] [Google Scholar]
- 30.Gira AK, Reisenauer AH, Hammock L, et al. Furunculosis due to Mycobacterium mageritense associated with footbaths at a nail salon. J Clin Microbiol. 2004;42(4):1813–1817. doi: 10.1128/JCM.42.4.1813-1817.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Redbord KP, Shearer DA, Gloster H, et al. Atypical Mycobacterium furunculosis occurring after pedicures. J Am Acad Dermatol. 2006;54(3):520–524. doi: 10.1016/j.jaad.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 32.Nagore E, Ramos P, Botella-Estrada R, Ramos-Niguez JA, Sanmartin O, Castejon P. Cutaneous infection with Mycobacterium fortuitum after localized microinjections (mesotherapy) treated successfully with a triple drug regimen. Acta Derm Venereol. 2001;81(4):291–293. doi: 10.1080/00015550152572967. [DOI] [PubMed] [Google Scholar]
- 33.Giannella M, Pistella E, Perciaccante A, Venditti M. Soft tissue infection caused by Mycobacterium chelonae following a liposculpture and lipofilling procedure. Ann Ital Med Int. 2005;20(4):245–247. [PubMed] [Google Scholar]
- 34.Meyers H, Brown-Elliott BA, Moore D, et al. An outbreak of Mycobacterium chelonae infection following liposuction. Clin Infect Dis. 2002;34(11):1500–1507. doi: 10.1086/340399. [DOI] [PubMed] [Google Scholar]
- 35.Murillo J, Torres J, Bofill L, et al. Skin and wound infection by rapidly growing mycobacteria: an unexpected complication of liposuction and liposculpture: the Venezuelan Collaborative Infectious and Tropical Diseases Study Group. Arch Dermatol. 2000;136(11):1347–1352. doi: 10.1001/archderm.136.11.1347. [DOI] [PubMed] [Google Scholar]
- 36.Ferringer T, Pride H, Tyler W. Body piercing complicated by atypical mycobacterial infections. Pediatr Dermatol. 2008;25(2):219–222. doi: 10.1111/j.1525-1470.2008.00638.x. [DOI] [PubMed] [Google Scholar]
- 37.Ara M, de Santamaria CS, Zaballos P, Yus C, Lezcano MA. Mycobacterium chelonae infection with multiple cutaneous lesions after treatment with acupuncture. Int J Dermatol. 2003;42(8):642–644. doi: 10.1046/j.1365-4362.2003.01639_3.x. [DOI] [PubMed] [Google Scholar]
- 38.Song JY, Sohn JW, Jeong HW, Cheong HJ, Kim WJ, Kim MJ. An outbreak of post-acupuncture cutaneous infection due to Mycobacterium abscessus. BMC Infect Dis. 2006;6:6. doi: 10.1186/1471-2334-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tortoli E. Clinical manifestations of nontuberculous mycobacteria infections. Clin Microbiol Infect. 2009;15(10):906–910. doi: 10.1111/j.1469-0691.2009.03014.x. [DOI] [PubMed] [Google Scholar]
- 40.Wagner D, Young LS. Nontuberculous mycobacterial infections: a clinical review. Infection. 2004;32(5):257–270. doi: 10.1007/s15010-004-4001-4. [DOI] [PubMed] [Google Scholar]
- 41.Cloud JL, Neal H, Rosenberry R, et al. Identification of Mycobacterium spp. by using a commercial 16S ribosomal DNA sequencing kit and additional sequencing libraries. J Clin Microbiol. 2002;40(2):400–406. doi: 10.1128/JCM.40.2.400-406.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Escalonilla P, Esteban J, Soriano ML, et al. Cutaneous manifestations of infection by nontuberculous mycobacteria. Clin Exp Dermatol. 1998;23(5):214–221. doi: 10.1046/j.1365-2230.1998.00359.x. [DOI] [PubMed] [Google Scholar]
- 43.Bartralot R, Pujol RM, Garcia-Patos V, et al. Cutaneous infections due to nontuberculous mycobacteria: histopathological review of 28 cases: comparative study between lesions observed in immunosuppressed patients and normal hosts. J Cutan Pathol. 2000;27(3):124–129. doi: 10.1034/j.1600-0560.2000.027003124.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.