Abstract
Mitochondria are responsible for the production of ATP, which drives cellular metabolic and biosynthetic processes. This is the first study to quantify the mtDNA copy number across all stages of oogenesis in a large monovulatory species, it includes assessment of the activity of mitochondria in germinal vesicle (GV) and metaphase II (MII) oocytes through JC1 staining. Primordial to early antral follicles (n = 249) were isolated from the sheep ovarian cortex following digestion at 37°C for 1 h and all oocytes were disaggregated from their somatic cells. Germinal vesicle oocytes (n = 133) were aspirated from 3- to 5-mm diameter antral follicles, and mature MII oocytes (n = 71) were generated following in vitro maturation (IVM). The mtDNA copy number in each oocyte was quantified using real-time PCR and showed a progressive, but variable increase in the amount of mtDNA in oocytes from primordial follicles (605 ± 205, n = 8) to mature MII oocytes (744 633 ± 115 799, n = 13; P < 0.05). Mitochondrial activity (P > 0.05) was not altered during meiotic progression from GV to MII during IVM. The observed increase in the mtDNA copy number across oogenesis reflects the changing ATP demands needed to orchestrate cytoskeletal and cytoplasmic reorganization during oocyte growth and maturation and the need to fuel the resumption of meiosis in mature oocytes following the pre-ovulatory gonadotrophin surge.
Keywords: JC1, mitochondria activity, mitochondrial DNA, meiotic maturation, sheep oocyte
Introduction
Improving the developmental competence or quality of human oocytes is a priority if the efficiency and success of assisted reproduction technology is to be improved. Mitochondria are derived exclusively from the oocyte (Giles et al., 1980; Cummins et al., 1997) and their characteristics and relationship to oocyte quality are relevant to assisted conception. There is no mitochondrial DNA (mtDNA) replication until post-implantation (Spikings et al., 2007) and potentially, there is a minimum mtDNA threshold in the oocyte required to meet the demands of meiotic progression (Steuerwald et al., 2000; Zeng et al., 2007).
Early research to quantify the number of mitochondria in murine oocytes has been conducted using electron microscopic morphometry. The estimated average number of stored mitochondria in individual, mature oocytes from this species was found to be 92 500 ± 7000 (SD) (Piko and Matsumoto, 1976). Studies of pooled, bovine oocytes revealed an average of 260 000 mtDNA copies per developing gamete (Michaels et al., 1982). The introduction of alternative PCR-based methods were developed for human oocytes with original reports showing an average of 138 000 mtDNA copies in metaphase II (MII) oocytes (Chen et al., 1995). Studies to quantify the mtDNA copy number in oocytes have been built on previous evidence suggesting that oocytes usually contain only one copy of the genome (Piko and Matsumoto, 1976; Piko and Taylor, 1987). The literature contains a number of studies looking at the number of mitochondria during oogenesis (Piko and Taylor, 1987; Steuerwald et al., 2000; Barritt et al., 2002; Santos et al., 2006; Zeng et al., 2007). None of the previous reports however have characterized the accumulation of mitochondrial across all stages of oocyte development in the same species. Efforts to quantify the amount of mtDNA in oocytes using PCR-based methodologies have shown that the number of mitochondria in human MII oocytes can be highly variable with results ranging from 20 000 to over 800 000 (Steuerwald et al., 2000; Reynier et al., 2001; Barritt et al., 2002; Santos et al., 2006). The total length of ovine mitochondrial DNA is 16.6 kb, and it codes for 13 of the proteins responsible for oxidative phosphorylation (Hiendleder et al., 1998).
Mitochondrial activity can be measured by ratio-metric analysis using the fluorescent reporter 5,5,6,6-tetrachoro-1,1,3,3-tetraethyl-benzimidazoyl-carbocyanine iodide (JC1) (Reers et al., 1995; Jones et al., 2004; Van Blerkom and Davis, 2006). In this assay, the polarity of the mitochondrial membrane is a reflection of the organelles activity, and in the presence of JC1, less polarized mitochondria will fluoresce green demonstrating low levels of activity, whereas highly polarized mitochondria will fluoresce orange due to the formation of J-aggregates (Picton et al., 2010).
The changes in the distribution and activity of the mitochondria are recognized as being important for oocyte cytoplasmic maturation and developmental competence (Jansen and de Boer, 1998; Torner et al., 2004; Van Blerkom, 2004). Mitochondrial production of ATP within oocytes is necessary for all obligatory oxidative biosynthetic and metabolic processes including transcription and translation during growth and preparation for nuclear and cytoplasmic maturation (Naviaux and McGowan, 2000; Harris and Picton, 2007). The failure of fertilization and early embryonic development can be attributed to a deficiency in mitochondrial number or activity (Wai et al., 2010), specific mitochondrial defects (Barritt et al., 1999), chromosomal errors (Harris et al., 2010; Picton et al., 2010) and metabolic abnormalities (Picton et al., 2010). Moreover, chromosome non-disjunction events leading to chaotic mosaicism have been associated with low mitochondrial membrane potential and aberrant meiotic spindle formation (Wilding et al., 2002). Mitochondrial distribution and ATP content of bovine oocytes before and after in vitro maturation (IVM) correlate with oocyte morphology and developmental competence following IVF (Stojkovic et al., 2001). Aging has been reported to have a negative impact on mitochondriogenesis (Lopez-Lluch et al., 2008) and ATP-related meiotic errors (Schon et al., 2000). Finally, although highly controversial, ooplasmic transfer has been promoted as a treatment for women who have intrinsic defects of oocyte cytoplasm and/or mitochondria (Cohen et al., 1997; Barritt et al., 1999; Barritt et al., 2001).
Given the relevance of mtDNA copy number and activity to oocyte development and quality we have sought to use a sensitive real-time PCR protocol to quantify the number of mtDNA in discrete stages of oogenesis and to map the progressive changes in mitochondrial number in the developing gamete. Secondly, we have assessed the activity of mitochondria at the germinal vesicle (GV) and MII stages of oocyte development. Sheep oocytes were used as a physiologically relevant model for human oogenesis (Lundy et al., 1999; Trounson et al., 2001; Campbell et al., 2003).
Materials and Methods
Follicle dissection
All reagents used in this series of experiments were purchased from Sigma (Dorset, UK), unless otherwise stated. Abattoir-derived sheep ovaries were collected and washed three times in phosphate-buffered saline (PBS) supplemented with penicillin G (100 IU/ml), streptomycin sulphate (100 µg/ml) and amphotericin B (250 ng/ml), and maintained in Hepes-buffered minimum essential medium at 37°C until processing. All stages of follicle development from primordial follicles to early antral stages (n = 249) were isolated from the ovarian cortex following digestion in collagenase 1B (740 U/ml) and DNAse (8 kU/ml) at 37°C for 1 h as previously described (Newton et al., 1999; Huntriss et al., 2002). The stages of follicle development were classified as described previously (Oktay et al., 1997), and used by Huntriss et al. (2002). All oocytes were disaggregated from their somatic cells by repeat pipetting during exposure to trypsin (0.6 mg/ml). Germinal vesicle stage oocytes (n = 133) surrounded by three to four layers of tightly compact cumulus cells were aspirated from 3- to 5-mm antral follicles, and MII oocytes (n = 71) were derived after IVM in the serum-free alpha MEM that contained pre-ovulatory surge levels of ovine LH (100 ng/ml) and ovine FSH (100 ng/ml), bovine transferrin (5 µg/ml), sodium pyruvate (0.47 mM), sodium selenite (5 ng/ml), l-glutamax (3 mM), 0.1% (w/v) BSA, insulin (10 ng/ml) and long R3 IGF-1 (10 ng/ml) (Cotterill et al., 2012). Cumulus enclosed oocytes were matured for 24 h at 39°C in 6% CO2, 5% O2 and 89% N2 in a humidified atmosphere (Cotterill et al., 2012). Oocytes used for molecular analysis were denuded and snap-frozen in liquid nitrogen in 10 µl of Dulbecco's PBS and kept at −80°C until use (Table I).
Table I.
Quantification of mtDNA copy number in denuded GV oocytes derived from defined stages of ovine folliculogenesis.
| Follicle stage (oocyte maturity) | Oocyte diameter (µm) (oocyte volume µm3) | Total no. of oocytes analysed | mtDNA copy no. (no. of repeats) | Range |
|---|---|---|---|---|
| Primordial (20–30 µm) (GV) | 29.6 ± 1.0a (14 241 ± 1372a) | 27 | 605 ± 205a (n = 8) | 74–1627 |
| Early primary (31–40 µm) (GV) | 40.5 ± 1.2b (36 214 ± 3440b) | 17 | 779 ± 256a (n = 7) | 313–1974 |
| Primary (41–100 µm) (GV) | 51.2 ± 1.8c (74 171 ± 8340c) | 14 | 15 626 ± 6637b (n = 8) | 170–57 594 |
| Secondary (101–200 µm) (GV) | 69.4 ± 2.2d (183,032 ± 17 629d) | 22 | 13 990 ± 4351b,c (n = 8) | 3064–33 292 |
| Pre-antral (201–300 µm) (GV) | 96.6 ± 1.3e (476 318 ± 20 600e) | 24 | 71 133 ± 14 437c,d (n = 8) | 23 151–131 881 |
| Early antral (301–500 µm) (GV) | 118.4 ± 1.4f (873 729 ± 30 954f) | 69 | 543 632 ± 240 415d,e (n = 10) | 4649–1 983 222 |
| Graafian (3–5 mm) (GV) | 120.3 ± 1.9f (921 032 ± 44 130f) | 76 | 708 463 ± 95 052e (n = 16) | 143 210–1 358 988 |
Follicle diameter, volume and oocyte nuclear status associated with each classification are shown from 15 repeat analyses. Within each follicle stage the range of values obtained was used to demonstrate the variability in mtDNA copy number throughout oogenesis. Values presented are the means ± SEM per oocyte for the number of replicate analyses shown; statistical comparisons are confined within columns and different superscripts indicate significant differences (P > 0.05).
Fluorimetric analysis of mitochondrial activity
The mitochondrial activity of GV oocytes aspirated from 3- to 5-mm Graafian follicles (n = 57) and MII (n = 49) denuded oocytes was assessed using JC1 staining (Harris et al., 2010). The oocytes were exposed to JC1 (1 µg/ml), for 30 min at 37°C, and then washed with Hank's-buffered salt solution supplemented with 0.47 mM pyruvate and 1 mg/ml of bovine serum albumin (Picton et al., 2010). JC1 monomers (low mitochondria polarization/low membrane potential) were detected with a green filter (wavelength 520–527 nm), and JC1 aggregates (high mitochondria polarization/high membrane potential) detected with an orange filter (wavelength 590 nm), using a micro-photometry Zeiss Axiovert 200 fluorescence microscope and photomultiplier detection system (Photon Technology International, Ford, West Sussex, UK). The ratio of orange and green fluorescence was calculated using Felix 32 software (Photon Technology international) (Harris et al., 2010; Picton et al., 2010).
DNA extraction and standard preparation
To evaluate the extraction efficiency, a 1-µl spike containing a construct of bovine DNA and the pGEM T-easy vector, at a concentration of 0.1 ng/µl was added to 19 repeat samples. The primer sequences used to amplify the spiked sample were 5′ ctagtgattgtgcgggagaga 3′ (forward) and 5′ ctttgaaattggctggatgtg 3′ (reverse). A standard curve was used to calculate the amount of the spike sample that remained after DNA extraction. To lyse each oocyte sample, 5 µl of KOH (200 mM) was added and heated at 65°C for 10 min. Once complete, 5 µl of HCl (200 mM) was added to neutralize each sample giving a final total volume of 20 µl. A PCR product of 165 bp was constructed from the cytochrome c oxidase I (COI) region in the mitochondrial genome using the forward primer 5′ acgtcgatacacgggcttac 3′ and the reverse primer 5′ agcctccgactgtgaaaaga 3′ (accession number: AF010406.1). A standard reaction mixture was made up as follows: 1.25 µl PCR buffer, 0.75 µl Mg2+ (50 mM), 0.1 µl Taq Polymerase (5 U/µl) (All supplied by BIOTAQ Polymerase Kit, Bioline Ltd, London, UK), 1 µl of each primer (25 µM), 2 µl dNTP (1.25 mM) and 6.1 µl H2O. The PCR conditions included 95°C for 5 min for 1 cycle, 35 cycles at 94°C for 30 s, 60°C for 30 s and 72°C for 30 s, 72°C for 5 min and maintained at 4°C. Quantification and quality of the product was confirmed using a nanodrop ND 1000 spectrophotometer (Thermo-Scientific, Wilmington, USA), and 10-fold serial dilutions were made in preparation for the real-time PCR. 1 ng of the 165 bp product was calculated to contain 5.61 × 109 molecules of DNA. The standard curve used to quantify each experimental sample included 6- and 10-fold serial dilutions (0.1–0.000001 ng/µl) of the 165 bp product of mtDNA.
Quantification of mtDNA copy number using real-time PCR
Real-time PCR analysis was performed using an ABI7900HT PCR analyser, using SYBR green technology (Applied Biosystems, CA, USA). The total amount of mtDNA was quantified and compared across all stages of oogenesis. The primers that were used were the same as those designed for the 165-bp product to ensure that the annealing efficiency was consistent. The reaction mixture for the real-time PCR (25 µl total reaction), comprised 12.5 µl of SYBR green master mix (Applied Biosystems, CA, USA), 1.25 µl specific primer (500pM), 10.25 µl H2O and 1 µl of each sample. All samples were analysed twice in triplicate and the middle four values were used for statistical analysis. All real-time analysis included a negative (no template) control. Each PCR reaction included a denaturation step of 15 s at 95°C and annealing for 1 min at 60°C for 40 cycles. A melting curve was routinely included to assess the quality of each amplicon for mispriming.
Statistical analysis
The number of mitochondria per oocyte was calculated for each stage of follicle development. All statistical analyses were performed using Minitab 15.0 software (Minitab Ltd, Coventry, UK). Data were tested for normality by the Anderson–Darling test, and the data that were not normally distributed were log transformed prior to analysis. Oocyte data were compared using ANOVA and when the data showed significance, the contrasts between the means were specified using Fisher's post hoc test. Mitochondrial activity data were assessed using the χ2 test. The values presented are means ± SEM corrected per oocyte for the number of replicate analyses shown. In all analyses, the P values of <0.05 were considered to be statistically significant.
Results
Quantification of oocyte mitochondrial DNA copy number
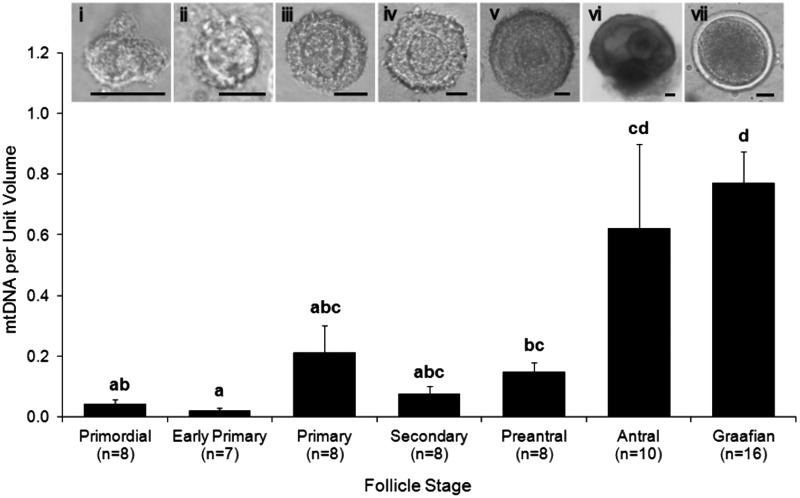
Analysis of mitochondrial DNA extraction efficiency yielded an average recovery of 99.7% over 19 repeat samples. The sample prior to extraction was calculated to contain 4.5 pg of spiked DNA (21 µl total volume) and the recovery of DNA after extraction was shown to be 4.5 pg ± 0.00031 pg. A total of 271 ovine oocytes were used for the experimental series. The average mtDNA copy number in oocytes increased with each progressive stage of follicle development from primordial to MII oocytes as shown in Table I. There was a significant increase (P < 0.05) in the oocyte mtDNA copy number from primordial follicles to primary follicles. Similarly, there were also significant differences (P < 0.05) between primary and pre-antral follicles, secondary to early antral follicles and pre-antral and Graafian follicles as follicle and oocyte development progressed (Table I). Considerable variability in the oocyte mtDNA copy number was apparent across all stages of folliculogenesis (Table I), with the greatest range of values being observed in early antral oocytes. When the oocyte mtDNA copy number from different staged follicles were compared on the basis of oocyte unit volume, there was a significant increase in mtDNA in oocytes from early antral and Graafian follicles compared with all other follicle stages (Fig. 1; P < 0.05). Comparison of the mtDNA copy number of in vivo grown GV oocytes with MII oocytes derived following IVM (Table II) (GV: 708 463 ± 95 052, n = 16 versus MII: 744 633 ± 115 799, n = 13), or mtDNA copy number per unit oocyte volume (GV: 0.77 ± 0.1, n = 16 versus MII: 0.47 ± 0.02, n = 16) showed no significant differences in either parameters between these two stages of nuclear maturity (P > 0.05).
Figure 1.
mtDNA copy number per unit volume in oocytes during discrete stages of folliculogenesis. Values are mean ± SEM for the number of replicate analyses shown; different letters indicate statistical significance (P < 0.05). The inserted images (i–vi) show the morphology of follicles isolated at primordial, early primary, primary, secondary, pre-antral and early antral stages of development, respectively. An example of a denuded GV oocyte from a Graafian follicle (vii) is also shown. Scale bars represent 300 m.
Table II.
In vitro matured MII oocytes.
| Oocyte maturity | Oocyte diameter (µm) (oocyte volume µm3) | Total no. of oocytes analysed | mtDNA copy no. (no. of repeats) | Range | Mt copy number per unit volume |
|---|---|---|---|---|---|
| MII | 121.0 ± 1.1 (930 233 ± 25 413) | 22 | 744 633 ± 115 799 (n = 13) | 25 059–1 348 657 | 0.47 |
Analysis of oocyte diameter and volume (Table II) highlighted the progressive and significant increase (P < 0.05) in both parameters at each follicle stage from primordial to early antral follicles. Furthermore, the majority of oocyte growth ceased following antral cavity formation so that neither the diameter nor the volume of oocytes from early antral follicles, GV oocytes from Graafian follicles or MII oocytes were significantly increased following antrum formation (P > 0.05).
Oocyte mitochondrial activity
The mitochondria from both in vivo-derived GV (n = 57) and in vitro-derived MII (n = 47) oocytes were exposed to JC1 to identify high potential, JC1-aggregates forming multimers that fluoresce orange in active mitochondria and green in inactive mitochondria (Harris et al., 2010). The ratio of both emissions is shown in Table III; lower values indicate increased mitochondrial activity. There was no significant difference (P > 0.05) between the mitochondrial activity measured between GV and MII oocytes after 24 h of IVM.
Table III.
Values for the ratio of JC1 are mean ± SEM of the number of oocytes used.
| Oocyte stage | n | JC1 ratio | SEM |
|---|---|---|---|
| GV | 57 | 12.78 | 0.93 |
| MII | 49 | 13.99 | 1.79 |
A lower JC1 ratio indicates increased mitochondrial activity. No significant differences were detected.
Discussion
The gradual accumulation of mitochondrial copy number and activity as oocyte development progresses is a prerequisite for the production of a fertile gamete capable of supporting embryogenesis. This study has quantified mtDNA across all stages of oogenesis and during the IVM of GV oocytes in a large monovulatory species with similar patterns of follicle development to humans (Lundy et al., 1999). The results revealed an average of 605 ± 205 mtDNA copies in oocytes obtained from primordial follicles and this number increased significantly (P < 0.05) to 708 463 ± 95 052 in GV oocytes and 744 633 ± 115 799 in in vitro matured MII oocytes. This represents over a thousand-fold increase in mtDNA copy number following growth activation of primordial oocytes to the completion of oocyte growth by the onset of antral cavity formation in the follicle (Picton et al., 1998). While the oocyte growth phase is clearly accompanied by mitochondrial replication, there is no change in oocyte nuclear status (Schultz et al., 1978). The observed 6-fold increase in mtDNA between oocytes from the secondary follicle and pre-antral stages (Table 1), and the significant increase (P < 0.05) between secondary follicle oocytes compared with the early antral stage (13 990 ± 4351 (n = 8) versus 543 632 ± 240 415 (n = 10), respectively), supports the increasing energy demands of the oocyte during antral cavity formation, as previously demonstrated in mice (Harris et al., 2007; Harris et al., 2009). During this time the oocyte is also involved in directing cumulus metabolism via GDF9 and BMP15 (Sugiura et al., 2005; Sugiura et al., 2007). There were no significant differences (P > 0.05) in mtDNA copy number between GV and IVM-derived MII oocytes. A similar lack of increase in mtDNA copy number from in vivo-derived GV and MII oocytes has also been shown in humans (Reynier et al., 2001; Barritt et al., 2002), further strengthening the suggestion that the majority of mtDNA replication has occurred by the completion of oocyte growth. Recent research in pigs showed in one experiment an average of 167 634 (GV) and 275 131 (MII) mtDNA copies and 185 004 (GV) and 239 392 (MII) copies in a second cohort of oocytes, demonstrating that the IVM of GV oocytes is not detrimental to mtDNA copy number (Mao et al., 2012). Research using cow oocytes (GV: 368 118 versus MII: 807 794) has shown a significant increase in mtDNA copy number after IVM (Iwata et al., 2011). The mitochondrial activity in our study during the GV to MII transition in vitro was not significantly different (P > 0.05) when assessed using the JC1 assay (Table III). This transition is known to be associated with the redistribution of mitochondria from a homogenous to heterogeneous pattern in the cytoplasm (Wilding et al., 2001; Van Blerkom, 2004; Martino et al., 2012). The redistribution of mitochondria to the subcortical and peri-nuclear regions in MII oocytes is correlated with an increase in ATP levels and has been shown to be critical for meiotic spindle formation and oocyte quality (Yu et al., 2010).
At the earliest stage of primordial follicle development in humans, estimates of oocyte mitochondrial numbers from electron micrographs (EM) have previously established a range between as little as 10 and 10 000 mtDNA copies per oocyte (Jansen and de Boer, 1998; Jansen, 2000). We have shown that the mtDNA copy number found in primordial oocytes using our fully quantitative method is 605 ± 205 (Table I). The majority of previous reports have quantified mtDNA copy number in only GV and MII oocytes. A semi-quantitative method (Chen et al., 1995) reported an average mtDNA content of 138 000 copies in human MII oocytes, which is lower than our findings in sheep. Unlike the present study, the previous reports did not factor in the efficiency of DNA extraction from each oocyte. Various studies have also attempted to quantify the mtDNA copy number using fully quantitative RT PCR methods (Steuerwald et al., 2000; Reynier et al., 2001; Barritt et al., 2002; Santos et al., 2006). In human studies, Santos et al. (2006) reported 163 698 mtDNA copies in MII human oocytes, Reynier et al. (2001) recorded 193 000 (range 20 000–598 000) and Barritt et al. (2002) reported an average of 795 534 mtDNA copies per mature human oocyte. In cows, 807 794 mtDNA copies in IVM-derived MII oocytes have been recorded (Iwata et al., 2011). In our study, in vitro-derived ovine MII oocytes yielded an mtDNA copy number of 744 633 ± 115 799, and is more consistent with the numbers found in human and cow oocytes reported by the latter two authors. The high oocyte variation in the mtDNA copy number is likely to account for this disparity. Also, the differences between the some published reports and the current data suggest either that sheep oocytes contain increased mtDNA, that the cohort of oocytes obtained from pre-pubertal and young adult sheep ovaries provide a more homogenous starting pool of oocytes, or that chronological age, or in vitro ageing affects mtDNA copy number. In support of the latter suggestion, previous human studies not only quantified mtDNA copy number on oocytes that had failed to undergo fertilization in vitro (Steuerwald et al., 2000; Reynier et al., 2001; Barritt et al., 2002) but also the oocytes were recovered from patients of ages ranging from 25 to 40. While one study showed no significant difference in the mtDNA copy number from women<35 to those >35 years of age (Barritt et al., 2002), recent bovine data support the notion that oocyte mtDNA copy number decreases with increasing maternal age (Iwata et al., 2011). Moreover, the suggestion that oocytes only contain one copy of the mitochondrial genome (Piko and Taylor, 1987) may require further verification. Unfortunately, the EM methods used to visualize and count actual numbers of mitochondria and the molecular methods used to quantify the mtDNA copy number are incompatible and therefore it is difficult to demonstrate equivalence in the same cell.
Sheep oocytes were used as a physiologically relevant model of human oocyte development in the present studies as ovine oogenesis is a protracted process (Campbell et al., 2003), sheep are predominantly monovular and follicle population dynamics of the ovine ovary parallels that in humans (Lundy et al., 1999). Also, the mature size of ovine oocytes is ∼120 µm in diameter as reported here (Table I), and is equivalent to that of human oocytes (Trounson et al., 2001). Furthermore, it was noted that oocyte growth ceased around the early antral follicle stage, and was not significantly different (P < 0.05) from GV and MII oocytes. There are however, acknowledged differences in the triglyceride content of ruminant and human oocytes (Ferguson and Leese, 2006). No significant changes (P > 0.05) were recorded in the mitochondrial activity during the IVM of GV oocytes and the ratio of active-to-inactive mitochondria remained constant throughout this period. The mitochondrial activity patterns reported in the present studies mirror those found in human GV and MII oocytes (Wilding et al., 2001; Harris et al., 2010). The JC1 staining strategy used here has previously been used to measure the mitochondrial activity in human and mouse GV and MII oocytes and early embryos (Van Blerkom et al., 2002; Van Blerkom et al., 2003; Van Blerkom and Davis, 2006; Harris et al., 2010; Picton et al., 2010). The present results for ovine oocyte mitochondrial activity correspond to previous murine data that has shown no significant correlation between mitochondrial activity and developmental competence of in vivo-derived MII oocytes (Van Blerkom et al., 2003). Reports in cows (Stojkovic et al., 2001) and pigs (Brevini et al., 2005) have shown that although activity remains unchanged, there are significant increases in ATP production during IVM.
The variable mtDNA copy number observed between cohorts of oocytes across all stages of ovine folliculogenesis in the present study (Table I) corresponds to the variability in mitochondrial DNA copy number previously recorded in humans (Steuerwald et al., 2000; Reynier et al., 2001; Barritt et al., 2002). It has been suggested that the biological variation in mtDNA in human oocytes is indicative of oocyte quality and attainment of a threshold of mitochondrial activity and/or ATP production necessary for fertilization and embryogenesis, such that oocytes containing a mtDNA copy number that falls below this arbitrary threshold are developmentally compromised (Reynier et al., 2001; Barritt et al., 2002; Santos et al., 2006; Zeng et al., 2007). While the validity of a mitochondrial threshold theory of oocyte quality remains to be proved, two reports in human oocytes (Santos et al., 2006; Zeng et al., 2007) suggest that the amount of mtDNA in fertile human oocytes is greater than in those that failed to undergo fertilization in vitro.
In summary, this work has shown that mtDNA copy number increases throughout ovine oogenesis, and that dramatic changes in mtDNA characterize the landmark events of oogenesis associated with primordial follicle growth activation, follicular antrum formation and Graafian follicle development. It is probable that the observed dramatic increase in the mtDNA copy number is essential to facilitate the production of sufficient ATP to orchestrate cytoskeletal and cytoplasmic reorganization in oocytes in preparation for the resumption of meiosis and the production of a fertile gamete.
Authors' roles
M.C.: experimental work including mtDNA copy number analysis, follicle isolation, data analysis and manuscript preparation. S.E.H.: study design and experimental work. E.C.F.: experimental work and mitochondrial activity assistance. J.D.H. and J.L.: experimental work. B.K.C.: co-applicant of grant that funded study. H.M.P.: research lead, grant holder, manuscript preparation.
Funding
This work was supported by the UK Medical Research Council Grant Reference Number G0800250. Esther Collado Fernandez was in receipt of a PhD studentship from the Infertility Research Trust. Funding to pay the Open Access publication charges for this article was provided by the UK Research Councils.
Conflict of interest
None declared.
References
- Barritt JA, Brenner CA, Cohen J, Matt DW. Mitochondrial DNA rearrangements in human oocytes and embryos. Mol Human Reprod. 1999;5:927–933. doi: 10.1093/molehr/5.10.927. [DOI] [PubMed] [Google Scholar]
- Barritt JA, Brenner CA, Malter HE, Cohen J. Mitochondria in human offspring derived from ooplasmic transplantation. Hum Reprod. 2001;16:513–516. doi: 10.1093/humrep/16.3.513. [DOI] [PubMed] [Google Scholar]
- Barritt JA, Kokot M, Cohen J, Steuerwald N, Brenner CA. Quantification of human ooplasmic mitochondria. Reprod Biomed Online. 2002;4:243–247. doi: 10.1016/s1472-6483(10)61813-5. [DOI] [PubMed] [Google Scholar]
- Brevini TAL, Vassena R, Francisci C, Gandolfi F. Role of adenosine triphosphate, active mitochondria, and microtubules in the acquisition of developmental competence of parthenogenetically activated pig oocytes. Biol Reprod. 2005;72:1218–1223. doi: 10.1095/biolreprod.104.038141. [DOI] [PubMed] [Google Scholar]
- Campbell BK, Souza C, Gong J, Webb R, Kendall N, Marsters P, Robinson G, Mitchell A, Telfer EE, Baird DT. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reproduction (Cambridge, England) Supplement. 2003;61:429–443. [PubMed] [Google Scholar]
- Chen X, Prosser R, Simonetti S, Sadlock J, Jagiello G, Schon EA. Rearranged mitochondrial genomes are present in human oocytes. Am J Hum Genet. 1995;57:239–247. [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Scott R, Schimmel T, Levron J, Willadsen S. Birth of infant after transfer of anucleate donor oocyte cytoplasm into recipient eggs. Lancet. 1997;350:186–187. doi: 10.1016/S0140-6736(05)62353-7. [DOI] [PubMed] [Google Scholar]
- Cotterill M, Catt SL, Picton HM. Characterisation of the cellular and molecular responses of ovine oocytes and their supporting somatic cells to pre-ovulatory levels of LH and FSH during in vitro maturation. Reproduction. 2012;144:195–207. doi: 10.1530/REP-12-0031. [DOI] [PubMed] [Google Scholar]
- Cummins JM, Wakayama T, Yanagimachi R. Fate of microinjected sperm components in the mouse oocyte and embryo. Zygote. 1997;5:301–308. doi: 10.1017/s0967199400003889. [DOI] [PubMed] [Google Scholar]
- Ferguson EM, Leese HJ. A potential role for triglyceride as an energy source during bovine oocyte maturation and early embryo development. Mol Reprod Dev. 2006;73:1195–1201. doi: 10.1002/mrd.20494. [DOI] [PubMed] [Google Scholar]
- Giles RE, Blanc H, Cann HM, Wallace DC. Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci USA. 1980;77:6715–6719. doi: 10.1073/pnas.77.11.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Picton HM. Metabolism of follicles and oocytes during growth and maturation. In: Tan SL, Chian RC, Bucket W, editors. In Vitro Maturation of Human Oocytes: Basic Science to Clinical Application. Abingdon: Informa UK Ltd; 2007. pp. 15–36. [Google Scholar]
- Harris SE, Adriaens I, Leese HJ, Gosden RG, Picton HM. Carbohydrate metabolism by murine ovarian follicles and oocytes grown in vitro. Reproduction. 2007;134:415–424. doi: 10.1530/REP-07-0061. [DOI] [PubMed] [Google Scholar]
- Harris SE, Leese HJ, Gosden RG, Picton HM. Pyruvate and oxygen consumption throughout the growth and development of murine oocytes. Mol Reprod Dev. 2009;76:231–238. doi: 10.1002/mrd.20945. [DOI] [PubMed] [Google Scholar]
- Harris SE, Maruthini D, Tang T, Balen AH, Picton HM. Metabolism and karyotype analysis of oocytes from patients with polycystic ovary syndrome. Hum Reprod. 2010;25:2305–2315. doi: 10.1093/humrep/deq181. [DOI] [PubMed] [Google Scholar]
- Hiendleder S, Lewalski H, Wassmuth R, Janke A. The complete mitochondrial DNA sequence of the domestic sheep (Ovis aries) and comparison with the other major ovine haplotype. J Mol Evol. 1998;47:441–448. doi: 10.1007/pl00006401. [DOI] [PubMed] [Google Scholar]
- Huntriss J, Gosden R, Hinkins M, Oliver B, Miller D, Rutherford AJ, Picton HM. Isolation, characterization and expression of the human factor in the germline alpha (FIGLA) gene in ovarian follicles and oocytes. Mol Human Reprod. 2002;8:1087–1095. doi: 10.1093/molehr/8.12.1087. [DOI] [PubMed] [Google Scholar]
- Iwata H, Goto H, Tanaka H, Sakaguchi Y, Kimura K, Kuwayama T, Monji Y. Effect of maternal age on mitochondrial DNA copy number, ATP content and IVF outcome of bovine oocytes. Reprod Fertil Dev. 2011;23:424–432. doi: 10.1071/RD10133. [DOI] [PubMed] [Google Scholar]
- Jansen RP. Germline passage of mitochondria: quantitative considerations and possible embryological sequelae. Hum Reprod. 2000;15(Suppl. 2):112–128. doi: 10.1093/humrep/15.suppl_2.112. [DOI] [PubMed] [Google Scholar]
- Jansen RPS, de Boer K. The bottleneck: mitochondrial imperatives in oogenesis and ovarian follicular fate. Mol Cell Endocrinol. 1998;145:81–88. doi: 10.1016/s0303-7207(98)00173-7. [DOI] [PubMed] [Google Scholar]
- Jones A, Van Blerkom J, Davis P, Toledo AA. Cryopreservation of metaphase II human oocytes effects mitochondrial membrane potential: implications for developmental competence. Hum Reprod. 2004;19:1861–1866. doi: 10.1093/humrep/deh313. [DOI] [PubMed] [Google Scholar]
- Lopez-Lluch G, Irusta PM, Navas P, de Cabo R. Mitochondrial biogenesis and healthy aging. Exp Gerontol. 2008;43:813–819. doi: 10.1016/j.exger.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundy T, Smith P, O'Connell A, Hudson NL, McNatty KP. Populations of granulosa cells in small follicles of the sheep ovary. J Reprod Fertil. 1999;115:251–262. doi: 10.1530/jrf.0.1150251. [DOI] [PubMed] [Google Scholar]
- Mao J, Whitworth KM, Spate LD, Walters EM, Zhao J, Prather RS. Regulation of oocyte mitochondrial DNA copy number by follicular fluid, EGF, and neuregulin 1 during in vitro maturation affects embryo development in pigs. Theriogenology. 2012;78:887–897. doi: 10.1016/j.theriogenology.2012.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino NA, Lacalandra GM, Filioli Uranio M, Ambruosi B, Caira M, Silvestre F, Pizzi F, Desantis S, Accogli G, Dell'Aquila ME. Oocyte mitochondrial bioenergy potential and oxidative stress: within-/between-subject, in vivo versus in vitro maturation, and age-related variations in a sheep model. Fertil Steril. 2012;97:720–728 e1. doi: 10.1016/j.fertnstert.2011.12.014. [DOI] [PubMed] [Google Scholar]
- Michaels GS, Hauswirth WW, Laipis PJ. Mitochondrial DNA copy number in bovine oocytes and somatic cells. Dev Biol. 1982;94:246–251. doi: 10.1016/0012-1606(82)90088-4. [DOI] [PubMed] [Google Scholar]
- Naviaux RK, McGowan KA. Organismal effects of mitochondrial dysfunction. Hum Reprod. 2000;15(Suppl. 2):44–56. doi: 10.1093/humrep/15.suppl_2.44. [DOI] [PubMed] [Google Scholar]
- Newton H, Picton H, Gosden RG. In vitro growth of oocyte-granulosa cell complexes isolated from cryopreserved ovine tissue. J Reprod Fertil. 1999;115:141–150. doi: 10.1530/jrf.0.1150141. [DOI] [PubMed] [Google Scholar]
- Oktay K, Briggs D, Gosden RG. Ontogeny of follicle-stimulating hormone receptor gene expression in isolated human ovarian follicles. J Clin Endocrinol Metab. 1997;82:3748–3751. doi: 10.1210/jcem.82.11.4346. [DOI] [PubMed] [Google Scholar]
- Picton H, Briggs D, Gosden R. The molecular basis of oocyte growth and development. Mol Cell Endocrinol. 1998;145:27–37. doi: 10.1016/s0303-7207(98)00166-x. [DOI] [PubMed] [Google Scholar]
- Picton HM, Elder K, Houghton FD, Hawkhead JA, Rutherford AJ, Hogg JE, Leese HJ, Harris SE. Association between amino acid turnover and chromosome aneuploidy during human preimplantation embryo development in vitro. Mol Human Reprod. 2010;16:557–569. doi: 10.1093/molehr/gaq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piko L, Matsumoto L. Number of mitochondria and some properites of mitochondrial-DNA in mouse egg. Dev Biol. 1976;49:1–10. doi: 10.1016/0012-1606(76)90253-0. [DOI] [PubMed] [Google Scholar]
- Piko L, Taylor KD. Amounts of mitochondrial-DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol. 1987;123:364–374. doi: 10.1016/0012-1606(87)90395-2. [DOI] [PubMed] [Google Scholar]
- Reers M, Smiley ST, MottolaHartshorn C, Chen A, Lin M, Chen LB. Mitochondrial membrane potential monitored by JC-1 dye. Mitochond Biogen Genet A. 1995;260:406–417. doi: 10.1016/0076-6879(95)60154-6. [DOI] [PubMed] [Google Scholar]
- Reynier P, May-Panloup P, Chretien MF, Morgan CJ, Jean M, Savagner F, Barriere P, Malthiery Y. Mitochondrial DNA content affects the fertilizability of human oocytes. Mol Human Reprod. 2001;7:425–429. doi: 10.1093/molehr/7.5.425. [DOI] [PubMed] [Google Scholar]
- Santos TA, El Shourbagy S, St John JC. Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril. 2006;85:584–591. doi: 10.1016/j.fertnstert.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Schon EA, Kim SH, Ferreira JC, Magalhaes P, Grace M, Warburton D, Gross SJ. Chromosomal non-disjunction in human oocytes: is there a mitochondrial connection? Hum Reprod (Oxford, England). 2000;15(Suppl. 2):160–172. doi: 10.1093/humrep/15.suppl_2.160. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Lamarca MJ, Wassarman PM. Absolute rates of protein synthesis during meiotic maturation of mammalian oocytes in vitro. Proc Natl Acad Sci USA. 1978;75:4160–4164. doi: 10.1073/pnas.75.9.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spikings EC, Alderson J, John JCS. Regulated mitochondrial DNA replication during oocyte maturation is essential for successful porcine embryonic development. Biol Reprod. 2007;76:327–335. doi: 10.1095/biolreprod.106.054536. [DOI] [PubMed] [Google Scholar]
- Steuerwald N, Barritt JA, Adler R, Malter H, Schimmel T, Cohen J, Brenner CA. Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote. 2000;8:209–215. doi: 10.1017/s0967199400001003. [DOI] [PubMed] [Google Scholar]
- Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: Correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–909. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Pendola FL, Eppig JJ. Oocyte control of metabolic cooperativity between oocytes and companion granulosa cells: energy metabolism. Dev Biol. 2005;279:20–30. doi: 10.1016/j.ydbio.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O'Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ. Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in cumulus cells. Development. 2007;134:2593–2603. doi: 10.1242/dev.006882. [DOI] [PubMed] [Google Scholar]
- Torner H, Brussow KP, Alm H, Ratky J, Pohland R, Tuchscherer A, Kanitz W. Mitochondrial aggregation patterns and activity in porcine oocytes and apoptosis in surrounding cumulus cells depends on the stage of pre-ovulatory maturation. Theriogenology. 2004;61:1675–1689. doi: 10.1016/j.theriogenology.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Trounson A, Anderiesz C, Jones G. Maturation of human oocytes in vitro and their developmental competence. Reproduction. 2001;121:51–75. doi: 10.1530/rep.0.1210051. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J. Mitochondria in human oogenesis and preimplantation embryogenesis: engines of metabolism, ionic regulation and developmental competence. Reproduction. 2004;128:269–280. doi: 10.1530/rep.1.00240. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P. High-polarized (Delta Psi m(HIGH)) mitochondria are spatially polarized in human oocytes and early embryos in stable subplasmalemmal domains: developmental significance and the concept of vanguard mitochondria. Reprod Biomed Online. 2006;13:246–254. doi: 10.1016/s1472-6483(10)60622-0. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406. doi: 10.1093/humrep/17.2.393. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Alexander S. Inner mitochondrial membrane potential (Delta Psi m), cytoplasmic ATP content and free Ca2+ levels in metaphase II mouse oocytes. Hum Reprod. 2003;18:2429–2440. doi: 10.1093/humrep/deg466. [DOI] [PubMed] [Google Scholar]
- Wai T, Ao A, Zhang X, Cyr D, Dufort D, Shoubridge EA. The role of mitochondrial DNA copy number in mammalian fertility. Biol Reprod. 2010;83:52–62. doi: 10.1095/biolreprod.109.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilding M, Dale B, Marino M, di Matteo L, Alviggi C, Pisaturo ML, Lombardi L, De Placido G. Mitochondrial aggregation patterns and activity in human oocytes and preimplantation embryos. Hum Reprod. 2001;16:909–917. doi: 10.1093/humrep/16.5.909. [DOI] [PubMed] [Google Scholar]
- Wilding M, Fiorentino A, De Simone ML, Infante V, De Matteo L, Marino M, Dale B. Energy substrates, mitochondrial membrane potential and human preimplantation embryo division. Reprod Biomed Online. 2002;5:39–42. doi: 10.1016/s1472-6483(10)61595-7. [DOI] [PubMed] [Google Scholar]
- Yu Y, Dumollard R, Rossbach A, Lai FA, Swann K. Redistribution of mitochondria leads to bursts of ATP production during spontaneous mouse oocyte maturation. J Cell Physiol. 2010;224:672–680. doi: 10.1002/jcp.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng HT, Ren Z, Yeung WSB, Shu YM, Xu YW, Zhuang GL, Liang XY. Low mitochondrial DNA and ATP contents contribute to the absence of birefringent spindle imaged with PolScope in in vitro matured human oocytes. Hum Reprod. 2007;22:1681–1686. doi: 10.1093/humrep/dem070. [DOI] [PubMed] [Google Scholar]