Abstract
Hyaluronic acid is a naturally occurring linear polysaccharide of the extracellular matrix of connective tissue, synovial fluid, and other tissues. Its use in the treatment of the inflammatory process is established in medical areas such as orthopedics, dermatology, and ophthalmology. The Pubmed/Medline database was searched for keywords “Hyaluronic acid and periodontal disease” and “Hyaluronic acid and gingivitis” which resulted in 89 and 22 articles respectively. Only highly relevant articles from electronic and manual search in English literature were selected for the present review article. In the field of dentistry, hyaluronic acid has shown anti-inflammatory and anti-bacterial effects in the treatment of periodontal diseases. Due to its tissue healing properties, it could be used as an adjunct to mechanical therapy in the treatment of periodontitis. Further studies are required to determine the clinical efficacy of hyaluronic acid in healing of periodontal lesion. The aim of the present review, article is to discuss the role of hyaluronic acid in periodontal therapy.
Keywords: Gingivitis and periodontitis, Hyaluronic acid, Periodontal healing
Introduction
Hyaluronic acid (HA) is a naturally occurring linear polysaccharide of the extracellular matrix of connective tissue, synovial fluid, and other tissues. It possesses various physiological and structural functions, which include cellular and extracellular interactions, interactions with growth factors and regulation of the osmotic pressure, and tissue lubrication. All these functions help in maintaining the structural and homeostatic integrity of the tissue. Extensive studies on the chemical and physicochemical properties of HA and its physiological role in humans have proved that it is an ideal biomaterial for cosmetic, medical, and pharmaceutical applications.
In the field of dentistry, preliminary clinical trials have been conducted by Pagnacco and Vangelisti in 1997.[1] HA has shown anti-inflammatory, anti-oedematous, and anti-bacterial effects for the treatment of periodontal disease, which is mainly caused by the microorganisms present in subgingival plaque. It has been found that the equilibrium between the free radicals/reactive oxygen species (ROS) and antioxidants is the major prerequisite for healthy periodontal tissue. Individuals suffering from the periodontitis might be at higher risk of developing other systemic inflammatory diseases like cardiovascular diseases and diabetes.[2] Sardi suggested that the co-existence of periodontal disease and diabetes could pathologically increase the effect of oxidative stress.[3] While, Pendyala et al. found that the total antioxidant capacity is inversely proportional to the severity of inflammation and can be used as an useful marker of periodontitis in health and diabetic patients.[4] However, it is also conceivable that HA administration to periodontal wound sites could achieve beneficial effects in periodontal tissue regeneration and periodontal disease treatment.[5]
The aim of this review, article is to discuss various physiochemical, biochemical, and pharmaco-therapeutic uses of HA, especially in relation to treatment of periodontal disease. The Pubmed/Medline database was searched for keywords “HA and periodontal disease,” which resulted in 89 articles and “HA and gingivitis” revealed 22 articles. Only highly relevant articles from electronic and manual search in English literature were selected for the present review article.
History
HA was discovered in 1934 by Meyer et al. John Palmer, scientists at Columbia University, New York, who isolated a chemical substance from the vitreous jelly of cow’s eyes.[6] They proposed the name HA as it was derived from the Greek word hyalos (glass) and contained two sugar molecules one of which was uronic acid.
Chemistry
The precise chemical structure of HA contains repeating units of d-glucoronic acid and N-acetyl-d-glucosamine. The primary structure of the polysaccharide comprises of an unbranched linear chain with the monosaccharides linked together through alternating β1,3 and β1,4 glycosidic bonds.[7] Hydrophobic faces exist within the secondary structure of HA, formed by the axial hydrogen atoms of about eight carbon-hydrogen (CH) groups on the alternating sides of the molecule. Such hydrophobic patches, energetically favor the formation of meshwork-like β-sheet tertiary structure as a result of molecular aggregation. The tertiary structure is stabilized by the presence of intermolecular hydrogen bonding. The hydrophobic and hydrogen bonding interactions in combination with the countering electrostatic repulsion enable large numbers of molecules to aggregate leading to the formation of molecular networks (matrices) of HA. The structural formula of HA has been explained in Figure 1.
Figure 1.
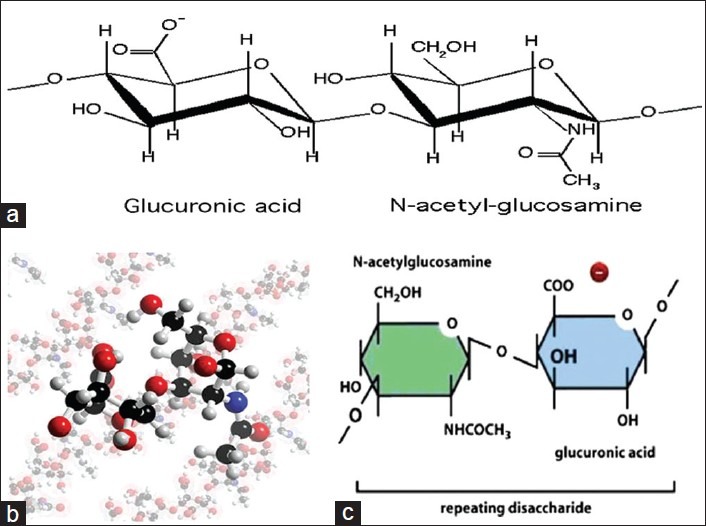
Structure of Hyaluronan. (a) Chemical structure of Hyaluronan. (b) 3D model of the Hyaluronan structure. (c) Hyaluronan is abundant, long, unsulfated glycosaminoglycan (up to 25,000 sugars), synthesized in the extra-cellular matrix by an enzyme complex in the plasma membrane
Origin, body reservoir and metabolism of HA
HA is found in almost all vertebrate organs, but most abundantly in the extracellular matrix of soft connective tissues. In the skin, it has a protective, structure stabilizing and shock-absorbing role. The estimated total amount of HA in human skin has been reported to be 5 g,[8] about a third of the total amount of HA believed to be present within the entire human body. The highest concentrations of HA are found in soft connective tissues (umbilical cord, synovial fluid, skin) and the lowest in blood serum.[9]
Most cells of the body are capable of synthesizing HA and synthesis take place in the cell membrane. HA is synthesized in the plasma membrane by a membrane-bound protein. Synthesized HA is directly secreted into the extracellular space. It is also produced by fibroblasts in the presence of endotoxins.
HA (Hyaluronan) has been identified in all periodontal tissues, being particularly prominent in the non-mineralized tissues such as gingiva and periodontal ligament and in only low quantities in mineralized tissues such as cementum and alveolar bone. The high molecular weight hyaluronan present in the periodontal tissues is synthesized by hyaluronan synthase (HAS) enzymes (HAS1, HAS2 and HAS3) in various cells from the periodontal tissues, including fibroblasts and keratinocytes in gingiva and periodontal ligament, cementoblasts in cementum and osteoblasts in alveolar bone.[10]
The turnover of HA content in the tissues occurs either by lymphatic drainage to the blood stream or by local metabolism. In skin and joints, some 20–30% of HA turnover occurs by the local metabolism, and the rest is removed by the lymphatic pathways. Upon reaching the blood stream, about 85-90% is eliminated in the liver. The kidneys extract about 10% but excrete only 1–2% in the urine. The tissue half-life of HA ranges from half a day to 2 or 3 days, regardless of its route of elimination.[11]
Properties of HA
HA has unique physiochemical and biological properties, which makes it useful in the treatment of the inflammatory process in medical areas such as orthopedics, dermatology, and ophthalmology [Figure 2].
Figure 2.
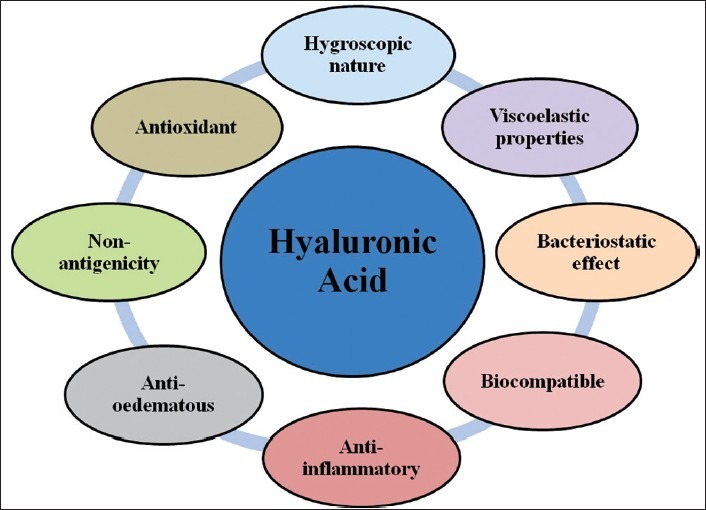
Properties of hyaluronic acid
Hygroscopic nature
HA is one of the most hygroscopic molecules known in nature. When HA is incorporated into aqueous solution, hydrogen bonding occurs between adjacent carboxyl and N-acetyl groups; this feature allows HA to maintain conformational stiffness and to retain water. One gram of HA can bind up to 6 L of water. As a physical background material, it has functions in space filling, lubrication, shock absorption and protein exclusion.[12]
Viscoelastic properties
Hyaluronan as a viscoelastic substance assists in periodontal regenerative procedures by maintaining spaces and protecting surfaces. Through recognition of its viscoelastic nature, HA can influence the cell functions that modify the surrounding cellular and the extracellular micro and macro environments. The viscoelastic properties of the material may slow the penetration of viruses, and bacteria, a feature of particular interest in the treatment of periodontal diseases.[12]
Bacteriostatic effect
Recent studies on regenerative surgical procedures indicate that reduction of bacterial burden at the wound site may improve the clinical outcome of regenerative therapy. The high concentration of medium and lower molecular weight HA has the greatest bacteriostatic effect, particularly on Aggregatibacter actinomycetemcomitans, Prevotella oris and Staphylococcus aureus strains, which are commonly found in oral gingival lesions and periodontal wounds. A clinical application of HA membranes, gels, and sponges during the surgical therapy may reduce the bacterial contamination of surgical wound site, thereby, lessening the risk of postsurgical infection and promoting more predictable regeneration.[13]
Biocompatibility and non-antigenicity
The highly biocompatible and non-immunogenic nature of HA has led to its use in a number of clinical applications, which include: The supplementation of joint fluid in arthritis; as a surgical aid in eye surgery; and to facilitate the healing and regeneration of bone, surgical wounds and periodontal tissue.
Modifications to Hyaluronan include esterification and cross-linking to provide some structure and rigidity to gel for cell-seeding purposes. These biopolymers are completely biodegradable and support the growth of fibroblasts, chondrocytes and mesenchymal stem cells.
Anti-inflammatory
HA has the anti-inflammatory effect, which may be due to the action of exogenous Hyaluronan as a scavenger by draining prostaglandins, metalloproteinases and other bio-active molecules.[14]
Anti-oedematous
The anti-oedematous effect of HA may also be related to the osmotic activity. Due to its acceleration in tissue healing properties, it could be used as an adjunct to mechanical therapy.[15]
Antioxidant
In a somewhat contradictory role, however, hyaluronan may regulate the inflammatory response, acting as an antioxidant by scavenging ROS. Thus, hyaluronan may help to stabilize the granulation tissue matrix.[16]
Functions and Uses of HA
HA has a lot of important physiological and biological functions. It plays a structural role in cartilage and other tissues. It associates with proteins that are enriched in the other types of glycosaminoglycans to form proteoglycans. HA is directly or indirectly related to many cell functions like cell proliferation, recognition, and locomotion, which will contribute to its tissue healing properties.[17] Because of its unique physiochemical properties and most importantly the non-immunogenicity of the highly purified form, Hyaluronan has already found medical applications for many years. Some important clinical applications are:
It is used as dermal filler in the field of cosmetic dermatology.[18]
Scar formation in the surgical wounds can be prevented by the administration of HA during surgery.[19]
Many reports have attested to the effects of exogenous Hyaluronan in producing beneficial wound healing outcomes.[20]
In orthopedics, for treatment osteoarthritis of the knee and rheumatoid arthritis.[21]
In ophthalmology, for treatment of cataract and xeropthalmia.
Hyaluronan has also been explored in the field of tissue engineering. Because of its significant role during organogenesis, cell migration and development in general.[22]
Modifications to Hyaluronan include esterification and cross-linking to provide some structure and rigidity to gel for the cell-seeding purpose.[23]
More recently, HA has been investigated as a drug delivery agent for various routes of administration, including ophthalmic, nasal, pulmonary, parenteral, and topical.[24]
HA and Periodontal Disease
HA is an essential component of the periodontal ligament matrix and plays various important roles in cell adhesion, migration and differentiation mediated by the various HA binding proteins and cell-surface receptors such as CD44.[25] HA has been studied as a metabolite or diagnostic marker of inflammation in the gingival crevicular fluid (GCF) as well as a significant factor in growth, development and repair of tissues.[26]
Based on current evidence in literature, it is now known that along with mechanical therapy, use of chemotherapeutic agents provide a better treatment strategy. The most common chemotherapeutic agents are antimicrobials and anti-inflammatory drugs. They are administered either systemically or topically. Topical antimicrobial agents for the treatment of periodontal diseases include chlorhexidine, tetracyclines, and metronidazole. HA is a recent addition to the local chemotherapeutic agents. It has shown a number of clinical therapeutic properties.
Role of HA in Periodontal Wound Healing
Healing of periodontal wound includes a series of highly reproducible and rigidly controlled biologic events (inflammation, granulation tissue formation, epithelium formation and tissue remodelling) which begin with chemo attraction of cells that accumulate and debride the injured tissue, foreign material, and microbial cells [Figure 3]. These events end with the formation and maturation of new extracellular matrix that restore resistance of tissue to functional stress.[27]
Figure 3.
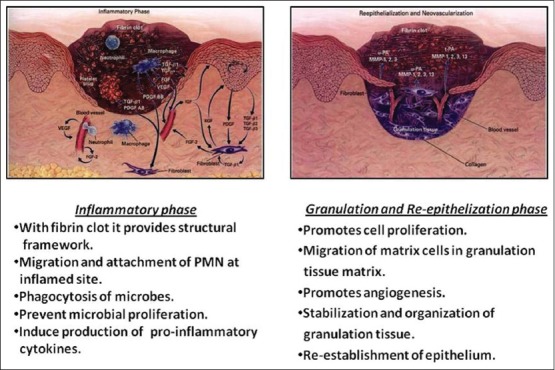
Role of hyaluronic acid in wound healing
Inflammatory phase
Hyaluronan has numerous roles in the initial inflammatory stages such as the provision of a structural framework via the interaction of Hyaluronan with the fibrin clot, which modulates host’s inflammatory and extracellular matrix cell infiltration into the inflamed site. Hakansson et al. suggested role of Hyaluronan in migration and adherence of polymorphonuclear leukocytes and macrophages at the inflamed site and the phagocytosis and killing of invading microbes. Such events would allow counteraction of colonization and proliferation of anaerobic pathogenic bacteria in the gingival crevice and adjacent periodontal tissues.[28]
Hyaluronan itself may also prevent periodontal pathogen colonization by directly preventing microbial proliferation. Hyaluronan may also indirectly act to moderate inflammation and stabilize the granulation tissue by preventing degradation of the extracellular matrix proteins by serine proteinases derived from inflammatory cells as healing progresses.[29]
Hyaluronan also induces the production of proinflammatory cytokines by fibroblasts, keratinocytes, cementoblasts and osteoblasts which promote the inflammatory response and consequently stimulate hyaluronan synthesis by endothelial cells.[30]
Granulation phase and re-epithelisation
During granulation phase, Hyaluronan promotes cell proliferation, migration of matrix cells into granulation tissue matrix and granulation tissue organization. In non-mineralized inflamed tissues, Hyaluronan is transiently elevated during the formation of granulation tissue and the re-establishment of the epithelium.[31] During the granulation tissue phase, HA in mineralized tissues is gradually replaced by a provisional mineralized callus.[32]
In later stage of the granulation phase, Hyaluronan synthesis ceases and existing Hyaluronan is depolymerized by hyaluronidases resulting in the formation of lower molecular weight Hyaluronan molecules and an alteration in the composition of the granulation tissue. Low molecular weight Hyaluronan fragments formed following hyaluronidase activity promote the formation of blood vessels (angiogenesis) within wound sites, although the precise mechanism of action is unknown.[33]
Bone regeneration
HA accelerates the bone regeneration by means of chemotaxis, proliferation and successive differentiation of mesenchymal cells. HA shares bone induction characteristics with osteogenic substances such as bone morphogenetic protein-2 and osteopontin.[34]
Effect on angiogenesis
It has been found that low molecular weight HA has marked angiogenic effect whereas, surprisingly, high molecular weight has the opposite effect.[35]
HA in Periodontics: Review of Literature
HA as a bactericidal agent is still controversial, but a study conducted by Pirnazar et al. suggested that HA in the Molecular Weight range of 1,300 kD may prove beneficial in minimizing bacterial contamination of surgical wounds when used in guided tissue regeneration surgery. Hyaluronan is identified in nearly all GCF samples, and circulating blood serum in patients with gingivitis but hyaluronan is absent in GCF samples from patients with acute necrotizing ulcerative gingivitis, owing to the high levels of bacterial enzymatic activity (hyaluronidases) associated with this condition.[13]
Engström et al. investigated the anti-inflammatory effect and the effect on bone regeneration of Hyaluronan in surgical and non-surgical groups. No statistical difference was found on radiographs in the non-surgical group, whereas the decrease in bone height was found for both groups after scaling. Probing depth (PD) reduced after the surgical treatment as well as after scaling and root planning (SRP). Hyaluronan in contact with bone and soft tissues had no influence on the immune system.[36]
Hyaluronan gel is effective in controlling inflammation and gingival bleeding. Studies have documented reduction in the depth of gingival pockets along with a significant reduction in epithelial and lymphocyte cell proliferation with the use of HA gel.[37] 0.2% Hyaluronan containing gel has a beneficial effect in the treatment of plaque induced gingivitis. All studied sites had a significant decrease in peroxidase and lysozyme activities after 7, 14, and 21 days.[15] Hyaluronan gel is also effective in controlling inflammation and gingival bleeding.
The topical application of an HA-containing preparation represents a potentially useful adjunct in the therapy of gingivitis, although its use does not diminish the need for plaque reduction as a primary therapeutic measure.[38] These results were in contradiction with the previous study carried out by Xu et al. who evaluated potential benefits of local subgingival application of HA gel adjunctive to SRP. They did not find any clinical or microbiological improvement by the adjunctive use of HA gel compared to SRP alone.[39] These contradictory results could be due to different inclusive criteria or different form of HA.
Pistorius Alixander evaluated the efficacy of topical application of HA for treatment of gingivitis and found that topical application of HA containing preparation was potentially useful adjunct in the therapy of gingivitis.[38]
Gengigel®(Ricerfarma S.r.l., Milano, Italy) contains high molecular weight fractions of HA in a gel formulation with 0.2% concentration for its effect in the treatment of plaque-induced gingivitis as an adjunct to SRP.[15] The adjunctive use of 0.8% Hyaluronan after thorough mechanical debridement potentially has major clinical benefits in terms of improved healing after non-surgical therapy.[40]
M de Arau’jo Nobre during the course of their study found that HA and chlorhexidine produced good results in maintaining a healthy peri-implant complex in immediate function implants for complete rehabilitations in the edentulous mandible. Statistically, significant differences were found in favour of the HA group in the modified bleeding index (BI) on the second observation.[41]
Ballini et al. found that autologous bone combined with an esterified low-molecular HA preparation seems to have good capabilities in accelerating new bone formation in the infra-bone defects.[42]
Leonardo in 2009 investigated the clinical efficacy of esterified HA (in the form of fibers) for treating deep periodontal defects. 18 infrabony and one mandibular molar furcation were selected for placing the HA fibers. After 1 year of treatment the mean probing pocket depth (PPD) was reduced by 5.8 mm and the attachment gain was 2.8 mm.[43]
Johannsen et al. conducted a split mouth design study to evaluate the adjunctive effect of local application of 0.8% Hyaluronan gel to SRP in the treatment of chronic periodontitis. They found a significant reduction in bleeding on probing (BOP) scores and PD in SRP + hyaluronan gel group as compared to SRP group.[44]
Pilloni et al., in their randomized controlled clinical pilot study, evaluated the efficacy of an esterified form of HA gel on periodontal clinical parameters. The periodontal clinical parameters were plaque index (PI), BOP, PPD, gingival index (GI), and probing attachment level. In the end of the study, they concluded that an esterified gel form of HA has shown an effect in reducing the gingival inflammation when used as an adjunct to mechanical home plaque control and that it could be successfully used to improve the periodontal clinical indexes.[45]
El-Sayed et al., in a randomized controlled trial, evaluated the effect of local application of 0.8% Hyaluronan gel in conjunction with periodontal surgery. After initial non-surgical periodontal therapy and re-evaluation, defects were randomly assigned to be treated with modified Widman flap surgery in conjunction with either 0.8% Hyaluronan gel (test) or placebo gel (control) application. Statistically, significant differences were noted for Clinical Attachment Level and gingival recession, (P < 0.05) in favor of the test sites. But non-significant results were found regarding PD, BOP and PI values (P > 0.05).[46]
Gontiya et al. investigated the clinical and histological outcomes of local subgingival application of 0.2% HA gel as an adjunct to SRP in chronic periodontitis patients. Clinical parameters such as GI, BI, PPD, and Relative Attachment Level were recorded at baseline (0 day), 4th, 6th, and 12th week. Finally, they concluded that subgingival placement of 0.2% HA gel along with SRP provides a significant improvement in gingival parameters, but no additional benefits were found in periodontal parameters. Histologically, experimental sites showed reduced inflammatory infiltrates, but the results were not statistically significant.[47]
Conclusion
Thus, it is evident that Hyaluronan has a multifunctional role in the wound healing process with a similar mechanism of healing potentially existing within periodontal tissues. As a consequence of the many functions attributed to Hyaluronan during wound healing, advances have been made in the development and application of Hyaluronan-based biomaterials in the treatment of various inflammatory conditions. Hence, further long-term studies with better standards such as application time, quantity of application, different forms and concentration needs to be carried out for better understanding of therapeutic effect of HA.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Pagnacco A, Vangelisti R, Erra C, Poma A. Double-blind clinical trial versus placebo of a new sodium-hyaluronate-based gingival gel. Attual Ter In. 1997;15:1–7. [Google Scholar]
- 2.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366:1809–20. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 3.Sardi Jde C. Oxidative stress in diabetes and periodontitis. N Am J Med Sci. 2013;5:58–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Pendyala G, Thomas B, Joshi SR. Evaluation of total antioxidant capacity of saliva in type 2 diabetic patients with and without periodontal disease: A case-control study. N Am J Med Sci. 2013;5:51–7. doi: 10.4103/1947-2714.106208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moseley R, Waddington RJ, Embery G. Hyaluronan and its potential role in periodontal healing. Dent Update. 2002;29:144–8. doi: 10.12968/denu.2002.29.3.144. [DOI] [PubMed] [Google Scholar]
- 6.Vedamurthy M. Soft tissue augmentation: Use of hyaluronic acid as dermal filler. Indian J Dermatol Venereol Leprol. 2004;70:383–7. [PubMed] [Google Scholar]
- 7.Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992;6:2397–404. [PubMed] [Google Scholar]
- 8.Banks J, Kreider JW, Bhavanandan VP, Davidson EA. Anionic polysaccharide production and tyrosinase activation in cultured human melanoma cells. C ancer Res. 1976;36:424–31. [PubMed] [Google Scholar]
- 9.Laurent TC, Fraser JR. The properties and turnover of hyaluronan. Ciba Found Symp. 1986;124:9–29. doi: 10.1002/9780470513385.ch2. [DOI] [PubMed] [Google Scholar]
- 10.Ijuin C, Ohno S, Tanimoto K, Honda K, Tanne K. Regulation of hyaluronan synthase gene expression in human periodontal ligament cells by tumour necrosis factor-alpha, interleukin-1beta and interferon-gamma. Arch Oral Biol. 2001;46:767–72. doi: 10.1016/s0003-9969(01)00032-2. [DOI] [PubMed] [Google Scholar]
- 11.Fraser JR, Laurent TC, Laurent UB. Hyaluronan: Its nature, distribution, functions and turnover. J Intern Med. 1997;242:27–33. doi: 10.1046/j.1365-2796.1997.00170.x. [DOI] [PubMed] [Google Scholar]
- 12.Sutherland IW. Novel and established applications of microbial polysaccharides. Trends Biotechnol. 1998;16:41–6. doi: 10.1016/S0167-7799(97)01139-6. [DOI] [PubMed] [Google Scholar]
- 13.Pirnazar P, Wolinsky L, Nachnani S, Haake S, Pilloni A, Bernard GW. Bacteriostatic effects of hyaluronic acid. J Periodontol. 1999;70:370–4. doi: 10.1902/jop.1999.70.4.370. [DOI] [PubMed] [Google Scholar]
- 14.Laurent TC, Laurent UB, Fraser JR. Functions of hyaluronan. Ann Rheum Dis. 1995;54:429–32. doi: 10.1136/ard.54.5.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jentsch H, Pomowski R, Kundt G, Göcke R. Treatment of gingivitis with hyaluronan. J Clin Periodontol. 2003;30:159–64. doi: 10.1034/j.1600-051x.2003.300203.x. [DOI] [PubMed] [Google Scholar]
- 16.Waddington RJ, Moseley R, Embery G. Reactive oxygen species: A potential role in the pathogenesis of periodontal diseases. Oral Dis. 2000;6:138–51. doi: 10.1111/j.1601-0825.2000.tb00325.x. [DOI] [PubMed] [Google Scholar]
- 17.Samuel SK, Hurta RA, Spearman MA, Wright JA, Turley EA, Greenberg AH. TGF-beta 1 stimulation of cell locomotion utilizes the hyaluronan receptor RHAMM and hyaluronan. J Cell Biol. 1993;123:749–58. doi: 10.1083/jcb.123.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monheit GD, Coleman KM. Hyaluronic acid fillers. Dermatol Ther. 2006;19:141–50. doi: 10.1111/j.1529-8019.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 19.Longaker MT, Harrison MR, Crombleholme TM, Langer JC, Decker M, Verrier ED, et al. Studies in fetal wound healing: I. A factor in fetal serum that stimulates deposition of hyaluronic acid. J Pediatr Surg. 1989;24:789–92. doi: 10.1016/s0022-3468(89)80538-x. [DOI] [PubMed] [Google Scholar]
- 20.King SR, Hickerson WL, Proctor KG. Beneficial actions of exogenous hyaluronic acid on wound healing. Surgery. 1991;109:76–84. [PubMed] [Google Scholar]
- 21.Balazs EA, Denlinger JL. Viscosupplementation: A new concept in the treatment of osteoarthritis. J Rheumatol Suppl. 1993;39:3–9. [PubMed] [Google Scholar]
- 22.Srisuwan T, Tilkorn DJ, Wilson JL, Morrison WA, Messer HM, Thompson EW, et al. Molecular aspects of tissue engineering in the dental field. Periodontol. 2000;2006(41):88–108. doi: 10.1111/j.1600-0757.2006.00176.x. [DOI] [PubMed] [Google Scholar]
- 23.Bartold PM, Xiao Y, Lyngstaadas SP, Paine ML, Snead ML. Principles and applications of cell delivery systems for periodontal regeneration. Periodontol. 2000;2006(41):123–35. doi: 10.1111/j.1600-0757.2006.00156.x. [DOI] [PubMed] [Google Scholar]
- 24.Brown MB, Jones SA. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J Eur Acad Dermatol Venereol. 2005;19:308–18. doi: 10.1111/j.1468-3083.2004.01180.x. [DOI] [PubMed] [Google Scholar]
- 25.Oksala O, Salo T, Tammi R, Häkkinen L, Jalkanen M, Inki P, et al. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995;43:125–35. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- 26.Pogrel MA, Lowe MA, Stern R. Hyaluronan (hyaluronic acid) in human saliva. Arch Oral Biol. 1996;41:667–71. doi: 10.1016/s0003-9969(96)00050-7. [DOI] [PubMed] [Google Scholar]
- 27.Bertolami CN, Day RH, Ellis DG. Separation and properties of rabbit buccal mucosal wound hyaluronidase. J Dent Res. 1986;65:939–44. doi: 10.1177/00220345860650061701. [DOI] [PubMed] [Google Scholar]
- 28.Håkansson L, Hällgren R, Venge P. Regulation of granulocyte function by hyaluronic acid. In vitro and in vivo effects on phagocytosis, locomotion, and metabolism. J Clin Invest. 1980;66:298–305. doi: 10.1172/JCI109857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisniewski HG, Vilcek J. TSG-6: An IL-1/TNF-inducible protein with anti-inflammatory activity. Cytokine Growth Factor Rev. 1997;8:143–56. doi: 10.1016/s1359-6101(97)00008-7. [DOI] [PubMed] [Google Scholar]
- 30.Larjava H, Heino J, Kähäri VM, Krusius T, Vuorio E. Characterization of one phenotype of human periodontal granulation-tissue fibroblasts. J Dent Res. 1989;68:20–5. doi: 10.1177/00220345890680010301. [DOI] [PubMed] [Google Scholar]
- 31.Bartold PM, Page RC. The effect of chronic inflammation on gingival connective tissue proteoglycans and hyaluronic acid. J Oral Pathol. 1986;15:367–74. doi: 10.1111/j.1600-0714.1986.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 32.Bertolami CN, Messadi DV. The role of proteoglycans in hard and soft tissue repair. Crit Rev Oral Biol Med. 1994;5:311–37. doi: 10.1177/10454411940050030601. [DOI] [PubMed] [Google Scholar]
- 33.Ruggiero SL, Bertolami CN, Bronson RE, Damiani PJ. Hyaluronidase activity of rabbit skin wound granulation tissue fibroblasts. J Dent Res. 1987;66:1283–7. doi: 10.1177/00220345870660071301. [DOI] [PubMed] [Google Scholar]
- 34.Mendes RM, Silva GA, Lima MF, Calliari MV, Almeida AP, Alves JB, et al. Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch Oral Biol. 2008;53:1155–62. doi: 10.1016/j.archoralbio.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Deed R, Rooney P, Kumar P, Norton JD, Smith J, Freemont AJ, et al. Early-response gene signalling is induced by angiogenic oligosaccharides of hyaluronan in endothelial cells. Inhibition by non-angiogenic, high-molecular-weight hyaluronan. Int J Cancer. 1997;71:251–6. doi: 10.1002/(sici)1097-0215(19970410)71:2<251::aid-ijc21>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 36.Engström PE, Shi XQ, Tronje G, Larsson A, Welander U, Frithiof L, et al. The effect of hyaluronan on bone and soft tissue and immune response in wound healing. J Periodontol. 2001;72:1192–200. doi: 10.1902/jop.2000.72.9.1192. [DOI] [PubMed] [Google Scholar]
- 37.Mesa FL, Aneiros J, Cabrera A, Bravo M, Caballero T, Revelles F, et al. Antiproliferative effect of topic hyaluronic acid gel. Study in gingival biopsies of patients with periodontal disease. Histol Histopathol. 2002;17:747–53. doi: 10.14670/HH-17.747. [DOI] [PubMed] [Google Scholar]
- 38.Pistorius A, Martin M, Willershausen B, Rockmann P. The clinical application of hyaluronic acid in gingivitis therapy. Quintessence Int. 2005;36:531–8. [PubMed] [Google Scholar]
- 39.Xu Y, Höfling K, Fimmers R, Frentzen M, Jervøe-Storm PM. Clinical and microbiological effects of topical subgingival application of hyaluronic acid gel adjunctive to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2004;75:1114–8. doi: 10.1902/jop.2004.75.8.1114. [DOI] [PubMed] [Google Scholar]
- 40.Koshal A, Patel P, Robert B, Galgut Peter N. A comparison in postoperative healing of sites receiving non-surgical debridement augemented with and without a single application of hyluronan 0.8% gel. Prev Dent. 2007;2:34–8. [Google Scholar]
- 41.de Araújo Nobre M, Cintra N, Maló P. Peri-implant maintenance of immediate function implants: A pilot study comparing hyaluronic acid and chlorhexidine. Int J Dent Hyg. 2007;5:87–94. doi: 10.1111/j.1601-5037.2007.00239.x. [DOI] [PubMed] [Google Scholar]
- 42.Ballini A, Cantore S, Capodiferro S, Grassi FR. Esterified hyaluronic acid and autologous bone in the surgical correction of the infra-bone defects. Int J Med Sci. 2009;6:65–71. doi: 10.7150/ijms.6.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanden Bogaerde L. Treatment of infrabony periodontal defects with esterified hyaluronic acid: Clinical report of 19 consecutive lesions. Int J Periodontics Restorative Dent. 2009;29:315–23. [PubMed] [Google Scholar]
- 44.Johannsen A, Tellefsen M, Wikesjö U, Johannsen G. Local delivery of hyaluronan as an adjunct to scaling and root planing in the treatment of chronic periodontitis. J Periodontol. 2009;80:1493–7. doi: 10.1902/jop.2009.090128. [DOI] [PubMed] [Google Scholar]
- 45.Pilloni A, Annibali S, Dominici F, Di Paolo C, Papa M, Cassini MA, et al. Evaluation of the efficacy of an hyaluronic acid-based biogel on periodontal clinical parameters. A randomized-controlled clinical pilot study. Ann Stomatol (Roma) 2011;2:3–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Fawzy El-Sayed KM, Dahaba MA, Aboul-Ela S, Darhous MS. Local application of hyaluronan gel in conjunction with periodontal surgery: A randomized controlled trial. Clin Oral Investig. 2012;16:1229–36. doi: 10.1007/s00784-011-0630-z. [DOI] [PubMed] [Google Scholar]
- 47.Gontiya G, Galgali SR. Effect of hyaluronan on periodontitis: A clinical and histological study. J Indian Soc Periodontol. 2012;16:184–92. doi: 10.4103/0972-124X.99260. [DOI] [PMC free article] [PubMed] [Google Scholar]


