Abstract
Background
Previous studies examining the teratogenic effects of antiherpetic medications have found no associations for birth defects overall but have not examined the risk of specific birth defects.
Methods
The National Birth Defects Prevention Study ascertains population-based cases with birth defects and live-born controls without birth defects in ten states across the United States for the purpose of identifying potential teratogenic risk factors. Mothers of cases and controls are interviewed within two years of their estimated date of delivery about demographic, medical and behavioral factors before and during pregnancy. This analysis examined the possible association between use of antiherpetic medications (acyclovir, valacyclovir or famciclovir) during early pregnancy and gastroschisis, a birth defect of the abdominal wall.
Results
The mothers of 1.1% (n=10) of 941 gastroschisis cases and 0.3% (n=27) of 8339 controls reported antiherpetic medication use during the month before conception through the third month of pregnancy. The adjusted odds ratios for such use in relation to gastroschisis were 4.68 (95% confidence interval [1.65, 13.28]) and 4.68 [1.15, 19.03] among women with and without self-reported genital herpes, respectively, when compared to women without antiherpetic use or herpes. Among women reporting no antiherpetic medication use, the odds ratio for self-reported genital herpes in relation to gastroschisis was 3.00 [1.58, 5.68].
Conclusions
Our study raises the possibility of an increased risk of gastroschisis due to either antiherpetic medication use during early pregnancy or the underlying genital herpes infection for which it was indicated.
Gastroschisis is a birth defect in which abdominal contents are located outside of the body wall and can lead to serious neonatal and long-term morbidity.1,2 The pathogenesis of gastroschisis is not known but the critical susceptibility window occurs during the first trimester.3 The increasing prevalence of gastroschisis within the United States (US) 4-7 suggests environmental factors may be important causes of this disease.3,8 However, the aetiology of gastroschisis remains elusive.
Previous studies have found no evidence for an increased risk of birth defects overall due to antiherpetic medication use.9-13 However, in an exploratory analysis, one recent Danish study observed a strong but imprecise association between first trimester antiherpetic medication use and abdominal wall defects in the aggregate (prevalence odds ratio 3.58, 95% confidence interval [0.84, 15.27]).9 Antiherpetic medications are nucleoside analogues that disrupt DNA replication and pharmacokinetic studies have shown that acyclovir crosses the placenta,12,14,15 therefore it is possible that antiherpetic medications could affect embryogenesis, a process that involves rapid mitotic divisions and DNA replication.
We examined the association between antiherpetic medication use and the risk of gastroschisis in the National Birth Defects Prevention Study (NBDPS), which includes the largest sample of gastroschisis cases with maternal exposure information in the US.
METHODS
Since 1997, the NBDPS has been ascertaining population-based cases with birth defects and live-born controls without birth defects in 10 US states for the purpose of identifying potential teratogenic risk factors.16 Cases were identified from birth defect registries and were classified by clinical geneticists using standardized criteria.17 Controls were infants born without any major structural malformation and were selected randomly from the source population.
Within two years of their estimated date of delivery, mothers took part in a computer-assisted telephone interview about demographic, medical and behavioral factors during pregnancy and the three months before conception. Interviews from women with an estimated date of delivery before January 2008 were included in this analysis. Institutional review boards at the Centers for Disease Control and Prevention and at each participating center have approved the study.
Gastroschisis cases
Cases included fetuses/infants diagnosed with gastroschisis during physical examination, surgery, or autopsy, or by prenatal high resolution ultrasound with view of the umbilicus.18
Antiherpetic medication use
Women were asked about occurrences of and treatments for infections and sexually transmitted infections. Women who reported medication use were asked about timing and frequency of use. Antiherpetic medications included acyclovir, valacyclovir or famciclovir.
Covariates
Reports of “herpes” illnesses were assumed to be genital herpes; reports of “cold sores” or “oral herpes” were classified separately as oral herpes. A priori, genital herpes was considered a confounder in the analysis. Antiherpetic medication is also indicated for other types of infections, therefore any reports of these infections were investigated jointly as a potential confounder.11 Additional potential confounders included maternal factors previously associated with antiherpetic medication use and/or gastroschisis as well as reported infections and medication use during the first trimester.8
Regression Analysis
We estimated the odds ratios of antiherpetic medication use in relation to gastroschisis with logistic regression models. Antiherpetic medication use during early pregnancy (the month before conception through the third month of pregnancy) was the primary exposure. Analyses examined women with and without self-reported herpes using a common reference group of women without antiherpetic medication use or herpes.
Assessment of recall bias
We performed two sensitivity analyses to explore possible recall bias. First, we explored the risk of gastroschisis in relation to antiherpetic medication use exclusively outside of early pregnancy, when there is no biologically plausible effect on the development of gastroschisis. If this estimate was elevated, it could indicate the primary model’s effect estimate might be elevated due to recall bias. Second, we repeated the analysis between early pregnancy antiherpetic medication use and gastroschisis using as controls infants with malformations other than gastroschisis, assuming mothers’ recall accuracy would be similar for both groups.
RESULTS
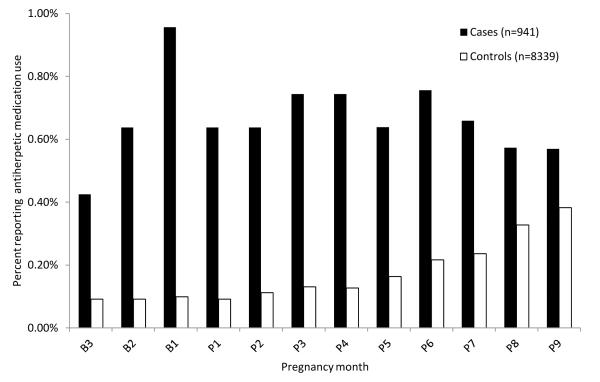
There were 941 gastroschisis cases and 8339 controls included in the analysis. Case mothers were more likely than controls to be young and to report periconceptional smoking, lower pre-pregnancy body mass index (BMI), illicit drug use and genital herpes (Table 1). Overall, use of antiherpetic medication during pregnancy and in the three months before conception was 1.5% (14/941) among case and 0.9% (71/8339) among control mothers. The monthly prevalence of antiherpetic medication use was higher among case than control mothers for all pregnancy months (Figure 1).
Table 1.
Maternal and pregnancy characteristics of gastroschisis cases and controls participating in the National Birth Defects Prevention study, 1997-2007
| Gastroschisis cases | (n=941) | Non-malformed controls (n=8339) |
||||||
|---|---|---|---|---|---|---|---|---|
| Maternal age (years) | < 25 n |
% | ≥ 25 n |
% | < 25 n |
% | ≥ 25 n |
% |
| TOTAL | 742 | 78.9 | 199 | 21.1 | 2772 | 33.2 | 5567 | 66.8 |
| Maternal age at delivery | ||||||||
| 13-19 years | 363 | 48.9 | 0 | 0.0 | 856 | 30.9 | 0 | 0.0 |
| 20-24 years | 379 | 51.1 | 0 | 0.0 | 1916 | 69.1 | 0 | 0.0 |
| 25-29 years | 0 | 0.0 | 136 | 68.3 | 0 | 0.0 | 2290 | 41.1 |
| 30-50 years | 0 | 0.0 | 63 | 31.7 | 0 | 0.0 | 3277 | 58.9 |
| Maternal race | ||||||||
| Non-Hispanic White | 361 | 48.7 | 122 | 61.3 | 1282 | 46.2 | 3664 | 65.8 |
| Non-Hispanic Black | 59 | 8.0 | 15 | 7.5 | 438 | 15.8 | 493 | 8.9 |
| Hispanic | 252 | 34.0 | 49 | 24.6 | 889 | 32.1 | 1032 | 18.5 |
| Asian/Pacific Islander | 26 | 3.5 | 4 | 2.0 | 40 | 1.4 | 205 | 3.7 |
| Native American | 7 | 0.9 | 0 | 0.0 | 18 | 0.6 | 24 | 0.4 |
| Other a | 37 | 5.0 | 9 | 4.5 | 105 | 3.8 | 147 | 2.6 |
| Body mass index (kg/m2) | ||||||||
|
Underweight (12 to
<18.5) |
73 | 9.8 | 7 | 3.5 | 229 | 8.3 | 204 | 3.7 |
| Normal (18.5 to <25) | 479 | 64.6 | 122 | 61.3 | 1331 | 48.0 | 2781 | 50.0 |
|
Overweight/obese
(≥25 ) |
170 | 22.9 | 65 | 32.7 | 1058 | 38.2 | 2395 | 43.0 |
| Missing | 20 | 2.7 | 5 | 2.5 | 154 | 5.6 | 187 | 3.4 |
| Periconceptional smoking | ||||||||
| Yes | 260 | 35.0 | 74 | 37.2 | 763 | 27.5 | 777 | 14.0 |
| No b | 482 | 65.0 | 125 | 62.8 | 2005 | 72.3 | 4789 | 86.0 |
|
Illicit drug use before or
during pregnancy |
||||||||
| Yes | 177 | 23.9 | 36 | 18.1 | 415 | 15.0 | 520 | 9.3 |
| No | 565 | 76.1 | 163 | 81.9 | 2357 | 85.0 | 5047 | 90.7 |
|
Self-reported genital
herpes |
||||||||
| Yes | 13 | 1.8 | 9 | 4.5 | 28 | 1.0 | 70 | 1.3 |
| No | 729 | 98.2 | 190 | 95.5 | 2744 | 99.0 | 5497 | 98.7 |
|
Other indicating
infections c |
||||||||
| Yes | 4 | 0.5 | 0 | 0.0 | 7 | 0.3 | 10 | 0.2 |
| No | 738 | 99.5 | 199 | 100 | 2765 | 99.7 | 5557 | 99.8 |
Includes 3 women missing maternal race information
Includes 5 women missing maternal smoking information
Includes oral herpes, mononucleosis and chicken pox/shingles
Figure 1.
Distribution of self-reported antiherpetic medication use among gastroschisis cases and controls by pregnancy month and standardized to age distribution in cases among participants of the National Birth Defects Prevention Study, 1997-2007
Abbreviations: “B”= months before conception, and “P”= months after conception
Antiherpetic medication exposure prevalence was 1.5% (14/941) among gastroschisis cases and 0.9% (71/8339) among controls.
Use of antiherpetic medication during early pregnancy was associated with an increased risk of gastroschisis among women with and without self-reported genital herpes (adjusted odds ratio [AOR] 4.68, 95% confidence interval [1.65, 13.28] and 4.68, [1.15, 19.03], respectively) when compared to women with neither genital herpes nor antiherpetic medication use (Table 2). Among women reporting no antiherpetic medication use, the AOR for self-reported genital herpes in relation to gastroschisis was 3.00 [1.58, 5.68]. Models were adjusted for maternal age and BMI; additional adjustment for other potential confounders, including other infections that might be treated with antiherpetics, did not substantially change estimates of association.
Table 2.
Multivariable logistic regression modeling of antiherpetic medication use on the risk of gastroschisis by pregnancy exposure window among participants of National Birth Defects Prevention Study, 1997-2007
|
| |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gastroschisis cases | Controls | Unadjusted | Adjusteda | ||||||
| Genital Herpes Antiherpetic use exposure windowb | n | % | n | % | OR | 95% CI | OR | 95% CI | |
|
| |||||||||
| All | All | 941 | 100.0 | 8339 | 100.0 | ||||
| No | No antiherpetic use | 911 | 96.8 | 8213 | 98.5 | Reference | |||
| No | Antiherpetic use during early pregnancy | 4 | 0.4 | 10 | 0.1 | 3.61 | [1.13, 11.53] | 4.68 | [1.15, 19.03] |
| No | Antiherpetic use exclusively outside of early pregnancy |
4 | 0.4 | 18 | 0.2 | 2.00 | [0.68, 5.93] | 2.69 | [0.81, 8.93] |
| Yes | No antiherpetic use | 16 | 1.7 | 55 | 0.7 | 2.62 | [1.50, 4.60] | 3.00 | [1.58, 5.68] |
| Yes | Antiherpetic use during early pregnancy | 6 | 0.6 | 17 | 0.2 | 3.18 | [1.25, 8.10] | 4.68 | [1.65, 13.28] |
| Yes | Antiherpetic use exclusively outside of early pregnancy |
0 | 0.0 | 26 | 0.3 | Not modeled | Not modeled | ||
The covariates for adjusted models included maternal age at delivery (13-19, 20-24, 25-29 [reference], 30-50 years) and BMI before conception (<18.5, 18.5-25 [reference], >25, missing)
Early pregnancy was defined as the month before conception through the third month of pregnancy.
Assessment of recall bias
Antiherpetic medication use exclusively outside of early pregnancy among women without genital herpes showed a possible elevated association with gastroschisis (AOR 2.69, [0.81, 8.93]); among women with genital herpes the OR for such use could not be calculated (0% among cases vs. 0.3% among controls). When the malformed controls were substituted for the non-malformed controls in the analysis, the association between early pregnancy antiherpetic medication use and gastroschisis remained elevated for both women with and without genital herpes (AOR 9.36 [3.50, 25.08] and 3.01 [0.96, 9.42], respectively) when compared to women with neither genital herpes nor antiherpetic medication use.
COMMENTS
Antiherpetic medication use during early pregnancy was uncommon but appeared to be associated with a four-fold increased risk for gastroschisis when compared to the risk among women reporting neither genital herpes nor antiherpetic medication use. Similar associations were observed for both women with and without self-reported genital herpes. That recall bias is unlikely to account for these association is supported by the fact that when the analysis was performed using malformed controls, risks remained elevated. It should be noted that antiherpetic medication use was rare in our study population (<1% prevalence), resulting in estimates of association that were imprecise and suggesting our study findings could be the result of random error. Notably, the elevated risk associated with early pregnancy antiherpetic mediation use was similar in magnitude to that associated with untreated genital herpes, suggesting that this underlying infection may be confounding the antiherpetic-gastroschisis association we observed.
Although several studies have examined the risk of birth defects overall in relation to antiherpetic medication during pregnancy and have found no association, no study has specifically focused on gastroschisis.9-13 Studies in rats have found teratogenic effects of high doses of acyclovir administered during organogenesis but no abdominal wall defects have been noted.19
Explanation for our findings
In an effort to disentangle the separate effects of antiherpetic medication use and genital herpes infection, we created mutually-exclusive categories with women who reported neither exposure as the comparison group. The elevated ORs for antiherpetic medication use and for genital herpes without such use could represent positive associations for both exposures. However, it is important to consider exposure measurement in this study. While reports of genital herpes were probably correct, women not reporting genital herpes may indeed have been diagnosed with genital herpes but did not report that in the interview. Women were not specifically asked about antiherpetic medication use or genital herpes in this study and both exposures were reported by smaller proportions of women (0.9% and 1.2%, respectively) than in another case-control birth defects study, the Slone Birth Defects Study20 (2.3% and 2.1%, respectively), which did specifically ask about medication use for genital herpes (based on an unpublished analysis by KAA and MMW). While 6% of women reported their antiherpetic medication use for indications other than genital herpes, 36% did not describe the infection treated, though it seems unlikely that an antiherpetic would be prescribed in the absence of an indication. With likely misclassification of both our primary exposure and its indication, assessment of their separate effects is further complicated. One possibility is that the medication use is a marker of underlying infection and the elevated ORs for such use represent an association with infection only. If this is the case, then the elevated OR for use outside the etiologic window might also represent an association with the herpes infection itself, rather than recall bias.
The NBDPS includes the largest sample of gastroschisis cases with maternal exposure information in the US, utilizes specific gastroschisis classification criteria, and collects information on a variety of maternal factors both before and during pregnancy. The results of our study suggest that antiherpetic medication use during pregnancy, or the infection for which it was indicated, may be associated with an increased risk of gastroschisis. This finding points to a possible infectious cause of gastroschisis and should direct future research into that arena.21 Specific information regarding antiherpetic medication use (including actual use patterns and indicating infection), genital herpes outbreak details, and serological testing for HSV types 1 and 2 and other herpes infections would allow for better classification of exposure and enhanced control for confounding by indication in future studies.
Acknowledgements
We thank the participants of the National Birth Defects Prevention Study. We also thank members of KAA’s doctoral committee, Kate Applebaum, Natasha Hochberg and Mike LaValley, and external reviewers, Kim Shea and Christina Chambers, for early review of the analysis. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Coding of drug information in the National Birth Defects Prevention Study used the Slone Drug Dictionary under license from the Slone Epidemiology Center of Boston University. This work was supported through cooperative agreements under PA 96043, PA 02081 and FOA DD09-001 from the Centers for Disease Control and Prevention to the Centers for Birth Defects Research and Prevention participating in the National Birth Defects Prevention Study. KAA’s work on this project was supported by NIH T32 HD052458 (Boston University Reproductive, Perinatal and Pediatric Epidemiology training program).
Footnotes
Details of contributors: KAA analyzed the data, prepared the initial draft and takes full responsibility for the accuracy of the data analysis. MMW conceived the project and guided the analysis and writing. MLF provided analytic support and expertise knowledge in drafting and revising the manuscript. AAM, MAC, and MTA reviewed the study design, analytic approach and revised the manuscript.
List of references
- 1.Chabra S, Gleason C. Gastroschisis: embryology, pathogenesis, epidemiology. NeoReviews. 2005;6(11):e493–499. [Google Scholar]
- 2.Swartz KR, Harrison MW, Campbell JR, Campbell TJ. Long-term follow-up of patients with gastroschisis. The American Journal of Surgery. 1986;151(5):546–549. doi: 10.1016/0002-9610(86)90540-4. [DOI] [PubMed] [Google Scholar]
- 3.Feldkamp M, Botto L. Developing a research and public health agenda for gastroschisis: How do we bridge the gap between what is known and what is not? American journal of medical genetics. Part C, Seminars in medical genetics. 2008;148C(3):155–161. doi: 10.1002/ajmg.c.30183. [DOI] [PubMed] [Google Scholar]
- 4.Roeper PJ, Harris J, Lee G, Neutra R. Secular rates and correlates for gastroschisis in California (1968- 1977) Teratology. 1987;35(2):203–210. doi: 10.1002/tera.1420350206. [DOI] [PubMed] [Google Scholar]
- 5.Castilla EE, Mastroiacovo P, Orioli IM. Gastroschisis: International epidemiology and public health perspectives. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2008;148C(3):162–179. doi: 10.1002/ajmg.c.30181. [DOI] [PubMed] [Google Scholar]
- 6.Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, Devine O, Petrini J, Ramadhani TA, Hobbs CA, Kirby RS. National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Research Part A: Clinical and Molecular Teratology. 2006;76(11):747–756. doi: 10.1002/bdra.20294. [DOI] [PubMed] [Google Scholar]
- 7.Parker SE, Mai CT, Canfield MA, Rickard R, Wang Y, Meyer RE, Anderson P, Mason CA, Collins JS, Kirby RS, Correa A, for the National Birth Defects Prevention N Updated national birth prevalence estimates for selected birth defects in the United States, 2004–2006. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88(12):1008–1016. doi: 10.1002/bdra.20735. [DOI] [PubMed] [Google Scholar]
- 8.Rasmussen S, Frías J. Non-genetic risk factors for gastroschisis. American journal of medical genetics. Part C, Seminars in medical genetics. 2008;148C(3):199–212. doi: 10.1002/ajmg.c.30175. [DOI] [PubMed] [Google Scholar]
- 9.Pasternak B, Hviid A. Use of acyclovir, valacyclovir, and famciclovir in the first trimester of pregnancy and the risk of birth defects. JAMA: the journal of the American Medical Association. 2010;304(8):859–866. doi: 10.1001/jama.2010.1206. [DOI] [PubMed] [Google Scholar]
- 10.Ratanajamit C, Skriver M, Jepsen P, Chongsuvivatwong V, Olsen J, Sørensen H. Adverse pregnancy outcome in women exposed to acyclovir during pregnancy: a population-based observational study. Scandinavian journal of infectious diseases. 2003;35(4):255–259. doi: 10.1080/00365540310000229. [DOI] [PubMed] [Google Scholar]
- 11.Reiff-Eldridge R, Heffner C, Ephross S, Tennis P, White A, Andrews E. Monitoring pregnancy outcomes after prenatal drug exposure through prospective pregnancy registries: a pharmaceutical company commitment. American journal of obstetrics and gynecology. 2000;182(1):159–163. doi: 10.1016/s0002-9378(00)70506-0. [DOI] [PubMed] [Google Scholar]
- 12.Stone K, Reiff-Eldridge R, White A, Cordero J, Brown Z, Alexander E, Andrews E. Pregnancy outcomes following systemic prenatal acyclovir exposure: Conclusions from the international acyclovir pregnancy registry, 1984-1999. Birth Defects Research Part A: Clinical and Molecular Teratology. 2004;70(4):201–207. doi: 10.1002/bdra.20013. [DOI] [PubMed] [Google Scholar]
- 13.Wilton L, Pearce G, Martin R, Mackay F, Mann R. The outcomes of pregnancy in women exposed to newly marketed drugs in general practice in England. BJOG: An International Journal of Obstetrics & Gynaecology. 1998;105(8):882–889. doi: 10.1111/j.1471-0528.1998.tb10234.x. [DOI] [PubMed] [Google Scholar]
- 14.Clercq E, Neyts J, Kräusslich H-G, Bartenschlager R. Handbook of Experimental Pharmacology. Vol. 189. Springer; Berlin Heidelberg: 2009. Antiviral Agents Acting as DNA or RNA Chain Terminators Antiviral Strategies; pp. 53–84. [DOI] [PubMed] [Google Scholar]
- 15.Brown Z. Antivirals during pregnancy. In: Sacks SL, Whitley RJ, Griffiths PD, editors. Clinical management of herpes viruses. IOS Press; 1995. p. 359. [Google Scholar]
- 16.Yoon P, Rasmussen S, Lynberg M, Moore C, Anderka M, Carmichael S, Costa P, Druschel C, Hobbs C, Romitti P. The National Birth Defects Prevention Study. Public Health Reports. 2001;116(Suppl 1):32–40. doi: 10.1093/phr/116.S1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen S, Olney R, Holmes L, Lin A, Keppler-Noreuil K, Moore C. Guidelines for case classification for the national birth defects prevention study. Birth Defects Research Part A: Clinical and Molecular Teratology. 2003;67(3):193–201. doi: 10.1002/bdra.10012. [DOI] [PubMed] [Google Scholar]
- 18.Moore C. Gastroschisis Classification. National Birth Defects Prevention Study. 2011 [Google Scholar]
- 19.Stahlmann R, Klug S, Lewandowski C, Bochert G, Chahoud I, Rahm U, Merker H, Neubert D. Prenatal toxicity of acyclovir in rats. Archives of toxicology. 1988;61(6):468–479. doi: 10.1007/BF00293693. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell AA, Gilboa SM, Werler MM, Kelley KE, Louik C, Hernández-Díaz S, Study NBDP Medication use during pregnancy, with particular focus on prescription drugs: 1976- 2008. Am J Obstet Gynecol. 2011;205(1):51.e1–8. doi: 10.1016/j.ajog.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werler M. Hypothesis: Could Epstein-Barr virus play a role in the development of gastroschisis? Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88(2):71–75. doi: 10.1002/bdra.20640. [DOI] [PubMed] [Google Scholar]