Abstract
BACKGROUND
Whether brain imaging can identify patients who are most likely to benefit from therapies for acute ischemic stroke and whether endovascular thrombectomy improves clinical outcomes in such patients remains unclear.
METHODS
In this study, we randomly assigned patients within 8 hours after the onset of large-vessel, anterior-circulation strokes to undergo mechanical embolectomy (Merci Retriever or Penumbra System) or receive standard care. All patients underwent pretreatment computed tomography or magnetic resonance imaging of the brain. Randomization was stratified according to whether the patient had a favorable penumbral pattern (substantial salvageable tissue and small infarct core) or a non-penumbral pattern (large core or small or absent penumbra). We assessed outcomes using the 90-day modified Rankin scale, ranging from 0 (no symptoms) to 6 (dead).
RESULTS
Among 118 eligible patients, the mean age was 65.5 years, the mean time to enrollment was 5.5 hours, and 58% had a favorable penumbral pattern. Revascularization in the embolectomy group was achieved in 67% of the patients. Ninety-day mortality was 21%, and the rate of symptomatic intracranial hemorrhage was 4%; neither rate differed across groups. Among all patients, mean scores on the modified Rankin scale did not differ between embolectomy and standard care (3.9 vs. 3.9, P = 0.99). Embolectomy was not superior to standard care in patients with either a favorable penumbral pattern (mean score, 3.9 vs. 3.4; P = 0.23) or a nonpenumbral pattern (mean score, 4.0 vs. 4.4; P = 0.32). In the primary analysis of scores on the 90-day modified Rankin scale, there was no interaction between the pretreatment imaging pattern and treatment assignment (P = 0.14).
CONCLUSIONS
A favorable penumbral pattern on neuroimaging did not identify patients who would differentially benefit from endovascular therapy for acute ischemic stroke, nor was embolectomy shown to be superior to standard care. (Funded by the National Institute of Neurological Disorders and Stroke; MR RESCUE ClinicalTrials.gov number, NCT00389467.)
Multiple randomized, controlled trials have shown the efficacy of the use of intravenous tissue plasminogen activator (t-PA), administered up to 4.5 hours after the onset of symptoms of acute ischemic stroke.1,2 However, the global effect of this therapy has been limited, largely because of the narrow time window available for treatment and the risk of symptomatic intracerebral hemorrhage. Although endovascular approaches, including thrombectomy devices, have been shown to achieve greater rates of recanalization than the use of intravenous t-PA, no randomized, controlled trial has been completed comparing clinical outcomes versus standard medical care. Moreover, the potential to benefit from interventions in late time windows (≥3 hours) may be increased when they are coupled with brain imaging to select patients who are the most likely to benefit.
Salvage of the ischemic penumbra has formed the theoretical basis of recanalization therapies designed to reverse or minimize the effects of acute ischemic stroke.3 For practical purposes, the ischemic penumbra can be defined as brain tissue with reduced blood flow that is at risk for infarction if flow is not restored. The use of multimodal computed tomography (CT) or magnetic resonance imaging (MRI) to identify patients with a favorable penumbral imaging pattern has been suggested to be particularly helpful in late time windows, when the proportion of patients with penumbral tissue steadily decreases over time.4 The hypothesis regarding penumbral-imaging selection presumes that some patients have substantial regions of salvageable brain tissue within several hours after a stroke, and it is this group of patients who would benefit from reperfusion treatments, whereas patients with nonpenumbral patterns (i.e., large core or small or absent penumbra) would not benefit and could even be harmed by reperfusion. It has been postulated that the modest rates of good outcomes (25 to 54%) observed in patients treated with endovascular recanalization despite high rates of recanalization (46 to 88%) are, in part, due to the treatment of patients who have large infarcts or those who do not have a clinically relevant volume of salvageable brain tissue.5–10
A number of studies have provided support for penumbral-imaging selection for the treatment of acute ischemic stroke11–14 However, to date, no randomized, controlled trial has shown that patients who are selected for revascularization on the basis of the penumbral-imaging pattern have better clinical outcomes than patients who are treated medically or those with nonpenumbral imaging patterns.
METHODS
STUDY DESIGN
The Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) trial was a phase 2b, randomized, controlled, open-label (blinded outcome), multicenter trial conducted at 22 study sites in North America. Details of the study design and rationale have been published previously.15 The study protocol, statistical analysis plan, and Supplementary Appendix with additional methodologic details are available with the full text of this article at NEJM.org.
In summary, patients between the ages of 18 and 85 years, with National Institutes of Health Stroke Scale (NIHSS) scores of 6 to 29 (on a scale ranging from 0 to 42, with higher scores indicating more severe neurologic deficits) who had a large-vessel, anterior-circulation ischemic stroke were randomly assigned within 8 hours after the onset of symptoms to undergo either mechanical embolectomy (Merci Retriever or Penumbra System) or standard medical care. Patients who were treated with intravenous t-PA without successful recanalization were eligible if magnetic resonance angiography or CT angiography after the treatment showed a persistent target occlusion. All patients underwent pretreatment multimodal CT or MRI of the brain, which permitted stratification according to the presence of a favorable penumbral pattern versus a nonpenumbral pattern during randomization with the use of a biased coin technique. A favorable penumbral pattern was defined as a predicted infarct core of 90 ml or less and a proportion of predicted infarct tissue within the at-risk region of 70% or less.16
STUDY OVERSIGHT
The study was performed under an investigational-device exemption approved by the Food and Drug Administration (FDA). The steering committee designed and oversaw the conduct of the trial, made the decision to submit the manuscript for publication, and vouches for the accuracy and completeness of the data and analysis and for the fidelity of this report to the study protocol. The first author drafted the manuscript without editorial assistance. Core laboratories completed primary neuroimaging analyses blinded to treatment assignment before database lock. Data analysis was undertaken by four authors. One author, a biostatistician, performed prespecified analyses after the database was cleaned and locked. Approval was obtained from the institutional review board at each study site. Patients or their legally authorized representatives provided written informed consent, except at one site that was exempted from the need for explicit consent by the FDA and the institutional review board.17
The trial was funded by the National Institute of Neurological Disorders and Stroke (NINDS). An independent medical monitor and a NINDS-appointed data and safety monitoring board oversaw the conduct of the trial. There were no confidentiality agreements between NINDS and the investigators. Concentric Medical provided study devices until August 2007; thereafter, costs were covered by study funds or third-party payers. Concentric Medical had no involvement in the study design or in the analysis or interpretation of the data. No other commercial support for the study was provided.
NEUROIMAGING ANALYSES
Baseline multimodal imaging was automatically processed on a dedicated site computer, generating a 4-digit code that assigned patients to treatment on the basis of separate permuted-block sequences for favorable penumbral and nonpenumbral patterns. If local postprocessing failed, the software generated a different 4-digit code indicating that penumbral status was unknown, and the patient underwent randomization with the use of an unknown-pattern permuted-block sequence. Final baseline imaging-pattern assignment was determined by the central imaging laboratory after quality checks and image postprocessing.
EMBOLECTOMY
Patients in the embolectomy group could be treated with any combination of FDA-cleared embolectomy devices, including the Merci Retriever (since trial initiation in 2004) and the Penumbra System (since 2009). The intraarterial administration of t-PA at a dose of as much as 14 mg was allowed as rescue therapy within 6 hours after symptom onset.
OUTCOME MEASURES
The primary study hypothesis was that the presence of substantial ischemic penumbral tissue and a small volume of predicted core infarct, as visualized on multimodal CT or MR imaging, would identify patients who were most likely to benefit from mechanical embolectomy for the treatment of acute ischemic stroke caused by a large-vessel occlusion up to 8 hours after symptom onset. Functional outcome was assessed with the modified Rankin scale, which ranges from 0 to 6, with higher scores indicating greater disability. The hypothesis was tested by analyzing whether the pretreatment penumbral pattern had a significant interaction with treatment assignment (embolectomy vs. standard medical care) as a determinant of functional outcome scores across all seven levels of the modified Rankin scale (shift in disability levels).
For secondary analyses, patients with scores of 0 to 2 on the modified Rankin scale were classified as having a good functional outcome. Successful revascularization was assessed with the use of the Thrombolysis in Cerebral Infarction (TICI) scale, which ranges from 0 (no perfusion) to 3 (full perfusion).18 Partial or complete revascularization was defined as a TICI score of 2a to 3. On 7-day CT or MRI perfusion imaging, successful reperfusion was defined as a reduction of 90% or more in the volume of the perfusion lesion from baseline with the time until the peak of the residue function of more than 6 seconds.
STATISTICAL ANALYSIS
We performed a nonparametric two-way analysis of variance using permutational methods for the primary outcome of the score on the modified Rankin scale. The analysis of variance interaction compared the mean treatment difference for the penumbral pattern versus the nonpenumbral pattern. All statistical analyses were performed with the use of SAS software, version 9.2 (SAS Institute), and STATA software, version 11.2.
RESULTS
STUDY POPULATION
Between 2004 and 2011, we randomly assigned 127 patients to the two study groups. Of these patients, 9 were excluded from the primary analyses (Fig. 1). This report focuses on the 118 patients who met the full eligibility criteria. Of these patients, 64 were assigned to undergo embolectomy and 54 to receive standard care. Table 1 shows the baseline characteristics of the patients and the four subgroups that were defined according to study-group assignment and penumbral-pattern status. Demographic and risk-factor characteristics were similar across subgroups, except for the baseline NIHSS, which was lower in both penumbral-pattern groups. Pairwise differences were also noted in congestive heart failure and alcohol use. For imaging characteristics, the median at-risk volumes and predicted core volumes were lower in the penumbral-pattern groups.
Figure 1. Enrollment and Outcomes.
The abbreviation t-PA denotes tissue plasminogen activator.
Table 1.
Characteristics of the Patients at Baseline.*
| Characteristic | All Patients (N = 118) | Study Group | P Value† | |||
|---|---|---|---|---|---|---|
| Embolectomy, Penumbral (N = 34) | Standard Care, Penumbral (N = 34) | Embolectomy, Nonpenumbral (N = 30) | Standard Care, Nonpenumbral (N = 20) | |||
| Age — yr | 65.5±14.6 | 66.4±13.2 | 65.8±16.9 | 61.6±12.0 | 69.4±15.9 | 0.11 |
|
| ||||||
| Male sex — no. (%) | 57 (48) | 17 (50) | 15 (44) | 13 (43) | 12 (60) | 0.64 |
|
| ||||||
| Hypertension — no. (%) | 95 (81) | 29 (85) | 24 (71) | 25 (83) | 17 (85) | 0.39 |
|
| ||||||
| Myocardial infarction — no. (%) | 24 (20) | 6 (18) | 8 (24) | 4 (13) | 6 (30) | 0.49 |
|
| ||||||
| Congestive heart failure — no. (%) | 19 (16) | 4 (12) | 8 (24) | 1 (3) | 6 (30) | 0.04‡ |
|
| ||||||
| Atrial fibrillation — no. (%) | 36 (31) | 11 (32) | 13 (38) | 5 (17) | 7 (35) | 0.27 |
|
| ||||||
| Diabetes mellitus — no. (%) | 26 (22) | 8 (24) | 8 (24) | 4 (13) | 6 (30) | 0.54 |
|
| ||||||
| Hyperlipidemia — no. (%) | 68 (58) | 19 (56) | 17 (50) | 17 (57) | 15 (75) | 0.34 |
|
| ||||||
| Previous ischemic stroke — no. (%) | 18 (15) | 6 (18) | 3 (9) | 4 (13) | 5 (25) | 0.43 |
|
| ||||||
| Smoking — no. (%) | 47 (40) | 15 (44) | 11 (32) | 12 (40) | 9 (45) | 0.74 |
|
| ||||||
| Alcohol use — no. (%) | 44 (37) | 12 (35) | 9 (26) | 10 (33) | 13 (65) | 0.04§ |
|
| ||||||
| Median score on NIHSS (interquartile range)¶ | 17 (13–21) | 16 (12–18) | 16 (11–18) | 19 (17–22) | 20.5 (17–23) | <0.001|| |
|
| ||||||
| Time to enrollment — hr | 5.5±1.4 | 5.3±1.6 | 5.8±1.0 | 5.2±1.4 | 5.7±1.4 | 0.49 |
|
| ||||||
| Administration of intravenous tissue plasminogen activator — no. (%) | 44 (37) | 16 (47) | 9 (26) | 12 (40) | 7 (35) | 0.36 |
|
| ||||||
| Use of magnetic resonance imaging — no. (%) | 94 (80) | 27 (79) | 31 (91) | 20 (67) | 16 (80) | 0.12 |
|
| ||||||
| Target occlusion site — no. (%) | 0.20 | |||||
|
| ||||||
| Internal carotid artery | 20 (17) | 6 (18) | 5 (15) | 7 (23) | 2 (10) | |
|
| ||||||
| M1 middle cerebral artery | 78 (66) | 18 (53) | 23 (68) | 21 (70) | 16 (80) | |
|
| ||||||
| M2 middle cerebral artery | 20 (17) | 10 (29) | 6 (18) | 2 (7) | 2 (10) | |
|
| ||||||
| Median at-risk volume (interquartile range) — ml | 177.6 (118.0–221.6) | 136.6 (103.4–178.9) | 126.2 (90.8–168.2) | 227.3 (194.0–260.0) | 230.8 (189.2–281.0) | <0.001 |
|
| ||||||
| Median predicted core volume (interquartile range) — ml | 60.2 (34.1–107.7) | 36.2 (23.6–50.9) | 37.1 (22.9–49.8) | 122.8 (96.9–171.4) | 107.5 (100.7–171.9) | <0.001 |
Plus–minus values are means ± SD.
P values are for the overall comparisons among the four subgroups. The alpha level for significance for pairwise comparisons was 0.05.
P = 0.01 for the pairwise comparison regarding a nonpenumbral pattern in the embolectomy group versus the standard-care group.
P = 0.04 for the pairwise comparison regarding a nonpenumbral pattern in the embolectomy group versus the standard-care group; P = 0.009 for the comparison in the standard-care group between patients with a favorable penumbral pattern and those with a nonpenumbral pattern.
Neurologic deficit was assessed with the National Institutes of Health Stroke Scale (NIHSS), which ranges from 0 to 42, with higher scores indicating more severe neurologic deficits.
P = 0.004 for the pairwise comparison in the embolectomy group between patients with a favorable penumbral pattern and those with a non-penumbral pattern; P = 0.005 for the comparison in the standard-care group between patients with a favorable penumbral pattern and those with a nonpenumbral pattern.
The study software successfully processed 74 of 127 cases (58%) in real time. At the core laboratory, 116 of 118 cases (98%) were successfully automatically processed by the software. Two cases required some manual processing to generate a pattern code. Final pattern assignment changed after core laboratory postprocessing in 10 of 118 cases (8%). Imbalances in the numbers of patients among the four randomization cells arose from the cases in which pattern categorization was not made in real time. Overall, 68 of 118 patients (58%) had a favorable penumbral pattern on final core laboratory review.
INTERVENTION
In the embolectomy group, adjunctive intraarterial t-PA was administered in eight patients (mean dose, 5.1 mg; range, 2 to 12). Revascularization (TICI score, 2a to 3), as determined on postprocedural angiography, was achieved in 67% of the patients. Seventeen procedural complications occurred, of which five were deemed to be serious adverse events (Tables S6, S7, and S8 in the Supplementary Appendix).
PRIMARY OUTCOMES
Table 2 shows clinical and imaging outcomes for the four subgroups. The primary analysis testing for an interaction between treatment assignment and penumbral pattern was not significant, with a mean difference of 0.88 between patients with a penumbral pattern versus those with a nonpenumbral pattern in the comparison between embolectomy and standard care on the basis of the 90-day score on the modified Rankin scale (P = 0.14).
Table 2.
Study Outcomes.*
| Outcome | Study Group | P Value† | |||
|---|---|---|---|---|---|
| Embolectomy, Penumbral (N = 34) | Standard Care, Penumbral (N = 34) | Embolectomy, Nonpenumbral (N = 30) | Standard Care, Nonpenumbral (N = 20) | ||
| Score on 90-day modified Rankin scale‡ | |||||
|
| |||||
| Unadjusted | 0.23 | ||||
|
| |||||
| Mean | 3.9 | 3.4 | 4.0 | 4.4 | |
|
| |||||
| Median | 4.0 | 3.0 | 4.0 | 4.0 | |
|
| |||||
| 95% CI | 3.3 to 4.4 | 2.8 to 4.0 | 3.4 to 4.6 | 3.6 to 5.2 | |
|
| |||||
| Adjusted§ | 0.30 | ||||
|
| |||||
| Mean | 3.8 | 3.4 | 4.3 | 4.2 | |
|
| |||||
| Median | 4.0 | 3.0 | 4.0 | 4.0 | |
|
| |||||
| 95% CI | 3.2 to 4.4 | 2.9 to 3.9 | 3.8 to 4.7 | 3.7 to 4.8 | |
|
| |||||
| Good outcome at 90 days | |||||
|
| |||||
| No. of patients (%) | 7 (21) | 9 (26) | 5 (17) | 2 (10) | 0.48 |
|
| |||||
| Adjusted analysis (%)§ | 14 | 23 | 9 | 10 | 0.39 |
|
| |||||
| Death — no. (%) | 6 (18) | 7 (21) | 6 (20) | 6 (30) | 0.75 |
|
| |||||
| Hemorrhage — no. (%) | |||||
|
| |||||
| Symptomatic | 3 (9) | 2 (6) | 0 | 0 | 0.24 |
|
| |||||
| Asymptomatic | 19 (56) | 14 (41) | 23 (77) | 12 (60) | 0.04 |
|
| |||||
| Final infarct volume | |||||
|
| |||||
| No. of patients evaluated | 32 | 32 | 30 | 19 | |
|
| |||||
| Median (interquartile range) — ml | 58.1 (34.5 to 138.2) | 37.3 (24.9 to 78.3) | 172.6 (84.6 to 273.8) | 217.1 (144.3 to 282.8) | <0.001¶ |
|
| |||||
| Absolute infarct growth | |||||
|
| |||||
| No. of patients evaluated | 32 | 32 | 30 | 19 | |
|
| |||||
| Median (interquartile range) — ml | 27.1 (−0.5 to 89.1) | 6.7 (−8.3 to 52.0) | 55.1 (−33.2 to 104.8) | 83.8 (24.1 to 137.5) | 0.009|| |
|
| |||||
| Reperfusion — no./total no. (%)** | 16/28 (57) | 14/27 (52) | 7/19 (37) | 6/12 (50) | 0.59 |
|
| |||||
| Partial or complete revascularization — no./total no. (%)†† | 20/30 (67) | 25/27 (93) | 20/26 (77) | 14/18 (78) | 0.13 |
CI denotes confidence interval.
P values are for the overall comparisons among the four groups. The alpha level for significance for pairwise comparisons was 0.05.
Functional outcome was assessed with the modified Rankin scale, which ranges from 0 to 6, with higher scores indicating greater disability.
This analysis was adjusted for an imbalance in age between groups.
P = 0.04 for the pairwise comparison between patients with a favorable penumbral pattern in the embolectomy group, as compared with those in the standard-care group; P<0.001 for the comparison in the standard-care group between patients with a favorable penumbral pattern and those with a nonpenumbral pattern; P = 0.001 for the comparison in the embolectomy group between patients with a favorable penumbral pattern and those with a nonpenumbral pattern.
P<0.001 for the pairwise comparison in the standard-care group between patients with a favorable penumbral pattern and those with a nonpenumbral pattern.
Reperfusion was assessed on day 7 with the use of perfusion MRI with contrast material and was defined as a reduction of 90% or more in the volume of the perfusion lesion from baseline with the time until the peak of the residue function of more than 6 seconds.
Revascularization was assessed with the use of magnetic resonance angiography or computed tomographic angiography on day 7 on the basis of the Thrombolysis in Cerebral Infarction (TICI) scale, which ranges from 0 (no perfusion) to 3 (full perfusion). Partial or complete revascularization was defined as a TICI score of 2a to 3.
Among all patients, mean scores on the modified Rankin scale did not differ between embolectomy and standard care (3.9 vs. 3.9, P = 0.99). In patients with a favorable penumbral pattern, embolectomy was not superior to standard care (mean score, 3.9 vs. 3.4; P = 0.23). Similarly, in patients with a nonpenumbral pattern, embolectomy was not superior (mean score, 4.0 vs. 4.4; P = 0.32). After adjustment for the only independent baseline prognostic factor (i.e., age), both the interaction and treatment-assignment analyses remained negative (P = 0.43 and P = 0.36, respectively).
SAFETY
Across the cohort, the rate of all-cause 90-day mortality was 21%, the rate of symptomatic hemorrhage was 4%, and the rate of asymptomatic hemorrhage was 58%. The rates did not differ significantly across groups in pairwise comparisons (Table 2).
SECONDARY OUTCOMES
In the prespecified age-adjusted analysis, 90-day scores on the modified Rankin scale were lower (indicating less disability) in patients with a penumbral pattern (3.6; 95% confidence interval [CI], 3.3 to 4.0) than in those with a nonpenumbral pattern (4.2; 95% CI, 3.8 to 4.7; P = 0.047), regardless of treatment assignment. There were no differences on the basis of treatment assignment alone in final infarct volume or lesion growth. Figure 2 shows the distribution of outcomes according to treatment assignment and imaging pattern across all scores on the modified Rankin scale. Good functional outcome (mean score, 0 to 2) according to subgroup is shown in Table 2. The final infarct volume was lower in patients with a favorable penumbral pattern regardless of treatment assignment. Across all four subgroups, there were no significant differences in the rates of reperfusion or recanalization on 7-day imaging (Table 2).
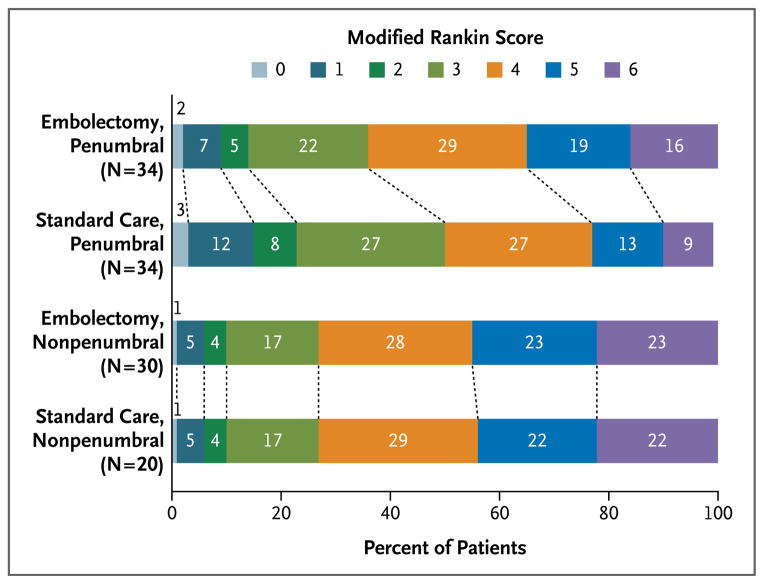
Figure 2. Functional Outcome at 90 Days in Four Subgroups of Patients, According to Score on the Modified Rankin Scale.
Shown are 90-day modified Rankin scores in patients undergoing embolectomy or receiving standard medical care for the treatment of acute ischemic stroke with a favorable penumbral pattern (substantial salvageable tissue and small infarct core) or a nonpenumbral pattern (large core or small or absent penumbra), after adjustment for age. The percentages of patients are shown in or above each cell. The modified Rankin scale ranges from 0 to 6, with higher scores indicating increased disability. Among all patients, mean modified Rankin scores did not differ between embolectomy and standard medical care (3.9 vs. 3.9, P = 0.99). Embolectomy was not superior to standard medical care in patients with either a favorable penumbral pattern (mean score, 3.9 vs. 3.4; P = 0.23) or a nonpenumbral pattern (mean score, 4.0 vs. 4.4, P = 0.32).
Table 3 shows analyses of final infarct volume and clinical outcome on the basis of assessments of revascularization at the time of 7-day imaging. A good 90-day clinical outcome, as well as attenuated infarct growth, was achieved more often in patients with substantial reperfusion (>90% reduction in tissue volume with >6-second delay in the time until the peak of the residue function)13 and in patients with 7-day revascularization (TICI score, 2a to 3).
Table 3.
Secondary Outcomes, According to Status Regarding Reperfusion and Revascularization.*
| Outcome and Measure | Patients with Reperfusion or Revascularization | Patients without Reperfusion or Revascularization | P Value |
|---|---|---|---|
| Reperfusion | |||
| No. of patients | 43 | 43 | |
| Mean score on 90-day modified Rankin scale (95% CI) | 3.2 (2.6 to 3.8) | 4.1 (3.7 to 4.5) | 0.04 |
| Median absolute infarct growth (interquartile range) — ml | 9.0 (−13.7 to 50.3) | 72.5 (5.6 to 120.7) | <0.001 |
| Partial or complete revascularization | |||
| No. of patients | 79 | 22 | |
| Mean score on 90-day modified Rankin scale (95% CI) | 3.5 (3.1 to 3.9) | 4.4 (4.0 to 4.8) | 0.04 |
| Median absolute infarct growth (interquartile range) — ml | 17.7 (−8.8 to 89.2) | 60.3 (19.9 to 93.3) | 0.10 |
Reperfusion was defined as a reduction of more than 90% in the volume of the perfusion lesion from baseline with the time until the peak of the residue function of more than 6 seconds. Revascularization was assessed with the use of the Thrombolysis in Cerebral Infarction (TICI) scale, which ranges from 0 (no perfusion) to 3 (full perfusion). Partial or complete revascularization was defined as a TICI score of 2a to 3.
Exploratory analysis with a receiver-operating-characteristic curve did not identify a threshold of predicted core volume that would have yielded a significant difference in outcomes on the basis of treatment assignment and a favorable penumbral pattern.
DISCUSSION
Our study did not confirm our primary hypothesis that penumbral imaging would identify patients who would differentially benefit from endovascular therapy for acute ischemic stroke within 8 hours after symptom onset. Moreover, among all enrolled patients regardless of penumbral-imaging pattern on study entry, no significant differences were noted in clinical and imaging outcomes for patients undergoing embolectomy, as compared with those receiving standard medical care.
These results raise important questions. There are several possible explanations for the neutral results independent of the validity of the imaging-selection hypothesis. One is the relatively low rate of substantial revascularization in the embolectomy group, which was perhaps associated with the use of first-generation embolectomy devices. In randomized trials, newer-generation stent retrievers have had higher revascularization rates and better clinical outcomes than has the Merci Retriever.7,8 It is possible that these newer-generation devices would show a treatment benefit (and a benefit in patients with a favorable penumbral pattern) because of both higher recanalization rates and lower complication rates.7,8 Other potential factors contributing to the neutral results include the extended time from imaging to embolectomy, the use of intravenous t-PA in some patients in the standard-care group, and heterogeneity of imaging approaches that were tested (both MRI and CT). Patients who were evaluated on CT tended to have larger predicted core volumes than those evaluated on MRI, which suggests that the two imaging approaches and predictive models may differ in some respects.
An alternative consideration is that the imaging-selection hypothesis is flawed as currently conceived. In our study, there was no difference in outcomes among patients with a favorable penumbral pattern who were treated with embolectomy, as compared with those treated with standard medical care.19 Moreover, data analysis with the use of a receiver-operating-characteristic curve did not show any threshold of predicted core volume that would have yielded a positive treatment effect in patients with a favorable penumbral pattern.
It is possible that patients with a favorable penumbral pattern, particularly in late time windows (i.e., ≥3 hours), may have a good functional outcome regardless of which recanalization treatment they undergo.20 In our study, patients with a favorable penumbral pattern had improved outcomes, smaller infarct volumes, and attenuated infarct growth, as compared with patients with a nonpenumbral pattern, regardless of treatment assignment. In early time windows (<3 hours), recanalization may be particularly beneficial in patients with large-vessel occlusions and poor collateral vessels. However, in later time windows, a favorable penumbral pattern may be a biomarker for a good outcome because of the presence of more vigorous collateral vessels and therefore greater tolerance of occlusion, increased likelihood of eventual spontaneous recanalization, and good final outcome.17,21 In patients with a favorable penumbral pattern without early recanalization, collateral flow may support penumbral tissue until spontaneous recanalization occurs.
Among patients with 7-day follow-up imaging, there were greater rates of good functional outcome as well as smaller infarct volumes in patients who had undergone reperfusion, recanalization, or both. Although the timing of follow-up imaging differed, these findings are similar to those of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE 2) trial and previous studies showing that reperfusion was associated with a better clinical outcome.13,19 However, unlike patients in the DEFUSE 2 trial, patients in our trial who had a nonpenumbral pattern showed a benefit in clinical outcome from late (but not early) reperfusion, albeit less pronounced. It is notable that if we had not included the control group in our study, we would not have been able to show that the benefit from reperfusion was not an effect of acute embolectomy. Unlike the DEFUSE 2 investigators, among patients who underwent embolectomy, we did not see a differential benefit in patients with a favorable penumbral pattern, as compared with those with a nonpenumbral pattern. However, our study differed from the DEFUSE 2 trial in that our patients had a longer time until treatment and larger predicted infarct cores, and we used varying approaches to predicting penumbral patterns, including a larger threshold for the predicted ischemic-lesion volume in the group with a favorable penumbral pattern.
Our study was also designed to explore outcomes in patients who were treated with embolectomy, as compared with standard medical care, regardless of imaging pattern. The trial found no evidence of benefit from embolectomy on clinical outcome, possibly because of the overall low rates of recanalization. This finding is unlikely to be explained by increased rates of procedural complications, since there were no significant between-group differences in the rates of death and symptomatic hemorrhage.
There are several limitations to this study. The trial was completed over an 8-year period, during which time there were advances in techniques and clinical practices. Study enrollment was also completed before the introduction of the new stent retrievers.7,8 Baseline-imaging prediction maps came from a single time point, and therefore the neuroimaging pattern may have changed by the time of recanalization in patients undergoing embolectomy. In addition, the time to groin puncture was more than 6 hours after the onset of symptoms, which is longer than in many previous trials of endovascular surgery.5,7,8,10,22 In our study, we used automated image-analysis software, allowing for the onsite identification of penumbral-pattern status in real time, which allowed the patients to be stratified according to pattern. However, real-time analysis was only modestly successful. An additional limitation, inherent to all studies of acute stroke, is that follow-up imaging was not available for all patients.
There are several important aspects of our study that may help guide the design of future trials. Despite FDA clearance of embolectomy devices and the relative lack of equipoise in the stroke community regarding the putative benefits on clinical outcomes of embolectomy versus standard medical care, we were able to complete a randomized clinical trial of embolectomy versus standard medical care, showing that true controlled trials of embolectomy are achievable (though arduous) for acute ischemic stroke. Our study also showed the feasibility and importance of performing trials that directly test the full spectrum of the imaging-selection hypothesis by enrolling patients with both favorable penumbral patterns and nonpenumbral patterns, rather than excluding patients with nonpenumbral patterns a priori.
In conclusion, our study did not show a treatment benefit in patients with a favorable penumbral pattern or an overall benefit from mechanical embolectomy versus standard medical care. Further randomized clinical trials that use new-generation devices are needed to test both the imaging-selection hypothesis and the clinical efficacy of mechanical embolectomy for the treatment of acute ischemic stroke. Our findings do not support the efficacy of using CT or MRI to select patients for acute stroke treatment or the efficacy of mechanical embolectomy with first-generation devices.
Supplementary Material
Acknowledgments
Supported by a grant (P50 NS044378) from NINDS. Concentric Medical provided study catheters and devices from the initiation of the study until August 2007; thereafter, costs for all study catheters and devices were covered by study funds or third-party payers.
APPENDIX
The authors’ affiliations are as follows; the Department of Neurology and the Stroke Center, Georgetown University, Washington, DC (C.S.K.); the Departments of Radiology and Neurosurgery (R.J.), Biomathematics (J. Gornbein), Neurology (J.R.A., J.L.S.), Neurosurgery (V.N.), and Emergency Medicine and Neurology (J. Guzy, S.S.), and the Stroke Center (R.J., J.R.A., J.L.S., J. Guzy, S.S.), University of California, Los Angeles; the Departments of Neurology (Z.A.) and Radiology (L.F.), Kaiser Permanente, Los Angeles; the Departments of Neurosciences (B.C.M.) and Radiology (S.O.) and the Stroke Center (B.C.M.), University of California, San Diego; and the Division of Neurosurgery, Scripps Clinic, La Jolla (S.O.) — all in California; the Departments of Neurology (L.H.S.) and Radiology (A.J.Y.), Harvard Medical School and Massachusetts General Hospital, Boston; the Departments of Neurology (R.S.M.) and Neurological Surgery and Radiology (P.M.M.), Columbia University College of Physicians and Surgeons, New York; the Departments of Neurology and Neurosurgery, University of Miami, Jackson Memorial Hospital, Miami (D.R.Y.); and the Department of Radiology, Neuroradiology Division, University of Virginia, Charlottesville (M.W.).
Footnotes
The authors’ affiliations are listed in the Appendix.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. . Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–7. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, et al. Thrombolysis with alteplase 3 to 4. 5 hours after acute ischemic stroke. N Engl J Med. 2008;359:1317–29. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.Fisher M. Characterizing the target of acute stroke therapy. Stroke. 1997;28:866–72. doi: 10.1161/01.str.28.4.866. [DOI] [PubMed] [Google Scholar]
- 4.Barber PA, Darby DG, Desmond PM, et al. Prediction of stroke outcome with echoplanar perfusion- and diffusion-weighted MRI. Neurology. 1998;51:418–26. doi: 10.1212/wnl.51.2.418. [DOI] [PubMed] [Google Scholar]
- 5.Smith WS, Sung G, Starkman S, et al. Safety and efficacy of mechanical embolectomy in acute ischemic stroke: results of the MERCI trial. Stroke. 2005;36:1432–8. doi: 10.1161/01.STR.0000171066.25248.1d. [DOI] [PubMed] [Google Scholar]
- 6.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke. 2008;39:1205–12. doi: 10.1161/STROKEAHA.107.497115. [DOI] [PubMed] [Google Scholar]
- 7.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): a randomised trial. Lancet. 2012;380:1231–40. doi: 10.1016/S0140-6736(12)61299-9. [Erratum, Lancet 2012; 380:1230.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- 9.Costalat V, Machi P, Lobotesis K, et al. Rescue, combined, and stand-alone thrombectomy in the management of large vessel occlusion stroke using the Solitaire device: a prospective 50-patient single-center study: timing, safety, and efficacy. Stroke. 2011;42:1929–35. doi: 10.1161/STROKEAHA.110.608976. [DOI] [PubMed] [Google Scholar]
- 10.The Penumbra Pivotal Stroke Trial Investigators. The Penumbra Pivotal Stroke Trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke. 2009;40:2761–8. doi: 10.1161/STROKEAHA.108.544957. [DOI] [PubMed] [Google Scholar]
- 11.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) study. Ann Neurol. 2006;60:508–17. doi: 10.1002/ana.20976. [DOI] [PubMed] [Google Scholar]
- 12.Lansberg MG, Kemp S, Straka M, et al. Results of DEFUSE 2: clinical endpoints. Stroke. 2012;43:A73. abstract. [Google Scholar]
- 13.Davis SM, Donnan GA, Parsons MW, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled randomised trial. Lancet Neurol. 2008;7:299–309. doi: 10.1016/S1474-4422(08)70044-9. [DOI] [PubMed] [Google Scholar]
- 14.Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med. 2012;366:1099–107. doi: 10.1056/NEJMoa1109842. [DOI] [PubMed] [Google Scholar]
- 15.Kidwell CS, Jahan R, Alger JR, et al. Design and rationale of the Mechanical Retrieval and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) Trial. Int J Stroke. 2012 Sep 13; doi: 10.1111/j.1747-4949.2012.00894.x. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kidwell CS, Wintermark M, De Silva DA, et al. Multiparametric MRI and CT models of infarct core and favorable penumbral imaging patterns in acute is-chemic stroke. Stroke. 2013;44:73–9. doi: 10.1161/STROKEAHA.112.670034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bateman BT, Meyers PM, Schumacher HC, Mangla S, Pile-Spellman J. Conducting stroke research with an exception from the requirement for informed consent. Stroke. 2003;34:1317–23. doi: 10.1161/01.STR.0000065230.00053.B4. [DOI] [PubMed] [Google Scholar]
- 18.Tomsick T, Broderick J, Carrozella J, et al. Revascularization results in the Interventional Management of Stroke II trial. AJNR Am J Neuroradiol. 2008;29:582–7. doi: 10.3174/ajnr.A0843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lansberg MG, Straka M, Kemp S, et al. MRI profile and response to endovascular reperfusion after stroke (DEFUSE 2): a prospective cohort study. Lancet Neurol. 2012;11:860–7. doi: 10.1016/S1474-4422(12)70203-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kidwell CS. MRI biomarkers in acute ischemic stroke: a conceptual framework and historical analysis. Stroke. 2012 Nov 6; doi: 10.1161/STROKEAHA.111.626093. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.Shuaib A, Butcher K, Mohammad AA, Saqqur M, Liebeskind DS. Collateral blood vessels in acute ischaemic stroke: a potential therapeutic target. Lancet Neurol. 2011;10:909–21. doi: 10.1016/S1474-4422(11)70195-8. [DOI] [PubMed] [Google Scholar]
- 22.Furlan A, Higashida R, Wechsler L, et al. Intra-arterial prourokinase for acute ischemic stroke — the PROACT II study: a randomized controlled trial. JAMA. 1999;282:2003–11. doi: 10.1001/jama.282.21.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.