Abstract
Taste buds are peripheral chemosensory organs situated in the oral cavity. Each taste bud consists of a community of 50–100 cells that interact synaptically during gustatory stimulation. At least three distinct cell types are found in mammalian taste buds – Type I cells, Receptor (Type II) cells, and Presynaptic (Type III) cells. Type I cells appear to be glial-like cells. Receptor cells express G protein-coupled taste receptors for sweet, bitter, or umami compounds. Presynaptic cells transduce acid stimuli (sour taste). Cells that sense salt (NaCl) taste have not yet been confidently identified in terms of these cell types. During gustatory stimulation, taste bud cells secrete synaptic, autocrine, and paracrine transmitters. These transmitters include ATP, acetylcholine (ACh), serotonin (5-HT), norepinephrine (NE), and GABA. Glutamate is an efferent transmitter that stimulates Presynaptic cells to release 5-HT. This chapter discusses these transmitters, which cells release them, the postsynaptic targets for the transmitters, and how cell–cell communication shapes taste bud signaling via these transmitters.
Keywords: Type I cell, Receptor (Type II) cell, Presynaptic (Type III) cell, ATP, Autocrine/paracrine/efferent transmitters, Pannexin-1
1. Introduction
As few as three decades ago, taste buds were merely considered inactive interfaces between flavorsome chemicals in the oral cavity and interpretive centers in the nervous system. These peripheral sensory organs were believed to passively convert gustatory stimuli into signals that sensory afferent fibers could propagate to the brain. The cytology of taste buds indicated that their constituent cell population was not homogeneous, but it was not known whether different cells responded to different tastants (e.g., sweet, bitter, etc. compounds), whether there were supporting versus sensory cells, or what transmitter(s) was/were released onto sensory afferent fibers. The intervening years have clarified many of these issues, principally through the efforts of researchers who have applied modern techniques to record functional activity in individual taste cells and who have used molecular biological methodology to identify the proteins found in specific taste bud cells. As a result of these efforts, we now have a tremendously expanded view of the distinct types of cells present in taste buds, how some of these cells contribute to taste reception, what proteins transduce different taste stimuli, and what neurotransmitters are released during taste stimulation. Most importantly, we now understand that the taste bud is a community of interacting cells with significant cell–cell communication taking place during gustatory excitation. Taste buds are no longer thought of as passive sensory structures.
This review tackles the following questions: what interactions take place within the taste bud during taste reception, what are the transmitters involved in these interactions, and how do these interactions shape the signal output from taste buds? I will review the evidence that taste bud cells release ATP, serotonin, norepinephrine, acetylcholine, and GABA to mediate feed-forward and feedback signaling, including excitation and inhibition. Presently, the functional consequences of the cell–cell interactions for taste detection and discrimination are not yet known, but I will hazard some predictions that might be the subject of future studies.
2. Anatomy of taste buds
There are between 2000 and 5000 taste buds in the human oral cavity, distributed on the tongue, the palate, and to a lesser extent the epiglottis, pharynx, and larynx [1–3]. There may be important differences between taste buds in different regions on the tongue and elsewhere, although histologically they appear quite similar. Taste buds on the anterior tongue are embedded in fungiform papilla. These taste buds are innervated by the chorda tympani nerve, a branch of the large facial nerve (N VII). Taste buds in the posterior tongue are located in circumvallate papillae. These taste buds are innervated by the glossopharyngeal nerve (N IX). There are also taste buds buried in folds in the lateral sides of the tongue, in the foliate papillae. These taste buds receive innervation from branches of the chorda tympani and glossopharyngeal nerves. Lastly, taste buds in the palate are innervated by the greater superficial petrosal nerve, another branch of the facial nerve. Although taste buds in all four regions (palate; fungiform, circumvallate, and foliate papillae) respond to sweet, salty, sour, bitter (and perhaps umami, though this has not been studied as thoroughly), there are differences in their sensitivities to these tastes [4]. Presumably this reflects differences in the taste cell populations from region to region.
Every taste bud in the above four regions is a community of approximately 100 cells. These cells fall into three major categories (Fig. 1), originally defined by their morphological appearances and more recently distinguished by their expression of specific proteins and responses to gustatory stimulation (reviewed in Chaudhari and Roper [5]).
Fig. 1.
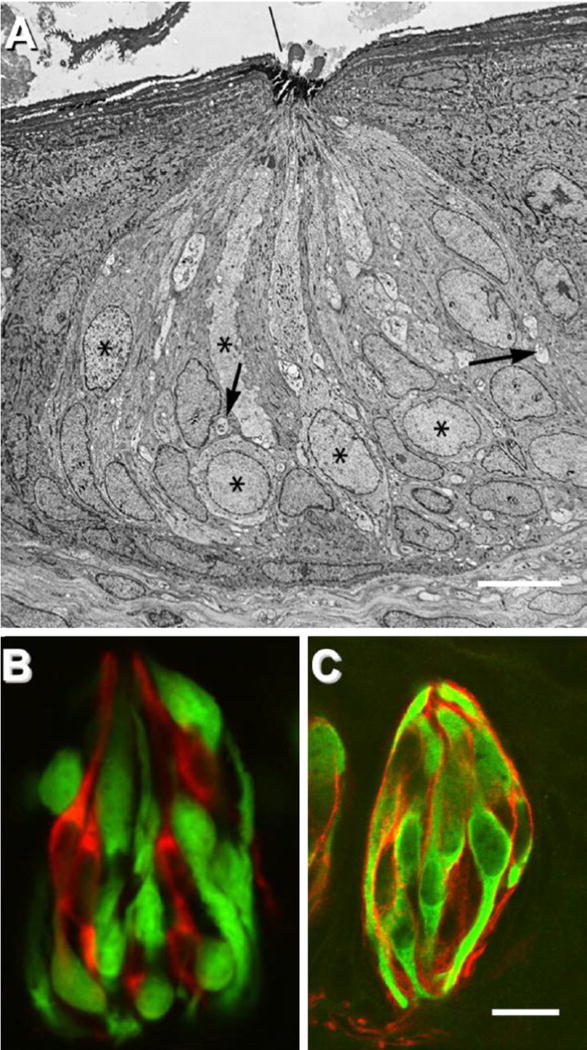
Cell types and synapses in the taste bud. (A) Electron micrograph of a rabbit taste bud showing cells with dark or light cytoplasm, and nerve profiles (arrows). Asterisks mark Type II (Receptor) cells. (B), A taste bud from a mouse, illustrating separate classes of taste cells – Receptor (Type II) and Presynaptic (Type III) cells. The tissue is from a transgenic mouse expressing GFP only in Receptor (Type II) cells (green). Presynaptic cells are immunostained (red) for aromatic amino acid decarboxylase (a neurotransmitter-synthesizing enzyme that is a marker for these cells). (C) Taste buds immunostained for NTPDase2 (an ectonucleotidase associated with the plasma membrane of Type I cells) reveal the thin lamellae (red) of Type I cells. These cytoplasmic extensions wraparound other cells in the taste bud. GFP (green) indicates Receptor cells as in B. Bar, 10 μm.
(A) Reprinted with permission from the Journal of Comparative Neurology (Royer and Kinnamon, 1991). (B) Reprinted with permission from the Journal of Neuroscience[9]. (C) Image courtesy of M. Sinclair and N. Chaudhari. Modified and reprinted from [5].
Type I cells
Type I cells, originally defined ultrastructurally as having electron-dense cytoplasm and elongate, pleomorphic nuclei, are believed to have glial-like functions [6]. These cells synthesize and deposit a powerful ecto-ATPase on their surfaces that degrades the transmitter released by other taste cells [7]. Type I cells also are characterized by extensive lamellar processes that enwrap other taste cells like blankets [8], perhaps to further limit the spread of transmitter(s) and prevent local changes in ion concentrations from reaching other regions of the taste bud during taste reception.
Type II (Receptor) cells
Type II cells feature large ovoid nuclei and electron-lucent cytoplasm [8]. At the molecular level, Type II cells have been identified as cells that express G protein-coupled receptors (GPCRs) for sweet, bitter, and umami taste compounds. Type II cells also express downstream effectors that couple to the taste GPCRs [5]. Consequently, these cells have been renamed taste Receptor cells [9]. Each Receptor cell responds mainly to a single taste quality, such as sweet, bitter, or umami [10,11].Thus, Receptor cells are relatively well “tuned” to specific taste stimuli. Curiously, these cells do not possess structurally identifiable synapses [12] and thus for many years it was unclear how Receptor cells communicated with sensory afferent fibers. Now, however, we know that Receptor cells secrete ATP as a neurotransmitter onto sensory afferent fibers, probably via pannexin1 hemichannels in their surface membrane [13–15, but see 16]. Thus, synaptic release of ATP from Receptor taste cells does not involve vesicular exocytosis or well-defined synapses.
Type III (Presynaptic) cells
Type III cells have ultrastructural features somewhat intermediate between Type I cells and Receptor (Type II) cells. Most importantly, though, Type III cells possess synapses [17–19]. The specific postsynaptic targets at these synapses have not been confidently identified. They may be sensory afferent fibers, but if so, they do not use ATP as a transmitter; Type III cells do not secrete ATP. They release serotonin, norepinephrine, and GABA [13,20–22]. Consistent with the identification of synapses, Type III cells express proteins associated with synaptic transmission, including SNAP 25, voltage-gated Ca2+ channels, biosynthetic enzymes for serotonin and GABA, and uptake transporters for biogenic amines [9,21,23]. Accordingly, Type III cells have been renamed Presynaptic cells [9]. When isolated as single cells, Presynaptic cells respond to acid (sour) stimuli [24]. Mice lacking Presynaptic taste cells do not respond to sour taste [25]. However, when studied in intact taste buds, Presynaptic cells also are excited by sweet, bitter, and umami stimuli even though they do not possess receptors for those compounds [11]. The explanation is cell–cell communication from Receptor to Presynaptic cells in situ. ATP secreted by Receptor cells during taste activation stimulates Presynaptic cells in addition to exciting sensory afferent fibers [5,13]. As a result, Presynaptic cells are indirectly excited by sweet, bitter, and umami compounds and appear to be some form of integrating element in taste bud signaling. This cell–cell interaction is discussed in greater detail below.
To summarize, Type I cells appear to be glial-like supporting cells in the taste bud. Receptor (Type II) cells are sensory cells for sweet, bitter, or umami tastes, all of which appear to be transduced by G protein coupled taste receptors. These stimuli trigger Receptor cells to secrete ATP which in turn excites sensory afferent fibers and adjacent Presynaptic taste cells. Presynaptic (Type III) taste cells are directly stimulated by sour taste and indirectly by sweet, bitter, and umami taste. Thus, these cells seem to integrate multiple gustatory stimuli in the taste bud. Presynaptic cells release serotonin, norepinephrine and GABA.
3. Physiology of taste buds
Many of the details of taste transduction have been illuminated in the past two decades, in large part through the use of genetically engineered mice, molecular biological studies, patch-clamp recordings, and functional imaging studies. G protein-coupled receptors for sweet, bitter and umami tastants have been identified. Sweet and umami tastants are detected by GPCRs from the small family of Tas1R genes (T1R proteins), and likely other genes as well [26–29]. Specifically, sugars and other sweet tastants (e.g., artificial sweeteners, and sweet proteins) bind to GPCR dimers comprised of T1R2plusT1R3 [30–32].There maybe additional, yet-undiscovered sweet receptors [30–32]. Monosodium glutamate, the prototypic umami tastant, binds to the GPCR dimer T1R1 plus T1R3, as well as to other receptors, possibly mGluR4, mGluR1, and others [33–37]. Bitter taste stimuli bind to a large family of about 2 dozen Tas2R genes (T2R proteins) [38]. The number of Tas2R genes varies from species to species (and not all species have all three Tas1R genes [39]). Presently, it is not known whether T2R receptors form multimers [40].
T1R and T2R taste GPCRs are expressed in the Type II (Receptor cell) class of taste bud cells. Each Receptor (Type II) cell mainly responds to a single taste quality (sweet, or bitter, or umami) [10,11], presumably because a single Receptor cell expresses GPCRs for that quality. However, there is not a strict one-cell-one taste receptor expression rule as appears the case in olfaction [41,42]. That is, some Type II taste receptor cells respond to two or more different tastes and express multiple taste GPCRs [11]; see Fig. 6 in [43].
Activation of taste GPCRs stimulates downstream enzymes and effectors in Receptor (Type II) cells. Specifically, when a tastant binds to a taste GPCR, this releases the G protein subunits Gβ3 and Gγ13 that activate a membrane-bound enzyme, phospholipase Cβ2 (PLCβ2) specifically expressed in Receptor cells. When activated, PLCβ2 digests plasma membrane phospholipids into diacylglycerol (DAG) and inositol trisphosphate (IP3). IP3 diffuses to nearby (intracellular) stores of calcium, binds to IP3 receptors (IP3R3 specifically) on those stores, and releases Ca2+ into the cytosol. This is the canonical PLC/IP3 Ca2+ release signaling pathway (http://stke.sciencemag.org/cgi/cm/stkecm;CMP_6680).
Over the years, all the elements of this pathway have been identified and localized to Receptor (Type II) taste cells (reviewed in [5]). Thus, the net effect of sweet, bitter, or umami tastant stimulation is to mobilize intracellular Ca2+. Intracellular mobilization of Ca2+ has been used to great advantage to record taste cell stimulation by using functional (Ca2+) imaging at the tissue and cellular levels.
The elevation of intracellular Ca2+ has at least two consequences for taste bud signaling. First, Ca2+ binds to and opens TRPM5 ion channels in Receptor cells, allowing Na+ ions to enter and depolarize the membrane (i.e., produce a receptor, or generator potential) [44,45]. Concurrently, Ca2+ binds to and is believed to open pannexin 1 (Panx1) gap junction hemichannels in the plasma membrane [13,46]. Membrane depolarization assists in this action and further promotes Panx1 channels to open [47]. Opening Panx1 channels allows the escape of ATP (and possibly Mg2+·ATP) from the cytosol into the extracellular spaces [13–16,48]. The total concentration of cytosolic ATP (free and Mg2+·ATP) can reach 1–5mM but varies markedly from cell to cell and tissue to tissue [49]. Thus, there is a powerful concentration gradient driving efflux of this nucleotide, given that extracellular ATP is normally micromolar. ATP is believed to be the excitatory neurotransmitter released by Receptor taste cells to activate postsynaptic primary sensory afferent fibers [13,50]. ATP released during taste stimulation also excites adjacent taste cells, as discussed below.
Stimulating taste GPCRs on Receptor cells also activates a taste-specific Gα protein subunit, gustducin. Gustducin is a Gαi protein related to transducin in photoreceptors [51]. The gustducin pathway, similar to the transducin pathway in photoreceptors, is linked to a phosphodiesterase present in taste Receptor cells, specifically PDE-3 [52–54]. In taste cells, PDE-3 degrades cAMP. Although the details are still murky, the general concept is that cytosolic cAMP exerts a tonic, low-level depression of Receptor cells by virtue of the ability of the cyclic nucleotide to inhibit the PLCβ2/IP3 pathway, above [55]. Concurrent activation of the PLCβ2/IP3 pathway by Gβ/Gγ subunits and reduction of cAMP by Gα (gustducin) boosts signaling along the aforementioned (parallel) PLC/IP3 pathway and assures reliable taste transduction for sweet, bitter, and umami tastes.
Sour taste stimuli (acids) activate the Type III category of taste bud cells, the so-called Presynaptic cells [24,25]. The specific transduction channels or receptors underlying sour taste remain an unsolved question. It is likely that cytosolic acidification of the Presynaptic cell by diffusion of the protonated acid (RCOOH) across the plasma membrane, followed by intracellular dissociation into the anion (RCOO−) and proton (H+) in the cytosol, is the proximate stimulus that opens ion channels and allows cation influx and thus membrane depolarization [24,56]. Intracellular acidification may, for example, close proton-sensitive potassium channels and thereby depolarize these cells [57]. Chang et al. [58] present evidence for a possible additional pathway involving direct H+ influx through proton channels. Presynaptic cells express voltage-gated Ca2+ channels, and thus membrane depolarization leads to Ca2+ influx. Sour taste stimulation of Presynaptic cells triggers these cells to release, serotonin (5-HT), GABA [22], and possibly norepinephrine [20]. Most likely, these transmitters are secreted via vesicular exocytosis initiated by Ca2+ influx [59].
Salt (specifically, Na+) taste remains an enigma. It is not yet known which cells in the taste bud are the targets for Na+ stimulation. There is evidence that epithelial Na channels (ENaC) underlie salt transduction in rodents [60,61] and that Type I taste cells may express ENaC [62]. This would lead to the conclusion that Type I cells are responsible for Na+ taste, but there is no definitive evidence for this to date.
A major conclusion is that different tastes initially activate different sets of taste bud cells and release different transmitters (Fig. 2). For instance, sugars stimulate sweet-sensitive Receptor (Type II) cells. Bitter and umami compounds excite other Receptor (Type II) cells. Acids stimulate Presynaptic (Type III) taste bud cells to elicit sour taste. Salt taste may activate Type I cells, but this is speculation. The different taste bud cells secrete different neurotransmitters. Receptor cells secrete ATP (and as will be described later, acetylcholine, ACh). Presynaptic cells secrete 5-HT, NE, and GABA. Little is known about which transmitters, if any, Type I cells secrete.
Fig. 2.
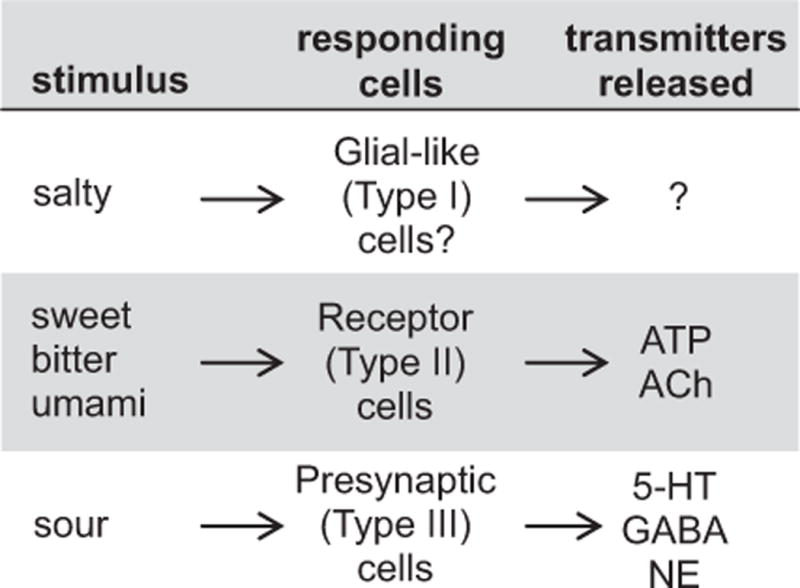
The cells that respond to taste stimuli and release transmitters in taste buds. Little is known about which cells respond to salt stimulation and which, if any, transmitters these cells secrete, as indicated by the question marks.
Key to appreciating how tastants activate taste buds is that the above taste transmitters, secreted during gustatory stimulation, act on other cells within the taste bud. That is, there is significant cell–cell communication within taste buds during chemosensory stimulation (Fig. 3). First, activation of Receptor cells triggers ATP release which excites adjacent Presynaptic taste cells. Second, when Presynaptic cells are stimulated, either by sour taste or by ATP during sweet, bitter and umami taste reception, they secrete transmitters that inhibit Receptor cells. These inhibitory transmitters include 5-HT and GABA. The function of a third Presynaptic cell transmitter, NE, is not yet understood. To add to this complexity, ATP itself exerts autocrine feedback onto Receptor cells (vide infra). This positive feedback apparently is critical for successful taste-evoked transmitter release. For instance, in the absence of certain purinoceptors in Receptor (Type II) cells (P2X2/P2X3 knockout mice), taste-evoked ATP secretion is greatly reduced and taste buds cease normal function. These transmitters and their functions in taste buds are explored in greater detail, next.
Fig. 3.
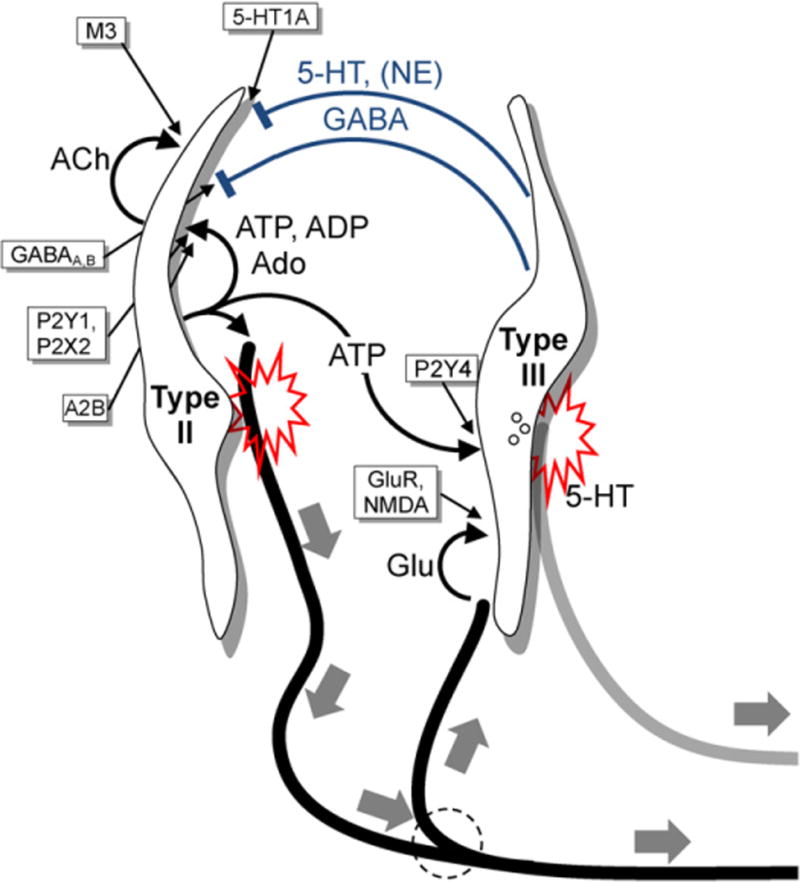
Summary of taste transmitters and synaptic interactions between Receptor (Type II) and Presynaptic (Type III) cells in taste buds. This diagram outlines synaptic, paracrine, autocrine, and efferent transmitters and their receptors identified in taste buds to date. Not shown are peptidergic transmitter interactions that are also likely to take place [88] but about which fewer details are known. Receptor (Type II) cells (left) respond to and transduce stimuli mediated by taste GPCRs (sweet, bitter, or umami) and secrete ATP to excite primary sensory afferent fibers (thick black lines, large solid gray arrows). ATP also excites neighboring Presynaptic (Type III) cells (right). ATP is degraded within the taste bud to ADP and these purines act as autocrine transmitters to boost further ATP release from Receptor cells via P2Y1 and P2X2 receptors. ADP is further degraded to adenosine (Ado) which excites A2B receptors expressed selectively on sweet-responsive Receptor cells. Lastly, Receptor cells also release acetylcholine (ACh) which stimulates muscarinic (M3) receptors on Type II cells. Presynaptic (Type III) cells respond directly to acid (sour) taste stimuli and indirectly, via ATP, to sweet, umami, and bitter stimuli. Presynaptic taste cells secrete serotonin (5-HT), norepinenephrine (NE) and GABA. 5-HT and GABA are inhibitory paracrine transmitters, acting on postsynaptic 5-HT1A and receptors on Type II cells, as shown. 5-HT is also believed to be the neurotransmitter at the synapses Type III cells form with axon terminals (solid gray line). The full identification of these putative serotonergic axon terminals remains to be established. Actions of NE have been investigated [90] but the cellular targets and functions for this transmitter are not well established.
4. Cell–cell communication and taste reception
The thesis of this review is that taste stimulation activates a series of highly orchestrated cell–cell interactions in the taste bud that modulate and shape the signal output from taste buds. The precise significance of the signal modulation is still under investigation and how these cell–cell interactions contribute to taste detection and taste discrimination is yet unknown. Nonetheless, there is no doubt that a certain degree of signal processing takes place in the peripheral sensory organs of taste and that the final output from taste buds is shaped by these cell–cell interactions. This unquestionably affects how taste signals are transmitted from the periphery to the brain and seriously challenges any notion that there is a simple labeled-line code for taste. Transmitters secreted in taste buds during gustatory stimulation are summarized in Fig. 3 and documented below.
4.1. ATP and the cycle of purinergic signaling during gustatory reception in taste buds
ATP is a major player in the family of taste bud neurotransmitters. Initially, ATP was intimated as a candidate transmitter because gustatory sensory afferents were immunopositive for receptors for this purine, specifically P2X2 and P2X3 [63,64]. Subsequently, lingual epithelial sheets containing taste buds were shown to secrete ATP when stimulated [50], and specific taste cells, namely Receptor (Type II) cells were identified as the source of taste-evoked ATP secretion [13,65]. Moreover, taste-evoked transmitter release was shown to be mediated by an unusual pathway – secretion through gap junction hemichannels likely comprised of pannexin 1 [13,14, but see 16]. Transmitter release via pannexin 1 channels is consistent with the microanatomy of taste buds. Ultrastructural evidence for conventional synapses (e.g., clusters of vesicles, pre- and postsynaptic thickenings) is not found in electron micrographs of Receptor cells [12]. Finally, genetic deletion of P2X2 and P2X3 leads to the disruption taste (with the exception of sour taste) in knockout mice [50]. Deleting P2X2/P2X3 was interpreted as removing the postsynaptic targets for the taste bud transmitter (ATP receptors on afferent fibers), hence supporting the notion that ATP was released by taste buds during gustatory stimulation.
However, the evidence that ATP is the only or even the primary excitatory taste bud transmitter acting on sensory afferent fibers is not watertight. Subsequent studies showed that P2X2 is expressed presynaptically in Receptor cells and that knocking out P2X2/P2X3 prevents normal taste-evoked transmitter secretion [66]. These studies indicated that P2X purinoceptors, and particularly P2X2, are required for taste transmitter release. P2X2 receptors exert positive autocrine feedback to boost and/or maintain taste-evoked ATP secretion (Fig. 4). Interestingly, such autocrine positive feedback has also been described for synaptic ATP release in the carotid body [67] and at terminals of neurosecretory cells in the posterior pituitary [68]. Thus, what was initially interpreted as being strictly a postsynaptic deficit of sensory fibers (absence of postsynaptic receptors in the P2X2/P2X3 knockout mice), is now understood to include a profound deficit in presynaptic release mechanisms in taste bud cells. Given this understanding, there may be other transmitters released from Receptor cells during taste transmission that contribute equally importantly as ATP. This remains to be investigated.
Fig. 4.

ATP exerts positive feedback via presynaptic autocrine receptors to boost its secretion during taste stimulation. This diagram shows a Receptor (Type II) cell releasing ATP next to a sensory afferent fiber. Receptor (Type II) taste cells secrete ATP likely via pannexin 1 gap junction hemichannels in the plasma membrane (Panx1). ATP excites postsynaptic targets as well as autocrine P2X2 and P2Y1 receptors on the Type II cell itself. Stimulation of P2X2 and P2Y1 receptors initiates Ca2+ influx and Ca2+ release from intracellular stores, respectively. Both these actions increase intracellular [Ca2+] which further enhances Panx1 activity and boosts ATP release. Genetic elimination of P2X2 (P2X2 knockout) greatly reduces taste-evoked ATP secretion from Receptor and taste reception [50,66].
Another important target for ATP released from Receptor cells is adjacent Presynaptic cells. Presynaptic cells express P2 purinoceptors, likely P2Y4 [69]. Thus, sweet (or bitter, umami) stimulation of taste buds directly excites Receptor (Type II) cells and indirectly activates Presynaptic (Type III) cells via purinergic cell–cell interactions. As a consequence, in intact taste buds Presynaptic cells are activated during sweet, bitter, umami or sour stimulation, even though in isolation Presynaptic cells are sensitive only to sour stimulation. In intact taste buds, Presynatic cells are also activated during salt (Na+) stimulation [11]. However, the proximate target for Na+ stimulation and how this excitation gets transferred to Presyaptic cells remains unsolved.
ATP follows an intriguing pathway of degradation after it is released from Receptor cells during taste stimulation. Taste bud cells, including Receptor cells, are enwrapped with lamellar processes of Type I taste cells [8]. Presumably, these lamellae restrict the diffusion of ATP within the taste bud and keep the transmitter confined to intercellular spaces immediately surrounding the active Receptor cell. Further, Type I cells express a powerful ecto-ATPase on their surface, NTPDase2 [7]. This ecto-ATPase quickly degrades ATP to ADP and AMP, further restricting diffusion of the secreted transmitter. Even as it is degraded, however, ATP continues to exert important actions. As described above, P2X2 receptors on Receptor taste cells are stimulated by the initial taste-evoked release of ATP, representing autocrine excitatory feedback. Additionally, ADP generated by degradation of ATP stimulates P2Y1 receptors that are also expressed on Receptor cells [69]. Finally, AMP generated by catabolism of ADP is degraded by other ectonucleotidases, specifically prostatic acid phosphatase (PAPP) and NTE5 expressed on Presynaptic (Type III) cells [43]. The endproduct of this degradation, adenosine, is also a neuromodulatory signal, acting on excitatory adenosine A2B receptors expressed selectively on sweet-sensitive Receptor cells [43,70]. The production of adenosine completes the autocrine neuromodulation of ATP and its byproducts.
Purines have been described up to this point as taste transmitters onto afferent fibers (ATP) and as autocrine excitatory compounds (ATP, ADP, and adenosine). Yet, it is equally plausible that they are paracrine excitatory modulators acting on surrounding Receptor taste cells. Thus, in principle, one Receptor cell could influence the activity of nearby Receptor cells by virtue of the spread, limited as it may be, of ATP, ADP, and adenosine. This may explain why individual Receptor cells have been observed to respond to more than a single taste [11]. Although the vast majority of Receptor cells is “tuned” to respond specifically only to sweet, bitter, or umami tastants, a small proportion respond to multiple taste qualities. This could be due to direct stimulation by one tastant followed by indirect activation by another tastant through ATP, ADP, and/or adenosine from an adjacent Receptor cell.
4.2. Acetylcholine signaling during gustatory reception in taste buds
Receptor (Type II) taste cells also secrete acetylcholine (ACh), a well-established transmitter at synapses throughout the nervous system. Taste-evoked ACh release from isolated mouse Receptor cells has been detected with highly sensitive biosensor cells [71]. ACh appears to stimulate muscarinic receptors, specifically M3, on the same (autocrine) or neighboring (paracrine) Receptor cells [72,73,74]. This muscarinic feedback augments taste-evoked release of ATP [71]. Muscarinic antagonists such as atropine blocks this positive feedback, and genetically mutant mice that lack M3 receptors (knockout mice) lack a taste-evoked muscarinic response [71].
4.3. Serotonin signaling during gustatory reception in taste buds
Transmitters other than ATP and ACh also contribute in a major fashion to cell–cell interactions and signal processing in taste buds. Serotonin (5-hydroxytryptamine, 5-HT) is one such transmitter, released by Presynaptic (Type III) cells in response to (a) sour (acid) taste stimulation; or (b) KCl depolarization [24,75]; or (c) purinergic (ATP) stimulation from nearby taste Receptor (Type II) cells (above) [13,69]. A prominent action of 5-HT is inhibition of Receptor cells via 5HT-1A receptors expressed by those cells [23,69,76]. Drugs such as paroxetine that interfere with the re-uptake of 5-HT greatly enhance inhibitory serotonergic feedback in the taste bud [69]. Conversely, drugs that block 5HT-1A receptors such as methysergide or WAY100635 reduce the inhibitory serotonergic feedback and augment Receptor cell function, especially ATP secretion [69].
When human subjects were treated with selective serotonin reuptake blockers (e.g., the antidepressant paroxetine), they manifest reduced taste thresholds for bitter and sweet compounds (i.e., the subjects became more sensitive to the taste of these compounds) [77]. The results were interpreted as the consequence of increased levels of 5-HT in the bloodstream reaching taste buds. At face value, this would seem to be counter to how serotonin inhibits Receptor cells in isolated mouse taste buds (above). Further, taste behavior tests on experimental animals (rats) failed to replicate the paroxetine data from humans [78]. However, in these experiments, there was no indication of how much circulating 5-HT had been altered by the antidepressant treatments. In short, psychophysical tests of how serotonin affects taste in vivo are incomplete and do not yet match with the physiological data from isolated mouse taste buds. There has been no resolution of this dilemma to date.
Because sour taste itself stimulates Presynaptic cells and triggers 5-HT release, one might anticipate that acid stimulation of taste buds might depress Receptor cell responses to sweet, bitter, or umami compounds. This has indeed been demonstrated in isolated mouse taste buds. Curiously, however, there are few reports that sour tastants interfere with sweet, bitter, or umami taste thresholds or taste perception in humans or animals. This remains an unresolved dilemma. A recent abstract from G. Delay’s laboratory indicates that the presence of lactic acid, for one, appears to change the taste sensation for amino acids in behavioral tests on mice [79]. Whether this is a manifestation of sour-evoked serotonergic interactions within the taste bud remains to be determined.
Another, unexplored possibility is that 5-HT functions as a transmitter between Presynaptic cells and the nerve fibers with which Presynaptic cells form synaptic contacts. Synapses between Presynaptic cells and postsynaptic axons may be serotonergic [80,81]. There is speculation that postsynaptic axons which innervate Presynaptic (Type III) cells represent a distinct population of sensory afferent fibers that carry information to the hindbrain in a separate pathway, i.e., that Presynaptic cells are part of a second pathway that is parallel to the presumed purinergic fibers innervating Receptor cells [82]. The speculation is that Presynaptic cells transmit information pertinent to physiological reflexes such as cephalic phase insulin secretion; In contrast, Receptor cells transmit information about taste quality recognition and discrimination – particularly sweet, bitter, umami, and salty.
4.4. GABA signaling during gustatory reception in taste buds
Presynaptic cells also synthesize and secrete GABA, another inhibitory transmitter in taste buds [21]. Sour taste stimulation and KCl depolarization trigger GABA release from Presynaptic cells [22]. Curiously, however, stimulating Presynaptic cells with ATP does not elicit GABA release. Why acid (sour) and KCl stimuli, but not ATP cause Presynaptic cells to secrete GABA is unknown. Presumably, the release mechanisms for GABA distinguish between Ca2+ arising from influx through voltage-gated divalent cation channels in the plasma membrane (opened by acid- or KCl-evoked depolarization) versus Ca2+ released from intracellular stores (resulting from ATP activation of Presynaptic cell P2Y4 receptors).
Targets for GABA include Receptor (Type II) and possibly Presynaptic (Type III) cells, themselves. The latter would represent autocrine feedback from GABA release. The best evidence is that GABA inhibits Receptor cells, and specifically, taste-evoked transmitter release (ATP release) from these cells. Receptor cells express GABAA and receptor subunits and both of these receptor subtypes appear to contribute to the GABAergic inhibition. Applying GABA to Type III cells evoked no obvious synaptic responses, inhibitory or otherwise. The GABA receptors on those cells may mediate long-term trophic responses, but this remains speculation to date.
4.5. Glutamate signaling during gustatory reception in taste buds
L-Glutamate is the primary neurotransmitter in auditory hair cells and retinal photoreceptors. Thus, it might be reasoned that this excitatory amino acid would figure prominently in chemosensory receptor cells. Glutamate is an established taste stimulus (umami) and this has complicated studies of this amino acid. Applying glutamate to isolated tissues and cells stimulates taste receptors as well as any presumptive synaptic glutamate receptors. Indeed, studies on isolated tissues have revealed that taste buds express receptors for glutamate taste (T1R1, T1R3, taste-mGluR4, taste-mGluR1) as well as ionotropic and metabotropic synaptic receptors for glutamate [33,35,83,84]. Caicedo et al. [85] used a novel approach to separate taste from synaptic receptors by recording responses of taste bud cells embedded in lingual slice preparations where glutamate could be differentially applied either to the apical chemosensory (taste) tips of cells, or to the basolateral (synaptic) regions. Those studies showed that ionotropic glutamate with properties consistent for synapses receptors (i.e., AMPA/Kaintate, NMDA receptors) are present on the basolateral surfaces of taste bud cells. Thus, taste buds represent a target for, not a source of glutamate release. More recent findings are consistent with those results. Namely, vesicular transporter for glutamate types I and 2 (VGLUT1,2) are present in afferent fibers that innervate taste buds [86]. The presence of VGLUTs typifies glutamate release sites elsewhere in the nervous system. Further, taste bud cells express specific subtypes of glutamate receptors, such as the ionotropic kainate receptor GluR7. Type III cells were identified as the likely targets of glutamate and glutamate was speculated to be an efferent transmitter in taste [86].
Most recently, Huang et al. [87] applied glutamate to isolated taste buds at concentrations appropriate for synaptic activation (micromolar) as opposed to concentrations that would stimulate taste receptors (mM). They found that when applied at synaptic concentrations, glutamate stimulates Type III (presynaptic) cells to secrete serotonin, an inhibitory paracrine taste transmitter. Although they did not test it, glutamate would also be expected to stimulate Type III cells to secrete GABA, another inhibitory transmitter. Huang et al. [87] concluded that glutamate was indeed an efferent transmitter, released from gustatory sensory afferents during taste stimulation via the mechanism of axon reflex. The net effect of efferent feedback from such release would be to inhibit further activation of taste cells (negative feedback) or to suppress neighboring taste buds via axon reflex activation of afferent branches, or both.
5. Summary and conclusions
This review has focused on the events that occur after taste stimuli have excited taste bud sensory cells, namely the release of taste transmitters. Sweet, bitter, and umami stimulation elicits release of ATP, perhaps as the primary, fast-acting excitatory synaptic transmitter. Knockout studies using mice lacking certain P2X purinoreceptors do not unambiguously identify ATP as the transmitter because synaptic release as a whole is defective in the taste buds of these mice. Instead, the knockout studies reveal that there are multiple positive feedback mechanisms (autocrine P2X and P2Y receptors) governing ATP release. Sour stimulation triggers serotonin release. Salt stimulation is not as well characterized regarding released transmitters. Taste stimulation also initiates negative feedback mechanisms, including release of serotonin and GABA. Negative feedback via efferent glutamatergic fibers further limits taste bud excitation. These interactions are summarized in Fig. 3.
What remains now is (1) to determine if there are additional neurotransmitters in taste buds (e.g., [88]) and (2) to unravel the existing tangle of taste transmitters–ATP, ADP, adenosine, ACh, serotonin, GABA, and glutamate. The individual actions of these transmitters has largely been revealed, but how they function during taste stimulation and how their actions are orchestrated to shape the final output from taste buds is largely unknown. Genetic manipulation via knockout mice and taste behavior studies may be helpful, but eliminating any given transmitter receptor in taste buds is also likely to affect synapses in the central nervous system. Thus, simple behavioral analyses will not be straightforward. Gustatory behavior is governed by sensory signals from peripheral sensory organs – taste buds – as well as by CNS circuitry.
Nonetheless, one important conclusion is that taste is highly unlikely to be coded by a simple “labeled line” mechanism. Such a mechanism postulates that there are dedicated sensory cells for a specific stimulus, such as sweet taste, that synapse with equally dedicated afferent fibers (“lines”). Moreover, the dedicated lines, in turn, ascend the gustatory pathway into the brain and to the primary sensory cortices, terminating in clusters of neurons that are dedicated solely to that initial stimulus such as sweet taste. To be sure, there are reports claiming taste is coded by labeled lines [89], but there is not consensus in the field of chemosensory sciences. To the contrary, the presence of so many interacting synaptic mechanisms at the initial point of sensation, peripheral taste buds, indicates that information processing and cellular interactions shape gustatory signals very early in taste reception. This is incompatible with labeled line coding. Instead, evidence suggests that taste is coded in a more elaborate mechanism such as combinatorial or temporal manner, or both (reviewed in Chaudhari and Roper [5].
Acknowledgments
This research has been supported by grants from the National Institutes of Health, National Institute on Deafness and Other Communication Disorders (5R01DC000374 and 1R01DC007630). I wish to thank Nirupa Chaudhari for fruitful discussions and productive collaboration on all the studies described in this report.
References
- 1.Lalonde ER, Eglitis JA. Number and distribution of taste buds on the epiglottis, pharynx, larynx, soft palate and uvula in a human newborn. Anatomical Record. 1961;140:91–5. doi: 10.1002/ar.1091400204. [DOI] [PubMed] [Google Scholar]
- 2.Miller IJ., Jr Variation in human fungiform taste bud densities among regions and subjects. Anatomical Record. 1986;216:474–82. doi: 10.1002/ar.1092160404. [DOI] [PubMed] [Google Scholar]
- 3.Miller IJ., Jr . Anatomy of the peripheral taste system. In: Doty RL, editor. Handbook of olfaction and gustation. NY: Marcel Dekker; 1995. pp. 521–47. [Google Scholar]
- 4.Collings VB. Human taste response as a function of locus of stimulation on the tongue and soft palate. Perception & Psychophysics. 1974;16:169–74. [Google Scholar]
- 5.Chaudhari N, Roper SD. The cell biology of taste. Journal of Cell Biology. 2010;190:285–96. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dvoryanchikov G, Sinclair MS, Perea-Martinez I, Wang T, Chaudhari N. Inward rectifier channel, ROMK, is localized to the apical tips of glial-like cells in mouse taste buds. Journal of Comparative Neurology. 2009;517 doi: 10.1002/cne.22152. spc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartel DL, Sullivan SL, Lavoie EG, Sevigny J, Finger TE. Nucleoside triphosphate diphosphohydrolase-2 is the ecto-ATPase of type I cells in taste buds. Journal of Comparative Neurology. 2006;497:1–12. doi: 10.1002/cne.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pumplin DW, Yu C, Smith DV. Light and dark cells of rat vallate taste buds are morphologically distinct cell types. Journal of Comparative Neurology. 1997;378:389–410. doi: 10.1002/(sici)1096-9861(19970217)378:3<389::aid-cne7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 9.DeFazio RA, Dvoryanchikov G, Maruyama Y, Kim JW, Pereira E, Roper SD, et al. Separate populations of receptor cells and presynaptic cells in mouse taste buds. Journal of Neuroscience. 2006;26:3971–80. doi: 10.1523/JNEUROSCI.0515-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshida R, Shigemura N, Sanematsu K, Yasumatsu K, Ishizuka S, Ninomiya Y. Taste responsiveness of fungiform taste cells with action potentials. Journal of Neurophysiology. 2006;96:3088–95. doi: 10.1152/jn.00409.2006. [DOI] [PubMed] [Google Scholar]
- 11.Tomchik SM, Berg S, Kim JW, Chaudhari N, Roper SD. Breadth of tuning and taste coding in mammalian taste buds. Journal of Neuroscience. 2007;27:10840–8. doi: 10.1523/JNEUROSCI.1863-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clapp TR, Yang R, Stoick CL, Kinnamon SC, Kinnamon JC. Morphologic characterization of rat taste receptor cells that express components of the phospholipase C signaling pathway. Journal of Comparative Neurology. 2004;468:311–21. doi: 10.1002/cne.10963. [DOI] [PubMed] [Google Scholar]
- 13.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell–cell communication in mouse taste buds. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:6436–41. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO Journal. 2007;26:657–67. doi: 10.1038/sj.emboj.7601526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dando R, Roper SD. Cell-to-cell communication in intact taste buds through ATP signalling from pannexin 1 gap junction hemichannels. Journal of Physiology. 2009;587:5899–906. doi: 10.1113/jphysiol.2009.180083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romanov RA, Bystrova MF, Rogachevskaya OA, Sadovnikov VB, Shestopalov VI, Kolesnikov SS. Dispensable ATP permeability of Pannexin 1 channels in a heterologous system and in mammalian taste cells. Journal of Cell Science. 2012 doi: 10.1242/jcs.111062. (September) [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murray RG. Cellular relations in mouse circumvallate taste buds. Microscopy Research and Technique. 1993;26:209–24. doi: 10.1002/jemt.1070260304. [DOI] [PubMed] [Google Scholar]
- 18.Yang R, Crowley HH, Rock ME, Kinnamon JC. Taste cells with synapses in rat circumvallate papillae display SNAP-25-like immunoreactivity. Journal of Comparative Neurology. 2000;424:205–15. doi: 10.1002/1096-9861(20000821)424:2<205::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Yee CL, Yang R, Bottger B, Finger TE, Kinnamon JC. “Type III” cells of rat taste buds: immunohistochemical and ultrastructural studies of neuron-specific enolase, protein gene product 9.5, and serotonin. Journal of Comparative Neurology. 2001;440:97–108. doi: 10.1002/cne.1372. [DOI] [PubMed] [Google Scholar]
- 20.Huang YA, Maruyama Y, Roper SD. Norepinephrine is coreleased with serotonin in mouse taste buds. Journal of Neuroscience. 2008;28:13088–93. doi: 10.1523/JNEUROSCI.4187-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dvoryanchikov G, Huang YA, Barro-Soria R, Chaudhari N, Roper SD. GABA, its receptors, and GABAergic inhibition in mouse taste buds. Journal of Neuroscience. 2011;31:5782–91. doi: 10.1523/JNEUROSCI.5559-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang YA, Pereira E, Roper SD. Acid stimulation (sour taste) elicits GABA and serotonin release from mouse taste cells. PLoS ONE. 2011;6:e25471. doi: 10.1371/journal.pone.0025471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dvoryanchikov G, Tomchik SM, Chaudhari N. Biogenic amine synthesis and uptake in rodent taste buds. Journal of Comparative Neurology. 2007;505:302–13. doi: 10.1002/cne.21494. [DOI] [PubMed] [Google Scholar]
- 24.Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. Journal of Physiology. 2008;586:2903–12. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–8. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–3. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 27.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–66. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 28.Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chemical Senses. 2006;31:351–7. doi: 10.1093/chemse/bjj039. [DOI] [PubMed] [Google Scholar]
- 29.Ohkuri T, Yasumatsu K, Horio N, Jyotaki M, Margolskee RF, Ninomiya Y. Multiple sweet receptors and transduction pathways revealed in knockout mice by temperature dependence and gurmarin sensitivity. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2009;296:R960–71. doi: 10.1152/ajpregu.91018.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS. Mammalian sweet taste receptors. Cell. 2001;106:381–90. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 31.Morini G, Bassoli A, Temussi PA. From small sweeteners to sweet proteins: anatomy of the binding sites of the human T1R2_T1R3 receptor. Journal of Medicinal Chemistry. 2005;48:5520–9. doi: 10.1021/jm0503345. [DOI] [PubMed] [Google Scholar]
- 32.Cui M, Jiang P, Maillet E, Max M, Margolskee RF, Osman R. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Current Pharmaceutical Design. 2006;12:4591–600. doi: 10.2174/138161206779010350. [DOI] [PubMed] [Google Scholar]
- 33.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nature Neuroscience. 2000;3:113–9. doi: 10.1038/72053. [DOI] [PubMed] [Google Scholar]
- 34.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. American Journal of Clinical Nutrition. 2009;90:738S–42S. doi: 10.3945/ajcn.2009.27462H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.San Gabriel A, Maekawa T, Uneyama H, Torii K. Metabotropic glutamate receptor type 1 in taste tissue. American Journal of Clinical Nutrition. 2009;90:743S–6S. doi: 10.3945/ajcn.2009.27462I. [DOI] [PubMed] [Google Scholar]
- 36.Nakashima K, Eddy MC, Katsukawa H, Delay ER, Ninomiya Y. Behavioral responses to glutamate receptor agonists and antagonists implicate the involvement of brain-expressed mGluR4 and mGluR1 in taste transduction for umami in mice. Physiology and Behavior. 2012;105:709–19. doi: 10.1016/j.physbeh.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 37.Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, et al. Umami taste in mice uses multiple receptors and transduction pathways. Journal of Physiology. 2012;590:1155–70. doi: 10.1113/jphysiol.2011.211920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–11. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 39.Shi P, Zhang J. Contrasting modes of evolution between vertebrate sweet/umami receptor genes and bitter receptor genes. Molecular Biology and Evolution. 2006;23:292–300. doi: 10.1093/molbev/msj028. [DOI] [PubMed] [Google Scholar]
- 40.Kuhn C, Bufe B, Batram C, Meyerhof W. Oligomerization of TAS2R bitter taste receptors. Chemical Senses. 2010;35:395–406. doi: 10.1093/chemse/bjq027. [DOI] [PubMed] [Google Scholar]
- 41.Mombaerts P. Odorant receptor gene choice in olfactory sensory neurons: the one receptor-one neuron hypothesis revisited. Current Opinion in Neurobiology. 2004;14:31–6. doi: 10.1016/j.conb.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Serizawa S, Miyamichi K, Sakano H. One neuron-one receptor rule in the mouse olfactory system. Trends in Genetics. 2004;20:648–53. doi: 10.1016/j.tig.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 43.Dando R, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. Adenosine enhances sweet taste through A2B receptors in the taste bud. Journal of Neuroscience. 2012;32:322–30. doi: 10.1523/JNEUROSCI.4070-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, et al. A transient receptor potential channel expressed in taste receptor cells. Nature Neuroscience. 2002;5:1169–76. doi: 10.1038/nn952. [DOI] [PubMed] [Google Scholar]
- 45.Liu D, Liman ER. Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:15160–5. doi: 10.1073/pnas.2334159100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Letters. 2006;580:239–44. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Huang YA, Roper SD. Intracellular Ca2+ and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. Journal of Physiology. 2010;588:2343–50. doi: 10.1113/jphysiol.2010.191106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Letters. 2004;572:65–8. doi: 10.1016/j.febslet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Ataullakhanov FI, Vitvitsky VM. What determines the intracellular ATP concentration. Bioscience Reports. 2002;22:501–11. doi: 10.1023/a:1022069718709. [DOI] [PubMed] [Google Scholar]
- 50.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–9. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 51.McLaughlin SK, McKinnon PJ, Margolskee RF. Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature. 1992;357:563–9. doi: 10.1038/357563a0. [DOI] [PubMed] [Google Scholar]
- 52.Borisy FF, Hwang PN, Ronnett GV, Snyder SH. High-affinity cAMP phosphodiesterase and adenosine localized in sensory organs. Brain Research. 1993;610:199–207. doi: 10.1016/0006-8993(93)91401-d. [DOI] [PubMed] [Google Scholar]
- 53.McLaughlin SK, McKinnon PJ, Spickofsky N, Danho W, Margolskee RF. Molecular cloning of G proteins and phosphodiesterases from rat taste cells. Physiology and Behavior. 1994;56:1157–64. doi: 10.1016/0031-9384(94)90360-3. [DOI] [PubMed] [Google Scholar]
- 54.Ruiz-Avila L, McLaughlin SK, Wildman D, McKinnon PJ, Robichon A, Spickofsky N, et al. Coupling of bitter receptor to phosphodiesterase through transducin in taste receptor cells. Nature. 1995;376:80–5. doi: 10.1038/376080a0. [DOI] [PubMed] [Google Scholar]
- 55.Clapp TR, Trubey KR, Vandenbeuch A, Stone LM, Margolskee RF, Chaudhari N, et al. Tonic activity of G alpha-gustducin regulates taste cell responsivity. FEBS Letters. 2008;582:3783–7. doi: 10.1016/j.febslet.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lyall V, Alam RI, Phan DQ, Ereso GL, Phan TH, Malik SA, et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. American Journal of Physiology – Cell Physiology. 2001;281:C1005–13. doi: 10.1152/ajpcell.2001.281.3.C1005. [DOI] [PubMed] [Google Scholar]
- 57.Richter TA, Dvoryanchikov GA, Chaudhari N, Roper SD. Acid-sensitive two-pore domain potassium (K2P) channels in mouse taste buds. Journal of Neurophysiology. 2004;92:1928–36. doi: 10.1152/jn.00273.2004. [DOI] [PubMed] [Google Scholar]
- 58.Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22320–5. doi: 10.1073/pnas.1013664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vandenbeuch A, Zorec R, Kinnamon SC. Capacitance measurements of regulated exocytosis in mouse taste cells. Journal of Neuroscience. 2010;30:14695–701. doi: 10.1523/JNEUROSCI.1570-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–5. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 61.Chandrashekar J, Kuhn C, Oka Y, Yarmolinsky DA, Hummler E, Ryba NJ, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vandenbeuch A, Clapp TR, Kinnamon SC. Amiloride-sensitive channels in type I fungiform taste cells in mouse. BMC Neuroscience. 2008;9:1. doi: 10.1186/1471-2202-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bo X, Alavi A, Xiang Z, Oglesby I, Ford A, Burnstock G. Localization of ATP-gated P2X2 and P2X3 receptor immunoreactive nerves in rat taste buds. Neuroreport. 1999;10:1107–11. doi: 10.1097/00001756-199904060-00037. [DOI] [PubMed] [Google Scholar]
- 64.Cheung KK, Burnstock G. Localization of P2X3 receptors and coexpression with P2X2 receptors during rat embryonic neurogenesis. Journal of Comparative Neurology. 2002;443:368–82. doi: 10.1002/cne.10123. [DOI] [PubMed] [Google Scholar]
- 65.Murata Y, Yasuo T, Yoshida R, Obata K, Yanagawa Y, Margolskee RF, et al. Action potential-enhanced ATP release from taste cells through hemichannels. Journal of Neurophysiology. 2010;104:896–901. doi: 10.1152/jn.00414.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang YA, Stone LM, Pereira E, Yang R, Kinnamon JC, Dvoryanchikov G, et al. Knocking out P2X receptors reduces transmitter secretion in taste buds. Journal of Neuroscience. 2011;31:13654–61. doi: 10.1523/JNEUROSCI.3356-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang M, Piskuric NA, Vollmer C, Nurse CA. P2Y2 receptor activation opens pannexin-1 channels in rat carotid body type II cells: potential role in amplifying the neurotransmitter ATP. Journal of Physiology. 2012;590:4335–50. doi: 10.1113/jphysiol.2012.236265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Custer EE, Knott TK, Cuadra AE, Ortiz-Miranda S, Lemos JR. P2X purinergic receptor knockout mice reveal endogenous ATP modulation of both vasopressin and oxytocin release from the intact neurohypophysis. Journal of Neuroendocrinology. 2012;24:674–80. doi: 10.1111/j.1365-2826.2012.02299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. Journal of Neuroscience. 2009;29:13909–18. doi: 10.1523/JNEUROSCI.2351-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kataoka S, Baquero A, Yang D, Shultz N, Vandenbeuch A, Ravid K, et al. A2BR adenosine receptor modulates sweet taste in circumvallate taste buds. PLoS ONE. 2012;7:e30032. doi: 10.1371/journal.pone.0030032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dando R, Huang YA, Roper SD. Acetylcholine, released from taste buds during gustatory stimulation, enhances taste responses. Chemical Senses. 2010;35:A2. [Google Scholar]
- 72.Simon SA, Baggett HC. Identification of muscarinic acetylcholine receptors in isolated canine lingual epithelia via voltage clamp measurements. Archives of Oral Biology. 1992;37:685–90. doi: 10.1016/0003-9969(92)90072-g. [DOI] [PubMed] [Google Scholar]
- 73.Ogura T, Lin W. Acetylcholine and acetylcholine receptors in taste receptor cells. Chemical Senses. 2005;30(Suppl. 1):i41. doi: 10.1093/chemse/bjh103. [DOI] [PubMed] [Google Scholar]
- 74.Eguchi K, Ohtubo Y, Yoshii K. Functional expression of M3, a muscarinic acetylcholine receptor subtype, in taste bud cells of mouse fungiform papillae. Chemical Senses. 2008;33:47–55. doi: 10.1093/chemse/bjm065. [DOI] [PubMed] [Google Scholar]
- 75.Huang YJ, Maruyama Y, Lu KS, Pereira E, Plonsky I, Baur JE, et al. Mouse taste buds use serotonin as a neurotransmitter. Journal of Neuroscience. 2005;25:843–7. doi: 10.1523/JNEUROSCI.4446-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaya N, Shen T, Lu SG, Zhao FL, Herness S. A paracrine signaling role for serotonin in rat taste buds: expression and localization of serotonin receptor subtypes. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2004;286:R649–58. doi: 10.1152/ajpregu.00572.2003. [DOI] [PubMed] [Google Scholar]
- 77.Heath TP, Melichar JK, Nutt DJ, Donaldson LF. Human taste thresholds are modulated by serotonin and noradrenaline. Journal of Neuroscience. 2006;26:12664–71. doi: 10.1523/JNEUROSCI.3459-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mathes CM, Spector AC. The selective serotonin reuptake inhibitor paroxetine does not alter consummatory concentration-dependent licking of prototypical taste stimuli by rats. Chemical Senses. 2011;36:515–26. doi: 10.1093/chemse/bjr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Delay E, Torres G. Society for Neuroscience. 2012. Taste qualities of dried-bonito dashi in wild type and T1R1 KO mice; p. 174.111. [Google Scholar]
- 80.Takeda M. Uptake of 5-hydroxytryptophan by gustatory cells in the mouse taste bud. Archivum Histologicum Japonicum Nippon Soshikigaku Kiroku. 1977;40:243–50. doi: 10.1679/aohc1950.40.243. [DOI] [PubMed] [Google Scholar]
- 81.Esakov AI, Golubtsov KV, Solov’eva NA. Significance of serotonin in the activity of the taste receptor apparatus of the frog Rana temporaria. Zhurnal Evoliutsionnoi Biokhimii i Fiziologii. 1983;19:62–7. [PubMed] [Google Scholar]
- 82.Roper SD. Parallel processing in mammalian taste buds? Physiology and Behavior. 2009;97:604–8. doi: 10.1016/j.physbeh.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 84.Lee SB, Lee CH, Kim SN, Chung KM, Cho YK, Kim KN. Type II and III taste bud cells preferentially expressed kainate glutamate receptors in rats. Korean Journal of Physiology and Pharmacology. 2009;13:455–60. doi: 10.4196/kjpp.2009.13.6.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Caicedo A, Jafri MS, Roper SD. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. Journal of Neuroscience. 2000;20:7978–85. doi: 10.1523/JNEUROSCI.20-21-07978.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vandenbeuch A, Tizzano M, Anderson CB, Stone LM, Goldberg D, Kinnamon SC. Evidence for a role of glutamate as an efferent transmitter in taste buds. BMC Neuroscience. 2010;11:77. doi: 10.1186/1471-2202-11-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang YA, Grant J, Roper S. Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds. PLoS ONE. 2012;7:e30662. doi: 10.1371/journal.pone.0030662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Herness S, Zhao FL. The neuropeptides CCK and NPY and the changing view of cell-to-cell communication in the taste bud. Physiology and Behavior. 2009;97:581–91. doi: 10.1016/j.physbeh.2009.02.043. [DOI] [PubMed] [Google Scholar]
- 89.Yarmolinsky DA, Zuker CS, Ryba NJ. Common sense about taste: from mammals to insects. Cell. 2009;139:234–44. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Herness S, Zhao FL, Kaya N, Lu SG, Shen T, Sun XD. Adrenergic signalling between rat taste receptor cells. Journal of Physiology. 2002;543:601–14. doi: 10.1113/jphysiol.2002.020438. [DOI] [PMC free article] [PubMed] [Google Scholar]


